Published online Nov 15, 2023. doi: 10.4251/wjgo.v15.i11.2017
Peer-review started: August 10, 2023
First decision: August 30, 2023
Revised: September 14, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 15, 2023
Processing time: 96 Days and 20 Hours
The global incidence of intrahepatic cholangiocarcinoma (ICCA) is soaring. Due to often delayed presentation, only a narrow spectrum of the disease is usually surgically resectable. To more accurately stage the disease, reduce recurrence, and improve overall survival, surgical teams are increasingly performing intraoperative lymph node dissection (LND) as well. This procedure has its associated morbidity, while there is no consensus or formal guidelines on its role in this setting. Hence, there is a need to better delineate the evidence for performing LND alongside surgical resection of the ICCA.
To perform a systematic review and meta-analysis on the role of LND in improving prognostication and survival post-resection of ICCA.
We performed a systematic literature search using Pubmed, Medline, Embase, and the Cochrane Library, for all studies involving LND, ICCA, and surgical resection using several keywords, Medical Subject Headings (MeSH) tags, and appropriate synonyms. All clinical studies comparing curative intent resection of ICCA with LND vs resection without LND were included, while single-arm case series, studies with insufficient data, and duplicates were excluded. We included all English-language studies from the different academic databases up till early December 2022. The primary outcome measures were set for overall survival (OS) and disease-free survival (DFS).
This systematic review and meta-analysis included 15 studies that fulfilled the selection criteria comprising 11413 patients with surgically-resectable ICCA, of whom 6424 (56.3%) underwent hepatectomy with LND while the remainder underwent hepatectomy only. In patients who underwent LND, on average, 27.7% of the resected lymph nodes were positive for metastatic disease. Overall, the results showed that performing LND did not significantly improve OS or DFS. However, the effect of LND on OS showed a degree of variability by geographical region, in Eastern and Western countries. As LND is increasingly being performed, further time-based analysis was undertaken to identify time-dependent changes in the role of LND. An increasing adoption of LND was not associated with improved OS. Furthermore, no roles were identified for neoadjuvant/adjuvant chemotherapy or increasing lymph node retrieval in improving OS either.
LND might aid in staging, prognosticating, and deciding further management of resected ICCA, but does not improve OS and DFS and is unsuitable for high-risk patients unlikely to benefit from further treatments.
Core Tip: The overall survival (OS) from surgically resectable intrahepatic cholangiocarcinoma remains poor. Lymph node dissection is increasingly being performed in the setting of hepatic resection with the aims of improving patient outcomes such as OS, minimising recurrence, as well as for accurate staging. However, no consensus exists in the literature regarding its use for these purposes. This systematic review and meta-analysis of hepatic resection with and without lymph node dissection for surgically resectable intrahepatic cholangiocarcinoma was performed with the primary outcome measures of OS and disease-free survival.
- Citation: Atif M, Borakati A, Mavroeidis VK. Role of routine lymph node dissection alongside resection of intrahepatic cholangiocarcinoma: Systematic review and meta-analysis. World J Gastrointest Oncol 2023; 15(11): 2017-2032
- URL: https://www.wjgnet.com/1948-5204/full/v15/i11/2017.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i11.2017
The incidence of intrahepatic cholangiocarcinoma (ICCA) continues to increase globally[1-3]. Unfortunately, due to late presentation and diagnosis at an advanced stage, only a narrow spectrum of the disease is amenable to hepatic resection[4-7]. While complete surgical resection is the only established treatment with curative intent, more recent studies have shown favourable results with liver transplantation in early ICCA, indicating that, under defined criteria, it may play a role in the future[6]. Considering that 5-year survival is only 5% for inoperable cases and reaches up to 30% after curative-intent resection, it is imperative to identify novel treatment strategies[8,9].
To precisely determine subsequent ICCA management and prognosis, it is important to accurately stage ICCA. In this regard, patients undergoing surgical resection may also undergo concomitant lymph node dissection (LND) of some degree[5,10,11]. This is based on the premise that ICCA dissemination occurs primarily through the lymphatics[3]. The presence of lymph node metastases is an established negative prognostic indicator for ICCA[5,10-14]. Further, confirming their presence by LND allows consideration of adjuvant chemotherapy. Although there is no consensus on the use of adjuvant chemotherapy, there is limited evidence that it may improve overall survival (OS) in ICCA[10,15-17]. In the event that a liver transplant is considered, the presence of lymph node metastases is an absolute contraindication[6]. Hence, a significant proportion of hepatobiliary surgeons, consider LND as an important stage of the resection procedure to restage disease, assess prognosis and, long-term survival.
Remarkably, the evidence base for LND during ICCA resection is conflicting regarding whether it adds any morbidity or confers long-term oncological benefit[10,11,14,18-23]. In the American Surveillance, Epidemiology, and End Results Program (SEER), 54.7% of patients underwent some form of concomitant LND during liver resection[24]. They had a mean of 4 (range 2-8) lymph nodes (LNs) resected of which, 41.3% were positive for metastatic disease. The presence of even one positive lymph node was associated with significantly poorer OS compared to those without nodal disease (18 mo vs 45 mo). This study also identified that increasing the total number of LNs examined (TNLE) to 6 or more was significantly associated with an increase in the detection of positive LNs. This study also suggested that in node-negative disease, TNLE > 6 had a trend towards increased OS vs TNLE < 6 (69.8 mo vs 39.5 mo, P = 0.069). TNLE had no association with OS where there was at least one confirmed lymph node metastasis. This was then taken further by another team who re-analysed the same data and found patients with nodal-positive disease had poorer median survival compared to those without (20 mo vs 52 mo)[10].
Similarly, in a larger multi-centre retrospective study of 449 patients undergoing resection of ICCA, 55% had a concomitant lymphadenectomy[1]. They had a median of 3 LNs harvested (range 1-76) of which, 29.8% were positive. These patients also had significantly poorer OS compared to those with node-negative disease (22.9 mo vs 30.1 mo). This was also identified in another study in which, patients who did not undergo LND had poorer OS compared to those with N0 (post-LND) disease[21]. In comparison, a smaller Japanese study performed a multivariate analysis of 44 patients with ICCA (of whom 30 underwent extended LND) and found no association between the presence of lymph node disease and OS (at 1 and 3 years)[14]. Broadly, these studies do not conclusively indicate LND as a procedure to improve OS. However, they do suggest it as a manner of identifying underlying metastatic disease and avoiding inaccurate staging of patients.
The upper abdominal lymphatic drainage is split into 17 lymph node stations based on anatomical location. They can be further divided depending on their direction of lymphatic drainage into hepatoduodenal and cardinal[25]. In a retrospective Korean study, stations 8 and 12 (both hepatoduodenal outflow) had the highest rates of likely positive nodal disease[26]. However, resecting both of these stations was not associated with improved OS.
In terms of consensus on performing LND, the international societies have expressed a range of perspectives. For example, the European Association for the Study of Liver (EASL) and the International Liver Cancer Association (ILCA) recommend that patients with ICCA who are amenable to surgical resection should have lymph node sampling performed for pre-operative staging[27]. For those that eventually undergo a resection, all should undergo an LND (of 6 nodes) for more precise disease staging and prognosis. Similarly, the National Comprehensive Cancer Network in the United States also recommends a regional lymphadenectomy be performed[28]. However, a minimum quantity of LNs to harvest is not specified. This procedure is to be performed for more precise prognosis estimation. In the event of positive lymph node identification, patients could be considered for adjuvant chemotherapy. Furthermore, the American Hepato-Pancreato-Biliary Association (AHPBA) recommends that a regional lymphadenectomy be considered rather than routinely performed. They also do not stipulate a minimum quantity of LNs to harvest and consider the procedure to be useful for prognostication as well[20]. Conversely, the Japanese Liver Cancer Study Group does not offer a definitive view on the role of LND[29]. This is especially the case if no clear evidence of lymphatic disease is identified on pre-operative imaging or staging laparoscopy.
As of today, the American Joint Committee on Cancer (AJCC) 8th edition recommends the evaluation of at least 6 regional LNs to improve the precision of disease staging[5].
Precise staging post-LND is also important to decide on offering adjuvant chemotherapy to patients. The AHPBA consensus guidelines suggest that patients undergoing an R1/R2 resection or with nodal-positive ICCA should be considered for gemcitabine, or 5-fluorouracil (5-FU)[20]. As mentioned above, sampling an adequate number of LNs is necessary to pick up the nodal disease. If a patient does not undergo LND, it is theoretically plausible that underlying nodal disease is not identified, leading to the patient not receiving adjuvant chemotherapy and thus negating their prognostic outlook. In a meta-analysis comprising 5060 patients across 15 retrospective studies, adjuvant chemotherapy was associated with improved OS post-R0 resection compared to the control (HR: 0.66, 0.55-0.79, P < 0.001)[30]. In particular, gemcitabine (not 5-FU) use led to significantly improved OS compared to surgical resection-only control (HR: 0.493, 0.34-0.72, P < 0.001). However, in comparison, a Cochrane review based on 4 randomised controlled trials involving approximately 900 patients with cholangiocarcinoma (intra- and extra-hepatic) and bile duct cancers found that adjuvant chemotherapy post-R0 resection did not affect 5-year all-cause mortality[16]. Notably, these trials consisted of patients with heterogeneous pathology and they were found to be at a high risk of detection bias due to the majority of trials being open-label. Overall, it is still unestablished whether adjuvant chemotherapy plays a role in promoting OS or preventing recurrence post-resection. Depending on local surgical practice, patients will have this performed routinely (or not), locally (or regionally) with varying quantities of LNs harvested. LND involves complex dissection around critical vessels that adds further operative morbidity to the patient. These patients are more likely to undergo a longer operation (hence, anaesthetic risk), develop wound infections as well as to develop a Class III Clavien-Dindo complication afterward[2,31]. A previous systematic review found that patients undergoing any operation (abdominal/neurosurgical/thoracic etc.) lasting more than 2 h had a twofold higher risk of developing any complication[32]. This risk increased by 21% for every hour. In the context of potential LND for ICCA, these factors need to be further balanced against the incidence of positive LNs. Nevertheless, LND may facilitate more precise disease staging and may improve OS[19,27] and the 8th Edition of the AJCC recommended harvesting of at least 6 LNs[34], despite the absence of any definitive evidence regarding this.
It is thus important to delineate whether routine LND is of any clinical value. In light of this, we performed a systematic review and meta-analysis of all published relevant studies, to better delineate the link between LND in the context of surgical resection of ICCA, and subsequent prognosis and survival.
This systematic review and meta-analysis was performed per the PRISMA framework and Cochrane Handbook for Systematic Reviews[35,36]. The study protocol was pre-emptively published on the PROSPERO database (CRD
We searched the following databases: Pubmed, Medline, Embase, and the Cochrane Library. All English-language studies from these databases were included up till the search period of early December 2022. All clinical studies comparing curative intent resection of ICCA with LND vs resection without LND were included. Single-arm case series were excluded. Studies without sufficient data for meta-analysis were excluded also. Where there were multiple publications about the same cohort of patients, only the most recent publication was included.
We used the following keywords and MeSH: “Intrahepatic AND cholangiocarcinoma”, “cholangiocarcinoma” as well as “bile duct/biliary” and “cancer”, “carcinoma”, “adenocarcinoma”, “lymphadenectomy”, “lymph node”, “dissection”, “resection”, “excision”, “removal”. We used the following search terms to exclude unrelated studies: “Gastric”, “periampullary”, “pancreaticoduodenectomy”, “Whipple’s”, “oesophageal”, “case report”, and “review”.
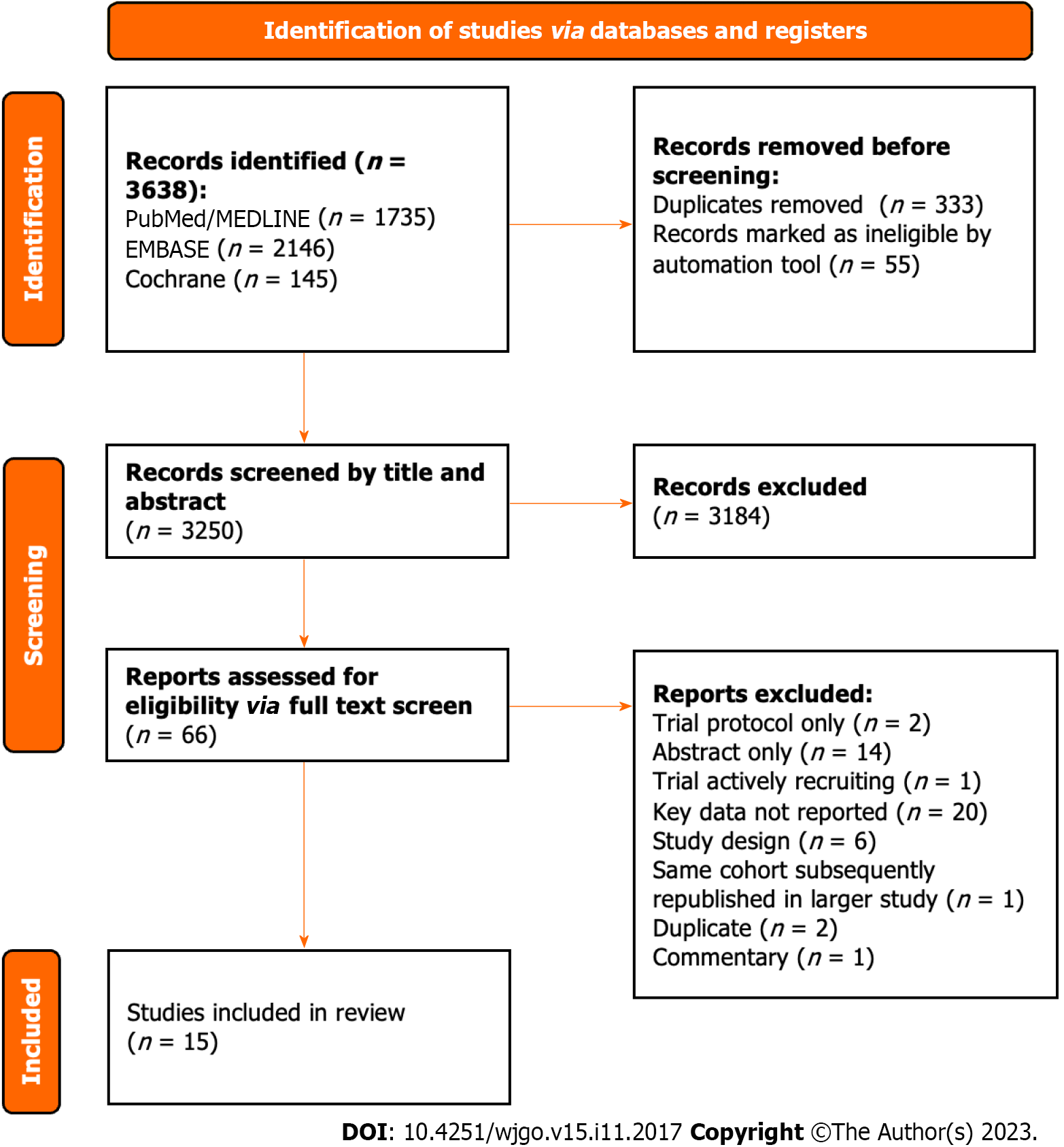
All 3638 studies were compiled into a single database on the Rayyan.ai online platform (Figure 1)[35].
After removing duplicates, all studies underwent an initial screen of the title and abstract independently by two authors (Atif M and Borakati A). After the initial screen, the remaining studies underwent a full-text screen again independently by the same authors. Any conflicts were resolved through mutual assessment and discussion.
Two authors (Atif M and Borakati A) extracted the following data fields from the included studies: Number of patients, country of origin, morphological characteristic of the tumour (periductal infiltrating or mass forming), neoadjuvant/adjuvant chemotherapy, the extent of resection (R0/R1), extent of LND (hilar, regional, distal), the number of LNs harvested, number of positive LNs and survival data (overall and disease-free).
The primary outcomes were hazard ratios for overall and disease-free survival (DFS) with LND vs no LND in curative intent resection of ICCA. Secondary outcomes were the effect of positive LNs, tumour morphology, and adjuvant therapies on survival.
Quality assessment was conducted using the ROBINS-I tool as recommended by the Cochrane Collaboration.
Data were analysed using RStudio (R 4.3.0; R Foundation, Austria) with meta and dmetar packages. Survival data was extracted if there was sufficient data available for meta-analysis of time-to-event outcomes and converted to log hazard ratios and standard errors for pooling of effect sizes using the methods described by Tierney et al[37]. Pooled hazard ratios (HR) with 95% confidence intervals (95%CI) for both overall and DFS were generated using the generic inverse variance method using random effects models. Fixed effect modelling is presented as a sensitivity analysis in a Supplementary file.
Statistical significance was considered at a level of P < 0.05. We used the I2 test to assess for heterogeneity between the included studies. Publication bias was assessed using funnel plots and Egger’s test.
Assessment of the effect of reported co-variates including the country of origin, morphological characteristics of the tumour, presence of lymph node metastases, and adjuvant chemotherapy was performed using sub-group analysis for categoric variables where survival is reported for each group. Otherwise, univariable meta-regression was used where only numbers of patients in each category were reported or for continuous variables to generate hazard ratios for survival for each co-variate.
Overall, we identified 15 eligible studies with a total of 11413 patients for meta-analysis, of whom 6424 (56.3%) underwent hepatectomy with LND while the remainder underwent hepatectomy only[21,38-50]. In patients who underwent LND, in the total of harvested LNs, the incidence of metastases was 27.7% overall (range 14.9%-42.5%)[31,38,42-46,48].
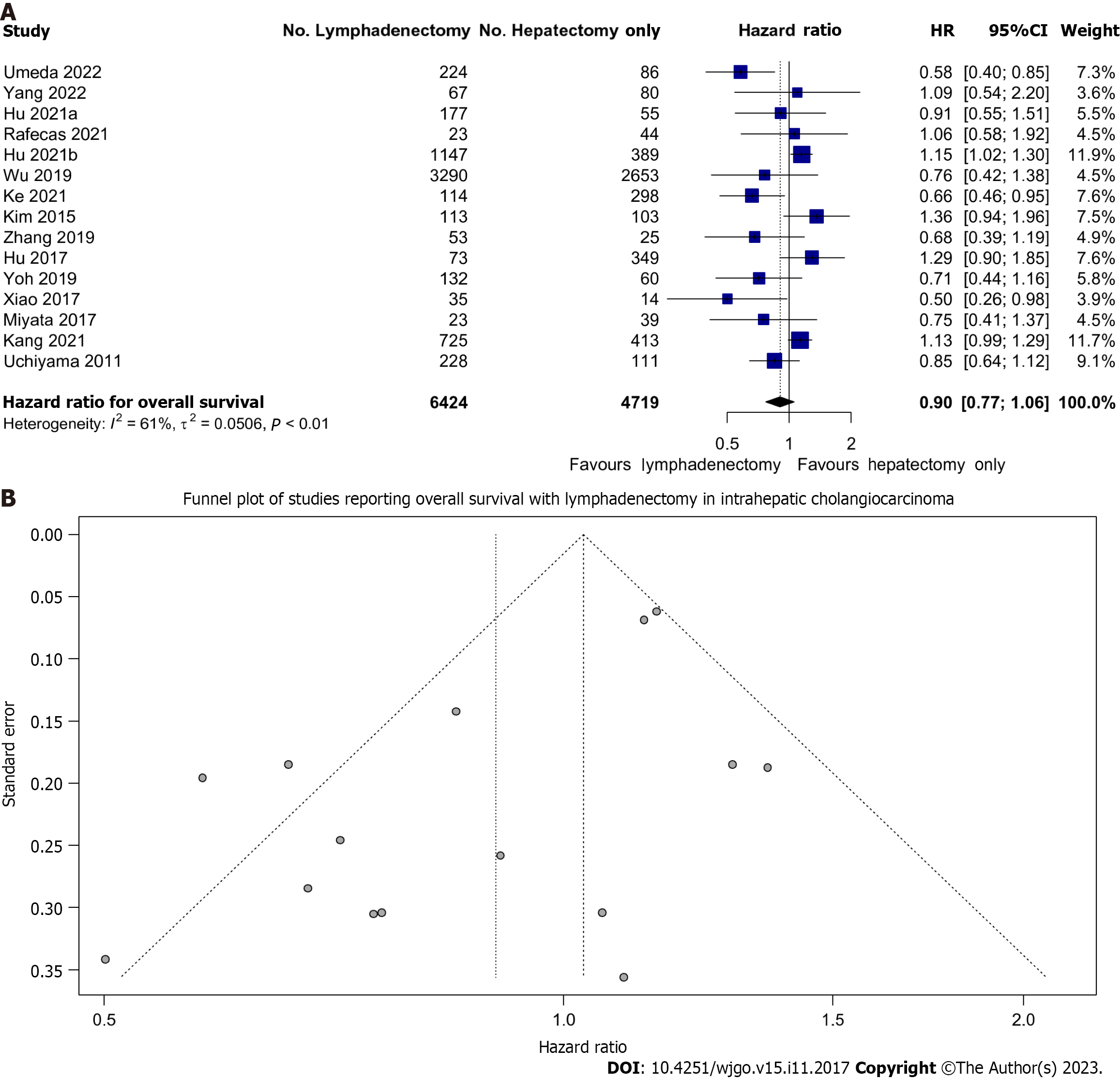
All studies reported on OS. We found that LND did not significantly improve OS in patients undergoing resection; HR: 0.90 (95%CI: 0.77-1.06) (Figure 2A). However, there may be a trend toward improved OS given the narrow confidence interval and minimal overlap with equivalence.
There was moderate heterogeneity (I2 = 61%) between the various cohorts. This data was not affected by publication bias as evidenced by the lack of asymmetry on Figure 2B.
Fixed effect modelling also showed no difference in OS (HR: 1.03, 95%CI: 0.96-1.11) (Supplementary Figure 1). The prediction interval of the hazard ratio of OS with lymphadenectomy was also wide (0.54-1.51) reflecting the uncertainty of the treatment effect of LND.
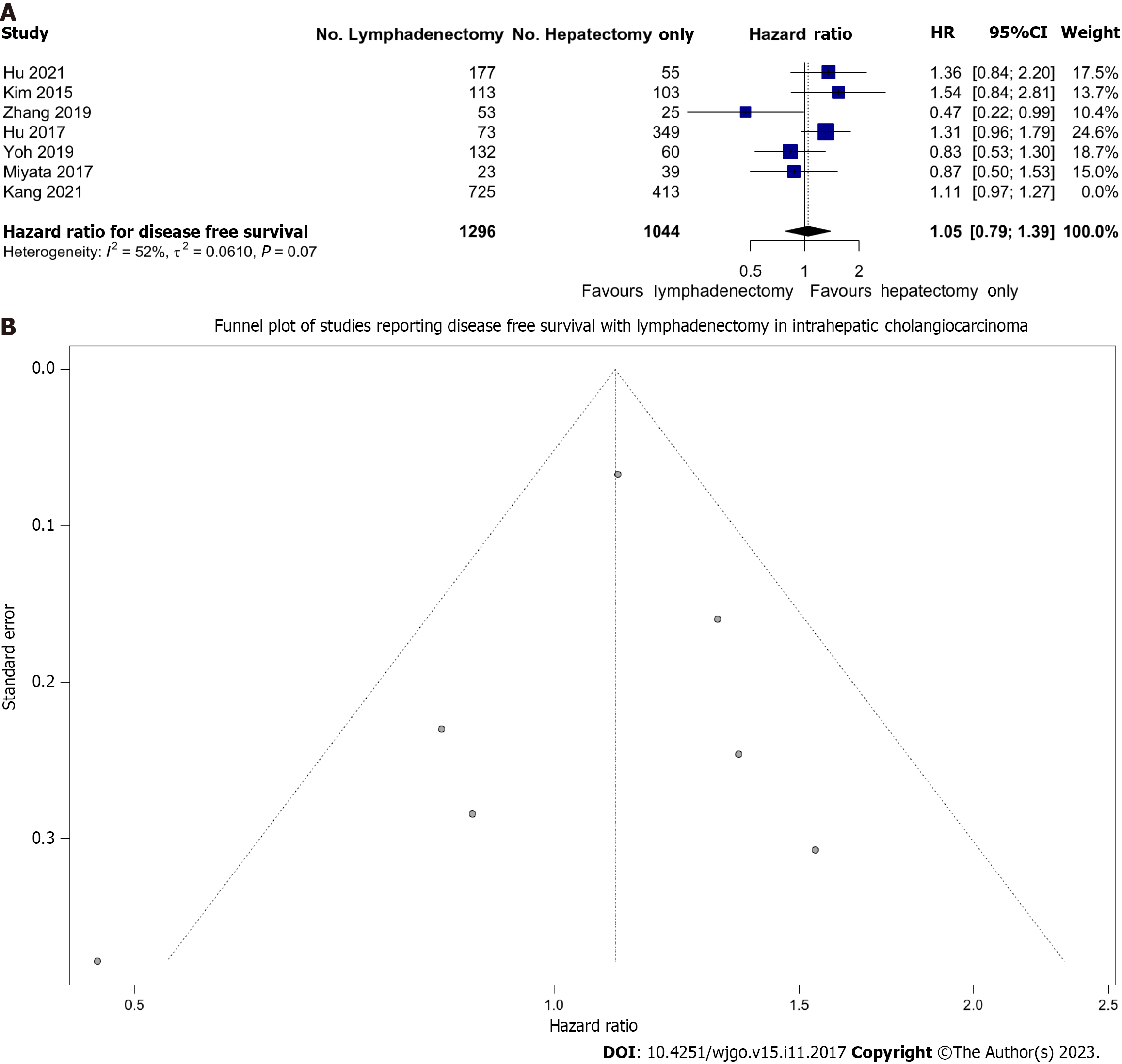
Overall, we pooled 2340 patients from 7 studies that reported on DFS survival, of whom 1296 (55.3%) underwent LND. There was moderate heterogeneity (I2 = 42%) between these studies. We found that LND was not associated with improved DFS; HR: 1.11 (0.99-1.23) (Figure 3A). This analysis was largely influenced by a single large study which contributed 68.1% of the weight (Kang et al)[46]. As a sensitivity analysis, this study was removed and the pooled effect remained non-significant with HR: 1.05 (95%CI: 0.79-1.39), however, heterogeneity increased to I2 = 52%.
Importantly, although there was no publication bias as evidenced by the lack of asymmetry of data on Figure 3B, fewer studies reported this measure compared to OS (7 vs 15).
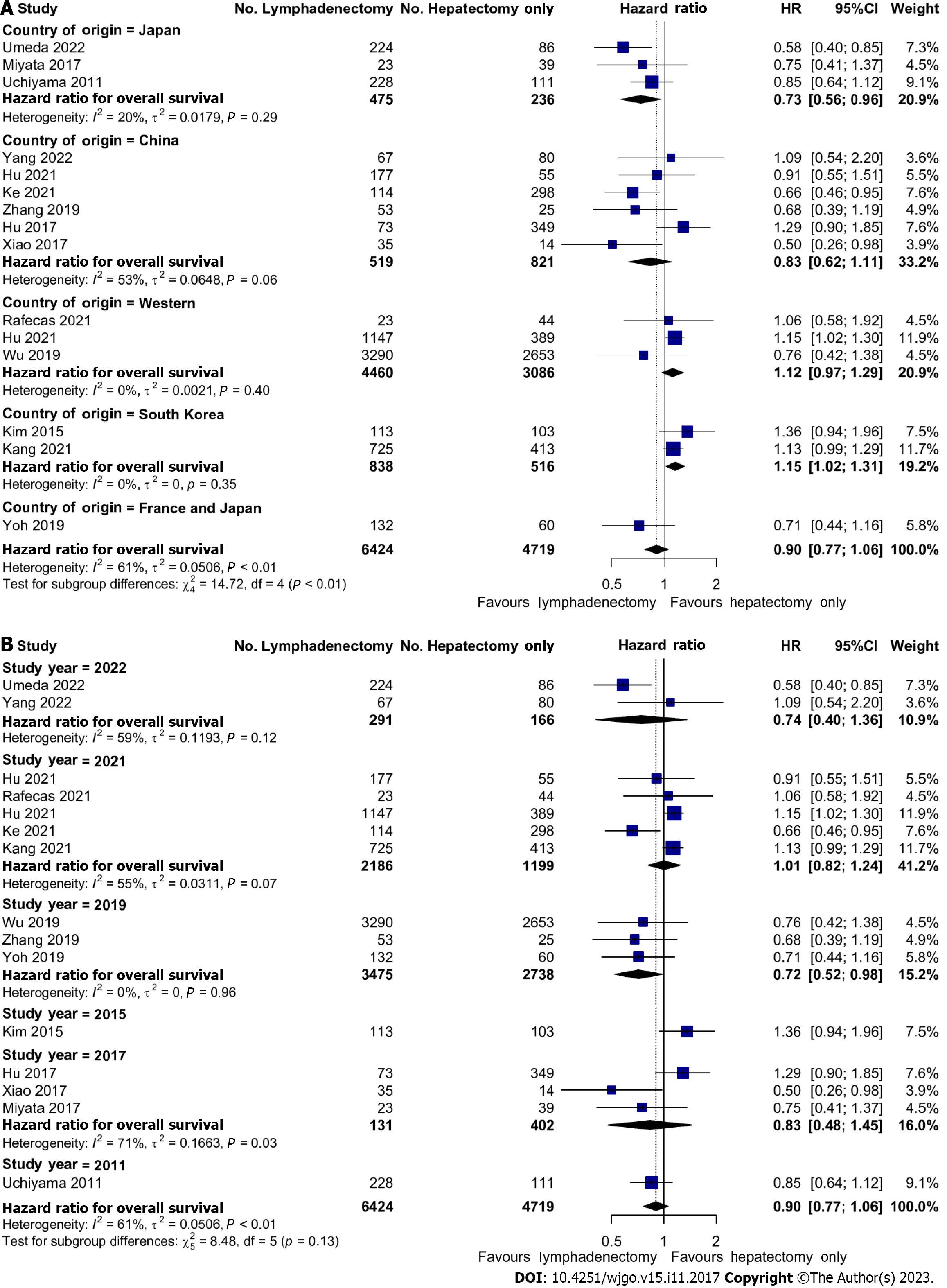
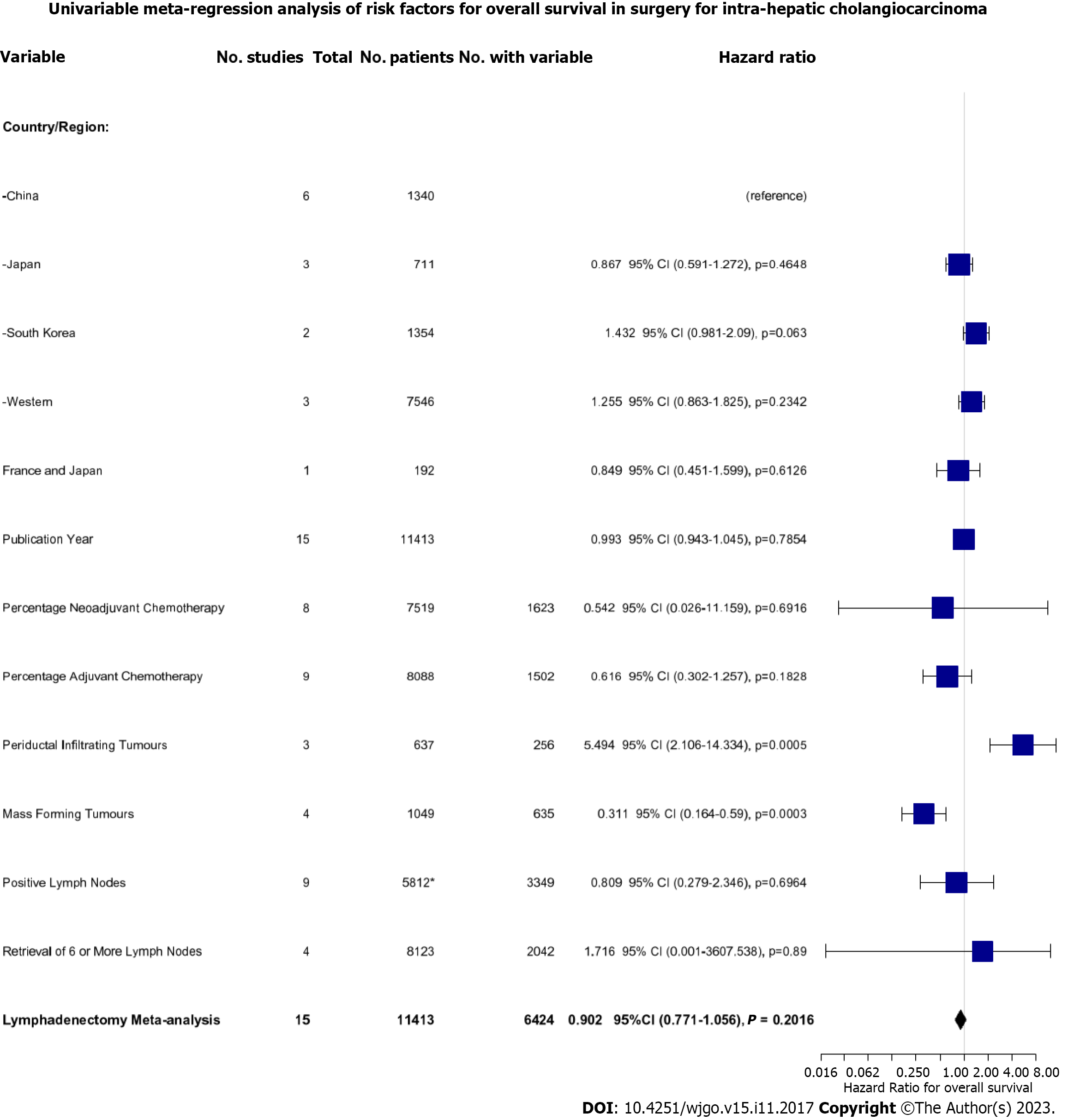
In a series of sub-group analyses, we studied the role of geographical region and time in the effects of LND on OS. Three studies reporting on 7546 (66.1%) patients were conducted on Western populations whilst the remainder of the studies were performed on Japanese, South Korean, or Chinese patients. In Japanese patients, there was improved OS with LND; HR: 0.73 (95%CI: 0.56-0.96). Conversely, in the two South Korean studies, there was reduced OS with HR 1.15 (95%CI: 1.02-1.31). There were no significant associations with OS in Chinese or Western studies (Figure 4A). There were also no significant associations with OS and the year of publication (Figure 4B).
To dissect the role of LND further, we performed a univariate meta-regression analysis based on a range of key patient- and tumour-related factors (Figure 5). Firstly, we found that performing LND in the Korean population demonstrated only a trend towards worse survival compared to the reference Chinese population (HR: 1.432, 95%CI: 0.981-1.272, P = 0.063). Secondly, we found that concomitant neoadjuvant and/or adjuvant chemotherapy with LND did not improve OS. Finally, we found that the effect of LND differed depending on tumour morphology. Resection of periductal infiltrating tumours was associated with significantly worsened OS (HR: 5.494, 95%CI: 2.106-14.334, P = 0.0005) whereas resection of mass-forming tumours was associated with improved OS (HR: 0.311, 95%CI: 0.164-0.590, P = 0.0003).
Neither the presence of positive LNs at lymphadenectomy (HR: 0.809, 95%CI: 0.279-2.346, P = 0.700) nor retrieval of > 6 LNs was associated with a difference in survival (HR: 1.716, 95%CI: 0.001-3607, P = 0.89). The latter was only reported in 4 studies, with high heterogeneity (I2 = 61%), however, meaning this estimate is likely to be inaccurate.
Few studies reported on operative complications of hepatectomy with LND. The most consistently reported complication was intra-operative blood loss, in 5 studies (Table 1). However, this was not reported in a consistent form to allow for meta-analysis. In general, those studies that reported intra-operative blood loss found significantly higher rates of blood loss with LND.
| Ref. | Country | Design | LND (n) | Hepatectomy only (n) | Lymph nodes retrieved (n) | Percentage of LND patients with ≥ 6 nodes retrieved | Percentage of LND patients with positive lymph nodes | Outcomes | Estimate of hazard ratio for overall survival (95%CI) | Blood loss LND (ml) | Blood loss hepatectomy only |
| Uchiyama et al[38], 2011 | Japan | Retrospective cohort | 228 | 111 | - | - | 61 | Neoadjuvant, adjuvant, tumour morphology | 0.85 (0.64-1.12) | - | - |
| Kim et al[39], 2015 | South Korea | Retrospective cohort | 113 | 103 | - | - | Disease free survival, neoadjuvant, adjuvant, tumour Morphology | - | - | ||
| 1.36 (0.94-1.96) | |||||||||||
| Hu et al[40], 2017 | China | Retrospective cohort | 73 | 349 | - | - | Disease free survival, adjuvant | 1.29 (0.90-1.85) | - | - | |
| Miyata et al[41], 2017 | Japan | Retrospective cohort | 23 | 39 | - | - | 26.1 | Disease free survival, tumour morphology, lymph node positivity | 0.75 (0.41-1.37) | Mean: 765 ± 116 | Mean: 552 ± 88 |
| Xiao et al[42], 2017 | China | Retrospective cohort | 35 | 14 | - | - | Tumour morphology | 0.50 (0.26-0.98) | - | - | |
| Wu et al[43], 2019 | United States | Retrospective cohort | 3290 | 2653 | - | 23.8 | 72.5 | Neoadjuvant, adjuvant, lymph node metastasis, number of lymph Nodes harvested | 0.76 (0.42-1.38) | - | - |
| Yoh et al[44], 2019 | France and Japan | Retrospective cohort | 132 | 60 | - | - | 16.7 | Disease free survival, neoadjuvant, lymph node metastasis | 0.71 (0.44-1.16) | - | - |
| Zhang et al[24], 2021 | China | Retrospective cohort | 53 | 25 | - | - | Disease free survival | 0.68 (0.39-1.19) | - | - | |
| Hu et al[46], 2021 | China | Retrospective cohort | 177 | 55 | - | 37.3 | 40.1 | Disease free survival, neoadjuvant, adjuvant, lymph node metastasis, number of lymph nodes harvested | 0.91 (0.55-1.51) | Percentage > 300 ml: 52% | Percentage > 300 ml: 30.9% |
| Hu et al[45], 2021 | United States | Retrospective cohort | 1147 | 389 | - | 27.6 | 33.9 | Lymph node metastasis, number of lymph nodes harvested | 1.15 (1.02-1.30) | ||
| Kang et al[47], 2021 | South Korea | Retrospective cohort | 725 | 413 | Mean: 5.9 (SD: 8.3) | - | 40.4 | Disease free survival, lymph node metastasis | 1.13 (0.99-1.29) | ||
| Ke et al[48], 2021 | China | Retrospective cohort | 114 | 298 | Median: 3.5 (IQR: 1–39) | 28.3 | Neoadjuvant, adjuvant, tumour morphology, number of lymph nodes harvested | 0.66 (0.46-0.95) | Percentage > 400 ml: 25.5% | Percentage > 400 ml: 3.6% | |
| Rafecas et al[49], 2021 | Spain | Retrospective cohort | 23 | 44 | - | - | 43.4 | Neoadjuvant, adjuvant, lymph node metastasis | 1.06 (0.58-1.92) | ||
| Umeda et al[50], 2022 | Japan | Retrospective cohort | 224 | 86 | - | - | Adjuvant, lymph node metastasis | 0.58 (0.40-0.85) | Median: 820 (IQR: 978) | Median: 525 (IQR: 773) | |
| Yang et al[51], 2022 | China | Retrospective cohort | 67 | 80 | Mean: 5 (Range: 3-9) | - | Neoadjuvant, adjuvant, lymph node metastasis | 1.09 (0.54-2.20) | Median: 200.0 (200.0-400.0) | Median: 200.0 (100.0-400.0) |
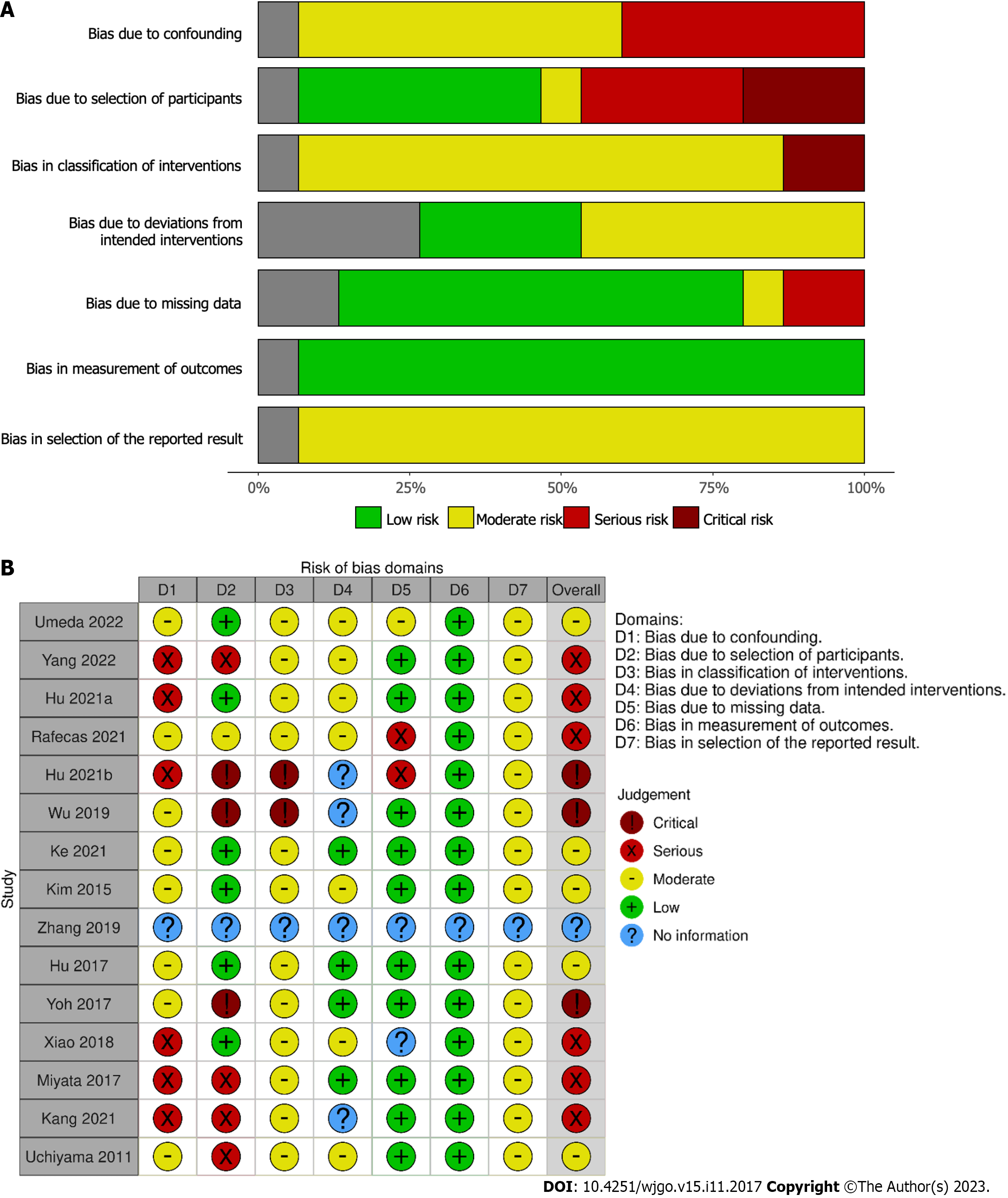
The majority of studies were assessed as having serious or critical risk of bias (Figure 6) using the ROBINS-I tool; this is predominantly due to the retrospective nature of all of the studies included and the lack of confounding information such as the use of neoadjuvant therapy and selection of LND based on pre-operative imaging suggesting lymphadenopathy.
Improving the OS and DFS of patients post-resection for ICCA remains a clinical challenge[51]. This systematic review and meta-analysis of 15 studies comprising 11413 patients demonstrated that performing LND for surgically-resectable ICCA did not significantly improve OS or DFS, overall. In sub-group analyses, however, the effect of LND on OS showed a degree of variability by geographical region, most characteristic of which was a positive effect in Japanese patients and conversely, a negative effect in South Korean studies. Furthermore, in sub-group analyses, OS was neither impacted by time nor concomitant chemotherapy. Importantly, tumour morphology was associated with divergent effects on OS, with the resection of mass-forming tumours being associated with improved OS compared to the resection of periductal infiltrating tumours.
Routine lymph node evaluation (radiological and/or pathological) is important in staging gastrointestinal, biliary, and hepatic cancers to inform subsequent treatment[20,51]. In ICCA, the presence of lymph node metastasis/es is both a reliable sign of malignancy dissemination and an established indicator of poorer prognosis[20,52]. An expert consensus statement from the AHPBA recommended that regional lymphadenectomy be “considered a standard part” of the resection procedure[20]. In comparison, the AJCC cancer staging guidelines also recommend harvesting a minimum of 6 regional LNs for completion of staging[5].
Similarly to ours, a previous systematic review of 1377 patients found no difference in OS between the LND and non-LND groups[53]. This was regardless of subsequent identification of lymph node metastases. Concerningly, the LND group had increased postoperative morbidity (odds ratio 2.67 LND vs non-LND.) A key contributor to this outcome was one study reporting that wound infections were significantly increased in the LND cohort with cirrhosis[2]. Such morbidity was also identified in another cohort where those undergoing LND were significantly more likely to undergo a longer operation, concomitant bile duct resection, and develop a complication requiring an invasive intervention to manage ( Clavien-Dindo III)[31]. Whilst the AJCC guidelines recommend evaluating at least 6 LNs for staging, our meta-analysis demonstrated that many studies reported the extent of the LND as “local”, “regional”[31,39,43,45,48,49], or “extended”[38] instead. In those studies, with the available quantity data, comparing > 6 vs < 6 LNs dissected was associated with no difference in overall or DFS[40,44-46,50]. In comparison, one multicentre retrospective Chinese study with 380 patients found improved OS in the LND group (HR: 0.66 95%CI: 0.46-0.95)[47]. This group had an average of 3.5 LNs resected. The same was also concluded in two further retrospective studies comprising of French, Japanese, and Chinese cohorts with improved OS and DFS post-LND[41,43]. However, these two studies did not quantify the mean/routine number of LNs resected or evaluated.
Despite this, our data suggests that the decisive parameter is not the quantity of LNs dissected but rather the identification of LNs with metastatic disease. Of the LNs evaluated, the incidence of metastases was 27.7% overall (range 14.9%-42.5%)[31,38,42-46,48,50]. In that regard, several studies report that regardless of how many LNs are resected, the subsequent identification of LN metastasis/es was associated with poorer patient survival[31,38,42-46,50]. Evaluating > 6 LNs has been associated with improved prognosis, especially for patients with node-negative disease[11,42]. Hence, one may consider it useful to perform routine LND to stratify those with node-negative disease and those with LN metastasis/es (and refer them for adjuvant chemotherapy or close surveillance for the development of recurrence).
Our finding that chemotherapy does not improve OS broadly reflects the available literature. In a Cochrane review, adjuvant chemotherapy was not associated with improved survival post-resection[16]. However, this work included only 5 trials with heterogeneity in design and outcomes reporting. In comparison, a meta-analysis involving 5060 patients, found adjuvant chemotherapy to be associated with improved OS instead[30]. This in particular favoured intravenous administration and a gemcitabine-based regime. With regards to neoadjuvant chemotherapy, very few patients presently receive it so a clear effect can only be identified through collating larger registry cohorts[54].
Although we found no difference overall in long-term survival between the Eastern and Western populations, it is notable that only a small proportion of the included studies originated from Asia[11]. Routine LND is also less commonly performed in that region.
Our review has some limitations. Firstly, the high level of heterogeneity between the included studies suggests a wide variation of the observed effect of LND. Not all studies defined the exact quantity of LNs dissected. This is important as the AJCC guidelines recommend the removal of at least 6 to improve the identification of potential metastases[5]. The included studies also had no inter-centre standardisation of local oncological and surgical protocols (e.g., provision of chemotherapy regimens, operative technique, patient selection). The retrospective nature of many studies may demonstrate underlying selection bias as well. Furthermore, the differences in reporting of outcomes were the key reason for excluding several studies from our regression analysis. For example, some studies reported a lymph node ratio instead of the number of LNs dissected. The AJCC TNM staging system recommends removing at least 6 LNs to accurately stage ICCA[5], which is not met if LND is insufficiently performed.
Finally, it was not possible to reliably assess for interactions in important variables in the regression analysis, such as the presence of lymph node positivity and adjuvant therapy and whether this improved survival in this group. This was due to the small number of studies that reported both such variables. For the same reason, it was not possible to reliably perform multivariable regression, and only univariable regression was conducted. Similarly, no regression or sub-group analysis was performed for DFS as there were significantly fewer studies reporting this.
Overall, the results of our meta-analysis show that LND does not improve OS or DFS in patients with ICCA. However, a significant proportion of patients undergoing LND are found to have LN metastases, suggesting that LND may result in a more accurate staging. Consequently, it may be beneficial for prognostication and stratification of patients to guide adjuvant treatments, a factor that may become more important in the future, should more effective chemotherapeutic agents be discovered for this type of cancer, ultimately leading to survival benefit. Notably, in high-risk patients unlikely to benefit from further treatments, the current evidence would not support the performance of LND as a standard part of the surgical resection of ICCA. There is an urgent clinical need for higher-quality studies to dissect the role of LND further.
Intraoperative lymph node dissection (LND) is increasingly being performed alongside hepatic resection for intrahepatic cholangiocarcinoma (ICCA) to more accurately stage the disease, reduce recurrence, and improve overall survival (OS). While this procedure may result in associated morbidity, there is no consensus or formal guidelines on its role in this setting.
There is a need to better delineate the evidence for performing LND alongside surgical resection of the ICCA in improving prognostication and survival post-resection of ICCA.
To compare curative intent resection of ICCA with LND vs resection without LND with the primary outcome measures of OS and disease-free survival (DFS).
A systematic review and meta-analysis was performed per the PRISMA framework and Cochrane Handbook for Systematic Reviews. A systematic literature search was performed using Pubmed, Medline, Embase, and the Cochrane Library, for all studies involving LND, ICCA, and surgical resection. All clinical studies comparing curative intent resection of ICCA with LND vs resection without LND were included from the different academic databases up till early December 2022. The primary outcome measures were set for OS and DFS. Quality assessment was conducted using the ROBINS-I tool. Data were analysed using RStudio (R 4.3.0; R Foundation, Austria) with meta and dmetar packages. Meta-analyses were conducted depending on feasibility.
In the total of harvested LNs in patients who underwent LND, the incidence of metastases was 27.7%. LND did not significantly improve OS and DFS in patients undergoing resection, however, there may be a trend toward improved OS. The effect of LND on OS showed a degree of variability by geographical region, in Eastern and Western countries. Concomitant neoadjuvant and/or adjuvant chemotherapy with LND did not improve OS. The effect of LND differed depending on tumour morphology with resection of periductal infiltrating tumours being associated with significantly worsened OS and resection of mass-forming tumours with improved OS. Positive lymph nodes (LNs) at lymphadenectomy or retrieval of > 6 LNs were not associated with a difference in survival.
Overall, the results of this meta-analysis show that LND does not improve OS or DFS in patients with ICCA. However, a significant proportion of patients undergoing LND are found to have LN metastases, suggesting that LND may result in a more accurate staging. Consequently, it may be beneficial for prognostication and stratification of patients to guide adjuvant treatments. Notably, in high-risk patients unlikely to benefit from further treatments, the current evidence would not support the performance of LND as a standard part of the surgical resection of ICCA.
The fact that LND may result in a more accurate staging and consequently aid the prognostication and stratification of patients to guide adjuvant treatments, may become more important in the future, should more effective chemotherapeutic agents be discovered for this type of cancer, ultimately leading to survival benefit.
There is an urgent clinical need for higher-quality studies to dissect the role of LND further.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: General Medical Council (United Kingdom), No. 7451513; Royal College of Surgeons of England, No. 9092145; American College of Surgeons, No. 03340060; International College of Surgeons, No. M21313; Faculty of Surgical Trainers of Edinburgh, Royal College of Surgeons of Edinburgh, No. 188646; Hellenic Surgical Society, No. 1974; Athens Medical Association, No. 061331; Institute of Clinical Research, No. 002934.
Specialty type: Surgery
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu K, China; Zeng YY, China S-Editor: Lin C L-Editor: A P-Editor: Zhang XD
| 1. | de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L, Ferrone CR, Zhu AX, Bauer TW, Walters DM, Gamblin TC, Nguyen KT, Turley R, Popescu I, Hubert C, Meyer S, Schulick RD, Choti MA, Gigot JF, Mentha G, Pawlik TM. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140-3145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 557] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 2. | Bagante F, Spolverato G, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Groot Koerkamp B, Guglielmi A, Itaru E, Ruzzenente A, Pawlik TM. Surgical Management of Intrahepatic Cholangiocarcinoma in Patients with Cirrhosis: Impact of Lymphadenectomy on Peri-Operative Outcomes. World J Surg. 2018;42:2551-2560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Sposito C, Droz Dit Busset M, Virdis M, Citterio D, Flores M, Bongini M, Niger M, Mazzaferro V. The role of lymphadenectomy in the surgical treatment of intrahepatic cholangiocarcinoma: A review. Eur J Surg Oncol. 2022;48:150-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Sposito C, Ratti F, Cucchetti A, Ardito F, Ruzzenente A, Di Sandro S, Maspero M, Ercolani G, Di Benedetto F, Guglielmi A, Giuliante F, Aldrighetti L, Mazzaferro V. Survival benefit of adequate lymphadenectomy in patients undergoing liver resection for clinically node-negative intrahepatic cholangiocarcinoma. J Hepatol. 2023;78:356-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 5. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4371] [Article Influence: 546.4] [Reference Citation Analysis (4)] |
| 6. | Borakati A, Froghi F, Bhogal RH, Mavroeidis VK. Liver transplantation in the management of cholangiocarcinoma: Evolution and contemporary advances. World J Gastroenterol. 2023;29:1969-1981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Saffioti F, Mavroeidis VK. Review of incidence and outcomes of treatment of cholangiocarcinoma in patients with primary sclerosing cholangitis. World J Gastrointest Oncol. 2021;13:1336-1366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Borakati A, Froghi F, Bhogal RH, Mavroeidis VK. Stereotactic radiotherapy for intrahepatic cholangiocarcinoma. World J Gastrointest Oncol. 2022;14:1478-1489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Krenzien F, Nevermann N, Krombholz A, Benzing C, Haber P, Fehrenbach U, Lurje G, Pelzer U, Pratschke J, Schmelzle M, Schöning W. Treatment of Intrahepatic Cholangiocarcinoma-A Multidisciplinary Approach. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 10. | Altman AM, Kizy S, Marmor S, Huang JL, Denbo JW, Jensen EH. Current survival and treatment trends for surgically resected intrahepatic cholangiocarcinoma in the United States. J Gastrointest Oncol. 2018;9:942-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Zhang XF, Chakedis J, Bagante F, Chen Q, Beal EW, Lv Y, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Groot Koerkamp B, Guglielmi A, Itaru E, Pawlik TM. Trends in use of lymphadenectomy in surgery with curative intent for intrahepatic cholangiocarcinoma. Br J Surg. 2018;105:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 12. | Zhang XF, Chen Q, Kimbrough CW, Beal EW, Lv Y, Chakedis J, Dillhoff M, Schmidt C, Cloyd J, Pawlik TM. Lymphadenectomy for Intrahepatic Cholangiocarcinoma: Has Nodal Evaluation Been Increasingly Adopted by Surgeons over Time?A National Database Analysis. J Gastrointest Surg. 2018;22:668-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 13. | Liu J, Zhong M, Feng Y, Zeng S, Wang Y, Xu H, Zhou H. Prognostic Factors and Treatment Strategies for Intrahepatic Cholangiocarcinoma from 2004 to 2013: Population-Based SEER Analysis. Transl Oncol. 2019;12:1496-1503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Nakagawa T, Kamiyama T, Kurauchi N, Matsushita M, Nakanishi K, Kamachi H, Kudo T, Todo S. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2005;29:728-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Altman AM, Kizy S, Marmor S, Hui JYC, Tuttle TM, Jensen EH, Denbo JW. Adjuvant chemotherapy for intrahepatic cholangiocarcinoma: approaching clinical practice consensus? Hepatobiliary Surg Nutr. 2020;9:577-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Luvira V, Satitkarnmanee E, Pugkhem A, Kietpeerakool C, Lumbiganon P, Pattanittum P. Postoperative adjuvant chemotherapy for resectable cholangiocarcinoma. Cochrane Database Syst Rev. 2021;9:CD012814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Lluís N, Asbun D, Wang JJ, Cao HST, Jimenez RE, Alseidi A, Asbun H. Lymph Node Dissection in Intrahepatic Cholangiocarcinoma: a Critical and Updated Review of the Literature. J Gastrointest Surg. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 18. | Xu-Feng Zhang. Routine Lymphadenectomy for Intrahepatic Cholangiocarcinoma [accessed 2021 Dec 9]. In: ClinicalTrials.gov [Internet]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: http://clinicaltrials.gov/show/NCT03796819 ClinicalTrials.gov Identifier: NCT03796819. |
| 19. | Ren T, Li YS, Dang XY, Li Y, Shao ZY, Bao RF, Shu YJ, Wang XA, Wu WG, Wu XS, Li ML, Cao H, Wang KH, Cai HY, Jin C, Jin HH, Yang B, Jiang XQ, Gu JF, Cui YF, Zhang ZY, Zhu CF, Sun B, Dai CL, Zheng LH, Cao JY, Fei ZW, Liu CJ, Li B, Liu J, Qian YB, Wang Y, Hua YW, Zhang X, Liu C, Lau WY, Liu YB. Prognostic significance of regional lymphadenectomy in T1b gallbladder cancer: Results from 24 hospitals in China. World J Gastrointest Surg. 2021;13:176-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Weber SM, Ribero D, O'Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015;17:669-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 339] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 21. | Zhang XF, Lv Y, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Koerkamp BG, Itaru E, Pawlik TM. Should Utilization of Lymphadenectomy Vary According to Morphologic Subtype of Intrahepatic Cholangiocarcinoma? Ann Surg Oncol. 2019;26:2242-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Aziz H, Pawlik TM. We Asked the Experts: Role of Lymphadenectomy in Surgical Management of Intrahepatic Cholangiocarcinoma. World J Surg. 2023;47:1530-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Li F, Jiang Y, Jiang L, Li Q, Yan X, Huang S, Chen J, Yuan S, Fu Y, Liu J. Effect of lymph node resection on prognosis of resectable intrahepatic cholangiocarcinoma: A systematic review and meta-analysis. Front Oncol. 2022;12:957792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Zhang XF, Xue F, Dong DH, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, Pulitano C, Bauer TW, Shen F, Poultsides GA, Soubrane O, Martel G, Koerkamp BG, Itaru E, Lv Y, Pawlik TM. Number and Station of Lymph Node Metastasis After Curative-intent Resection of Intrahepatic Cholangiocarcinoma Impact Prognosis. Ann Surg. 2021;274:e1187-e1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 25. | Miyazaki M, Ohtsuka M, Miyakawa S, Nagino M, Yamamoto M, Kokudo N, Sano K, Endo I, Unno M, Chijiiwa K, Horiguchi A, Kinoshita H, Oka M, Kubota K, Sugiyama M, Uemoto S, Shimada M, Suzuki Y, Inui K, Tazuma S, Furuse J, Yanagisawa A, Nakanuma Y, Kijima H, Takada T. Classification of biliary tract cancers established by the Japanese Society of Hepato-Biliary-Pancreatic Surgery: 3(rd) English edition. J Hepatobiliary Pancreat Sci. 2015;22:181-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 26. | Kim SH, Han DH, Choi GH, Choi JS, Kim KS. Extent of Lymph Node Dissection for Accurate Staging in Intrahepatic Cholangiocarcinoma. J Gastrointest Surg. 2022;26:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | European Association for the Study of the Liver. EASL-ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma. J Hepatol. 2023;79:181-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 124] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 28. | Benson AB, D'Angelica MI, Abrams T, Abbott DE, Ahmed A, Anaya DA, Anders R, Are C, Bachini M, Binder D, Borad M, Bowlus C, Brown D, Burgoyne A, Castellanos J, Chahal P, Cloyd J, Covey AM, Glazer ES, Hawkins WG, Iyer R, Jacob R, Jennings L, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Ronnekleiv-Kelly S, Sahai V, Singh G, Stein S, Turk A, Vauthey JN, Venook AP, Yopp A, McMillian N, Schonfeld R, Hochstetler C. NCCN Guidelines® Insights: Biliary Tract Cancers, Version 2.2023. J Natl Compr Canc Netw. 2023;21:694-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 90] [Reference Citation Analysis (0)] |
| 29. | Kubo S, Shinkawa H, Asaoka Y, Ioka T, Igaki H, Izumi N, Itoi T, Unno M, Ohtsuka M, Okusaka T, Kadoya M, Kudo M, Kumada T, Kokudo N, Sakamoto M, Sakamoto Y, Sakurai H, Takayama T, Nakashima O, Nagata Y, Hatano E, Harada K, Murakami T, Yamamoto M. Liver Cancer Study Group of Japan Clinical Practice Guidelines for Intrahepatic Cholangiocarcinoma. Liver Cancer. 2022;11:290-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 30. | Ma KW, Cheung TT, Leung B, She BWH, Chok KSH, Chan ACY, Dai WC, Lo CM. Adjuvant chemotherapy improves oncological outcomes of resectable intrahepatic cholangiocarcinoma: A meta-analysis. Medicine (Baltimore). 2019;98:e14013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Miyata T, Yamashita YI, Yamao T, Umezaki N, Tsukamoto M, Kitano Y, Yamamura K, Arima K, Kaida T, Nakagawa S, Imai K, Hashimoto D, Chikamoto A, Ishiko T, Baba H. Clinical Benefits of Lymph Node Dissection in Intrahepatic Cholangiocarcinoma: A Retrospective Single-institution Study. Anticancer Res. 2017;37:2673-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Cheng H, Clymer JW, Po-Han Chen B, Sadeghirad B, Ferko NC, Cameron CG, Hinoul P. Prolonged operative duration is associated with complications: a systematic review and meta-analysis. J Surg Res. 2018;229:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 522] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 33. | Kim SH, Han DH, Choi GH, Choi JS, Kim KS. Oncologic Impact of Lymph Node Dissection for Intrahepatic Cholangiocarcinoma: a Propensity Score-Matched Study. J Gastrointest Surg. 2019;23:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 34. | Lee AJ, Chun YS. Intrahepatic cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin Oncol. 2018;7:52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 35. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 39485] [Article Influence: 9871.3] [Reference Citation Analysis (2)] |
| 36. | Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.3. Cochrane. Dec 2022. Available from: https://training.cochrane.org/handbook/.. |
| 37. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 4941] [Article Influence: 274.5] [Reference Citation Analysis (0)] |
| 38. | Uchiyama K, Yamamoto M, Yamaue H, Ariizumi S, Aoki T, Kokudo N, Ebata T, Nagino M, Ohtsuka M, Miyazaki M, Tanaka E, Kondo S, Uenishi T, Kubo S, Yoshida H, Unno M, Imura S, Shimada M, Ueno M, Takada T. Impact of nodal involvement on surgical outcomes of intrahepatic cholangiocarcinoma: a multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci. 2011;18:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Kim DH, Choi DW, Choi SH, Heo JS, Kow AW. Is there a role for systematic hepatic pedicle lymphadenectomy in intrahepatic cholangiocarcinoma? A review of 17 years of experience in a tertiary institution. Surgery. 2015;157:666-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Hu J, Chen FY, Zhou KQ, Zhou C, Cao Y, Sun HC, Fan J, Zhou J, Wang Z. Intrahepatic cholangiocarcinoma patients without indications of lymph node metastasis not benefit from lymph node dissection. Oncotarget. 2017;8:113817-113827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Xiao J, Zhu J, Liu Z, Wan R, Li Y, Xiao W. Role of surgical treatment for hepatolithiasis-associated intrahepatic cholangiocarcinoma: A retrospective study in a single institution. J Cancer Res Ther. 2017;13:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Wu L, Tsilimigras DI, Paredes AZ, Mehta R, Hyer JM, Merath K, Sahara K, Bagante F, Beal EW, Shen F, Pawlik TM. Trends in the Incidence, Treatment and Outcomes of Patients with Intrahepatic Cholangiocarcinoma in the USA: Facility Type is Associated with Margin Status, Use of Lymphadenectomy and Overall Survival. World J Surg. 2019;43:1777-1787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 43. | Yoh T, Cauchy F, Le Roy B, Seo S, Taura K, Hobeika C, Dokmak S, Farges O, Gelli M, Sa Cunha A, Adam R, Uemoto S, Soubrane O. Prognostic value of lymphadenectomy for long-term outcomes in node-negative intrahepatic cholangiocarcinoma: A multicenter study. Surgery. 2019;166:975-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 44. | Hu H, Zhao H, Cai J. The role of lymph node dissection and a new N-staging system for intrahepatic cholangiocarcinoma: a study from the SEER database. J Int Med Res. 2021;49:3000605211012209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Hu H, Xu G, Du S, Luo Z, Zhao H, Cai J. The role of lymph node dissection in intrahepatic cholangiocarcinoma: a multicenter retrospective study. BMC Surg. 2021;21:359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 46. | Kang CM, Suh KS, Yi NJ, Hong TH, Park SJ, Ahn KS, Hayashi H, Choi SB, Jeong CY, Takahara T, Shiozaki S, Roh YH, Yu HC, Fukumoto T, Matsuyama R, Naoki U, Hashida K, Seo HI, Okabayashi T, Kitajima T, Satoi S, Nagano H, Kim H, Taira K, Kubo S, Choi DW. Should Lymph Nodes Be Retrieved in Patients with Intrahepatic Cholangiocarcinoma? A Collaborative Korea-Japan Study. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Ke Q, Wang L, Lin Z, Lou J, Zheng S, Bi X, Wang J, Guo W, Li F, Zheng Y, Li J, Cheng S, Zhou W, Zeng Y. Prognostic Value of Lymph Node Dissection for Intrahepatic Cholangiocarcinoma Patients With Clinically Negative Lymph Node Metastasis: A Multi-Center Study From China. Front Oncol. 2021;11:585808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Rafecas A, Torras J, Fabregat J, Lladó L, Secanella L, Busquets J, Serrano T, Ramos E. Intrahepatic cholangiocarcinoma: Prognostic factors for recurrence and survival in a series of 67 patients treated surgically at a single center. Cir Esp (Engl Ed). 2021;99:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Umeda Y, Mitsuhashi T, Kojima T, Satoh D, Sui K, Endo Y, Inagaki M, Oishi M, Yagi T, Fujiwara T. Impact of lymph node dissection on clinical outcomes of intrahepatic cholangiocarcinoma: Inverse probability of treatment weighting with survival analysis. J Hepatobiliary Pancreat Sci. 2022;29:217-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Yang F, Wu C, Bo Z, Xu J, Yi B, Li J, Qiu Y. The clinical value of regional lymphadenectomy for intrahepatic cholangiocarcinoma. Asian J Surg. 2022;45:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 51. | Cloyd JM, Ejaz A, Pawlik TM. The Landmark Series: Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2020;27:2859-2865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Ercolani G, Grazi GL, Ravaioli M, Grigioni WF, Cescon M, Gardini A, Del Gaudio M, Cavallari A. The role of lymphadenectomy for liver tumors: further considerations on the appropriateness of treatment strategy. Ann Surg. 2004;239:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 53. | Zhou R, Lu D, Li W, Tan W, Zhu S, Chen X, Min J, Shang C, Chen Y. Is lymph node dissection necessary for resectable intrahepatic cholangiocarcinoma? A systematic review and meta-analysis. HPB (Oxford). 2019;21:784-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 54. | Miura JT, Johnston FM, Tsai S, George B, Thomas J, Eastwood D, Banerjee A, Christians KK, Turaga KK, Pawlik TM, Clark Gamblin T. Chemotherapy for Surgically Resected Intrahepatic Cholangiocarcinoma. Ann Surg Oncol. 2015;22:3716-3723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |