Published online Feb 15, 2023. doi: 10.4251/wjgo.v15.i2.352
Peer-review started: October 10, 2022
First decision: October 20, 2022
Revised: October 23, 2022
Accepted: November 28, 2022
Article in press: November 28, 2022
Published online: February 15, 2023
Processing time: 127 Days and 9.1 Hours
Immune checkpoint inhibitors (ICIs) have shown promising efficacy in treatment and clinical management of advanced gastric and gastroesophageal junction cancer. However, the inhibitors also cause immune-related adverse events (irAEs). The current systematic review and meta-analysis study aimed to investigate the incidence and nature of irAEs caused by ICIs.
To investigate the incidence and nature of irAEs in advanced gastric and gastroesophageal junction cancer.
This systematic review was registered with PROSPERO (Reg. number: CRD42020152291). Data included in this study were collected from patients diagnosed with advanced gastric cancer or gastroesophageal junction cancer and treated with ICIs. A systematic literature search was conducted using the PubMed, EMBASE, and Cochrane Library databases. Meta-analysis was carried out using the single sample rate method. Synthesis and analysis of the data was conducted using Stata/SE and Review Manager Software.
The patients enrolled in the present study included 14 patients from 14 case reports, 326 patients from 6 case series, and 1249 patients from 8 clinical trials. It was found that the overall incidence of irAEs was 16% [95% confidence interval (CI): 11-20] for all grades and 3% (95%CI: 2-4) for the severe grade. It was evident that the incidence of irAEs varied with the type of inhibitor and organs. A comparative study of the anti-programmed cell death receptor-1 (PD-1) and anti-programmed death receptor-ligand 1 (PD-L1) treatments showed that the anti-PD-1 group had a higher overall incidence of irAEs (20%) as compared with that of the anti-PD-L1 group (13%). Results of this study showed that the endocrine system experienced the highest incidence of organ-specific irAEs (7.4%), including hypothyroidism, hyperthyroidism, thyroiditis, diabetes, and adrenal insufficiency, followed by gastroenterology (2.2%), pulmonology (1.8%), neurology (1.4%), dermatology (1.4%), hematology (0.8%), and hepatology (0.7%). In clinical trials, it was found that the incidence of death related to irAEs was 1% (95%CI: 0-2.0), whereby colitis and interstitial lung diseases were the leading causes of death.
It was evident that the incidence and nature of irAEs are both organ- and inhibitor-specific. The anti-PD-1 group had the highest incidence of all irAEs grades including the severe grades of irAEs. Early identification and management of irAEs allows clinical oncologists to effectively consider the pros and cons and hence enables them to strike a balance.
Core Tip: This systematic review shows that there is an increasing number of immune-related adverse events (irAEs) associated with immune checkpoint inhibitors that are being reported in patients with gastric cancer or gastroesophageal junction cancer. This is particularly severe organ-specific irAEs and death because of irAEs, which poses significant challenges for clinical oncologists. Therefore, to help clinicians effectively identify and manage irAEs as well as strike a balance, a comprehensive understanding, systematic prediction, and appropriate management of the adverse events are critical.
- Citation: Pei WG, Chen WZ, Wu YK, Tan SX, Jie ZG. Immune-related adverse events associated with immune checkpoint inhibitors for advanced gastric and gastroesophageal junction cancer: A meta-analysis. World J Gastrointest Oncol 2023; 15(2): 352-367
- URL: https://www.wjgnet.com/1948-5204/full/v15/i2/352.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i2.352
Gastric cancer (GC) is one of the most prevalent malignancies of the digestive tract in the world whereby the global incidence and mortality of GC ranks fifth and fourth of the malignancies, respectively[1]. Furthermore, it has been found that the global incidence and mortality rates of GC are 15.59 per 100000 and 11.88 per 100000, respectively, as well as 30.64 per 100000 and 21.72 per 100000, respectively in China[2]. Although the most effective treatment for GC or gastroesophageal junction cancer (GEJC) is a surgical operation, the majority of patients cannot undergo radical surgery because of the advanced stage of the disease at the time of diagnosis. Instead, the patients receive chemotherapy, targeted therapy, and other medical treatment. Recently, immune checkpoint inhibitors (ICIs) have also made significant progress in the treatment and management of GC/GEJC.
The first ICI, ipilimumab [Yervoy, anti-cytotoxic T lymphocyte-associated antigen-4 (CTLA-4)], was approved by the Food and Drug Administration (FDA) in 2011 for the treatment of metastatic melanoma[3]. Following the approval of the first programmed cell death receptor-1 (PD-1)/pro
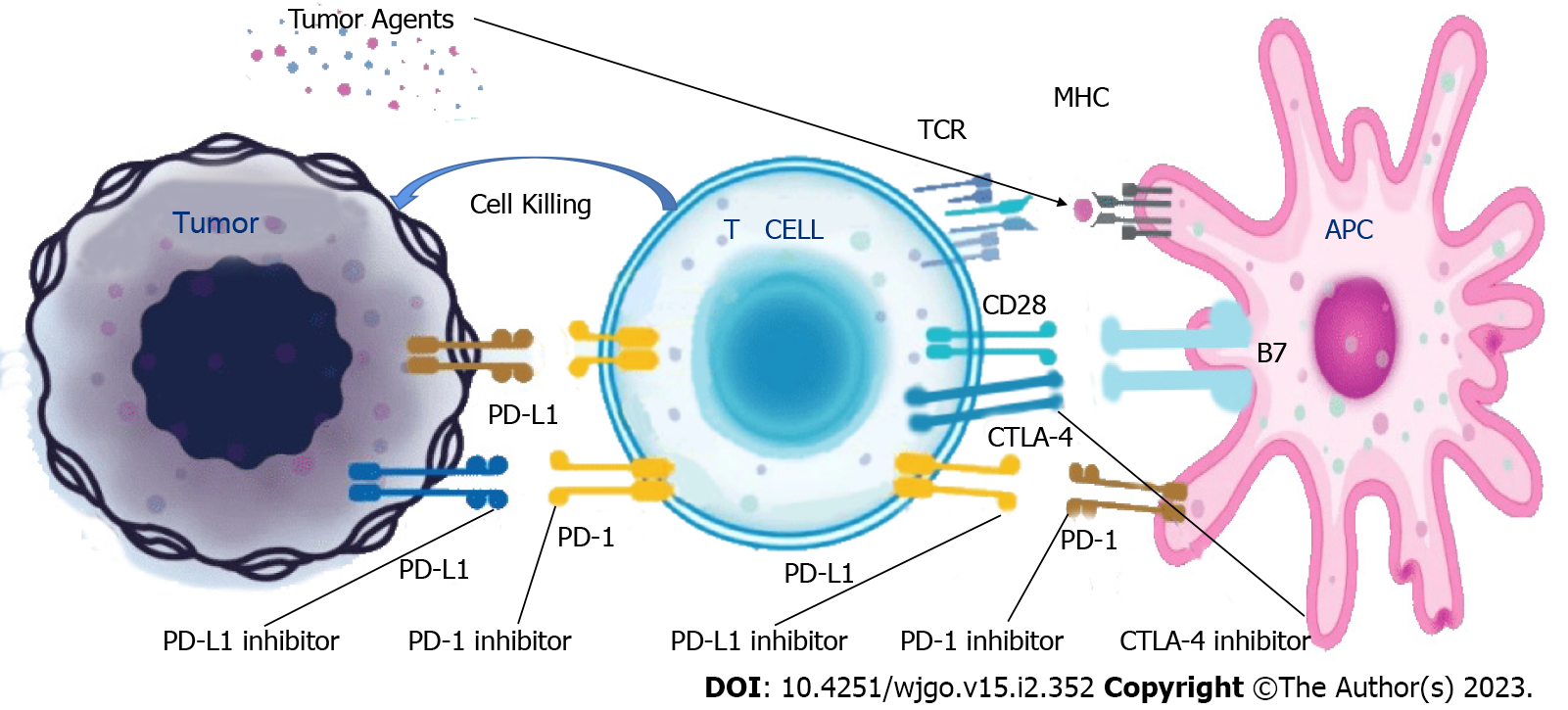
CTLA-4 possesses structural homology with CD28 and binds to the B7 molecules on APC with a higher affinity than the CD28. This results in a competitive inhibition of costimulatory CD28 signaling and damage to the T cell signaling[7-8]. ICIs exert anti-tumor effects by damaging co-inhibitory T cell signaling (Figure 1, source: Beida Pharmaceutical official website).
ICIs offer patients with GC or GEJC a glimmer of hope. A previous study suggested that pembrolizumab monotherapy may provide a potential treatment benefit for GC or GEJC[9]. However, ICIs result in severe or even fatal immune-related adverse events (irAEs) whereby they cause immune system hyperactivation in the normal tissues, which may be the underlying cause of irAEs[10]. Organ-specific irAEs associated with ICIs mainly occur in endocrinopathy, gastroenterology, hepatology, neurology, hematology, dermatology, pulmonology, nephrology, cardiology, and rheumatic immunology.
The irAEs can result in a reduction in dosage, drug withdrawal, a decrease in compliance, delayed treatment, organ function damage, and eventual death. These adverse events have been reported in other tumors, but there has been no systematic review of the events in GC or GEJC. Therefore, this meta-analysis was aimed to assess the incidence and nature of irAEs by conducting a systematic review of their adverse events in patients with GC or GEJC. The objective of the current systematic review and meta-analysis study was to assist clinicians in effective identification, and strike a balance by considering the pros and cons in management approaches of irAEs.
Three major databases (PubMed, EMBASE, and Cochrane Library) were used to perform a systematic literature search for the present study. The search was conducted for the studies published between January, 2000 and January, 2022. Population, Intervention, Comparison, Outcome, and Study design was utilized as a framework to conduct the literature search (Table 1). The relevant searching terms corresponded to terms of the Medical Subject Heading. In addition, the searches were immediately repeated before the final analyses to identify any additional studies for inclusion[11]. This study adhered to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and was registered with PROSPERO (Registration number: CRD42020152291)[12]. Detailed search strategies in the three major databases (PubMed, EMBASE, and Cochrane Library) were as shown in Supplementary Tables 1-3. The retrieved documents were lastly managed using the EndNote 20.
| Query | Search term |
| #1 | P (Neoplasm, Stomach[Title/Abstract] OR Stomach oplasm[Title/Abstract] OR Neoplasms, Stomach[Title/Abstract] OR Gastric Neoplasms[Title/Abstract] OR Gastric Neoplasm[Title/Abstract] OR Neoplasm, Gastric[Title/Abstract] OR Cancer of Stomach[Title/Abstract] OR Stomach Cancers[Title/Abstract] OR Gastric Cancer[Title/Abstract] OR Cancer, Gastric[Title/Abstract] OR gastroesophageal junction cancer[Title/Abstract] OR gastroesophageal junction adenocarcinoma[Title/Abstract] OR adenocarcinoma gastroesophageal junction[Title/Abstract]) |
| #2 | I (Checkpoint Inhibitors, Immune[Title/Abstract] OR Immune Checkpoint Inhibitor[Title/Abstract] OR PD-L1 Inhibitors[Title/Abstract] OR PD L1 Inhibitors[Title/Abstract] OR PD-L1 Inhibitor[Title/Abstract] OR PD L1 Inhibitor[Title/Abstract] OR CTLA-4 Inhibitors[Title/Abstract] OR CTLA 4 Inhibitors[Title/Abstract] OR ipilimumab[Title/Abstract] OR ticilimumab[Title/Abstract] OR nivolumab[Title/Abstract] OR pembrolizumab[Title/Abstract] OR pidilizumab[Title/Abstract] OR atezolizumab[Title/Abstract] OR durvalumab[Title/Abstract] OR avelumab[Title/Abstract]) |
| #3 | O (immune-related adverse events[Title/Abstract] OR immune related adverse events checkpoint inhibitors[Title/Abstract] OR immune related adverse events checkpoint blockade[Title/Abstract] OR management of immune related adverse events[Title/Abstract] OR immune related adverse events in patients[Title/Abstract] OR immune related adverse events systemic immunosuppression[Title/Abstract]) |
| #4 | S ("randomized controlled trial"[pt] OR "controlled clinical trial"[pt] OR randomized[tiab] OR placebo[tiab] OR "drug therapy"[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab] OR "randomized controlled trial"[pt] OR "controlled clinical trial"[pt] OR "clinical trials as topic"[mesh] OR "random allocation"[mesh] OR "double-blind method"[mesh] OR "single-blind method"[mesh]) |
| #5 | #1 AND #2 AND #3 AND #4 |
Inclusion criteria of the participants of this systematic review and meta-analysis study were: Adults diagnosed with advanced GC, GEJC, and treated with ICIs. On the other hand, the included studies were randomized controlled trials (RCTs), case series, and case reports published in peer-reviewed journals without language or time restrictions. In addition, there were no set restrictions on sex, race, ethnicity, education, and economic status in the study.
Exclusion criteria of this systematic review and meta-analysis study were: Patients receiving other therapies such as chemotherapy, radiotherapy, targeted therapy, or other immunotherapy. Further, the studies excluded were: Cohort studies, case-control studies, cross-sectional studies, and other nonrandomized studies.
First, duplications were filtered using the automatic screening function of EndNote 20. The unqualified documents were then filtered after reading the title and abstract. Finally, the studies were further filtered by reading their full text via the online databases and school libraries. Corresponding authors were also contacted for further clarification during the filtering process. The search was carried out by two independent reviewers. Differences were resolved through consensus after discussion and consultation with a senior third party.
Incidences of irAEs and organ-specific adverse events associated with ICIs in the treatment of GC/GEJC were documented in the present meta-analysis. The irAEs were described using version 2, 3, or 4 of the Common Terminology Criteria for Adverse Events of the National Cancer Institute. Adverse events were graded on a scale of 1 to 5 and grades 3 to 5 were regarded as the severe grade.
The data for each study were independently extracted and recorded by the two reviewers. The data collected for clinical trials were: Author(s), year, clinical trial information, study design, enrollment size, types of tumors, type and dose of monoclonal antibodies, version of the Common Terminology Criteria for Adverse Events, frequency of irAEs and organ-specific irAEs, and the median time.
The data collected for case reports and case series were: Patient characteristics, previous oncologic treatment, cancer outcome (oncologic response or progressive disease), the nature of each irAE, as well as irAE onset, treatment, and outcome. The final results were cross-checked and any disagreements (Kappa score: 0.76) were resolved through consensus after discussion or consultation with a senior third party.
The Cochrane Risk of Bias Tool was used to assess the risk of bias and quality of the RCTs[13]. The tool consists of seven aspects: Random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases. Each aspect of the Cochrane Risk of Bias Tool was assigned a high, low, or unclear risk of bias[13]. Quality assessment was conducted using Review Manager Software (version 5.4.1). This quality assessment was independently conducted by two reviewers who reached an agreement through consensus.
All analyses in the present systemic review and meta-analysis were performed using Stata/SE (version 12.0) and Review Manager (version 5.4.1) software. The following was the procedure involved in conducting the statistical analysis in the study.
First, the incidence of irAEs was determined in each study based on the sample size and total number of irAEs. The incidence of irAEs was then combined, and the effect value was determined based on a meta-analysis of sample rate and standard error. Stata/SE software (version 12.0) was used to draw the forest map and obtain the 95% confidence intervals (CIs) for the weighted average of all studies[14]. The combined effect value was conducted using Stata/SE (version 12.0) with the metan and metafunnel commands of meta-analysis.
Statistical heterogeneity between the selected studies was analyzed using the Q test and I² statistic. When the P-value of Q statistic was > 0.10 or I² ≤ 50%, there was no heterogeneity or acceptable heterogeneity between the studies. Further, when the P value was ≤ 0.10 or I² > 50%, there was a greater degree of heterogeneity between the studies[15]. The random-effect model, which accounts for the heterogeneity between the studies was used to examine the effect size because the heterogeneity between the studies was greater[16]. A Galbraith plot for heterogeneity was drawn to evaluate heterogeneity in the present study. The heterogeneity test was conducted using Stata/SE with the galbr command of meta-analysis.
Initially, the risk of publication bias was evaluated using a funnel plot with pseudo 95% confidence limits and the publication bias was then assessed in the present study by observing the symmetry of the funnel plot. Furthermore, the funnel plot was evaluated using both the Begg and Egger methods. Therefore, the funnel plot was quantified and publication bias was assessed by examining the P-value. The test of publication bias was conducted using Stata/SE with the metabias command of meta-analysis.
Subgroup analysis is a common method for addressing heterogeneity. The studies in the present review were grouped according to the types of ICIs and organ-specific adverse events studied. The analysis was conducted using Stata/SE with the metan command of meta-analysis.
A new meta-analysis was conducted to determine whether the effect size had changed whenever research was deleted. However, the deleted study was considered when result of the new meta-analysis differed from that of the previous one to influence the total effect size. Influence analysis was conducted using Stata/SE with the metaninf command of meta-analysis.
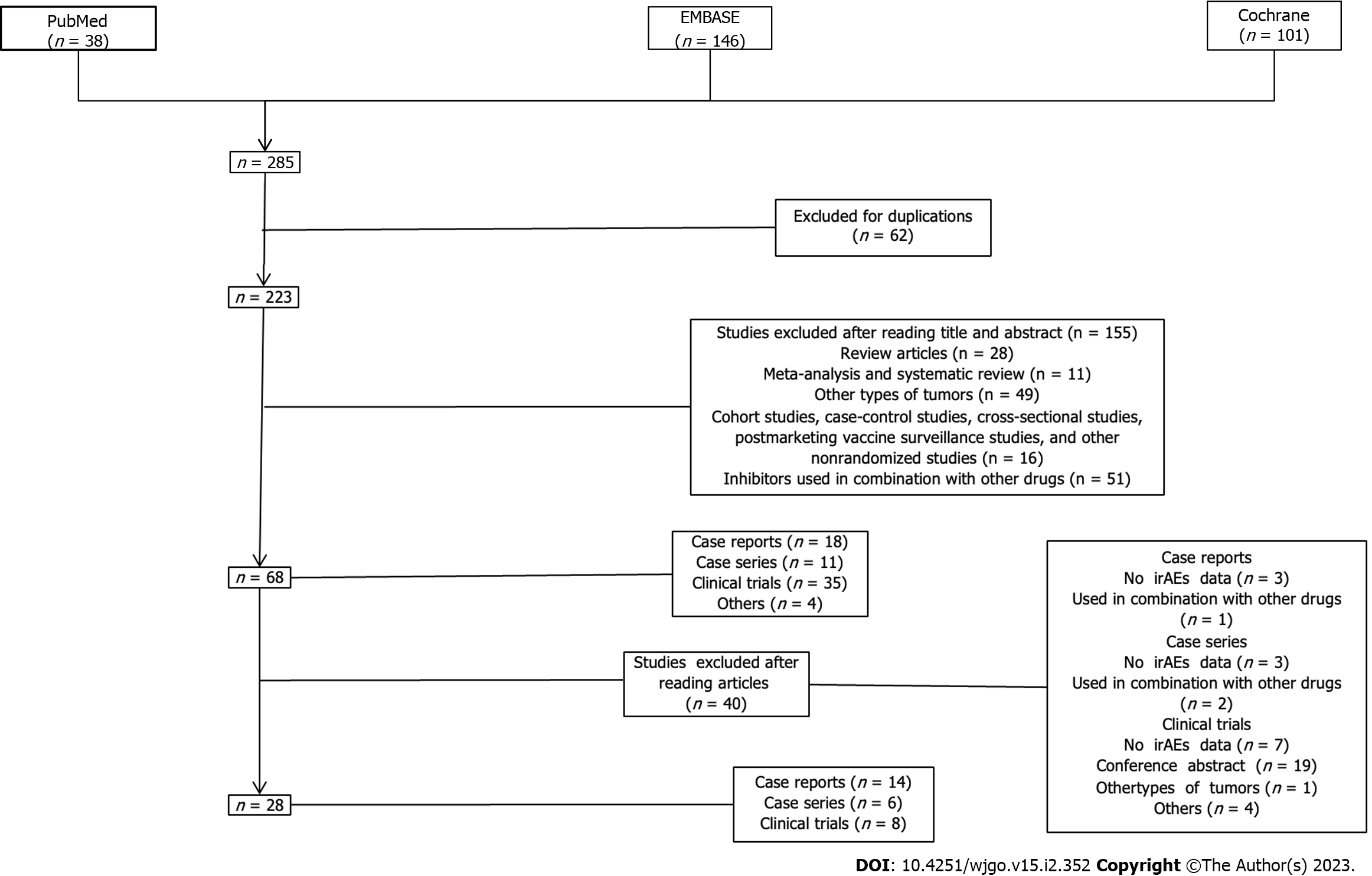
The literature search was conducted in the current systematic review based on the pre-established strategy. A total of 285 pieces of literature were searched including 38, 146, and 101 in the PubMed, EMBASE, and Cochrane databases, respectively. Initially, a total of 62 duplicated literature were excluded. A total of 155 articles that did not meet the criteria were then excluded after reading their titles and abstracts. A total of 28 articles (8 clinical trials, 14 case reports, and 6 case series) that met the inclusion criteria were finally selected after reading the full text (Figure 2). A reference list of all the excluded studies and reasons for their exclusion was as shown in the Supplementary Table 4.
General characteristics: A total of 8 clinical trials were included in this meta-analysis including 2 PD-1 (pembrolizumab)[17-18], 5 PD-L1 (avelumab)[19-23], and 1 CTLA-4 (ipilimumab)[24]. All reviewed trials showed total irAEs, with 6 of them describing organ-specific irAEs. The remaining 2 trials only reported total irAEs. The general characteristics of the included studies were as shown in Table 2, which included a total of 1249 participants from 8 clinical trials. All trials included in the meta-analysis were open-label, multicenter, and randomized trials. Further, it was found that there was only one phase II clinical trial, three phase I clinical trials, and four phase III clinical trials. The average immunotherapy duration for the all included trials was 2.9 mo [interquartile range (IQR): 2.4 to 3.1 mo], whereas the median follow-up time was 15.5 mo (IQR: 9.9 to 20.5 mo). The median overall survival (OS) of these trials ranged between 4.6 (95%CI: 3.6 to 5.7) to 12.7 mo (95%CI: 10.5 to 18.9) whereas the median PFS ranged between 1.4 (95%CI: 1.4 to 1.5) and 3.2 mo (95%CI: 2.8 to 4.1).
| Trial | Design | Design details | Cancer types | Enrollment size, n | ICIs | Dose, mg/kg | IrAEs, all grades, n | IrAEs, severe grades, n | mOS | mPFS |
| Shitara et al[17], 2018 | RCT | Open-label, multicenter, phase III | Advanced GC/GEJC | 294 | PD-1 (pembrolizumab) | 200 mg, q3w | 61 | 10 | 9.1 mo (95%CI: 6.2 to 10.7 mo) | 1.5 mo (95%CI: 1.4 to 2.0 mo) |
| Fuchs et al[18], 2022 | RCT | Open-label, multicenter, phase III | Advanced GC/GEJC | 294 | PD-1 (pembrolizumab) | 200 mg, q3w | 55 | 11 | NA | NA |
| Moehler et al[19], 2021 | RCT | Open-label, multicenter, phase III | Advanced GC/GEJC | 249 | PD-L1 (avelumab) | 10, q2w | 32 | 8 | 10.4 mo (95%CI: 9.1 to 12.0 mo) | 3.2 mo (95%CI: 2.8 to 4.1 mo) |
| Doi et al[20], 2019 | RCT | Open-label, multicenter, phase I | Advanced GC/GEJC | 40 | PD-L1 (avelumab) | 10, q2w | 9 | 0 | 9.1 mo (95%CI: 7.2 to 11.2 mo) | 2.4 mo (95%CI: 1.4 to 2.8 mo) |
| Doi et al[21], 2018 | RCT | Open-label, multicenter, phase I | Advanced GC/GEJC | 40 | PD-L1 (avelumab) | 10, q2w | 5 | 0 | 9.1 mo (95%CI: 7.2 to 11.2 mo) | 2.5 mo (95%CI: 1.4 to 2.8 mo) |
| Chung et al[22], 2019 | RCT | Open-label, multicenter, phase I | Advanced GC/GEJC | 90 | PD-L1 (avelumab) | 10, q2w | 17 | 2 | NA | NA |
| Bang et al[23], 2018 | RCT | Open-label, multicenter, phase III | Advanced GC/GEJC | 185 | PD-L1 (avelumab) | 10, q2w | 12 | 4 | 4.6 mo (95%CI: 3.6 to 5.7 mo) | 1.4 mo (95%CI: 1.4 to 1.5 mo) |
| Bang et al[24], 2017 | RCT | Open-label, multicenter, phase II | Advanced GC/GEJC | 57 | CTLA-4 (ipilimumab) | 10, q3w | 10 | 0 | 12.7 mo (95%CI: 10.5 to 18.9 mo) | 2.7 mo |
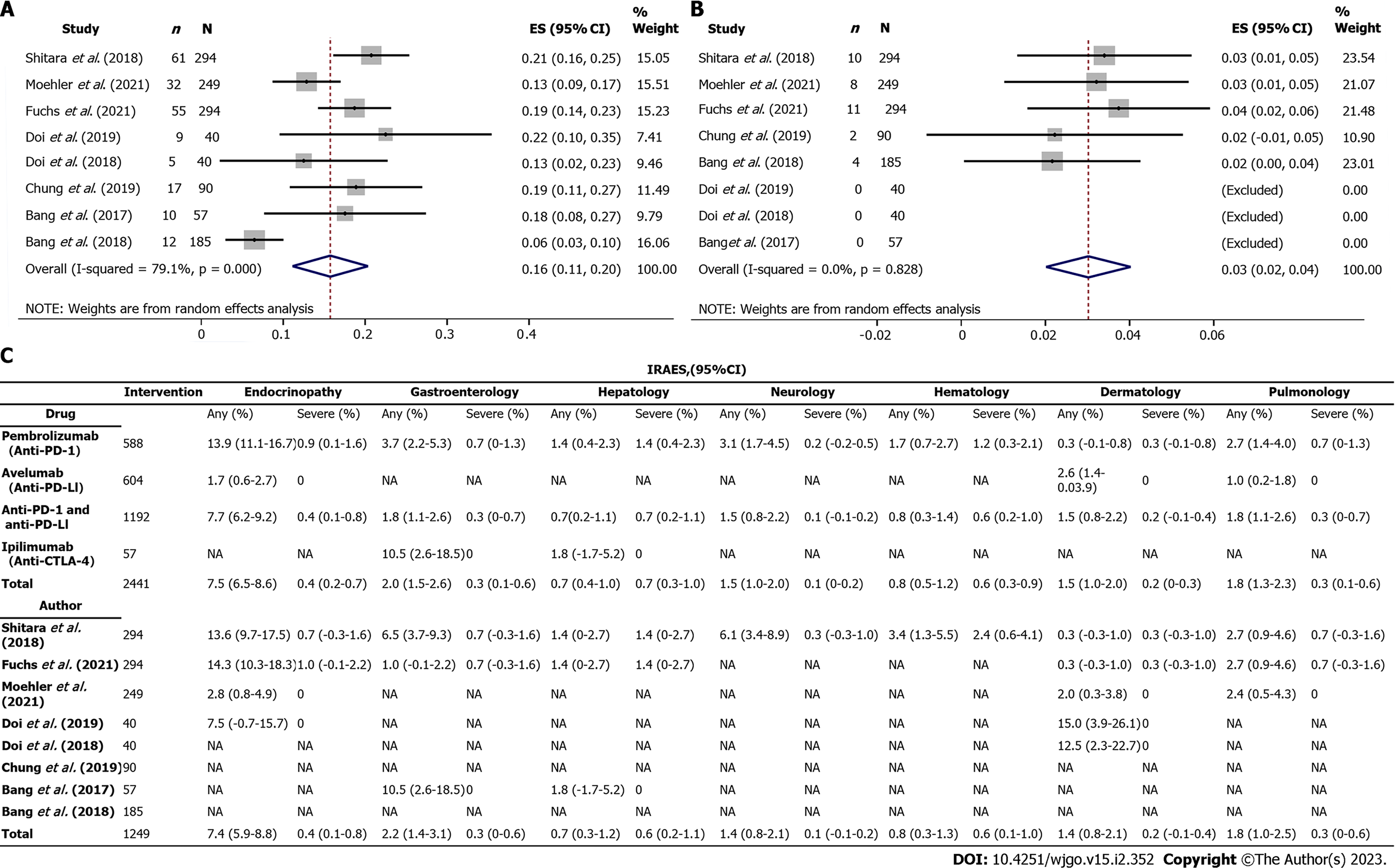
Global incidence of irAEs: The global incidence of irAEs for overall grades was 20% (95%CI: 16 to 23) in the anti-PD-1 group, 13% (95%CI: 8 to 19) in the anti-PD-L1 group, and 18% (95%CI: 8 to 27) in the anti-CTLA-4 group, whereas 4% (95%CI: 2 to 5) in the anti-PD-1 group and 3% (95%CI: 1 to 4) in the anti-PD-L1 group for severe grade (Supplementary Figures 1-3). It was found that the anti-PD-1 group had the highest incidence of irAEs at all grades and severe grades as compared with that of the other three inhibitors. In addition, the overall incidence of irAEs was 16% (95%CI: 11 to 21) for all grades and 3% (95%CI: 2 to 4) for severe grade in the anti-PD-1 combined with the anti-PD-L1 group (Supplementary Figure 4), which was comparable with that in the anti-PD-1 combined with anti-PD-L1, and anti-CTLA-4 groups (Figure 3A and B).
Incidence of organ-specific irAEs: Organ-specific irAEs and their incidence were described as shown in Figure 3C. It was found that only one article documented an irAEs associated with dermatology[21]. In addition, it was noted that there were only two articles that showed the incidence of total irAEs without describing organ-specific irAEs[22-23]. The most common organ-specific irAEs occurred in the endocrine system, accounting for 7.4% (95%CI: 5.9-8.8), and included hypothyroidism, hyperthyroidism, thyroiditis and diabetes, followed by gastroenterology, pulmonology, neurology, and dermatology, accounting for 2.2% (95%CI: 1.4-3.1), 1.8% (95%CI: 1.0-2.5), 1.4% (95%CI: 0.8-2.1) and 1.4% (95%CI: 0.8-2.1), respectively.
On the other hand, it was found that organ-specific irAEs with a lower incidence occurred in hematology and hepatology, accounting for 0.8% (95%CI: 0.3-1.3) and 0.7% (95%CI: 0.3-1.2), respectively. However, it was evident that the incidence of severe grade organ-specific irAEs was higher in hematology and hepatology than in other systems which was comparable with the results observed in the anti-PD-1 group (Figure 3C). In the group of anti-PD-1 combined with the anti-PD-L1, it was found that the most common organ-specific irAEs occurred in endocrinology accounting for 7.7% (95%CI: 6.2-9.2), whereas the rarest organ-specific irAEs occurred in hepatology and accounted for 0.7% (95%CI: 0.4-1.0). Incidence of organ-specific irAEs related to endocrinopathy, gastroenterology, hepatology, neurology, hematology, dermatology, pulmonology, nephrology, cardiology, and rheumatic immunology was displayed in forest plots for all grade and severe grade in anti-PD-1, anti-PD-L1, anti-CTLA-4, or anti-PD-1 combined with anti-PD-L1 groups (Supplementary Figures 5-23).
Incidence of death related to irAEs: Results of this study found that the incidence of death related to irAEs was 1% (95%CI: 0-2.0) in all the included trials and a total of 6 deaths were reported in the anti-PD-1 group (Supplementary Figure 24). Further, it was evident that the main causes of death were colitis and interstitial lung disease.
General characteristics: A total of 14 case reports[25-38] and 6 case series[39-44] were included in this meta-analysis. In the case of reports, one patient received pembrolizumab treatment and thirteen received nivolumab treatment. The general characteristics of the patients were as shown in Attached file 4 (Supplementary Table 5). The average age of the enrolled patients was 70 years and 79% of them were male. Before receiving the anti-PD-1 therapy, it was found that 13 patients (93%) had failed at least one course of chemotherapy. Twelve patients (86%) reported occurrence of irAEs in a single system[25,27-33,35-38], whereas two patients (14%) reported occurrence of irAEs in more than one system.
Results of case reports indicated an average immunotherapy duration of 14.6 cycles (IQR: 5.5 to 17.5) and a mean onset time of 8.2 mo (IQR: 3.0 to 6.0). Four patients (29%) continued to receive anti-PD-1 treatment despite irAEs[25,27,29-30]. In the case series, all 326 patients received nivolumab treatment and their general characteristics were as shown in Table 3. Furthermore, the median OS in these case series ranged from 2.5 mo (95%CI: 0 to 5.0) to 7.9 mo (95%CI: 5.9 to 13.5), and the median PFS ranged from 1.0 mo (95%CI: 0.9 to 1.1) to 2.3 mo (95%CI: 0.5 to 24.8).
| Case series | Enrollment size, n | Cancer types | ICIs | IrAEs, all grades, n | IrAEs, severe grades, n | mOS | mPFS |
| Suzuki et al[44], 2021 (Low ascites burden) | 50 | AGC | PD-1 (nivolumab) | 9 | 1 | 5.3 mo (95%CI: 3.4 to 7.3 mo) | 1.5 mo (95%CI: 1.0 to 2.0 mo) |
| Suzuki et al[44], 2021 (High ascites burden) | 22 | AGC | PD-1 (nivolumab) | 5 | 0 | 2.5 mo (95%CI: 0 to 5.0 mo) | 1.0 mo (95%CI: 0.9 to 1.1 mo) |
| Ohta et al[43], 2020 | 15 | AGC | PD-1 (nivolumab) | 5 | 0 | 6.3 mo | NA |
| Namikawa et al[42], 2020 | 29 | AGC | PD-1 (nivolumab) | 10 | 0 | 5.6 mo (95%CI: 0.6 to 26.8 mo) | 2.3 mo (95%CI: 0.5 to 24.8 mo) |
| Kono et al[41], 2021 | 52 | AGC | PD-1 (nivolumab) | 13 | 1 | 7.9 mo (95%CI: 5.9 to 13.5 mo) | 1.9 mo (95%CI: 1.4 to 3.0 mo) |
| Booka et al[40], 2021 | 50 | GEA/ESCC | PD-1 (nivolumab) | 13 | 5 | NA | NA |
| Ando et al[39], 2021 | 108 | AGC | PD-1 (nivolumab) | 17 | 5 | 3.6 mo (95%CI: 3.0 to 5.3 mo) | 1.4 mo (95%CI: 1.2 to 1.8 mo) |
Incidence and nature of irAEs: Results of the present study showed that organ-specific irAEs in the case of reports were as described in the Supplementary Table 6. It was found that the endocrine system had the highest incidence of organ-specific irAEs, accounting for 36% (n = 5), including hyperthyroidism (n = 1)[37], thyroiditis (n = 2)[34,38], ACTH deficiency (n = 2)[30,38], and diabetes (n = 1)[31]. On the other hand, neurology and dermatology had the lowest incidence of organ-specific irAEs, accounting for 7% for each (n = 2)[26,29], including dizziness, nausea, truncal ataxia, rash, and sequential herpes zoster virus activation.
One patient experienced irAEs in multiple systems[26], including hematology, nephrology, dermatology, cardiology, and pulmonology. Although the patient was cured of irAEs, after receiving steroid treatment, he later suffered severe irAEs[38], developed grade 3 thyroiditis, and ACTH deficiency. It was found that treatment with anti-PD-1 induced an oncologic response in three patients (21%), and disease progression in five patients (36%). A total of 11 (79%) patients with irAEs were treated with steroids, 6 (43%) had cured irAEs, 3 (21%) had persistent irAEs, and 2 (14%) remained uncertain. Two (14%) of the 3 patients who were not treated with steroids developed persistent irAEs, and 1 (7%) died.
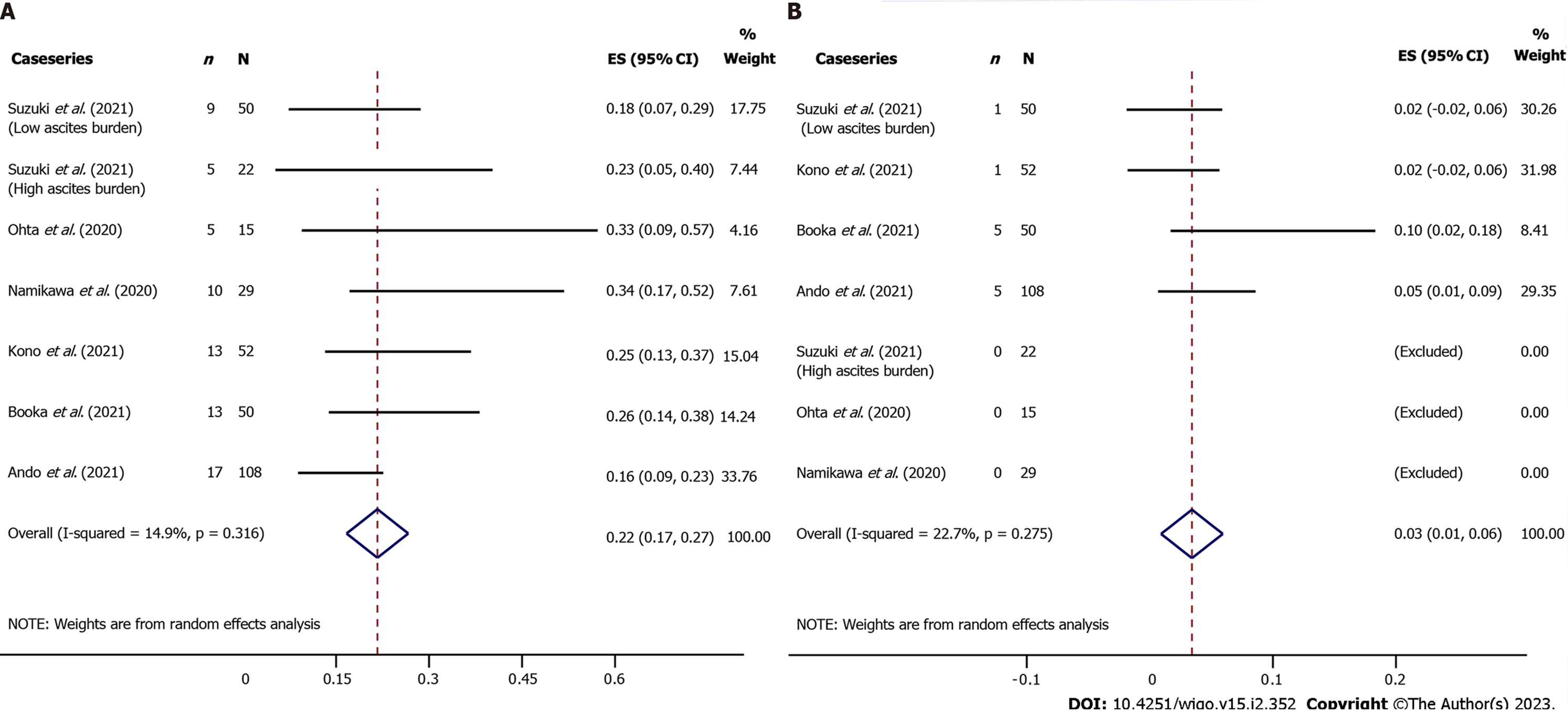
Results showed that the overall incidence of irAEs in the case series was 22% (95%CI: 17 to 27) for all grades and 3% (95%CI: 1 to 6) for severe grade (Figure 4). It was noted that[39] the overall incidence of organ-specific irAEs was reported in only one article but did not describe organ-specific irAEs. Further, 7.1% of all grade organ-specific irAEs occurred in the endocrine system, including hypothyroidism (n = 6), hyperthyroidism (n = 2), thyroiditis (n = 3), hypopituitarism (n = 2), hyperglycemia (n = 1), thyroid insufficiency (n = 3), type 1 diabetes mellitus (n = 1) and others (n = 5). This was then followed by pulmonology (4.3%, n = 14), gastroenterology (3.7%, n = 12), and dermatology (3.4%, n = 11), whereas organ-specific irAEs with a lower incidence included, myocarditis, infusion reaction, arthritis, liver insufficiency, loss of appetite, taste disorder, myopathy, adrenal insufficiency, and mucositis.
Interstitial pneumonia and myocarditis were the most common organ-specific irAEs for severe grade. It was found that two patients died due to severe myocarditis and interstitial pneumonia. Furthermore, one article[43] reported that the incidence of irAEs in patients with advanced GC and a high ascites burden was 23% (95%CI: 5 to 40), as compared with 18% (95%CI: 7 to 29) in patients with a low ascites burden. In addition, the median OS in the high and low ascites burden groups was 2.5 mo (95%CI: 0 to 5.0) and 5.3 mo (95%CI: 3.4 to 7.3), respectively. Comparatively, the median PFS in the high and low ascites burden groups were 1.0 mo (95%CI: 0.9 to 1.1) and 1.5 mo (95%CI: 1.0 to 2.0), respectively.
Quality of included studies and sensitivity analysis: Quality assessment: The risk of bias in each of the included RCTs was as shown in the attached file 5. The risk of selection bias was rated as high in 3 studies (37.5%) whereas the risk of reporting bias was rated as high in 5 studies (62.5%) (Supplementary Figures 25 and 26).
Sensitivity analysis: The sensitivity analysis of all clinical trials was as shown in Attached file 5. It was found that the influence of a single study on the total merger effect was not significant except for one study[22] (Supplementary Figure 27).
Heterogeneity test: Galbraith plot indicated that there existed heterogeneity between the included studies (Supplementary Figure 28). Therefore, sensitivity analysis was used to explain the source of heterogeneity and the random effect model was used to determine the effect quantity.
Publication bias test: The Begg’s funnel plot and Egger’s publication bias plot showed that there was existence of publication bias among the included studies (Supplementary Figures 29 and 30).
This meta-analysis analyzed the irAEs of ICIs for advanced GC/GEJC according to different targets, tumor types, drug types, doses, and organ specificity to improve the understanding of the safety and efficacy of the emerging cancer drugs. A total of 8 clinical trials, 14 case reports, and 6 case series were included in this study. It was evident that the overall incidence of irAEs was high in patients with advanced GC/GEJC, at a rate of 16% (95%CI: 11 to 21) in clinical trials and 22% (95%CI: 17 to 27) in case series. It noted that the most common organ-specific irAEs were endocrine system disorders, including hypothyroidism, hyperthyroidism, thyroiditis, and diabetes. The incidence of irAEs for severe grade (3%), and especially the death rate (1%) were relatively low whereas the interstitial pneumonia was the leading cause of death.
Consistent with results of a previous study, it was found that treatment with anti-PD-1 was significantly associated with a higher prevalence of all irAEs grades and severe grade irAEs as compared with that of anti-PD-L1 treatment[45]. This could be because the variation in the irAEs associated with anti-PD-1 and anti-PD-L1. Anti-PD-1 drugs may increase the risk of immune-related pneumonia whereas anti-PD-L1 drugs may increase the risk of hypothyroidism[45]. However, results of the current research showed that both anti-PD-1 and anti-PD-L1 drugs were associated with an increase in risk of endocrinopathy, which could be caused by different types of cancer.
In addition, findings of a previous meta-analysis showed that the overall incidence of irAEs with anti-CTLA-4 treatment was 72% (95%CI: 65 to 79) for all grade and 24% (95%CI: 18 to 30) for severe grade[46], which was higher than 18% (95%CI: 8 to 27) and 0% in the present meta-analysis. This conclusion may have been caused by insufficient sample sizes of our study, or that the definition of irAEs required to be further clarified. Based on the findings of this study, there is need for additional research on irAEs with a particular focus on comparing anti-PD-1 and anti-PD-L1 medications to provide future guidance for clinical practices.
The relationship between irAEs and efficacy of ICI is the subject of current debate. IrAEs have been associated with improved outcomes and high heterogeneity[47]. A previous meta-analysis showed that anti-PD-1 or anti-PD-L1 treatment improved the clinical benefits of long-term OS and prolonged duration of response in the patients as compared with that of chemotherapy[48]. The median OS for these trials was 9.2 mo and the median PFS was 2.3 mo which was higher than the best supportive therapy or placebo. However, irAEs cannot be ignored when ICIs improve the clinical outcome of oncology. It was found that the overall incidence of irAEs was particularly high in patients with advanced GC/GEJC. In addition, more than 50% of patients experienced intolerable toxicity caused by the reduction of irAEs or discontinuation of their medication. Therefore, it is essential to predict and manage irAEs in cancer immunotherapy.
The findings of the current study showed that incidence of all grade organ-specific irAEs in hematology and hepatology was low. However, the incidence of severe grade irAEs was high. Although hypothyroidism is the most common irAE of the endocrine system, its specific pathophysiological mechanism is still unknown. Furthermore, there was no association between hypothyroidism and cancer outcomes and the strongest associations for hypothyroidism were higher baseline thyroid-stimulating hormone and female sex[49]. Therefore, there is a need for positive clinical tests, such as thyroid function tests (T3, T4, and TSH) which should be performed before and during treatment. Further standardization and improvement are also required for the clinical indicators of other irAEs.
Increasing numbers of drugs targeting immunotherapy and molecular pathways are moving from clinical trials to the clinic. However, the selection of the most appropriate therapy, timing of drug administration, and management of adverse events remain a challenge for severe toxicity and disease progression. Meanwhile, patients are treated with steroids and it has been found that the irAEs either persists or disappears.
Several studies have demonstrated that the use of steroids may inhibit the anti-tumor immune response and hence cause poor prognosis[50-51]. Drug withdrawal and decrease in compliance of patients may also contribute to occurrence of a poor prognosis. On the contrary, a different study has indicated that groups with poor prognoses were more likely to receive steroid treatment and that steroids were less likely to affect the efficacy of immunotherapy[52]. Therefore, there is a need for more research to show the relationship between toxicity and clinical outcomes.
In this systemic review, 14 case reports and 6 case series were included to qualitatively supplement the quantitative findings of the meta-analysis. The statistical analysis is usually constrained because the case studies typically report only novel or rare irAEs. Nonetheless, case studies included in the present review provide an opportunity to assess and study the incidence and nature of irAEs.
Case studies demonstrated that endocrine-related irAEs were the most common and this was in agreement with the findings of another previous meta-analysis[53]. It was evident that the incidence of irAEs was comparable in both case studies and clinical trials of anti-PD-1 therapy. Similar situations apply to deaths caused by irAEs. IrAEs resulted in a 2% mortality rate in case series and a 1% mortality rate in clinical trials, with colitis, myocarditis, and interstitial lung disease being the leading causes of death. This meta-analysis showed a higher mortality rate than a previous one which involved 112 trials and 19,217 patients whereby toxicity-related deaths occurred at 0.36% (anti-PD-1), 0.38% (anti-PD-L1), and 1.08% (anti-CTLA-4)[54].
This study had some advantages. First, it systematically evaluated the incidence of global irAEs and organ-specific irAEs associated with the ICIs monotherapy for advanced GC or GEJC. There are currently very few meta-analyses on irAEs in patients with GC and GEJC. Second, the trials selected for this meta-analysis were RCTs, with large samples, and a high evidence-based value. In addition, a random-effect model and subgroup analysis was used based on different targets, tumor types, drug types, organ specificity, and irAE grade to reduce both variance and bias. Third, the study included both case reports and case series, as well as a comprehensive evaluation of the occurrence, treatment, and prognosis of irAEs. Therefore, these improved the quality of the results and in strengthening the validity of the conclusions made in this study.
This study also had some limitations. First, there were selection, reporting, and publication biases among the included studies. Second, common symptoms such as fatigue, nausea, infusion reactions, and malaise were more likely to be diagnosed as treatment-related adverse events (trAEs) rather than irAEs and hence missed the diagnoses. Therefore, there is urgent need for standardization of the quantifiable standards between irAEs and trAEs, irAEs and non-irAEs. To effectively diagnose and manage irAEs and trAEs, clinicians must also avoid confusing clinical symptoms with test indicators. Third, our study was a meta-analysis of irAEs with a single sample rate. Therefore, odds ratio could not be used for statistical analysis. Lastly, the number of articles included in our analysis is limited. Numerous indicators may be heterogeneous, and the outcome may readily amplify research findings and inaccuracies. This was because of the limited number of published clinical studies on immunotherapy for GC/GEJC and even fewer studies describing irAEs. Consequently, the results of this study should be interpreted with caution and there is additional research to validate the obtained results.
This systematic review shows that there is an increasing number of irAEs associated with ICIs that are being reported in patients with GC or GEJC. This is particularly severe organ-specific irAEs and death because of irAEs, which poses significant challenges for clinical oncologists. Therefore, to help clinicians effectively identify and manage irAEs as well as strike a balance, a comprehensive understanding, systematic prediction, and appropriate management of the adverse events are critical.
In recent years, there has been a steep rise in the development and implementation of anti-cancer immunotherapies. Although there has been a large amount of research focusing on adverse events associated with immune checkpoint inhibitors (ICIs), few studies have focused specifically on advanced gastric cancer (GC) and gastroesophageal junction cancer (GEJC).
By unbalancing the immune system, these new immunotherapies also generate dysimmune toxicities, called immune-related adverse events (irAEs) that mainly involve the gut, skin, endocrine glands, liver, and lung but can potentially affect any tissue. Although steroids can be used to treat these IRAEs, the associated immunosuppression may compromise the antitumor response. To help clinicians effectively identify and manage irAEs as well as strike a balance are critical.
This study focuses on the mechanisms of irAEs generation, putative relationship between dysimmune toxicity and antitumor efficacy.
In the study, we systematically evaluated the incidence of global irAEs and organ-specific irAEs and proposed a random-effect model and subgroup analysis based on different targets, tumor types, drug types, organ specificity, and irAE grade to reduce variance and bias.
It was found that the overall incidence of irAEs was 16% (95%CI: 11-20) for all grades and 3% (95%CI: 2-4) for the severe grade. It was evident that the incidence of irAEs varied with the type of inhibitor and organs. In clinical trials, it was found that the incidence of death related to irAEs was 1% (95%CI: 0-2.0) whereby colitis and interstitial lung diseases were the leading causes of death.
This systematic review shows that there is an increasing number of irAEs associated with ICIs that are being reported in patients with GC or GEJC. This is particularly severe for organ-specific irAEs and death because of irAEs, which poses significant challenges for clinical oncologists. Therefore, to help clinicians effectively identify and manage irAEs as well as strike a balance, a comprehensive understanding, systematic prediction, and appropriate management of the adverse events are critical.
In the study, we systematically evaluated the incidence of global irAEs and organ-specific irAEs and proposed a random-effect model and subgroup analysis based on different targets, tumor types, drug types, organ specificity, and irAE grade to reduce variance and bias. Another strength of our study is that both case reports and case series were included, as well as a comprehensive evaluation of the occurrence, treatment, and prognosis of irAEs. The study would be of great interest to a broad range of readers including oncologists, clinical researchers, patients, and other researchers in related fields.
Authors are grateful to the scholars who participated in this study for their contributions. Thanks to the reviewers and editors of this work for their valuable suggestions on this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khayyat YM, Saudi Arabia; Mehta V, India; Zha B, China S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64551] [Article Influence: 16137.8] [Reference Citation Analysis (176)] |
| 2. | He Y, Wang Y, Luan F, Yu Z, Feng H, Chen B, Chen W. Chinese and global burdens of gastric cancer from 1990 to 2019. Cancer Med. 2021;10:3461-3473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 3. | Bagchi S, Yuan R, Engleman EG. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu Rev Pathol. 2021;16:223-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 1335] [Article Influence: 267.0] [Reference Citation Analysis (0)] |
| 4. | Karlsson AK, Saleh SN. Checkpoint inhibitors for malignant melanoma: a systematic review and meta-analysis. Clin Cosmet Investig Dermatol. 2017;10:325-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Liu B, Song Y, Liu D. Recent development in clinical applications of PD-1 and PD-L1 antibodies for cancer immunotherapy. J Hematol Oncol. 2017;10:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 6. | Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. 2018;6:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 932] [Cited by in RCA: 953] [Article Influence: 136.1] [Reference Citation Analysis (0)] |
| 7. | Alegre ML, Noel PJ, Eisfelder BJ, Chuang E, Clark MR, Reiner SL, Thompson CB. Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J Immunol. 1996;157:4762-4770. [PubMed] |
| 8. | Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1642] [Cited by in RCA: 1695] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 9. | Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1413] [Cited by in RCA: 1461] [Article Influence: 208.7] [Reference Citation Analysis (0)] |
| 10. | Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 3149] [Article Influence: 449.9] [Reference Citation Analysis (0)] |
| 11. | Baumann N. How to use the medical subject headings (MeSH). Int J Clin Pract. 2016;70:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 12. | Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011;39:91-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 583] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 13. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24811] [Article Influence: 1772.2] [Reference Citation Analysis (3)] |
| 14. | Chaimani A, Mavridis D, Salanti G. A hands-on practical tutorial on performing meta-analysis with Stata. Evid Based Ment Health. 2014;17:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46478] [Article Influence: 2112.6] [Reference Citation Analysis (3)] |
| 16. | Nikolakopoulou A, Mavridis D, Salanti G. Demystifying fixed and random effects meta-analysis. Evid Based Ment Health. 2014;17:53-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic C, Chung HC, Muro K, Goekkurt E, Mansoor W, McDermott RS, Shacham-Shmueli E, Chen X, Mayo C, Kang SP, Ohtsu A, Fuchs CS; KEYNOTE-061 investigators. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 998] [Article Influence: 142.6] [Reference Citation Analysis (0)] |
| 18. | Fuchs CS, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, Fornaro L, Olesinski T, Caglevic C, Chung HC, Muro K, Van Cutsem E, Elme A, Thuss-Patience P, Chau I, Ohtsu A, Bhagia P, Wang A, Shih CS, Shitara K. Pembrolizumab versus paclitaxel for previously treated PD-L1-positive advanced gastric or gastroesophageal junction cancer: 2-year update of the randomized phase 3 KEYNOTE-061 trial. Gastric Cancer. 2022;25:197-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 19. | Moehler M, Dvorkin M, Boku N, Özgüroğlu M, Ryu MH, Muntean AS, Lonardi S, Nechaeva M, Bragagnoli AC, Coşkun HS, Cubillo Gracian A, Takano T, Wong R, Safran H, Vaccaro GM, Wainberg ZA, Silver MR, Xiong H, Hong J, Taieb J, Bang YJ. Phase III Trial of Avelumab Maintenance After First-Line Induction Chemotherapy Versus Continuation of Chemotherapy in Patients With Gastric Cancers: Results From JAVELIN Gastric 100. J Clin Oncol. 2021;39:966-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 180] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 20. | Doi T, Iwasa S, Muro K, Satoh T, Hironaka S, Esaki T, Nishina T, Hara H, Machida N, Komatsu Y, Shimada Y, Otsu S, Shimizu S, Watanabe M. Phase 1 trial of avelumab (anti-PD-L1) in Japanese patients with advanced solid tumors, including dose expansion in patients with gastric or gastroesophageal junction cancer: the JAVELIN Solid Tumor JPN trial. Gastric Cancer. 2019;22:817-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 21. | Doi T, Iwasa S, Muro K, Satoh T, Hironaka S, Esaki T, Nishina T, Hara H, Machida N, Komatsu Y, Shimada Y, Otsu S, Shimizu S, Chand V, Watanabe M. Avelumab (anti-PD-L1) in Japanese patients with advanced gastric or gastroesophageal junction cancer (GC/GEJC): Updated results from the phase Ib JAVELIN solid tumour JPN trial. Ann Oncol. 2018;21:viii205-viii270. [RCA] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | Chung HC, Arkenau HT, Lee J, Rha SY, Oh DY, Wyrwicz L, Kang YK, Lee KW, Infante JR, Lee SS, Kemeny M, Keilholz U, Melichar B, Mita A, Plummer R, Smith D, Gelb AB, Xiong H, Hong J, Chand V, Safran H. Avelumab (anti-PD-L1) as first-line switch-maintenance or second-line therapy in patients with advanced gastric or gastroesophageal junction cancer: phase 1b results from the JAVELIN Solid Tumor trial. J Immunother Cancer. 2019;7:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 23. | Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, Alsina M, Ryu MH, Chung HC, Evesque L, Al-Batran SE, Park SH, Lichinitser M, Boku N, Moehler MH, Hong J, Xiong H, Hallwachs R, Conti I, Taieb J. Phase III, randomised trial of avelumab versus physician's choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol. 2018;29:2052-2060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 413] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 24. | Bang YJ, Cho JY, Kim YH, Kim JW, Di Bartolomeo M, Ajani JA, Yamaguchi K, Balogh A, Sanchez T, Moehler M. Efficacy of Sequential Ipilimumab Monotherapy versus Best Supportive Care for Unresectable Locally Advanced/Metastatic Gastric or Gastroesophageal Junction Cancer. Clin Cancer Res. 2017;23:5671-5678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 25. | Besaw RJ, Smith MP, Zerillo JA, Bullock AJ. Chronic intestinal pseudo-obstruction in a patient with metastatic gastro-oesophageal junction cancer receiving treatment with pembrolizumab. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Ai L, Gao J, Zhao S, Li Q, Cui YH, Liu Q, Wu D, Wang Y, Jin X, Ji Y, Li J, Yu Y, Liu T. Nivolumab-associated DRESS in a genetic susceptible individual. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Higashi T, Miyamoto H, Yoshida R, Furuta Y, Nagaoka K, Naoe H, Naito H, Nakayama H, Tanaka M. Sjögren's Syndrome as an Immune-related Adverse Event of Nivolumab Treatment for Gastric Cancer. Intern Med. 2020;59:2499-2504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Kimura A, Sakai D, Kudo T, Nishida N, Katou A, Inagaki C, Otsuru T, Miyazaki Y, Tanaka K, Makino T, Takahashi T, Kurokawa Y, Yamasaki M, Mori M, Doki Y, Satoh T. Nivolumab-induced interstitial lung disease in a patient with gastric cancer. Oxf Med Case Reports. 2019;2019:omz007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Maetani Y, Nezu T, Ueno H, Aoki S, Hosomi N, Maruyama H. Steroid-responsive Nivolumab-induced Involuntary Movement with Anti-thyroid Antibodies. Intern Med. 2019;58:3577-3581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Namikawa T, Shimizu S, Yokota K, Tanioka N, Fukudome I, Munekage M, Uemura S, Maeda H, Kitagawa H, Hanazaki K. Isolated adrenocorticotropic hormone deficiency induced by nivolumab treatment for advanced gastric cancer. Clin J Gastroenterol. 2021;14:988-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Ohara N, Kobayashi M, Ikeda Y, Hoshi T, Morita S, Kanefuji T, Yagi K, Suda T, Takada T, Hasegawa G, Sato Y, Hirano K, Kosugi SI. Non-insulin-dependent Diabetes Mellitus Induced by Immune Checkpoint Inhibitor Therapy in an Insulinoma-associated Antigen-2 Autoantibody-positive Patient with Advanced Gastric Cancer. Intern Med. 2020;59:551-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Sawada K, Shonaka T, Nishikawa Y, Hasegawa K, Hayashi H, Hasebe T, Nakajima S, Ikuta K, Fujiya M, Furukawa H, Okumura T. Successful Treatment of Nivolumab-related Cholangitis with Prednisolone: A Case Report and Review of the Literature. Intern Med. 2019;58:1747-1752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Shiraishi K, Tokoro Y, Kanai Y, Hayashi F, Marusawa H, Kanaya S, Tsumura T. [A Case of Gastric Cancer with Pericardial Effusion as an Immune-Reactive Adverse Events]. Gan To Kagaku Ryoho. 2020;47:1237-1240. [PubMed] |
| 34. | Taira K, Kimura A, Nakata A, Nadatani Y, Fukunaga S, Otani K, Hosomi S, Tanaka F, Kamata N, Nagami Y, Watanabe T, Fujiwara Y. A case of nivolumab-induced cervical lymphadenopathy in a patient with gastric cancer. J Gastrointest Oncol. 2021;12:880-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Takeda Y, Kubota T, Choda Y, Toi Y, Ichimura K, Ishida M, Yano T, Sato D, Yoshimitsu M, Nakano K, Harano M, Matsukawa H, Idani H, Shiozaki S, Okajima M. [Two Cases of Stevens-Johnson Syndrome after Nivolumab Therapy for Gastric Cancer]. Gan To Kagaku Ryoho. 2021;48:154-156. [PubMed] |
| 36. | Tanabe K, Kanzaki H, Wada T, Nakashima Y, Sugiyama H, Okada H, Wada J. Nivolumab-induced IgA nephropathy in a patient with advanced gastric cancer: A case report. Medicine (Baltimore). 2020;99:e20464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Yamada H, Okajima F, Onda T, Fujimori S, Emoto N, Sugihara H. New-onset graves' disease after the initiation of nivolumab therapy for gastric cancer: a case report. BMC Endocr Disord. 2020;20:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 38. | Yatsuda Y, Hirose S, Ito Y, Onoda T, Sugiyama Y, Nagafuchi M, Suzuki H, Niisato Y, Tange Y, Ikeda T, Yamada T, Yamamoto Y, Ohyama Osawa M, Sakamoto N, Moriwaki T, Mizokami Y. A Durable Response after the Discontinuation of Nivolumab in an Advanced Gastric Cancer Patient. Intern Med. 2021;60:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Ando T, Ueda A, Ogawa K, Motoo I, Kajiura S, Nakajima T, Hirano K, Okumura T, Tsukada K, Hara T, Suzuki N, Nakada N, Horikawa N, Fujii T, Yasuda I. Prognosis of Immune-related Adverse Events in Patients With Advanced Gastric Cancer Treated With Nivolumab or Pembrolizumab: A Multicenter Retrospective Analysis. In Vivo. 2021;35:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Booka E, Kikuchi H, Haneda R, Soneda W, Kawata S, Murakami T, Matsumoto T, Hiramatsu Y, Takeuchi H. Impact of Immune-related Adverse Events on Nivolumab Efficacy in Patients With Upper Gastrointestinal Cancer. In Vivo. 2021;35:2321-2326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Kono Y, Choda Y, Nakagawa M, Miyahara K, Ishida M, Kubota T, Seo K, Hirata T, Obayashi Y, Gotoda T, Moritou Y, Okikawa Y, Iwamoto Y, Okada H. Association Between Immune-Related Adverse Events and the Prognosis of Patients with Advanced Gastric Cancer Treated with Nivolumab. Target Oncol. 2021;16:237-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 42. | Namikawa T, Yokota K, Tanioka N, Fukudome I, Iwabu J, Munekage M, Uemura S, Maeda H, Kitagawa H, Kobayashi M, Hanazaki K. Systemic inflammatory response and nutritional biomarkers as predictors of nivolumab efficacy for gastric cancer. Surg Today. 2020;50:1486-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 43. | Ohta A, Komatsu S, Tsuji R, Tanaka S, Kumano T, Imura K, Shimomura K, Ikeda J, Taniguchi F, Doi T, Yamada S, Tomatsuri N, Yoshida N, Shioaki Y. [Clinical Evaluation of the Efficacy and Adverse Effects of Nivolumab Treatment for Patients with Advanced Gastric Cancer]. Gan To Kagaku Ryoho. 2020;47:725-727. [PubMed] |
| 44. | Suzuki H, Yamada T, Sugaya A, Ueyama S, Yamamoto Y, Moriwaki T, Hyodo I. Retrospective analysis for the efficacy and safety of nivolumab in advanced gastric cancer patients according to ascites burden. Int J Clin Oncol. 2021;26:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Huang YF, Xie WJ, Fan HY, Du J. Comparative Safety of PD-1/PD-L1 Inhibitors for Cancer Patients: Systematic Review and Network Meta-Analysis. Front Oncol. 2019;9:972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 46. | Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 511] [Cited by in RCA: 520] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 47. | Dall'Olio FG, Rizzo A, Mollica V, Massucci M, Maggio I, Massari F. Immortal time bias in the association between toxicity and response for immune checkpoint inhibitors: a meta-analysis. Immunotherapy. 2021;13:257-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 48. | Chen C, Zhang F, Zhou N, Gu YM, Zhang YT, He YD, Wang L, Yang LX, Zhao Y, Li YM. Efficacy and safety of immune checkpoint inhibitors in advanced gastric or gastroesophageal junction cancer: a systematic review and meta-analysis. Oncoimmunology. 2019;8:e1581547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 49. | Muir CA, Clifton-Bligh RJ, Long GV, Scolyer RA, Lo SN, Carlino MS, Tsang VHM, Menzies AM. Thyroid Immune-related Adverse Events Following Immune Checkpoint Inhibitor Treatment. J Clin Endocrinol Metab. 2021;106:e3704-e3713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 50. | Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, Cohen J, Sullivan RJ. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124:3706-3714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 347] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 51. | Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, Martínez-Bernal G, Ferrara R, Lai WV, Hendriks LEL, Sabari JK, Caramella C, Plodkowski AJ, Halpenny D, Chaft JE, Planchard D, Riely GJ, Besse B, Hellmann MD. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36:2872-2878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 748] [Article Influence: 106.9] [Reference Citation Analysis (0)] |
| 52. | Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, Brambilla M, Baglivo S, Grossi F, Chiari R. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145:479-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 264] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 53. | Zhong L, Wu Q, Chen F, Liu J, Xie X. Immune-related adverse events: promising predictors for efficacy of immune checkpoint inhibitors. Cancer Immunol Immunother. 2021;70:2559-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 54. | Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun-Vignes B, Eroglu Z, Hassel JC, Menzies AM, Sosman JA, Sullivan RJ, Moslehi JJ, Johnson DB. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4:1721-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1846] [Article Influence: 263.7] [Reference Citation Analysis (0)] |