Published online Mar 15, 2023. doi: 10.4251/wjgo.v15.i3.389
Peer-review started: October 28, 2022
First decision: January 3, 2023
Revised: January 17, 2023
Accepted: February 7, 2023
Article in press: February 7, 2023
Published online: March 15, 2023
Processing time: 137 Days and 7.4 Hours
Chronic inflammation, through a variety of mechanisms, plays a key role in the occurrence and development of digestive system malignant tumors (DSMTs). In this study, we feature and provide a comprehensive understanding of DSMT prevention strategies based on preventing or controlling chronic inflammation. The development and evaluation of cancer prevention strategies is a longstanding process. Cancer prevention, especially in the early stage of life, should be emphasized throughout the whole life course. Issues such as the time interval for colon cancer screening, the development of direct-acting antiviral drugs for liver cancer, and the Helicobacter pylori vaccine all need to be explored in long-term, large-scale experiments in the future.
Core Tip: Chronic inflammation plays an important role in the development of digestive system malignant tumors (DSMTs). The prevention and control of chronic inflammation is the key process of pre-disease prevention of DSMTs. This article summarizes the current prevention strategies of DSMTs based on chronic inflammation control. Health management throughout the life course significantly reduces the risk of cancer, especially in the early stages of life.
- Citation: Zhang YH, Chen XL, Wang YR, Hou YW, Zhang YD, Wang KJ. Prevention of malignant digestive system tumors should focus on the control of chronic inflammation. World J Gastrointest Oncol 2023; 15(3): 389-404
- URL: https://www.wjgnet.com/1948-5204/full/v15/i3/389.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i3.389
Cancer is the leading cause of death and an important obstacle to increasing world life expectancy. According to the latest global report, the global cancer burden is projected to increase by 47% in 2040 over 2020, reaching 28.4 million cases. Global cancer morbidity and mortality are increasing rapidly[1]. Digestive system malignant tumors (DSMTs) account for more than one-third of all cancer deaths, and mainly include colorectal (9.4%), liver (8.3%), gastric (7.7%), esophageal (5.5%) and pancreatic (4.7%) cancer[1].
Inflammation represents the host's immune response to destructive stimuli caused by irritants or pathogens. While most pathogens stir up an acute inflammatory response that completely clears irritants from suitable hosts, insufficient resolution of inflammation and an unrestricted inflammatory response can trigger chronic inflammation, destroy host immunity, and predispose the host to various diseases, including cancer[2]. The link between chronic inflammation and DSMTs has been established for more than a century[3,4]. Approximately 25% of cancer cases have been estimated to be related to chronic inflammation[5]. In fact, chronic inflammation tends to lead to tumor formation in various gastrointestinal organs, including hepatocellular carcinoma (HCC) caused by chronic hepatitis induced by hepatitis B virus (HBV) or hepatitis C virus (HCV), gastric cancer (GC) caused by chronic gastritis associated with Helicobacter pylori (H. pylori), and colorectal cancer (CRC) caused by inflammatory bowel disease (IBD).
Much research evidence suggests that chronic inflammation contributes greatly to tumorigenesis, but the underlying molecular mechanisms are intricate. The innate immune system has pro-tumor and anti-tumor effects on tumorigenesis. The innate immune response can protect the host from tumors induced by viruses by inhibiting or eliminating viral infections. On the other hand, swift removal of pathogens and suppression of inflammation will establish an appropriate inflammatory microenvironment for tumor formation[6-8]. Recent studies have found that when persistent inflammation occurs, the inflammasome complex begins to activate, causing it to assemble and further activate caspase, produce pro-inflammatory cytokines, and induce pyroptosis[9]. Apoptosis-associated speck-like protein containing a CARD promotes tumors through the nuclear factor kappa B (NF-κB) signaling pathway in GC[10]. Nucleotide-binding oligomerization domain, leucine-rich repeat, and pyrin domain-containing protein have the ability to support rapid migration of cancer cells in vitro and metastasis in vivo in CRC[11].
Because DSMT diagnosis usually occurs in the late stages of disease development, more work is needed to realize the enormous potential of primary prevention and early detection. This article summarizes the latest progress in DSMT prevention strategies based on chronic inflammation.
The global incidence of DSMTs has decreased gradually, while DSMT mortality has remained steadily at 35.6%. Major DSMTs are colorectal (1.14 million new cases in 2020), gastric (1.08 million), and liver (906577) cancers. In the early stage, most DSMT patients are asymptomatic or have chronic inflammation, which is not easy to detect. When clinical symptoms appear, they are usually in the late stage of the disease. Due to the late diagnosis of DSMTs, the 5-year survival rate is usually very low[12].
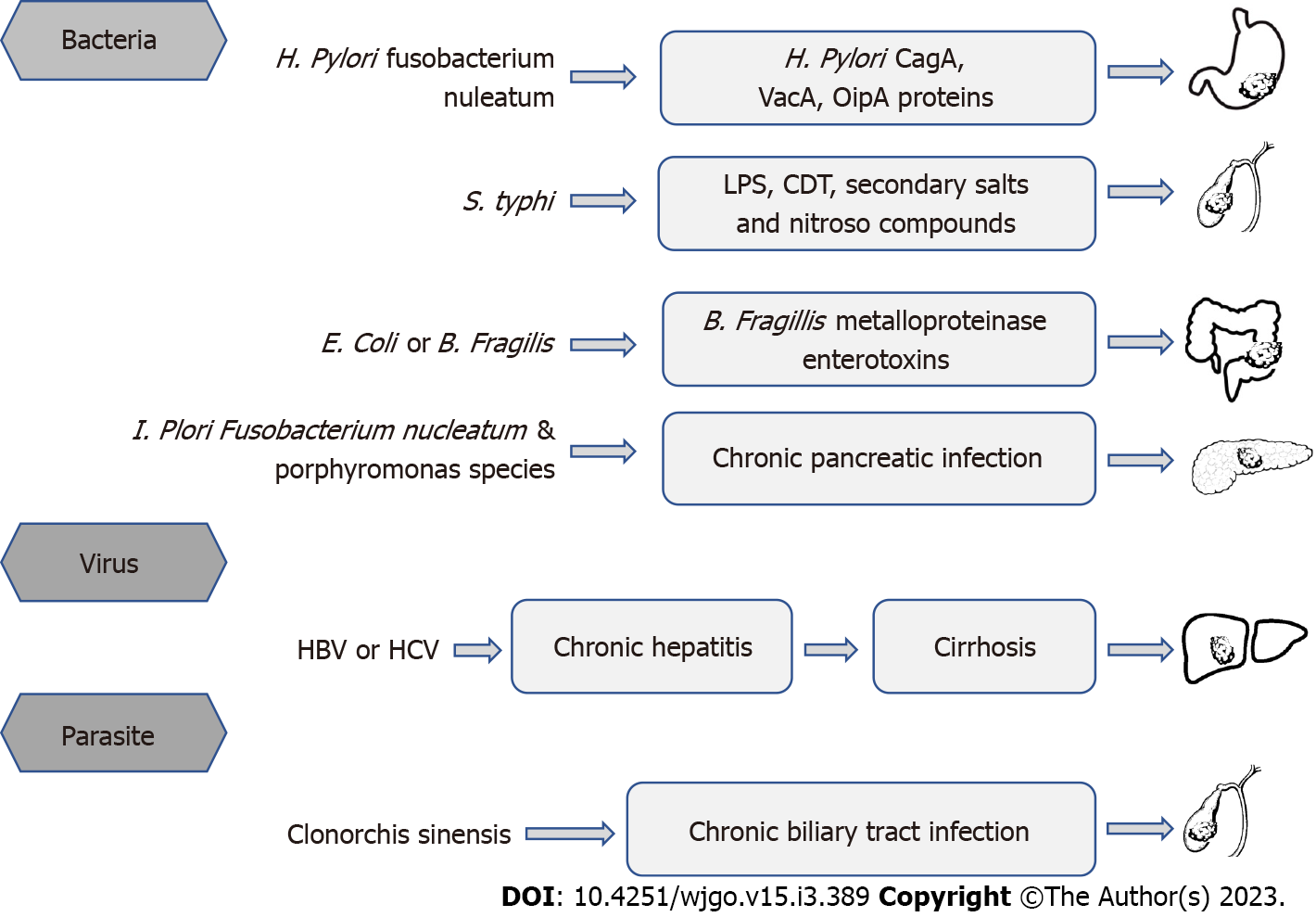
Chronic inflammation caused by infection with potential carcinogens is a major risk factor for DSMTs (Figure 1). Parkin DM’s study showed that more than one-quarter of cancers were attributable to infection[13]. In 2018, an estimated 2.2 million diagnosed cancer cases were attributed to infections worldwide[12]. Primary causes were H. pylori (810000 cases), HBV (360000), and HCV (160000). The highest infection-attributable age-standardized incidence rates (ASIR) was in eastern Asia (37.9 cases per 100000 person-years) and sub-Saharan Africa (33.1), and the lowest ASIR was in northern Europe (13.6) and western Asia (13.8). China accounts for one-third of worldwide cancer cases attributable to infection, driven by the high ASIR of H. pylori (15.6) and HBV (11.7) infection[12]. The overall burden of cancer caused by infection is the same in men and women. In Asia, among six major infectious agents, new cancer cases were mostly attributed to H. pylori (31.5%), followed by HBV (28.6%), and HPV (22.0%).
More than half of all DSMTs were caused by modifiable risk factors, including alcohol consumption and smoking, chronic inflammation caused by infection, diet, and obesity[14]. The marked temporal variations in the main DSMT incidence over the past decades are largely ascribed to changes in the prevalence of these risk factors[15,16]. Since most DSMT diagnoses[17,18] occur at an advanced stage with poor prognosis, mortality trends at specific sites usually reflect incidence trends. In 2040, the number of new DSMT cases and deaths worldwide are expected to increase by 58% and 73%, respectively[12]. This estimated burden level highlights the necessity of identifying the best clinical approaches for DSMTs, as well as the need to prioritize and implement preventative strategies. DSMT incidence is declining, while mortality and the number of cancer deaths are still high.
To date, many studies have linked chronic inflammation to DSMTs, including tumors in the colon, liver, stomach, and other parts of the digestive tract[19-22]. Chronic inflammation can generate the production of chemokines, growth factors, reactive oxygen species (ROS), and reactive nitrogen species (RNS). These mediators trigger inflammatory pathways in digestive system epithelial cells including the cyclooxygenase-2 (COX-2), NF-κB, signal transducer and activator of transcription 3 (STAT3), and inducible nitric oxide synthase pathways. Subsequently, activation promotes tumor initiation by increasing cell circulation, inhibiting tumor inhibitory pathways, and activating oncogenes[3]. As soon as tumors are established, inflammation promotes tumor progression by suppressing apoptosis, promoting cell proliferation and angiogenesis, regulating cellular adhesion, and promoting metastasis[23]. Due to their high activity against cancer and inflammation, natural products and their derivatives have been studied, such as berberine[24]. Chronic inflammation caused by exposure to long-term environmental irritants or associated with infection or autoimmune disease precedes tumor devel
The gastrointestinal tract not only has the function of digesting food and absorbing nutrients but also provides a physical barrier for the body to resist a large number of pathogenic or commensal microorganisms in the gastrointestinal cavity[28]. Through many innate immune mechanisms, the gastrointestinal tract prevents and clears pathogenic microbial infections of the intestine[29]. Host inflammation affects gut microbiome composition and functional capacity[30]. Chronic inflammation can target and induce the expansion of gut microbes[31]. Gut microbiota forms the host immune system and is essential for human health[32]. The appearance of gut inflammatory diseases, such as colitis, is associated with changes in the gut microbes[33]. Colitis occurs when microbes change from a “eubiotic” to a “dysbiotic” state, which is a risk factor of CRC development[34]. Compelling evidence are supporting that alteration of gut microbiota, particularly the dysbiosis condition might produce enrichment in pro-inflammatory opportunistic pathogens and a decrease in butyrate-producing bacteria, which may lead to an imbalance in intestinal homeostasis that could ultimately lead to tumor formation[23,35,36]. The relationship between H. pylori and GC is the most iconic relationship between individual microbial species and cancer[37]. When H. pylori is present, making up 40%-90% of the stomach microbiome, it becomes the richest organism in the stomach microbiome[38]. H. pylori contains toxins like cytotoxin-associated gene A (CagA) and cytotoxin-associated gene A pathogenicity island (VacA), which can manipulate cell survival and chronic inflammation that leads to cancer[39]. Many mechanisms could explain how microbes influence colorectal oncogenesis. Mucosa-associated Escherichia coli and Bacteroides fragilis (B. fragilis) are often found in tissues of patients with Crohn's disease and CRC[40]. B. fragilis produces a zinc-dependent metalloproteinase toxin called Bifidobacterium fragilis toxin, which cleaves the extracellular domain of the cell surface protein E-cadherin, resulting in the complete degradation of E-cadherin[41]. Long-term colonization of the colonic epithelium by B. fragilis increases the risk of CRC. Loss of surface barrier function in CRC triggers commensal bacteria-induced tumors that promote inflammation. The commensal bacteria themselves can also invade tumor tissue, induce tumor-infiltrating myeloid cells to produce inflammatory cytokines, and promote CRC oncogenesis[23]. The resistance response induced by Fusobacterium nucleatum (F. nucleatum) in the host induces an inflammatory environment and promotes the recruitment of inflammatory cells as well as the secretion of inflammatory factors[42]. This effect of F. nucleatum creates a microenvironment conducive to tumor growth. About 3%-5% of acute Salmonella Typhi (S. Typhi) infected people become chronic carriers. Since S. Typhi infection persists in the biliary system, leading to chronic infection of the gallbladder[43]. A case-control study showed that chronic typhoid carriers died from hepatobiliary cancer six times more frequently than in the control group[44]. A study conducted in Chile, a country with the highest infection rate in the world, showed that early detection of S. Typhi is critical for the development of gallbladder cancer prevention strategies[45].
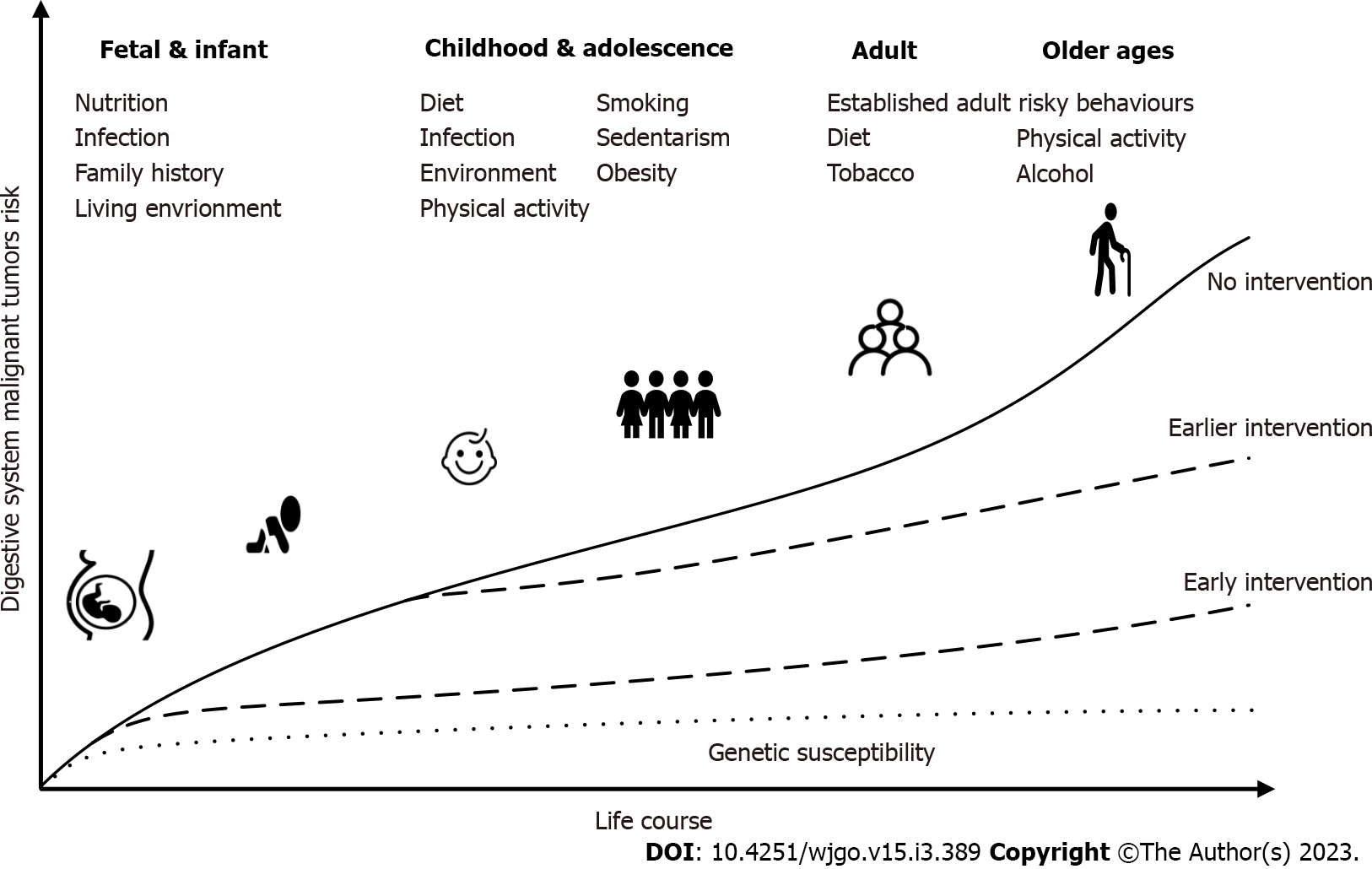
Many studies have shown that several chronic diseases, such as cancer, which are more common in later adulthood, are influenced by social and psychological environments at birth, during childhood, in adolescence, and during early adulthood[46]. Studies have shown that an accumulation of damage over the course of life is the cause of disease, not simply what happens at a certain point in life. DSMT is a multifactorial complex systemic chronic disease. Most of the underlying exposures cannot be considered individual factors, and exposures cannot be treated as separate or isolated factors because of their role at different levels, which vary from time to time. Life-course epidemiology[47] attempts to combine the entire biological and social risk processes leading to chronic disease. It studies how exposure to social patterns at various stages of life (childhood, adolescence, and early adulthood) affects disease risk and socioeconomic status in adulthood, which may lead to social inequality in adult health and mortality[48]. Current research in life course epidemiology has focused on chronic infectious diseases, psychological diseases, cardiovascular diseases, and other aspects. Cancer is also regarded as a noninfectious chronic disease, and many related life course epidemiological studies have been carried out on cancer, such as aerodigestive cancer[49], HCC[50], and CRC[51]. Most of these studies focus on early life variable risk factors such as chronic inflammation and diet. The time from susceptibility to disability, death, or recovery is variable. In many nonpersistent infections, symptoms of the disease occur within days (more over a longer period) of the initial infection. For example, HBV and HCV persist and replicate in the body, and may not cause disease (primary liver cancer in this case) until 50 years after infection. Age at the time of infection also affects the severity and progression of the disease. Epstein-Barr virus infection hardly causes any symptoms in childhood, whereas it results in glandular fever in adolescence. With increasing age, hepatitis A has become an increasingly serious disease. In terms of the origin of diseases (such as cancer), the core of the life course health development model is to find the causes from the life course framework, focus on identifying high-risk phenotypes and risk markers in early life, and then take measures to prevent diseases and promote health. We should look at the healthy development trajectory from the perspective of the whole life course (Figure 2). Disease prevention and health promotion are important throughout the whole life course, but in the “window of opportunity” period, such as fetal development, childhood, and adolescence, the intervention is more effective[52]. The earlier an intervention occurs, the better the effect of disease prevention.
Population-wide prevention includes avoidance of known carcinogens, enhancement of host defense mechanisms, lifestyle changes, and chemoprevention. In cancers associated with chronic inflammation, eradication of the relevant pathogen must be considered a population-wide preventative measure. Target population prevention includes screening and treatment of precancerous lesions or early cancers.
According to the Global Cancer Statistics 2020 report, CRC is the third most common cancer worldwide, and its incidence has been increasing despite some advances in screening and treatment[53]. Due to the lack of effective treatment, the 5-year survival rate of stage four CRC patients at diagnosis is less than 10%[23]. Although developed countries have the highest CRC incidence and mortality around the world, CRC incidence has recently shown an increasing trend in low-income and middle-income nations[54]. This trend reflects changes in lifestyle factors and diet: increased intake of animal-derived foods and sedentary lifestyles lead to reduced physical activity and a higher prevalence of excess body weight[1]. However, complex reasons behind this reflect both population ageing and global population growth, as well as changes in the prevalence and distribution of major cancer risk factors. The study found that in countries undergoing major transitions, such as Eastern Europe, Southern Europe, Central and South Asia, and South America, incidence tends to increase consistently with the human development index[55].
CRC has long been considered to be closely related to chronic inflammation, which can occur in the early stages of tumor onset. Studies have shown that[56] IBD, including Crohn’s disease and ulcerative colitis (UC), dramatically increases the risk of colitis-associated CRC (CAC). UC patients have a 2.4-fold increased risk of CAC[57]. Currently, the relationship between IBD patients and CRC has been widely confirmed. Compared with the general population, patients with long-term colonic IBD have a higher risk of CRC[58]. Any event that promotes and maintains inflammation may be considered a potential carcinogen[59]. Under healthy conditions, there is a strictly controlled interaction between enterocytes and intestinal immune system cells to maintain the balance between proinflammatory mediators [e.g., tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6] and anti-inflammatory signals (e.g., IL-10 and transforming growth factor-β)[60]. In IBD, an imbalance leads to changes in cell behavior. In CAC, various inflammatory mediators (e.g., TNF, IL-17A, and IL-23) and genotoxic substances (i.e., ROS and RNS) generated by different cellular systems (immune cells and mesenchymal cells) synergistically introduce genetic and epigenetic modifications, eventually leading to tumorgenesis[61]. The genetic mechanisms of tumorigenesis in CAC are similar to that in sporadic CRC, including chromosomal instability, microsatellite instability, mutations in pivotal tumor suppressor genes, and aneuploidy. But the timing and frequency of these conditions are different between CAC and sporadic CRC[62]; TP53 variants and aneuploidy were detected earlier in CAC, than sporadic CRCs, while KRAS and APC variants showed a lower prevalence at later stages of tumorigenesis[60]. Recently, some scholars[42] have found that F. nucleatum promotes the occurrence of CRC through several virulence mechanisms: adhesion to the intestinal epithelium or induction of host inflammation and host immune response.
Population-wide prevention: Population-wide prevention is an important supplement to CRC screening and prevention. In addition to some inherent risk or protective factors, such as sex, age, family history, and genetic predisposition, epidemiological studies have revealed some potentially modifiable factors related to the increase or decrease in CRC risk, which all point out the challenges and potential opportunities for prevention.
Various nutrients with proinflammatory or anti-inflammatory activity may affect CRC risk through intertwined pathways, such as intestinal microbial metabolism[63]. In recent years, there have been many studies on the prevention measures of CRC based on the prevention and control of chronic inflammation. Smoking, excessive drinking, being overweight or obese, Western diets and processed meats, and inflammatory eating patterns are risk factors that have been recognized in recent years. On the other hand, physical activity, regular use of aspirin and hormone replacement therapy, non-steroidal anti-inflammatory drugs (NSAIDs), probiotics, and the Mediterranean diet were found to be associated with decreased CRC risk. There are signs that consuming milk and whole grains may also protect against CRC[64]. Inflammation may be a potential mechanism linking dietary patterns to CRC development[65]. A large cohort study of 121050 adults who were followed up for 26 years found that the risk of CRC increased significantly with the intake of a proinflammatory diet. Reducing the adverse effects of an inflammatory diet may reduce the risk of CRC[66]. Physical activity can reduce systemic inflammation, reduce the level of proinflammatory cytokines related to CRC, or affect the inflammatory microenvironment, which may play a crucial role in reducing the risk of CRC[67]. With respect to the use of aspirin in the general population, the incidence of CRC decreased by 26% after 23 years of follow-up. The use of NSAIDs, such as aspirin and celecoxib (COX-2 inhibitors), has a protective effect on the occurrence of CRC[68]. Studies have shown that the potential mechanisms related to the use of probiotics are alterations in the gut microbiota and physicochemical conditions, the production of antioxidant metabolites, a reduction in intestinal inflammation, and the production of harmful enzymes to support the prevention of colon cancer[69].
Targeted screening: CRC usually develops slowly over a period of several decades after normal colonic epithelium is transformed into an adenoma, providing ample time for intervention to prevent CRC[70]. In 1980, the American Cancer Society recommended the use of fecal occult blood tests for early diagnosis of CRC every year for people over 50 years old[71]. Since then, inspection technology has developed rapidly[72] and the screening strategy has been continuously updated[73-76]. Currently, common screening techniques include colonoscopy, rectal endoscopy, and fecal occult blood tests. At the same time, many new technologies, such as color endoscopy, are being studied as complementary technologies to improve the early detection of dysplasia and cancer in high-risk populations[77]. One study evaluated colonoscopy and regular endoscopic follow-up of patients who had undergone precursor resection. Compared with the external control group, the incidence, and mortality of rectal cancer decreased by 31% and 18%, respectively, after 20 years of follow-up[78]. Researchers at the Harvard School of Public Health (HPFS) found that 40% of CRC can be prevented if people undergo colonoscopies regularly[79]. Target population screening can effectively reduce the incidence and mortality of CRC. The incidence and mortality of screening are reduced by approximately 50% and 53%, respectively, but the gap can be filled by modifying the prevention strategy of attributable risk factors in the whole population. However, there are several aspects of CRC screening problems in clinical practice that require specific attention: Who should be provided with CRC screening? When should the first screening test be given? Is the screening interval the same for everyone? How screening strategies be developed? The burden of disease and the overall socioeconomic situation vary from region to region, and each region should propose screening recommendations suitable for its population based on various international standards and consensus.
Population-wide prevention requires a lifelong perspective and may have benefits in the long run. Since CRC and other common chronic diseases have many of the same risk and protective factors, long-term universal prevention efforts aimed at reducing CRC risk factors may provide far more than just the expected benefits[80]. Changing dietary and lifestyle factors may have a significant overall impact on the risk of CRC. In previous HPFS studies, up to 70% of the total burden of colon cancer in the United States population could be prevented by moderately changing diet and lifestyle[81]. To further integrate the combined effects of modifiable risk factors, a comprehensive model of colon cancer incidence was developed that took into account the changes in risk factors throughout the life course. The study found that women with “high-risk” lifestyle factors were nearly four times more likely to develop colon cancer than women in the “low-risk” group[82]. Although endoscopic screening can reduce the incidence of cancer in these high-risk women, the CRC risk after this reduction is still significantly higher than that of medium- and low-risk women. Therefore, a population-wide prevention strategy of changing lifestyle in early life is an important supplement to CRC screening.
HCC is the most common form of primary liver cancer (75%-85% of cases). The incidence and mortality of liver cancer have declined in many high-risk countries in eastern and southeastern Asia since the 1970s and in Japan since the 1990s. Vaccination against HBV had been a major public health success. It was first introduced to high-risk countries in East Asia in the early 1980s and greatly reduced the prevalence of HBV infection and the incidence of HCC[83]. It is believed that the aetiology of HCC is mainly related to cirrhosis, viral hepatitis, alcoholic liver disease, metabolic-related fatty liver disease, aflatoxin infection, heavy drinking, being overweight, type 2 diabetes, and smoking[1]. HBV infection and HCV infection account for 56% and 20% of global liver cancer deaths, respectively. Although nonviral risk factors are increasingly important for the burden of liver cancer, the elimination of viral hepatitis is still a key strategy for the primary prevention of liver cancer worldwide[84]. Viral hepatitis is the main pathogenic factor, the most common of which is chronic HBV and HCV infection. Compared with HCV infection alone, cirrhosis and HCC are more likely to occur in patients with HBV/HCV coinfection[85]. Because the chronic infection is usually asymptomatic, many infected people are not diagnosed[86]. As of 2015, an estimated 290 million people worldwide remained undiagnosed, and more than 80% of liver cancer patients are diagnosed at an advanced stage[87].
HCC usually occurs in tissues that experience chronic inflammation[88]. Although the underlying molecular mechanisms of the aetiology are different, in most cases, chronic liver inflammation and the resulting cirrhotic microenvironment promote the initiation and development of HCC[89]. Repeated liver inflammatory injury can lead to liver cell damage, cirrhosis, and ultimately hepatocellular carcinoma[90]. The microbiome profile, consuming a high-fat Western diet, and a high intake of alcohol[91] are associated with various forms of inflammations, which will promote the onset of HCC. The main trigger of inflammation associated with liver cancer is epithelial cell death. Pathways contributing to inflammation-mediated hepatocarcinogenesis mainly include cytokine signaling (TNF-α, IL-6, NF-κB, JNK, and STAT3), innate immune signaling, and adaptive immunity[89]. Experimental evidence indicates that HCV may also contribute to hepatocarcinogenesis directly through the interference of viral proteins with host cell signaling pathways involved in cell survival, transformation, proliferation, and angiogenesis[92]. Benkheil’s studies have shown that activation of the epidermal growth factor receptor (EGFR) and downstream signaling through mitogen-activated protein kinase contribute to the expression of various proinflammatory and angiogenic proteins involved in the pathogenesis of liver cancer. These data suggest that sustained activation of EGFR in patients with chronic HCV infection may be a mechanism by which HCV contributes to the pathogenesis of liver cancer[93].
To reduce the burden of global HCC, the global HCC management strategy[94] points out that four major areas need to be improved: prevention of HBV and HCV infection; treatment of chronic hepatitis B, hepatitis C, and liver disease; reduction in exposure to dietary and metabolic risk factors; and improvement in the detection, diagnosis, and treatment of liver cancer. Three of them are related to the prevention and treatment mechanisms of chronic hepatitis.
Population-wide prevention: Population-wide prevention includes universal coverage of the hepatitis B vaccine, control of chronic viral hepatitis through antiviral therapy, and a reduction in environmental and lifestyle-related risk factors[94]. Since the World Health Organization proposed a hepatitis B immunization mid-course target trial for liver cancer prevention in 1983, 189 countries had introduced hepatitis B vaccines into their national infant immunization programs by the end of 2019, with an estimated 85% global coverage of three doses[1]. Increasing evidence showed that this large-scale vaccination greatly reduces the burden of hepatitis B virus-related diseases. This campaign in China has reduced the prevalence of new HBV infections by 90%. It will prevent approximately 2.8 to 3.5 million HBV-related deaths in the future[95]. Mother-to-child transmission (MTCT) is the main route of HBV transmission and its prevention is very important to eliminate HBV. Strengthening the standardized management of pregnant women and their infants with chronic HBV infection is an effective measure to eliminate HBV-MTCT[96]. There is evidence that antiviral therapy for pregnant women with a high HBV load in late pregnancy can reduce the risk of MTCT[97]. A population-based study in Taiwan showed that the HCC incidence in a birth cohort without the hepatitis B vaccine was four times higher than that in a birth cohort with the hepatitis B vaccine[98].
In addition to vaccination against HBV, screening for HBV and HCV in high-risk populations, and universal access to medication for chronic hepatitis B and hepatitis C infection in infected patients will reduce the burden of global liver cancer. Currently, there is no vaccine available to prevent HCV infection. Direct-acting antiviral agents (DAAs) are short-course (8-12 wk) oral drugs[94,99]. The emergence of DAAs as a new HCV drug with a high cure rate (> 95%) offers hope for the treatment of chronic hepatitis C. At present, the impact of DAA treatment of HCV infection on HCC, tumor recurrence, and progression has become a hot topic. Successful DAA treatment does seem to reduce the risk of HCC, but studies have shown that DAAs may increase the risk of HCC recurrence after treatment. Therefore, well-designed prospective multicenter studies are needed to fully characterize the clinical effect of DAA treatment on the risk of HCC recurrence. The countries with the highest prevalence of HCV are mainly low-income and middle-income countries, where a large proportion of infections occur in healthcare settings through unsafe injections and other invasive procedures. Strengthening infection control through safety measures, such as screening blood transfusions, preventing MTCT, and providing clean needles and medical facilities, is a key aspect of HCV control[100]. Although viral therapy has been improved through DAA therapy, cases of HCV-induced HCC are expected to increase at least until 2030[101]. The increase is mainly attributed to the increase in chronic HCV infection before 1992 when HCV screening was implemented and the disease progressed slowly[93]. People with known risk factors must be regularly monitored to detect early cancer lesions (monitoring and final treatment). Early detection and diagnosis of HCC can significantly improve the survival rate of patients.
For 400 million chronic HBV-infected patients, the hepatitis B vaccine is ineffective in preventing HCC[102]. Increasing evidence shows that persistent HBV replication is an important risk factor for HCC. For CHB patients, antiviral therapy to control viral replication may reduce the risk of HCC. Interferon or nucleoside analogues are effective antiviral drugs to prevent disease progression to cirrhosis and HCC[102]. Additionally, promoting a healthy diet and physical activity, reducing environmental and lifestyle exposure, and preventing metabolic syndrome, nonalcoholic fatty liver disease, and nonalcoholic steatohepatitis are ways to prevent HCC.
Targeted screening: Targeted screening includes early detection through HCC surveillance programs. The current practice guidelines recommend regular HCC screening by ultrasonography every two years for α-fetoprotein (AFP) in people with or without a clinically identifiable HCC risk above a certain threshold[103]. A series of cohort studies and model-based simulation studies have shown that HCC screening is cost-effective when more than 34% of high-risk patients can be screened for HCC, and is associated with improved early cancer detection rates, cure rates, and survival rates[104,105]. International and national liver cancer management guidelines have also been developed[106]. Most liver cancers found in China are advanced. Chinese experts have developed consensus guidelines for secondary prevention[107] that provide guidelines for the prevention, monitoring, and early diagnosis of primary liver cancer in patients with chronic liver disease. To improve the early diagnosis of liver cancer. With the rise in big data and bioinformatics, predicting individual liver cancer risk is critical to implementing effective and feasible liver cancer screening. At present, some studies have evaluated the combination of existing clinical symptoms and laboratory variables to develop HCC risk prediction scores. However, due to its limited performance, it has not been used in clinical practice[101]. New serum/plasma biomarkers (such as tumor-associated antigens)[108,109] have been explored as possible alternatives to AFP. To improve diagnostic performance, a comprehensive score combining serum biomarkers and clinical variables has been proposed and is awaiting clinical validation for further development and application.
Only a few people infected with HBV will continue to be infected, and the main determinant is the age of first contact with the virus[109]. Approximately 90% of children born to carrier mothers are infected, and perinatal infections are associated with about 90% of the risk of becoming carriers[110]. Infections are most common in children in Asia and many other parts of the Sahara and South Africa. For example, in Gambia, 35 to 70% of children were found to be infected at the age of five[111]. The prevalence among infected mothers in this population is much lower than that in China, but the chronic infection rate in this population remains high because children are approximately 20-30% more likely to become carriers after early infection. In contrast, the risk of becoming a carrier during adolescence or adulthood is less than 10%. This model makes the choice of prevention more clearer, and primary prevention of persistent infection must be carried out in the early life course[112].
GC remains a globally important malignant tumor, with more than one million new cases in 2020 and an estimated one GC death in every 13 deaths[12]. Although GC is often reported as a single entity, it can generally be divided into two topographical subsites: cardia GC and non-cardia GC. Since H. pylori was found in 1982, it has been closely related to a variety of digestive system diseases[113]. H. pylori infection is regarded as a risk factor for GC and is classified as a human class I carcinogen[114]. The prevalence of H. pylori infection is extraordinarily high, infecting 50% of the world’s population[115], and it mainly occurs in developing countries[116]. Moreover, less than 5% of infected hosts will develop cancer, likely due to differences in bacterial genetics, host genetics, age of infection acquisition, and environmental factors[117]. Based on world population data, the attributable fraction of noncardiac GC attributable to H. pylori infection is 89%[12,118], and the burden varies widely among regions[119].
H. pylori infection can cause the gastric mucosa to be susceptible to atrophic gastritis, intestinal metaplasia, dysplasia, and ultimately GC[120]. It is usually acquired in early life, followed by a long quiescent period, when chronic gastritis of different intensities is present, and the symptoms are not obvious[121]. Only 10%-15% of individuals with H. pylori infection will develop peptic ulcers, and it is estimated that the risk of GC is approximately 5%[1]. Peptic ulcers tend to be chronic infections in 20 to 30 years old patients, and GC occurs decades later. Currently, studies on the pathogenesis of H. pylori infection and GC are mainly classified into two main categories. One study found that H. pylori act on gastric epithelial cells by releasing virulence factors, namely, CagA and its pathogenicity island and VacA, which deregulates host intracellular signaling pathways and lowers the threshold for neoplastic transformation[116]. However, other related research has concentrated on H. pylori inducing inflammatory responses by recruiting circulating immune cells to the site of infection, which results in an active inflammatory microenvironment. Tumor-infiltrating lymphocytes interact with tumor cells via chemokines (IL-1, IL-6, IL-8, TNF-α, and TNF-β), inflammatory molecules, and matrix metalloproteinases to form an inflammatory network[122]. Overactivation of the NF-ĸB transcription factor and dysregulation of JAK/STAT pathway is considered to be the classical pathways[123] in the progression of H. pylori infection and GC-related research. These processes may play an important role in the progression of gastritis and GC, but their key regulators are not fully defined.
A continuous decline in noncardia GC incidence and mortality worldwide has been observed in the last half-century. However, due to the expected growing elderly population, the absolute number of patients is increasing and remains an important global health problem[124]. After H. pylori infection in early life, chronic gastritis develops slowly after the Correa cascade, i.e., chronic gastritis, atrophy, intestinal metaplasia, intraepithelial neoplasia, and GC[125]. The extremely long interval (approx. decades) of the developmental process offers us the chance to interrupt the carcinogenic cascade to prevent GC. Multimodal primary secondary GC prevention relies on 4 main pillars: the eradication of H. pylori infection, excellent endoscopies as “digitized eyes”, diagnostic reliability among pathologists, a structured health care system, and clinical specialists familiar with the management of high-risk patients[126].
Population-wide prevention: Prevention of GC includes the prevention and eradication of H. pylori infection, lifestyle modification, and chemoprevention. Clinical studies have shown that children aged 6-15 years provided with an oral H. pylori vaccine compared to those one year after vaccination had good protection against H. pylori infection with 71.8% prophylactic protection. From vaccine experiments to clinical application, there is still a long way to go, and longer follow-up studies are still needed to confirm its immune competence in the future[127]. Studies have suggested that the risk of recurrent peptic ulcer, peptic ulcer-related adverse events, and GC increases significantly with increasing latency to H. pylori eradication. All patients with peptic ulcers and confirmed H. pylori infection should receive eradication therapy as soon as possible[128]. To reduce the incidence of GC and related diseases, H. pylori eradication has begun nationwide in Japan and South Korea to save future medical burdens[129]. The 2016 Japanese guidelines for the management of H. pylori infections suggest the eradication of H. pylori in adolescence to control infections in the next generation[130]. Following H. pylori eradication, genetic and epigenetic markers have shown promise in GC risk stratification, but require further validation in prospective studies[124,131]. H. pylori eradication cannot regress all precancerous lesions, which may depend on the extent and extent of precancerous lesions at the time of eradication[132]. Once people are diagnosed with H. pylori infection, untreated infection will persist throughout the patient’s lifetime[133]. Lifestyle changes early in life or the establishment of good lifestyle habits and diets are adequate for low-risk individuals. Since the implementation of lifestyle prevention recommendations such as improvements in the preservation and storage of food, adequate hygienic housing, and the consumption of fresh vegetables and fruits, GC incidence has decreased substantially[134]. H. pylori eradication has been evaluated as a form of chemoprevention of GC by antimicrobial therapy with additional administration of NSAIDs, such as aspirin[135]. Chronic inflammatory mediators may serve as potential therapeutic targets for the prevention of GC[121]. Currently, there are no global guidelines for the management of H. pylori infection, but consensus guidelines for the management of H. pylori have been successively developed from region to region[136-139].
Targeted screening: Targeted screening includes the screening and treatment of precancerous lesions. Screening for timely detection and treatment of these epithelial tissue changes is equally important to prevent[123] in addition to the eradication of H. pylori infection. Selecting the most effective timing of screening and intervention has important effects on tissue gastric carcinogenesis[140]. Recently, guidelines related to GC screening have been developed in some countries such as China and the United Kingdom[124,141,142]. The statement of the Kyoto Consensus report[130] suggested that after the age of 12 in the infected area, the screening and treatment of infection should start to prevent the subsequent occurrence of precancerous lesions such as atrophy and intestinal metaplasia. The latest guidelines in China set the age for the start of screening at 45 for high-risk populations[142]. The screening interval should be formulated according to the disease burden, medical level, and social and economic conditions of GC in each region. The screening and treatment of H. pylori infection have potential cost-effectiveness to prevent GC, especially in the target high-risk population[143].
Studies need to explore effective interventions to eliminate infection and inflammation in pediatric populations. Some studies have introduced a third new family-based H. pylori eradication strategy[130,144] and this method can control H. pylori infection in family members and reduce the long-term complications by screening, identifying, treating, and tracking all H. pylori infected persons in the whole family[145]. In the long run, it is difficult to control H. pylori infection from the source due to the dynamic and gradual nature of the infection, thus increasing the medical burden in the later stage of the disease. However, a family-based eradication strategy will help solve the above problems. The 2016 H. pylori infection management guidelines revised in Japan (2019) suggested that individuals should receive eradication treatment before becoming parents to prevent infection within the family and transmission to the next generation[130]. The 2018 Bangkok Consensus report[144] states that it is recommended to receive screening and treatment for families of GC patients. These guidelines reflect that control of family infection is important to prevent H. pylori-induced diseases. Regular follow-up home treatment should be used to detect the infection status of children as soon as possible once parents are diagnosed with infection. Eliminating the risk factors or early life infection plays a key role in preventing gastric tumor genesis.
The prevention and control of chronic inflammation is count for much of the malignant digestive system tumor genesis. The whole life course management of human health is of great significance in combating chronic inflammation to prevent the occurrence of DSMTs. This article summarized the prevention strategies for DSMTs and their overall implementation based on the prevention or control of chronic inflammation. Although we have an understanding of the research status of chronic inflammation and DSMTs, we still have some questions to answer in the future. For example, how long is the interval between colonoscopy and screening, and how can interval cancer be prevented? Does DAA therapy play a role in the prevention of HCC? How can drug resistance and reinfection be avoided after eradication of H. pylori infection? Health education on cancer prevention knowledge still needs to be continued. The higher the disease awareness rate of people is, the greater the success in disease control. Cancer prevention should be given attention throughout the life course, especially in early life. Control and intervention related to infection and modifiable lifestyle changes in the early life course play an important role in cancer prevention.
We are very grateful to the other members of the research team for their careful review and suggestions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Kiuchi J, Japan; Rojas A, Chile; Sipos F, Hungary S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 63793] [Article Influence: 15948.3] [Reference Citation Analysis (174)] |
| 2. | Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1530] [Cited by in RCA: 1778] [Article Influence: 136.8] [Reference Citation Analysis (1)] |
| 3. | Nagai N, Kudo Y, Aki D, Nakagawa H, Taniguchi K. Immunomodulation by Inflammation during Liver and Gastrointestinal Tumorigenesis and Aging. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763-2779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 545] [Cited by in RCA: 629] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 5. | Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 515] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 6. | Nagarsheth N, Wicha MS, Zou W. Chemokines in the cancer microenvironment and their relevance in cancer immunotherapy. Nat Rev Immunol. 2017;17:559-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 964] [Cited by in RCA: 1533] [Article Influence: 191.6] [Reference Citation Analysis (0)] |
| 7. | Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol. 2018;18:309-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1132] [Cited by in RCA: 1901] [Article Influence: 271.6] [Reference Citation Analysis (0)] |
| 8. | Wang J, Li D, Cang H, Guo B. Crosstalk between cancer and immune cells: Role of tumor-associated macrophages in the tumor microenvironment. Cancer Med. 2019;8:4709-4721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 234] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 9. | Balahura LR, Selaru A, Dinescu S, Costache M. Inflammation and Inflammasomes: Pros and Cons in Tumorigenesis. J Immunol Res. 2020;2020:2549763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Deswaerte V, Nguyen P, West A, Browning AF, Yu L, Ruwanpura SM, Balic J, Livis T, Girard C, Preaudet A, Oshima H, Fung KY, Tye H, Najdovska M, Ernst M, Oshima M, Gabay C, Putoczki T, Jenkins BJ. Inflammasome Adaptor ASC Suppresses Apoptosis of Gastric Cancer Cells by an IL18-Mediated Inflammation-Independent Mechanism. Cancer Res. 2018;78:1293-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Deng Q, Geng Y, Zhao L, Li R, Zhang Z, Li K, Liang R, Shao X, Huang M, Zuo D, Wu Y, Ma Q. NLRP3 inflammasomes in macrophages drive colorectal cancer metastasis to the liver. Cancer Lett. 2019;442:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 12. | de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180-e190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1273] [Article Influence: 254.6] [Reference Citation Analysis (0)] |
| 13. | Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030-3044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1946] [Cited by in RCA: 1967] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 14. | Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, McCullough ML, Patel AV, Ma J, Soerjomataram I, Flanders WD, Brawley OW, Gapstur SM, Jemal A. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68:31-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 995] [Article Influence: 142.1] [Reference Citation Analysis (2)] |
| 15. | Arnold M, Pandeya N, Byrnes G, Renehan PAG, Stevens GA, Ezzati PM, Ferlay J, Miranda JJ, Romieu I, Dikshit R, Forman D, Soerjomataram I. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 667] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 16. | Song M, Giovannucci E. Preventable Incidence and Mortality of Carcinoma Associated With Lifestyle Factors Among White Adults in the United States. JAMA Oncol. 2016;2:1154-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 17. | Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2711] [Cited by in RCA: 3371] [Article Influence: 481.6] [Reference Citation Analysis (1)] |
| 18. | Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TM, Myklebust TÅ, Tervonen H, Thursfield V, Ransom D, Shack L, Woods RR, Turner D, Leonfellner S, Ryan S, Saint-Jacques N, De P, McClure C, Ramanakumar AV, Stuart-Panko H, Engholm G, Walsh PM, Jackson C, Vernon S, Morgan E, Gavin A, Morrison DS, Huws DW, Porter G, Butler J, Bryant H, Currow DC, Hiom S, Parkin DM, Sasieni P, Lambert PC, Møller B, Soerjomataram I, Bray F. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20:1493-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 718] [Article Influence: 119.7] [Reference Citation Analysis (0)] |
| 19. | Pereira JFS, Bessa C, Matos P, Jordan P. Pro-Inflammatory Cytokines Trigger the Overexpression of Tumour-Related Splice Variant RAC1B in Polarized Colorectal Cells. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Giraud J, Saleh M. Host-Microbiota Interactions in Liver Inflammation and Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Cai WY, Lin LY, Wang L, Yang L, Ye GD, Zeng Q, Cheng J, Xie YY, Chen ML, Luo QC. Inhibition of Bcl6b promotes gastric cancer by amplifying inflammation in mice. Cell Commun Signal. 2019;17:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Ishimoto T, Arima K, Bu L, Uchihara T, Miyake K, Eto T, Itoyama R, Baba H. Abstract 1987: Expansion of pancreatic cancer stem-like cells through PGE2 accumulation in inflammatory environment. Cancer Res. 2018;78. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Schmitt M, Greten FR. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. 2021;21:653-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 407] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 24. | McGowan EM, Lin Y, Chen S. Targeting Chronic Inflammation of the Digestive System in Cancer Prevention: Modulators of the Bioactive Sphingolipid Sphingosine-1-Phosphate Pathway. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8135] [Article Influence: 542.3] [Reference Citation Analysis (0)] |
| 26. | Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2609] [Cited by in RCA: 2781] [Article Influence: 146.4] [Reference Citation Analysis (0)] |
| 27. | Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 2078] [Article Influence: 129.9] [Reference Citation Analysis (1)] |
| 28. | Blyth GAD, Connors L, Fodor C, Cobo ER. The Network of Colonic Host Defense Peptides as an Innate Immune Defense Against Enteropathogenic Bacteria. Front Immunol. 2020;11:965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Gong W, Yang K, Zhao W, Zheng J, Yu J, Guo K, Sun X. Intestinal Gasdermins for regulation of inflammation and tumorigenesis. Front Immunol. 2022;13:1052111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 30. | Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, Huttenhower C. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1756] [Cited by in RCA: 2048] [Article Influence: 157.5] [Reference Citation Analysis (0)] |
| 31. | Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1374] [Cited by in RCA: 1649] [Article Influence: 126.8] [Reference Citation Analysis (1)] |
| 32. | Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1596] [Cited by in RCA: 1942] [Article Influence: 176.5] [Reference Citation Analysis (0)] |
| 33. | Sethi V, Kurtom S, Tarique M, Lavania S, Malchiodi Z, Hellmund L, Zhang L, Sharma U, Giri B, Garg B, Ferrantella A, Vickers SM, Banerjee S, Dawra R, Roy S, Ramakrishnan S, Saluja A, Dudeja V. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology. 2018;155:33-37.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 295] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 34. | Wang X, Yang Y, Huycke MM. Microbiome-driven carcinogenesis in colorectal cancer: Models and mechanisms. Free Radic Biol Med. 2017;105:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 35. | Zeng MY, Inohara N, Nuñez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 571] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 36. | de Oliveira GLV, Leite AZ, Higuchi BS, Gonzaga MI, Mariano VS. Intestinal dysbiosis and probiotic applications in autoimmune diseases. Immunology. 2017;152:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 199] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 37. | Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, Liu WD, Hu Y, Han ZX, Crystal-Mansour S, Pee D, Blot WJ, Fraumeni JF Jr, You WC, Gail MH. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012;104:488-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 352] [Article Influence: 27.1] [Reference Citation Analysis (1)] |
| 38. | Chen CC, Liou JM, Lee YC, Hong TC, El-Omar EM, Wu MS. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes. 2021;13:1-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (3)] |
| 39. | Wroblewski LE, Peek RM Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 1005] [Article Influence: 67.0] [Reference Citation Analysis (1)] |
| 40. | Martin HM, Campbell BJ, Hart CA, Mpofu C, Nayar M, Singh R, Englyst H, Williams HF, Rhodes JM. Enhanced Escherichia coli adherence and invasion in Crohn's disease and colon cancer. Gastroenterology. 2004;127:80-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 558] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 41. | Haghi F, Goli E, Mirzaei B, Zeighami H. The association between fecal enterotoxigenic B. fragilis with colorectal cancer. BMC Cancer. 2019;19:879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (1)] |
| 42. | Wu J, Li Q, Fu X. Fusobacterium nucleatum Contributes to the Carcinogenesis of Colorectal Cancer by Inducing Inflammation and Suppressing Host Immunity. Transl Oncol. 2019;12:846-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 43. | Dutta U, Garg PK, Kumar R, Tandon RK. Typhoid carriers among patients with gallstones are at increased risk for carcinoma of the gallbladder. Am J Gastroenterol. 2000;95:784-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 159] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 44. | Welton JC, Marr JS, Friedman SM. Association between hepatobiliary cancer and typhoid carrier state. Lancet. 1979;1:791-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 103] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Koshiol J, Wozniak A, Cook P, Adaniel C, Acevedo J, Azócar L, Hsing AW, Roa JC, Pasetti MF, Miquel JF, Levine MM, Ferreccio C; Gallbladder Cancer Chile Working Group. Salmonella enterica serovar Typhi and gallbladder cancer: a case-control study and meta-analysis. Cancer Med. 2016;5:3310-3235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 46. | Olvera Alvarez HA, Kubzansky LD, Campen MJ, Slavich GM. Early life stress, air pollution, inflammation, and disease: An integrative review and immunologic model of social-environmental adversity and lifespan health. Neurosci Biobehav Rev. 2018;92:226-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 47. | Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C. Life course epidemiology. J Epidemiol Community Health. 2003;57:778-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1234] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 48. | Ó Hartaigh B, Gill TM, Shah I, Hughes AD, Deanfield JE, Kuh D, Hardy R. Association between resting heart rate across the life course and all-cause mortality: longitudinal findings from the Medical Research Council (MRC) National Survey of Health and Development (NSHD). J Epidemiol Community Health. 2014;68:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Gupta B, Lalloo R, Johnson NW. Life course models for upper aero-digestive tract cancer. Int Dent J. 2015;65:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Yang B, Petrick JL, Kelly SP, Graubard BI, Freedman ND, McGlynn KA. Adiposity across the adult life course and incidence of primary liver cancer: The NIH-AARP cohort. Int J Cancer. 2017;141:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Husson O, Vissers PA, Denollet J, Mols F. The role of personality in the course of health-related quality of life and disease-specific health status among colorectal cancer survivors: A prospective population-based study from the PROFILES registry. Acta Oncol. 2015;54:669-677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Halfon N, Hochstein M. Life course health development: an integrated framework for developing health, policy, and research. Milbank Q. 2002;80:433-479, iii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 578] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 53. | Song M. Global epidemiology and prevention of colorectal cancer. Lancet Gastroenterol Hepatol. 2022;7:588-590. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 54. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2526] [Cited by in RCA: 2905] [Article Influence: 363.1] [Reference Citation Analysis (3)] |
| 55. | Ionica E, Gaina G, Tica M, Chifiriuc MC, Gradisteanu-Pircalabioru G. Contribution of Epithelial and Gut Microbiome Inflammatory Biomarkers to the Improvement of Colorectal Cancer Patients' Stratification. Front Oncol. 2021;11:811486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Wang Z, Guo K, Liu Y, Huang C, Wu M. Dynamic impact of virome on colitis and colorectal cancer: Immunity, inflammation, prevention and treatment. Semin Cancer Biol. 2022;86:943-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 57. | Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 658] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 58. | Marabotto E, Kayali S, Buccilli S, Levo F, Bodini G, Giannini EG, Savarino V, Savarino EV. Colorectal Cancer in Inflammatory Bowel Diseases: Epidemiology and Prevention: A Review. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 59. | Nebbia M, Yassin NA, Spinelli A. Colorectal Cancer in Inflammatory Bowel Disease. Clin Colon Rectal Surg. 2020;33:305-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 60. | Fantini MC, Guadagni I. From inflammation to colitis-associated colorectal cancer in inflammatory bowel disease: Pathogenesis and impact of current therapies. Dig Liver Dis. 2021;53:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 61. | Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 851] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 62. | Baker AM, Cross W, Curtius K, Al Bakir I, Choi CR, Davis HL, Temko D, Biswas S, Martinez P, Williams MJ, Lindsay JO, Feakins R, Vega R, Hayes SJ, Tomlinson IPM, McDonald SAC, Moorghen M, Silver A, East JE, Wright NA, Wang LM, Rodriguez-Justo M, Jansen M, Hart AL, Leedham SJ, Graham TA. Evolutionary history of human colitis-associated colorectal cancer. Gut. 2019;68:985-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 63. | Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148:1244-60.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 465] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 64. | Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010;138:2029-2043.e10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 475] [Cited by in RCA: 437] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 65. | Printz C. Study finds link between inflammation and colorectal cancer. Cancer. 2018;124:13. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 66. | Tabung FK, Liu L, Wang W, Fung TT, Wu K, Smith-Warner SA, Cao Y, Hu FB, Ogino S, Fuchs CS, Giovannucci EL. Association of Dietary Inflammatory Potential With Colorectal Cancer Risk in Men and Women. JAMA Oncol. 2018;4:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 67. | Chang WY, Chiu HM. Beyond colonoscopy: Physical activity as a viable adjunct to prevent colorectal cancer. Dig Endosc. 2023;35:33-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 68. | Haq S, Ali S, Mohammad R, Sarkar FH. The complexities of epidemiology and prevention of gastrointestinal cancers. Int J Mol Sci. 2012;13:12556-12572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Molska M, Reguła J. Potential Mechanisms of Probiotics Action in the Prevention and Treatment of Colorectal Cancer. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 70. | Hossain MS, Karuniawati H, Jairoun AA, Urbi Z, Ooi J, John A, Lim YC, Kibria KMK, Mohiuddin AKM, Ming LC, Goh KW, Hadi MA. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 390] [Article Influence: 130.0] [Reference Citation Analysis (1)] |
| 71. | Lefall LD Jr. Colorectal cancer--Prevention and detection. Cancer. 1981;47:1170-1172. [PubMed] [DOI] [Full Text] |
| 72. | Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, Parkin DM, Wardle J, Duffy SW, Cuzick J; UK Flexible Sigmoidoscopy Trial Investigators. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1134] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 73. | Selby JV. Screening sigmoidoscopy for colorectal cancer. Lancet. 1993;341:728-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 74. | Winawer SJ, St John DJ, Bond JH, Rozen P, Burt RW, Waye JD, Kronborg O, O'Brien MJ, Bishop DT, Kurtz RC. Prevention of colorectal cancer: guidelines based on new data. WHO Collaborating Center for the Prevention of Colorectal Cancer. Bull World Health Organ. 1995;73:7-10. [PubMed] |
| 75. | Rex DK; ACG Board of Trustees. American College of Gastroenterology action plan for colorectal cancer prevention. Am J Gastroenterol. 2004;99:574-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Rex DK, Lieberman D; ACG. ACG colorectal cancer prevention action plan: update on CT-colonography. Am J Gastroenterol. 2006;101:1410-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 77. | Wang ZH, Fang JY. Colorectal Cancer in Inflammatory Bowel Disease: Epidemiology, Pathogenesis and Surveillance. Gastrointest Tumors. 2014;1:146-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Shams AZ, Haug U. Strategies for prevention of gastrointestinal cancers in developing countries: a systematic review. J Glob Health. 2017;7:020405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Printz C. Regular colonoscopy could prevent 40% of colorectal cancers. Cancer. 2014;120:156-157. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 80. | Brenner H, Chen C. The colorectal cancer epidemic: challenges and opportunities for primary, secondary and tertiary prevention. Br J Cancer. 2018;119:785-792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 189] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 81. | Platz EA, Willett WC, Colditz GA, Rimm EB, Spiegelman D, Giovannucci E. Proportion of colon cancer risk that might be preventable in a cohort of middle-aged US men. Cancer Causes Control. 2000;11:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 198] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 82. | Wei EK, Colditz GA, Giovannucci EL, Fuchs CS, Rosner BA. Cumulative risk of colon cancer up to age 70 years by risk factor status using data from the Nurses' Health Study. Am J Epidemiol. 2009;170:863-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 83. | Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1328] [Cited by in RCA: 1194] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 84. | Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609-e616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1068] [Cited by in RCA: 1010] [Article Influence: 112.2] [Reference Citation Analysis (0)] |
| 85. | Wands JR. Prevention of hepatocellular carcinoma. N Engl J Med. 2004;351:1567-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 86. | Cooke GS, Andrieux-Meyer I, Applegate TL, Atun R, Burry JR, Cheinquer H, Dusheiko G, Feld JJ, Gore C, Griswold MG, Hamid S, Hellard ME, Hou J, Howell J, Jia J, Kravchenko N, Lazarus JV, Lemoine M, Lesi OA, Maistat L, McMahon BJ, Razavi H, Roberts T, Simmons B, Sonderup MW, Spearman CW, Taylor BE, Thomas DL, Waked I, Ward JW, Wiktor SZ; Lancet Gastroenterology & Hepatology Commissioners. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2019;4:135-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 389] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 87. | Zhang X, Guan L, Tian H, Zeng Z, Chen J, Huang D, Sun J, Guo J, Cui H, Li Y. Risk Factors and Prevention of Viral Hepatitis-Related Hepatocellular Carcinoma. Front Oncol. 2021;11:686962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 88. | Benbow JH, Thompson KJ, Cope HL, Brandon-Warner E, Culberson CR, Bossi KL, Li T, Russo MW, Gersin KS, McKillop IH, deLemos AS, Schrum LW. Diet-Induced Obesity Enhances Progression of Hepatocellular Carcinoma through Tenascin-C/Toll-Like Receptor 4 Signaling. Am J Pathol. 2016;186:145-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 89. | Yang YM, Kim SY, Seki E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets. Semin Liver Dis. 2019;39:26-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 302] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 90. | Khalyfa AA, Punatar S, Yarbrough A. Hepatocellular Carcinoma: Understanding the Inflammatory Implications of the Microbiome. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 91. | Strathearn LS, Stepanov AI, Font-Burgada J. Inflammation in Primary and Metastatic Liver Tumorigenesis-Under the Influence of Alcohol and High-Fat Diets. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 92. | Hoshida Y, Fuchs BC, Bardeesy N, Baumert TF, Chung RT. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J Hepatol. 2014;61:S79-S90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 93. | Benkheil M, Paeshuyse J, Neyts J, Van Haele M, Roskams T, Liekens S. HCV-induced EGFR-ERK signaling promotes a pro-inflammatory and pro-angiogenic signature contributing to liver cancer pathogenesis. Biochem Pharmacol. 2018;155:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 94. | Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 2862] [Article Influence: 477.0] [Reference Citation Analysis (17)] |
| 95. | Alqahtani SA, Colombo M. Treatment for Viral Hepatitis as Secondary Prevention for Hepatocellular Carcinoma. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 96. | Liu Z, Chen Z, Cui F, Ding Y, Gao Y, Han G, Jia J, Li J, Li Z, Liu Y, Mao Q, Wang A, Wang W, Wei L, Xia J, Xie Q, Yang X, Yin X, Zhang H, Zhang L, Zhang W, Zhuang H, Dou X, Hou J. Management Algorithm for Prevention of Mother-to-child Transmission of Hepatitis B Virus (2022). J Clin Transl Hepatol. 2022;10:1004-1010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 97. | Jourdain G, Ngo-Giang-Huong N, Harrison L, Decker L, Khamduang W, Tierney C, Salvadori N, Cressey TR, Sirirungsi W, Achalapong J, Yuthavisuthi P, Kanjanavikai P, Na Ayudhaya OP, Siriwachirachai T, Prommas S, Sabsanong P, Limtrakul A, Varadisai S, Putiyanun C, Suriyachai P, Liampongsabuddhi P, Sangsawang S, Matanasarawut W, Buranabanjasatean S, Puernngooluerm P, Bowonwatanuwong C, Puthanakit T, Klinbuayaem V, Thongsawat S, Thanprasertsuk S, Siberry GK, Watts DH, Chakhtoura N, Murphy TV, Nelson NP, Chung RT, Pol S, Chotivanich N. Tenofovir versus Placebo to Prevent Perinatal Transmission of Hepatitis B. N Engl J Med. 2018;378:911-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 98. | Chang MH, You SL, Chen CJ, Liu CJ, Lai MW, Wu TC, Wu SF, Lee CM, Yang SS, Chu HC, Wang TE, Chen BW, Chuang WL, Soon MS, Lin CY, Chiou ST, Kuo HS, Chen DS; Taiwan Hepatoma Study Group. Long-term Effects of Hepatitis B Immunization of Infants in Preventing Liver Cancer. Gastroenterology. 2016;151:472-480.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 99. | Soriano V, Tefferi A. Prevention of liver cancer with new curative hepatitis C antivirals: Real-world challenges. Cancer. 2018;124:1647-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 100. | Lanini S, Easterbrook PJ, Zumla A, Ippolito G. Hepatitis C: global epidemiology and strategies for control. Clin Microbiol Infect. 2016;22:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 101. | Fujiwara N, Friedman SL, Goossens N, Hoshida Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J Hepatol. 2018;68:526-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 523] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 102. | Kim MN, Han KH, Ahn SH. Prevention of hepatocellular carcinoma: beyond hepatitis B vaccination. Semin Oncol. 2015;42:316-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 103. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 2999] [Article Influence: 428.4] [Reference Citation Analysis (3)] |
| 104. | Cadier B, Bulsei J, Nahon P, Seror O, Laurent A, Rosa I, Layese R, Costentin C, Cagnot C, Durand-Zaleski I, Chevreul K; ANRS CO12 CirVir and CHANGH groups. Early detection and curative treatment of hepatocellular carcinoma: A cost-effectiveness analysis in France and in the United States. Hepatology. 2017;65:1237-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 105. | Mittal S, Kanwal F, Ying J, Chung R, Sada YH, Temple S, Davila JA, El-Serag HB. Effectiveness of surveillance for hepatocellular carcinoma in clinical practice: A United States cohort. J Hepatol. 2016;65:1148-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 106. | Howell J, Chan HLY, Feld JJ, Hellard ME, Thompson AJ. Closing the Stable Door After the Horse Has Bolted: Should We Be Treating People With Immune-Tolerant Chronic Hepatitis B to Prevent Hepatocellular Carcinoma? Gastroenterology. 2020;158:2028-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 107. | Nan Y, Xu X, Gao Y, Wang R, Li W, Yang M, Liu L, Duan Z, Jia J, Wei L, Zhuang H; Chinese Society of Hepatology, Chinese Medical Association. Consensus on the secondary prevention of primary liver cancer. Hepatol Int. 2021;15:1289-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 108. | Wang K, Li M, Qin J, Sun G, Dai L, Wang P, Ye H, Shi J, Cheng L, Yang Q, Qiu C, Jiang D, Wang X, Zhang J. Serological Biomarkers for Early Detection of Hepatocellular Carcinoma: A Focus on Autoantibodies against Tumor-Associated Antigens Encoded by Cancer Driver Genes. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 109. | Wu J, Wang P, Han Z, Li T, Yi C, Qiu C, Yang Q, Sun G, Dai L, Shi J, Wang K, Ye H. A novel immunodiagnosis panel for hepatocellular carcinoma based on bioinformatics and the autoantibody-antigen system. Cancer Sci. 2022;113:411-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 110. | Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol. 1977;105:94-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 377] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 111. | Whittle H, Inskip H, Bradley AK, McLaughlan K, Shenton F, Lamb W, Eccles J, Baker BA, Hall AJ. The pattern of childhood hepatitis B infection in two Gambian villages. J Infect Dis. 1990;161:1112-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 112. | Hall AJ, Smith PG. Prevention of hepatocellular cancer: one of the most cost-effective ways to reduce adult mortality? Br J Cancer. 1999;81:1097-1098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 113. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3302] [Cited by in RCA: 3257] [Article Influence: 79.4] [Reference Citation Analysis (1)] |
| 114. | Malfertheiner P, Sipponen P, Naumann M, Moayyedi P, Mégraud F, Xiao SD, Sugano K, Nyrén O; Lejondal H. pylori-Gastric Cancer Task Force. Helicobacter pylori eradication has the potential to prevent gastric cancer: a state-of-the-art critique. Am J Gastroenterol. 2005;100:2100-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 115. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2031] [Article Influence: 253.9] [Reference Citation Analysis (0)] |
| 116. | Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 567] [Article Influence: 51.5] [Reference Citation Analysis (1)] |
| 117. | Kidd M, Lastovica AJ, Atherton JC, Louw JA. Heterogeneity in the Helicobacter pylori vacA and cagA genes: association with gastroduodenal disease in South Africa? Gut. 1999;45:499-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 118. | Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 692] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 119. | Ji X, He G, Wang K, Zhang Y, Yin J. Estimation of gastric cancer burden attributable to Helicobacter pylori infection in Asia. J Public Health (Oxf). 2022;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 120. | Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2220] [Cited by in RCA: 1969] [Article Influence: 246.1] [Reference Citation Analysis (1)] |
| 121. | Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 587] [Cited by in RCA: 610] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 122. | Ma HY, Liu XZ, Liang CM. Inflammatory microenvironment contributes to epithelial-mesenchymal transition in gastric cancer. World J Gastroenterol. 2016;22:6619-6628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 123. | Yang H, Wei B, Hu B. Chronic inflammation and long-lasting changes in the gastric mucosa after Helicobacter pylori infection involved in gastric cancer. Inflamm Res. 2021;70:1015-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 124. | Liou JM, Malfertheiner P, Lee YC, Sheu BS, Sugano K, Cheng HC, Yeoh KG, Hsu PI, Goh KL, Mahachai V, Gotoda T, Chang WL, Chen MJ, Chiang TH, Chen CC, Wu CY, Leow AH, Wu JY, Wu DC, Hong TC, Lu H, Yamaoka Y, Megraud F, Chan FKL, Sung JJ, Lin JT, Graham DY, Wu MS, El-Omar EM; Asian Pacific Alliance on Helicobacter and Microbiota (APAHAM). Screening and eradication of Helicobacter pylori for gastric cancer prevention: the Taipei global consensus. Gut. 2020;69:2093-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 297] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 125. | Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 731] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 126. | Rugge M. Big Data on Gastric Dysplasia Support Gastric Cancer Prevention. Clin Gastroenterol Hepatol. 2022;20:1226-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 127. | Zeng M, Mao XH, Li JX, Tong WD, Wang B, Zhang YJ, Guo G, Zhao ZJ, Li L, Wu DL, Lu DS, Tan ZM, Liang HY, Wu C, Li DH, Luo P, Zeng H, Zhang WJ, Zhang JY, Guo BT, Zhu FC, Zou QM. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1457-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 128. | Yip HC, Teoh AYB. Importance of timely eradication of Helicobacter pylori to prevent peptic ulcer recurrence and gastric cancer. Gastrointest Endosc. 2018;88:251-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 129. | Koh JS, Joo MK. Helicobacter pylori eradication in the treatment of gastric hyperplastic polyps: beyond National Health Insurance. Korean J Intern Med. 2018;33:490-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 130. | Kato M, Ota H, Okuda M, Kikuchi S, Satoh K, Shimoyama T, Suzuki H, Handa O, Furuta T, Mabe K, Murakami K, Sugiyama T, Uemura N, Takahashi S. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 Revised Edition. Helicobacter. 2019;24:e12597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 200] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 131. | Duan F, Song C, Wang P, Ye H, Dai L, Zhang J, Wang K. Polygenic Risk Scores for Prediction of Gastric Cancer Based on Bioinformatics Screening and Validation of Functional lncRNA SNPs. Clin Transl Gastroenterol. 2021;12:e00430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 132. | Lu B, Li M. Helicobacter pylori eradication for preventing gastric cancer. World J Gastroenterol. 2014;20:5660-5665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 133. | Stemmermann GN, Fenoglio-Preiser C. Gastric carcinoma distal to the cardia: a review of the epidemiological pathology of the precusors to a preventable cancer. Pathology. 2002;34:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 134. | Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 542] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 135. | Ji XK, Madhurapantula SV, He G, Wang KY, Song CH, Zhang JY, Wang KJ. Genetic variant of cyclooxygenase-2 in gastric cancer: More inflammation and susceptibility. World J Gastroenterol. 2021;27:4653-4666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 136. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1322] [Cited by in RCA: 1168] [Article Influence: 116.8] [Reference Citation Analysis (0)] |
| 137. | Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 1013] [Article Influence: 126.6] [Reference Citation Analysis (1)] |
| 138. | El-Serag HB, Kao JY, Kanwal F, Gilger M, LoVecchio F, Moss SF, Crowe SE, Elfant A, Haas T, Hapke RJ, Graham DY. Houston Consensus Conference on Testing for Helicobacter pylori Infection in the United States. Clin Gastroenterol Hepatol. 2018;16:992-1002.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 139. | Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, Gasbarrini A, Hunt RH, Leja M, O'Morain C, Rugge M, Suerbaum S, Tilg H, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study group. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 630] [Article Influence: 210.0] [Reference Citation Analysis (0)] |
| 140. | Malfertheiner P. Helicobacter pylori Treatment for Gastric Cancer Prevention. N Engl J Med. 2018;378:1154-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 141. | Banks M, Graham D, Jansen M, Gotoda T, Coda S, di Pietro M, Uedo N, Bhandari P, Pritchard DM, Kuipers EJ, Rodriguez-Justo M, Novelli MR, Ragunath K, Shepherd N, Dinis-Ribeiro M. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019;68:1545-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 393] [Article Influence: 65.5] [Reference Citation Analysis (1)] |
| 142. | He J, Chen WQ, Li ZS, Li N, Ren JS, Tian JH, Tian WJ, Hu FL, Peng J; Expert Group of China Guideline for the Screening,Early Detection and Early Treatment of Gastric Cancer; ; Working Group of China Guideline for the Screening, Early Detection and Early Treatment of Gastric Cancer. [China guideline for the screening, early detection and early treatment of gastric cancer (2022, Beijing)]. Zhonghua Zhong Liu Za Zhi. 2022;44:634-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 143. | Chen Q, Liang X, Long X, Yu L, Liu W, Lu H. Cost-effectiveness analysis of screen-and-treat strategy in asymptomatic Chinese for preventing Helicobacter pylori-associated diseases. Helicobacter. 2019;24:e12563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 144. | Mahachai V, Vilaichone RK, Pittayanon R, Rojborwonwitaya J, Leelakusolvong S, Maneerattanaporn M, Chotivitayatarakorn P, Treeprasertsuk S, Kositchaiwat C, Pisespongsa P, Mairiang P, Rani A, Leow A, Mya SM, Lee YC, Vannarath S, Rasachak B, Chakravuth O, Aung MM, Ang TL, Sollano JD, Trong Quach D, Sansak I, Wiwattanachang O, Harnsomburana P, Syam AF, Yamaoka Y, Fock KM, Goh KL, Sugano K, Graham D. Helicobacter pylori management in ASEAN: The Bangkok consensus report. J Gastroenterol Hepatol. 2018;33:37-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 145. | Ding SZ. Global whole family based-Helicobacter pylori eradication strategy to prevent its related diseases and gastric cancer. World J Gastroenterol. 2020;26:995-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |