Published online Feb 15, 2023. doi: 10.4251/wjgo.v15.i2.343
Peer-review started: October 29, 2022
First decision: December 30, 2022
Revised: January 2, 2023
Accepted: January 23, 2023
Article in press: January 23, 2023
Published online: February 15, 2023
Processing time: 108 Days and 13.8 Hours
Claudin 18.2 (CLDN18.2) is a cell surface protein expressed by gastric cancer cells. The monoclonal antibody zolbetuximab binds CLDN18.2-positive cancer cells and causes cancer cell death. A few studies researched the prognostic effect of CLDN18.2 expression in metastatic gastric adenocarcinoma.
To identify the prognostic value of CLDN18.2 expression in patients with metastatic gastric adenocarcinoma.
This study was conducted with 65 patients over the age of 18 who were diagnosed with metastatic gastric adenocarcinoma. We investigated the effect of CLDN18.2 expression on clinicopathological characteristics (age, sex, histological grade, Lauren classification, family history, metastatic site, HER2 expression) and prognosis for patients with metastatic gastric adenocarcinoma.
CLDN18.2 expression was positive in 73.8% (48) of the patients. During the median 17.7-mo follow-up period, 89.2% (58) of the patients died. Median progression-free survival and overall survival (OS) were 6 mo (95% confidence interval: 1.6-10.4) and 12 mo (95% confidence interval: 7.5-16.5). There was no statistically significant correlation between CLDN18.2 expression and clinicopathological characteristics of the patients. In univariate and multivariate Cox regression analysis, there was no correlation between clinicopathological characteristics of patients and progression-free survival or OS.
CLDN18.2 expression was quite high in patients with gastric adenocarcinoma, identifying the proportion of the patients in whom zolbetuximab would be efficacious. There is no statistically significant correlation with clinicopathological characteristics and OS. CLDN18.2 is not a prognostic marker in patients with gastric adenocarcinoma, although it is predictive.
Core Tip: Zolbetuximab is a new antibody drug targeting the cell surface protein claudin 18.2 (CLDN18.2) expressed by gastric cancer cells. CLDN18.2 expression, identifying the patient population who are susceptible to zolbetuximab, is discordant in different studies. The present study aimed to research the expression ratio of CLDN18.2 and its prognostic value for overall survival in patients with gastric adenocarcinoma in a single center located in Turkey.
- Citation: Kayikcioglu E, Yüceer RO, Cetin B, Yüceer K, Karahan N. Prognostic value of claudin 18.2 expression in gastric adenocarcinoma. World J Gastrointest Oncol 2023; 15(2): 343-351
- URL: https://www.wjgnet.com/1948-5204/full/v15/i2/343.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i2.343
Stomach cancer represents the third most common cause of cancer-related mortality globally and caused 768793 deaths in 2020 (7.7% of all cancer deaths)[1]. Most people with stomach cancer in its early stages show no symptoms. The majority of patients (60%) receive diagnosis at the advanced stage following the emergence of symptoms[2]. In light of phase 2 and 3 studies from Europe, perioperative chemotherapy (ChT) has become standard for patients with stage 2 and 3 gastric cancer, but the 5-year overall survival (OS) is still approximately 36%[3,4]. The prognosis for locally advanced, unresectable, or metastatic gastric cancer is poor; in clinical trials evaluating the effectiveness of ChT, the median survival time was typically less than 1 year[5].
Claudin (CLDN) 18, a member of the cell surface protein claudin family, has two isoforms: CLDN18.1 expressed in lung tissue and CLDN18.2 expressed specifically in gastric tissue. CLDN18.2 is also expressed by gastric cancer cells, showing that it is not lost during malignant transformation[6]. The monoclonal antibody zolbetuximab binds CLDN18.2-positive cancer cells and causes cancer cell death by antibody-dependent cellular toxicity and complement-dependent cytotoxicity. In MONO phase 2a study of zolbetuximab as a single agent, CLDN18.2-positive patients with metastatic gastric and gastroesophageal junction (G/GEJ) adenocarcinoma received a minimum of one line of ChT and showed a 23% response rate[7]. The phase 2 FAST study of zolbetuximab plus ChT (epirubicin, oxaliplatin, capecitabine) vs ChT (epirubicin, oxaliplatin, capecitabine) showed superior OS and progression-free survival (PFS), defining CLDN18.2 as a new target for cancer therapy[8].
We investigated the effect of CLDN18.2 expression on clinicopathological characteristics and prognosis of patients with metastatic gastric adenocarcinoma undergoing ChT.
Patients admitted to the medical oncology clinic of Suleyman Demirel University hospital between January 2013 and December 2021 with metastatic gastric adenocarcinoma were enrolled in this study. All cases were histopathologically confirmed according to the 5th edition of the World Health Organization classification of digestive system tumors[9]. The Protocol for the Examination of Specimens from Patients with Cancers of the Stomach 2022 of the College of American Pathologists was used to identify histopathologic subtype, tumor location, tumor grade, and HER2 for gastric adenocarcinoma[10]. From the hospital database, the following clinical data were obtained: age, sex, histological type and grade, family history of gastric cancer, metastatic site, HER2 expression, PFS, and OS. The ethics committee of Suleyman Demirel University approved the study with date and number 01/04/2022-102. Patients who accepted participation in the study, who were older than 18-years-old, followed up in the medical oncology clinic of Suleyman Demirel University hospital, and whose paraffin blocks for diagnosis of gastric adenocarcinoma could be reached were enrolled in the study.
Hematoxylin and eosin sections representing the tumor of patients diagnosed with gastric adenocarcinoma were re-examined. The best paraffin block was selected for immunohistochemistry staining. Sections with 4-micron thickness were taken from paraffin blocks and transferred onto an adhesive coated slide system. The following method was used for immunohistochemical staining with streptavidin-biotin. Sections were incubated at 56 °C for 12 h for deparaffinization. Three percent hydrogen peroxide was used to block endogenous peroxidase. Antigen retrieval was performed in a microwave oven for 20 min using 0.01 mol/L Tris/EDTA buffer pH 9.0. Sections were coated with primary antibodies including CLDN18.2 (rabbit monoclonal antibody, Clone EPR19202, at 1:500 dilution, Abcam, United Kingdom) and incubated at room temperature for 2 h. Sections were incubated for another 20 min at room temperature after the addition of binding (secondary) antibody (Goat Anti-Rabbit IgG H&L (HRP) kit, Abcam, United Kingdom). The streptavidin-biotin complex was added. 3,3′-Diaminobenzidine was used as chromogen for visualization. CLDN18.2 non-tumor gastric tissues were used as positive controls for each staining session.
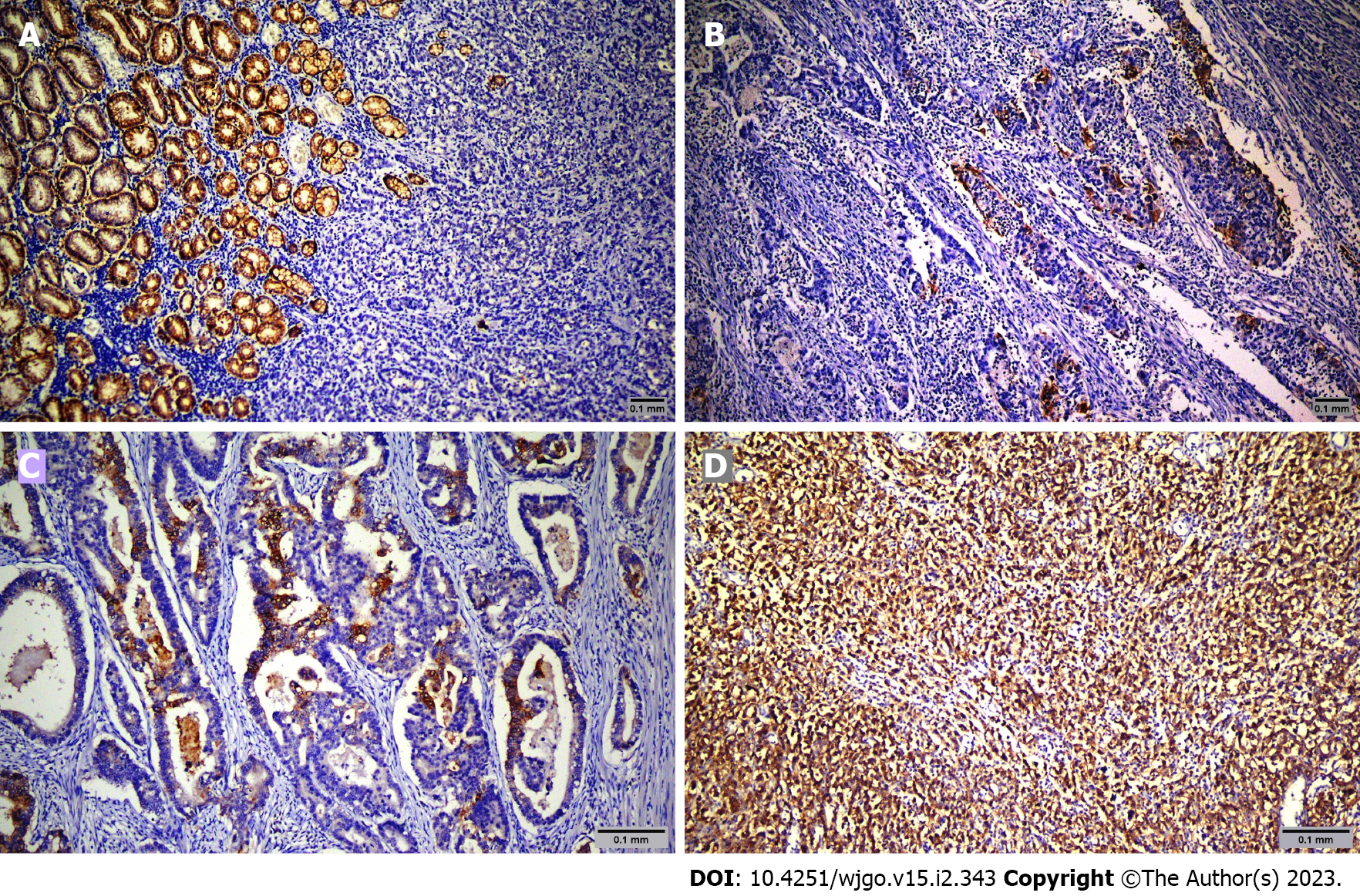
Pathology slides were reviewed by two expert pathologists (ROY and NK) who did not know patient treatments and outcomes. Tumor cells were scored positive for CLDN18.2 if they showed definite membranous staining and negative if tumor nuclei and cell membrane did not have immunoreactivity. Staining intensity was scored between 0 and 3 (absent: 0, weak: 1, moderate: 2, strong: 3).
Data analysis was performed using the Statistical Package for the Social Sciences 26.0 (SPSS Inc., Chicago, IL, United States). Age and clinical characteristics were compared between patients with expression of CLDN18.2 using the Mann-Whitney U-test for individual samples. In patient tumor samples with expression of CLDN18.2, sex, localization, family history, Lauren classification, grade, sites of metastasis, liver and lung metastases, and history of adjuvant and neoadjuvant ChT were compared using Pearson’s χ2 test. The correlation between CLDN18.2 and HER2 was determined with the Spearman correlation test. OS and PFS were estimated using the Kaplan-Meier method, and a log-rank test was used to compare study groups in terms of survival. Multivariate analyses were performed using Cox regression analysis. A P value of < 0.05 was considered statistically significant.
Sixty-nine patients were screened, and sixty-five were included in the study. The mean age was 64.6 years ± 12.9 years. Among the patients, 49 (75.4%) were male, and 16 (24.6%) were female. Table 1 shows the demographic and clinicopathologic characteristics of the patients according to CLDN18.2 expression. Immunohistochemical staining was used to screen 65 metastatic gastric adenocarcinoma cases for the pathological significance of CLDN18.2 expression (Figure 1). CLDN18.2 expression was positive in 73.8% (48) of the patients.
| Parameter | Number of cases | CLDN18.2 score | P value | ||||||||
| 0 | 1 | 2 | 3 | ||||||||
| n | % | n | % | n | % | n | % | n | % | ||
| Age in yr | |||||||||||
| < 65 | 30 | 46.2 | 4 | 13.3 | 10 | 33.3 | 9 | 30.0 | 7 | 23.3 | 0.091 |
| ≥ 65 | 35 | 53.8 | 13 | 37.1 | 10 | 28.6 | 5 | 14.3 | 7 | 20.0 | |
| Sex | |||||||||||
| Male | 49 | 75.4 | 14 | 28.6 | 15 | 30.6 | 11 | 22.4 | 9 | 18.4 | 0.314 |
| Female | 16 | 24.6 | 3 | 18.8 | 5 | 31.3 | 3 | 18.8 | 5 | 31.3 | |
| Lauren classification | |||||||||||
| Intestinal | 41 | 63.1 | 9 | 22.0 | 12 | 29.3 | 10 | 24.4 | 10 | 24.4 | 0.221 |
| Diffuse | 24 | 39.9 | 8 | 33.3 | 8 | 33.3 | 4 | 16.7 | 4 | 16.7 | |
| Tumor grade | |||||||||||
| G1 | 30 | 46.2 | 9 | 30.0 | 8 | 26.7 | 7 | 23.3 | 6 | 20.0 | 0.889 |
| G2 | 8 | 12.3 | 1 | 12.5 | 3 | 37.5 | 2 | 25.0 | 2 | 25.0 | |
| G3 | 27 | 41.5 | 7 | 25.9 | 9 | 33.3 | 5 | 18.5 | 6 | 22.2 | |
| Localization | |||||||||||
| Cardia | 18 | 27.7 | 3 | 16.7 | 9 | 50.0 | 3 | 16.0 | 3 | 16.7 | 0.307 |
| Corpus | 10 | 18.5 | 4 | 33.3 | 5 | 41.7 | 1 | 8.3 | 2 | 16.7 | |
| Antrum | 12 | 15.4 | 3 | 30.0 | 3 | 30.0 | 2 | 20.0 | 2 | 20.0 | |
| Pylorus | 2 | 2.1 | 0 | 0.0 | 0 | 0.0 | 2 | 100.0 | 0 | 0.0 | |
| Antropyloric | 23 | 35.4 | 7 | 30.4 | 3 | 13.0 | 6 | 26.1 | 7 | 30.4 | |
| Her2Neu | |||||||||||
| Negative | 54 | 83.1 | 13 | 24.1 | 15 | 27.8 | 13 | 24.1 | 13 | 24.1 | 0.116 |
| Positive | 11 | 16.9 | 4 | 36.4 | 5 | 45.5 | 1 | 9.1 | 1 | 9.1 | |
| Family history | |||||||||||
| No | 40 | 61.5 | 13 | 32.5 | 9 | 22.5 | 10 | 25.0 | 8 | 20.0 | 0.751 |
| Yes | 14 | 21.5 | 2 | 14.3 | 6 | 42.9 | 2 | 14.3 | 4 | 28.6 | |
| Unknown | 11 | 16.9 | 2 | 18.2 | 5 | 45.5 | 2 | 18.2 | 2 | 18.2 | |
| Liver metastasis | |||||||||||
| No | 33 | 50.8 | 8 | 25.0 | 10 | 31.3 | 6 | 18.8 | 8 | 25.00 | 0.703 |
| Yes | 32 | 49.2 | 9 | 27.3 | 10 | 30.3 | 8 | 24.2 | 6 | 18.2 | |
| Lung metastasis | |||||||||||
| No | 48 | 73.2 | 9 | 52.9 | 3 | 17.6 | 2 | 11.8 | 3 | 17.6 | 0.053 |
| Yes | 17 | 26.8 | 8 | 16.7 | 17 | 35.4 | 12 | 25.0 | 11 | 22.9 | |
| Metastasis sites | |||||||||||
| Liver | 16 | 24.6 | 2 | 12.5 | 7 | 43.8 | 2 | 12.5 | 5 | 31.3 | 0.050 |
| Lung | 4 | 6.2 | 2 | 50.0 | 0 | 0.0 | 0 | 0.0 | 2 | 50.0 | |
| Peritoneum | 11 | 16.9 | 0 | 0.0 | 5 | 45.5 | 3 | 27.3 | 3 | 27.3 | |
| LAP | 14 | 21.5 | 4 | 28.6 | 2 | 14.3 | 6 | 42.90 | 2 | 14.30 | |
| Brain | 2 | 3.1 | 2 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Liver + lung | 17 | 26.2 | 7 | 41.2 | 6 | 35.0 | 2 | 11.8 | 2 | 11.8 | |
| Ovary | 1 | 1.5 | 0 | 0.0 | 0 | 0.0 | 1 | 100.0 | 0 | 0.0 | |
| Adjuvant chemotherapy | |||||||||||
| No | 29 | 44.6 | 8 | 27.6 | 9 | 31.0 | 6 | 20.7 | 6 | 20.7 | 0.793 |
| Yes | 36 | 55.4 | 9 | 25.0 | 11 | 30.6 | 8 | 22.2 | 8 | 22.2 | |
| Neoadjuvant chemotherapy | |||||||||||
| No | 61 | 91.8 | 14 | 23.0 | 20 | 32.8 | 13 | 21.3 | 14 | 23.0 | 0.097 |
| Yes | 4 | 8.2 | 3 | 75.0 | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 | |
| Exitus | |||||||||||
| No | 7 | 10.8 | 0 | 0.0 | 3 | 42.9 | 3 | 42.9 | 1 | 14.3 | 0.401 |
| Yes | 58 | 89.2 | 17 | 29.3 | 17 | 29.3 | 11 | 19.0 | 13 | 22.4 | |
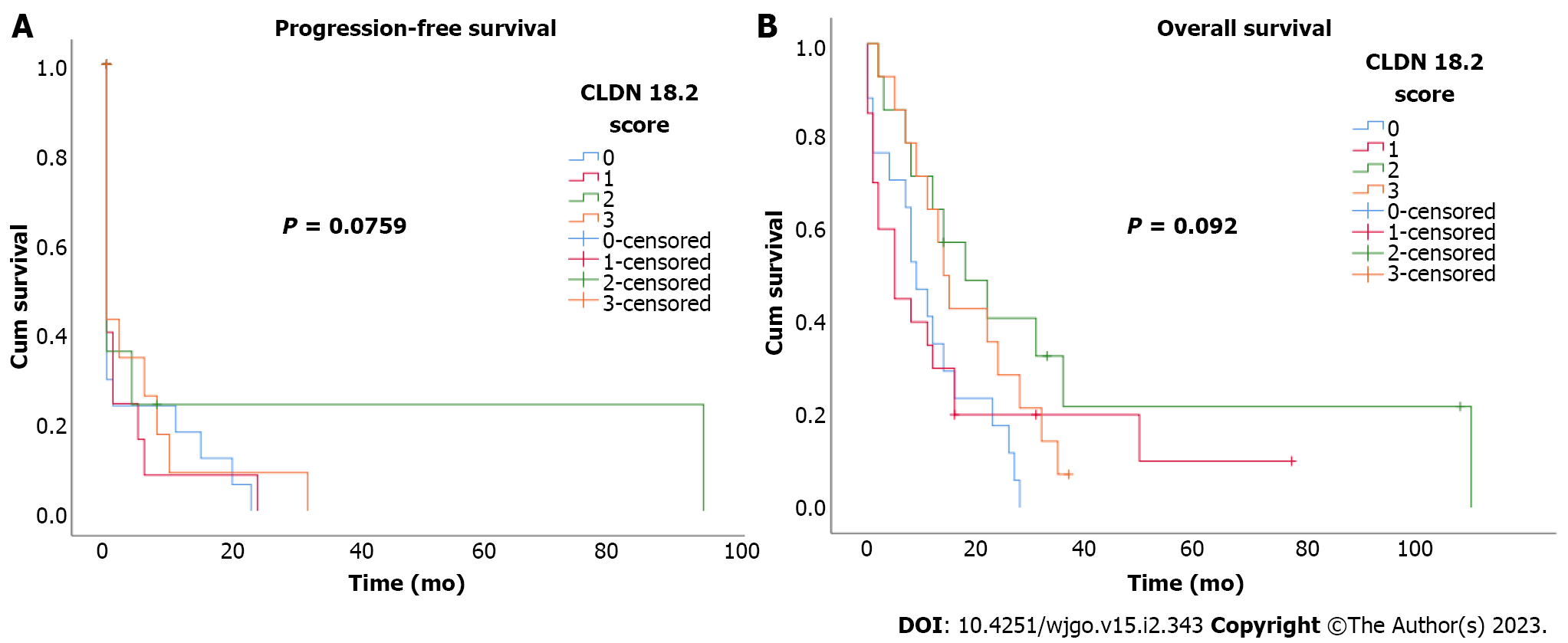
During the median 17.7-mo follow-up period, 89.2% (58) of the patients died. Median PFS and OS were 6 mo (95% confidence interval: 1.6-10.4) and 12 mo (95% confidence interval: 7.5-16.5). There was no statistically significant correlation between CLDN18.2 expression and clinicopathological characteristics of the patients (Figure 2). In univariate and multivariate Cox regression analysis for PFS, there was no correlation between clinicopathological characteristics of patients and PFS (Table 2). In univariate and multivariate Cox regression analysis for OS, older age was an independent risk factor for poor OS (Table 3).
| Parameter | Progression-free survival univariate analysis | Progression-free survival multivariate analysis | |||||
| HR | 95%CI | P | HR | 95%CI | P | ||
| Age in yr | 1.36 | 0.80-2.30 | 0.26 | Age in yr | 1.29 | 0.71-2.33 | 0.41 |
| Sex | 1.40 | 0.76-2.56 | 0.28 | Sex | 1.49 | 0.73-3.05 | 0.28 |
| Lauren classification | 0.89 | 0.52-1.53 | 0.67 | Lauren classification | 0.93 | 0.39-2.22 | 0.87 |
| Tumor grade | 0.85 | 0.49-1.47 | 0.56 | Tumor grade | 0.91 | 0.58-1.43 | 0.69 |
| Family history | 0.87 | 0.45-1.77 | 0.75 | Family history | 0.51 | 0.20-1.28 | 0.15 |
| Liver metastasis | 1.09 | 0.64-1.85 | 0.75 | Liver metastasis | 1.07 | 0.60-1.91 | 0.82 |
| Lung metastasis | 0.93 | 0.52-1.66 | 0.79 | Lung metastasis | 0.98 | 0.49-1.94 | 0.95 |
| Localization | 0.94 | 0.42-2.11 | 0.88 | Localization | 1.01 | 0.81-1.24 | 0.98 |
| Metastasis sites | 0.83 | 0.27-2.60 | 0.75 | Metastasis sites | 0.99 | 0.84-1.18 | 0.94 |
| Her2Neu | 0.81 | 0.40-1.64 | 0.56 | Her2Neu | 0.85 | 0.37-1.93 | 0.69 |
| CLDN18.2 | 1.22 | 0.54-2.32 | 0.77 | CLDN18.2 | 1.30 | 0.54-3.19 | 0.56 |
| Paragraph | Overall survival univariate analysis | Overall survival multivariate analysis | |||||
| HR | 95%CI | P | HR | 95%CI | P | ||
| Age in yr | 2.46 | 1.39-4.33 | 0.01 | Age in yr | 3.17 | 1.45-6.92 | 0.01 |
| Sex | 1.10 | 0.61-1.99 | 0.75 | Sex | 0.65 | 0.26-1.59 | 0.34 |
| Lauren classification | 1.28 | 0.66-1.94 | 0.66 | Lauren classification | 1.23 | 0.24-6.16 | 0.81 |
| Tumor grade | 0.41 | 0.15-1.07 | 0.07 | Tumor grade | 0.29 | 0.08-1.06 | 0.06 |
| Family history | 0.83 | 0.36-1.97 | 0.67 | Family history | 2.14 | 0.68-6.71 | 0.19 |
| Liver metastasis | 0.94 | 0.56-1.59 | 0.82 | Liver metastasis | 0.74 | 0.33-1.65 | 0.46 |
| Lung metastasis | 0.71 | 0.39-1.27 | 0.25 | Lung metastasis | 0.58 | 0.22-1.52 | 0.27 |
| Localization | 0.91 | 0.46-1.79 | 0.78 | Localization | 2.14 | 0.68-6.71 | 0.19 |
| Metastasis sites | 1.36 | 0.30-6.09 | 0.69 | Metastasis sites | 1.88 | 0.52-6.82 | 0.34 |
| Her2Neu | 1.11 | 0.56-2.22 | 0.77 | Her2Neu | 0.90 | 0.35-2.34 | 0.83 |
| CLDN18.2 | 1.68 | 0.81-3.50 | 0.12 | CLDN18.2 | 2.78 | 0.85-9.07 | 0.09 |
Gastric cancer is common and fatal. With targeted agents and immunotherapy, the median OS of patients with metastatic gastric cancer has reached 13.8-14.4 mo[11,12]. Novel therapies are critical for extending the survival of gastric adenocarcinoma patients. CLDN18.2 is a tight junction molecule found on the surface of gastric mucosa epithelium and gastric cancer cells[6]. In metastatic gastric cancer patients, the monoclonal antibody zolbetuximab targeting CLDN18.2 contributes to OS alone and when combined with ChT. It had tolerable side effects such as nausea and vomiting[7,8]. Worldwide clinical trials of zolbetuximab in the first-line setting, in combination with ChT and immunotherapy, are ongoing for G/GEJ adenocarcinoma (NCT03505320, NCT03504397, and NCT03653507).
Histopathological subtype was diffuse in 36.9% (24) of patients and intestinal in 63.1% (41), and there was no correlation with CLDN18.2 expression. In a study including 481 patients with gastric cancer, there was no correlation between histopathological subtype per Lauren classification and CLDN18.2 expression, as in our study[13]. However, in a study including 263 Japanese patients with gastric adenocarcinoma, diffuse histopathological subtype was associated with strong CLDN18.2 expression[14]. In another study of 85 patients with gastric adenocarcinoma, intestinal subtype was associated with strong CLDN18.2 expression[15]. There was no correlation between grades of tumors and CLDN18.2 expression in a study including 485 patients with esophageal adenocarcinoma[16]; however, grade 3 tumors were associated with strong CLDN18.2 expression in two studies[13,14].
HER2 expression was positive in 16.9% (11) of patients, and there was no correlation between HER2 and CLDN18.2 expression. In three different studies, there was no correlation between HER2 and CLDN18.2 expression[13,15,17], while there was an inverse correlation in a study including patients with esophageal adenocarcinoma[16].
In the present study, CLDN18.2 expression was detected in 73.8% (48) of patients, with moderate to strong expression (≥ 2+) in 43.1% (n = 28). CLDN18.2 expression was detected in 87%, with moderate and strong expression in 51.5%, of Japanese patients in a study conducted by Rohde et al[14], and moderate to strong expression was present in 49% of patients with G/GEJ adenocarcinoma in the FAST study conducted by Sahin et al[6]. There was no correlation between clinicopathological characteristics of the patients and OS in the present study, consistent with other studies[13,15,16].
Inconsistent with the present study, Türeci et al[7] and Sahin et al[8] detected CLDN18.2 expression in only 17.1% and 14.1% of patients, respectively. This could be due to the different patient cohorts in the studies as well as the different kits used to detect CLDN18.2 expression. Few studies have been published regarding the expression of CLDN18.2 in gastric adenocarcinoma. Conflicting results exist about the CLDN18.2 expression ratios and the relationship between these parameters and the clinicopathological characteristics of patients with gastric adenocarcinoma; however, the studies are consistent in showing there is no correlation between CLDN18.2 expression and OS, as in the present study. The proportion of patients with gastric adenocarcinoma in whom zolbetuximab was efficacious was determined by the MONO and FAST trials. Our findings are consistent with these studies.
The limitations of this study included the relatively small number of patients analyzed and the retrospective character. Additional studies with a larger number of patients are needed to define the effect of CLDN18.2 expression on OS.
CLDN18.2 expression is quite high in patients with gastric adenocarcinoma, identifying the proportion of the patients in whom zolbetuximab would be efficacious. There is no statistically significant correlation with clinicopathological characteristics and OS. CLDN18.2 is not a prognostic marker in patients with gastric adenocarcinoma.
Claudin 18.2 (CLDN18.2) is a cell surface protein expressed by gastric cancer cells and a new target for the monoclonal antibody named zolbetuximab.
It is unknown whether CLDN18.2 expression on gastric cancer cells is prognostic.
To identify the prognostic value of CLDN18.2 expression in patients with metastatic gastric adenocarcinoma.
We investigated the effect of CLDN18.2 expression on clinicopathological characteristics (age, sex, histological grade, Lauren classification, family history, metastatic site, and HER2 expression) and prognosis for patients with metastatic gastric adenocarcinoma.
There was no statistically significant correlation between CLDN18.2 expression and clinicopathological characteristics of the patients. In univariate and multivariate Cox regression analysis, there was no correlation between clinicopathological characteristics of patients and progression-free survival or overall survival. The expression of CLDN18.2 was predictive for zolbetuximab in metastatic gastric adenocarcinoma, but it is not prognostic.
CLDN18.2 expression is high in metastatic gastric adenocarcinoma and predictive for zolbetuximab, but it is not prognostic.
Detection of new prognostic and predictive markers will make gastric cancer more manageable.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Obando A, Nicaragua; Vieth M, Germany S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64628] [Article Influence: 16157.0] [Reference Citation Analysis (176)] |
| 2. | Allum W, Lordick F, Alsina M, Andritsch E, Ba-Ssalamah A, Beishon M, Braga M, Caballero C, Carneiro F, Cassinello F, Dekker JW, Delgado-Bolton R, Haustermans K, Henning G, Hutter B, Lövey J, Netíková IŠ, Obermannová R, Oberst S, Rostoft S, Saarto T, Seufferlein T, Sheth S, Wynter-Blyth V, Costa A, Naredi P. ECCO essential requirements for quality cancer care: Oesophageal and gastric cancer. Crit Rev Oncol Hematol. 2018;122:179-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4609] [Article Influence: 242.6] [Reference Citation Analysis (0)] |
| 4. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Genève J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1504] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 5. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2856] [Article Influence: 571.2] [Reference Citation Analysis (5)] |
| 6. | Sahin U, Koslowski M, Dhaene K, Usener D, Brandenburg G, Seitz G, Huber C, Türeci O. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14:7624-7634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 300] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 7. | Türeci O, Sahin U, Schulze-Bergkamen H, Zvirbule Z, Lordick F, Koeberle D, Thuss-Patience P, Ettrich T, Arnold D, Bassermann F, Al-Batran SE, Wiechen K, Dhaene K, Maurus D, Gold M, Huber C, Krivoshik A, Arozullah A, Park JW, Schuler M. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: the MONO study. Ann Oncol. 2019;30:1487-1495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 8. | Sahin U, Türeci Ö, Manikhas G, Lordick F, Rusyn A, Vynnychenko I, Dudov A, Bazin I, Bondarenko I, Melichar B, Dhaene K, Wiechen K, Huber C, Maurus D, Arozullah A, Park JW, Schuler M, Al-Batran SE. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021;32:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 263] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 9. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2439] [Article Influence: 487.8] [Reference Citation Analysis (3)] |
| 10. | Shi C, Badgwell BD, Grabsch HI, Gibson MK, Hong SM, Kumarasinghe P, Lam AK, Lauwers G, O'Donovan M, van der Post RS, Tang L, Ushiku T, Vieth M, Selinger CI, Webster F, Nagtegaal ID. Data Set for Reporting Carcinoma of the Stomach in Gastrectomy. Arch Pathol Lab Med. 2022;146:1072-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5324] [Article Influence: 354.9] [Reference Citation Analysis (3)] |
| 12. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1898] [Article Influence: 474.5] [Reference Citation Analysis (1)] |
| 13. | Dottermusch M, Krüger S, Behrens HM, Halske C, Röcken C. Expression of the potential therapeutic target claudin-18.2 is frequently decreased in gastric cancer: results from a large Caucasian cohort study. Virchows Arch. 2019;475:563-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 14. | Rohde C, Yamaguchi R, Mukhina S, Sahin U, Itoh K, Türeci Ö. Comparison of Claudin 18.2 expression in primary tumors and lymph node metastases in Japanese patients with gastric adenocarcinoma. Jpn J Clin Oncol. 2019;49:870-876. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 15. | Hong JY, An JY, Lee J, Park SH, Park JO, Park YS, Lim HY, Kim KM, Kang WK, Kim ST. Claudin 18.2 expression in various tumor types and its role as a potential target in advanced gastric cancer. Transl Cancer Res. 2020;9:3367-3374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Moentenich V, Gebauer F, Comut E, Tuchscherer A, Bruns C, Schroeder W, Buettner R, Alakus H, Loeser H, Zander T, Quaas A. Claudin 18.2 expression in esophageal adenocarcinoma and its potential impact on future treatment strategies. Oncol Lett. 2020;19:3665-3670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Arnold A, Daum S, von Winterfeld M, Berg E, Hummel M, Rau B, Stein U, Treese C. Prognostic impact of Claudin 18.2 in gastric and esophageal adenocarcinomas. Clin Transl Oncol. 2020;22:2357-2363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |