Published online Feb 15, 2023. doi: 10.4251/wjgo.v15.i2.215
Peer-review started: September 28, 2022
First decision: December 14, 2022
Revised: December 18, 2022
Accepted: January 9, 2023
Article in press: January 9, 2023
Published online: February 15, 2023
Processing time: 139 Days and 16.9 Hours
With the establishment of the immune surveillance mechanism since the 1950s, attempts have been made to activate the immune system for cancer treatment through the discovery of various cytokines or the development of antibodies up to now. The fruits of these efforts have contributed to the recognition of the 3rd generation of anticancer immunotherapy as the mainstream of cancer treatment. However, the limitations of cancer immunotherapy are also being recognized through the conceptual establishment of cold tumors recently, and colorectal cancer (CRC) has become a major issue from this therapeutic point of view. Here, it is emphasized that non-clinical strategies to overcome the immunosuppressive environment and clinical trials based on these basic investigations are being made on the journey to achieve better treatment outcomes for the treatment of cold CRC.
Core Tip: There have been continuing attempts to treat colorectal cancer (CRC) with immunotherapies, and various methods of converting cold into hot tumors have gone through trial and error up to now. Based on this background, this editorial introduces the concept of cold CRC and various strategies across non-clinical and clinical for enhancing immunotherapeutic efficacy and further encourages the journey to an advanced level of immunotherapies targeting cold CRC.
- Citation: Jeong KY. Challenges to addressing the unmet medical needs for immunotherapy targeting cold colorectal cancer. World J Gastrointest Oncol 2023; 15(2): 215-224
- URL: https://www.wjgnet.com/1948-5204/full/v15/i2/215.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i2.215
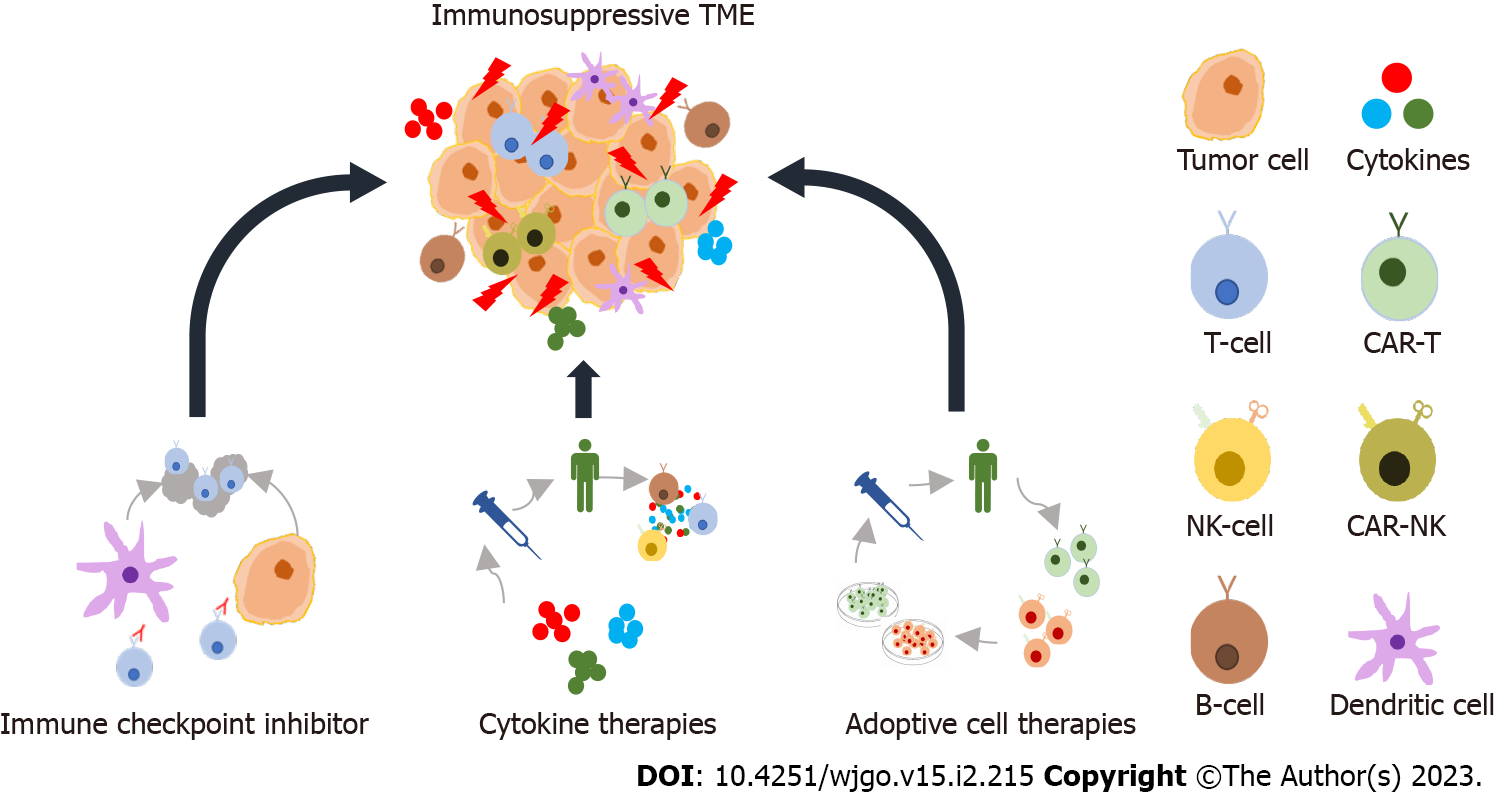
Cancer immunotherapy provides a basis for activating the components of the immune system of cancer patients. Recently spotlighted methods of cancer immunotherapy utilize antibodies and peptides that bind to and inhibit the proteins related to immune evasion (e.g., immune checkpoint inhibitors and cytokine therapies), DNA and RNA vaccines, and immune cell therapies such as chimeric antigen receptor natural killer (CAR-NK) and CAR-T cells (Figure 1)[1]. The idea of using immunotherapy for cancer treatment emerged with the first proposed theory of cancer immunosurveillance in the 1950s, which suggested that lymphocytes act as a monitoring system to identify and eliminate cells harboring somatic mutations[2]. However, due to a lack of non-clinical data to support these theories, it took a long time to establish a bridgehead for clinical applications[2]. Eventually, the identification of T-cell growth factor interleukin 2 (IL-2) in the 1970s allowed improved T-cell production through IL-2 exposure and led to positive results in patients with metastatic cancer[2,3]. Milstein and Köhler pioneered the production of monoclonal antibodies by fusion of lymphocytes around the same time, and antibody-based therapies led to the development of rituximab, which targets immature B cells-based NK cell activation[2,4]. After this discovery, development was stagnant because it was difficult to devise clinically effective cancer immunotherapy strategies until 2010. Ipilimumab [targeting cytotoxic T lymphocyte antigen 4 (CTLA-4)], nivolumab, and atezolizumab [targeting programmed cell death-1 (PD-1) or its ligand (PD-L1), respectively] have been approved in the 2010s as a result of ongoing research for the discovery of immune checkpoint molecules[1,5]. More recently, six CAR-T cell therapies have been approved for the treatment of lymphoma, some forms of leukemia, and multiple myeloma[6]. Such rapid development over the past decade established immunotherapy as the mainstream of cancer treatment as third-generation cancer treatment next to second-generation targeted therapies.
Meanwhile, it would be an erroneous attempt to follow in the footsteps of approaches focusing on only its potential while looking at the rapid development of immunotherapy. Given the extensive non-clinical research and clinical investigation efforts dedicated to advancing different immunotherapy approaches, such efforts should be accompanied by those focusing on the various prominent issues that emerge. A discussion may be required on the optimal model that can accurately reflect the human immune system by replacing the immunodeficient mouse used in the non-clinical efficacy evaluation studies or on the concerns about synthetic rather than endogenous immunity. However, here I would like to highlight organ-specific tumor immunity, especially in colorectal cancer (CRC), as a key concern among the multiple issues involved in the resistance to immunotherapies. The widely accepted concept of ‘cold tumor’ focuses on tumors that are unlikely to elicit a strong immune response due to the heterogeneity of the tumor microenvironment (TME)[7].
The advancements in the knowledge of the interactions among different types of cells in the TME have enabled the establishment of the basis of therapeutic strategies focused on the immune system. Patient stratification with an immune score can be performed according to the types or densities of immune cells within the tumor, and it could be possible to make a more accurate prediction of prognosis compared with TNM staging[8]. This concept is based on the quantification of CD3+ and CD8+ T-cells abundance in and around the TME. The immune score ranges from I0 (immune score 0) to I4, where I0 denotes the absence of both CD3+ and CD8+ T-cell types, and I4 indicates a high density of immune cells positive for the expression of the T-cell types[9,10]. Such a system was proposed for immune-based tumor classification and allowed the discrimination between high-invasive immune score I4 (hot tumor) and non-invasive immune score I0 (cold tumor)[8-10]. The feasibility of the immune score has been proved in CRC and is recognized as having a greater relative prognostic value compared with pathological staging, lymphatic invasion, tumor differentiation, and microsatellite status[8,11]. Currently, the definition of cold tumor is routinely used to refer to tumors with little or no T-cell infiltration, inflamed but non-T-cell infiltrated, or non-inflammatory tumors[12]. In addition to the analysis of tumor-infiltrating lymphocytes, it is characterized by the regulation of antigen-presenting machinery markers, such as low expression levels of PD-L1 or reduced presentation of neoantigens[13]. At this point, it is possible to characterize the immune signature in CRC represented by the propensity for cold tumors. A neoantigen is an abnormal peptide mainly generated by a genetic mutation or gene fusion and is encoded by mutant genes in tumor cells[14]. Tumor-associated antigens, a type of neoantigen, are proteins that are overexpressed in tumor cells but also expressed in normal cells, limiting specific immune responses[14]. For example, carcinoembryonic antigen (CEA) is an important tumor-associated antigen in CRC and is frequently found on the surfaces of most metastatic CRC cells, but it induces immune tolerance since CEA is also expressed at the embryonic stage[14,15]. Further, the presence of various mechanisms that interfere with antigen presentation is a hallmark of metastatic CRC, typically with low microsatellite instability (MSI)/DNA mismatch repair deficient molecular characteristics[16,17]. Such mechanisms interfere with antigen presentation and proteasome processing of antigens, impede transporter functions involved in antigen processing and inhibit the expression of major histocompatibility complex (MHC) structural components through genetic mutations[16]. In particular, loss of β-2-microglobulin heterozygosity may affect antigen presentation of the MHC-I, which is known to induce resistance to T-cell invasion[18]. Furthermore, the immune signature of CRC indicates that it could interfere with the recruitment or activation of T-cells through various molecular biological changes as a result of the inter-communication between the constituents of the TME[19]. It has been reported that activation of Wnt/β-catenin signaling is associated with T-cell exclusion and inversely proportional to T-cell infiltration in CRC tissues[20]. Signal transducer and activator of transcription 3 (STAT3) can reduce the expression of interferon-gamma (IFN-γ) in CD8-positive T-cells[21]. This, in turn, inhibits chemokine (C-X-C motif) ligand secretion by tumor-associated myeloid cells and interferes with T-cell recruitment[21,22]. The mitogen-activated protein kinase (MAPK) signaling cascade upregulates the expression levels of the immunosuppressive cytokines such as vascular endothelial growth factor (VEGF) and IL-8, suppressing T-cell function and its infiltration into the TME[23-25]. These immune signatures in cold CRC by their genetic and molecular complexity may be a major cause of resistance to cancer therapies (especially in immunotherapy). Therefore, a variety of attempts are currently being made to overcome these obstacles through non-clinical and clinical studies.
Non-clinical strategies are being designed to overcome the obstacles in cancer immunotherapy, and these strategies can be classified into key categories as follows: Increasing the number of antigen-specific T-cells, T-cell priming, and promoting T-cell trafficking and infiltration[26-29]. First, the method of increasing the number of antigen-specific T-cells and T-cell priming includes the application of adoptive cell therapy[30,31], adjuvant immunotherapy[32,33], epigenetic modification inhibitors[34,35], cancer vaccines[36,37], oncolytic viruses[38,39], and their combination with conventional therapies[40-43] (Table 1). Adoptive cell therapy enhances the immune response through CAR-T or CAR-NK cells. Utilization of CAR-T or CAR-NK cells involves the genetic modification of T lymphocytes or NK cells to express specific antigens to target the tumor cells. The activities of CAR-T or CAR-NK cells are not limited by the presence or absence of MHC and can further enhance the immune response against tumor cells through the addition of costimulatory molecules such as CD28, OX40, or 4-1BB[30,44]. The strategy utilizes the direct recognition of tumor antigens by CARs and has the potential to treat cold CRC[30]. Adjuvant immunotherapy is based on innate immune responses through the activity of the pattern recognition receptor family[45]. The pattern recognition receptor family includes Toll-like receptors, nucleotide oligomerization domain-like receptors, retinoic acid-inducible gene-I-like receptors, and type C lectin receptors. Agonistic activation of these receptors can generate a variety of proinflammatory cytokines including type I IFNs to promote T-cell priming[45]. Targeting DNA methyltransferase and histone deacetylase activities to inhibit epigenetic modifications has been shown to enhance the expression levels of tumor antigens and other immune-related genes, as a specific therapy for tumors with low antigen expression[46]. Cancer vaccines enhance the treatment efficacy and overcome the limitations of immunotherapy by increasing the number of specific effector T-cells. They include molecular-based vaccines using peptides, protein, DNA and mRNA prepared with isolated cancer cells and adenovirus for the expression of cancer-specific antigens[47]. Oncolytic viruses capable of selectively targeting and destroying cancer cells contribute to the maturation of antigen-presenting cells that carry out the activation of antigen-specific CD4+ and CD8+ T-cell responses and activate both innate and adaptive immune responses to convert a cold tumor into a hot tumor[38]. Chemotherapy and radiotherapy can exert anti-tumor effects by directly killing tumor cells while contributing to immune system stimulation[41,42]. Radiotherapy promotes the activation of dendritic cells and the expression of cell adhesion molecules that promote the attraction of immune cells[42,45]. Chemotherapy regulates immunogenicity and increases T-cell infiltration. 5-fluorouracil and oxaliplatin-based chemotherapies and MAPK and epidermal growth factor receptor inhibitors are some examples[41,48,49]. Methods for promoting T-cell trafficking and infiltration include the application of transforming growth factor (TGF)-β suppression[50,51], oncogenic pathway inhibitors[52,53], angiogenesis inhibitors, CXC chemokine receptors (CXCR) inhibitors[54,55], and immune cytokines[56] (Table 1). TGF-β is associated with a lack of immune responses in the noninflamed T-cell phenotype with a deterioration in the ability to produce type I IFNs in tumor-associated dendritic cells, leading to STAT3 up-regulation and an imbalance in T-cell infiltration. Non-clinical studies have shown that a combination of TGF-β blocking antibodies induces T-cell penetration into tumors, allowing for anti-tumor immunity and tumor regression[50,51]. Targeting oncogenic pathways helps to reverse intrinsic T cell exclusion in tumors. Inhibition of the WNT/β-catenin pathway by p21-activated kinase 4 inhibitors or the endogenous Dickkopf family binding to lipoprotein receptor-associated proteins may increase tumor invasion of cytotoxic T lymphocytes[52,53]. Inhibition of well-established biochemical pathways, CDK4/6, phosphoinositide 3-kinase (PI3K)/AKT, or MAPK, involved in tumor growth and differentiation can lead to a significant upregulation of tumor-infiltrating T lymphocytes with the regulation of granzyme B and CC chemokine ligand 4/5[49,57,58]. Angiogenesis inhibitors play a role in the normalization of the unregulated balance between angiogenesis-promoting and antiangiogenic signals by upregulation of the leukocyte adhesion molecules in tumor endothelial cells resulting in amelioration of tumor vascular abnormalities, improved tissue perfusion, and increased infiltration of immune effector cells[54,59]. CXCR4 is a receptor for CXC ligand (CXCL) 12 and is overexpressed in tumors, and it can reduce the infiltration of cytotoxic T lymphocytes into the TME and mediate the invasion of immunosuppressive cells, such as regulatory T-cells (Treg), into the tumor. Regulation of CXCL12 by inhibiting CXCR4 can promote the infiltration of T lymphocytes into the tumor and reverse immune resistance[60,61]. Finally, since immune cytokines mediate the influx and expansion of leukocytes at tumor sites, cognate receptor expression on tumor and immune cells may induce an antitumor effect. IL-2, IFN, tumor necrosis factor, IL-12, granulocyte-macrophage colony-stimulating factor, promotion of MHC-I expression, and T-cell activation and infiltration enhance antitumor immunity[56,62].
| Strategy | Therapeutic approach | Ref. |
| Increase in antigen-specific T-cells and T-cell priming | Adoptive cell therapy (CAR-T and -NK) | [30-44,49] |
| Adjuvant immunotherapy | ||
| Epigenetic modification inhibitors | ||
| Cancer vaccine | ||
| Oncolytic viruses | ||
| Combined with conventional therapies | ||
| Promoting T-cell trafficking and infiltration | TGF-β suppression | [50-58,60,61] |
| Oncogenic pathway inhibitors | ||
| Angiogenesis inhibitors | ||
| CXCR4 inhibitors | ||
| Immunocytokines |
Over the past two decades, a multidisciplinary approach to graft novel therapeutic modalities onto the backbone of fluoropyrimidine-based chemotherapy in local and advanced CRC has achieved significant improvements in the therapeutic efficiency of immunotherapy[63]. However, the expected overall survival of patients with microsatellite stable (MSS) CRC is only about 30 mo, indicating an unmet medical need[64]. Therefore, several clinical trials evaluating immune checkpoint inhibitors have focused on designs that can overcome resistance and achieve clinically meaningful responses, but mono and combination therapies utilizing immune checkpoint inhibitors as the mainstay have not yet shown significant clinical success[65-68]. For example, studies using the single agent of pembrolizumab and nivolumab did not find any objective response rates (ORR)[65-67]. In a study of a combination of ipilimumab, nivolumab, and anti-CTLA-4 antibody in CRC patients with high MSI and MSS, the median progression-free survival (PFS) was only 1.4 mo, and no ORR was observed[68]. These results represent the limitations of approaches that do not target multiple molecular pathways involved in immune exclusion. Strategies for converting the cold CRC into hot CRC, which can enhance the responses to immune checkpoint inhibitors by promoting activation or recruitment of cytotoxic T lymphocytes in TME, should have been included in clinical trials. Recently, several trials have been conducted in favor of strategies to enhance immune activity and T lymphocyte infiltration into the TME to achieve substantial anti-tumor immune responses targeting CRC (Table 2). The list of completed clinical trials reflecting the non-clinical strategies includes the following: A phase I study (NCT02650713) in which a T-cell bispecific antibody and CEA combined with atezolizumab (targeting PD-L1) in CEA-positive solid tumors, indicating 20% partial response (PR) and 50% stable disease (SD)[69]; a phase I/II study (NCT03711058) with a combination of copanlisib (PI3K inhibitor) and nivolumab (anti-PD-1 antibody) targeting relapsed/refractory MSS CRC, with a decreasing trend of CD4+ T-lymphocytes mainly comprised of Treg and helper subsets[70]; a phase Ib study (NCT03977090) evaluating the safety and preliminary efficacy of fruquintinib (VEGF inhibitor) with geptanolimab (anti-PD-1 antibody) targeting metastatic CRC, indicating 26.7% ORR, 80% disease control rate (DCR), and 7.33 mo median PFS[71]; a phase Ib/II study (NCT03946917) of regorafenib plus toripalimab (anti-PD-1 antibody) targeting CRC, with 15.2% ORR and the 36.4% DCR[72]; a phase II randomized study (NCT02870920) of durvalumab (anti-PD-L1 antibody) plus tremelimumab (anti-CTLA-4 antibody) in patients with refractory CRC, resulting in 22% DCR, 1.8 mo PFA, and 6.6 mo overall survival[73]; a phase Ib study (NCT02947165) of the anti-TGF-β monoclonal antibody combined with spartalizumab (anti-PD-1 antibody) in patients with MSS CRC, providing a clinical proof of concept with 2 PR cases[74]; a phase II study (NCT03638297) to assess the efficacy of pembrolizumab (anti-PD-1 antibody) combined with celebrex (COX inhibitor) in patients with high MSI metastatic CRC, with 83.3% ORR, 12.5% SD, and 4.2% progressive disease[75]; and a phase I/II study (NCT03202758) to determine the safety and efficacy of durvalumab (anti-PD-L1 antibody) and tremelimumab (anti-CTLA-4 antibody) in combination with folinic acid, fluorouracil, and oxaliplatin in patients with metastatic CRC, with 31.2% PR and CR, 25% SD, and 6 mo PFS[76]. In summary, positive results were obtained targeting cold CRC through a variety of strategies for increasing immune responses, therefore, follow-up studies continue to be performed for treatment found to show significant results. Further, many clinical trials with various combinatory strategies by tyrosine kinase inhibitors, TGF-β inhibitors, Wnt signaling inhibitors, chemotherapies, and cancer vaccines to enhance immunotherapeutic efficacy are also ongoing (Table 3).
| Regimen | NCT number | Outcome | Completion |
| T-cell bispecific antibody and CEA combined with atezolizumab | NCT02650713 | 20% PR and 50% SD | January 2020 |
| Copanlisib plus nivolumab | NCT03711058 | No results available | January 2022 |
| Fruquintinib plus geptanolimab | NCT03977090 | 26.7% ORR, 80% DCR, and 7.33 mo median PFS | December 2021 |
| Regorafenib plus toripalimab | NCT03946917 | 15.2% ORR and the 36.4% DCR | November 2021 |
| Durvalumab plus tremelimumab | NCT02870920 | 2% DCR, 1.8 mo PFS, and 6.6 mo OS | December 2021 |
| Anti-TGF-β antibody plus spartalizumab | NCT02947165 | Clinical proof of concept with 2 PR cases | June 2021 |
| Pembrolizumab plus celebrex | NCT03638297 | 83.3% ORR, 12.5% SD, and 4.2% PD | August 2021 |
| Durvalumab and tremelimumab plus FOLFOX | NCT03202758 | 31.2% PR and CR, 25% SD, and 6 mo PFS | August 2020 |
| Strategy | NCT number | Intervention |
| Targeting tyrosine kinase | NCT04764006 | Surufatinib (VEGFR1, VEGFR2, VEGFR3, FGFR1, and CSF-1R inhibitor) |
| NCT04819516 | High-intensity focused ultrasound therapy; toripalimab (anti-PD-1 antibody) | |
| NCT04963283 | Cabozantinib (anti-VEGFR2) plus nivolumab (anti-PD-1 antibody) | |
| Targeting TGF-β | NCT03724851 | TEW-7197 (TGF-β receptor ALK4/ALK5 inhibitor) |
| Targeting Wnt signaling | NCT02521844 | ETC-159 (Porcupine inhibitor) plus Pembrolizumab (anti-PD-1 antibody) |
| Combination with chemotherapy | NCT04301557 | Pembrolizumab plus binimetinib (MEK 1/2 inhibitor) plus FOLFOX plus irinotecan |
| NCT04895137 | FOLFOX6 plus bevacizumab (anti-VEGF A) plus anti-PD-1 antibody | |
| NCT03374254 | Anti-PD-1 antibody plus oxaliplatin plus capecitabine plus radiotherapy then mesorectal excision | |
| Cancer vaccine | NCT04046445 | ATP128 (chimeric recombinant protein vaccine) plus BI754091 (IgG4Pro antibody inhibitor) plus VSV-GP128 (recombinant vesicular stomatitis virus) |
| NCT04117087 | KRAS peptide vaccine plus nivolumab (anti-PD-1 antibody) plus ipilimumab (anti-CTLA4 inhibitor) | |
| NCT04912765 | Neoantigen dendritic cell vaccine plus nivolumab |
Recently, several attempts have been made to conquer CRC with immunotherapies, but poor clinical outcomes were obtained due to the non-immunogenic characteristics of cold CRC. However, a variety of methods of converting cold into hot tumors were obtained through trial and error, and positive results have been drawn based on this background. We will have to carry our journey to a higher level to target cold CRC by discovering useful biomarkers through various efforts that span non-clinical and clinical studies in the future.
Provenance and peer review: Invited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu Z, China; Rathnaswami A, India; Xu X, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651-668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2409] [Cited by in RCA: 2663] [Article Influence: 532.6] [Reference Citation Analysis (0)] |
| 2. | Dobosz P, Dzieciątkowski T. The Intriguing History of Cancer Immunotherapy. Front Immunol. 2019;10:2965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 268] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 3. | Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Pérez-Gracia JL, Rodríguez-Ruiz ME, Ponz-Sarvise M, Castañón E, Melero I. Cytokines in clinical cancer immunotherapy. Br J Cancer. 2019;120:6-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 598] [Cited by in RCA: 812] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 4. | Pierpont TM, Limper CB, Richards KL. Past, Present, and Future of Rituximab-The World's First Oncology Monoclonal Antibody Therapy. Front Oncol. 2018;8:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 250] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 5. | Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 429] [Cited by in RCA: 837] [Article Influence: 139.5] [Reference Citation Analysis (0)] |
| 6. | Sengsayadeth S, Savani BN, Oluwole O, Dholaria B. Overview of approved CAR-T therapies, ongoing clinical trials, and its impact on clinical practice. EJHaem. 2022;3:6-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 7. | Kamal Y, Schmit SL, Frost HR, Amos CI. The tumor microenvironment of colorectal cancer metastases: opportunities in cancer immunotherapy. Immunotherapy. 2020;12:1083-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Malka D, Lièvre A, André T, Taïeb J, Ducreux M, Bibeau F. Immune scores in colorectal cancer: Where are we? Eur J Cancer. 2020;140:105-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 9. | Trabelsi M, Farah F, Zouari B, Jaafoura MH, Kharrat M. An Immunoscore System Based On CD3(+) And CD8(+) Infiltrating Lymphocytes Densities To Predict The Outcome Of Patients With Colorectal Adenocarcinoma. Onco Targets Ther. 2019;12:8663-8673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol. 2013;25:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 420] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 11. | Angell HK, Bruni D, Barrett JC, Herbst R, Galon J. The Immunoscore: Colon Cancer and Beyond. Clin Cancer Res. 2020;26:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 274] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 12. | Bonaventura P, Shekarian T, Alcazer V, Valladeau-Guilemond J, Valsesia-Wittmann S, Amigorena S, Caux C, Depil S. Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front Immunol. 2019;10:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 812] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 13. | Mpakali A, Stratikos E. The Role of Antigen Processing and Presentation in Cancer and the Efficacy of Immune Checkpoint Inhibitor Immunotherapy. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 14. | Jiang T, Shi T, Zhang H, Hu J, Song Y, Wei J, Ren S, Zhou C. Tumor neoantigens: from basic research to clinical applications. J Hematol Oncol. 2019;12:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 265] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 15. | Kass E, Schlom J, Thompson J, Guadagni F, Graziano P, Greiner JW. Induction of protective host immunity to carcinoembryonic antigen (CEA), a self-antigen in CEA transgenic mice, by immunizing with a recombinant vaccinia-CEA virus. Cancer Res. 1999;59:676-683. [PubMed] |
| 16. | Randrian V, Evrard C, Tougeron D. Microsatellite Instability in Colorectal Cancers: Carcinogenesis, Neo-Antigens, Immuno-Resistance and Emerging Therapies. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 17. | Lizardo DY, Kuang C, Hao S, Yu J, Huang Y, Zhang L. Immunotherapy efficacy on mismatch repair-deficient colorectal cancer: From bench to bedside. Biochim Biophys Acta Rev Cancer. 2020;1874:188447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 18. | Middha S, Yaeger R, Shia J, Stadler ZK, King S, Guercio S, Paroder V, Bates DDB, Rana S, Diaz LA Jr, Saltz L, Segal N, Ladanyi M, Zehir A, Hechtman JF. Majority of B2M-Mutant and -Deficient Colorectal Carcinomas Achieve Clinical Benefit From Immune Checkpoint Inhibitor Therapy and Are Microsatellite Instability-High. JCO Precis Oncol. 2019;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Plundrich D, Chikhladze S, Fichtner-Feigl S, Feuerstein R, Briquez PS. Molecular Mechanisms of Tumor Immunomodulation in the Microenvironment of Colorectal Cancer. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Xue J, Yu X, Xue L, Ge X, Zhao W, Peng W. Intrinsic β-catenin signaling suppresses CD8(+) T-cell infiltration in colorectal cancer. Biomed Pharmacother. 2019;115:108921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 21. | Gargalionis AN, Papavassiliou KA, Papavassiliou AG. Targeting STAT3 Signaling Pathway in Colorectal Cancer. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 22. | Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting STAT3 in Cancer Immunotherapy. Mol Cancer. 2020;19:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 681] [Article Influence: 136.2] [Reference Citation Analysis (0)] |
| 23. | Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front Immunol. 2018;9:978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 446] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 24. | Kumar S, Principe DR, Singh SK, Viswakarma N, Sondarva G, Rana B, Rana A. Mitogen-Activated Protein Kinase Inhibitors and T-Cell-Dependent Immunotherapy in Cancer. Pharmaceuticals (Basel). 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Fousek K, Horn LA, Palena C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol Ther. 2021;219:107692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (1)] |
| 26. | Hont AB, Powell AB, Sohai DK, Valdez IK, Stanojevic M, Geiger AE, Chaudhary K, Dowlati E, Bollard CM, Cruz CRY. The generation and application of antigen-specific T cell therapies for cancer and viral-associated disease. Mol Ther. 2022;30:2130-2152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Westcott PMK, Sacks NJ, Schenkel JM, Ely ZA, Smith O, Hauck H, Jaeger AM, Zhang D, Backlund CM, Beytagh MC, Patten JJ, Elbashir R, Eng G, Irvine DJ, Yilmaz OH, Jacks T. Low neoantigen expression and poor T-cell priming underlie early immune escape in colorectal cancer. Nat Cancer. 2021;2:1071-1085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 28. | Qiao G, Kone LB, Phillips EH, Lee SS, Brown GE, Khetani SR, Thakur A, Lum LG, Prabhakar BS, Maker AV. LIGHT enhanced bispecific antibody armed T-cells to treat immunotherapy resistant colon cancer. Oncogene. 2022;41:2054-2068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 29. | Bai Z, Zhou Y, Ye Z, Xiong J, Lan H, Wang F. Tumor-Infiltrating Lymphocytes in Colorectal Cancer: The Fundamental Indication and Application on Immunotherapy. Front Immunol. 2021;12:808964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 112] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 30. | Li H, Yang C, Cheng H, Huang S, Zheng Y. CAR-T cells for Colorectal Cancer: Target-selection and strategies for improved activity and safety. J Cancer. 2021;12:1804-1814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 31. | Xiao L, Cen D, Gan H, Sun Y, Huang N, Xiong H, Jin Q, Su L, Liu X, Wang K, Yan G, Dong T, Wu S, Zhou P, Zhang J, Liang W, Ren J, Teng Y, Chen C, Xu XH. Adoptive Transfer of NKG2D CAR mRNA-Engineered Natural Killer Cells in Colorectal Cancer Patients. Mol Ther. 2019;27:1114-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 221] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 32. | Ju H, Xing W, Yang J, Zheng Y, Jia X, Zhang B, Ren H. An effective cytokine adjuvant vaccine induces autologous T-cell response against colon cancer in an animal model. BMC Immunol. 2016;17:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Dai Y, Zhao W, Yue L, Dai X, Rong D, Wu F, Gu J, Qian X. Perspectives on Immunotherapy of Metastatic Colorectal Cancer. Front Oncol. 2021;11:659964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 34. | Nagarsheth N, Peng D, Kryczek I, Wu K, Li W, Zhao E, Zhao L, Wei S, Frankel T, Vatan L, Szeliga W, Dou Y, Owens S, Marquez V, Tao K, Huang E, Wang G, Zou W. PRC2 Epigenetically Silences Th1-Type Chemokines to Suppress Effector T-Cell Trafficking in Colon Cancer. Cancer Res. 2016;76:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 217] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 35. | Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, Sun Y, Zhao E, Vatan L, Szeliga W, Kotarski J, Tarkowski R, Dou Y, Cho K, Hensley-Alford S, Munkarah A, Liu R, Zou W. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527:249-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 662] [Cited by in RCA: 917] [Article Influence: 91.7] [Reference Citation Analysis (0)] |
| 36. | Jia W, Zhang T, Huang H, Feng H, Wang S, Guo Z, Luo Z, Ji X, Cheng X, Zhao R. Colorectal cancer vaccines: The current scenario and future prospects. Front Immunol. 2022;13:942235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 37. | Corulli LR, Cecil DL, Gad E, Koehnlein M, Coveler AL, Childs JS, Lubet RA, Disis ML. Multi-Epitope-Based Vaccines for Colon Cancer Treatment and Prevention. Front Immunol. 2021;12:729809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 38. | Ren Y, Miao JM, Wang YY, Fan Z, Kong XB, Yang L, Cheng G. Oncolytic viruses combined with immune checkpoint therapy for colorectal cancer is a promising treatment option. Front Immunol. 2022;13:961796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 39. | Wang N, Wang J, Zhang Z, Cao H, Yan W, Chu Y, Chard Dunmall LS, Wang Y. A novel vaccinia virus enhances anti-tumor efficacy and promotes a long-term anti-tumor response in a murine model of colorectal cancer. Mol Ther Oncolytics. 2021;20:71-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Taniura T, Iida Y, Kotani H, Ishitobi K, Tajima Y, Harada M. Immunogenic chemotherapy in two mouse colon cancer models. Cancer Sci. 2020;111:3527-3539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Guan Y, Kraus SG, Quaney MJ, Daniels MA, Mitchem JB, Teixeiro E. FOLFOX Chemotherapy Ameliorates CD8 T Lymphocyte Exhaustion and Enhances Checkpoint Blockade Efficacy in Colorectal Cancer. Front Oncol. 2020;10:586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 42. | Witek M, Blomain ES, Magee MS, Xiang B, Waldman SA, Snook AE. Tumor radiation therapy creates therapeutic vaccine responses to the colorectal cancer antigen GUCY2C. Int J Radiat Oncol Biol Phys. 2014;88:1188-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Ji D, Song C, Li Y, Xia J, Wu Y, Jia J, Cui X, Yu S, Gu J. Combination of radiotherapy and suppression of Tregs enhances abscopal antitumor effect and inhibits metastasis in rectal cancer. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 44. | Ebert LM, Yu W, Gargett T, Brown MP. Logic-gated approaches to extend the utility of chimeric antigen receptor T-cell technology. Biochem Soc Trans. 2018;46:391-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 45. | Bai L, Li W, Zheng W, Xu D, Chen N, Cui J. Promising targets based on pattern recognition receptors for cancer immunotherapy. Pharmacol Res. 2020;159:105017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 46. | Liu Z, Ren Y, Weng S, Xu H, Li L, Han X. A New Trend in Cancer Treatment: The Combination of Epigenetics and Immunotherapy. Front Immunol. 2022;13:809761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 47. | Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress. J Hematol Oncol. 2022;15:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 412] [Article Influence: 137.3] [Reference Citation Analysis (0)] |
| 48. | Ebert PJR, Cheung J, Yang Y, McNamara E, Hong R, Moskalenko M, Gould SE, Maecker H, Irving BA, Kim JM, Belvin M, Mellman I. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity. 2016;44:609-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 566] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 49. | Napolitano S, Matrone N, Muddassir AL, Martini G, Sorokin A, De Falco V, Giunta EF, Ciardiello D, Martinelli E, Belli V, Furia M, Kopetz S, Morgillo F, Ciardiello F, Troiani T. Triple blockade of EGFR, MEK and PD-L1 has antitumor activity in colorectal cancer models with constitutive activation of MAPK signaling and PD-L1 overexpression. J Exp Clin Cancer Res. 2019;38:492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Céspedes MV, Sevillano M, Nadal C, Jung P, Zhang XH, Byrom D, Riera A, Rossell D, Mangues R, Massagué J, Sancho E, Batlle E. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 900] [Article Influence: 69.2] [Reference Citation Analysis (0)] |
| 51. | Wei N, Li J, Fang C, Chang J, Xirou V, Syrigos NK, Marks BJ, Chu E, Schmitz JC. Targeting colon cancer with the novel STAT3 inhibitor bruceantinol. Oncogene. 2019;38:1676-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 52. | Abril-Rodriguez G, Torrejon DY, Liu W, Zaretsky JM, Nowicki TS, Tsoi J, Puig-Saus C, Baselga-Carretero I, Medina E, Quist MJ, Garcia AJ, Senapedis W, Baloglu E, Kalbasi A, Cheung-Lau G, Berent-Maoz B, Comin-Anduix B, Hu-Lieskovan S, Wang CY, Grasso CS, Ribas A. PAK4 inhibition improves PD-1 blockade immunotherapy. Nat Cancer. 2020;1:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 53. | Qi L, Sun B, Liu Z, Li H, Gao J, Leng X. Dickkopf-1 inhibits epithelial-mesenchymal transition of colon cancer cells and contributes to colon cancer suppression. Cancer Sci. 2012;103:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 54. | Rahbari NN, Kedrin D, Incio J, Liu H, Ho WW, Nia HT, Edrich CM, Jung K, Daubriac J, Chen I, Heishi T, Martin JD, Huang Y, Maimon N, Reissfelder C, Weitz J, Boucher Y, Clark JW, Grodzinsky AJ, Duda DG, Jain RK, Fukumura D. Anti-VEGF therapy induces ECM remodeling and mechanical barriers to therapy in colorectal cancer liver metastases. Sci Transl Med. 2016;8:360ra135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 55. | Jung K, Heishi T, Incio J, Huang Y, Beech EY, Pinter M, Ho WW, Kawaguchi K, Rahbari NN, Chung E, Kim JK, Clark JW, Willett CG, Yun SH, Luster AD, Padera TP, Jain RK, Fukumura D. Targeting CXCR4-dependent immunosuppressive Ly6C(low) monocytes improves antiangiogenic therapy in colorectal cancer. Proc Natl Acad Sci U S A. 2017;114:10455-10460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 56. | Chen X, Xu J, Guo Q, Wang L, Yang Y, Guo H, Gu N, Zhang D, Qian W, Hou S, Li J, Dai J, Guo Y, Wang H. Therapeutic efficacy of an anti-PD-L1 antibody based immunocytokine in a metastatic mouse model of colorectal cancer. Biochem Biophys Res Commun. 2016;480:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 57. | Tong J, Tan X, Song X, Gao M, Risnik D, Hao S, Ermine K, Wang P, Li H, Huang Y, Yu J, Zhang L. CDK4/6 Inhibition Suppresses p73 Phosphorylation and Activates DR5 to Potentiate Chemotherapy and Immune Checkpoint Blockade. Cancer Res. 2022;82:1340-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 58. | Santiago L, Castro M, Sanz-Pamplona R, Garzón M, Ramirez-Labrada A, Tapia E, Moreno V, Layunta E, Gil-Gómez G, Garrido M, Peña R, Lanuza PM, Comas L, Jaime-Sanchez P, Uranga-Murillo I, Del Campo R, Pelegrín P, Camerer E, Martínez-Lostao L, Muñoz G, Uranga JA, Alcalde A, Galvez EM, Ferrandez A, Bird PI, Metkar S, Arias MA, Pardo J. Extracellular Granzyme A Promotes Colorectal Cancer Development by Enhancing Gut Inflammation. Cell Rep. 2020;32:107847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 59. | Lee WS, Yang H, Chon HJ, Kim C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp Mol Med. 2020;52:1475-1485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 415] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 60. | Khare T, Bissonnette M, Khare S. CXCL12-CXCR4/CXCR7 Axis in Colorectal Cancer: Therapeutic Target in Preclinical and Clinical Studies. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 61. | D'Alterio C, Zannetti A, Trotta AM, Ieranò C, Napolitano M, Rea G, Greco A, Maiolino P, Albanese S, Scognamiglio G, Tatangelo F, Tafuto S, Portella L, Santagata S, Nasti G, Ottaiano A, Pacelli R, Delrio P, Botti G, Scala S. New CXCR4 Antagonist Peptide R (Pep R) Improves Standard Therapy in Colorectal Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 62. | Runbeck E, Crescioli S, Karagiannis SN, Papa S. Utilizing Immunocytokines for Cancer Therapy. Antibodies (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 63. | Gmeiner WH. Fluoropyrimidine Modulation of the Anti-Tumor Immune Response-Prospects for Improved Colorectal Cancer Treatment. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 64. | Sahin IH, Ciombor KK, Diaz LA, Yu J, Kim R. Immunotherapy for Microsatellite Stable Colorectal Cancers: Challenges and Novel Therapeutic Avenues. Am Soc Clin Oncol Educ Book. 2022;42:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 65. | Asaoka Y, Ijichi H, Koike K. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;373:1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 320] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 66. | Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, Diaz LA Jr. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3799] [Cited by in RCA: 4953] [Article Influence: 619.1] [Reference Citation Analysis (0)] |
| 67. | Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167-3175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2330] [Cited by in RCA: 2414] [Article Influence: 160.9] [Reference Citation Analysis (0)] |
| 68. | Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, Ready NE, Gerber DE, Chow LQ, Juergens RA, Shepherd FA, Laurie SA, Geese WJ, Agrawal S, Young TC, Li X, Antonia SJ. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 775] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 69. | Tabernero J, Melero I, Ros W, Argilés G, Marabelle A, Rodriguez-Ruiz ME, Albanell J, Calvo E, Moreno V, Cleary JM, Eder JP, Karanikas V, Bouseida S, Sandoval F, Sabanes D, Sreckovic S, Hurwitz H, Paz-Ares LG, Saro J, Segal NH. Phase Ia and Ib studies of the novel carcinoembryonic antigen (CEA) T-cell bispecific (CEA CD3 TCB) antibody as a single agent and in combination with atezolizumab: Preliminary efficacy and safety in patients with metastatic colorectal cancer (mCRC). J Clin Oncol. 2017;35:3002-3002. [RCA] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 70. | Jakubowski C, Collins NB, Sugar EA, Berg M, Cao H, Giannakis M, Jaffee EM, Azad NS. A phase I/II study of PI3Kinase inhibition with copanlisib combined with the anti-PD-1 antibody nivolumab in relapsed/refractory solid tumors with expansions in MSS colorectal cancer. J Clin Oncol. 2020;38:TPS4114-TPS4114. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 71. | Bai Y, Xu N, An S, Chen W, Gao C, Zhang D. A phase ib trial of assessing the safety and preliminary efficacy of a combination therapy of geptanolimab (GB 226) plus fruquintinib in patients with metastatic colorectal cancer (mCRC). J Clin Oncol. 2021;39:e15551-e15551. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Wang F, He MM, Yao YC, Zhao X, Wang ZQ, Jin Y, Luo HY, Li JB, Wang FH, Qiu MZ, Lv ZD, Wang DS, Li YH, Zhang DS, Xu RH. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: a phase Ib/II clinical trial and gut microbiome analysis. Cell Rep Med. 2021;2:100383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 73. | Loree JM, Topham JT, Kennecke HF, Feilotter H, Lee YS, Virk S, Kopetz S, Duose DY, Manyam GC, Morris JS, Maru DM, Renouf D, Jonker DJ, Tu D, O'Callaghan CJ, Chen EX. Impact of consensus molecular subtyping (CMS) on survival in the CO.26 trial of durvalumab plus tremelimumab vs best supportive care (BSC) in metastatic colorectal cancer (mCRC). J Clin Oncol. 2022;40:3551-3551. [RCA] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 74. | Bauer TM, Lin CC, Greil R, Goebeler ME, Huetter-Kroenke ML, Garrido-Laguna I, Santoro A, Perotti A, Spreafico A, Yau T, Joerger M, Cremasco V, Dostalek M, Pelletier M, Barys L, Lu D, Katsanou V, Fabre C, Doi T. Phase Ib study of the anti-TGF-β monoclonal antibody (mAb) NIS793 combined with spartalizumab (PDR001), a PD-1 inhibitor, in patients (pts) with advanced solid tumors. J Clin Oncol. 2021;39:2509-2509. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Wu Z, Hu H, Zhang J, Cai Y, Xie X, Ling J, Li W, Deng Y. PD-1 blockade combined with COX inhibitor in patients with MSI-H/dMMR or high TMB, advanced or metastatic colorectal cancer (PCOX study). J Clin Oncol. 2020;38:111-111. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 76. | Ghiringhelli F, Chibaudel B, Taieb J, Bennouna J, Martin-Babau J, Fonck M, Borg C, Cohen R, Thibaudin M, Limagne E, Fumet J-D. Durvalumab and tremelimumab in combination with FOLFOX in patients with RAS-mutated, microsatellite-stable, previously untreated metastatic colorectal cancer (MCRC): Results of the first intermediate analysis of the phase Ib/II MEDETREME trial. J Clin Oncol. 2020;38:3006-3006. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |