Published online Nov 15, 2023. doi: 10.4251/wjgo.v15.i11.1891
Peer-review started: February 13, 2023
First decision: April 25, 2023
Revised: May 28, 2023
Accepted: June 13, 2023
Article in press: June 13, 2023
Published online: November 15, 2023
Processing time: 275 Days and 4.2 Hours
The presence of Spigelman stage (SS) IV duodenal polyposis is considered the most significant risk factor for duodenal cancer in patients with MUTYH-associated polyposis (MAP). However, advanced SS disease is rarely reported in MAP patients, and no clear recommendations on small bowel (SB) surveillance have been proposed in this patient setting.
To research more because that case reports of duodenal cancers in MAP suggest that they may develop in the absence of advanced benign SS disease and often involve the distal portion of the duodenum.
We describe a series of MAP patients followed up at the Regina Elena National Cancer Institute of Rome (Italy). A literature overview on previously reported SB cancers in MAP is also provided.
We identified two (6%) SB adenocarcinomas with no previous history of duodenal polyposis. Our observations, supported by literature evidence, suggest that the formula for staging duodenal polyposis and predicting risk factors for distal duodenum and jejunal cancer may need to be adjusted to take this into account rather than focusing solely on the presence or absence of SS IV disease.
Our study emphasizes the need for further studies to define appropriate upper gastrointestinal surveillance programs in MAP patients.
Core Tip: Case reports of duodenal cancers in MUTYH-associated polyposis suggest that they may develop in the absence of advanced Spigelman stage (SS) benign disease and often involve the distal portion of the duodenum. In our case series, we identified two (6%) small-bowel adenocarcinomas with no previous history of duodenal polyposis. Our observations, supported by literature evidence, suggest that the formula for staging duodenal polyposis and predicting risk factors for distal duodenum and jejunal cancer should be adjusted to take into consideration the presence of SS IV disease, rather than focusing only on this feature.
- Citation: Sanchez-Mete L, Mosciatti L, Casadio M, Vittori L, Martayan A, Stigliano V. MUTYH-associated polyposis: Is it time to change upper gastrointestinal surveillance? A single-center case series and a literature overview. World J Gastrointest Oncol 2023; 15(11): 1891-1899
- URL: https://www.wjgnet.com/1948-5204/full/v15/i11/1891.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i11.1891
MUTYH-associated polyposis (MAP) is an autosomal recessive inherited disease caused by a biallelic pathogenic germline variant in the MUTYH gene. It was first described in 2002 in patients who presented clinical features similar to familial adenomatous polyposis/attenuated FAP (FAP/AFAP) but without APC gene mutation, with lesser polyps, later onset and a lower cancer lifetime risk[1,2]. MAP is typically associated with a dozen to a few hundred colonic adenomatous polyps, most frequently located in the right colon. However, colorectal cancer (CRC) can develop in some individuals in the absence of polyposis. Serrated adenomas, hyperplastic/sessile serrated polyps, and mixed (hyperplastic and adenomatous) polyps can also occur[3]. The lifetime risk of CRC in patients with MAP without surveillance is 80%-90% at a median age of onset of 48 years[3,4]. Given this substantial risk, MAP patients are advised to receive intensive colonoscopy surveillance every 1-2 years depending on the polyp burden, beginning at 25-30 years, and/or prophylactic surgery if the burden polyps are not manageable endoscopically[5-7].
Like FAP/AFAP, patients with MAP present an increased risk of extra-intestinal manifestations[8]. Among them, a frequent extra-colonic manifestation is a duodenal polyposis[9]. Even if duodenal polyposis develops less frequently than in FAP (14%-34% versus 65%-90%, respectively) and at a later age[9], the risk of developing duodenal cancer is comparable between the two conditions[5,9-11].
The Spigelman five-stage (0-IV) system (SS) is traditionally used to classify the duodenal polyposis severity, predict the risk of duodenal cancer, define the frequency of endoscopic surveillance and the timing of prophylactic duodenectomy. Equal relevance is given to each of the criteria considered in calculating the SS score and stage - size, number, histology, and degree of dysplasia[12]. Histological evaluation of duodenal polyps is required to obtain all criteria of the SS score. However, the biopsy is not performed routinely because it could interfere with the optical diagnosis using narrow-band imaging or create fibrosis that interferes with subsequent endoscopic resection.
When endoscopic removal is not performed (i.e., small adenomas < 10 mm), SS could be determined by using optical diagnosis like narrow-band imaging[13,14]. The occurrence of SS IV duodenal polyposis is reported as the main risk factor for duodenal cancer[5,15]. However, advanced SS disease is rarely reported in MAP patients, and adenomas and adenocarcinoma beyond Treitz’s ligament are described anecdotally[9]. Consequently, no clear recommendations on SB surveillance in MAP patients have been proposed (Table 1). The frequency of surveillance is mostly determined by the SS classification, with increasing frequency only if advanced-stage polyps are discovered[5].
| SS | Surveillance interval (yr/mo) | ||||
| ESGE[13,14] 2022, 2019 | EMG[35] 2008 | ESMO[36] 2019 | ASGE[5] 2020 | NCCN[7] (v. 2.2022) | |
| Duodenum | |||||
| 0 | 5 yr | 5 yr | 5 yr | 5 yr | 3-5 yr |
| I | 5 yr | 5 yr | 5 yr | 5 yr | 2-3 yr |
| II | 3 yr | 3 yr | 3 yr | 3 yr | 1-2 yr |
| III | 1 yr | 1-2 yr | 1-2 yr | 6-12 mo | 6-12 mo |
| IV | 6 mo, consider treatment | Surgical evaluation | 6 mo or consider prophylactic surgery | 3-6 mo, surgical evaluation | Expert surveillance 3-6 mo |
| Rest of SB | |||||
| ESGE 2019 do not mention SB. ESGE 2022: CE and/or cross-sectional imaging techniques may be considered when an investigation of the mid-distal small bowel is clinically indicated | Not mentioned | Carry out a first endoscopy at 25-30 yr and continue depending on the SS. In FAP, the risk of cancer in the jejunum and ileum is extremely low; therefore, routine surveillance is not recommended | Suggested in SS IV with CE or MRE. Enteroscopy is not recommended routinely but only in positive CE or MRE and pre-duodenal surgery to avoid reconstruction with an SB segment with a high-density adenoma | High evidence supporting SB screening distal to the duodenum is lacking. Consider it, especially if advanced duodenal polyposis | |
Notably, case reports of duodenal cancers in MAP suggest that they may develop in the absence of advanced benign SS disease, even without coexisting adenomas, and often involve the distal portion of the duodenum, out of the reach of conventional esophagogastroduodenoscopy[9,11].
To increase our knowledge regarding MAP and its associated duodenal polyposis, in light of recent literature evidence, this paper describes a series of MAP patients followed up at the Regina Elena National Cancer Institute of Rome, Italy. In particular, the presence of SB cancers was assessed in relation to the history of duodenal polyposis. In addition, a literature overview on previously reported SB cancers in MAP is provided.
Clinical records of thirty-eight MAP patients followed up at the Regina Elena National Cancer Institute between 2003 and 2021 were considered. In addition, a literature revision by a PubMed search was carried out on previously reported SB cancers in MAP, without any limitations in terms of publication date and language.
The baseline characteristics of patients are summarized in Table 2. The missense pathogenic variant c.452A>G;p.Tyr151Cys (NM_001048174.2)[16] (previously known as c.536A>G;p.Tyr179Cys, NM_001128425.1) was the most reported one (n = 13; 33%). Upper gastrointestinal endoscopy was performed in 33 out of 38 patients (87%); the median age (SD) at first duodenoscopy was 49 (10) years. The first and last duodenoscopy showed a similar endoscopic feature: In 30 out of 33 patients (90%), no polyps were found, whereas a SS grade I polyposis was found in two patients (6%), and a SS II polyposis in one patient (3%).
| Characteristics | n (%) |
| Male | 28 (74) |
| Age at diagnosis (yr), mean ± SD | 48 ± 10 |
| Pathogenic MUTYH variants | |
| Homozygotes | 7 (18) |
| Compound heterozygotes | 31 (82) |
| Most reported variant1 | c.452A>G;p.Tyr151Cys |
| Colectomy | |
| No | 11 (29) |
| Total | 14 (37) |
| Subtotal | 13 (34) |
| Colorectal cancer | 18 (47) |
| Duodenal adenomas | 32 (9) |
| Spigelman stage I | 2 (6) |
| Spigelman stage II | 1 (3) |
| Spigelman stage III | 0 |
| Spigelman stage IV | 0 |
| Extracolic tumors | 11 (29) |
| Thyroid carcinoma/papillary thyroid carcinoma | 4 (36) |
| Breast cancer | 2 (18) |
| Small bowel cancer | 2 (18) |
| Bladder cancer | 1 (9) |
| Desmoid tumor | 1 (9) |
| Kidney tumor | 1 (9) |
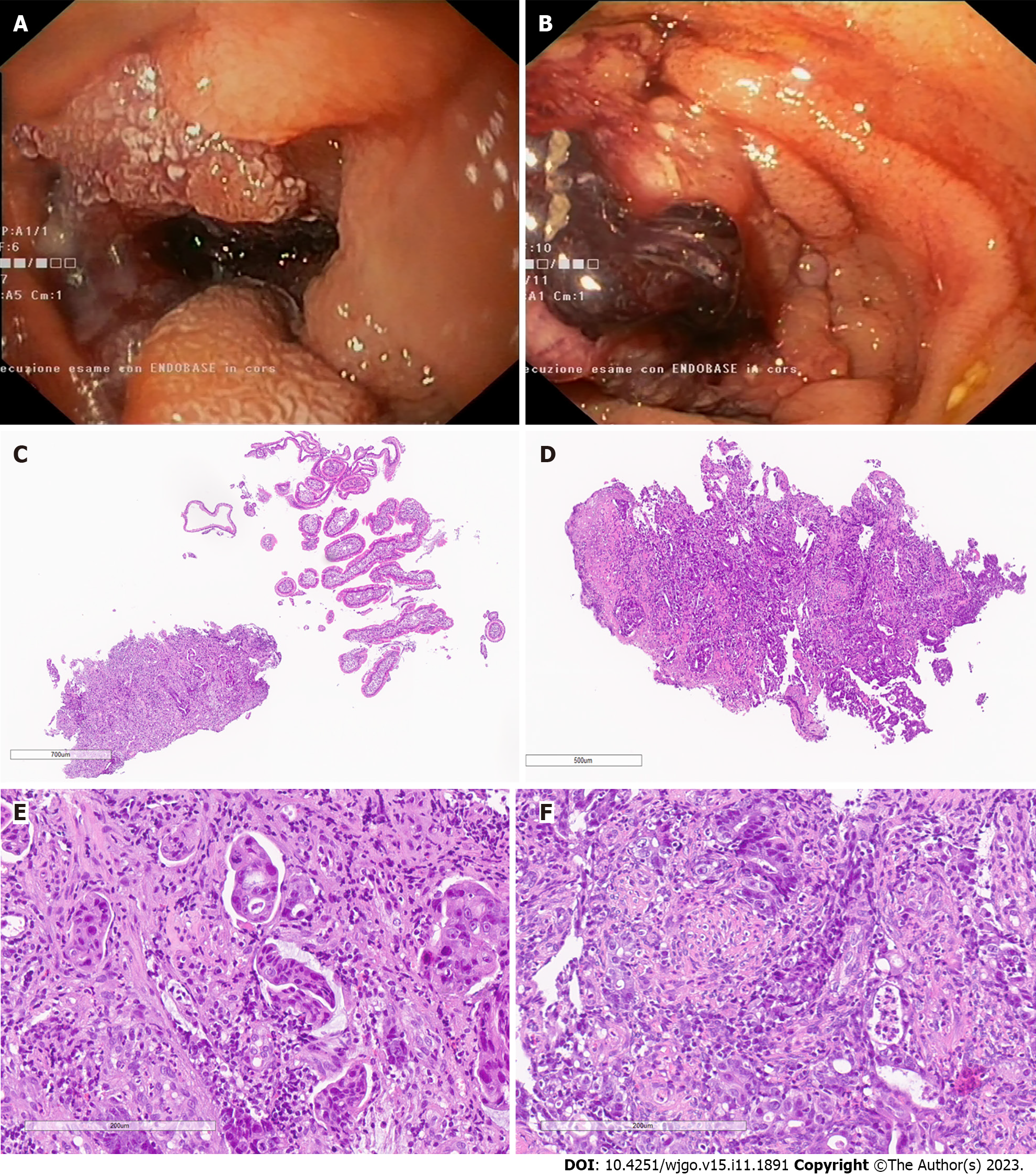
SB cancer was found in two out of 33 patients (6%) with no previous history of duodenal polyposis. The first patient, a 54-year-old man, was a compound heterozygote for the MUTYH pathogenic variants c.452A>G;p.Tyr151Cys (previously known as c.536A>G;p.Tyr179Cys) and c.849+3A>C;p.? (previously known as c.933+3A>C;p.?). During routine surveillance, an advanced, metastatic adenocarcinoma of the distal duodenum was found (Figures 1A, 1C and 1E).
The second patient, a 58-year-old man, was homozygote for the MUTYH pathogenic variant c.452A>G;p.Tyr151Cys (previously known as c.536A>G;p.Tyr179Cys). He underwent capsule endoscopy and subsequent push enteroscopy for anemia. A proximal jejunal adenocarcinoma was found and treated with surgery. In his anamnestic history, this patient presented a papillary tumor of the thyroid gland, adrenal adenoma, and a duodenal ampullary adenoma, diagnosed after surgery during a subsequent duodenoscopy (Figures 1B, 1D and 1E).
After the first description of MAP as an adenomatous colorectal polyposis in 2002, several extracolonic manifestations, particularly duodenal polyposis, have been reported in MAP patients[8,17,18]. Although different studies suggested that SS IV disease strongly predicts future cancer[15], duodenal cancers lacking prior stage IV disease have been reported in MAP patients[9-11,19].
Large cohort studies can be useful in describing features and frequency of duodenal cancers in MAP (Table 3). In a cohort of 276 MAP patients recruited from a European multicenter study, duodenal polyposis occurred in 26 out of 150 patients (17%) who underwent esophagogastroduodenoscopy. SS classification was not presented. Among this cohort, two duodenal cancers were reported (about 1%)[10].
| Study design | Number of MAP patients | Main findings | Ref. |
| Cohort study | 394 | None of the four MAP-associated duodenal cancers (about 1%) reported in this cohort study developed in the context of prior stage IV disease. Three of them involved the distal duodenum | [11] |
| Retrospective study | 92 | One duodenal and one ampullary cancer occurred in this cohort (about 2%), none in the context of prior stage IV disease | [9] |
| European multicenter study | 276 | Duodenal polyposis occurred in 17% of patients who underwent esophagogastroduodenoscopy. SS classification was not presented. Two duodenal cancers were reported (about 1%) | [10] |
In a retrospective study on 92 MAP patients undergoing surveillance esophagogastroduodenoscopy, 34% (n = 31) reported duodenal polyposis. Of them, 29 (32%) developed SS I-III disease and only 2 (2%) SS IV disease. One duodenal and one ampullary cancer occurred in this cohort (about 2%), none in the context of prior stage IV disease[9].
In a recent cohort study on 394 MAP patients, 21% of patients had duodenal polyposis, and the incidence of SS IV duodenal polyposis was 1.5%[11]. None of the four MAP-associated duodenal cancers (about 1%) reported in this cohort study developed in the context of prior stage IV disease, and three of four duodenal cancers involved the distal duodenum[11].
In our series, we identified two (6%) SB adenocarcinomas with no previous history of duodenal polyposis. Both cancer patients carried the most reported pathogenic variant (c.452A>G;p.Tyr151Cys; NM_001048174.2), known to be associated with more aggressive disease[20]. Prior studies on larger series reported a lower percentage (about 1%) of duodenal cancers. Nonetheless, in most of them, no previous history of SS IV duodenal polyposis was reported; the distal duodenum/jejunum was mostly involved, as we observed in our series[9-11]. This suggests that SS can fail in identifying patients with MAP at risk of future cancer.
It is still unclear if SS should also be considered a duodenal cancer predictor in FAP. Although several studies have shown a good correlation between these two parameters[21], a recent systematic review assessing the risk factors for non-ampullary duodenal carcinoma in FAP patients reported three cohort studies characterized by an increased incidence in SS[22]; in only one case-control study, the inconsistency of SS as a duodenal cancer prediction risk indicator was suggested since more than half of FAP patients diagnosed with duodenal cancer lacked SS IV duodenal polyposis[23]. The authors found that only two out of four SS components (large duodenal polyp size and degree of dysplasia) were positively associated with duodenal cancer and reported advanced papilla pathology as an important feature[23].
Of note, duodenal adenomas in MAP appear to display a more aggressive molecular pattern. Recent molecular analyses suggest they have a high mutational burden and likely harbor oncogenic driver mutations, such as those in KRAS[24]. These features of the biology and natural history of duodenal polyposis in MAP, together with the debate over the utility of all the SS components, challenge the current upper gastrointestinal tract surveillance guidelines in this patient setting, as recently observed[25].
Current International guidelines do not suggest any specific surveillance of small bowel (SB) in MAP for FAP. More recent guidelines suggest performing an SB study with capsule endoscopy or magnetic resonance enterography only in cases of advanced duodenal polyposis and limiting device-assisted enteroscopy to pre-duodenal surgery[5,26]. As a matter of fact, capsule endoscopy has shown better diagnostic yield for detecting smaller jejunum-ileal polyps than other imaging modalities; therefore, when indicated, it should be the first-choice examination. Moreover, it has a similar detection rate to device-assisted enteroscopy but a lower diagnostic yield for SB tumors/polyps located in the first tract of the SB, especially the periampullary area and the proximal jejunum. This could probably be due to the rapid transit[27-33].
In our MAP series, the SB cancer identified were both in the distal duodenum/jejunum, within reach of push enteroscopy. According to international guidelines, MAP patients regularly undergo front and side view upper endoscopy, so deeper exploration of the proximal jejunum, using push enteroscopy, could be considered instead of conventional gastroscopy.
This study presents some limitations, such as the small sample and the retrospective analysis. However, some strengths can be described, such as the focus on some peculiar features of MAP which emerged in the last years, that consider MAP a distinct clinical entity characterized by a higher susceptibility to extra-colonic malignancies than APC-associated polyposis, with a different and more aggressive behavior[8,25,34]. However, taken together, our observations and literature evidence suggest that further studies are needed to define appropriate upper gastrointestinal surveillance programs in MAP patients.
Although larger studies are needed to validate the overall findings, our observations suggest that the formula for staging duodenal polyposis and predicting risk factors for distal duodenum and jejunal cancer may need to be adjusted to take this into account rather than focusing solely on the presence or absence of SS IV disease. Moreover, the biological pattern and behavior of SB adenomas in MAP compared with FAP ones should be investigated. Push enteroscopy and side view upper endoscopy could be considered/hypothesized to better examine the proximal SB. In conclusion, a revision of upper gastrointestinal/SB surveillance guidelines may be required to better prevent SB cancer in MAP.
Patients with MUTYH-associated polyposis (MAP) present an increased risk of extra-intestinal manifestations. Among them, a frequent extra-colonic manifestation is duodenal polyposis, which severity is traditionally classified with the Spigelman five-stage system (SS). The occurrence of SS IV duodenal polyposis is reported as the main risk factor for duodenal cancer. However, case reports of duodenal cancers in MAP suggest that they may develop in the absence of advanced benign SS disease, even without coexisting adenomas, and often involve the distal portion of the duodenum, out of the reach of conventional esophagogastroduodenoscopy.
Further studies are needed to define appropriate upper gastrointestinal surveillance programs in MAP patients.
To increase the knowledge regarding MAP and its associated duodenal polyposis, in light of recent literature evidence, we describe a series of MAP patients followed up at the Regina Elena National Cancer Institute of Rome, Italy. In addition, a literature revision on previously reported small bowel (SB) cancers in MAP was carried out.
Clinical records of thirty-eight MAP patients followed up at the Regina Elena National Cancer Institute between 2003 and 2021 were considered. A literature revision by a PubMed search was carried out on previously reported SB cancers in MAP, without any limitations in terms of publication date and language.
In our case series, we identified two (6%) SB adenocarcinomas with no previous history of duodenal polyposis.
Our observations suggest that the formula for staging duodenal polyposis and predicting risk factors for distal duodenum and jejunal cancer should be adjusted to take in consideration the presence of SS IV disease, rather than focusing only on this feature.
A revision of upper gastrointestinal/SB surveillance guidelines may be required to better prevent SB cancer in MAP.
Editorial and graphical assistance was provided by Simonetta Papa, PhD, Massimiliano Pianta, Valentina Attanasio, and Aashni Shah (Polistudium SRL, Milan, Italy). This assistance was supported by internal funds.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Serban ED, Romania; Yuan Y, China S-Editor: Wang JJ L-Editor: A P-Editor: Yu HG
| 1. | Al-Tassan N, Chmiel NH, Maynard J, Fleming N, Livingston AL, Williams GT, Hodges AK, Davies DR, David SS, Sampson JR, Cheadle JP. Inherited variants of MYH associated with somatic G:C-->T:A mutations in colorectal tumors. Nat Genet. 2002;30:227-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 960] [Cited by in RCA: 929] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 2. | Patel R, McGinty P, Cuthill V, Hawkins M, Moorghen M, Clark SK, Latchford A. MUTYH-associated polyposis - colorectal phenotype and management. Colorectal Dis. 2020;22:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Nielsen M, Morreau H, Vasen HF, Hes FJ. MUTYH-associated polyposis (MAP). Crit Rev Oncol Hematol. 2011;79:1-16. [RCA] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Kanth P, Grimmett J, Champine M, Burt R, Samadder NJ. Hereditary Colorectal Polyposis and Cancer Syndromes: A Primer on Diagnosis and Management. Am J Gastroenterol. 2017;112:1509-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 5. | Yang J, Gurudu SR, Koptiuch C, Agrawal D, Buxbaum JL, Abbas Fehmi SM, Fishman DS, Khashab MA, Jamil LH, Jue TL, Law JK, Lee JK, Naveed M, Qumseya BJ, Sawhney MS, Thosani N, Wani SB, Samadder NJ. American Society for Gastrointestinal Endoscopy guideline on the role of endoscopy in familial adenomatous polyposis syndromes. Gastrointest Endosc. 2020;91:963-982.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 6. | Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW; American College of Gastroenterology. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110:223-62; quiz 263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 1087] [Article Influence: 108.7] [Reference Citation Analysis (0)] |
| 7. | National Comprehensive Cancer Network. NCCN Guidelines: Genetic/Familial High-Risk Assessment: Colorectal. Mutyh Associated Polyposis. Version 1.2023. [cited 15 December 2022]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=2&id=1436. |
| 8. | Magrin L, Fanale D, Brando C, Corsini LR, Randazzo U, Di Piazza M, Gurrera V, Pedone E, Bazan Russo TD, Vieni S, Pantuso G, Russo A, Bazan V. MUTYH-associated tumor syndrome: The other face of MAP. Oncogene. 2022;41:2531-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Walton SJ, Kallenberg FG, Clark SK, Dekker E, Latchford A. Frequency and Features of Duodenal Adenomas in Patients With MUTYH-Associated Polyposis. Clin Gastroenterol Hepatol. 2016;14:986-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Vogt S, Jones N, Christian D, Engel C, Nielsen M, Kaufmann A, Steinke V, Vasen HF, Propping P, Sampson JR, Hes FJ, Aretz S. Expanded extracolonic tumor spectrum in MUTYH-associated polyposis. Gastroenterology. 2009;137:1976-85.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 223] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 11. | Collaborative Group on Duodenal Polyposis in MAP; Thomas LE, Hurley JJ, Sanchez AA, Aznárez MR, Backman AS, Bjork J, Capella G, Clark SK, Colas C, Dekker E, Dolwani S, Ghorbanoghli Z, Gonn M, Gonzalez Romero S, Hes FJ, Jundi H, Kelland S, Latchford AR, Brito HL, Lynch PM, Meuser E, Mork ME, Mort M, Garcia MN, Nielsen M, Parc Y, Ricci MT, Saurin JC, Tuin KV, Vasen H, Vilar E, Vinet O, Vitellaro M, Walton SJ, West HD, Sampson JR. Duodenal Adenomas and Cancer in MUTYH-associated Polyposis: An International Cohort Study. Gastroenterology. 2021;160:952-954.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 473] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 13. | van Leerdam ME, Roos VH, van Hooft JE, Dekker E, Jover R, Kaminski MF, Latchford A, Neumann H, Pellisé M, Saurin JC, Tanis PJ, Wagner A, Balaguer F, Ricciardiello L. Endoscopic management of polyposis syndromes: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2019;51:877-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 14. | Pennazio M, Rondonotti E, Despott EJ, Dray X, Keuchel M, Moreels T, Sanders DS, Spada C, Carretero C, Cortegoso Valdivia P, Elli L, Fuccio L, Gonzalez Suarez B, Koulaouzidis A, Kunovsky L, McNamara D, Neumann H, Perez-Cuadrado-Martinez E, Perez-Cuadrado-Robles E, Piccirelli S, Rosa B, Saurin JC, Sidhu R, Tacheci I, Vlachou E, Triantafyllou K. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2022. Endoscopy. 2023;55:58-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 146] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 15. | Groves CJ, Saunders BP, Spigelman AD, Phillips RK. Duodenal cancer in patients with familial adenomatous polyposis (FAP): results of a 10 year prospective study. Gut. 2002;50:636-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 239] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | Morales J, Pujar S, Loveland JE, Astashyn A, Bennett R, Berry A, Cox E, Davidson C, Ermolaeva O, Farrell CM, Fatima R, Gil L, Goldfarb T, Gonzalez JM, Haddad D, Hardy M, Hunt T, Jackson J, Joardar VS, Kay M, Kodali VK, McGarvey KM, McMahon A, Mudge JM, Murphy DN, Murphy MR, Rajput B, Rangwala SH, Riddick LD, Thibaud-Nissen F, Threadgold G, Vatsan AR, Wallin C, Webb D, Flicek P, Birney E, Pruitt KD, Frankish A, Cunningham F, Murphy TD. A joint NCBI and EMBL-EBI transcript set for clinical genomics and research. Nature. 2022;604:310-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 282] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 17. | Sutcliffe EG, Bartenbaker Thompson A, Stettner AR, Marshall ML, Roberts ME, Susswein LR, Wang Y, Klein RT, Hruska KS, Solomon BD. Multi-gene panel testing confirms phenotypic variability in MUTYH-Associated Polyposis. Fam Cancer. 2019;18:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Win AK, Reece JC, Dowty JG, Buchanan DD, Clendenning M, Rosty C, Southey MC, Young JP, Cleary SP, Kim H, Cotterchio M, Macrae FA, Tucker KM, Baron JA, Burnett T, Le Marchand L, Casey G, Haile RW, Newcomb PA, Thibodeau SN, Hopper JL, Gallinger S, Winship IM, Lindor NM, Jenkins MA. Risk of extracolonic cancers for people with biallelic and monoallelic mutations in MUTYH. Int J Cancer. 2016;139:1557-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Nielsen M, Poley JW, Verhoef S, van Puijenbroek M, Weiss MM, Burger GT, Dommering CJ, Vasen HF, Kuipers EJ, Wagner A, Morreau H, Hes FJ. Duodenal carcinoma in MUTYH-associated polyposis. J Clin Pathol. 2006;59:1212-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Zhu LH, Dong J, Li WL, Kou ZY, Yang J. Genotype-Phenotype Correlations in Autosomal Dominant and Recessive APC Mutation-Negative Colorectal Adenomatous Polyposis. Dig Dis Sci. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 21. | Alderlieste YA, Rauws EA, Mathus-Vliegen EM, Fockens P, Dekker E. Prospective enteroscopic evaluation of jejunal polyposis in patients with familial adenomatous polyposis and advanced duodenal polyposis. Fam Cancer. 2013;12:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Yabuuchi Y, Yoshida M, Kakushima N, Kato M, Iguchi M, Yamamoto Y, Kanetaka K, Uraoka T, Fujishiro M, Sho M; Japan Duodenal Cancer Committee. Risk Factors for Non-Ampullary Duodenal Adenocarcinoma: A Systematic Review. Dig Dis. 2022;40:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Thiruvengadam SS, Lopez R, O'Malley M, LaGuardia L, Church JM, Kalady M, Walsh RM, Burke CA. Spigelman stage IV duodenal polyposis does not precede most duodenal cancer cases in patients with familial adenomatous polyposis. Gastrointest Endosc. 2019;89:345-354.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Thomas LE, Hurley JJ, Meuser E, Jose S, Ashelford KE, Mort M, Idziaszczyk S, Maynard J, Brito HL, Harry M, Walters A, Raja M, Walton SJ, Dolwani S, Williams GT, Morgan M, Moorghen M, Clark SK, Sampson JR. Burden and Profile of Somatic Mutation in Duodenal Adenomas from Patients with Familial Adenomatous- and MUTYH-associated Polyposis. Clin Cancer Res. 2017;23:6721-6732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Aelvoet AS, Buttitta F, Ricciardiello L, Dekker E. Management of familial adenomatous polyposis and MUTYH-associated polyposis; new insights. Best Pract Res Clin Gastroenterol. 2022;58-59:101793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 26. | Weiss JM, Gupta S, Burke CA, Axell L, Chen LM, Chung DC, Clayback KM, Dallas S, Felder S, Gbolahan O, Giardiello FM, Grady W, Hall MJ, Hampel H, Hodan R, Idos G, Kanth P, Katona B, Lamps L, Llor X, Lynch PM, Markowitz AJ, Pirzadeh-Miller S, Samadder NJ, Shibata D, Swanson BJ, Szymaniak BM, Wiesner GL, Wolf A, Yurgelun MB, Zakhour M, Darlow SD, Dwyer MA, Campbell M. NCCN Guidelines® Insights: Genetic/Familial High-Risk Assessment: Colorectal, Version 1.2021. J Natl Compr Canc Netw. 2021;19:1122-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 27. | Burke CA, Santisi J, Church J, Levinthal G. The utility of capsule endoscopy small bowel surveillance in patients with polyposis. Am J Gastroenterol. 2005;100:1498-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Gupta A, Postgate AJ, Burling D, Ilangovan R, Marshall M, Phillips RK, Clark SK, Fraser CH. A prospective study of MR enterography versus capsule endoscopy for the surveillance of adult patients with Peutz-Jeghers syndrome. AJR Am J Roentgenol. 2010;195:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Iaquinto G, Fornasarig M, Quaia M, Giardullo N, D'Onofrio V, Iaquinto S, Di Bella S, Cannizzaro R. Capsule endoscopy is useful and safe for small-bowel surveillance in familial adenomatous polyposis. Gastrointest Endosc. 2008;67:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Lewis BS, Eisen GM, Friedman S. A pooled analysis to evaluate results of capsule endoscopy trials. Endoscopy. 2005;37:960-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Zagorowicz ES, Pietrzak AM, Wronska E, Pachlewski J, Rutkowski P, Kraszewska E, Regula J. Small bowel tumors detected and missed during capsule endoscopy: single center experience. World J Gastroenterol. 2013;19:9043-9048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Kim ER. Roles of Capsule Endoscopy and Device-Assisted Enteroscopy in the Diagnosis and Treatment of Small-Bowel Tumors. Clin Endosc. 2020;53:410-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Han JW, Hong SN, Jang HJ, Jeon SR, Cha JM, Park SJ, Byeon JS, Ko BM, Kim ER, Choi H, Chang DK. Clinical Efficacy of Various Diagnostic Tests for Small Bowel Tumors and Clinical Features of Tumors Missed by Capsule Endoscopy. Gastroenterol Res Pract. 2015;2015:623208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Curia MC, Catalano T, Aceto GM. MUTYH: Not just polyposis. World J Clin Oncol. 2020;11:428-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (4)] |
| 35. | Vasen HF, Möslein G, Alonso A, Aretz S, Bernstein I, Bertario L, Blanco I, Bülow S, Burn J, Capella G, Colas C, Engel C, Frayling I, Friedl W, Hes FJ, Hodgson S, Järvinen H, Mecklin JP, Møller P, Myrhøi T, Nagengast FM, Parc Y, Phillips R, Clark SK, de Leon MP, Renkonen-Sinisalo L, Sampson JR, Stormorken A, Tejpar S, Thomas HJ, Wijnen J. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut. 2008;57:704-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 470] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 36. | Stjepanovic N, Moreira L, Carneiro F, Balaguer F, Cervantes A, Balmaña J, Martinelli E; ESMO Guidelines Committee. Hereditary gastrointestinal cancers: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1558-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |