Published online Mar 15, 2023. doi: 10.4251/wjgo.v15.i3.562
Peer-review started: October 1, 2022
First decision: January 9, 2023
Revised: January 15, 2023
Accepted: March 3, 2023
Article in press: March 3, 2023
Published online: March 15, 2023
Processing time: 164 Days and 3.6 Hours
Carcinosarcomas of the common bile duct (CBD) are an extremely rare finding in the clinical setting. Based on a review of 12 literatures, 3 cases had the imaging features of ossification. Carcinosarcomas are prone to distant metastasis, as they possess clinical features of both carcinoma and sarcoma, and generally have with a poor prognosis. Due to the small number of cases reported, clinical experience in the diagnosis and treatment of the disease is lacking.
The patient was a 75-year-old woman who had experienced recurrent chills with nausea and vomiting for 3 mo. Computed tomography, magnetic resonance imaging, endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography led to the diagnosis of malignant tumor of the CBD. The patient ultimately underwent cholecystectomy, CBD resection, and choledochojejunostomy. Postoperative pathological examination revealed carcinosarcoma of the CBD, and the latest follow-up showed that the patient is recovering well. Based on previous case reports, some carcinosarcoma has ossification characteristics in imaging. If it is misdiagnosed as biliary calculi, the use of laser lithotripsy in surgery may lead to tumor diffusion. Choledochoscopy and narrow band staining of mucosa are very important for diagnosis.
We herein present a rare case of carcinosarcomas of the CBD, we found the tumours may have imaging features of polypoid growth and ossification only when the sarcomal components are bone differentiation, while show soft tissue shadow when non bone differentiation. Confirmation of diagnosis depends greatly upon postoperative pathological examination and the adjuvant treatment has not been established, which leads to the poor prognosis.
Core Tip: Carcinosarcomas of the common bile duct are extremely rare. Polypoid growth and ossification in the tumor could be representative features in cases of the sarcomal components being differentiated from bone. If misdiagnosed as biliary calculi, the use of laser lithotripsy may lead to tumor diffusion. Our case showed soft tissue exclusively on imaging and was initially misdiagnosed as acute cholangitis, highlighting the critical dependence of diagnosis confirmation on postoperative pathological examination, which itself is important for establishment of adjuvant treatment and the consequent prognosis. Other critical investigations to avoid misdiagnosis are choledochoscopy and narrow band staining of mucosa.
- Citation: Yao Y, Xiang HG, Jin L, Xu M, Mao SY. Carcinosarcoma of common bile duct: A case report. World J Gastrointest Oncol 2023; 15(3): 562-570
- URL: https://www.wjgnet.com/1948-5204/full/v15/i3/562.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i3.562
Carcinosarcomas of the common bile duct (CBD) are an extremely rare finding in the clinical setting, as they are mixture of carcinoma and sarcoma. Polypoid growth and ossification in the tumor could be representative features of carcinosarcomas of the extrahepatic bile duct[1]. For the case discussed herein, the patient’s clinical presentation suggested recurrent cholangitis, and the follow-up examination showed no obvious ossification. Confirmation of diagnosis almost always depends on postoperative pathological examination so, currently, the main treatment is surgery. However, these tumors are prone to recurrence and distant metastasis, as they possess clinical features of both carcinoma and sarcoma.
A 75-year-old woman was admitted to our practice for recurrent chills with nausea and vomiting.
The patient’s symptoms had started 3 mo prior.
The patient was healthy in the past and did not disclose any long-term drug use, smoking or alcohol abuse, or any history of operations.
The patient did not disclose any family genetic or aggregation diseases.
Slight yellowing of skin and sclera, the abdomen is soft, without tenderness or obvious mass.
The patient’s liver function profile showed the following: Total bilirubin, 40.0 μmol/L (normal range: 0-17.1 μmol/L); direct bilirubin, 31.4 μmol/L (normal range: 0-5.1 μmol/L); aspartate aminotransferase, 99 IU/L (normal range: 0-40 IU/L); alanine aminotransferase, 85 IU/L (normal range: 0-40 IU/L); gamma-glutamyl transferase, 377 U/L (normal range: 6-71 U/L); and carbohydrate antigen 19-9 (CA19-9), 25.4 IU/mL (normal range: 0-37 IU/mL). The patient’s white blood cell count was 13.67 × 109/L [normal range: (3.5-9.5) × 109/L]; her neutrophils were 89.8% and her C-reactive protein level was 80.25 mg/L (normal range: 0-10 mg/L).
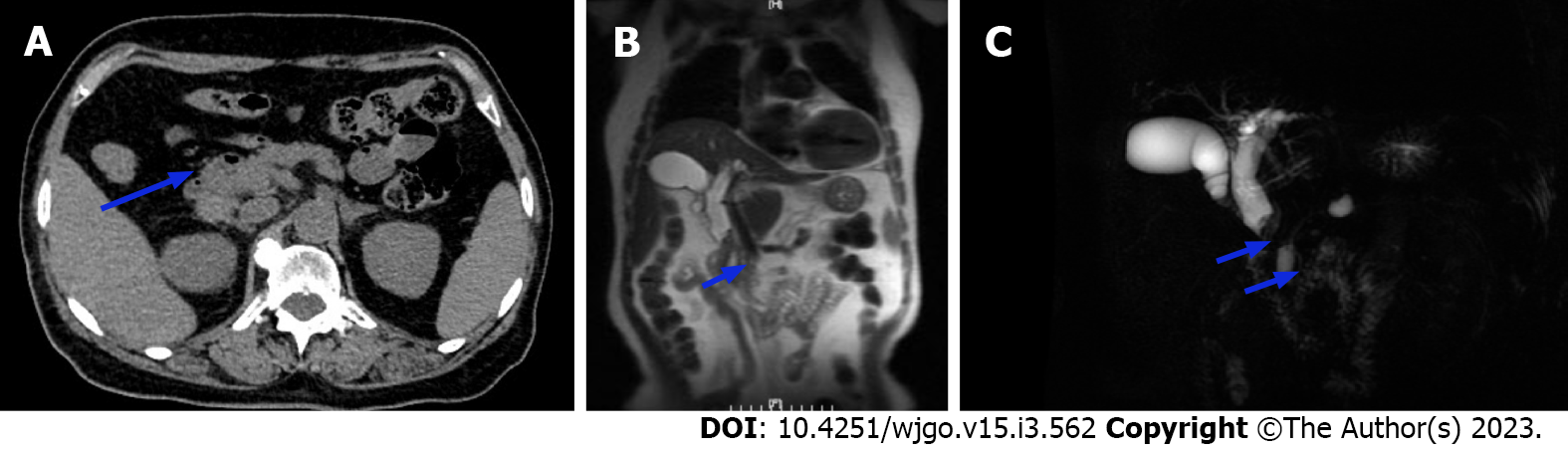
Computed tomography (CT) showed localized thickening with iso-low signal nodules in the middle part of the CBD, approximately 12 mm × 13 mm in size. The nodules were significantly enhanced heterogeneously. Magnetic resonance imaging (MRI) revealed dilation of intrahepatic and CBDs, soft tissue mass signal at the lower end of CBD, with rough edge, limited diffusion of dispersion weighting, low signal of apparent dispersion coefficient (Figure 1).
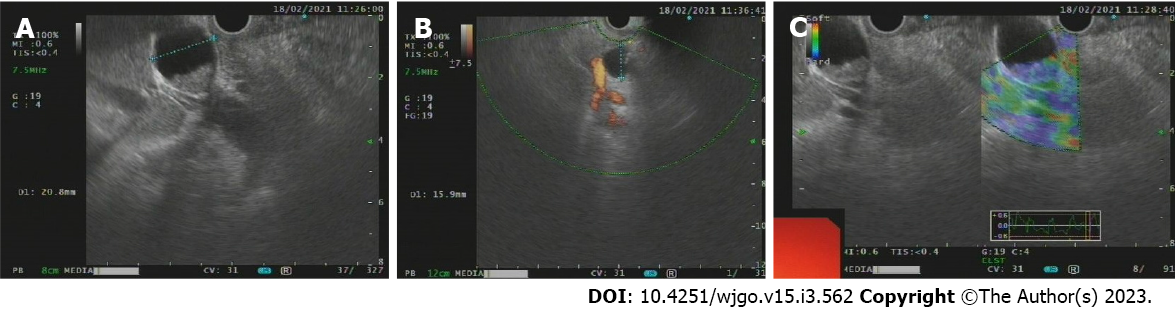
Endoscopic ultrasound (Figure 2) showed a solid occupancy in the middle part of the CBD with medium elasticity and imaging quality, partial compression of the portal vein, and an enlarged lymph node next to the CBD, approximately 12.9 mm in size. The intrahepatic bile duct was widened, to approximately 6 mm in diameter. We found a cystic occupancy in the neck of the pancreas, approximately 15.9 mm in size, with a clear border, no communication with the main pancreatic duct, and no significant dilatation of the pancreatic duct. Endoscopic retrograde cholangiopancreatography (ERCP) was recommended before reaching the final diagnosis (Figure 3).
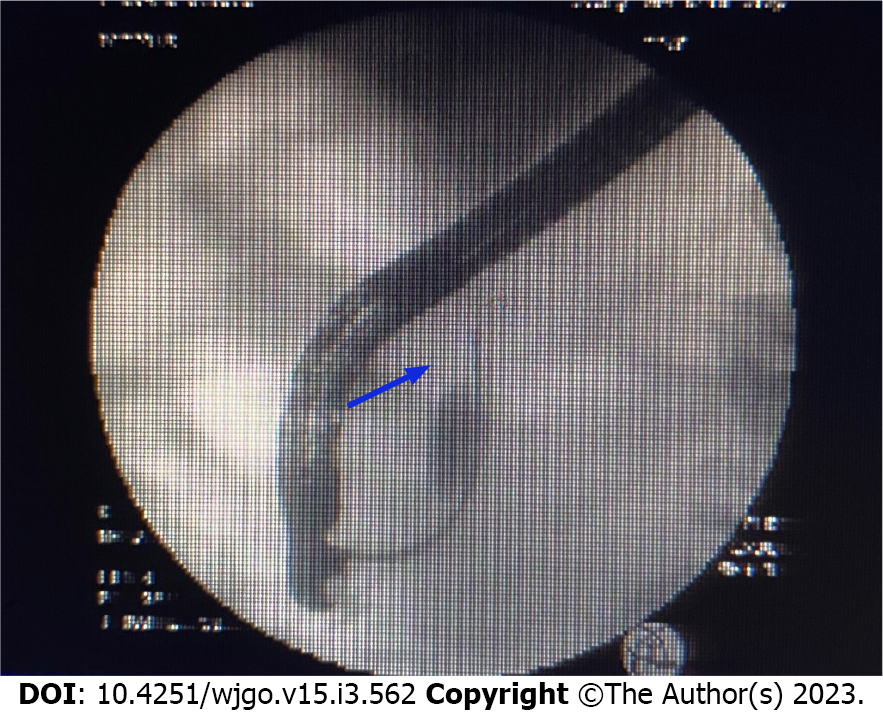
Bile duct biopsy was performed, and a 7.5 Fr × 7 cm bile duct plastic stent was implanted during ERCP. Postoperative bile duct biopsy revealed small fibrous tissue with inflammatory cell infiltration and individual glands, which was considered tumor marginal tissue. Immunohistochemistry (IHC) revealed the following findings: Ki-67 (+ 20%); P53 (+), CD68 (+); ALK (-); Des (-); CD34 (vascular +); vimentin (+); and S-100 (-). Therefore, intraoperative frozen sectioning was recommended.
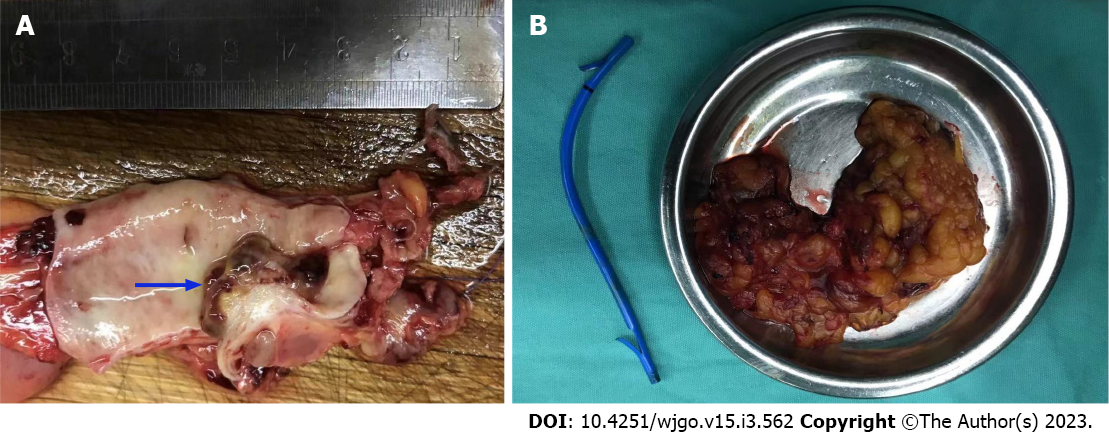
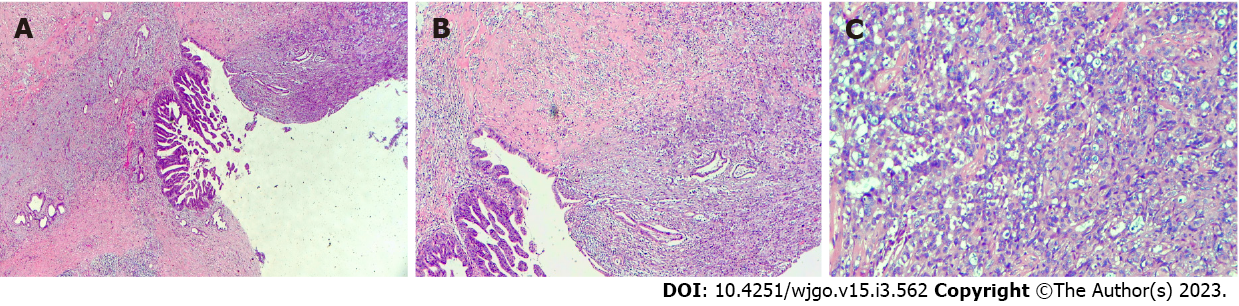
The patient was referred for general surgery. After general anesthesia, in the supine position, intraoperative exploration revealed a tumor located in the middle and lower part of the CBD (approximately 2 cm × 1.5 cm in size) with a hard texture and relatively limited, so cholecystectomy, CBD resection, and choledochojejunostomy were performed. The lesser omentum was cut at the lower edge of the liver, and the hepatoduodenal ligament and the lymph nodes near the common hepatic artery were cleaned. The gallbladder was removed and the hilar lymph nodes were cleaned. The common hepatic duct above the cystic duct was cut off and the lymph nodes around the CBD and behind the pancreatic head were cleaned. The CBD was cut 1 cm away from the edge of the tumor and the distal bile duct was ligated and sutured. The jejunum was cut 20 cm away from the flexor ligament, and end-to-side intermittent anastomosis was performed between the distal jejunum and the common hepatic duct. The jejunal stump was then closed. The distal jejunum (50 cm away from the anastomosis) was anastomosed side-to-side with the severed proximal jejunum (Roux-en-Y anastomosis with Ankang linear cutting stapler, Changzhou Ankang Medical Instrument Co., Ltd). Intraoperative frozen section revealed CBD mesenchymal tumor inclined to sarcoma with mild dysplasia of the CBD epithelium, negative upper and lower margins of CBD (Figure 4).
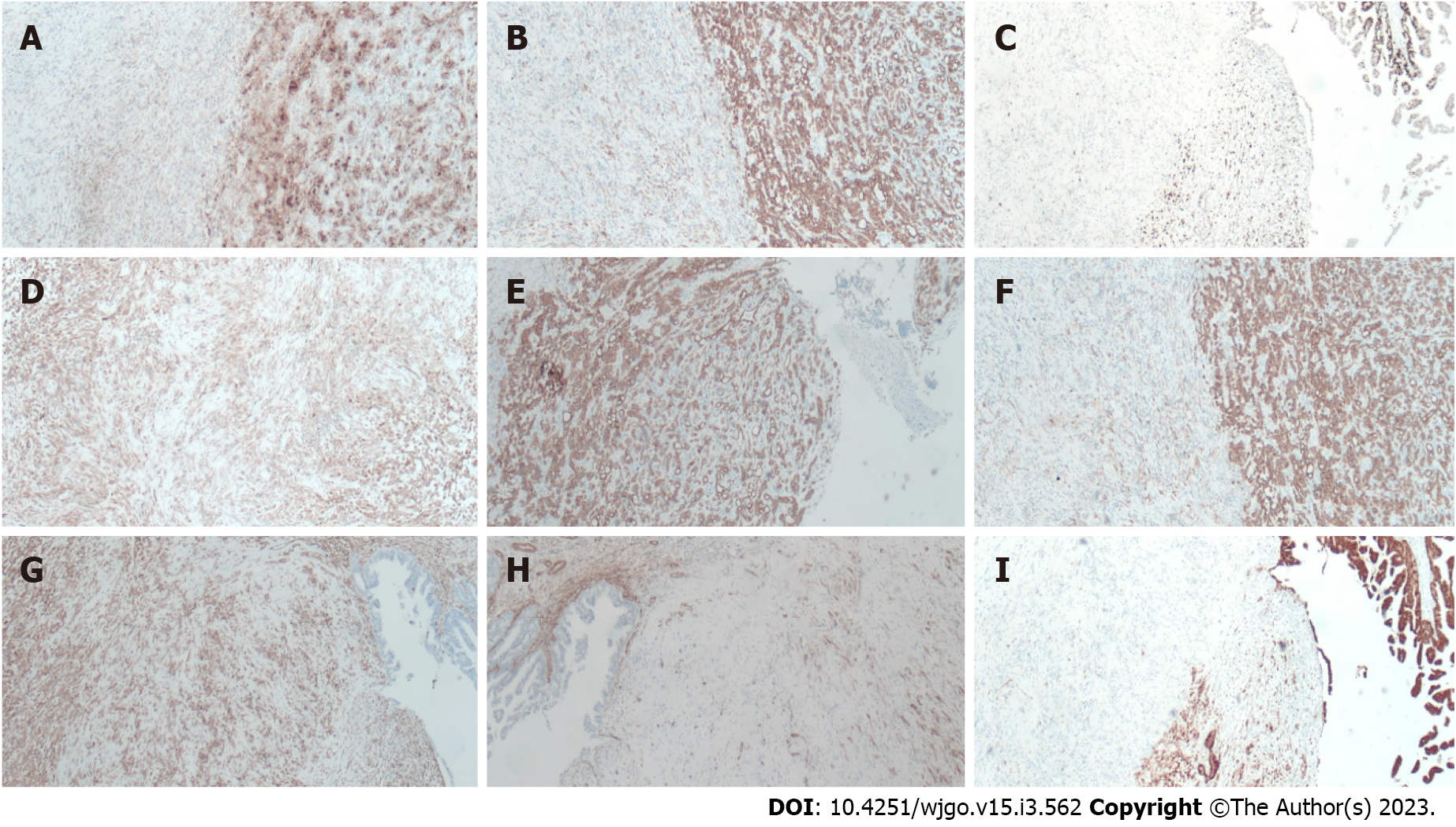
Postoperative pathological examination revealed carcinosarcoma of the CBD with a tumor volume of 1.0 cm × 0.6 cm × 0.6 cm. The tumor had invaded the muscular layer and did not involve the outer membrane. A small amount of pancreatic tissue was observed at the edge of the CBD with no tumor involvement. The upper and lower margins of the CBD were negative (Figure 5). IHC examination revealed the following: CK7 (+); CK19 (+); CK18 (+); Ki-67 (approximately 40%); carcinoembryonic antigen (+); CA19-9 (+); ventralis intermedius (+); s-100p (+); diethylstilbestrol (-); Calponin (+); spinal muscular atrophy (SMA) (-); and CKpan (+). Seven lymph nodes were detected in the twelfth and eighth groups, and no tumor metastasis was observed in any of the nodes (0/7) (Figure 6).
In this case, the postoperative pathology showed a negative margin and no clear lymph node metastasis. After consulting the literature, the patient was not given additional radiotherapy or chemotherapy. In the outpatient follow-up of 6 mo and telephone follow-up of 8 mo after the operation, the patient was in good condition.
Carcinosarcomas are rare malignant tumors consisting of a mixture of carcinoma and sarcoma, first named by Virchow (1864). According to the 1990 World Health Organization (WHO) histologic classification of tumors[2], a carcinosarcoma is defined as a neoplastic organism containing both carcinoma and sarcoma components with no detectable epithelial markers on the sarcoma component by IHC and a sarcoma component with a clear mesenchymal tissue cell origin. According to the 2006 WHO classification of tumors[3], carcinosarcoma is classified as a subset of sarcomatoid carcinoma and defined as a mixed tumor with cancer and differentiated sarcomatous components (e.g., malignant cartilage, bone, or rhabdomyosarcoma). The mechanism of its development is currently discussed in several ways: (1) Collision theory: Two independent malignancies colliding; (2) Combination theory: The tumor is thought to have a dual origin[4], wherein the cancer is derived from epithelial cells and the sarcoma from mesenchymal tissue, and that the stem cells develop into both cancer and sarcoma and infiltrate each other at the same location in the same organ to form a carcinosarcoma; (3) Conversion theory: The sarcomatous part is derived from the carcinomatous part via metaplastic transformation; and (4) Composition theory: The carcinoma drives a pseudosarcomatous stromal reaction[5]. The current study proposes an alternative hypothesis[6]: That carcinosarcomas are monoclonal tumors with heterologous differentiation, in which cancer and sarcoma components are derived from a subpopulation of epithelial stem cells that de-differentiate and express mesenchymal markers. Evidence supporting this hypothesis is the migratory transition between the carcinoma and heterologous mesenchymal components of carcinosarcoma, the maintenance of consistent genetic alterations, and the positivity of both epithelial markers. Molecular biology studies have shown that the cumulative effect of multiple oncogenic events, including genetic alterations and genetic instability, leads to tumor formation followed by epithelial-to-mesenchymal transition (EMT), resulting in a sarcomatoid transformation. The EMT pathway activation mechanism is currently considered to be closely associated with the development and progression of sarcomatoid carcinoma of the lung[7].
The pathological presentation of this case was carcinosarcoma, which is extremely rare in solid hepatic cholangiocarcinoma. Histologically, carcinosarcoma is characterized by the presence of a mixture of malignant epithelial and mesenchymal components, with no transition between carcinoma and sarcoma. The epithelial component is mainly adenocarcinoma, squamous carcinoma, or both; the mesenchymal components of the tumor include fibrosarcoma, smooth muscle sarcoma, rhabdomyosarcoma, angiosarcoma, osteosarcoma, and chondrosarcoma. The immunohistochemical markers such as CD34, CD68, SMA, etc. can be used to identify the components of sarcoma. Studies have shown that the most common malignant epithelial component is adenocarcinoma, followed by a mixed state of adenocarcinoma and squamous carcinoma, which is less common; the malignant mesenchymal component most commonly shows spindle cells, while osteoid cells are less common. The mesenchymal component can have both spindle cells and chondrocytes. In such cases, the differential diagnosis should be sarcomatoid carcinoma, which belongs to the epithelial-derived tumor and is essentially a poorly differentiated carcinoma. The morphology may be all single sarcomatoid spindle or pleomorphic cells, or it may have both carcinoma and sarcomatoid morphology, with a predominant sarcomatoid component. In typical cases, the carcinoma and sarcomatoid forms have a migratory transition; IHC staining of both carcinoma and sarcomatoid components reveals epithelial markers such as creatine kinase, and the sarcomatoid component also expresses mesenchymal markers such as vimentin, which indicates the migratory pattern between malignant epithelial cells and sarcomatoid cells typical of sarcomatoid carcinoma[8].
Carcinosarcomas occurring in the CBD are extremely rare. Through literature review, we found only 12 case reports (Table 1). Through these 12 cases[1,5,9-18], we found that the prevalence is consistent between male and female. Among them, 9 patients were more than 70 years old. The chief complaint of 8 patients was jaundice, and others were abdominal pain and liver dysfunction. Six cases were diagnosed as tumor before operation, three were diagnosed as CBD stone, and two were cholangitis. All 11 patients received surgical treatment, of which 6 patients died, with an average survival time of 8.3 mo. 2 patients survived, but the follow-up time was short, only 7 mo. 4 patients lost follow-up. In general, these cases indicate that the tumor can occur throughout the bile duct and has a poor prognosis; even after surgery, they are prone to local recurrence and liver or lung metastasis (the clinical features of both carcinomas and sarcomas).
| Ref. | Age/sex | Main complaints | Location | Preoperative diagnosis | Surgery | Outcome at time of last follow-up |
| Sasamoto et al[5], 2021 | 79/male | General malaise | Lower bile duct | Bile duct cancer | PD | Death, 26 mo |
| Moreno Moraleda et al[9], 2020 | 39/male | Jaundice | CBD | CBD cancer | NA | Death, 2 mo |
| Xie et al[1], 2020 | 76/male | Abdominal pain | Distal | CBD stone | Lap-C | NA |
| Usui et al[10], 2019 | 74/male | High γ-glutamyl transferase level | Right intrahepatic and CBD | Bile duct cancer | Right hepatectomy | NA |
| Lee et al[11], 2016 | 91/female | Liver dysfunction | Hilar | Bile duct cancer | Resection of the hilar cholangiocarcinoma | Death, 2 mo |
| Kumei et al[12]2015 | 73/female | Abdominal pain, jaundice | Middle | Bile duct cancer | SSPPD | Death, 6 mo |
| Hoshino et al[13], 2013 | 73/male | Abdominal pain, jaundice | NA | Cholangitis | PD | Death, 4 mo |
| Tanaka et al[14], 2012 | 71/male | Appetite loss, jaundice | Distal | Repeated cholangitis | PD | Death, 10 mo |
| Aurello et al[15], 2008 | 73/female | Jaundice | Distal | Intrapancreatic | PD | Alive, 7 mo |
| Xia and Xu[16], 2006 | 57/female | Abdominal pain, jaundice | Distal | CBD cancer | BDR | NA |
| Kadono et al[17]2005 | 75/female | Jaundice | Middle-distal | CBD stone | PD | Alive, 7 mo |
| Loud et al[18], 1997 | 35/female | Jaundice | Hilar | CBD stone | BDR | NA |
A recent report from China described the case of cholangiocarcinoma sarcoma who had been initially diagnosed with bile duct stones by B-ultrasound and had undergone a biliary stent implantation after failed ERCP lithotripsy[1]. During the subsequent laparoscopic choledochotomy, blood vessels were observed at the root of the stone, which prompted further investigation by narrow band imaging (NBI); histological staining confirmed the presence of blood vessels, supporting the possibility that the stone-like mass could actually be a tumor. The clinical care team, therefore, performed an open pancreaticoduodenectomy; the postoperative pathological and IHC findings confirmed a CBD carcinosarcoma. The authors concluded that polypoid growth and ossification are likely typical features of extrahepatic cholangiocarcinoma sarcomas. Such tumors can be easily misdiagnosed as a bile duct stone. Unfortunately, the well-established treatment of laser lithotripsy for stones can lead to undesirable events if targeting tumorous tissue (i.e., tumor spread). Thus, it is critical to understand this uncommon differential diagnosis between bile duct stones and carcinosarcomas, since their treatments are not interchangeable. Other critical investigations to avoid misdiagnosis are choledochoscopy and NBI.
The other two cases which were misdiagnosed by CT or B-ultrasound as CBD stone before operation were also diagnosed by CT or B-ultrasound. But our case and other cases with correct preoperative diagnosis showed only a soft tissue shadow on imaging. Therefore, we propose the possibility that carcinosarcoma only shows ossification when the sarcomal components involve malignant cartilage and/or are differentiated from bone.
Early diagnosis of cholangiocarcinoma sarcoma and sarcomatoid carcinoma is challenging due to few early clinical manifestations and unspecific imaging features. Confirmation of diagnosis largely depends on postoperative pathological examination. Regardless of whether the cancer is in an early-stage or has progressed to the stages of invasion and metastasis, pathological examination of carcinosarcomas should be performed using adequate and extensive sampling of the biopsy samples and should be supplemented with IHC staining to avoid misdiagnosis. If choledochoscopy is available, observation through NBI and performance of a biopsy under direct vision will be beneficial for clinical decision-making.
Carcinosarcomas of the CBD may have imaging features of polypoid growth and ossification only when the sarcomal components are bone differentiation, while show soft tissue shadow when non bone differentiation. Confirmation of diagnosis depends greatly upon postoperative pathological examination and the adjuvant treatment has not been established, which leads to the poor prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: El-Arabey AA, Egypt; Ker CG, Taiwan; Shekouhi R, Iran; Takemura N, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Xie JM, Li W, Chen W. Hepatobiliary and pancreatic: A case of stone-like carcinosarcoma of the common bile duct. J Gastroenterol Hepatol. 2020;35:711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Jass JR, Sobin LH, Watanabe H. The World Health Organization's histologic classification of gastrointestinal tumors. A commentary on the second edition. Cancer. 1990;66:2162-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J Thorac Oncol. 2015;10:1240-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 1095] [Article Influence: 109.5] [Reference Citation Analysis (0)] |
| 4. | Li YJ, Li WY, Wang J, Gao ZQ, Qi F, Jiang H. [Clinic features of laryngeal carcinosarcoma and sarcomatoid carcinoma]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017;52:385-387. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Sasamoto S, Aoki T, Tashiro Y, Matsuda K, Koizumi T, Kusano T, Wada Y, Shibata H, Tomioka K, Yamashita T, Date H, Ariyoshi T, Goto S, Yamazaki K, Fujimori A, Watanabe M, Enami Y, Otsuka K, Norose T, Ohike N, Yamochi T, Takimoto M, Murakami M. Experience of the pancreas duodenectomy for so-called carcinosarcoma of the common bile duct:a case report and review of literature. Int Cancer Conf J. 2021;10:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Zhang Y, Weinberg RA. Epithelial-to-mesenchymal transition in cancer: complexity and opportunities. Front Med. 2018;12:361-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 483] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 7. | Zhang Z, Li LH, Wang HX. Epithelial-mesenchymal transition in carcinosarcoma. Linchuang Yu Shiyan Bingli Xue Za Zhi. 2011;27:79-82. [DOI] [Full Text] |
| 8. | Ren YM, Hu WM. Clinicopathological analysis of two cases of sarcoma and sarcomatoid carcinoma of the gallbladder. Linchuang Yu Bingli Za Zhi. 2018;38:456-641. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Moreno Moraleda I, Delgado Maroto A, Barrientos Delgado A, López González J. Carcinosarcoma of the extrahepatic bile duct: an unusual cause of obstructive jaundice. Rev Esp Enferm Dig. 2021;113:298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Usui G, Hashimoto H, Kusakabe M, Shirota G, Sugiura Y, Fujita Y, Satou S, Harihara Y, Horiuchi H, Morikawa T. Intrahepatic Carcinosarcoma With Cholangiocarcinoma Elements and Prominent Bile Duct Spread. Int J Surg Pathol. 2019;27:900-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Lee SY, Shia J, Kingham TP, Jarnagin WR. Carcinosarcoma of the bile duct: a case report and review of literature. Hepatobiliary Surg Nutr. 2016;5:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Kumei S, Onishi Y, Ogura T, Kusumoto C, Matsuno Y, Nishigami T, Maeda M, Harada M. Carcinosarcoma of the Extrahepatic Bile Duct Presenting with Stone-like Radiological Findings. Intern Med. 2015;54:1747-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Hoshino T, Naganuma A, Takagi H, Koitabashi E, Sakamoto N, Inui M, Souma H, Kudo T, Ishihara H, Ogawa A, Mizuide M. [A case of so-called carcinosarcoma of the middle portion of the bile duct diagnosed preoperatively]. Nihon Shokakibyo Gakkai Zasshi. 2013;110:263-270. [PubMed] |
| 14. | Tanaka M, Ajiki T, Matsumoto I, Asari S, Fukumoto T, Masuda A, Shiomi H, Hayakumo T, Ku Y. Duodenal protrusion by carcinosarcoma of the extrahepatic bile duct. Dig Endosc. 2012;24:484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Aurello P, Milione M, Dente M, D'Angelo F, Nigri G, Del Gaudio M, Valabrega S, Ramacciato G. Synchronous carcinosarcoma of the intrapancreatic bile duct and carcinoma in situ of wirsung duct: a case report. Pancreas. 2008;36:95-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Xia XG, Xu H. [Carcinosarcoma of common bile duct: report of a case]. Zhonghua Bing Li Xue Za Zhi. 2006;35:192. [PubMed] |
| 17. | Kadono J, Hamada N, Higashi M, Ishizaki N, Nakamura N, Sakata R. Carcinosarcoma of the extrahepatic bile duct. J Hepatobiliary Pancreat Surg. 2005;12:328-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Loud PA, Warshauer DM, Woosley JT, Hartmann TM. Carcinosarcoma of the extrahepatic bile ducts: cholangiographic and CT appearance. Abdom Imaging. 1997;22:85-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |