Published online Mar 15, 2023. doi: 10.4251/wjgo.v15.i3.405
Peer-review started: September 18, 2022
First decision: February 4, 2023
Revised: February 11, 2023
Accepted: February 27, 2023
Article in press: February 27, 2023
Published online: March 15, 2023
Processing time: 177 Days and 10.4 Hours
Hepatocellular carcinoma (HCC) is associated with high morbidity and mortality, and is prone to intra- and extrahepatic metastasis due to the anatomical and functional characteristics of the liver. Due to the complexity and high relapse rate associated with radical surgery or radiofrequency ablation, immune checkpoint inhibitors (ICIs) are increasingly being used to treat HCC. Several immunotherapeutic agents, along with their combinations, have been clinically approved to treat advanced or recurrent HCC. This review discusses the leading ICIs in practice and those currently undergoing randomized phase 1–3 trials as monotherapy or combination therapy. Furthermore, we summarize the rapidly developing alternative strategies such as chimeric antigen receptor-engineered T cell therapy and tumor vaccines. Combination therapy is a promising potential treatment option. These immunotherapies are also summarized in this review, which provides insights into the advantages, limitations, and novel angles for future research in establishing viable and alternative therapies against HCC.
Core Tip: The high recurrence rate of hepatocellular carcinoma (HCC) following radical treatment remains challenging; therefore, immune checkpoint inhibitors (ICIs) are increasingly being used to treat HCC. Herein, we discuss the ICIs in practice and those undergoing trials, and summarize the alternative strategies such as chimeric antigen receptor-engineered T cell therapy and tumor vaccines. Combination therapy is also a promising potential treatment option. We believe our study significantly contributes to the literature as it addresses the current state of immunotherapy against HCC and provides insights into the advantages and limitations, thereby facilitating future research.
- Citation: Luo YZ, Zhu H. Immunotherapy for advanced or recurrent hepatocellular carcinoma. World J Gastrointest Oncol 2023; 15(3): 405-424
- URL: https://www.wjgnet.com/1948-5204/full/v15/i3/405.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i3.405
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy (75%-85% of cases), sixth most diagnosed cancer, and the third most common cause of cancer-related deaths worldwide in 2020[1]. The incidence and main risk factors for HCC vary from area to area. Traditionally, the highest epidemic of HCC is mainly in East and South-East Asia; however, the incidence of HCC has increased in the United States and Europe[2]. The key risk factors of HCC include chronic hepatitis B virus or hepatitis C virus (HCV) infection, aflatoxin-contaminated foods, excessive drinking, obesity, and smoking[3,4].
Hepatic resection is the best method for treating early-stage HCC[5-7]. Radiofrequency ablation (RFA) is also considered a radical treatment in many patients with small HCC and is the recommended treatment for patients with a single tumor < 2 cm or 2–3 nodules of ≤ 3 cm[5-8]. Less than 30% of patients with HCC can be treated with surgery and RFA due to distant metastases, anatomical location limitations, hepatic insufficiency, and neurovascular invasion[9,10]. Besides, patients with HCC who receive radical treatment have a high recurrence rate, typically manifesting as recurrence in liver remnants[11]. The recurrence rate in early HCC patients remains high at 5 years post curable excision[11-13]. Most HCCs (> 70%) are diagnosed at an advanced stage[14]. Radical treatment of recurrent HCC includes repeated hepatic resection and liver transplantation; these radical treatments are complex due to the shortage of donors, small residual areas of liver after hepatectomy, hepatic dysfunction, and multiple metastases. Due to the particularity of advanced and recurrent HCC, radiotherapy alone is not recommended. Systematic chemotherapy is also rarely recommended due to resistance to multiple cytotoxic drugs and abnormal liver function[15]. Therefore, local interventional therapies have been developed to treat recurrence, including transcatheter arterial chemoembolization (TACE)[16] and hepatic artery infusion chemotherapy (HAIC)[17]. Locoregional therapy is, for the most part, not a radical treatment, with recurrence and local disease progression being typical. For patients undergoing these, there is an urgent need to explore new therapies to treat recurrent HCC.
Sorafenib, which was been recommended as a first-line treatment for liver cancer with Child-Pugh type A liver function and Barcelona Clinic Liver Cancer-C in 2007, is a multi- tyrosine kinase inhibitor (TKI) that can extend median overall survival (mOS) and the time to radiologic progression by 3 mo[18]. Lenvatinib, which is an alternative first-line treatment for advanced HCC[19], is not inferior to sorafenib. However, lenvatinib is associated with significant improvements compared with sorafenib in terms of higher objective response rate (ORR), prolonged progression-free survival (PFS), and prolonged time to progression[20,21]. Regorafenib[22], cabozantinib[23], and ramucirumab[24,25] are recommended as second-line treatments for advanced HCC[26]. These licensed systemic multi-TKIs may be poorly tolerated due to their significant side effects, drug resistance, and modest benefits in mOS[21,27-29]. Since nivolumab was approved as a second-line treatment for advanced HCC in 2017, immunotherapy for recurrent or advanced HCC has witnessed rapid development. Nivolumab, pembrolizumab, atezolizumab, durvalumab, ipilimumab, tremelimumab, tislelizumab, sintilimab, and camrelizumab and their combinations have been approved in succession for HCC treatment[8]. The advent of cancer immunotherapy has completely changed the traditional treatment concept for HCC by stimulating the immune system of individuals to kill tumor cells selectively. Other immunotherapy strategies, such as chimeric antigen receptor-engineered T cells (CAR-Ts) and therapeutic cancer vaccines, have matured to the stage of clinical trials, offering new hope for HCC patients[30-32]. This article reviews approved immunotherapies and those in clinical development for HCC treatment.
The liver, which receives arterial and venous blood is exposed to pathogens in the systemic circulation (mainly from the gut). Liver immunosurveillance is one of the most critical lines of defense. The liver contains a variety of immune cells, some of which are innate immune cells including neutrophils, macrophages, natural killer cells (NKs), NK T cells (NKTs), dendritic cells (DCs), and Kupffer cells, all of which are essential immune sentinels and antigen-presenting cells (APCs)[33-37]. Kupffer cells can capture antigens under flowing conditions, whereas NKs and NKTs can be activated upon detection of antigens and directly release granulosin and perforase to act on target cells or release large amounts of cytokines (e.g., interferon gamma [IFN-g]) to direct the immune response[38-40]. DCs are the most potent APCs, which can effectively take up, process, and present antigens. As important immune cells, DCs can participate in the development and activation of T and B cells. DCs can also secrete a variety of cytokines (interleukin [IL], IFN, and tumor necrosis factor) and chemokines to participate in the immune function regulation and mediate the chemotaxis of other immune cells[33,41,42]. Neutrophils promote the progression of HCC by interacting with macrophages and regulatory T cells (Tregs). Large numbers of neutrophils predict poor tumor status[43,44]. Conversely, adaptive immune cells include B cells, plasma cells, and effector T cells. A normal liver provides a tolerant microenvironment that inhibits innate and adaptive immunity in homeostasis and prevents inflammation or tissue damage in the liver[45,46].
The immune system of the liver plays a vital role in controlling the occurrence and development of HCC. The interaction between innate and adaptive immunity can lead to effective antitumor immunosurveillance[47]. Tumor cells, Tregs, inhibitory B lymphocytes, and other inhibitory cells mediate the tumor microenvironment by regulating negative costimulatory molecules to achieve immune escape[48]. In addition, myeloid suppressor cells (MDSCs) or M2-polarized tumor-associated macrophages generate an inflammatory microenvironment, which can also serve as a medium for tumor initiation, angiogenesis, and metastasis[49]. Transforming growth factor beta (TGF-β) is the primary mediator for this activity[50] and plays a central role in inflammation, fibrogenesis, and immunomodulation in the HCC microenvironment[51,52]. Therefore, controlling the synthesis and activation of TGF-β during tumor progression is important.
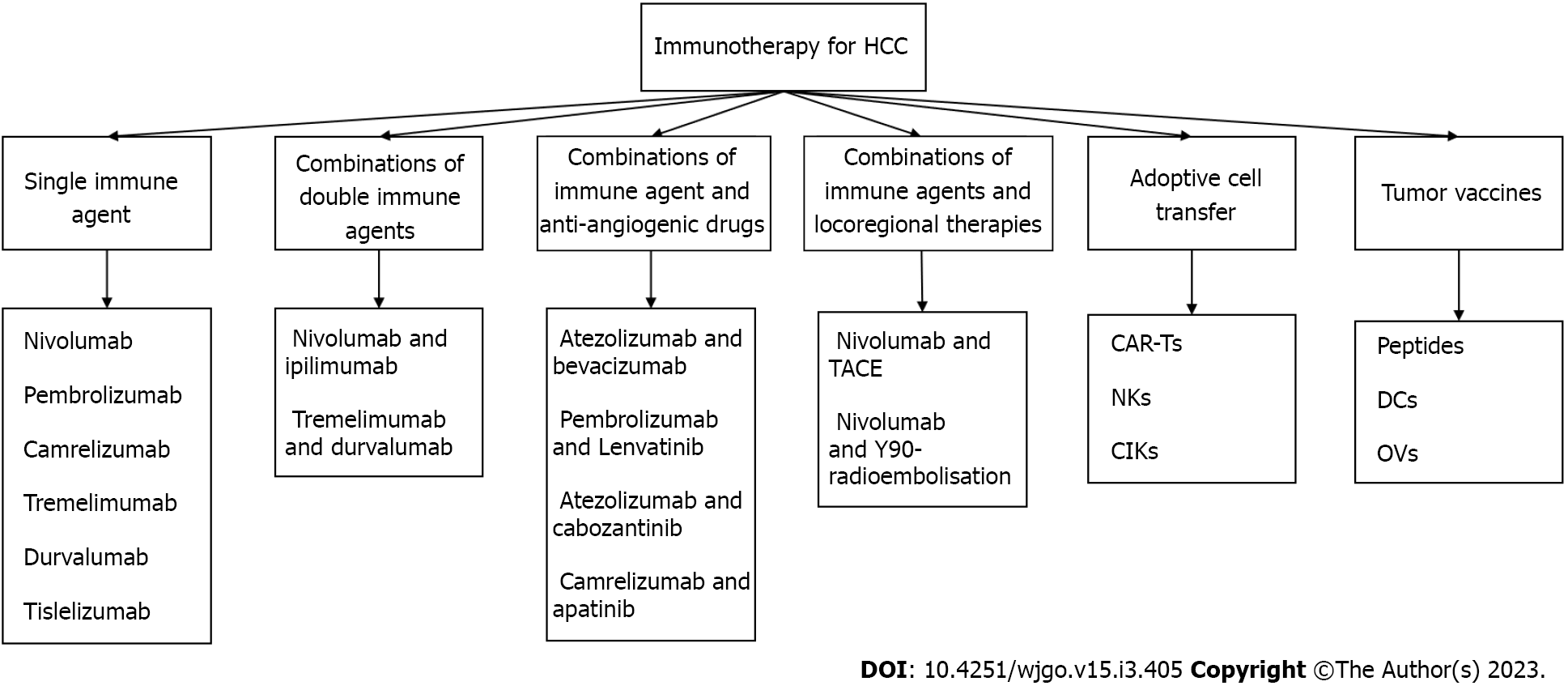
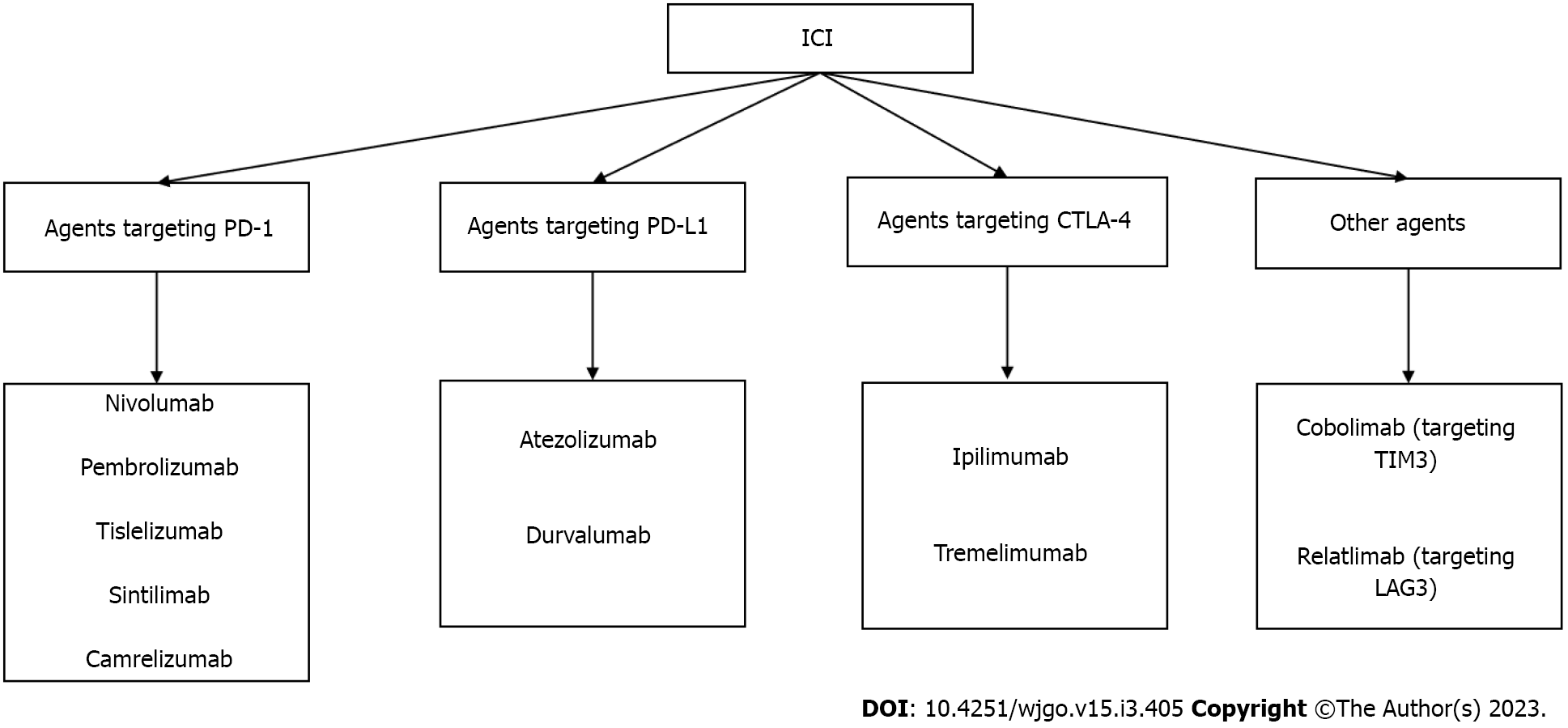
Tumor cells inhibit immune checkpoint overactivation and express corresponding ligands to achieve an immune escape[53]. We previously studied various immunosuppressive receptors, including programmed cell death protein 1 (PD-1), cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), lymphocyte-activation gene 3, T cell immunoglobulin and mucin domain containing-3, and B- and T-lymphocyte attenuator[54,55]. For example, the inhibitory receptor on T cells, PD-1, can be expressed in various immune cell types and binds to programmed death ligand-1 (PD-L1) of the corresponding target cells to inhibit the effects of T cells. CTLA-4 is expressed on the surface of activated T cells by competing with cluster of differentiation 28 (CD28) and binding to CD80 and CD86 to reduce the co-inhibitory signal of CD28 and induce T cell apoptosis. Meanwhile, CTLA-4, an essential gene in Treg differentiation, development, and maintenance of cell functions is highly expressed in Tregs[56]. The concept of blocking inhibitory immune receptors and activating the antitumor function of reinvigorated immune cells has been experimentally demonstrated and translated into the clinical treatment of many types of tumors[57]. Inhibitors of PD-1, PD-L1, and CTLA-4, known as immune checkpoint inhibitors (ICIs), are an essential part of immunotherapy for many tumors including melanoma, non-small cell lung cancer, and colorectal cancer[58]. ICIs, which can block the influence of negative immune costimulatory molecules, can exhibit antitumor activity and kill tumor cells by promoting and upregulating the activation of T cells, thereby restoring normal physiological functions of the human body[59]. ICIs have shown that effective immune response can exterminate tumor cells. Current approaches of immunotherapy were shown in Figure 1. Some ICIs and their related targets are summarized in Figure 2.
Nivolumab was approved in 2017 for patients with recurrent HCC who showed no respond to sorafenib treatment[60]. Nivolumab showed noble safety and tolerability in the phase of escalation (0.1-10 mg/kg) in the CheckMate 040 study. Only 12 of 48 patients (25%) experienced grade 3 or 4 AEs, and no deaths linked to nivolumab treatment were confirmed. In the phase of dose expansion (3 mg/kg), ORR, disease control rate (DCR), and mPFS were 20%, 40%, and 4 mo, respectively. Compared with the phase of escalation, the indices of the dose-expansion phase were significantly improved[61]. In the CheckMate 040 study (NCT01658878) (Table 1), a single nivolumab showed an enduring response, controlled safety, and satisfactory survival in patients with advanced HCC. As the CheckMate 040 study lacked a randomized control, the CheckMate 459 randomized trial (NCT02576509) (Table 1) was conducted to evaluate the efficacy of nivolumab vs sorafenib in a first-line setting. Although nivolumab did not significantly improve mOS (16.4 mo vs 14.7 mo, hazard ratio [HR]: 0.85; P = 0.075) compared with sorafenib, a lower proportion of grade 3 or 4 treatment-related adverse events (AEs), persistent response frequency, and clinical activity make nivolumab a broader treatment prospect[62].
| Drugs (dose) | Other treatment | Targets | Trial identifier | Patient group | n | mOS in mo | ORR, % | DCR, % | mPFS in mo | Phase | Setting |
| Nivolumab (3 mg/kg every 2 wk) | No | PD-1 | NCT01658878 | Advanced HCC | 214 | NR | 20.0 | 64.0 | 4.00 | I-II | 1L |
| Nivolumab (240 mg every 2 wk) | vs Sorafenib | PD-1 | NCT02576509 | Advanced HCC | 371 | 16.40 | 15.0 | 55.0 | 3.70 | III | 1L |
| Pembrolizumab (200 mg every 3 wk) | No | PD-1 | NCT02702414 | Advanced HCC | 104 | 12.90 | 17.0 | 62.0 | 4.90 | II | 2L |
| Pembrolizumab (200 mg every 3 wk) | vs Placebo | PD-1 | NCT02702401 | Advanced HCC | 278 | 13.90 | 18.3 | 62.2 | 3.00 | III | 2L |
| Pembrolizumab (200 mg every 3 wk) | No | PD-1 | NCT02658019 | Advanced HCC | 29 | 11.00 | 32.0 | 46.0 | 4.50 | II | 2L |
| Camrelizumab (200 mg every 2 wk) | vs Camrelizumab (200 mg q3w) | PD-1 | NCT02989922 | Advanced HCC | 109 | 14.20 | 11.9 | 47.7 | 2.30 | II | 2L |
| Camrelizumab (200 mg every 3 wk) | vs Camrelizumab (200 mg q2w) | PD-1 | NCT02989922 | Advanced HCC | 108 | 13.20 | 17.6 | 44.0 | 2.00 | II | 2L |
| Durvalumab (1500 mg every 4 wk) | vs T300+D and tremelimumab | PD-L1 | NCT02519348 | Unresectable HCC | 104 | 13.60 | 10.6 | 37.5 | 2.07 | II | Mix |
| Durvalumab (1500 mg every 4 wk) | vs T300+D and sorafenib | PD-L1 | NCT03298451 | Unresectable HCC | 389 | 16.56 | 17.0 | 54.8 | 3.65 | III | 1L |
| Tremelimumab (750 mg every 4 wk) | vs T300+D and durvalumab | CTLA-4 | NCT02519348 | Unresectable HCC | 69 | 15.10 | 7.2 | 49.3 | 2.69 | II | Mix |
| Tislelizumab (5 mg/kg every 3 wk) | No | PD-1 | NCT02407990 | Advanced HCC | 50 | 12.2 | 51.0 | Ib | 2L |
Pembrolizumab, an anti-PD-1 monoclonal antibody (mAb), has demonstrated promising antineoplastic effects and safety in a variety of malignant tumors[63]. KEYNOTE-224 study (NCT02702414) (Table 1) was conducted to evaluate the efficacy and safety of pembrolizumab in patients with recurrent HCC with no response to sorafenib. The results included ORR of 17%, DCR of 62%, mPFS of 4.9 mo, mOS of 12.9 mo, and grade 3 or 4 AEs that occurred in 25% of the clinical trial participants. Therefore, the Food and Drug Administration (FDA) approved pembrolizumab for treating unresectable intermediate and advanced HCC in November 2018[64]. Pembrolizumab showed good efficacy and a controllable safety profile in patients with advanced HCC who had previously received sorafenib; therefore a worldwide phase 3 study of pembrolizumab (KEYNOTE-240) (NCT02702401) (Table 1) was conducted. In the second-line treatment of advanced HCC, mOS of pembrolizumab and placebo were 13.9 mo vs 10.6 mo (HR: 0.77), mPFS was 3.3 mo vs 2.8 mo (HR: 0.70), and OS and PFS did not meet the specified criteria for statistical significance. Improvements in ORR, DCR, PFS, and OS with pembrolizumab treatment were consistent with the results of the single-cohort KEYNOTE-224 study (Table 1). The difference in ORR (18.4% vs 4.4%) favored pembrolizumab[65]. Accelerated FDA approval was acquired for pembrolizumab use for treating advanced HCC in patients who failed to respond to prior sorafenib therapy.
Camrelizumab, an immunoglobulin G4 (IgG4) anti-PD-1 mAb, is used to treat several cancers including lymphoma, lung cancer, esophageal cancer, and HCC[66,67]. Camrelizumab showed significant antitumor efficacy and tolerance in patients with advanced solid tumors in phase 1 trials[68-70]. To continue evaluating the activity and safety of camrelizumab as a second-line or higher treatment for advanced or recurrent HCC, a randomized phase 2 trial (NCT02989922) (Table 1) was conducted. A total of 217 patients with advanced HCC were randomly assigned in a 1:1 ratio to two groups, including 2 wk of camrelizumab (3 mg/kg) (n = 109) treatment and 3 wk of camrelizumab (3 mg/kg) (n = 108) treatment. At the end of data cutoff, survival metrics from the 2- or 3-wk group, including mOS (14.2 mo vs 13.2 mo), mPFS (2.3 mo vs 2 mo), DCR (47.7% vs 44 %) and ORR (11.9% vs 17.6%) showed good antitumor activity. In terms of safety, grade 3 or 4 AEs occurred in 47 patients (22%)[71].
Compared with other PD-1 inhibitors, camrelizumab experienced a significantly lower DCR (44.2% vs 55% with nivolumab[62] in sorafenib-patients and 47.7% vs 62% with pembrolizumab[64] in the second-line setting after sorafenib use) and shorter mPFS (2.1 mo vs 4.9 mo with pembrolizumab and 3.7 mo with nivolumab). Overall, camrelizumab demonstrated potential antitumor efficacy and safety. However, the efficacy of single camrelizumab was limited; hence, a combination with targeted agents and other ICIs are needed to improve the efficacy.
In March 2020, camrelizumab was approved by the Chinese Food and Drug Administration for treating patients with advanced HCC who had received sorafenib or chemotherapy with oxaliplatin. Camrelizumab is also the first PD-1 inhibitor with HCC indications approved in China, which is a breakthrough in immunotherapy in China.
Tremelimumab is a human IgG2 mAb that blocks the binding of CTLA-4[72]. ORR was 17.6% with a DCR of 76.4% in a clinical trial of tremelimumab in patients with HCC and chronic HCV. Surprisingly, tremelimumab showed satisfactory antitumor activity, antiviral activity, and safety in patients with advanced HCC developed from HCV-induced liver cirrhosis. However, the first trial of tremelimumab for HCC included only 20 patients and therefore could not account for chance results caused by multiple clinical covariates[73]. In a phase 2 clinical trial of tremelimumab in combination with durvalumab for HCC (NCT02519348) (Tables 1 and 2), 326 patients were assigned to four cohorts, namely the tremelimumab monotherapy arm (750 mg once every 4 wk [seven doses] and then once every 12 wk), durvalumab monotherapy arm and T300+D arm (tremelimumab 300 mg plus durvalumab 1500 mg [one dose each during the first cycle] followed by durvalumab 1500 mg once every 4 wk), and T75+D arm (750 mg once every 4 wk [seven doses] and then once every 12 wk). The tremelimumab monotherapy arm represented the first large cohort of HCC patients receiving anti-CTLA-4 monotherapy. The ORR was 7.2%, DCR was 49.3%, mOS was 15.1 mo, and mPFS was 2.69 mo. Although the ORR of this cohort was the lowest (7.2%), the mOS was the second longest, and the median duration of response (mDOR) was prolonged (23.95 mo). However, the grade 1-4 AEs of T300+D were highest (82.4%), whereas that of grade ≥ 3 AEs of tremelimumab monotherapy was the highest (43.5%). Among the four arms, the tremelimumab monotherapy received the highest dose of tremelimumab; therefore, serious AEs were considered to be dose-related to tremelimumab. Compared with tremelimumab monotherapy, the combination of T300+D significantly enhanced antitumor efficacy[74].
| Drugs | Targets | Other treatment | Trial identifier | Patient group | n | mOS in mo | ORR, % | DCR, % | mPFS in mo | Phase | Setting |
| Nivolumab + ipilimumab | PD-1; CTLA-4 | No | NCT01658878 | Advanced HCC | 50 | 22.80 | 32.0 | 54.0 | I/II | 1L | |
| Durvalumab + tremelimumab | PD-L1; CTLA4 | vs Durvalumab and tremelimumab | NCT02519348 | Unresectable HCC | 75 | 18.70 | 24.0 | 45.3 | 2.17 | I/II | 2L |
| Durvalumab + tremelimumab | PD-L1; CTLA4 | vs Durvalumab and sorafenib | NCT03298451 | Unresectable HCC | 393 | 16.40 | 20.1 | 60.1 | 3.78 | III | 1L |
In the phase 2 clinical trial of tremelimumab in combination with durvalumab for HCC (NCT02519348) (Tables 1 and 2) mentioned before, 104 patients with HCC who had progressed on, were intolerant to, or refused sorafenib were randomly assigned to receive durvalumab monotherapy; ORR was 10.6%, DCR was 37.5%, mOS was 13.6 mo, and mPFS was 2.07 mo[74]. Meanwhile, in a phase 3 trial (NCT03298451) (Table 1) of tremelimumab in combination with durvalumab for HCC, the durvalumab monotherapy arm was non-inferior to sorafenib in ORR (17% vs 5.1%) and mOS (16.56 mo vs 13.77 mo). Compared with durvalumab monotherapy in the phase 2 study, durvalumab in this phase 3 study had significantly increased activity with an ORR of 17%, DCR of 54.8%, mPFS of 3.65 mo, and mOS of 16.56 mo[75].
Tislelizumab (BGB-A317) is a humanized IgG4 mAb with high affinity and binding specificity for PD-1. Unlike nivolumab and pembrolizumab, tislelizumab evades the efficacy mediated by Fc gamma R1 (FcγR1) and minimizes the binding of macrophages to FcγR; this may mitigate potential adverse interactions with other immune cells, including macrophages and MDSCs[76-78]. Tislelizumab has demonstrated satisfactory tolerability and significant antitumor activity in patients with advanced HCC. Fifty advanced HCC patients who had previously received other antitumor therapies were reported in the HCC cohort, with an ORR of 12.2% (95% confidence interval [CI]: 4.6-24.8), a DCR of 51% (95%CI: 36.3-65.6), and an average DOR of 15.7 mo. Preliminary safety and antitumor activity support the continued exploration and development of tislelizumab in patients with advanced HCC[79,80]. Therefore, the phase 2 open-label clinical trial of tislelizumab (NCT03419897) further explored the efficacy and safety of Tislelizumab in the second-line treatment of advanced HCC and a phase 3 randomized controlled trial (NCT03412773) is currently evaluating the efficacy and safety of tislelizumab and sorafenib as a first-line treatment for unresectable HCC. These results will provide more options for treating advanced and recurrent HCC.
Nivolumab and pembrolizumab have demonstrated antitumor properties in treating advanced HCC. PD-1/PD-L1 inhibitors and CTLA-4 inhibitors influence T cell response through a complementary mechanism to enhance antitumor efficacy[81]. These positive results inspired the study of the combination of PD-1/PD-L1 inhibitors with CTLA-4 inhibitors with an aim of longer survival and higher response rates. Several combinations of ICIs have been tested to prove their efficacy (Table 2), whereas some remain in the experimental research and development stage (Table 3). According to the preliminary results of the nivolumab and ipilimumab combination compared with nivolumab monotherapy, the ORR (34%) and mOS (22.8 mo) significantly increased with the combination of PD-1 and CTLA-4[82] (NCT01658878) (Table 2). The rate of AEs was significantly higher with the combination of nivolumab and ipilimumab than with nivolumab monotherapy. More than 50% of patients in the Checkmate 040 study required corticosteroids, and the discontinuation rate was 22%, due to tolerably high immunotoxicity[61]. Similar results that showed the antitumor activity of dual immunoblockers being superior to that of single drug were also observed in tremelimumab and durvalumab for patients with unresectable HCC (NCT02519348). Compared with tremelimumab or durvalumab monotherapy, T300+D showed the most encouraging benefit-risk profile[74], which promotes T300+D to enter into phase 3 clinical trial (NCT03298451). For the 393 patients, the ORR was 20.1%, DCR was 60.1%, mOS was 16.4 mo, and mPFS was 3.7 mo (Table 2). Durvalumab was not inferior to tremelimumab; however, the combination of T300+D showed superior efficacy and a favorable benefit-risk profile compared with durvalumab and tremelimumab monotherapy. Compared with the combination of nivolumab and ipilimumab, the incidence of immunotoxicity requiring systemic corticosteroids in the T300+D regimen was 24.3%. The discontinuation rate was only 10.8% due to AEs[75]. Overall, the results of these two studies demonstrated that PD-1/PD-L1 and CTLA-4 had different and complementary antitumor mechanisms.
| Drugs | Other treatment | Targets | Trial identifier | Patient group | n | mOS in mo | ORR, % | DCR, % | mPFS in mo | Phase | Setting |
| Atezolizumab + bevacizumab | vs Sorafenib | PD-L1; VEGF | NCT03434379 | Unresectable HCC | 326 | 19.20 | 27.3 | 74.0 | 6.90 | III | 1L |
| Pembrolizumab + lenvatinib | No | PD-1; VEGFR | NCT03006926 | Unresectable HCC | 104 | 22.00 | 36.0 | 88.0 | 8.60 | Ib | 1L |
| Sintilimab + IBI305 | vs Sorafenib | PD-1; VEGF | NCT03794440 | Unresectable HCC | 380 | NR | 21.0 | 72.0 | 4.60 | III | 1L |
| Atezolizumab + cabozantinib | vs Sorafenib | PD-L1; VEGFR | NCT03755791 | Advanced HCC | 432 | 15.40 | 11.0 | 78.0 | 6.10 | III | 1L |
| Camrelizumab + apatinib | No | PD-1; VEGFR | NCT03463876 | Advanced HCC | 70 | NR | 34.3 | 77.1 | 5.70 | II | 1L |
| Camrelizumab + apatinib | No | PD-1; VEGFR | NCT03463876 | Advanced HCC | 120 | NR | 22.5 | 75.8 | 5.50 | II | 2L |
The overexpression of vascular endothelial growth factor (VEGF) is important in the occurrence and development of HCC. Anti-angiogenic drugs, including sorafenib, lenvatinib, and bevacizumab, are capable of targeting platelet-derived growth factor receptor, VEGF receptor (VEGFR), fibroblast growth factor receptor, hepatocyte factor receptor (c-KIT), and other proteins to inhibit tumor angiogenesis. Anti-VEGFR drugs (sorafenib and lenvatinib) effectively reduce VEGFR-mediated immune suppression and promote T cell activity in the tumor environment[83,84]. Sorafenib was the first anti-VEGFR drug used to treat advanced HCC in the past decade. Since then, until the emergence of atezolizumab in combination with bevacizumab, no treatment has surpassed the first-line efficacy of sorafenib[18,85]. In a phase 1b randomized cohort trial comprising 119 patients, atezolizumab in combination with bevacizumab resulted in significantly higher mPFS (7 mo) and ORR (36%) than atezolizumab monotherapy[86]. In the IMbrave150 clinical trial (NCT03434379) (Table 4), compared with sorafenib, the combination of atezolizumab (PD-L1) and bevacizumab (a vascular epidermal growth factor inhibitor) reduced the risk of death by 42% and extended mPFS and mOS by 2.5 and 5.8 mo (median follow-up 15.6 mo), respectively. The results showed an ORR of 27.3%, DCR of 74%, mPFS of 6.9 mo, and mOS of 19.2 mo. Notably, the ORR of this combination even reached more than twice that of sorafenib[85]. With long-term follow-up, to the best of our knowledge, this combination had the longest mOS observed in a phase 3 trial for HCC until now. In terms of safety, the grade ≥ 3 AEs of the combination occurred in 160 patients (49%), which were consistent with the known AEs of each drug[87]. The combination of atezolizumab and bevacizumab was approved by the FDA for treating patients with advanced or recurrent HCC who had not previously received systemic treatment[88].
| Drugs | Other treatment | Targets | Trial identifier | Patient group | Status | n | Estimated completion date | Phase | Setting |
| Single ICI | |||||||||
| Pembrolizumab | Placebo | PD-1 | NCT03062358 | Advanced HCC | Active, not recruiting | 454 | June 30, 2023 | III | 2L |
| Tislelizumab | vs Sorafenib | PD-1 | NCT03412773 | Advanced HCC | Active, not recruiting | 674 | May 1, 2022 | III | 1L |
| Durvalumab | No | PD-L1 | NCT04294498 | Advanced HCC | Recruiting | 43 | December 31, 2023 | II | 2L |
| Tislelizumab | vs Sorafenib | PD-1 | NCT03419897 | Unresectable HCC | Active, not recruiting | 249 | June 30, 2022 | II | 2L |
| Combination of ICIs | |||||||||
| Nivolumab + ipilimumab | vs Sorafenib and lenvatinib | CTLA-4, PD-1 | NCT04039607 | Advanced HCC | Recruiting | 728 | September 30, 2019 | III | 1L |
| Sintilimab + IBI310 | vs Sorafenib | PD-1, CTLA-4 | NCT04720716 | Advanced HCC | Recruiting | 490 | February 7, 2021 | III | 1L |
| Combination of ICIs and antiangiogenic drugs | |||||||||
| Nivolumab + regorafenib | No | PD-1, VEGFR | NCT04310709 | Unresectable HCC | Recruiting | 42 | May 30, 2023 | II | 1L |
| Pembrolizumab + lenvatinib | Placebo and lenvatinib | PD-1, VEGFR | NCT03713593 | Advanced HCC | Recruiting | 750 | December 31, 2023 | III | 1L |
| Pembrolizumab + futibatinib | No | PD-1, FGFR | NCT04828486 | Advanced HCC | Recruiting | 25 | May 6, 2024 | II | 2L |
| Pembrolizumab + regorafenib | No | PD-1, VEGFR | NCT03347292 | HCC | Active, not recruiting | 57 | September 26, 2022 | I | 1L |
| Pembrolizumab + sorafenib | No | PD-1, VEGFR | NCT03211416 | Advanced or metastatic HCC | Recruiting | 41 | December 7, 2022 | I/II | 1L |
| Pembrolizumab + cabozantinib | No | PD-1 VEGFR | NCT04442581 | Advanced HCC | Recruiting | 29 | September 13, 2024 | II | 1L |
| Camrelizumab + apatinib | No | PD-1, VEGFR | NCT04826406 | HCC | Recruiting | 40 | August 30, 2023 | II | 1L |
| Camrelizumab + lenvatinib | No | PD-1, VEGFR | NCT04443309 | Advanced HCC | Recruiting | 53 | August 1, 2023 | I/II | 1L |
| Camrelizumab + apatinib | vs Sorafenib | PD-1 VEGFR | NCT03764293 | Advanced HCC | Active, not recruiting | 543 | June 1, 2022 | III | 1L |
| Toripalimab + lenvatinib | No | PD-1, VEGFR | NCT04368078 | Advanced HCC | Recruiting | 76 | April 1, 2023 | II | 2L |
| Tislelizumab + regorafenib | No | PD-1, VEGFR | NCT04183088 | Advanced HCC | Recruiting | 125 | March 1, 2025 | II | 1L |
| Tislelizumab + lenvatinib | No | PD-1, VEGFR | NCT04401800 | Locally advanced or Unresectable HCC | Recruiting | 66 | December 1, 2022 | II | 1L |
| Sintilimab + lenvatinib | No | PD-1, VEGFR | NCT04042805 | Advanced HCC | Recruiting | 36 | August 30, 2024 | II | 1L |
| Sintilimab + anlotinib | No | PD-1, VEGFR | NCT04052152 | Advanced HCC | Recruiting | 20 | December 30, 2021 | II | 1L |
| Sintilimab + IBI305 | vs Sorafenib | PD-1, VEGFR | NCT03794440 | Advanced HCC | Active, not recruiting | 595 | December 1, 2022 | II/III | 1L |
| Sintilimab + regorafenib | vs Regorafenib | PD-1, VEGFR | NCT04718909 | Unresectable HCC | Recruiting | 180 | December 31, 2022 | II | 1L |
| Sintilimab + donafenib | No | PD-1, VEGFR | NCT05162352 | Advanced HCC | Recruiting | 30 | May 1, 2023 | II | 1L |
| Atezolizumab + lenvatinib or sorafenib | vs Sorafenib or lenvatinib | PD-L1, VEGFR | NCT04770896 | Unresectable HCC | Recruiting | 554 | October 8, 2024 | III | 2L |
| Atezolizumab + bevacizumab | No | PD-L1, VEGFR | NCT04829383 | Unresectable HCC | Recruiting | 50 | July 1, 2024 | II | 1L |
| Atezolizumab + bevacizumab | No | PD-L1, VEGFR | NCT04732286 | Unresectable HCC | Active, not recruiting | 100 | September 25, 2023 | III | 1L |
| Atezolizumab + bevacizumab | No | PD-L1, VEGFR | NCT04487067 | Unresectable HCC | Active, not recruiting | 152 | July 31, 2023 | IIIb | 1L |
| Durvalumab + tivozanib | No | PD-L1, VEGFR | NCT03970616 | Advanced HCC | Recruiting | 42 | August 1, 2022 | I/II | Mix |
| Durvalumab + lenvatinib | No | PD-L1, VEGFR | NCT05312216 | Unresectable HCC | Not yet recruiting | 25 | April 1, 2022 | II | 1L |
| Durvalumab + bevacizumab | Placebo | PD-L1, VEGFR | NCT03847428 | High risk of recurrence HCC | Active, not recruiting | 877 | May 31, 2024 | III | 1L |
Lenvatinib was not statistically inferior to sorafenib in a phase 3 trial comparing lenvatinib with sorafenib as the first-line treatment for unresectable HCC. Compared with sorafenib, lenvatinib showed significant and clinically significant improvements in ORR, PFS, and TTP[21]. However, pembrolizumab also exhibited substantial antitumor activity and safety. Lenvatinib, in combination with pembrolizumab, has received accelerated approval for the treatment of advanced tumors that do not have high microsatellite instability or mismatch repair defects[89]. The encouraging preliminary trial data has led to a phase 1b study for the combinations of lenvatinib and pembrolizumab to treat unresectable HCC (NCT03006926) (Table 4). Surprisingly, the combination achieved an ORR of 46.0% (95%CI: 36.0%–56.3%, mRECIST standard) and a DCR of > 85% (regardless of the RECIST category). mPFS and mOS were 9.3 and 22 mo, respectively. The combination of lenvatinib and pembrolizumab showed no new AEs[90]. Based on the interim data from this study, the FDA granted lenvatinib in combination with pembrolizumab as first-line therapy for advanced HCC. The combination is being studied in a randomized phase 3 trial (NCT03713593) and compared with the first-line treatment of unresectable or metastatic HCC using lenvatinib (Table 3).
ICIs combined with anti-angiogenic agents open a new avenue for treating HCC. In contrast, FDA-approved first-line combination therapies for HCC are only available in a few regions worldwide. Therefore, alternative therapies need to be developed and approved. Currently, PD-1/PD-L1 checkpoint inhibitors, CTLA-4 checkpoint inhibitors, TKI, along with other antitumor agents are undergoing randomized phase 1–3 trials as monotherapy or combination therapy (Table 3). Cabozantinib, approved in 2019 by the FDA as a second-line treatment of sorafenib, has shown promising antitumor activity. COSMIC312 (NCT03755791) evaluated the combination of cabozantinib and atezolizumab vs sorafenib as first-line systemic therapy for HCC. Compared with sorafenib, the combination arm significantly improved PFS (HR: 0.63; 99%CI: 0.44–0.91; P = 0.0012; mPFS 6.8 mo vs 4.2 mo). However, OS was not improved[91] (HR: 0.90; 96%CI: 0.69-1.18; P = 0.438). At the end of 2020, the ORIENT-32 trial, which enrolled 571 HCC patients without systemic therapy, reported that combination of sintilizumab (PD-1) and bevacizumab biosimilar (IBI305) was significantly superior to sorafenib in terms of OS and PFS, as shown in Table 4. After a median follow-up of 10 mo, the mOS was not achieved in the combination line (sintilizumab and IBI305), while it was 10.4 mo in the sorafenib group (HR: 0.57; 95%CI: 0.43–0.75; P < 0.0001); mPFS (4.6 mo, 95%CI: 4.1–5.7) was significantly prolonged (HR: 0.56, 95%CI: 0.46-0.70; P < 0.0001)[92]. In early 2021, camrelizumab (PD-1) in combination with apatinib (a selective VEGFR-2 tyrosine kinase inhibitor) was assessed in phase 2 (NCT03463876) as the first- and second-line treatment for advanced HCC. Significant antitumor activity was achieved in ORR, DOR, and OS for both first- and second-line treatments[93] (Table 4). Encouraging antitumor properties continue to emerge in the new combination therapies with ICIs and TKIs, which will provide options for recurrent HCC treatment.
Some locoregional therapies for HCC, including radiotherapy, RFA, TACE and HAIC, can release or produce altering substances from cancer cells to stimulate the aggregation of DCs into tumor tissues. This can upregulate the expression and antigenicity of tumor-associated antigens (TAAs) and trigger injury-related molecular patterns to induce “immunogenic cell death”[94-96]. Locoregional therapies can induce the release of proinflammatory cytokines to activate and expand innate and adaptive immune cells (NK and cytotoxic T cells) and reduce the activity of immunosuppressive cells (Tregs and MDSCs)[97-100]. Meanwhile, immunotherapy can not only improve the hypoxic microenvironment in tumors and enhance the effect of radiotherapy by inducing vascular normalization through a T cell-dependent pathway but also enhance the immune induction effect of radiotherapy to slow the growth of distant tumors (abscopal effect). Radiotherapy and immunotherapy synergize to exert more potent local effects in the irradiated tumors[101,102]. The IMMUTACE trial initially evaluated the efficacy of nivolumab plus TACE in 49 patients with mid-stage HCC; the ORR was 71.4% (95%CI: 56.8%-83.4%), including 16.3% complete responses (CRs) and 55.1% partial responses. Despite the small number of patients in each group, subgroup analyses did not reveal differences in treatment responses[103]. In the CA 209-678 study (NCT03033446) of Y90-radioembolisation followed by nivolumab in 36 patients with advanced HCC, the ORR of 30.6% compared favorably with an ORR of approximately 20% noted with Y90-radioembolisation. Notably, 81% of patients showed regression of radiation-field target lesions. This combination is safe and tolerable with grade 3–4 treatment-related AEs or serious AEs noted in 14% of patients[104]. Many clinical trials of locoregional therapies combined with ICIs are being conducted successively (Table 5). This combination is expected to become the mainstream treatment for HCC in the future.
| Main intervention methods | Comparison arms | Trial identifier | Status | Estimated or actual enrollment | Patient group | Phase |
| Pembrolizumab + RAF/MWA/brachytherapy/TACE | vs Pembrolizumab + RAF/MWA/brachytherapy/TACE | NCT03753659 | Active, not recruiting | 30 | Early-stage HCC | II |
| Nivolumab + TACE | No | NCT03572582 | Active, not recruiting | 49 | Intermediate-stage HCC | II |
| Pembrolizumab + TACE | No | NCT03397654 | Active, not recruiting | 26 | HCC | I/II |
| Durvalumab + tremelimumab + TACE/RAF/cryoablation | vs Durvalumab + tremelimumab | NCT02821754 | Active, not recruiting | 54 | Advanced HCC | II |
| Durvalumab + tremelimumab + TACE | No | NCT03638141 | Recruiting | 30 | Intermediate-stage HCC | II |
| Durvalumab + tremelimumab + bevacizumab + TACE | No | NCT03937830 | Recruiting | 22 | Advanced HCC | II |
| Durvalumab + bevacizumab + TACE | vs Durvalumab + TACE vs TACE | NCT03778957 | Active, not recruiting | 724 | Intermediate-stage HCC | III |
| Apatinib + camrelizumab + HAIC | No | NCT04191889 | Recruiting | 84 | Advanced HCC | II |
| Pembrolizumab + SBRT | No | NCT03316872 | Recruiting | 30 | Advanced HCC | II |
| Durvalumab + tremelimumab + SBRT | No | NCT03482102 | Recruiting | 70 | Advanced HCC | II |
| Nivolumab + curative resection/RAF | vs Curative resection/RAF | NCT03383458 | Active, not recruiting | 545 | Resected HCC | III |
| Durvalumab + bevacizumab + curative resection/RAF | vs Durvalumab + curative resection/RAF vs Curative resection/RAF | NCT03847428 | Active, not recruiting | 877 | Resected HCC | III |
| Ipilimumab + nivolumab + TACE | vs Nivolumab + TACE + placebo vs TACE + placebo + placebo | NCT04340193 | Active, not recruiting | 26 | Intermediate-stage HCC | III |
| Lenvatinib + pembrolizumab + TACE | vs Placebo + placebo + TACE | NCT04246177 | Active, not recruiting | 950 | Incurable/non-metastatic HCC | III |
| Nivolumab + DEB TACE | vs DEB TACE | NCT04268888 | Recruiting | 522 | Intermediate-stage HCC | II/III |
Adoptive cell transfer is a form of passive therapy in which immune cells are activated and expanded in vitro and then reinfused into the patient. These immune cells commonly used include NKs, tumor-infiltrating lymphocytes, lymphokine-activated killer cells, cytokine-induced killer cells (CIKs), and CAR-T cells.
NKs can recognize tumor cells based on the expression of ligands for inhibitory and stimulant NK receptors[40]. Encouraging clinical trials results in which autologous lymphocytes containing NK cells were transfused into HCC patients after ablation or resection had shown that extended NKs have significant cytotoxic effects on HCC cells[105]. Furthermore, extended NKs significantly enhanced the anti-HCC cytotoxicity of sorafenib[106]. Multiple phase 2 trials are being conducted to evaluate the use of NKs in patients after hepatectomy (NCT02008929) or TACE (NCT02854839). However, how to cultivate high purity NKs is still a problem to be solved.
CIKs are heterogeneous cells with non-major histocompatibility complex-restricted tumor killing activity. After being cultured in vitro, CIKs can secrete a variety of cytokines to improve the internal microenvironment of tissues and organs and enhance the killing activity of immune cells[107]. A phase 3 clinical trial (NCT00699816) including 230 patients showed that adjuvant CIK immunotherapy improved PFS and OS in patients with HCC after curable surgical resection, RFA, or percutaneous ethanol injection[105]. CIKs may have a significant impact on adoptive immunotherapy regimens in patients with primary HCC.
CAR-T therapy is a developing immunotherapy approach for treating malignant tumors. Due to the great success of CAR-T therapy in the treatment of CD19-positive hematological malignancies, such as a CR rate of up to 90% with anti-CD19 CAR-T cells in B-cell acute lymphoblastic leukemia[108-111], two CAR-T cell therapies, Kymriah® and Yescarta®, were approved by the FDA for lymphoma studies in 2018 and 2017, respectively. Because of this lymphoma breakthrough, CAR-T’s application in treating solid tumors, such as HCC, has also been explored. Glypican-3 (GPC-3), a member of the GPC family, is a 70 kDa heparan sulfate proteoglycan overexpressed in HCC and associated with poor diagnosis and prognosis[112-115]. Several clinical trials have evaluated the safety and efficacy of GPC-3 CAR-T cells. Shanghai Renji Hospital combined lymphodepleting chemotherapy with GPC-3 CAR-T cells in 13 patients with GPC3-positive HCC and confirmed the antitumor efficacy and safety of GPC3 CAR-T cells (NCT02395250)[116]. GPC-3 CAR-T cells combined with sorafenib may be a promising option for treating of HCC[117].Chongqing Xinqiao Hospital has attempted to combine TACE with CAR-T to treat GPC3-positive advanced HCC (NCT03084380). Other clinical trials are recruiting patients to improve the efficacy of intratumoral or intravenous administration of GPC3-CART cells (NCT03130712, NCT02715362, NCT04951141, NCT03198546, and NCT05155189). In conclusion, GPC-3 is a promising target for future therapeutic strategies in HCC. Mucin 1 glycoprotein (MUC-1)[118,119] and epithelial cell adhesion molecule (EpCAM)[120] are two transmembrane glycoproteins that can be overexpressed during the occurrence and development of HCC and can be used as biomarkers and therapeutic targets for HCC. One clinical trial of MUC-1 CAR-T cells (NCT02587689) and two clinical trials of EpCAM CAR-T cells (NCT03013712 and NCT02729493) are ongoing. Alpha-fetoprotein (AFP), which is overexpressed in HCC, is another potential therapeutic target being explored. However, AFP is a glycoprotein of the cellular endocrine system and expression and is therefore considered inappropriate for the CAR. Some researchers have designed a highly specific antibody (Ab) of the (AFP)-MHC complex to be expressed as the CAR and found that CAR-T cells of this Ab had an apparent inhibitory effect on HCC; this provided a promising new approach for HCC immunotherapy[121].
Tumor vaccines are active immunotherapies that require the injection of tumor antigens, including viruses, DNA, peptides, and tumor cell-expressed genes, into patients to trigger TAA-specific immune responses and mediate powerful antitumor effects[122]. Therapeutic tumor vaccines include peptides, DCs, whole-cell vaccines, oncolytic viruses, and DNA reagents.
Several peptide-based cancer vaccines have been assessed for HCC treatment. As a biomarker of HCC, AFP was constructed as a peptide vaccine, used in 2 patients with AFP-expressing tumors and showed high levels of AFP-specific CD8+T cell expression and apparent safety (NCT00093548). GPC-3 is highly expressed in most malignant tumors and is rarely in normal tissues; therefore, GPC-3 is considered an ideal TAA for developing cancer vaccines[114,123]. The GPC-3 vaccine is well-tolerated and safe[124,125]. Similarly, multidrug resistance-associated protein 3 (MRP3), a vector-type transporter, highly expressed and associated with various cancers[126], is a great potential candidate for tumor vaccine development. In a phase 1 trial, the MRP3-derived peptides (MRP3765) showed promising safety and antitumor properties in 12 HLA-A24-positive HCC patients. MRP3-specific T-cell responses were induced in 8 patients (72.7%) and the mOS was 14 mo (95%CI: 9.6-18.5)[127]. Other TAAs, including synovial sarcoma X breakpoint 2, NY-ESO-1, human telomerase reverse transcriptase and melanoma-associated antigens family A, can also be valuable targets for HCC immunotherapy, but no clinical trials have verified the clinical response to these antigens in HCC[128]. Although peptide vaccines have achieved some success in terms of safety, tolerability, and mOS improvement, they have fewer clinical benefits and more stringent screening conditions than ICIs.
DCs as APCs can stimulate T cells and increase the antitumor effect[129]. Peripheral monocytes were isolated in vitro and the DC population was expanded by adding cofactors (granulocyte-macrophage colony-stimulating factor or IL-4). Mature DCs are activated with autologous tumor lysates (TLs) or specific TAAs. Finally, these cells are reinfused into the patient to stimulate the adaptive cells to mount an antitumor immune response[36,130,131]. Currently, several clinical trials have confirmed the immunogenicity and safety of DCs. In a phase 1 trial of 17 patients with HCC treated with immunoprimers (ilixadencel), 73% had an increased frequency of tumor-specific CD8+-T cells in their peripheral blood[132]. Meanwhile, a phase 1 clinical trial in Japan injected DCs pulsed with TLs into 10 patients with unresectable HCC. All patients had an excellent immune tolerance; 1 patient experienced significant tumor shrinkage, while two experienced considerable tumor marker decrease[133]. In another phase 2 study, the intravenous administration of mature DCs pulsed with tumor lysate (HepG2) showed promising antitumor properties and safety in 35 patients with HCC[134]. When DCs were combined with TACE, tumor-specific immune responses were enhanced more effectively than when TACE was used alone[135]. Multiple clinical trials on DCs are in progress (NCT01821482, NCT02638857, NCT02882659, NCT03674073, and NCT03203005). A growing body of evidence suggests that DC vaccines have general safety and antitumor properties as primary therapy and adjunct to other established therapies. DC vaccines are promising mainstream immunotherapy for HCC.
An oncolytic virus (OV) is a specially modified intracellular pathogen that can achieve an antitumor response by massive replication in tumor cells, leading to direct lysis of tumor cells to produce soluble TAAs[136,137]. OVs have been shown to improve ORR and mOS in advanced melanoma (NCT00769704)[138]. Currently, adenovirus and vesicular stomatitis virus are the main oncolytic viruses used to treat HCC, which can preferentially infect HCC tumor cells, followed by the herpes simplex virus and vaccinia virus[139]. In a recent randomized phase 2 trial (NCT00554372), JX-549 (Pexa-Vec) was injected into the tumors of 30 HCC patients, and mOS was significantly longer in the high-dose group than in the low-dose group (14.1 mo vs 6.7 mo) (HR: 0.39; P = 0.020)[140]. Unfortunately, the phase 2b trial (NCT01387555), which compared Pexa-Vec to placebo as second-line therapy in patients with advanced HCC with no response to sorafenib therapy, did not achieve its OS[141]. A phase 3 trial (NCT02562755) is currently underway, which compares the safety and efficacy of sorafenib with Pexa-Vec against sorafenib alone in HCC. Currently, two clinical trials are underway to evaluate the efficacy of the combination of OVs and ICIs in HCC (NCT03647163 and NCT03071094)[142].
The rapid development of immunotherapy has changed the traditional treatment modalities for recurrent HCC. Immunotherapy can play a unique role in the comprehensive treatment of HCC, including prolonging and improving quality of life and even curing HCC. Several clinical trials have attempted to evaluate the antitumor properties and safety of ICIs and their combinations in recurrent HCC, and have reported encouraging results. Although ICIs are the leading immunotherapy for recurrent HCC, other immunotherapy modalities including CAR-T cells, DC vaccines, and OVs are rapidly evolving. Among the multiple treatment options for recurrent HCC, achieving satisfactory results with single immunotherapy has become challenging. The development of synergistic immunotherapy may be a promising direction for HCC treatment in the future. In addition, immunosuppression of HCC remains a significant obstacle for immunotherapy drugs in which they must exert their antitumor properties. Another priority is to actively exploring the mechanisms of immunotherapy resistance or overcoming immune drug resistance through multiple antitumor drugs. Immunotherapy can lead to future breakthroughs and progress in treating recurrent HCC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu L, China; Suda T, Japan; Xie Y, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64652] [Article Influence: 16163.0] [Reference Citation Analysis (176)] |
| 2. | Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 3. | Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 2907] [Article Influence: 484.5] [Reference Citation Analysis (17)] |
| 4. | Balogh J, Victor D 3rd, Asham EH, Burroughs SG, Boktour M, Saharia A, Li X, Ghobrial RM, Monsour HP Jr. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma. 2016;3:41-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 755] [Cited by in RCA: 807] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 5. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3029] [Article Influence: 432.7] [Reference Citation Analysis (3)] |
| 6. | Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, Nault JC, Neumann U, Ricke J, Sangro B, Schirmacher P, Verslype C, Zech CJ, Arnold D, Martinelli E; ESMO Guidelines Committee. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv238-iv255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 723] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 7. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6060] [Article Influence: 865.7] [Reference Citation Analysis (3)] |
| 8. | Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, Chahal P, Chang DT, Cloyd J, Covey AM, Glazer ES, Goyal L, Hawkins WG, Iyer R, Jacob R, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Sahai V, Schefter T, Singh G, Stein S, Vauthey JN, Venook AP, Yopp A, McMillian NR, Hochstetler C, Darlow SD. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:541-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 575] [Article Influence: 143.8] [Reference Citation Analysis (0)] |
| 9. | Zhu F, Chang Q, Duan S, Leng W. Efficacy and safety of radiofrequency ablation versus laparoscopic hepatectomy for small hepatocellular carcinoma: A protocol for a randomized controlled trial. Medicine (Baltimore). 2021;100:e23678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Han J, Fan YC, Wang K. Radiofrequency ablation versus microwave ablation for early stage hepatocellular carcinoma: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e22703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 728] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 12. | Ringe B, Pichlmayr R, Wittekind C, Tusch G. Surgical treatment of hepatocellular carcinoma: experience with liver resection and transplantation in 198 patients. World J Surg. 1991;15:270-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 419] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Iwatsuki S, Starzl TE, Sheahan DG, Yokoyama I, Demetris AJ, Todo S, Tzakis AG, Van Thiel DH, Carr B, Selby R. Hepatic resection versus transplantation for hepatocellular carcinoma. Ann Surg. 1991;214:221-8; discussion 228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 470] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 14. | Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, Gores G, Kerlan R, Merle P, O'Neil B, Poon R, Schwartz L, Tepper J, Yao F, Haller D, Mooney M, Venook A. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994-4005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 323] [Article Influence: 21.5] [Reference Citation Analysis (1)] |
| 15. | Asghar U, Meyer T. Are there opportunities for chemotherapy in the treatment of hepatocellular cancer? J Hepatol. 2012;56:686-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 16. | Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 423] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 17. | Chen CT, Liu TH, Shao YY, Liu KL, Liang PC, Lin ZZ. Revisiting Hepatic Artery Infusion Chemotherapy in the Treatment of Advanced Hepatocellular Carcinoma. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10270] [Article Influence: 604.1] [Reference Citation Analysis (2)] |
| 19. | Kudo M. Lenvatinib in Advanced Hepatocellular Carcinoma. Liver Cancer. 2017;6:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Al-Salama ZT, Syed YY, Scott LJ. Lenvatinib: A Review in Hepatocellular Carcinoma. Drugs. 2019;79:665-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 204] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 21. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3827] [Article Influence: 546.7] [Reference Citation Analysis (1)] |
| 22. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2715] [Article Influence: 339.4] [Reference Citation Analysis (0)] |
| 23. | Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1630] [Cited by in RCA: 1769] [Article Influence: 252.7] [Reference Citation Analysis (0)] |
| 24. | Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1249] [Article Influence: 208.2] [Reference Citation Analysis (0)] |
| 25. | Zhu AX, Finn RS, Kang YK, Yen CJ, Galle PR, Llovet JM, Assenat E, Brandi G, Motomura K, Ohno I, Daniele B, Vogel A, Yamashita T, Hsu CH, Gerken G, Bilbruck J, Hsu Y, Liang K, Widau RC, Wang C, Abada P, Kudo M. Serum alpha-fetoprotein and clinical outcomes in patients with advanced hepatocellular carcinoma treated with ramucirumab. Br J Cancer. 2021;124:1388-1397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 26. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3883] [Article Influence: 970.8] [Reference Citation Analysis (3)] |
| 27. | Alves RC, Alves D, Guz B, Matos C, Viana M, Harriz M, Terrabuio D, Kondo M, Gampel O, Polletti P. Advanced hepatocellular carcinoma. Review of targeted molecular drugs. Ann Hepatol. 2011;10:21-27. [PubMed] |
| 28. | Keating GM. Sorafenib: A Review in Hepatocellular Carcinoma. Target Oncol. 2017;12:243-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 29. | Olsen SK, Brown RS, Siegel AB. Hepatocellular carcinoma: review of current treatment with a focus on targeted molecular therapies. Therap Adv Gastroenterol. 2010;3:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:525-543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 856] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 31. | Johnston MP, Khakoo SI. Immunotherapy for hepatocellular carcinoma: Current and future. World J Gastroenterol. 2019;25:2977-2989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 121] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 32. | Zongyi Y, Xiaowu L. Immunotherapy for hepatocellular carcinoma. Cancer Lett. 2020;470:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 33. | Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 780] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 34. | Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21:311-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 562] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 35. | Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54-S62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 968] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 36. | Gardner A, de Mingo Pulido Á, Ruffell B. Dendritic Cells and Their Role in Immunotherapy. Front Immunol. 2020;11:924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 339] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 37. | Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 586] [Cited by in RCA: 617] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 38. | Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 743] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 39. | Notas G, Kisseleva T, Brenner D. NK and NKT cells in liver injury and fibrosis. Clin Immunol. 2009;130:16-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Kumar N, Khakoo SI. Hepatocellular carcinoma: Prospects for natural killer cell immunotherapy. HLA. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Ouyang FZ, Wu RQ, Wei Y, Liu RX, Yang D, Xiao X, Zheng L, Li B, Lao XM, Kuang DM. Dendritic cell-elicited B-cell activation fosters immune privilege via IL-10 signals in hepatocellular carcinoma. Nat Commun. 2016;7:13453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 42. | Roderburg C, Wree A, Demir M, Schmelzle M, Tacke F. The role of the innate immune system in the development and treatment of hepatocellular carcinoma. Hepat Oncol. 2020;7:HEP17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 43. | Li X, Xing YF, Lei AH, Xiao Q, Lin ZH, Hong YF, Wu XY, Zhou J. Neutrophil count is associated with myeloid derived suppressor cell level and presents prognostic value of for hepatocellular carcinoma patients. Oncotarget. 2017;8:24380-24388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | Personeni N, Giordano L, Abbadessa G, Porta C, Borbath I, Daniele B, Van Laethem JL, Van Vlierberghe H, Trojan J, De Toni EN, Gasbarrini A, Lencioni M, Lamar ME, Wang Y, Shuster D, Schwartz B, Santoro A, Rimassa L. Prognostic value of the neutrophil-to-lymphocyte ratio in the ARQ 197-215 second-line study for advanced hepatocellular carcinoma. Oncotarget. 2017;8:14408-14415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 45. | Aravalli RN. Role of innate immunity in the development of hepatocellular carcinoma. World J Gastroenterol. 2013;19:7500-7514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 46. | Sutti S, Albano E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol. 2020;17:81-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 280] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 47. | Heymann F, Tacke F. Immunology in the liver--from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 827] [Article Influence: 91.9] [Reference Citation Analysis (0)] |
| 48. | Jacobs JF, Nierkens S, Figdor CG, de Vries IJ, Adema GJ. Regulatory T cells in melanoma: the final hurdle towards effective immunotherapy? Lancet Oncol. 2012;13:e32-e42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 49. | Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1209] [Cited by in RCA: 1236] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 50. | Gold LI. The role for transforming growth factor-beta (TGF-beta) in human cancer. Crit Rev Oncog. 1999;10:303-360. [PubMed] |
| 51. | Chen J, Gingold JA, Su X. Immunomodulatory TGF-β Signaling in Hepatocellular Carcinoma. Trends Mol Med. 2019;25:1010-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 52. | Caja L, Dituri F, Mancarella S, Caballero-Diaz D, Moustakas A, Giannelli G, Fabregat I. TGF-β and the Tissue Microenvironment: Relevance in Fibrosis and Cancer. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 257] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 53. | Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2216] [Cited by in RCA: 2278] [Article Influence: 189.8] [Reference Citation Analysis (0)] |
| 54. | Liu S, Liu F, Zhou Y, Jin B, Sun Q, Guo S. Immunosuppressive Property of MSCs Mediated by Cell Surface Receptors. Front Immunol. 2020;11:1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 55. | He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 851] [Article Influence: 170.2] [Reference Citation Analysis (0)] |
| 56. | Han Y, Chen Z, Yang Y, Jiang Z, Gu Y, Liu Y, Lin C, Pan Z, Yu Y, Jiang M, Zhou W, Cao X. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology. 2014;59:567-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 57. | Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293-12297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2103] [Cited by in RCA: 2455] [Article Influence: 106.7] [Reference Citation Analysis (0)] |
| 58. | Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4180] [Cited by in RCA: 5239] [Article Influence: 523.9] [Reference Citation Analysis (0)] |
| 59. | Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, Lichtor T, Decker WK, Whelan RL, Kumara HMCS, Signori E, Honoki K, Georgakilas AG, Amin A, Helferich WG, Boosani CS, Guha G, Ciriolo MR, Chen S, Mohammed SI, Azmi AS, Keith WN, Bilsland A, Bhakta D, Halicka D, Fujii H, Aquilano K, Ashraf SS, Nowsheen S, Yang X, Choi BK, Kwon BS. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35 Suppl:S185-S198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 1116] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 60. | Callahan MK, Postow MA, Wolchok JD. Targeting T Cell Co-receptors for Cancer Therapy. Immunity. 2016;44:1069-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 386] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 61. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3310] [Article Influence: 413.8] [Reference Citation Analysis (1)] |
| 62. | Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Sieghart W, Assenat E, Zaucha R, Furuse J, Abou-Alfa GK, El-Khoueiry AB, Melero I, Begic D, Chen G, Neely J, Wisniewski T, Tschaika M, Sangro B. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 744] [Article Influence: 186.0] [Reference Citation Analysis (0)] |
| 63. | Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2870] [Cited by in RCA: 3580] [Article Influence: 358.0] [Reference Citation Analysis (0)] |
| 64. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1899] [Article Influence: 271.3] [Reference Citation Analysis (0)] |
| 65. | Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL; KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1341] [Article Influence: 268.2] [Reference Citation Analysis (0)] |
| 66. | Mo H, Huang J, Xu J, Chen X, Wu D, Qu D, Wang X, Lan B, Zhang H, Chi Y, Yang Q, Xu B. Safety, anti-tumour activity, and pharmacokinetics of fixed-dose SHR-1210, an anti-PD-1 antibody in advanced solid tumours: a dose-escalation, phase 1 study. Br J Cancer. 2018;119:538-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 67. | Markham A, Keam SJ. Camrelizumab: First Global Approval. Drugs. 2019;79:1355-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 175] [Article Influence: 29.2] [Reference Citation Analysis (1)] |
| 68. | Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, Zhang G, Zhao C, Chen C, Wang Y, Yi X, Hu Z, Zou J, Wang Q. Anti-PD-1 Antibody SHR-1210 Combined with Apatinib for Advanced Hepatocellular Carcinoma, Gastric, or Esophagogastric Junction Cancer: An Open-label, Dose Escalation and Expansion Study. Clin Cancer Res. 2019;25:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 359] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 69. | Fang W, Yang Y, Ma Y, Hong S, Lin L, He X, Xiong J, Li P, Zhao H, Huang Y, Zhang Y, Chen L, Zhou N, Zhao Y, Hou X, Yang Q, Zhang L. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 2018;19:1338-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 357] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 70. | Huang J, Xu B, Mo H, Zhang W, Chen X, Wu D, Qu D, Wang X, Lan B, Yang B, Wang P, Zhang H, Yang Q, Jiao Y. Safety, Activity, and Biomarkers of SHR-1210, an Anti-PD-1 Antibody, for Patients with Advanced Esophageal Carcinoma. Clin Cancer Res. 2018;24:1296-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 71. | Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, Bai Y, Yang L, Zhu H, Fang W, Lin X, Chen X, Li E, Wang L, Chen C, Zou J. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 425] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 72. | Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 750] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 73. | Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, Riezu-Boj JI, Larrea E, Alfaro C, Sarobe P, Lasarte JJ, Pérez-Gracia JL, Melero I, Prieto J. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 750] [Article Influence: 62.5] [Reference Citation Analysis (0)] |
| 74. | Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, Qin S, Tai DW, Lim HY, Yau T, Yong WP, Cheng AL, Gasbarrini A, Damian S, Bruix J, Borad M, Bendell J, Kim TY, Standifer N, He P, Makowsky M, Negro A, Kudo M, Abou-Alfa GK. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients With Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J Clin Oncol. 2021;39:2991-3001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 330] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 75. | Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV. FcγRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. Cancer Cell. 2015;28:285-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 293] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 76. | Zhang T, Song X, Xu L, Ma J, Zhang Y, Gong W, Zhou X, Wang Z, Wang Y, Shi Y, Bai H, Liu N, Yang X, Cui X, Cao Y, Liu Q, Song J, Li Y, Tang Z, Guo M, Wang L, Li K. The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol Immunother. 2018;67:1079-1090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 207] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 77. | Zhang T, Song J, Li Y, Ma J, Shi Y, Wu X, Xu L, Liu Q, Cao Y, Wang Y, Peng H, Hou H, Zhao X, Song X, Wang L, Wei M, Luo L, Li K. Abstract 2226: Anti-human PD-1 antibody BGB-A317 exhibits potent immune cell activation. Cancer Res. 2016;76:2226-2226. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 78. | Yen CJ, Markman B, Chao Y, Hill A, Kang J, Wang L, Li K, Qi Q, Wu Z, Gan H. Preliminary results of a phase 1A/1B study of BGB-A317, an anti-PD-1 monoclonal antibody (mAb), in patients with advanced hepatocellular carcinoma (HCC). Ann Oncol. 2017;28:iii54. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 79. | Desai J, Voskoboynik M, Markman B, Hou J, Zeng D, Meniawy T. Phase 1/2 study investigating safety, tolerability, pharmacokinetics, and preliminary antitumor activity of anti-PD-L1 monoclonal antibody bgb-A333 alone and in combination with anti-PD-1 monoclonal antibody tislelizumab in patients with advanced solid tumors. J Clin Oncol. 2018;36:TPS3113-TPS3113. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 80. | Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H, Callahan M, Wolchok JD, Halaban R, Dhodapkar MV, Dhodapkar KM. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol. 2015;194:950-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 369] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 81. | Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 908] [Cited by in RCA: 970] [Article Influence: 194.0] [Reference Citation Analysis (0)] |
| 82. | Abou-Alfa GK, Chan, Stephen Lam, Kudo, Masatoshi, Lau, George, Kelley, Robin Kate, Furuse, Junji, Sukeepaisarnjaroen, Wattana, Kang, Yoon-Koo, Dao, Tu V, De Toni, Enrico N, Rimassa, Lorenza, Breder, Valeriy Vladimirovich, Vasilyev, Alexander, Heurgue, Alexandra, Tam, Vincent, Mody, Kabir, Thungappa, Satheesh Chiradoni, He, Philip, Negro, Alejandra, Sangro B. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J Clin Oncol. 2022;40:379-379. [RCA] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 83. | Zhu AX, Duda DG, Sahani DV, Jain RK. HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol. 2011;8:292-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 429] [Cited by in RCA: 442] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 84. | Morse MA, Sun W, Kim R, He AR, Abada PB, Mynderse M, Finn RS. The Role of Angiogenesis in Hepatocellular Carcinoma. Clin Cancer Res. 2019;25:912-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 387] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 85. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4698] [Article Influence: 939.6] [Reference Citation Analysis (2)] |
| 86. | Hsu CH, Lee MS, Lee KH, Numata K, Stein S, Verret W, Hack S, Spahn J, Liu B, Huang C, He R, Ryoo BY. LBA7 - Randomised efficacy and safety results for atezolizumab (Atezo) + bevacizumab (Bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30:ix187. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 87. | Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 960] [Article Influence: 320.0] [Reference Citation Analysis (0)] |
| 88. | Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim TY, Kudo M, Breder V, Merle P, Kaseb A, Li D, Mulla S, Verret W, Xu DZ, Hernandez S, Ding B, Liu J, Huang C, Lim HY, Cheng AL, Ducreux M. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:991-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 236] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 89. | Marabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2020;38:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2120] [Cited by in RCA: 2021] [Article Influence: 404.2] [Reference Citation Analysis (0)] |
| 90. | Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38:2960-2970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 876] [Article Influence: 175.2] [Reference Citation Analysis (0)] |
| 91. | Kelley RK, Yau T, Cheng AL, Kaseb A, Qin S, Zhu A X, Chan S, Sukeepaisarnjaroen W, Breder V, Verset G, Gane E, Borbath I, Gomez Rangel JD, Merle P, Benzaghou FM, Banerjee K, Hazra S, Fawcett J, Rimassa L. VP10-2021: Cabozantinib (C) plus atezolizumab (A) vs sorafenib (S) as first-line systemic treatment for advanced hepatocellular carcinoma (aHCC): Results from the randomized phase III COSMIC-312 trial. Ann Oncol. 2022;33:114-116. [RCA] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 92. | Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, Li Q, Lu Y, Chen Y, Guo Y, Chen Z, Liu B, Jia W, Wu J, Wang J, Shao G, Zhang B, Shan Y, Meng Z, Gu S, Yang W, Liu C, Shi X, Gao Z, Yin T, Cui J, Huang M, Xing B, Mao Y, Teng G, Qin Y, Xia F, Yin G, Yang Y, Chen M, Wang Y, Zhou H, Fan J; ORIENT-32 study group. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol. 2021;22:977-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 671] [Article Influence: 167.8] [Reference Citation Analysis (1)] |
| 93. | Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, Shao G, Xu L, Yin T, Liu J, Ren Z, Xiong J, Mao X, Zhang L, Yang J, Li L, Chen X, Wang Z, Gu K, Pan Z, Ma K, Zhou X, Yu Z, Li E, Yin G, Zhang X, Wang S, Wang Q. Camrelizumab in Combination with Apatinib in Patients with Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-label, Phase II Trial. Clin Cancer Res. 2021;27:1003-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 399] [Article Influence: 99.8] [Reference Citation Analysis (0)] |
| 94. | Yang D, Han Z, Oppenheim JJ. Alarmins and immunity. Immunol Rev. 2017;280:41-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 296] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 95. | Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1356] [Cited by in RCA: 2056] [Article Influence: 228.4] [Reference Citation Analysis (0)] |
| 96. | Cheng AL, Hsu C, Chan SL, Choo SP, Kudo M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol. 2020;72:307-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 373] [Article Influence: 74.6] [Reference Citation Analysis (1)] |
| 97. | Greten TF, Mauda-Havakuk M, Heinrich B, Korangy F, Wood BJ. Combined locoregional-immunotherapy for liver cancer. J Hepatol. 2019;70:999-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 98. | Han JW, Yoon SK. Immune Responses Following Locoregional Treatment for Hepatocellular Carcinoma: Possible Roles of Adjuvant Immunotherapy. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 99. | Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, Lencioni R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 594] [Article Influence: 148.5] [Reference Citation Analysis (0)] |
| 100. | Singh P, Toom S, Avula A, Kumar V, Rahma OE. The Immune Modulation Effect of Locoregional Therapies and Its Potential Synergy with Immunotherapy in Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2020;7:11-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 101. | Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 856] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 102. | Arina A, Gutiontov SI, Weichselbaum RR. Radiotherapy and Immunotherapy for Cancer: From "Systemic" to "Multisite". Clin Cancer Res. 2020;26:2777-2782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 103. | IMMUTACE: A Phase 2 Single-Arm, Open-Label Study of Transarterial Chemoembolization in Combination With Nivolumab Performed for Intermediate-Stage Hepatocellular Carcinoma. Gastroenterol Hepatol (N Y). 2021;17:16-17. [PubMed] |
| 104. | Tai D, Loke K, Gogna A, Kaya NA, Tan SH, Hennedige T, Ng D, Irani F, Lee J, Lim JQ, Too CW, Ng MCH, Tham CK, Lam J, Koo SL, Chong HS, Goh GB, Huang HL, Venkatanarasimha N, Lo R, Chow PKH, Goh BKP, Chung A, Toh HC, Thng CH, Lim TKH, Yeong J, Zhai W, Chan CY, Choo SP. Radioembolisation with Y90-resin microspheres followed by nivolumab for advanced hepatocellular carcinoma (CA 209-678): a single arm, single centre, phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:1025-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 105. | Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383-91.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 388] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 106. | Kamiya T, Chang YH, Campana D. Expanded and Activated Natural Killer Cells for Immunotherapy of Hepatocellular Carcinoma. Cancer Immunol Res. 2016;4:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 107. | Wang FS, Liu MX, Zhang B, Shi M, Lei ZY, Sun WB, Du QY, Chen JM. Antitumor activities of human autologous cytokine-induced killer (CIK) cells against hepatocellular carcinoma cells in vitro and in vivo. World J Gastroenterol. 2002;8:464-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 77] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 108. | Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ, Gopal AK, Pagel JM, Lindgren CG, Greenberg PD, Riddell SR, Press OW. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261-2271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 559] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 109. | Holstein SA, Lunning MA. CAR T-Cell Therapy in Hematologic Malignancies: A Voyage in Progress. Clin Pharmacol Ther. 2020;107:112-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 122] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 110. | Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, Qu J, Wasielewska T, He Q, Fink M, Shinglot H, Youssif M, Satter M, Wang Y, Hosey J, Quintanilla H, Halton E, Bernal Y, Bouhassira DC, Arcila ME, Gonen M, Roboz GJ, Maslak P, Douer D, Frattini MG, Giralt S, Sadelain M, Brentjens R. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6:224ra25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1670] [Cited by in RCA: 1984] [Article Influence: 180.4] [Reference Citation Analysis (0)] |
| 111. | Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2061] [Cited by in RCA: 2323] [Article Influence: 232.3] [Reference Citation Analysis (0)] |
| 112. | Iglesias BV, Centeno G, Pascuccelli H, Ward F, Peters MG, Filmus J, Puricelli L, de Kier Joffé EB. Expression pattern of glypican-3 (GPC3) during human embryonic and fetal development. Histol Histopathol. 2008;23:1333-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 113. | Zhou F, Shang W, Yu X, Tian J. Glypican-3: A promising biomarker for hepatocellular carcinoma diagnosis and treatment. Med Res Rev. 2018;38:741-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 257] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 114. | Shirakawa H, Suzuki H, Shimomura M, Kojima M, Gotohda N, Takahashi S, Nakagohri T, Konishi M, Kobayashi N, Kinoshita T, Nakatsura T. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci. 2009;100:1403-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 211] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 115. | Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 683] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 116. | Zhai B, Shi, D, Gao H, Qi X, Jiang H, Zhang Y, Chi J, Ruan H, Wang H, Ru Q, Cindy, Li Z. A phase I study of anti-GPC3 chimeric antigen receptor modified T cells (GPC3 CAR-T) in Chinese patients with refractory or relapsed GPC3+ hepatocellular carcinoma (r/r GPC3+ HCC). J Clin Oncol. 2017;35:3049-3049. [RCA] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 117. | Trinh TL, Wu Q, Chang L, Ho M, Liu C. Abstract 2316: GPC3-specific chimeric antigen receptor T cell in combination with Sorafenib as a novel therapeutic treatment for hepatocellular carcinoma. Cancer Res. 2016;76:2316-2316. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 118. | Kufe DW. Mucins in cancer: function, prognosis and therapy. Nat Rev Cancer. 2009;9:874-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1026] [Cited by in RCA: 1119] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 119. | Nath S, Mukherjee P. MUC1: a multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med. 2014;20:332-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 612] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 120. | Terris B, Cavard C, Perret C. EpCAM, a new marker for cancer stem cells in hepatocellular carcinoma. J Hepatol. 2010;52:280-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 121. | Liu H, Xu Y, Xiang J, Long L, Green S, Yang Z, Zimdahl B, Lu J, Cheng N, Horan LH, Liu B, Yan S, Wang P, Diaz J, Jin L, Nakano Y, Morales JF, Zhang P, Liu LX, Staley BK, Priceman SJ, Brown CE, Forman SJ, Chan VW, Liu C. Targeting Alpha-Fetoprotein (AFP)-MHC Complex with CAR T-Cell Therapy for Liver Cancer. Clin Cancer Res. 2017;23:478-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 172] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 122. | Aranda F, Vacchelli E, Eggermont A, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Peptide vaccines in cancer therapy. Oncoimmunology. 2013;2:e26621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 123. | Haruyama Y, Kataoka H. Glypican-3 is a prognostic factor and an immunotherapeutic target in hepatocellular carcinoma. World J Gastroenterol. 2016;22:275-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 124. | Sawada Y, Yoshikawa T, Nobuoka D, Shirakawa H, Kuronuma T, Motomura Y, Mizuno S, Ishii H, Nakachi K, Konishi M, Nakagohri T, Takahashi S, Gotohda N, Takayama T, Yamao K, Uesaka K, Furuse J, Kinoshita T, Nakatsura T. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res. 2012;18:3686-3696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 233] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 125. | Sawada Y, Yoshikawa T, Ofuji K, Yoshimura M, Tsuchiya N, Takahashi M, Nobuoka D, Gotohda N, Takahashi S, Kato Y, Konishi M, Kinoshita T, Ikeda M, Nakachi K, Yamazaki N, Mizuno S, Takayama T, Yamao K, Uesaka K, Furuse J, Endo I, Nakatsura T. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016;5:e1129483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 126. | Kiuchi Y, Suzuki H, Hirohashi T, Tyson CA, Sugiyama Y. cDNA cloning and inducible expression of human multidrug resistance associated protein 3 (MRP3). FEBS Lett. 1998;433:149-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 145] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 127. | Mizukoshi E, Nakagawa H, Kitahara M, Yamashita T, Arai K, Sunagozaka H, Iida N, Fushimi K, Kaneko S. Phase I trial of multidrug resistance-associated protein 3-derived peptide in patients with hepatocellular carcinoma. Cancer Lett. 2015;369:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 128. | Charneau J, Suzuki T, Shimomura M, Fujinami N, Nakatsura T. Peptide-Based Vaccines for Hepatocellular Carcinoma: A Review of Recent Advances. J Hepatocell Carcinoma. 2021;8:1035-1054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 129. | Palucka K, Ueno H, Fay J, Banchereau J. Dendritic cells and immunity against cancer. J Intern Med. 2011;269:64-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 130. | Mastelic-Gavillet B, Balint K, Boudousquie C, Gannon PO, Kandalaft LE. Personalized Dendritic Cell Vaccines-Recent Breakthroughs and Encouraging Clinical Results. Front Immunol. 2019;10:766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 131. | Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20:7-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 1707] [Article Influence: 284.5] [Reference Citation Analysis (0)] |
| 132. | Rizell M, Sternby Eilard M, Andersson M, Andersson B, Karlsson-Parra A, Suenaert P. Phase 1 Trial With the Cell-Based Immune Primer Ilixadencel, Alone, and Combined With Sorafenib, in Advanced Hepatocellular Carcinoma. Front Oncol. 2019;9:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 133. | Iwashita Y, Tahara K, Goto S, Sasaki A, Kai S, Seike M, Chen CL, Kawano K, Kitano S. A phase I study of autologous dendritic cell-based immunotherapy for patients with unresectable primary liver cancer. Cancer Immunol Immunother. 2003;52:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 134. | Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 135. | Mizukoshi E, Nakamoto Y, Arai K, Yamashita T, Mukaida N, Matsushima K, Matsui O, Kaneko S. Enhancement of tumor-specific T-cell responses by transcatheter arterial embolization with dendritic cell infusion for hepatocellular carcinoma. Int J Cancer. 2010;126:2164-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 136. | Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nat Med. 2001;7:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 377] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 137. | Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 350] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 138. | Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, Milhem M, Cranmer L, Curti B, Lewis K, Ross M, Guthrie T, Linette GP, Daniels GA, Harrington K, Middleton MR, Miller WH Jr, Zager JS, Ye Y, Yao B, Li A, Doleman S, VanderWalde A, Gansert J, Coffin RS. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol. 2015;33:2780-2788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1591] [Article Influence: 159.1] [Reference Citation Analysis (0)] |
| 139. | Yoo SY, Badrinath N, Woo HY, Heo J. Oncolytic Virus-Based Immunotherapies for Hepatocellular Carcinoma. Mediators Inflamm. 2017;2017:5198798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 140. | Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, Cho M, Lim HY, Chung HC, Kim CW, Burke J, Lencioni R, Hickman T, Moon A, Lee YS, Kim MK, Daneshmand M, Dubois K, Longpre L, Ngo M, Rooney C, Bell JC, Rhee BG, Patt R, Hwang TH, Kirn DH. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 614] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 141. | Moehler M, Heo J, Lee HC, Tak WY, Chao Y, Paik SW, Yim HJ, Byun KS, Baron A, Ungerechts G, Jonker D, Ruo L, Cho M, Kaubisch A, Wege H, Merle P, Ebert O, Habersetzer F, Blanc JF, Rosmorduc O, Lencioni R, Patt R, Leen AM, Foerster F, Homerin M, Stojkowitz N, Lusky M, Limacher JM, Hennequi M, Gaspar N, McFadden B, De Silva N, Shen D, Pelusio A, Kirn DH, Breitbach CJ, Burke JM. Vaccinia-based oncolytic immunotherapy Pexastimogene Devacirepvec in patients with advanced hepatocellular carcinoma after sorafenib failure: a randomized multicenter Phase IIb trial (TRAVERSE). Oncoimmunology. 2019;8:1615817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 142. | Oh CM, Chon HJ, Kim C. Combination Immunotherapy Using Oncolytic Virus for the Treatment of Advanced Solid Tumors. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |