Published online Feb 15, 2023. doi: 10.4251/wjgo.v15.i2.240
Peer-review started: September 22, 2022
First decision: November 30, 2022
Revised: December 18, 2022
Accepted: January 5, 2023
Article in press: January 5, 2023
Published online: February 15, 2023
Processing time: 145 Days and 8.5 Hours
The advent of immunotherapy and the development of immune checkpoint inhibitors (ICIs) are changing the way we think about cancer treatment. ICIs have shown clinical benefits in a variety of tumor types, and ICI-based immunotherapy has shown effective clinical outcomes in immunologically “hot” tumors. However, for immunologically “cold” tumors such as colorectal cancer (CRC), only a limited number of patients are currently benefiting from ICIs due to limitations such as individual differences and low response rates. In this review, we discuss the classification and differences between hot and cold CRC and the current status of research on cold CRC, and summarize the treatment strategies and challenges of immunotherapy for cold CRC. We also explain the mechanism, biology, and role of immunotherapy for cold CRC, which will help clarify the future development of immunotherapy for cold CRC and discovery of more emerging strategies for the treatment of cold CRC.
Core Tip: Immune checkpoint inhibitors (ICIs) are usually produced by antibodies, and their effectiveness relies on the antitumor effects of immune cells (especially T cells). Colorectal cancer (CRC) is one of the most common forms of cancer worldwide. Only a limited number of patients are currently benefiting from ICIs due to limitations such as individual differences and low response rates. In this review, we discuss the classification and differences between hot and cold CRC and the current status of research on cold CRC, and summarize the treatment strategies and challenges of immunotherapy for cold CRC.
- Citation: Liu JL, Yang M, Bai JG, Liu Z, Wang XS. “Cold” colorectal cancer faces a bottleneck in immunotherapy. World J Gastrointest Oncol 2023; 15(2): 240-250
- URL: https://www.wjgnet.com/1948-5204/full/v15/i2/240.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i2.240
Colorectal cancer (CRC) is one of the most common forms of cancer worldwide[1]. Globally, CRC is the second most common cancer in women and the third most common in men[2]. More than half of the development of CRC can be attributed to modifiable risk factors such as smoking, unhealthy diet, heavy alcohol consumption, lack of physical activity, and overweight; therefore, the disease is preventable[3]. Despite some progress in the diagnosis and treatment of CRC, it remains a significant cause of cancer-related deaths[2]. The global burden of CRC is expected to increase by 60% by 2030[4]. Therefore, there is an urgent need to develop new preventive and treatment strategies for this disease[1].
In contrast to traditional cancer therapies that affect the proliferation, survival, and metabolic activities of tumor cells[5], immunotherapy mainly works by modulating the tumor microenvironment (TME), restoring anticancer immunity, and stimulating or suppressing the immune system to play an antitumor role[6]. Immune checkpoint inhibitors (ICIs) are usually produced by antibodies, and their effectiveness relies on the antitumor effects of immune cells (especially T cells)[7].
However, most solid tumors have little T-cell infiltration and are defined as non-T-cell inflammatory or “cold” tumors[8]. In CRC, it has been shown that only patients with mismatch repair deficiency (dMMR) or microsatellite instability (MSI) high (dMMR/MSI-H) tumor subpopulations respond to treatment with ICIs[9-11]. Clinical trials related to ICIs have been conducted for the treatment of CRC (Table 1). In these patients, there is an urgent need to improve the efficacy of tumor immunotherapy by improving intratumoral T-cell infiltration and converting cold tumors into “hot” or T-cell inflammatory tumors.
| Name | Targets | Phase | Settings | Trial identifier |
| Nivolumab and ipilimumab | PD-1 and CTLA4 | II | dMMR and/or MSI mCRC | NCT04730544 |
| Camrelizumab and apatinib | PD-L1 and VEGF | II | Locally advanced dMMR/MSI-H CRC | NCT04715633 |
| Toripalimab with or without celecoxib | PD-1 and COX | I and II | Resectable non-metastatic dMMR/MSI-H CRC | NCT03926338 |
| Cetuximab-avelumab | PD-1 and EGFR | II | mCRC | NCT04561336 |
| Nivolumab + relatlimab | PD-1 and LAG3 | II | MSS colorectal adenocarcinomas | NCT03642067 |
| Obinutuzumab + atezolizumab + cibisatamab + tocilizumab | CD20, PD-L1, CEA + CD3 and IL-6R | Ib | MSS mCRC | NCT03866239 |
In this review, we discuss the classification and differences between hot and cold CRC and the current state of research on cold CRC, the therapeutic strategies and challenges of immunotherapy, and the pathological mechanisms of cold CRC.
Tumor-immune system interactions provide a basis for patient stratification and treatment strategies for various cancers, which can more accurately predict survival in CRC[12]. An immune scoring system for tumor classification was developed based on the quantification of two lymphocyte populations (cluster of differentiation 3 [CD3] and CD8)[13,14] at the tumor center and aggressive margins[15-19]. The scoring system has four levels (immune score 0 [i0], i1, i2, i3, and i4). The concepts of hot (highly invasive immune score i4) and cold (noninvasive immune score i0) tumors were introduced[15]. In colon cancer, the consensus immune scoring system has a greater relative prognostic value than pathological T staging, pathological N staging, lymphovascular infiltration, tumor differentiation, and MSI status[20].
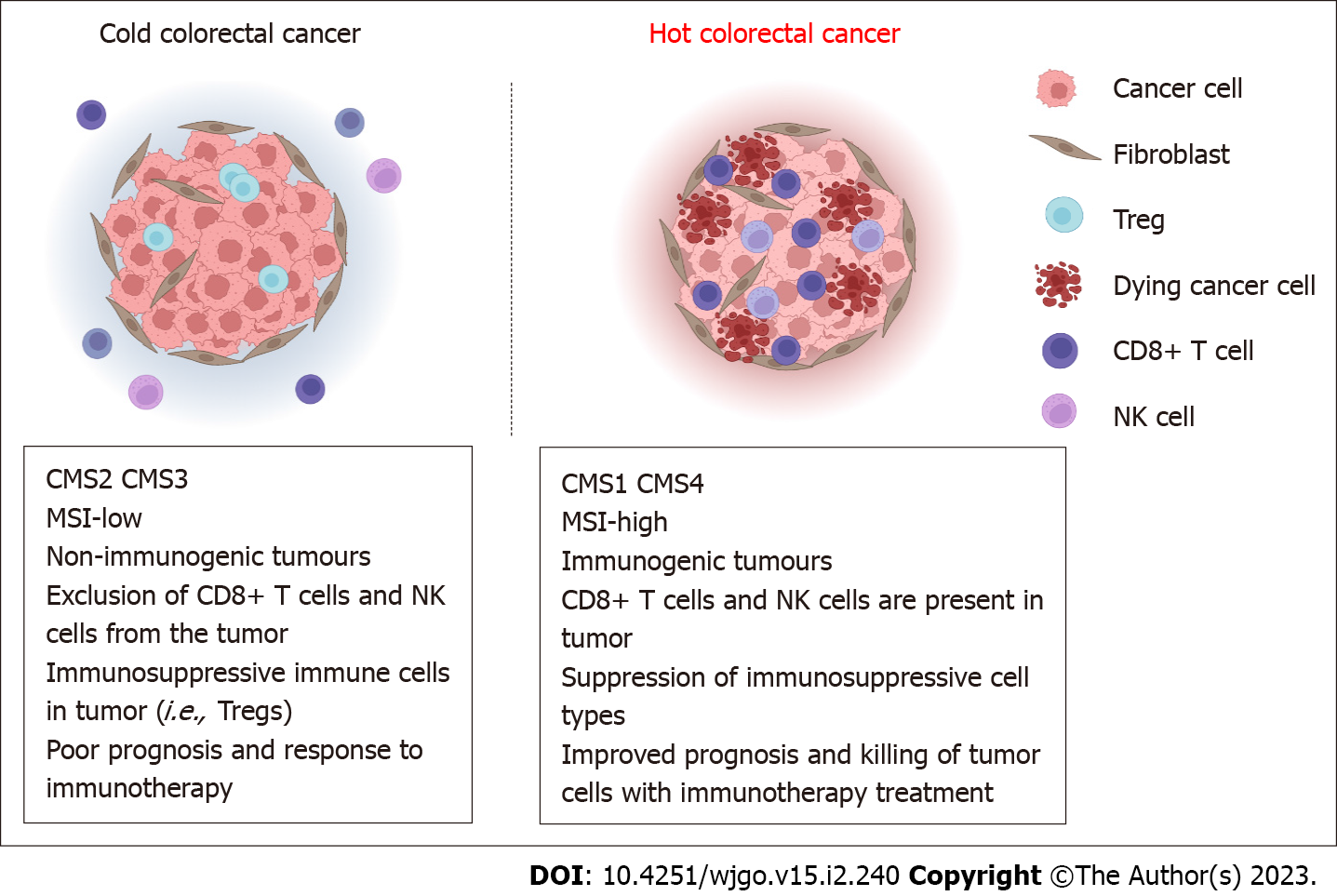
Currently, hot and cold tumors are typically referred to as T-cell infiltrated, inflammatory but noninfiltrating, and noninflammatory tumors[15]. This immune classification has been validated in melanoma and breast cancer[21,22]. In addition to the presence of tumor-infiltrating lymphocytes (TILs), other features are the consensus molecular subtype (CMS) classification developed through a comprehensive reassessment and comparison of CRC molecular gene expression profiles: CMS1 and CMS4 are hot tumors (Figure 1); they are considered immunoreactive and highly infiltrated by immune cells. These tumors are immunoreactive and highly infiltrated by immune cells, as opposed to CMS2 and CMS3, which are cold tumors[23]. A small group of CRCs with dMMR/MSI-H benefits from immunotherapy. dMMR/MSI-H in solid tumors, including CRC, suggests a good tumor response to immunotherapy[7]; however, the majority of patients with skilled MMR (pMMR) or microsatellite stable (MSS) CRC do not respond well to this treatment[24]. However, immune scoring is a better predictor of prognosis in CRC patients than MSI testing alone[25], and MSI has been used to predict the response to anti-programmed cell death protein 1 therapy in cancer[26]. The expression of anti-programmed cell death ligand 1 (PD-L1) on tumor-associated immune cells, possible genomic instability, and the pre-existing antitumor immune response are characteristics of hot tumors[27].
Currently, the most comprehensive approach to define hot and cold tumors remains the immune scoring system, but there are still some tumors with characteristics intermediate between hot and cold tumors, and the four main categories of tumor classification, namely hot, altered exclusion, altered immunosuppression, and cold, provide classification of the four major tumor categories[28]. This system provides a more comprehensive approach to classification and helps to suggest new ideas for immunotherapy strategies.
With the development of immunotherapy and its achievements, it is important to determine how to use immune scoring to classify tumors and help and guide the choice of treatment. A blanket use of parameters to score may produce bias, which reinforces the need to incorporate the details of each individual case and to adequately integrate clinical practice to develop a rational, standardized, and coordinated scoring approach to guide treatment decisions. For immunotherapy to overcome the bottleneck of cold CRC, a general consensus is still required.
Immunotherapy has made significant progress in cancer treatment[29]. In particular, hot tumors with an immune microenvironment of highly TILs are highly responsive to most immunotherapies, a property that plays a key role in obtaining good antitumor responses to immunotherapy[30-33]. The discovery and development of ICs and IC-related drugs are of importance in cancer immunotherapy. This immunotherapy approach has excellent long-term regression efficacy in hot tumors; however, hot tumors have a low response rate to immunocooled tumors lacking predominant infiltration of tumor immune cells[34-40]. Therefore, the absence or low number of lymphocytes in the TME also serves as a biomarker for cold tumor unresponsiveness to ICIs[41].
Therefore, it is important to consider a proper treatment plan for cold CRC. Classifying tumors according to their immunophenotypes is too homogeneous; an emphasis on tumor heterogeneity can enable us to have a better understanding of individualized cancer treatment[6]. Most solid tumors are non-T-cell inflammatory or cold tumors[8,42], and CRC is no exception. Therefore, there is an urgent need to improve the TME to convert cold tumors into hot or T-cell inflammatory tumors to improve the efficacy of tumor immunotherapy.
It is important to elucidate the mechanisms involved in cold CRC that do not respond to immunotherapy to provide additional insights into the therapeutic strategies that can be developed. In this section, we outline the mechanisms and approaches related to the possible modulation of non-immune-responsive cold CRC to improve the efficacy of treatment approaches against non-immune-responsive tumors.
Establishing an inflammatory response in the TME is a key goal of immunomodulatory approaches for all cold tumors, including CRC. Infection by pathogens can activate the immune system, thus stimulating a series of immune attacks[43]. The involvement of such pattern recognition receptors can activate immune cells and lead to an immune system-mediated antitumor response[44]. Interventional radiology has enabled the precision treatment of local tumors, and a variety of therapeutic substances, including pattern recognition receptor agonists, such as tumor lysing peptides or lysing viruses, cytokines, encoded nucleic acid sequences, bispecific T-cell participants, nanoparticles or particles, and immune cells, can be delivered locally[45]. The immunogenic cell death pathway induced by precise radiotherapy and cryoablation or radiofrequency ablation that produces massive tumor antigen release can convert tumors into in situ vaccines, which provides us with new insights and options[45,46].
Although a growing number of studies has demonstrated the effectiveness of radiotherapy[47-50], the benefits obtained in these trials cannot be attributed exclusively to radiotherapy. Explicit demonstration of the contribution of radiation to the immunotherapeutic response is challenging but crucial. The optimal integration of radiobiology and tumor immunology may lead to potentially significant clinical benefits.
Precision therapy is limited by several operational, clinical, and biological factors, in addition to the numerous complications that may arise from injecting drugs or biologics directly into the tumor. Therefore, a systemic approach to tumor-specific therapy remains attractive. Chemotherapeutic regimens as systemic treatments can induce immunogenic cell death by releasing damage-associated molecular patterns[51,52] and activating necrotic or apoptotic pathways[53]. Some studies have indicated that drugs such as 5-fluorouracil can induce apoptosis of myeloid-derived suppressor cells (MDSCs) and increase CD8 cell function to enhance inflammatory immunity[54].
Immune microenvironment analysis of patients with liver metastases from CRC has revealed that cytotoxicity and memory T-cell density are significantly higher in patients who received preoperative chemotherapy than in patients with untreated metastasis[55-57], suggesting that the use of chemotherapy can induce tumor inflammation to some extent, providing insight into the transformation of cold to hot CRC. Epidermal growth factor receptor (EGFR), vascular EGFR kinase, and mitogen-activated protein kinase kinase (MEK) inhibitors are widely used in the clinical solid tumor routine, and the clinical effects and related mechanisms of EGFR (cetuximab and panitumumab) and angiogenesis (bevacizumab, afliximab, or ramucizumab) as first- and second-line targeted agents for metastatic CRC are being actively investigated[58-61]. Although there is a lack of knowledge regarding the detailed molecular mechanisms of action between targeted drugs and immunity, in vivo studies have shown that the activated mitogen-activated protein kinase (MAPK) signaling pathway can inhibit major histocompatibility class I components and antigen-presentation mechanisms. Use of MAPK inhibitors enhances T-cell-mediated killing of tumor cells[62].
MEK inhibitors may also be involved in the immune effects of tumors[63,64]. This effect promotes antigen presentation on the surface of tumor cells to activate the recognition of CD8 T lymphocytes, which then kills tumor cells. Additionally, it has been found that inhibition of the phosphoinositide 3-kinase/AKT/mammalian target of rapamycin pathway can prevent the activation of immunosuppressive pathways[65], thereby affecting the immune microenvironment.
There is a large body of literature that strongly supports the idea that drugs targeting oncogenes and non-oncogenes can beneficially affect the TME in a wide range of tumors and thus enhance tumor immune responses. However, many therapeutic methods have not yet been used in the routine management of cancer patients, as their preclinical and clinical development has only recently begun.
For example, toll-like receptors (TLRs) are highly expressed in immune cells in the TME, and in clinical development, the involvement of TLR agonists can activate antitumor immune responses[66]. However, the complexity of the TLR system challenges the selection of agonists. Different agonists may cause different types of inflammatory responses. TLR agonists are currently being used as monotherapy and in combination with ICIs[66,67].
Stimulator of interferon (IFN) genes is an endoplasmic reticulum transmembrane protein, whose mechanism of action is to sense cytosolic DNA, induce type I IFN gene transcription, and promote antigen cross-presentation[68]. IFN gene-stimulating factor agonists increase CD8 T cells in the TME[69] and, when combined with anti-PD-L1, reduce local immunosuppression to mediate a systemic antitumor response[70]. This combination may be used as a method of local immunosuppression, and the study of its agonist is in the clinical development stage.
Cytokines and chemokines are molecular messengers of the immune system, and many cytokines (e.g., interleukin 2 [IL-2], IL-7, IL-15, IL-21, granulocyte macrophage colony-stimulating factor, and IFN-α) in the immune TME[71-76] regulate the function of T cells, and studies have reported their use as single agents or in combination with other drugs[77]. Accumulation of MDSCs as a group of immature myeloid cells with immunosuppressive functions in tumors attenuates the regulation of immune responses. Regulatory T cells (Tregs) can promote tumor growth by suppressing cytotoxic immune responses. Therefore, therapeutic strategies that specifically eliminate MDSCs or Tregs have been proposed[78]. It has been suggested that monoclonal antibodies against immune checkpoints or agonists of the tumor necrosis factor receptor superfamily may have a modulatory effect on Tregs[79].
Immunosuppressive soluble ligands play an important role in the immune microenvironment. For example, prostaglandin E2 promotes tumor growth and exerts immunosuppressive effects[80]. The TME is also rich in adenosine. The TME is also enriched in adenosine, which is released owing to the death of tumor cells through CD73 and CD39 ectonucleases[81]. The increase in adenosine, a substance enriched in the TME, impairs the implantation and activation of immune cells in the TME. Antagonizing adenosine or its pathway can block CD39 to enhance T-cell proliferation and induce proinflammatory cytokines, which can control tumor growth[82].
Since 1993, engineered T cells targeting artificial chimeric antigen receptors (CARs) on the surface molecules of tumor cells were first proposed[83], and in 2010, their nature as anticancer drugs was revealed[84]. CAR T-cell-based therapies have shown specific, rapid, high success rates, and long-lasting effects[85]. The principle of action is mainly through T cells with novel properties to induce a tumor rejection response. Patient-derived TILs that can be expanded in vitro with recombinant IL-2 have been demonstrated and have made important advances in the treatment of metastatic melanoma and other types of tumors[86-88].
To some extent, the efficacy of T-cell therapy depends on the potency of the T cells themselves; however, T cells can be influenced by the dose (absolute number of T cells injected) as well as the characteristics of the tumor-specific T cells administered. Controlling the dose of tumor-specific T cells is crucial for activation of the endothelial complement by IFN-γ to overcome the vascular endothelial barrier[89]. Therefore, the effect of pericyte therapy alone may be limited, and patients may benefit more from its combination with tumor-targeted interventions aimed at reprogramming the TME. A combination of many factors (intervention of tumor-intrinsic pathways, local inflammatory response, or intercellular messaging) to ensure proper engraftment and function of relayed metastatic T cells may be beneficial for cold tumors. Repeated stimulation of tumor-specific T cell expansion by cell lines established in lesions from patients with resected melanoma has been shown to be effective[90]. Melanoma is the best solid cancer type to respond to adoptive cell therapy[91].
In summary, peritoneal cellular immunotherapy is a promising treatment modality for CRC. The approach is based on the collection T cells from patients, which are expanded in vitro and then transfused back into the patient. These T cells are designed to express CARs, which can be designed to recognize not only tumor antigens but also to produce anticancer cytokines or ICIs. However, despite the results of CAR-T therapy in the treatment of hematological tumors such as B-cell malignancies, the effectiveness and applicability of the approach to convert cold tumors into hot tumors, and whether it can be successful in CRC and other solid tumors remains to be elucidated[7,92].
As a branch of hyperthermia, modulated electro-hyperthermia (mEHT) has been gradually applied in the treatment of various cancers in recent years[93,94]. The principle of mEHT is delivering locoregional clinical hyperthermia generated by 13.56 MHz amplitude-modulated radiofrequency[95]. A series of studies have shown the effect of mEHT in the treatment of CRC[96-99], and related mechanism studies have also shown its relevance in immunity[100,101]. This treatment may be a good candidate for transforming cold CRC.
In addition, some experiments are being conducted to make tumors hotter, such as combining oncolytic bacteria or viruses or peptides, tumor, virus or dendritic cell antigens with various adjuvants, with the goal of improving CRC immunogenicity. Tumor-associated macrophages, as a key driver of inflammation that facilitates tumor progression, are attractive targets to complement current immunotherapy[102].
As a key target of tumor-associated macrophages, colony-stimulating factor 1 receptor (CSF1R) can bind to CSF1 or IL-34 to activate macrophage proliferation and function[103,104]. CSF1R-specific inhibitors and other macrophage modulators are currently being studied in clinical trials in solid tumors[105].
Despite the widespread use of immunotherapy, poor clinical response to cold tumors is a current challenge[22]. Prior to immunotherapy, the resected tumor (primary or metastatic) should be classified as hot, altered, or cold. Although the tumor sample is valuable in providing information about the disease, it is limited in that it is not representative of the entire tumor landscape[28]. Recently, it was noted that, in addition to the CMS of CRC, the underlying epithelial cell diversity of CRC was summarized in a large transcriptome into two intrinsic subtypes, iCMS2 and iCMS3. This finding refines the CMS[106]. Because of genomic and immune heterogeneity, each sample can be considered an individual tumor[56]. Moreover, immune parameters change over the course of the disease[107].
The concept of personalized cancer immunotherapy is being increasingly promoted. A major challenge for immunotherapy of cold tumors is the need to identify key immune- or tumor-related features at the time of diagnosis to establish a reliable classification strategy to support immunotherapy for maximum efficiency[108].
With an increasing number of studies conducted on cold tumors, personalized cancer immunotherapy for individual patients is gaining ground. A major challenge hindering the development of therapeutic strategies may be the lack of mastery of the relationship between cancer and immunity. Even though a great deal of technological innovation and related research has been conducted to achieve some progress, the variability of cancer among individual patients cannot be generalized[108]. Identifying key phenotypic features is of interest when developing treatment strategies, and considerable progress has been made with ICIs approved by the United States Food and Drug Administration for the treatment of patients with dMMR/MSI-HmCRC. Notably, subtype dMMR/MSI-H CRC represents only a small fraction of all CRCs, and most pMMR/MSS mCRC patients do not benefit from ICI treatment alone. Therefore, further tumor states need to be identified, which has led to the continued reporting of new biomarkers, such as comprehensive immune scoring and complete CMS classification, and these results have led to a better understanding of the immune mechanisms of CRC and their relationship to tumor treatment strategies.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Herold Z, Hungary; Mohammadi S, Iran S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3024] [Article Influence: 504.0] [Reference Citation Analysis (3)] |
| 2. | Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3585] [Cited by in RCA: 4899] [Article Influence: 699.9] [Reference Citation Analysis (1)] |
| 3. | Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, McCullough ML, Patel AV, Ma J, Soerjomataram I, Flanders WD, Brawley OW, Gapstur SM, Jemal A. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68:31-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 1002] [Article Influence: 143.1] [Reference Citation Analysis (2)] |
| 4. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3306] [Article Influence: 413.3] [Reference Citation Analysis (3)] |
| 5. | Groeneveldt C, van Hall T, van der Burg SH, Ten Dijke P, van Montfoort N. Immunotherapeutic Potential of TGF-β Inhibition and Oncolytic Viruses. Trends Immunol. 2020;41:406-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 6. | Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2373] [Cited by in RCA: 3600] [Article Influence: 450.0] [Reference Citation Analysis (0)] |
| 7. | Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH, Diaz LA Jr. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol. 2019;16:361-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1114] [Cited by in RCA: 1225] [Article Influence: 204.2] [Reference Citation Analysis (0)] |
| 8. | Gajewski TF. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Semin Oncol. 2015;42:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 390] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 9. | Asaoka Y, Ijichi H, Koike K. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;373:1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 320] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 10. | Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, Desai J, Hill A, Axelson M, Moss RA, Goldberg MV, Cao ZA, Ledeine JM, Maglinte GA, Kopetz S, André T. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1775] [Cited by in RCA: 2090] [Article Influence: 261.3] [Reference Citation Analysis (0)] |
| 11. | Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine JM, Cao ZA, Kamble S, Kopetz S, André T. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol. 2018;36:773-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1013] [Cited by in RCA: 1474] [Article Influence: 210.6] [Reference Citation Analysis (0)] |
| 12. | Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pagès F, Galon J. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 794] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 13. | Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944-5951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 740] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 14. | Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, Nagtegaal ID, Palmqvist R, Masucci GV, Botti G, Tatangelo F, Delrio P, Maio M, Laghi L, Grizzi F, Asslaber M, D'Arrigo C, Vidal-Vanaclocha F, Zavadova E, Chouchane L, Ohashi PS, Hafezi-Bakhtiari S, Wouters BG, Roehrl M, Nguyen L, Kawakami Y, Hazama S, Okuno K, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Wang Y, Kopetz S, Sinicrope FA, Scripcariu V, Ascierto PA, Marincola FM, Fox BA, Pagès F. Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J Pathol. 2014;232:199-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1121] [Cited by in RCA: 1075] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 15. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4318] [Cited by in RCA: 4910] [Article Influence: 258.4] [Reference Citation Analysis (0)] |
| 16. | Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 664] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 17. | Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 420] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 18. | Galluzzi L, Senovilla L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial watch: Dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2012;1:1111-1134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 19. | Galon J, Fridman WH, Pagès F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 2007;67:1883-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 319] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 20. | Pagès F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, Vink-Börger E, Hartmann A, Geppert C, Kolwelter J, Merkel S, Grützmann R, Van den Eynde M, Jouret-Mourin A, Kartheuser A, Léonard D, Remue C, Wang JY, Bavi P, Roehrl MHA, Ohashi PS, Nguyen LT, Han S, MacGregor HL, Hafezi-Bakhtiari S, Wouters BG, Masucci GV, Andersson EK, Zavadova E, Vocka M, Spacek J, Petruzelka L, Konopasek B, Dundr P, Skalova H, Nemejcova K, Botti G, Tatangelo F, Delrio P, Ciliberto G, Maio M, Laghi L, Grizzi F, Fredriksen T, Buttard B, Angelova M, Vasaturo A, Maby P, Church SE, Angell HK, Lafontaine L, Bruni D, El Sissy C, Haicheur N, Kirilovsky A, Berger A, Lagorce C, Meyers JP, Paustian C, Feng Z, Ballesteros-Merino C, Dijkstra J, van de Water C, van Lent-van Vliet S, Knijn N, Mușină AM, Scripcariu DV, Popivanova B, Xu M, Fujita T, Hazama S, Suzuki N, Nagano H, Okuno K, Torigoe T, Sato N, Furuhata T, Takemasa I, Itoh K, Patel PS, Vora HH, Shah B, Patel JB, Rajvik KN, Pandya SJ, Shukla SN, Wang Y, Zhang G, Kawakami Y, Marincola FM, Ascierto PA, Sargent DJ, Fox BA, Galon J. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1567] [Cited by in RCA: 1487] [Article Influence: 212.4] [Reference Citation Analysis (0)] |
| 21. | Gajewski TF, Corrales L, Williams J, Horton B, Sivan A, Spranger S. Cancer Immunotherapy Targets Based on Understanding the T Cell-Inflamed Versus Non-T Cell-Inflamed Tumor Microenvironment. Adv Exp Med Biol. 2017;1036:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 208] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 22. | Song X, Zhou Z, Li H, Xue Y, Lu X, Bahar I, Kepp O, Hung MC, Kroemer G, Wan Y. Pharmacologic Suppression of B7-H4 Glycosylation Restores Antitumor Immunity in Immune-Cold Breast Cancers. Cancer Discov. 2020;10:1872-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 23. | Becht E, de Reyniès A, Giraldo NA, Pilati C, Buttard B, Lacroix L, Selves J, Sautès-Fridman C, Laurent-Puig P, Fridman WH. Immune and Stromal Classification of Colorectal Cancer Is Associated with Molecular Subtypes and Relevant for Precision Immunotherapy. Clin Cancer Res. 2016;22:4057-4066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 409] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 24. | Huyghe N, Baldin P, Van den Eynde M. Immunotherapy with immune checkpoint inhibitors in colorectal cancer: what is the future beyond deficient mismatch-repair tumours? Gastroenterol Rep (Oxf). 2020;8:11-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 25. | Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T, Sasso M, Bilocq AM, Kirilovsky A, Obenauf AC, Hamieh M, Berger A, Bruneval P, Tuech JJ, Sabourin JC, Le Pessot F, Mauillon J, Rafii A, Laurent-Puig P, Speicher MR, Trajanoski Z, Michel P, Sesboüe R, Frebourg T, Pagès F, Valge-Archer V, Latouche JB, Galon J. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity. 2016;44:698-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 757] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 26. | Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073-2087.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1677] [Cited by in RCA: 1545] [Article Influence: 103.0] [Reference Citation Analysis (0)] |
| 27. | Hegde PS, Karanikas V, Evers S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin Cancer Res. 2016;22:1865-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 685] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 28. | Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 2220] [Article Influence: 370.0] [Reference Citation Analysis (0)] |
| 29. | Chen L, Chen H, Ye J, Ge Y, Wang H, Dai E, Ren J, Liu W, Ma C, Ju S, Guo ZS, Liu Z, Bartlett DL. Intratumoral expression of interleukin 23 variants using oncolytic vaccinia virus elicit potent antitumor effects on multiple tumor models via tumor microenvironment modulation. Theranostics. 2021;11:6668-6681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 30. | Haanen JBAG. Converting Cold into Hot Tumors by Combining Immunotherapies. Cell. 2017;170:1055-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 198] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 31. | Ruan H, Hu Q, Wen D, Chen Q, Chen G, Lu Y, Wang J, Cheng H, Lu W, Gu Z. A Dual-Bioresponsive Drug-Delivery Depot for Combination of Epigenetic Modulation and Immune Checkpoint Blockade. Adv Mater. 2019;31:e1806957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 32. | Wang C, Wang J, Zhang X, Yu S, Wen D, Hu Q, Ye Y, Bomba H, Hu X, Liu Z, Dotti G, Gu Z. In situ formed reactive oxygen species-responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 428] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 33. | Noman MZ, Parpal S, Van Moer K, Xiao M, Yu Y, Viklund J, De Milito A, Hasmim M, Andersson M, Amaravadi RK, Martinsson J, Berchem G, Janji B. Inhibition of Vps34 reprograms cold into hot inflamed tumors and improves anti-PD-1/PD-L1 immunotherapy. Sci Adv. 2020;6:eaax7881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 190] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 34. | Feng B, Zhou F, Hou B, Wang D, Wang T, Fu Y, Ma Y, Yu H, Li Y. Binary Cooperative Prodrug Nanoparticles Improve Immunotherapy by Synergistically Modulating Immune Tumor Microenvironment. Adv Mater. 2018;30:e1803001. [PubMed] [DOI] [Full Text] |
| 35. | Wang D, Wang T, Yu H, Feng B, Zhou L, Zhou F, Hou B, Zhang H, Luo M, Li Y. Engineering nanoparticles to locally activate T cells in the tumor microenvironment. Sci Immunol. 2019;4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 178] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 36. | He C, Duan X, Guo N, Chan C, Poon C, Weichselbaum RR, Lin W. Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy. Nat Commun. 2016;7:12499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 481] [Cited by in RCA: 563] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 37. | Dong X, Pan P, Zheng DW, Bao P, Zeng X, Zhang XZ. Bioinorganic hybrid bacteriophage for modulation of intestinal microbiota to remodel tumor-immune microenvironment against colorectal cancer. Sci Adv. 2020;6:eaba1590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 38. | Wang Y, Gao Z, Du X, Chen S, Zhang W, Wang J, Li H, He X, Cao J. Co-inhibition of the TGF-β pathway and the PD-L1 checkpoint by pH-responsive clustered nanoparticles for pancreatic cancer microenvironment regulation and anti-tumor immunotherapy. Biomater Sci. 2020;8:5121-5132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 39. | Chao Y, Xu L, Liang C, Feng L, Xu J, Dong Z, Tian L, Yi X, Yang K, Liu Z. Combined local immunostimulatory radioisotope therapy and systemic immune checkpoint blockade imparts potent antitumour responses. Nat Biomed Eng. 2018;2:611-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 356] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 40. | Xu J, Lv J, Zhuang Q, Yang Z, Cao Z, Xu L, Pei P, Wang C, Wu H, Dong Z, Chao Y, Yang K, Peng R, Cheng Y, Liu Z. A general strategy towards personalized nanovaccines based on fluoropolymers for post-surgical cancer immunotherapy. Nat Nanotechnol. 2020;15:1043-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 368] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 41. | Ochoa de Olza M, Navarro Rodrigo B, Zimmermann S, Coukos G. Turning up the heat on non-immunoreactive tumours: opportunities for clinical development. Lancet Oncol. 2020;21:e419-e430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 42. | van der Woude LL, Gorris MAJ, Halilovic A, Figdor CG, de Vries IJM. Migrating into the Tumor: a Roadmap for T Cells. Trends Cancer. 2017;3:797-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 220] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 43. | Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3387] [Cited by in RCA: 3102] [Article Influence: 100.1] [Reference Citation Analysis (0)] |
| 44. | Shekarian T, Valsesia-Wittmann S, Brody J, Michallet MC, Depil S, Caux C, Marabelle A. Pattern recognition receptors: immune targets to enhance cancer immunotherapy. Ann Oncol. 2017;28:1756-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 45. | Erinjeri JP, Fine GC, Adema GJ, Ahmed M, Chapiro J, den Brok M, Duran R, Hunt SJ, Johnson DT, Ricke J, Sze DY, Toskich BB, Wood BJ, Woodrum D, Goldberg SN. Immunotherapy and the Interventional Oncologist: Challenges and Opportunities-A Society of Interventional Oncology White Paper. Radiology. 2019;292:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 46. | Whiteside TL, Demaria S, Rodriguez-Ruiz ME, Zarour HM, Melero I. Emerging Opportunities and Challenges in Cancer Immunotherapy. Clin Cancer Res. 2016;22:1845-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 248] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 47. | Floudas CS, Brar G, Mabry-Hrones D, Duffy AG, Wood B, Levy E, Krishnasamy V, Fioravanti S, Bonilla CM, Walker M, Morelli MP, Kleiner DE, Steinberg SM, Figg WD, Greten TF, Xie C. A Pilot Study of the PD-1 Targeting Agent AMP-224 Used With Low-Dose Cyclophosphamide and Stereotactic Body Radiation Therapy in Patients With Metastatic Colorectal Cancer. Clin Colorectal Cancer. 2019;18:e349-e360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 48. | Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, Duggan R, Wang Y, Barber GN, Fitzgerald KA, Alegre ML, Gajewski TF. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 1370] [Article Influence: 124.5] [Reference Citation Analysis (0)] |
| 49. | Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, Huang X, Gajewski TF, Chen ZJ, Fu YX, Weichselbaum RR. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1055] [Cited by in RCA: 1562] [Article Influence: 142.0] [Reference Citation Analysis (0)] |
| 50. | Bonvalot S, Le Pechoux C, De Baere T, Kantor G, Buy X, Stoeckle E, Terrier P, Sargos P, Coindre JM, Lassau N, Ait Sarkouh R, Dimitriu M, Borghi E, Levy L, Deutsch E, Soria JC. First-in-Human Study Testing a New Radioenhancer Using Nanoparticles (NBTXR3) Activated by Radiation Therapy in Patients with Locally Advanced Soft Tissue Sarcomas. Clin Cancer Res. 2017;23:908-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 51. | Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1808] [Cited by in RCA: 1896] [Article Influence: 158.0] [Reference Citation Analysis (0)] |
| 52. | Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1434] [Cited by in RCA: 2242] [Article Influence: 172.5] [Reference Citation Analysis (0)] |
| 53. | Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1356] [Cited by in RCA: 2056] [Article Influence: 228.4] [Reference Citation Analysis (0)] |
| 54. | Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052-3061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 963] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 55. | Van den Eynde M, Mlecnik B, Bindea G, Fredriksen T, Church SE, Lafontaine L, Haicheur N, Marliot F, Angelova M, Vasaturo A, Bruni D, Jouret-Mourin A, Baldin P, Huyghe N, Haustermans K, Debucquoy A, Van Cutsem E, Gigot JF, Hubert C, Kartheuser A, Remue C, Léonard D, Valge-Archer V, Pagès F, Machiels JP, Galon J. The Link between the Multiverse of Immune Microenvironments in Metastases and the Survival of Colorectal Cancer Patients. Cancer Cell. 2018;34:1012-1026.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 207] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 56. | Mlecnik B, Van den Eynde M, Bindea G, Church SE, Vasaturo A, Fredriksen T, Lafontaine L, Haicheur N, Marliot F, Debetancourt D, Pairet G, Jouret-Mourin A, Gigot JF, Hubert C, Danse E, Dragean C, Carrasco J, Humblet Y, Valge-Archer V, Berger A, Pagès F, Machiels JP, Galon J. Comprehensive Intrametastatic Immune Quantification and Major Impact of Immunoscore on Survival. J Natl Cancer Inst. 2018;110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 57. | Tanis E, Julié C, Emile JF, Mauer M, Nordlinger B, Aust D, Roth A, Lutz MP, Gruenberger T, Wrba F, Sorbye H, Bechstein W, Schlag P, Fisseler A, Ruers T. Prognostic impact of immune response in resectable colorectal liver metastases treated by surgery alone or surgery with perioperative FOLFOX in the randomised EORTC study 40983. Eur J Cancer. 2015;51:2708-2717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 58. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7730] [Article Influence: 368.1] [Reference Citation Analysis (1)] |
| 59. | Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy DC, Van Cutsem E, Grothey A, Prausová J, Garcia-Alfonso P, Yamazaki K, Clingan PR, Lonardi S, Kim TW, Simms L, Chang SC, Nasroulah F; RAISE Study Investigators. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 664] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 60. | Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Williams R, Rong A, Wiezorek J, Sidhu R, Patterson SD. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1610] [Cited by in RCA: 1731] [Article Influence: 144.3] [Reference Citation Analysis (0)] |
| 61. | Van Cutsem E, Lenz HJ, Köhne CH, Heinemann V, Tejpar S, Melezínek I, Beier F, Stroh C, Rougier P, van Krieken JH, Ciardiello F. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 632] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 62. | Brea EJ, Oh CY, Manchado E, Budhu S, Gejman RS, Mo G, Mondello P, Han JE, Jarvis CA, Ulmert D, Xiang Q, Chang AY, Garippa RJ, Merghoub T, Wolchok JD, Rosen N, Lowe SW, Scheinberg DA. Kinase Regulation of Human MHC Class I Molecule Expression on Cancer Cells. Cancer Immunol Res. 2016;4:936-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 63. | Ebert PJR, Cheung J, Yang Y, McNamara E, Hong R, Moskalenko M, Gould SE, Maecker H, Irving BA, Kim JM, Belvin M, Mellman I. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity. 2016;44:609-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 565] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 64. | Liu L, Mayes PA, Eastman S, Shi H, Yadavilli S, Zhang T, Yang J, Seestaller-Wehr L, Zhang SY, Hopson C, Tsvetkov L, Jing J, Zhang S, Smothers J, Hoos A. The BRAF and MEK Inhibitors Dabrafenib and Trametinib: Effects on Immune Function and in Combination with Immunomodulatory Antibodies Targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res. 2015;21:1639-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 370] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 65. | Xue G, Zippelius A, Wicki A, Mandalà M, Tang F, Massi D, Hemmings BA. Integrated Akt/PKB signaling in immunomodulation and its potential role in cancer immunotherapy. J Natl Cancer Inst. 2015;107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 66. | Bourquin C, Pommier A, Hotz C. Harnessing the immune system to fight cancer with Toll-like receptor and RIG-I-like receptor agonists. Pharmacol Res. 2020;154:104192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 67. | Ribas A, Medina T, Kummar S, Amin A, Kalbasi A, Drabick JJ, Barve M, Daniels GA, Wong DJ, Schmidt EV, Candia AF, Coffman RL, Leung ACF, Janssen RS. SD-101 in Combination with Pembrolizumab in Advanced Melanoma: Results of a Phase Ib, Multicenter Study. Cancer Discov. 2018;8:1250-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 240] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 68. | Rivera Vargas T, Benoit-Lizon I, Apetoh L. Rationale for stimulator of interferon genes-targeted cancer immunotherapy. Eur J Cancer. 2017;75:86-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 69. | Jing W, McAllister D, Vonderhaar EP, Palen K, Riese MJ, Gershan J, Johnson BD, Dwinell MB. STING agonist inflames the pancreatic cancer immune microenvironment and reduces tumor burden in mouse models. J Immunother Cancer. 2019;7:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 70. | Moore E, Clavijo PE, Davis R, Cash H, Van Waes C, Kim Y, Allen C. Established T Cell-Inflamed Tumors Rejected after Adaptive Resistance Was Reversed by Combination STING Activation and PD-1 Pathway Blockade. Cancer Immunol Res. 2016;4:1061-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 71. | Jiang T, Zhou C, Ren S. Role of IL-2 in cancer immunotherapy. Oncoimmunology. 2016;5:e1163462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 363] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 72. | Ochoa MC, Mazzolini G, Hervas-Stubbs S, de Sanmamed MF, Berraondo P, Melero I. Interleukin-15 in gene therapy of cancer. Curr Gene Ther. 2013;13:15-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 499] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 74. | Al-Chami E, Tormo A, Khodayarian F, Rafei M. Therapeutic utility of the newly discovered properties of interleukin-21. Cytokine. 2016;82:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 75. | Hong IS. Stimulatory versus suppressive effects of GM-CSF on tumor progression in multiple cancer types. Exp Mol Med. 2016;48:e242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 76. | Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015;14:847-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1250] [Cited by in RCA: 1752] [Article Influence: 175.2] [Reference Citation Analysis (1)] |
| 77. | García-Martínez E, Smith M, Buqué A, Aranda F, de la Peña FA, Ivars A, Cánovas MS, Conesa MAV, Fucikova J, Spisek R, Zitvogel L, Kroemer G, Galluzzi L. Trial Watch: Immunostimulation with recombinant cytokines for cancer therapy. Oncoimmunology. 2018;7:e1433982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 78. | Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, Umansky V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120:16-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 602] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 79. | Sharabi A, Tsokos MG, Ding Y, Malek TR, Klatzmann D, Tsokos GC. Regulatory T cells in the treatment of disease. Nat Rev Drug Discov. 2018;17:823-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 234] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 80. | Adams JL, Smothers J, Srinivasan R, Hoos A. Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Discov. 2015;14:603-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 324] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 81. | Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17:709-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 533] [Article Influence: 66.6] [Reference Citation Analysis (0)] |
| 82. | Li XY, Moesta AK, Xiao C, Nakamura K, Casey M, Zhang H, Madore J, Lepletier A, Aguilera AR, Sundarrajan A, Jacoberger-Foissac C, Wong C, Dela Cruz T, Welch M, Lerner AG, Spatola BN, Soros VB, Corbin J, Anderson AC, Effern M, Hölzel M, Robson SC, Johnston RL, Waddell N, Smith C, Bald T, Geetha N, Beers C, Teng MWL, Smyth MJ. Targeting CD39 in Cancer Reveals an Extracellular ATP- and Inflammasome-Driven Tumor Immunity. Cancer Discov. 2019;9:1754-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 206] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 83. | Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015;125:3335-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 994] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 84. | Rosenbaum L. Tragedy, Perseverance, and Chance - The Story of CAR-T Therapy. N Engl J Med. 2017;377:1313-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 85. | Gomes-Silva D, Ramos CA. Cancer Immunotherapy Using CAR-T Cells: From the Research Bench to the Assembly Line. Biotechnol J. 2018;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 86. | Dafni U, Michielin O, Lluesma SM, Tsourti Z, Polydoropoulou V, Karlis D, Besser MJ, Haanen J, Svane IM, Ohashi PS, Kammula US, Orcurto A, Zimmermann S, Trueb L, Klebanoff CA, Lotze MT, Kandalaft LE, Coukos G. Efficacy of adoptive therapy with tumor-infiltrating lymphocytes and recombinant interleukin-2 in advanced cutaneous melanoma: a systematic review and meta-analysis. Ann Oncol. 2019;30:1902-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 87. | Tran E, Robbins PF, Lu YC, Prickett TD, Gartner JJ, Jia L, Pasetto A, Zheng Z, Ray S, Groh EM, Kriley IR, Rosenberg SA. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med. 2016;375:2255-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 1061] [Article Influence: 117.9] [Reference Citation Analysis (0)] |
| 88. | Stevanović S, Draper LM, Langhan MM, Campbell TE, Kwong ML, Wunderlich JR, Dudley ME, Yang JC, Sherry RM, Kammula US, Restifo NP, Rosenberg SA, Hinrichs CS. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33:1543-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 491] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 89. | Facciabene A, De Sanctis F, Pierini S, Reis ES, Balint K, Facciponte J, Rueter J, Kagabu M, Magotti P, Lanitis E, DeAngelis RA, Buckanovich RJ, Song WC, Lambris JD, Coukos G. Local endothelial complement activation reverses endothelial quiescence, enabling t-cell homing, and tumor control during t-cell immunotherapy. Oncoimmunology. 2017;6:e1326442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 90. | Verdegaal EM, de Miranda NF, Visser M, Harryvan T, van Buuren MM, Andersen RS, Hadrup SR, van der Minne CE, Schotte R, Spits H, Haanen JB, Kapiteijn EH, Schumacher TN, van der Burg SH. Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature. 2016;536:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 363] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 91. | Kato D, Yaguchi T, Iwata T, Morii K, Nakagawa T, Nishimura R, Kawakami Y. Prospects for personalized combination immunotherapy for solid tumors based on adoptive cell therapies and immune checkpoint blockade therapies. Nihon Rinsho Meneki Gakkai Kaishi. 2017;40:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, Alaparthy S, Berman D, Jure-Kunkel M, Siemers NO, Jackson JR, Shahabi V. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother. 2012;61:1019-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 627] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 93. | Andocs G, Renner H, Balogh L, Fonyad L, Jakab C, Szasz A. Strong synergy of heat and modulated electromagnetic field in tumor cell killing. Strahlenther Onkol. 2009;185:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 94. | Andocs G, Szasz O, Szasz A. Oncothermia treatment of cancer: from the laboratory to clinic. Electromagn Biol Med. 2009;28:148-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 95. | Szasz AM, Minnaar CA, Szentmártoni G, Szigeti GP, Dank M. Review of the Clinical Evidences of Modulated Electro-Hyperthermia (mEHT) Method: An Update for the Practicing Oncologist. Front Oncol. 2019;9:1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 96. | Kuo IM, Lee JJ, Wang YS, Chiang HC, Huang CC, Hsieh PJ, Han W, Ke CH, Liao ATC, Lin CS. Potential enhancement of host immunity and anti-tumor efficacy of nanoscale curcumin and resveratrol in colorectal cancers by modulated electro- hyperthermia. BMC Cancer. 2020;20:603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 97. | You SH, Kim S. Feasibility of modulated electro-hyperthermia in preoperative treatment for locally advanced rectal cancer: Early phase 2 clinical results. Neoplasma. 2020;67:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 98. | Kim S, Lee JH, Cha J, You SH. Beneficial effects of modulated electro-hyperthermia during neoadjuvant treatment for locally advanced rectal cancer. Int J Hyperthermia. 2021;38:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 99. | Vancsik T, Kovago C, Kiss E, Papp E, Forika G, Benyo Z, Meggyeshazi N, Krenacs T. Modulated electro-hyperthermia induced loco-regional and systemic tumor destruction in colorectal cancer allografts. J Cancer. 2018;9:41-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 100. | Tsang YW, Huang CC, Yang KL, Chi MS, Chiang HC, Wang YS, Andocs G, Szasz A, Li WT, Chi KH. Improving immunological tumor microenvironment using electro-hyperthermia followed by dendritic cell immunotherapy. BMC Cancer. 2015;15:708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 101. | Krenacs T, Meggyeshazi N, Forika G, Kiss E, Hamar P, Szekely T, Vancsik T. Modulated Electro-Hyperthermia-Induced Tumor Damage Mechanisms Revealed in Cancer Models. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 102. | Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1778] [Cited by in RCA: 2851] [Article Influence: 356.4] [Reference Citation Analysis (0)] |
| 103. | Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, Hollenbaugh D, Linnemann T, Qin M, Wong J, Chu K, Doberstein SK, Williams LT. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 616] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 104. | Otero K, Turnbull IR, Poliani PL, Vermi W, Cerutti E, Aoshi T, Tassi I, Takai T, Stanley SL, Miller M, Shaw AS, Colonna M. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and beta-catenin. Nat Immunol. 2009;10:734-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 249] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 105. | Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017;114:206-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 602] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 106. | Joanito I, Wirapati P, Zhao N, Nawaz Z, Yeo G, Lee F, Eng CLP, Macalinao DC, Kahraman M, Srinivasan H, Lakshmanan V, Verbandt S, Tsantoulis P, Gunn N, Venkatesh PN, Poh ZW, Nahar R, Oh HLJ, Loo JM, Chia S, Cheow LF, Cheruba E, Wong MT, Kua L, Chua C, Nguyen A, Golovan J, Gan A, Lim WJ, Guo YA, Yap CK, Tay B, Hong Y, Chong DQ, Chok AY, Park WY, Han S, Chang MH, Seow-En I, Fu C, Mathew R, Toh EL, Hong LZ, Skanderup AJ, DasGupta R, Ong CJ, Lim KH, Tan EKW, Koo SL, Leow WQ, Tejpar S, Prabhakar S, Tan IB. Single-cell and bulk transcriptome sequencing identifies two epithelial tumor cell states and refines the consensus molecular classification of colorectal cancer. Nat Genet. 2022;54:963-975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 203] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 107. | Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T, Lafontaine L, Berger A, Bruneval P, Fridman WH, Becker C, Pagès F, Speicher MR, Trajanoski Z, Galon J. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1792] [Cited by in RCA: 2955] [Article Influence: 246.3] [Reference Citation Analysis (0)] |
| 108. | Blank CU, Haanen JB, Ribas A, Schumacher TN. CANCER IMMUNOLOGY. The "cancer immunogram". Science. 2016;352:658-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 578] [Article Influence: 64.2] [Reference Citation Analysis (0)] |