Published online Feb 15, 2023. doi: 10.4251/wjgo.v15.i2.332
Peer-review started: November 20, 2022
First decision: December 14, 2022
Revised: December 21, 2022
Accepted: December 30, 2022
Article in press: December 30, 2022
Published online: February 15, 2023
Processing time: 86 Days and 14.1 Hours
The overexpression of the MYC gene plays an important role in the occurrence, development and evolution of colorectal cancer (CRC). Bromodomain and extraterminal domain (BET) inhibitors can decrease the function BET by recognizing acetylated lysine residues, thereby downregulating the expression of MYC.
To investigate the inhibitory effect and mechanism of a BET inhibitor on CRC cells.
The effect of the BET inhibitor JAB-8263 on the proliferation of various CRC cell lines was studied by CellTiter-Glo method and colony formation assay. The effect of JAB-8263 on the cell cycle and apoptosis of CRC cells was studied by propidium iodide staining and Annexin V/propidium iodide flow assay, respectively. The effect of JAB-8263 on the expression of c-MYC, p21 and p16 in CRC cells was detected by western blotting assay. The anti-tumor effect of JAB-8263 on CRC cells in vivo and evaluation of the safety of the compound was predicted by constructing a CRC cell animal tumor model.
JAB-8263 dose-dependently suppressed CRC cell proliferation and colony formation in vitro. The MYC signaling pathway was dose-dependently inhibited by JAB-8263 in human CRC cell lines. JAB-8263 dose-dependently induced cell cycle arrest and apoptosis in the MC38 cell line. SW837 xenograft model was treated with JAB-8263 (0.3 mg/kg for 29 d), and the average tumor volume was significantly decreased compared to the vehicle control group (P < 0.001). The MC38 syngeneic murine model was treated with JAB-8263 (0.2 mg/kg for 29 d), and the average tumor volume was significantly decreased compared to the vehicle control group (P = 0.003).
BET could be a potential effective drug target for suppressing CRC growth, and the BET inhibitor JAB-8263 can effectively suppress c-MYC expression and exert anti-tumor activity in CRC models.
Core Tip: After treating colorectal cancer (CRC) cells with the bromodomain and extraterminal domain (BET) inhibitor JAB-8263, we found that MC38 cells undergo cell cycle arrest and apoptosis. In multiple human CRC cell lines, we found that JAB-8263 downregulated c-MYC expression and upregulated p21 and p16 expression, which is associated with the highly potent antiproliferative effects of JAB-8263. JAB-8263 effectively inhibited CRC growth with acceptable tolerance in tumor mouse models. Our studies suggested that BET can be a potential effective drug target for suppressing CRC growth, and JAB-8263 can effectively suppress c-MYC expression and exert anti-tumor activity in CRC models.
- Citation: Liu XM, Xia SY, Long W, Li HJ, Yang GQ, Sun W, Li SY, Du XH. Potent bromodomain and extraterminal domain inhibitor JAB-8263 suppresses MYC expression and exerts anti-tumor activity in colorectal cancer models. World J Gastrointest Oncol 2023; 15(2): 332-342
- URL: https://www.wjgnet.com/1948-5204/full/v15/i2/332.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i2.332
Colorectal cancer (CRC) is one of the most common malignant tumors, and its morbidity and mortality ranks third among all tumor patients[1], which seriously threatens human health. Traditional treatment methods include surgery, chemotherapy and radiotherapy. However, these treatments are invasive and are often accompanied by side effects[2]. In recent years, targeted therapy and immunotherapy have also developed rapidly as new treatment methods. With the deepening of tumor research, it has been found that the occurrence and development of colorectal tumors are related to the dysregulation of the epigenome[3], and one of the major areas of interest in epigenetic targets is the bromodomain and extraterminal domain (BET).
BET proteins belong to the acetyl-lysine-binding bromodomain (BRD) protein family and have four members, BRD2, BRD3, BRD4 and BRDT[4,5]. BET proteins have two N-terminal bromodomains (BD1 and BD2) that interact with acetylated lysine residues in histones. Then, it binds to transcription factor P-TEFb and RNA polymerase II and induces transcription[6]. BET protein acts as an epigenetic regulator and transcriptional cofactor, and it is closely associated with gene transcription, cell cycle and apoptosis, invasion and metastasis. BET proteins promote aberrant expression of many oncogenes such as MYC, CCND1 and BCL2L1[7,8].
MYC is a proto-oncogene that is activated by amplification and chromosomal translocation rearrangement. The overexpression of MYC plays an important role in the occurrence, development and evolution of CRC[9,10]. Overexpression of MYC and dysregulation of MYC target genes can be found in most CRC cells[11]. BET inhibitors bind to the BET protein, occupying the space where it binds to acetylated lysines, thus inhibiting the transcription of its downstream MYC oncogenes and MYC-dependent genes[12,13]. A study showed the small molecule BET inhibitor JQ1 occupies the bromodomain pocket of BRD4, resulting in downregulation of MYC mRNA and MYC protein[14]. This provides a rationale for the idea that BET inhibitors may exert anti-tumor activity in CRC cells.
The BET inhibitor JAB-8263 used in this study is a new type of BET inhibitor, which has a strong affinity with BET proteins and can significantly inhibit BET downstream signals c-MYC and N-MYC at a concentration of less than 1 nmol/L. It can significantly inhibit the proliferation of various tumor cells and induce the expression of cleaved PARP and the activation of caspase3/7, thereby inhibiting the proliferation of tumor cells and inducing apoptosis. Previous in vivo studies have shown that JAB-8263 has strong anti-tumor effects in various tumor models such as hematological tumors and small cell lung cancer through the MYC pathway. The pharmacology tests on safety show that JAB-8263 has no adverse effects on the cardiovascular system, respiratory system and central nervous system.
We predicted that JAB-8263 can suppress CRC cells in vitro and in vivo, and the purpose of this study was to explore the mechanism of its inhibitory effect on CRC cells.
All CRC cell lines (HT29, DLD1, Colo205, H716, SW837, H508 and MC38) used in this study were purchased from ATCC and kept in our laboratory. The CellTiter-Glo method was used in this experiment. CRC cells were plated in cell culture plates and cultured in a cell culture incubator at 37 °C, 5% CO2 or 100% air and 95% humidity. Compounds were added the next day and incubated for 5 d, and cell viability was detected with the CellTiter-Glo kit. The data were analyzed using GraphPad Prism software, and a four-parameter equation was used to fit a concentration-response curve, from which the IC50 of the compound concentration corresponding to 50% cell viability on the curve was calculated. Cell viability (%) = (Lumitest compound-Lumiblank control)/(Lumisolvent control-Lumiblank control) × 100%. Compound information: BET inhibitor JAB-8263 (Jacobio Pharmaceuticals, Beijing, China), purity: 99.10%, storage condition: 4 °C.
The cell suspension was serially diluted, and 1000 cells were inoculated in each group of cells per dish, cultured in a cell incubator at 37 °C, 5% CO2 or 100% air and 95% humidity and stained with crystal violet solution after 5 d. Cells exposed to the drug were compared to controls (treated with DMSO) assayed in triplicate.
Six-well plates were seeded with MC38 cells in logarithmic growth phase, 5 × 105 cells per well. Diluted JAB-8263 compound was added to each well, and 0.1% DMSO was added to the control group; the incubation time was 3 d and 5 d, respectively. Cells were then trypsinized, washed with PBS and stained with propidium iodide (PI) solution for 30 min in a dark room. Cell DNA content was analyzed by flow cytometry in triplicate.
Six-well plates were seeded with MC38 cells in logarithmic growth phase, 5 × 105 cells per well. Diluted JAB-8263 compound was added to each well, and 0.1% DMSO was added to the control group; the incubation time was 3 d and 5 d, respectively. Cells were then trypsinized and washed with PBS. Cells were stained (Thermo Annexin V Apoptosis Detection Kit, APC) and incubated for 30 min at room temperature in a dark room. Analysis was performed in triplicate using a drain cytometer in triplicate.
Cells were harvested, and cellular protein collection was performed after addition of lysate. The protein concentration was detected according to the BCA instructions. The samples added to loading buffer were electrophoresed by discontinuous SDS-PAGE denaturing gel. The protein was transferred to PVDF membrane and detected by ECL exposure. Antibodies information: Anti-c-MYC antibody (ab32072, Abcam, United Kingdom); p21 Waf1/Cip1 (12D1) Rabbit mAb (#2947, GST, United States); p16 INK4A (E6N8P) Rabbit mAb (#18769, GST, United States); GAPDH (D16H11) XP® Rabbit mAb (#18769, GST, United States).
All animal care and use-related experimental protocols and changes to the experimental protocols of animals in this experiment were reviewed, approved and guided by the Jacobio Animal Care and Use Management Committee.
SW837 xenograft mouse model: 12 female NOD-SCID mice were subcutaneously inoculated with 1 × 107 SW837 cells on the right back. When the tumor grew to an average of 121 mm3, the mice were randomly divided into two groups according to tumor size and body weight. The experiment was divided into a vehicle control group and a JAB-8263 0.3 mg/kg treatment group. The JAB-8263 0.3 mg/kg treatment group and vehicle control group were administered by gavage once every 2 d. The anti-tumor activity was evaluated according to the relative tumor growth inhibition (TGI) rate. TGI (%) = (1-TRTV))/CRTV × 100% (TRTV: mean RTV of the treatment group; CRTV: mean RTV of the vehicle control group; RTV = Vt-V0, V0 is the volume of the subcutaneous transplanted tumor of the mouse at the time of grouping, and Vt is the volume of the subcutaneous tumor of the mouse after treatment). The safety was evaluated according to the changes in animal body weight, drug withdrawal and death.
MC38 syngeneic murine model: 16 female C57BL/6 mice were subcutaneously inoculated with 1 × 106 MC38 cells on the right back. When the tumors grew to an average of 103 mm3, they were randomly divided into two groups according to the tumor size and the weight of the mice. The experiment was divided into a vehicle control group and a JAB-8263 0.2 mg /kg treatment group. JAB-8263 0.2 mg/kg treatment group and vehicle control group were administered by gavage once every 2 d. The anti-tumor activity was evaluated according to the relative TGI rate, and the safety was evaluated according to the changes in animal body weight, drug withdrawal and death.
A single-dose MC38 model: In addition, we used the above method to establish a single-dose MC38 model. Nine female C57BL/6 mice were randomly divided into two groups according to the tumor size and the weight of the mice. The experiment was divided into a vehicle control group, a JAB-8263 0.1 mg/kg treatment group and a JAB-8263 0.2 mg/kg treatment group. One hour after the treatments were administered, the experiment was terminated, all mice were euthanized, and tumor tissues were collected.
All experimental results were expressed as mean ± SD. The t-test method was used to compare the data of the treatment group and the control group for statistical differences. All data were analyzed with SPSS 22.0, and P < 0.05 was considered statistically significant.
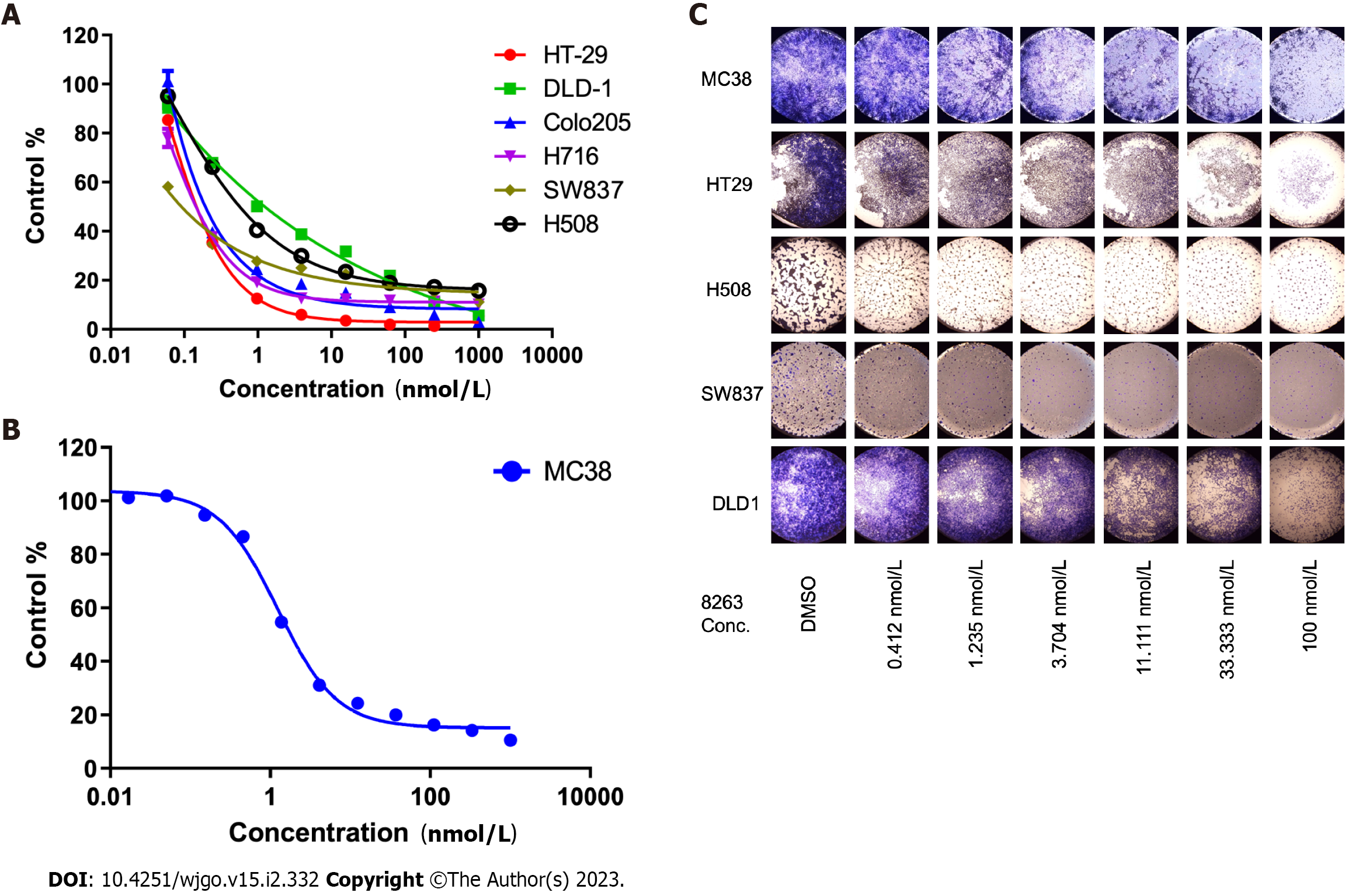
We found seven CRC cell lines that were sensitive to JAB-8263 in cell proliferation assays, including human CRC cell lines (HT29, DLD1, Colo205, H716, SW837 and H508, Figure 1A) and murine CRC cell line (MC38, Figure 1B). The IC50 values of six human CRC cell lines including HT-29, DLD-1, Colo205, H716, SW837 and H508 were 0.09-1.24 nmol/L, and the IC50 of the murine CRC cell line MC38 was 1.25 nmol/L.
In the colony formation assay, five groups of CRC cell lines were sensitive to the JAB-8263 compound. Compared with the control group (DMSO), the colony formation of the cell lines in each group was significantly reduced with increasing drug concentration (Figure 1C). Taken together, these data suggest that JAB-8263 dose-dependently suppressed CRC cell proliferation and colony formation in vitro.
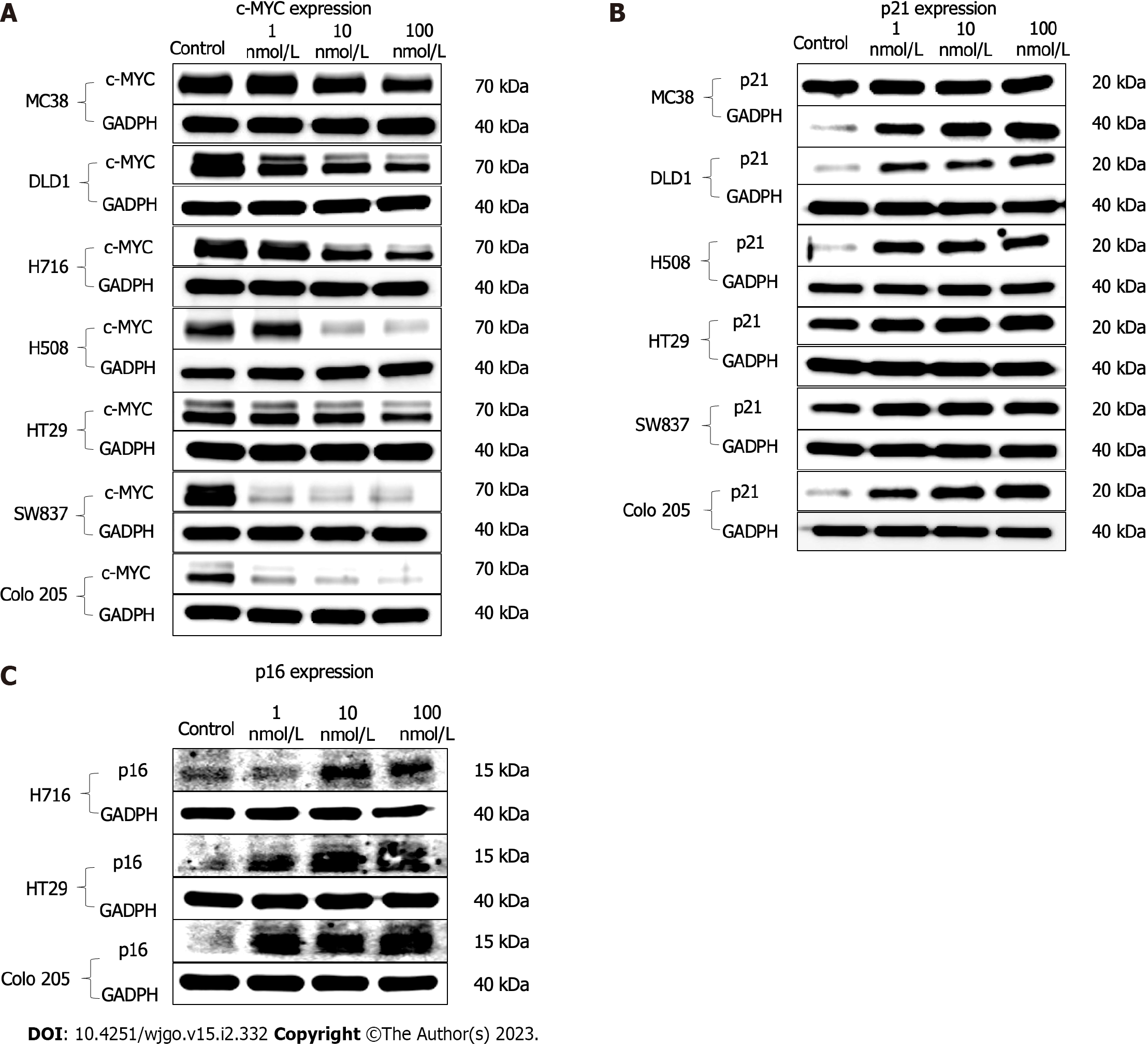
Western blot assays on MYC, p21 and p16 levels were performed in human CRC cell lines with JAB-8263 treatment. Compared with the control group (DMSO), the expression of MYC was downregulated in all cell lines with the treatment of different concentrations of JAB-8263 (1 nmol/L, 10 nmol/L and 100 nmol/L). The p21 expression of MC38, DLD-1, H508, HT29, SW837 and Colo205 was upregulated, and the expression of p16 in H716, HT29 and colo205 was upregulated (Figure 2A-C). This data suggest that JAB-8263 dose-dependently downregulated the expression of c-MYC in CRC cells and upregulated the expression of p21 and p16 in some of the CRC cell lines.
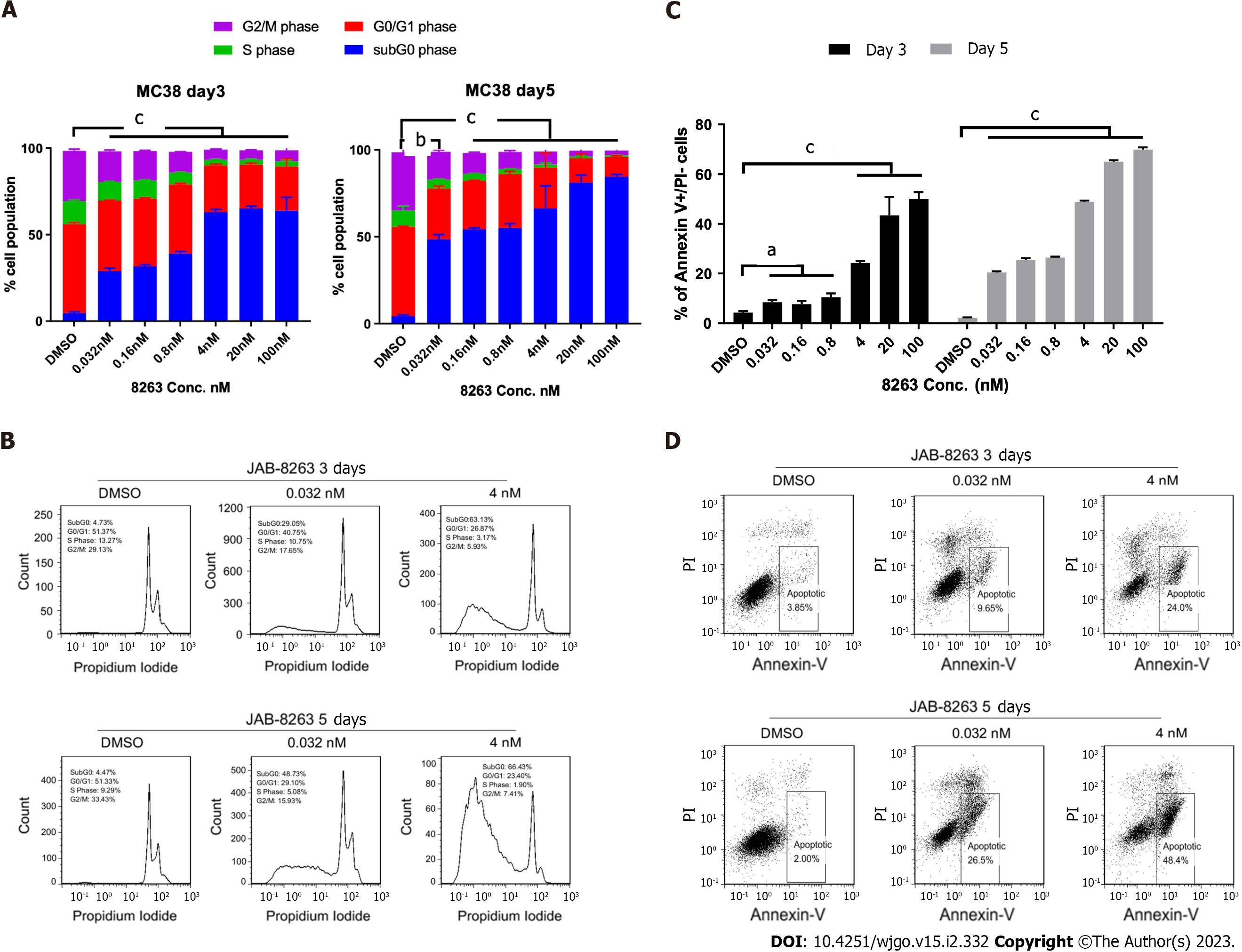
We conducted further cell cycle and apoptosis assays on the murine CRC cell line MC38 to explore the mechanism of JAB-8263 suppressed CRC cell proliferation. In the cell cycle assay, the MC38 cell cycle was arrested in the subG0 phase compared with the control group after 3 d and 5 d of treatment with JAB-8263 in different concentrations. JAB-8263 dose-dependently decreased the G2/M phase ratio and increased the subG0 prophase ratio in MC38 cells, indicating that JAB-8263 induced cell cycle arrest in the G0 phase. (Figure 3A and B). In the apoptosis assay, the apoptotic ratio of MC38 was increased compared with the control group after 3 d and 5 d of treatment with JAB-8263. Furthermore, the apoptotic ratio increased with the compound concentration (Figure 3C and D). This data indicates that JAB-2485 suppressed tumor cell activity in two ways by inducing MC38 cell cycle arrest and apoptosis.
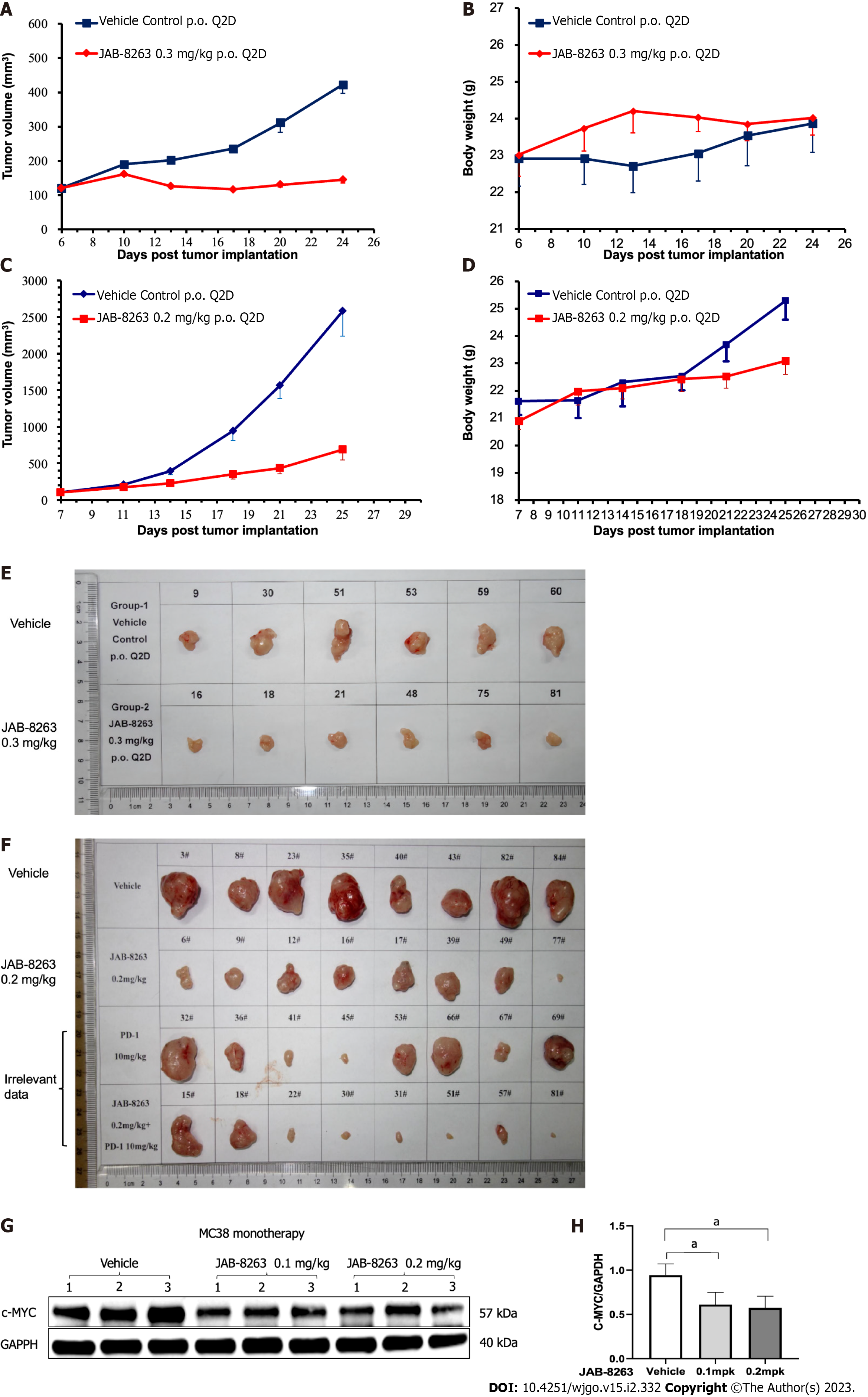
After 29 d of treatment in the SW837 xenograft model, the average tumor volume in the vehicle control group was 895 mm3, and the average tumor volume in the JAB-8263 0.3 mg/kg treatment group was 283 mm3, which was statistically significant compared to the vehicle control group. The relative tumor inhibition rate TGI (%) was 79.0% (Figure 4A). Only one animal in the JAB-8263 treatment group lost 16.6% of body weight at the end of the trial, and animals in the other groups tolerated it well without discontinuation or death (Figure 4B). After 18 d of treatment in the MC38 syngeneic model, the average tumor volume in the vehicle control group was 2580 mm3, and the average tumor volume in the JAB-8263 0.2 mg/kg treatment group was 686 mm3. Compared with the vehicle control group, there was a significant statistical difference (P = 0.003) (Figure 4C), and the relative tumor inhibition rate TGI (%) was 76.5%. The body weight change of each treatment group was controlled within 15%, no drug discontinuation or death occurred, and the animals tolerated the treatment well (Figure 4D).
The tumor tissue of the single-dose MC38 model was further subjected to the western blot assay to evaluate the underlying mechanism, and it was found that the expression of c-MYC was significantly decreased by a single dose of JAB-8263 administration (P = 0.013 and P = 0.011) (Figure 4G and H). All data showed that JAB-8263 downregulated the expression of c-MYC in tumor tissue from the single-dose MC38 model.
In recent years, BET protein inhibitors have received extensive attention in the application of tumors, and many BET inhibitors have been used in clinical trials, but most are focused on hematological tumors and some solid tumors such as lung cancer and prostate cancer[15-19]. Some previous studies have used JQ1 and other compounds in the study of CRC cells[20,21], but due to the short half-life of most compounds, they are quite challenging for further clinical application. JAB-8263 used in this study has stronger protein affinity, high affinity for BET protein in vitro, and the IC50 is less than 1 nmol/L.
In the in vitro cell proliferation and colony formation experiments, we found that JAB-8263 had an inhibitory effect on CRC cells. To further study its mechanism of action, we performed cell cycle and apoptosis experiments. However, only the mouse CRC cell line MC38 obtained ideal positive results, and the human CRC cell lines did not have a significant difference from the control group. We conducted the western blot experiments to further explore this result.
MYC plays an important role in the cell cycle, cell death, cellular senescence, and tumorigenesis of CRC cells[9]. Myc-related lnc-RNAs such as MYCLo-2 are overexpressed in CRC cells and have oncogenic functions[14]. Through the in vitro and in vivo studies of this study, we found that JAB-8263 can effectively suppress the expression of c-MYC and finally suppress CRC cells.
The tumor suppressor genes p21 and p16 are regulated by the MYC gene[14]. Therefore, we further investigated whether the expression of these two genes was affected by BET inhibitors. p21 (CDKN1A) is involved in the regulation of cell cycle and cellular senescence[22]. In 1993, it was reported that p21 can suppress multiple tumors such as CRC by activating wild-type p53[23]. Moreover, studies have shown that p21 can also suppress tumor growth by inhibiting cyclin kinase complexes and proliferating cell nuclear antigen[24]. JAB-8263 achieves an anti-tumor effect by inducing CRC cell cycle arrest by upregulating p21. However, at the same time, some studies have suggested that p21 has an anti-apoptotic effect, and the apoptosis of hCT116 colon cancer cells can be inhibited by inhibiting p21[25,26]. This might be one reason why JAB-8263 did not have ideal results in the apoptosis experiments, which also requires further study.
p16 (CDKN2A) can inhibit the function of CDK4, and the combination of CDk4 and cyclin D1 plays a key regulatory role in the G1→S phase of the cell cycle, thereby suppressing the malignant proliferation of cells[27]. The inactivation or decreased expression of the p16 gene can lead to the malignant proliferation of cells and lead to tumorigenesis[28,29]. JAB-8263 inhibits CDK4 function by upregulation of p16, thereby suppressing CRC cells.
Finally, we verified that JAB-8263 has a significant tumor inhibitory effect compared with the control group in the SW837 and MC38 animal models. The animals in the treatment group tolerated the drug well. Since c-MYC expression is disturbed in long-term dosing models, we established a single-dose model. The detection of tumor tissue in single-dose MC38 model also showed that c-MYC was downregulated. This is consistent with the conclusions we obtained in the in vitro studies.
According to the conclusion of this study, the BET inhibitor JAB-8263 can inhibit CRC cells mainly by inhibiting the expression of c-MYC. But at the same time, we found that the inhibition of BET inhibitors on CRC has many mechanisms other than the MYC gene. Further directions include whether the BET inhibitors still have an anti-tumor effect in cells that do not overexpress MYC, which will provide a theoretical basis for the indications of CRC treatment in future clinical applications.
The JAB-8263 compound inhibited the BET target. The expression of BET downstream signaling protein MYC was repressed by JAB-8263, resulting in downregulation of c-MYC and upregulation of p21 and p16. It induced cell cycle arrest, promoted apoptosis of CRC cells and displayed anti-tumor activity. In vivo, JAB-8263 was effective in CRC models.
The overexpression of the MYC gene plays an important role in the occurrence, development and evolution of colorectal cancer (CRC). Bromodomain and extraterminal domain (BET) inhibitors decrease the function of BET, which is the recognition of acetylated lysine residues, thereby downregulating the expression of MYC.
BET proteins are an important target in solid tumors, hematologic tumors and myelofibrosis. The development of BET small-molecule inhibitors has promising therapeutic value.
The study aimed to investigate the inhibitory effect and mechanism of a BET inhibitor on CRC cells.
The effect of the BET inhibitor JAB-8263 on the proliferation of various CRC cell lines was studied by the CellTiter-Glo method and colony formation assay. The effect of JAB-8263 on the cell cycle and apoptosis of CRC cells was studied by propidium iodide staining and Annexin V/propidium iodide flow assay, respectively. The effect of JAB-8263 on the expression of c-MYC, p21 and p16 in CRC cells was detected by western blot. To predict the anti-tumor effect of JAB-8263 on CRC cells in vivo and to evaluate the safety of the compound, a CRC cell animal tumor model was developed.
JAB-8263 dose-dependently suppressed CRC cell proliferation and colony formation in vitro. The MYC signaling pathway was dose-dependently inhibited by JAB-8263 in human CRC cell lines. JAB-8263 dose-dependently induced cell cycle arrest and apoptosis in the MC38 cell line. The SW837 xenograft model was treated with JAB-8263 0.3 mg/kg for 29 d. The average tumor volume was significantly decreased compared to the vehicle control group, P < 0.001. The MC38 syngeneic murine model was treated with JAB-8263 0.2 mg/kg for 29 d. The average tumor volume was significantly decreased compared to the vehicle control group, P = 0.003.
BET can be a potential effective drug target for suppressing CRC growth, and the BET inhibitor JAB-8263 can effectively suppress c-MYC expression and exert anti-tumor activity in CRC models.
BET proteins are an important target in solid tumors, hematologic tumors and myelofibrosis. The development of BET small-molecule inhibitors has promising therapeutic value. Our study results are encouraging and will motivate further clinical evolution.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kalofonos HP, Greece; Rajagopalan A, Australia S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Zhang XD
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4235] [Cited by in RCA: 11429] [Article Influence: 3809.7] [Reference Citation Analysis (4)] |
| 2. | Venook A. Critical evaluation of current treatments in metastatic colorectal cancer. Oncologist. 2005;10:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 3. | Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5906] [Cited by in RCA: 5600] [Article Influence: 466.7] [Reference Citation Analysis (0)] |
| 4. | Xu Y, Vakoc CR. Targeting Cancer Cells with BET Bromodomain Inhibitors. Cold Spring Harb Perspect Med. 2017;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 5. | Lombardi PM, Cole KE, Dowling DP, Christianson DW. Structure, mechanism, and inhibition of histone deacetylases and related metalloenzymes. Curr Opin Struct Biol. 2011;21:735-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 6. | Pervaiz M, Mishra P, Günther S. Bromodomain Drug Discovery - the Past, the Present, and the Future. Chem Rec. 2018;18:1808-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 7. | Chen H, Liu H, Qing G. Targeting oncogenic Myc as a strategy for cancer treatment. Signal Transduct Target Ther. 2018;3:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 580] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 8. | Lovén J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, Bradner JE, Lee TI, Young RA. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1873] [Cited by in RCA: 2263] [Article Influence: 188.6] [Reference Citation Analysis (0)] |
| 9. | Dang CV. MYC on the path to cancer. Cell. 2012;149:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1984] [Cited by in RCA: 2588] [Article Influence: 199.1] [Reference Citation Analysis (0)] |
| 10. | Dow LE, O'Rourke KP, Simon J, Tschaharganeh DF, van Es JH, Clevers H, Lowe SW. Apc Restoration Promotes Cellular Differentiation and Reestablishes Crypt Homeostasis in Colorectal Cancer. Cell. 2015;161:1539-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 412] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 11. | Duan Y, Guan Y, Qin W, Zhai X, Yu B, Liu H. Targeting Brd4 for cancer therapy: inhibitors and degraders. Medchemcomm. 2018;9:1779-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 12. | Donati B, Lorenzini E, Ciarrocchi A. BRD4 and Cancer: going beyond transcriptional regulation. Mol Cancer. 2018;17:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 506] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 13. | Otto C, Schmidt S, Kastner C, Denk S, Kettler J, Müller N, Germer CT, Wolf E, Gallant P, Wiegering A. Targeting bromodomain-containing protein 4 (BRD4) inhibits MYC expression in colorectal cancer cells. Neoplasia. 2019;21:1110-1120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Kim T, Jeon YJ, Cui R, Lee JH, Peng Y, Kim SH, Tili E, Alder H, Croce CM. Role of MYC-regulated long noncoding RNAs in cell cycle regulation and tumorigenesis. J Natl Cancer Inst. 2015;107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 15. | Zhou X, Sun T, Meng Y, Luo J, Chen J, Xia B, Zhang Z, Cheng Z, Wang X. BET inhibitors combined with chemotherapy synergistically inhibit the growth of NSCLC cells. Oncol Rep. 2021;45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 16. | Alcitepe İ, Salcin H, Karatekin İ, Kaymaz BT. HDAC inhibitor Vorinostat and BET inhibitor Plx51107 epigenetic agents' combined treatments exert a therapeutic approach upon acute myeloid leukemia cell model. Med Oncol. 2022;39:257. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Roussel MF, Jonchere B, Williams J, Zindy F, Liu J, Robinson S, Farmer DM, Min J, Yang L, Stripay JL, Wang Y, Freeman BB, Yu J, Shelat AA, Rankovic Z. Combination of ribociclib with BET-bromodomain and PI3K/mTOR inhibitors for medulloblastoma treatment in vitro and in vivo. Mol Cancer Ther. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Dawson MA, Borthakur G, Huntly B, Karadimitris A, Alegre A, Chaidos A, Vogl DT, Pollyea DA, Davies FE, Morgan GJ, Glass J, Kamdar M, Mateos Manteca MV, Tovar N, Yeh P, García Delgado R, Basheer F, Marando L, Gallipoli P, Wyce A, Krishnatry AS, Barbash O, Bakirtzi E, Ferron-Brady G, Karpinich NO, McCabe MT, Foley SW, Horner T, Dhar A, Kremer BE, Dickinson M. A phase I/II open-label study of molibresib for the treatment of relapsed/refractory hematologic malignancies. Clin Cancer Res. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Alqahtani A, Choucair K, Ashraf M, Hammouda DM, Alloghbi A, Khan T, Senzer N, Nemunaitis J. Bromodomain and extra-terminal motif inhibitors: a review of preclinical and clinical advances in cancer therapy. Future Sci OA. 2019;5:FSO372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 196] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 20. | Shi C, Yang EJ, Liu Y, Mou PK, Ren G, Shim JS. Bromodomain and extra-terminal motif (BET) inhibition is synthetic lethal with loss of SMAD4 in colorectal cancer cells via restoring the loss of MYC repression. Oncogene. 2021;40:937-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Cheng X, Huang Z, Long D, Jin W. BET inhibitor bromosporine enhances 5-FU effect in colorectal cancer cells. Biochem Biophys Res Commun. 2020;521:840-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Cao N, Yu Y, Zhu H, Chen M, Chen P, Zhuo M, Mao Y, Li L, Zhao Q, Wu M, Ye M. SETDB1 promotes the progression of colorectal cancer via epigenetically silencing p21 expression. Cell Death Dis. 2020;11:351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 23. | el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6088] [Cited by in RCA: 6307] [Article Influence: 197.1] [Reference Citation Analysis (0)] |
| 24. | Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2178] [Cited by in RCA: 2125] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 25. | Tian H, Wittmack EK, Jorgensen TJ. p21WAF1/CIP1 antisense therapy radiosensitizes human colon cancer by converting growth arrest to apoptosis. Cancer Res. 2000;60:679-684. [PubMed] |
| 26. | Jänicke RU, Sohn D, Essmann F, Schulze-Osthoff K. The multiple battles fought by anti-apoptotic p21. Cell Cycle. 2007;6:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Sherr CJ. Cancer cell cycles. Science. 1996;274:1672-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3965] [Cited by in RCA: 4006] [Article Influence: 138.1] [Reference Citation Analysis (0)] |
| 28. | Ye X, Mo M, Xu S, Yang Q, Wu M, Zhang J, Chen B, Li J, Zhong Y, Huang Q, Cai C. The hypermethylation of p16 gene exon 1 and exon 2: potential biomarkers for colorectal cancer and are associated with cancer pathological staging. BMC Cancer. 2018;18:1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Bihl MP, Foerster A, Lugli A, Zlobec I. Characterization of CDKN2A(p16) methylation and impact in colorectal cancer: systematic analysis using pyrosequencing. J Transl Med. 2012;10:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |