Published online Nov 15, 2023. doi: 10.4251/wjgo.v15.i11.1900
Peer-review started: May 21, 2023
First decision: August 15, 2023
Revised: September 14, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 15, 2023
Processing time: 177 Days and 19.7 Hours
A well-recognized class effect of immune checkpoint inhibitors (ICI) is immune-related adverse events (IrAEs) ranging from low grade toxicities to life-threatening end organ damage requiring permanent discontinuation of ICI. Deaths are reported in < 5% of patients treated with ICI. There are, however, no reliable markers to predict the onset and severity of IrAEs. We tested the association between neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) at baseline with development of clinically significant IrAEs (grade ≥ 2) in hepatocellular carcinoma (HCC) patients treated with ICI.
To test the association between NLR and PLR at baseline with development of clinically significant IrAEs (grade ≥ 2) in HCC patients treated with ICI.
Data was extracted from an international database from a consortium of 11 tertiary-care referral centers. NLR = absolute neutrophil count/absolute lymphocyte count (ALC) and PLR = platelet count/ALC. Cutoff of 5 was used for NLR and 300 for PLR based on literature. We also tested the association between antibiotic and steroid exposure to IrAEs.
Data was collected from 361 patients treated between 2016-2020 across the United States (67%), Asia (14%) and Europe (19%). Most patients received Nivolumab (n = 255, 71%). One hundred sixty-seven (46%) patients developed at least one IrAE, highest grade 1 in 80 (48%), grade ≥ 2 in 87 (52%) patients. In a univariable regression model PLR > 300 was significantly associated with a lower incidence of grade ≥ 2 IrAEs (OR = 0.40; P = 0.044). Similarly, a trend was observed between NLR > 5 and lower incidence of grade ≥ 2 IrAEs (OR = 0.58; P = 0.097). Multivariate analyses confirmed PLR > 300 as an independent predictive marker of grade ≥ 2 IrAEs (OR = 0.26; P = 0.011), in addition to treatment with programmed cell death ligand 1 (PD-1)/cytotoxic T lymphocyte-associated protein-4 (OR = 2.57; P = 0.037) and PD-1/tyrosine kinase inhibitor (OR = 3.39; P = 0.01) combinations. Antibiotic use was not associated with IrAE incidence (OR = 1.02; P = 0.954). Patients treated with steroids had a > 2-fold higher incidence of grade ≥ 2 IrAEs (OR = 2.74; P < 0.001), although 74% were prescribed steroids for the treatment of IrAEs.
Given that high baseline NLR and PLR are associated with a decreased incidence of IrAEs, lower baseline NLR and PLR may be predictive biomarkers for the appearance of IrAEs in HCC treated with ICI. This finding is in keeping with several studies in solid tumors that have shown that baseline NLR and PLR appear predictive of IrAEs.
Core Tip: In this study of hepatocellular carcinoma (HCC) patients treated with immune checkpoint inhibitors (ICI), the association between two biomarkers, neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR), and immune-related adverse events (IrAEs) was examined. Data from 361 patients showed that a higher PLR (> 300) was significantly associated with a lower incidence of grade ≥ 2 IrAEs. A trend was observed between a higher NLR (> 5) and lower incidence of grade ≥ 2 IrAEs. Multivariate analyses confirmed PLR as an independent predictive marker for grade ≥ 2 IrAEs. These findings suggest that NLR and PLR could be potential predictive markers for IrAEs in HCC patients receiving ICI treatment.
- Citation: Dharmapuri S, Özbek U, Jethra H, Jun T, Marron TU, Saeed A, Huang YH, Muzaffar M, Pinter M, Balcar L, Fulgenzi C, Amara S, Weinmann A, Personeni N, Scheiner B, Pressiani T, Navaid M, Bengsch B, Paul S, Khan U, Bettinger D, Nishida N, Mohamed YI, Vogel A, Gampa A, Korolewicz J, Cammarota A, Kaseb A, Galle PR, Pillai A, Wang YH, Cortellini A, Kudo M, D’Alessio A, Rimassa L, Pinato DJ, Ang C. Baseline neutrophil-lymphocyte ratio and platelet-lymphocyte ratio appear predictive of immune treatment related toxicity in hepatocellular carcinoma. World J Gastrointest Oncol 2023; 15(11): 1900-1912
- URL: https://www.wjgnet.com/1948-5204/full/v15/i11/1900.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i11.1900
Hepatocellular carcinoma (HCC) arises in a precancerous milieu of chronic inflammation heralded by oncoviral infection, alcohol consumption or steatohepatitis in up to 90% of patients[1-3]. Systemic inflammatory response driven by pro-inflammatory cytokines such as vascular endothelial growth factor (VEGF), matrix metalloproteinases and interleukin-18 plays a pivotal role in oncogenesis as well as tumor progression and metastasis[3-5].
Immune checkpoint inhibitors (ICI) and their combinations can induce robust and durable anti-tumor responses in a subset of patients with advanced Hepatocellular Carcinoma (aHCC). The IMbrave-150 and HIMALAYA trials both showed significantly improved progression free and overall survival with ICI combinations compared to sorafenib, making atezolizumab/bevacizumab and durvalumab/tremelimumab standard front-line options[6]. ICI monotherapies such as nivolumab and pembrolizumab remain an integral part of the treatment paradigm owing to their safety, tolerability and improved quality of life over tyrosine kinase inhibitors[6-9]. With the advent of ICI as the mainstay of treatment of aHCC, considerable interest has been generated towards studying the relationship between inflammation and its impact on outcomes of patients treated with ICIs.
A well-recognized class effect of these drugs is immune treatment-related adverse events (IrAEs) ranging from low grade toxicities managed supportively with or without steroids or other immunosuppressive agents to potentially life-threatening end organ damage requiring hospitalization and permanent discontinuation of ICI. Treatment related deaths are reported in less than 5% of patients treated with ICIs [Yervoy® (ipilimumab) prescribing information. Bristol-Myers Squibb Company. Princeton, NJ, United States (2013)][10-12]. At present, there are no reliable markers to predict the onset and severity of IrAEs. This study was undertaken with the objective of examining the relationship between inflammatory ratios such as neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) with the incidence of IrAEs in aHCC patients treated with ICIs. In addition, given recent data demonstrating that steroids can modulate NLR[13] and that antibiotics may increase the risk of IrAEs[14,15], we also aimed to explore the interaction between these drugs, NLR, PLR and IrAEs.
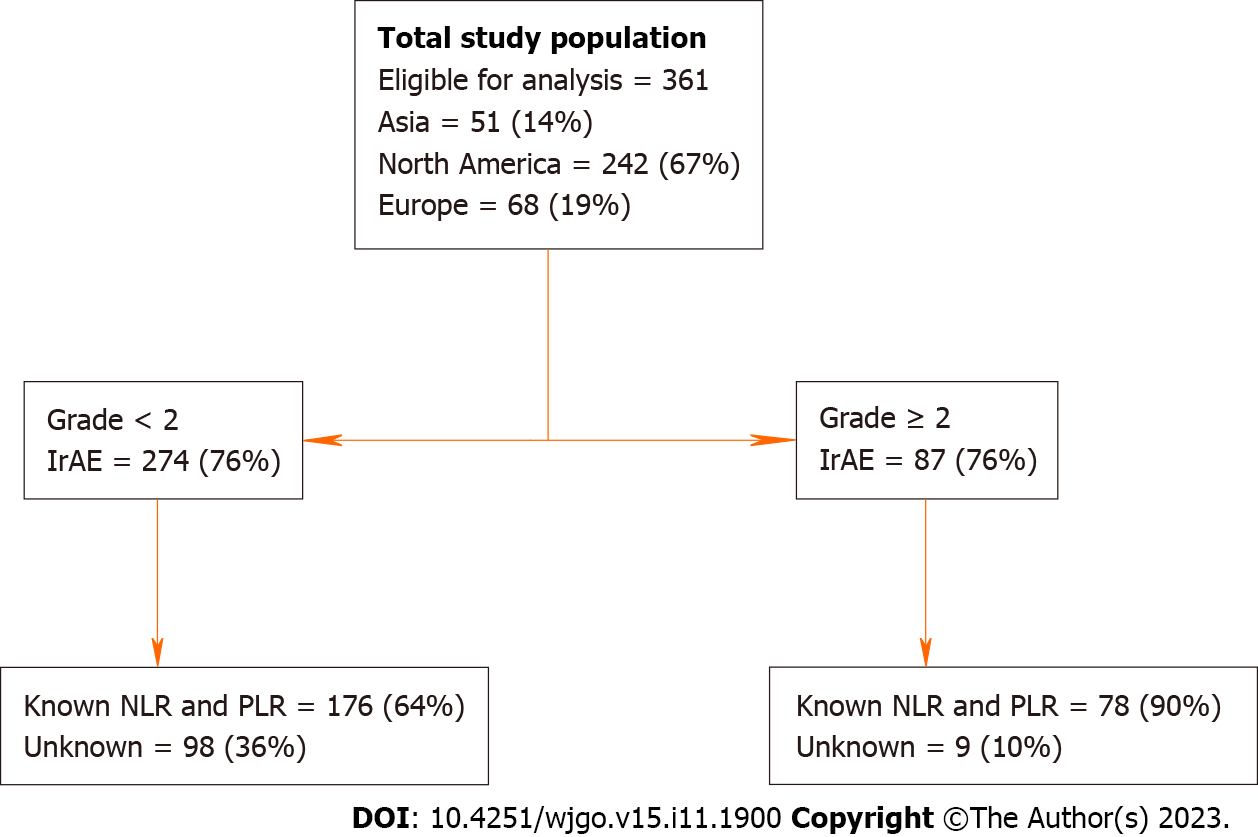
Data was extracted from a large international database from a consortium of 11 tertiary-care referral centers located in the United States, Europe and Asia. To be eligible patients had to have: (1) A diagnosis of HCC made by histopathology or imaging criteria according to international guidelines; (2) be candidate for ICI monotherapy or combinations for HCC; (3) not amenable to curative or loco-regional therapy following local multidisciplinary tumor board review, and (4) have measurable disease according to RECIST 1.1 criteria. Between June 2016 and September 2020, 427 patients were included in the database. In our final analysis we excluded 66 patients due to incomplete IrAE data (final n = 361). Of the 361 patients included in the final analysis, 242 (67%) were treated in the United States, 51 (14%) in Asia and 68 (19%) in Europe. Institutional review board approval was obtained at Mount Sinai Hospital and in each participating institution. All study-related procedures and data collection were conducted in accordance with the Declaration of Helsinki and in accordance with good clinical practice.
NLR was calculated as the ratio of absolute neutrophil count to absolute lymphocyte count (ALC), and PLR was calculated as the ratio of platelet count to ALC from blood draws at baseline. A cutoff of 5 was used for NLR groups and 300 for PLR based on literature[16-20]. Information regarding IrAEs was extracted by manual review of clinical documentation at each cycle and records of hospitalizations. Each IrAE was defined and documented per the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) v5.0 per the treating physician and validated by a member of the study group. Grade ≥ 2 IrAEs were considered clinically significant since these patients typically require close monitoring and/or initiation of corticosteroid therapy and/or permanent discontinuation of ICI, while grade 1 toxicities only require supportive measures.
Patients were considered exposed to corticosteroid if > 10 mg of prednisone (or equivalent) was administered for > 24 h within 30 d prior to or concomitantly until permanent cessation of immunotherapy. Similarly, patients were considered to have been exposed to antibiotic therapy if they were received within 30 d from starting ICI therapy and up to 30 d after cycle 1 of immunotherapy were based on literature which suggests early antibiotic exposure as a detrimental factor influencing ICI efficacy[21,22].
Descriptive statistics were calculated to summarize baseline status, including demographics, disease characteristics, and treatment characteristics. Univariable logistic models were conducted to identify associations between adverse events, inflammatory scores and other baseline characteristics. Continuous variables were presented as medians and minimum-maximum values, and categorical variables as frequency and proportions. Clinically relevant variables such as baseline alpha-fetoprotein (AFP), barcelona clinic liver cancer stage, presence of cirrhosis, child-turcotte pugh class, eastern cooperative oncology group performance status, etiology of chronic liver disease, and use of corticosteroids and antibiotics for IrAE management were considered as predictors of toxicity and were analyzed by univariable and multivariable logistic regression models. P values of less than 0.05 were considered to indicate statistical significance. All analyses were performed using R statistical package version 4.0.3 (R Core Team 2020)[23].
A total of 361 patients had documented IrAE data and were included in the final analysis, of whom 242 (67%) were treated in the United States, 51 (14%) in Asia and 68 (19%) in Europe (Figure 1). Table 1 shows the breakdown of key clinicopathologic characteristics in patients who had none or grade < 2 and grade ≥ 2 IrAEs. A majority had underlying cirrhosis (n = 263, 73%). Two hundred and seventy-six (76%) patients had child-pugh class A and 74 (20%) had class B liver function. The most common etiologies were hepatitis C virus infection in 153 (42%) patients, followed by hepatitis B virus infection in 77 (21%). Most patients received programmed cell death ligand 1 (PD-1) monotherapy (n = 305, 84%; nivolumab = 255; pembrolizumab = 20), followed by combination PD-1/cytotoxic T lymphocyte-associated protein-4 (CTLA-4) (n = 28, 8%), PD-1/tyrosine kinase inhibitor (TKI) (n = 24, 7%) and others in 4 (1%).
| Variable | Category | No AEs or AEs grade < 2 | AEs grade ≥ 2 | OR | 95%CI | P value |
| n = 361 | 274 (76)1 | 87 (24) | ||||
| Age (Median) | 65 | 64 | 0.99 | (0.97, 1.01) | 0.450 | |
| Sex | Male | 222 (61) | 65 (18) | 1.00 | ||
| Female | 52 (14) | 22 (7) | 1.44 | (0.82, 2.56) | 0.206 | |
| AFP (Median) | 115 | 29 | 1.00 | (1, 1) | 0.850 | |
| Known cirrhosis | Yes | 201 (56) | 62 (17) | 0.90 | (0.53, 1.54) | 0.702 |
| No | 73 (20) | 25 (7) | 1.00 | |||
| Known PVT | Yes | 61 (17) | 18 (5) | 0.60 | (0.33, 1.1) | 0.100 |
| No | 124 (34) | 61 (17) | 1.00 | |||
| NLR | NLR ≤ 5 | 122 (34) | 62 (17) | 1.00 | ||
| NLR > 5 | 54 (15) | 16 (5) | 0.58 | (0.31, 1.1) | 0.096 | |
| Unknown | 98 (27) | 9 (2) | ||||
| PLR | PLR ≤ 300 | 145 (40) | 72 (20) | 1.00 | ||
| PLR > 300 | 31 (9) | 6 (2) | 0.39 | (0.16, 0.98) | 0.044 | |
| Unknown | 98 (27) | 9 (2) | ||||
| Child pugh class | A | 202 (56) | 74 (20) | 1.00 | ||
| B | 61 (17) | 13 (4) | 0.58 | (0.3, 1.12) | 0.105 | |
| C | 7 (2) | 0 (0) | 0.00 | (0, Inf) | 0.986 | |
| Unknown | 4 (1) | 0 (0) | ||||
| HCV | Yes | 112 (31) | 41 (11) | 1.29 | (0.79, 2.09) | 0.305 |
| No | 162 (45) | 46 (13) | 1.00 | |||
| HBV | Yes | 59 (16) | 18 (5) | 0.95 | (0.53, 1.72) | 0.867 |
| No | 215 (60) | 69 (19) | 1.00 | |||
| EtOH | Yes | 48 (13) | 17 (5) | 1.14 | (0.62, 2.11) | 0.669 |
| No | 226 (63) | 70 (19) | 1.00 | |||
| NASH | Yes | 37 (10) | 8 (2) | 0.65 | (0.29, 1.45) | 0.292 |
| No | 237 (66) | 79 (22) | 1.00 | |||
| BCLCs | A | 9 (2) | 1 (1) | 1.00 | ||
| B | 66 (18) | 25 (7) | 3.41 | (0.41, 28.31) | 0.256 | |
| C | 194 (53) | 61 (17) | 2.83 | (0.35, 22.79) | 0.328 | |
| D | 4 (1) | 0 (0) | 0.00 | (0, Inf) | 0.985 | |
| Unknown | 1 (1) | 0 (0) | ||||
| Extra Hepatic Disease | Yes | 100 (28) | 36 (10) | 0.73 | (0.43, 1.23) | 0.239 |
| No | 87 (24) | 43 (12) | 1.00 | |||
| Unknown | 87 (24) | 8 (2) | ||||
| ECOG | 0 | 168 (47) | 77 (21) | 1.00 | ||
| 1 | 18 (5) | 2 (1) | 0.24 | (0.05, 1.07) | 0.062 | |
| Unknown | 88 (24) | 8 (2) | ||||
| Steroid use | Yes | 57 (16) | 37 (10) | 2.74 | (1.61, 4.64) | 0.0002 |
| No | 190 (53) | 45 (12) | ||||
| Unknown | 27 (7) | 5 (2) | ||||
| Antibiotic use | Yes | 42 (12) | 14 (4) | 1.01 | (0.52, 1.98) | 0.95 |
| No | 205 (56) | 67 (19) | ||||
| Unknown | 27 (7) | 6 (2) | ||||
| Treatment | PD-1 | 244 (68) | 61 (17) | |||
| PD-1/CLTA-4 | 16 (4) | 12 (3) | 3.00 | (1.35, 6.67) | 0.007 | |
| PD-1/TKI | 12 (3) | 12 (3) | 4.00 | (1.71, 9.34) | 0.001 | |
| PD-1/other | 1 (0) | 1 (0) | 4.00 | (0.25, 64.86) | 0.329 | |
| CTLA-4 | 1 (0) | 1 (0) | 4.00 | (0.25, 64.86) | 0.32 |
One hundred and sixty-seven (46%) patients were documented as having developed at least one IrAE, highest grade 1 in 80 (48%), grade 2 or higher in 87 (52 %) patients. There was one treatment related death reported. The most common IrAE was hepatitis (41%) followed by constitutional symptoms including fatigue, anorexia, chills (40%). Table 2 details the IrAE incidence by groups and severity. No significant differences in age, sex, risk factors including Ethanol, hepatitis B and C, presence of cirrhosis, portal vein thrombosis and median AFP were noted between patients who developed IrAEs of grade ≥ 2 and those who did not.
| IrAE by organ system n = 167 | Grade < 2 | Grade ≥ 2 |
| Dermatological | 43 (26) | 15 (9) |
| Colitis | 19 (11) | 10 (6) |
| Hepatitis | 40 (24) | 29 (17) |
| Endocrine | 18 (11) | 11 (7) |
| Polyarthritis | 3 (1) | 1 (0) |
| Pneumonitis | 10 (6) | 9 (5) |
| Constitutional | 52 (31) | 15 (9) |
| Other | 15 (9) | 22 (13) |
Treatment with combinations PD-1/CTLA-4 and PD-1/TKI were associated with a 3 (OR = 3; P = 0.007) and 4-fold (OR = 4; P = 0.001) higher incidence of grade ≥ 2 IrAEs over PD-1 only. In a multivariable regression analysis, treatment with both PD-1/CTLA-4 (OR = 2.57; P = 0.037) and PD-1/TKI (OR = 3.39; P = 0.01) were independently associated with incidence of grade ≥ 2 IrAEs.
NLR was ≤ 5 in 184 (51%) patients and > 5 in 70 (20%) patients. The proportion of patients with an NLR < 5 or > 5 did not differ significantly between the IrAE groups. PLR was ≤ 300 in 217 (60%) and > 300 in 37 (11%) patients (Table 1). In a univariable regression model, baseline PLR > 300 was significantly associated with a lower incidence of grade ≥ 2 IrAEs (OR = 0.40; P = 0.044). Similarly, NLR > 5 was associated with a trend toward lower incidence of grade ≥ 2 IrAEs (OR = 0.58; P = 0.097). The multivariable regression analysis showed an independent association between PLR > 300 (OR = 0.26; P = 0.011) with a lower incidence of grade 2 or higher IrAEs (Table 3).
| Odds ratio | 95%CI | P value | |
| Univariable model: | |||
| PLR (> 300) | 0.40 | (0.16, 0.98) | 0.044 |
| NLR (> 5) | 0.58 | (0.31, 1.10) | 0.097 |
| Steroid use | 2.74 | (1.62, 4.64) | < 0.001 |
| Antibiotics use | 1.02 | (0.52, 1.98) | 0.954 |
| Treatment | |||
| PD-1 CLTA-4 | 3 | (1.35, 6.67) | 0.007 |
| PD-1 TKI | 4 | (1.71, 9.34) | 0.001 |
| Other1 | 4 | (0.55, 28.97) | 0.170 |
| Multivariable model: | |||
| PLR (> 300) | 0.26 | (0.09, 0.74) | 0.011 |
| Steroid use | 4.43 | (2.21, 8.88) | < 0.001 |
| Treatment | |||
| PD-1/CLTA-4 | 2.57 | (1.06, 6.24) | 0.037 |
| PD-1/TKI | 3.39 | (1.34, 8.56) | 0.01 |
| Other1 | 3.50 | (0.21, 57.86) | 0.381 |
A total of 94 patients received corticosteroids, including 47 patients who developed an IrAE. Steroid use was associated with a greater than 2-fold higher incidence of grade ≥ 2 IrAEs (OR = 2.74; P < 0.001) and maintained a significant association in a multivariable regression analysis (OR = 4.43; P < 0.001). Notably, the indication for steroids in 35 (74%) of the 47 patients who developed an IrAE, was for the treatment of IrAEs.
Fifty-six patients received antibiotics for treatment of an infection or as pre-transarterial chemoembolisation prophylaxis. Twenty-nine of these patients had documented IrAEs. Antibiotics use was not associated with IrAE incidence (OR = 1.02; P = 0.954) (Table 3).
NLR and PLR did not differ significantly between patients who did and did not receive steroids [(NLR; HR = 1.03; P = 0.69) (PLR; HR = 1.04; P = 0.53)] or antibiotics [(NLR; HR = 1.13; P = 0.35) (PLR; HR = 1.21; P = 0.10)].
ICIs induce a diverse array of toxicities, presenting as single organ inflammatory disease such as hepatitis to multi system toxicity[24]. Most IrAEs present within the first few months of therapy, though they can manifest throughout the course of treatment[25,26]. They most frequently affect barrier tissues such as the skin (-20%)[26], gastrointestinal tract (-50%)[27] and the respiratory epithelium (-35%), followed by endocrine organs (6%-12%)[28] and less frequently cause joint inflammation (-10%), neurological, cardiovascular or hematologic toxicity (1%-5%). Treatment related deaths are also reported in < 5% of patients treated with ICI[24,25].
Currently, there are no prospectively validated biomarkers to predict the onset or the severity of IrAEs though several have been studied such as preexisting autoimmune disease, body-mass index, and gut microbiome among others[29,30]. Onset of IrAEs however, closely correlates with clinical benefit from ICI therapy based on several reports across disease types[31-38], including from our consortium[39]. Conversely, inflammatory ratios such as NLR and PLR have consistently demonstrated an inverse relationship with response and survival outcomes from ICI therapy[40,41]. Considering the correlation between these inflammatory ratios and IrAEs, as well as the broad accessibility, ease of execution, and rapidity of these tests, it is pertinent to study the relationship between these inflammatory ratios and IrAEs.
Our study demonstrates, for the first time, a potential inverse association between baseline PLR and NLR with incidence of clinically significant (i.e., grade ≥ 2) IrAEs in aHCC treated with ICI, particularly for PLR which was an independent predictive marker of grade ≥ 2 IrAEs. Such an association has also been described in other diseases, particularly in non-small cell lung cancer[42-44]. While the individual relationships between NLR, PLR, IrAE incidence and ICI response directionally align with the existing literature, the mechanisms linking the systemic inflammatory response and the development of IrAEs require further study.
An elevated NLR in a chronic inflammatory state such as cancer, has been shown to be associated with an increased concentration of polymorphonuclear myeloid-derived suppressor cells (PMN-MDSCs) in the peripheral blood[45] as well as tumor-infiltrating neutrophils[43]. The role of PMN-MDSCs in immune tolerance by means of upregulated expression of arginase-1, increased production of reactive oxygen species, Nitrous Oxide, prostaglandin E2 among others is well described in literature[46]. It may therefore be reasonable to hypothesize that the suppression of the anti-tumor T lymphocyte responses in the tumor microenvironment heralded by PMN-MDSCs likely contributes to the poor clinical outcomes and a lower incidence of IrAEs. Additionally, Lymphocytes are an integral components in the immune response against tumors, and an elevated presence of tumor-infiltrating lymphocytes within the neoplastic tissue holds prognostic value, correlating with improved survival outcomes[47]. Patients with higher NLR and PLR have a state of relative lymphopenia, signifying an impaired immune response. This likely accounts for both a reduced incidence of IrAEs as well as poorer prognostic outcomes on ICI.
The mechanistic rationale for the predictive nature of PLR is less well understood, though may be partly explained by the hypercortisolemic state of chronic inflammatory disease[48]. Hypercortisolemia results in elevated platelet counts, heightened platelet activation, and concurrently lowers lymphocyte counts[49-51]. Thrombocytosis has been demonstrated to exert pro-tumor effects, facilitating tumor progression and metastasis through the production of VEGF and platelet-derived growth factor production. These factors subsequently recruit neutrophils and monocytes, a phenomenon that ultimately culminates in adverse prognostic outcomes[40,52]. Beyond their role in hemostasis, platelets modulate the immune response via both pro and anti-inflammatory mechanisms. Platelets have been shown to express functional CD154, a molecule critical for primary and memory T cell responses[53]. In addition to acting as antigen presenting cells[54], platelets also augment CD8 T cell responses via CD154 mediated secondary signaling[55]. Their anti-inflammatory effect is exerted via platelet-derived cytokine TGF β which inhibits FoxP3(-) T-cells, among other mechanisms[56]. The vast role of platelets in immune modulation remains underrepresented in literature.
Consistent with published literature[24,57], our study shows a 2 to 3-fold higher incidence of grade ≥ 2 IrAEs in patients receiving ICI combinations over monotherapy. In the HIMALAYA trial, the incidence of any grade toxicity was 76% in the combination arm, as opposed to 52% in the durvalumab only arm[58]. Similarly, combination Atezolizumab-bevacizumab led to any grade toxicity rate of 98% in the IMbrave-150 study[6]. Understanding how to balance the toxicity of ICI and its combinations, while maximizing its anti-tumor activity remains a critical unmet need and may hinge on identifying early predictors of impending toxicity.
Our study also demonstrates that the use of steroids was strongly associated with a 2-fold increase in the incidence of grade > 2 IrAEs. However, of the 47 patients with IrAEs who received steroids, 74% were prescribed them for treatment of IrAEs. Thus, the association is likely explained by steroids being used to treat IrAEs and does not indicate a causal relationship. Due to the small number of patients treated with steroids, a distinct analysis of the impact of steroid exposure before ICI therapy vs its use in the management of IrAEs is not feasible.
Antibiotic exposure has been shown to impair efficacy of ICI and increase the risk of IrAEs by inducing loss of gut microbial diversity and dysbiosis[59,60]. Our study showed no association between antibiotic exposes and IrAE risk. Another study published from this consortium also showed an improved progression free survival in patients with early antibiotic exposure while on ICI therapy[21], contrasting with studies in other solid tumors. HCC is associated with an immunosuppressive microbiome in the setting of underlying cirrhosis, which may explain the differential effect of antibiotics compared to other solid tumors[21]. We also noted no significant differences in NLR and PLR between patients who did and did not receive steroids and antibiotics.
We understand the limitations of our study include its retrospective design, a relatively small sample size and lack of correlative studies. Our non-prospective design precludes definitive conclusions regarding the predictive value of NLR and PLR but can be considered hypothesis-generating and warrants further validation in larger, prospective cohorts. Despite the fact that most patients in our study were treated with ICI monotherapy, which contrasts with the current standard of care of atezolizumab-bevacizumab, the findings remain significant as ICIs are a crucial component of the treatment paradigm for HCC. Data were collected and entered manually; some missing IrAE data were noted in our analyses. History regarding other chronic inflammatory conditions that could affect NLR and PLR were not collected during chart review and therefore cannot be evaluated. The influence of other confounding factors such as thrombocytopenia from chronic liver disease on NLR and PLR remains a subject of investigation to be explored in a larger prospective cohort. The documentation of IrAEs was based on the NCI-CTCAE v5.0 per the treating physician and validated by a member of the study group, however inconsistencies may exist due to the subjective nature of some groups of IrAEs.
In conclusion, our study demonstrates an interesting association between baseline inflammatory ratios and onset of IrAEs in a real-world cohort of HCC patients, although this conclusion warrants prospective validation. Given that high baseline NLR and PLR are associated with a lower incidence of clinically significant IrAEs, lower baseline NLR and PLR may be predictive biomarkers for the appearance of IrAEs in HCC treated with ICI. These findings have potentially important implications, as identifying and monitoring patients at high risk of developing IrAEs may help decrease mortality and morbidity associated with IrAE. Our findings also provide real world evidence for increased toxicity of ICI combinations over monotherapy in HCC.
In conclusion, our study demonstrates an interesting association between baseline inflammatory ratios and onset of IrAEs in a real-world cohort of HCC patients, although this conclusion warrants prospective validation. Given that high baseline NLR and PLR are associated with a lower incidence of clinically significant IrAEs, lower baseline NLR and PLR may be predictive biomarkers for the appearance of IrAEs in HCC treated with ICI. These findings have potentially important implications, as identifying and monitoring patients at high risk of developing IrAEs may help decrease mortality and morbidity associated with IrAE. Our findings also provide real world evidence for increased toxicity of ICI combinations over monotherapy in HCC.
Immune checkpoint inhibitors (ICI) are known to cause immune-related adverse events (IrAEs) ranging from mild to severe, sometimes leading to treatment discontinuation, with less than 5% of patients experiencing fatal outcomes. Nevertheless, there are currently no dependable markers to forecast the occurrence and seriousness of IrAEs.
Nevertheless, there are currently no dependable markers to forecast the occurrence and seriousness of IrAEs.
In this study, we examined whether baseline neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) are linked to the development of clinically significant IrAEs (grade ≥ 2) in patients with hepatocellular carcinoma (HCC) undergoing ICI treatment.
Data was gathered from a global database comprising data from 11 specialized medical centers. NLR was calculated as the absolute neutrophil count divided by the absolute lymphocyte count (ALC), while PLR was calculated as the platelet count divided by ALC, with predefined cutoff values of 5 for NLR and 300 for PLR as per existing literature. Additionally, we explored the connection between the use of antibiotics and steroids with the occurrence of IrAEs.
Data was collected from 361 patients treated between 2016 and 2020 across the United States (67%), Asia (14%), and Europe (19%), with the majority receiving Nivolumab (71%). Of these patients, 46% experienced at least one IrAE, with 48% being grade 1 and 52% grade ≥ 2. In a univariable regression analysis, a PLR > 300 was significantly linked to a lower incidence of grade ≥ 2 IrAEs (OR = 0.40; P = 0.044), while there was a trend towards lower incidence with NLR > 5 (OR = 0.58; P = 0.097). Multivariate analysis confirmed PLR > 300 as an independent predictor of grade ≥ 2 IrAEs (OR = 0.26; P = 0.011), along with treatment involving programmed cell death ligand 1 (PD-1)/cytotoxic T lymphocyte-associated protein-4 (OR = 2.57; P = 0.037) and PD-1/tyrosine kinase inhibitor combinations (OR = 3.39; P = 0.01). The use of antibiotics did not show a significant association with IrAE incidence (OR = 1.02; P = 0.954), while patients treated with steroids had more than a twofold increased risk of grade ≥ 2 IrAEs (OR = 2.74; P < 0.001), although a majority of them received steroids for IrAE treatment.
Considering that elevated baseline NLR and PLR are correlated with a reduced occurrence of IrAEs, lower baseline NLR and PLR levels could potentially serve as predictive biomarkers for the development of IrAEs in HCC patients undergoing ICI treatment.
Considering that elevated baseline NLR and PLR are correlated with a reduced occurrence of IrAEs, lower baseline NLR and PLR levels could potentially serve as predictive biomarkers for the development of IrAEs in HCC patients undergoing ICI treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Dang SS, China; Liu K, China; Șurlin VM, Romania; Wu SZ, China S-Editor: Qu XL L-Editor: A P-Editor: Qu XL
| 1. | Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv Cancer Res. 2021;149:1-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 558] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 2. | Schütte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma--epidemiological trends and risk factors. Dig Dis. 2009;27:80-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 309] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 3. | Dhanasekaran R, Bandoh S, Roberts LR. Molecular pathogenesis of hepatocellular carcinoma and impact of therapeutic advances. F1000Res. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 4. | Tangkijvanich P, Thong-Ngam D, Mahachai V, Theamboonlers A, Poovorawan Y. Role of serum interleukin-18 as a prognostic factor in patients with hepatocellular carcinoma. World J Gastroenterol. 2007;13:4345-4349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493-e503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 951] [Cited by in RCA: 1627] [Article Influence: 162.7] [Reference Citation Analysis (0)] |
| 6. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4708] [Article Influence: 941.6] [Reference Citation Analysis (2)] |
| 7. | Finn RS, Ryoo B-Y, Merle P, Kudo M, Bouattour M, Lim H-YJ, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). Journal of Clinical Oncology. 2019;37 Suppl 15:4004-4004. [RCA] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 8. | Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 908] [Cited by in RCA: 971] [Article Influence: 194.2] [Reference Citation Analysis (0)] |
| 9. | Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Han KH, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Begic D, Chen G, Neely J, Anderson J, Sangro B. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Annals of Oncology. 2019;30:v874-v5. [RCA] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 433] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 10. | Opdivo (nivolumab) prescribing information. Bristol-Myers Squibb Company. Princeton, NJ, United States; 2015. |
| 11. | Yervoy® (ipilimumab) prescribing information. Bristol-Myers Squibb Company. Princeton, NJ, United States; 2013. |
| 12. | Inno A, Metro G, Bironzo P, Grimaldi AM, Grego E, Di Nunno V, Picasso V, Massari F, Gori S. Pathogenesis, clinical manifestations and management of immune checkpoint inhibitors toxicity. Tumori. 2017;103:405-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Marté JL, Toney NJ, Cordes L, Schlom J, Donahue RN, Gulley JL. Early changes in immune cell subsets with corticosteroids in patients with solid tumors: implications for COVID-19 management. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Jing Y, Chen X, Li K, Liu Y, Zhang Z, Chen Y, Wang Y, Lin SH, Diao L, Wang J, Lou Y, Johnson DB, Liu H, Han L. Association of antibiotic treatment with immune-related adverse events in patients with cancer receiving immunotherapy. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 15. | Mohiuddin JJ, Chu B, Facciabene A, Poirier K, Wang X, Doucette A, Zheng C, Xu W, Anstadt EJ, Amaravadi RK, Karakousis GC, Mitchell TC, Huang AC, Shabason JE, Lin A, Swisher-McClure S, Maity A, Schuchter LM, Lukens JN. Association of Antibiotic Exposure With Survival and Toxicity in Patients With Melanoma Receiving Immunotherapy. J Natl Cancer Inst. 2021;113:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 16. | Fiala O, Hosek P, Korunkova H, Hora M, Kolar J, Windrichova J, Sorejs O, Topolcan O, Travnicek I, Sedlackova H, Finek J. Enzalutamide or Abiraterone Acetate With Prednisone in the Treatment of Metastatic Castration-resistant Prostate Cancer in Real-life Clinical Practice: A Long-term Single Institution Experience. Anticancer Res. 2023;43:463-471. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Song A, Eo W, Lee S. Comparison of selected inflammation-based prognostic markers in relapsed or refractory metastatic colorectal cancer patients. World J Gastroenterol. 2015;21:12410-12420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Pinato DJ, Shiner RJ, Seckl MJ, Stebbing J, Sharma R, Mauri FA. Prognostic performance of inflammation-based prognostic indices in primary operable non-small cell lung cancer. Br J Cancer. 2014;110:1930-1935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 19. | Giordano G, Vaccaro V, Lucchini E, Bertocchi P, Bergamo F, Musettini G, Santoni M, Lo Re G, Giommoni E, Russano M, Campidoglio S, Santini D, Vasile E, Cascinu S, Zagonel V, Zaniboni A, Melisi D, Milella M, Febbraro A. Analysis of prognostic factors in advanced pancreatic cancer (APDAC) patients (pts) undergoing to first-line nab-paclitaxel (Nab-P) and gemcitabine (G) treatment. Journal of Clinical Oncology. 2015;33 Suppl 3:412-412. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Cedrés S, Torrejon D, Martínez A, Martinez P, Navarro A, Zamora E, Mulet-Margalef N, Felip E. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin Transl Oncol. 2012;14:864-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 21. | Fessas P, Naeem M, Pinter M, Marron TU, Szafron D, Balcar L, Saeed A, Jun T, Dharmapuri S, Gampa A, Wang Y, Khan U, Muzaffar M, Navaid M, Lee PC, Bulumulle A, Yu B, Paul S, Nimkar N, Bettinger D, Hildebrand H, Abugabal YI, Pressiani T, Personeni N, Nishida N, Kudo M, Kaseb A, Huang YH, Ang C, Pillai A, Rimassa L, Naqash AR, Sharon E, Cortellini A, Pinato DJ. Early Antibiotic Exposure Is Not Detrimental to Therapeutic Effect from Immunotherapy in Hepatocellular Carcinoma. Liver Cancer. 2021;10:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Pinato DJ, Gramenitskaya D, Altmann DM, Boyton RJ, Mullish BH, Marchesi JR, Bower M. Antibiotic therapy and outcome from immune-checkpoint inhibitors. J Immunother Cancer. 2019;7:287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 23. | R Development Core Team. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2017;. [DOI] [Full Text] |
| 24. | Dougan M, Luoma AM, Dougan SK, Wucherpfennig KW. Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell. 2021;184:1575-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 192] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 25. | Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. 2020;70:86-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 885] [Article Influence: 177.0] [Reference Citation Analysis (36)] |
| 26. | Sibaud V. Dermatologic Reactions to Immune Checkpoint Inhibitors : Skin Toxicities and Immunotherapy. Am J Clin Dermatol. 2018;19:345-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 432] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 27. | Bureš J, Kohoutová D, Zavoral M. Gastrointestinal toxicity of systemic oncology immunotherapy. Klin Onkol. 2022;35:346-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 28. | Król A, Gawlik T, Jarząb B. Endocrine complications of cancer immunotherapy. Endokrynol Pol. 2018;69:722-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Tison A, Quéré G, Misery L, Funck-Brentano E, Danlos FX, Routier E, Robert C, Loriot Y, Lambotte O, Bonniaud B, Scalbert C, Maanaoui S, Lesimple T, Martinez S, Marcq M, Chouaid C, Dubos C, Brunet-Possenti F, Stavris C, Chiche L, Beneton N, Mansard S, Guisier F, Doubre H, Skowron F, Aubin F, Zehou O, Roge C, Lambert M, Pham-Ledard A, Beylot-Barry M, Veillon R, Kramkimel N, Giacchero D, De Quatrebarbes J, Michel C, Auliac JB, Gonzales G, Decroisette C, Le Garff G, Carpiuc I, Vallerand H, Nowak E, Cornec D, Kostine M; Groupe de Cancérologie Cutanée, Groupe Français de Pneumo-Cancérologie, and Club Rhumatismes et Inflammations. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer and Preexisting Autoimmune Disease: A Nationwide, Multicenter Cohort Study. Arthritis Rheumatol. 2019;71:2100-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 30. | Smithy JW, Faleck DM, Postow MA. Facts and Hopes in Prediction, Diagnosis, and Treatment of Immune-Related Adverse Events. Clin Cancer Res. 2022;28:1250-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 31. | Maher VE, Fernandes LL, Weinstock C, Tang S, Agarwal S, Brave M, Ning YM, Singh H, Suzman D, Xu J, Goldberg KB, Sridhara R, Ibrahim A, Theoret M, Beaver JA, Pazdur R. Analysis of the Association Between Adverse Events and Outcome in Patients Receiving a Programmed Death Protein 1 or Programmed Death Ligand 1 Antibody. J Clin Oncol. 2019;37:2730-2737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 245] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 32. | Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, Brambilla M, Baglivo S, Grossi F, Chiari R. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol. 2019;145:479-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 265] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 33. | Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, Kaneda H, Hasegawa Y, Tanaka K, Takeda M, Nakagawa K. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol. 2018;4:374-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 766] [Article Influence: 127.7] [Reference Citation Analysis (0)] |
| 34. | Sato K, Akamatsu H, Murakami E, Sasaki S, Kanai K, Hayata A, Tokudome N, Akamatsu K, Koh Y, Ueda H, Nakanishi M, Yamamoto N. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer. 2018;115:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 314] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 35. | Lisberg A, Tucker DA, Goldman JW, Wolf B, Carroll J, Hardy A, Morris K, Linares P, Adame C, Spiegel ML, Wells C, McKenzie J, Ledezma B, Mendenhall M, Abarca P, Bornazyan K, Hunt J, Moghadam N, Chong N, Nameth D, Marx C, Madrigal J, Vangala S, Shaverdian N, Elashoff D, Garon EB. Treatment-Related Adverse Events Predict Improved Clinical Outcome in NSCLC Patients on KEYNOTE-001 at a Single Center. Cancer Immunol Res. 2018;6:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 36. | Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res. 2016;22:886-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 672] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 37. | Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, Luiten RM. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 458] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 38. | Cortellini A, Buti S, Agostinelli V, Bersanelli M. A systematic review on the emerging association between the occurrence of immune-related adverse events and clinical outcomes with checkpoint inhibitors in advanced cancer patients. Semin Oncol. 2019;46:362-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 39. | Pinato DJ, Marron TU, Mishra-Kalyani PS, Gong Y, Wei G, Szafron D, Sharon E, Saeed A, Jun T, Dharmapuri S, Naqash AR, Peeraphatdit T, Gampa A, Wang Y, Khan U, Muzaffar M, Navaid M, Lee CJ, Lee PC, Bulumulle A, Yu B, Paul S, Nimkar N, Bettinger D, Hildebrand H, Abugabal YI, Pressiani T, Personeni N, D'Alessio A, Kaseb AO, Huang YH, Ang C, Schneider J, Pillai A, Rimassa L, Goldberg KB, Pazdur R, Theoret M, Lemery S, Fashoyin-Aje ', Cortellini A, Pelosof L. Treatment-related toxicity and improved outcome from immunotherapy in hepatocellular cancer: Evidence from an FDA pooled analysis of landmark clinical trials with validation from routine practice. Eur J Cancer. 2021;157:140-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 40. | Dharmapuri S, Özbek U, Lin JY, Sung M, Schwartz M, Branch AD, Ang C. Predictive value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in advanced hepatocellular carcinoma patients treated with anti-PD-1 therapy. Cancer Med. 2020;9:4962-4970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 41. | Muhammed A, D'Alessio A, Enica A, Talbot T, Fulgenzi CAM, Nteliopoulos G, Goldin RD, Cortellini A, Pinato DJ. Predictive biomarkers of response to immune checkpoint inhibitors in hepatocellular carcinoma. Expert Rev Mol Diagn. 2022;22:253-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 42. | Pavan A, Calvetti L, Dal Maso A, Attili I, Del Bianco P, Pasello G, Guarneri V, Aprile G, Conte P, Bonanno L. Peripheral Blood Markers Identify Risk of Immune-Related Toxicity in Advanced Non-Small Cell Lung Cancer Treated with Immune-Checkpoint Inhibitors. Oncologist. 2019;24:1128-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 43. | Lee PY, Oen KQX, Lim GRS, Hartono JL, Muthiah M, Huang DQ, Teo FSW, Li AY, Mak A, Chandran NS, Tan CL, Yang P, Tai ES, Ng KWP, Vijayan J, Chan YC, Tan LL, Lee MB, Chua HR, Hong WZ, Yap ES, Lim DK, Yuen YS, Chan YH, Aminkeng F, Wong ASC, Huang Y, Tay SH. Neutrophil-to-Lymphocyte Ratio Predicts Development of Immune-Related Adverse Events and Outcomes from Immune Checkpoint Blockade: A Case-Control Study. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 44. | Fujimoto A, Toyokawa G, Koutake Y, Kimura S, Kawamata Y, Fukuishi K, Yamazaki K, Takeo S. Association between pretreatment neutrophil-to-lymphocyte ratio and immune-related adverse events due to immune checkpoint inhibitors in patients with non-small cell lung cancer. Thorac Cancer. 2021;12:2198-2204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 45. | Basu A, Kollengode KA, Rafatnia A, Manoli H, Danenberg G, Chakravartty E, Epstein AL, Pinski JK. Relationship between neutrophil lymphocyte ratio (NLR) and MDSC concentration in localized and metastatic castration resistant prostate cancer (mCRPC) patients. Journal of Clinical Oncology. 2018;36 Suppl 6:338-338. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 1359] [Article Influence: 194.1] [Reference Citation Analysis (0)] |
| 47. | Mao Y, Qu Q, Chen X, Huang O, Wu J, Shen K. The Prognostic Value of Tumor-Infiltrating Lymphocytes in Breast Cancer: A Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0152500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 48. | Väyrynen JP, Väyrynen SA, Sirniö P, Minkkinen I, Klintrup K, Karhu T, Mäkelä J, Herzig KH, Karttunen TJ, Tuomisto A, Mäkinen MJ. Platelet count, aspirin use, and characteristics of host inflammatory responses in colorectal cancer. J Transl Med. 2019;17:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Szczepanek-Parulska E, Adamska M, Korda O, Kosicka W, Skowrońska D, Świejkowska A, Tuzimek D, Dadej D, Krygier A, Ruchała M. Changes in complete blood count parameters influenced by endocrine disorders. Endokrynol Pol. 2021;72:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 50. | Gasparyan AY, Ayvazyan L, Mukanova U, Yessirkepov M, Kitas GD. The Platelet-to-Lymphocyte Ratio as an Inflammatory Marker in Rheumatic Diseases. Ann Lab Med. 2019;39:345-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 357] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 51. | Mischler K, Fischer JE, Zgraggen L, Kudielka BM, Preckel D, von Känel R. The effect of repeated acute mental stress on habituation and recovery responses in hemoconcentration and blood cells in healthy men. Life Sci. 2005;77:1166-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Tazzyman S, Lewis CE, Murdoch C. Neutrophils: key mediators of tumour angiogenesis. Int J Exp Pathol. 2009;90:222-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 243] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 53. | van Kooten C, Banchereau J. CD40-CD40 Ligand. J Leukoc Biol. 2000;67:2-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1040] [Cited by in RCA: 1081] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 54. | Chapman LM, Aggrey AA, Field DJ, Srivastava K, Ture S, Yui K, Topham DJ, Baldwin WM 3rd, Morrell CN. Platelets present antigen in the context of MHC class I. J Immunol. 2012;189:916-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 55. | Ali RA, Wuescher LM, Worth RG. Platelets: essential components of the immune system. Curr Trends Immunol. 2015;16:65-78. [PubMed] |
| 56. | Zhu L, Huang Z, Stålesen R, Hansson GK, Li N. Platelets provoke distinct dynamics of immune responses by differentially regulating CD4+ T-cell proliferation. J Thromb Haemost. 2014;12:1156-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Pauken KE, Dougan M, Rose NR, Lichtman AH, Sharpe AH. Adverse Events Following Cancer Immunotherapy: Obstacles and Opportunities. Trends Immunol. 2019;40:511-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 204] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 58. | Abou-Alfa Ghassan K, Lau G, Kudo M, Chan Stephen L, Kelley Robin K, Furuse J, Sukeepaisarnjaroen W, Kang YK, Dao TV, De Toni EN, Rimassa L, Breder V, Vasilyev A, Heurgué A, Tam VC, Mody K, Thungappa SCT, Ostapenko Y, Yau T, Azevedo S, Varela M, Cheng AL, Qin S, Galle PR, Ali S, Marcovitz M, Makowsky M, He P, Kurland JF, Negro A, Sangro B. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evidence. 2022;1: EVIDoa2100070. [RCA] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 663] [Article Influence: 221.0] [Reference Citation Analysis (0)] |
| 59. | Pierrard J, Seront E. Impact of the gut microbiome on immune checkpoint inhibitor efficacy-a systematic review. Curr Oncol. 2019;26:395-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 60. | Herbst DA, Reddy KR. Risk factors for hepatocellular carcinoma. Clin Liver Dis (Hoboken). 2012;1:180-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |