Published online Nov 15, 2023. doi: 10.4251/wjgo.v15.i11.1864
Peer-review started: August 25, 2023
First decision: September 5, 2023
Revised: September 15, 2023
Accepted: October 16, 2023
Article in press: October 16, 2023
Published online: November 15, 2023
Processing time: 82 Days and 4.9 Hours
Studies evaluating the characteristics of dual primary gastric and colorectal cancer (CRC) (DPGCC) are limited.
To analyze the clinicopathologic characteristics and prognosis of synchronous and metachronous cancers in patients with DPGCC.
From October 2010 to August 2021, patients with DPGCC were retrospectively reviewed. The patients with DPGCC were divided into two groups (synchronous and metachronous). We compared the overall survival (OS) between the groups using Kaplan-Meier survival methods. Univariate and multivariate analyses were performed using Cox’s proportional hazards model to identify the independent prognostic factors for OS.
Of the 76 patients with DPGCC, 46 and 30 had synchronous and metachronous cancers, respectively. The proportion of unresectable CRC in patients with synchronous cancers was higher than that in patients with metachronous cancers (28.3% vs 3.3%, P = 0.015). The majority of the second primary cancers had occurred within 5 years. Kaplan-Meier survival analysis showed that the patients with metachronous cancers had a better prognosis than patients with synchronous cancers (P = 0.010). The patients who had undergone gastrectomy (P < 0.001) or CRC resection (P < 0.001) had a better prognosis than those who had not. In the multivariate analysis, synchronous cancer [hazard ratio (HR) = 6.8, 95% confidence interval (95%CI): 2.0-22.7, P = 0.002)] and stage III-IV gastric cancer (GC) [HR = 10.0, 95%CI: 3.4-29.5, P < 0.001)] were risk prognostic factor for OS, while patients who underwent gastrectomy was a protective prognostic factor for OS [HR = 0.2, 95%CI: 0.1-0.6, P = 0.002].
Regular surveillance for metachronous cancer is necessary during postoperative follow-up. Surgical resection is the mainstay of therapy to improve the prognosis of DPGCC. The prognosis appears to be influenced by the stage of GC rather than the stage of CRC. Patients with synchronous cancer have a worse prognosis, and its treatment strategy is worth further exploration.
Core Tip: We conducted a retrospective analysis of the patients with dual primary gastric and colorectal cancer from our hospital database. We found some interesting results. Firstly, the majority of the second primary cancers had happened within 5 years, suggesting that surveillance for metachronous cancer is necessary during the postoperative follow-up. Secondly, the patients with metachronous cancers had a better prognosis than patients with synchronous cancers. Thirdly, the prognosis appears to be influenced by the stage of gastric cancer rather than the stage of colorectal cancer. Therefore, the treatment strategy for synchronous cancers is worth further exploring. In conclusion, the findings in the study are valuable for clinical practice.
- Citation: Lin YJ, Chen HX, Zhang FX, Hu XS, Huang HJ, Lu JH, Cheng YZ, Peng JS, Lian L. Features of synchronous and metachronous dual primary gastric and colorectal cancer. World J Gastrointest Oncol 2023; 15(11): 1864-1873
- URL: https://www.wjgnet.com/1948-5204/full/v15/i11/1864.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i11.1864
Colorectal cancer (CRC) and gastric cancer (GC) are the most common gastrointestinal malignancies and leading causes of cancer-related death[1]. With the advancement and widespread use of cancer screening, the detection rate of synchronous tumors is increasing. In addition, the development of treatment modalities results in delayed cancer progression and prolonged survival, and the incidence of metachronous tumors has increased. Knowing the outcomes of these multiple primary cancers is valuable. A study has shown that CRC is the most common second primary cancer in patients with GC[2]. For GC patients with a second primary cancer, a study reported that synchronous cancers have a worse prognosis than metachronous cancers[3]. CRC and GC show high morbidity and high mortality in China[4], but studies on dual primary gastric and CRC (DPGCC) are limited. The present study analyses the clinicopathologic characteristics and prognosis of patients with DPGCC. These data will provide important information to further our under
A total of 131 patients with DPGCC at the Sixth Affiliated Hospital of Sun Yat-Sen University from October 2010 to August 2021 were retrospectively included in the study cohort. The requirement for informed consent was waived in this retrospective study, and approval was obtained by the Ethical Committee of the Sixth Affiliated Hospital of Sun Yat-Sen University (No. 2022ZSLYEC-209).
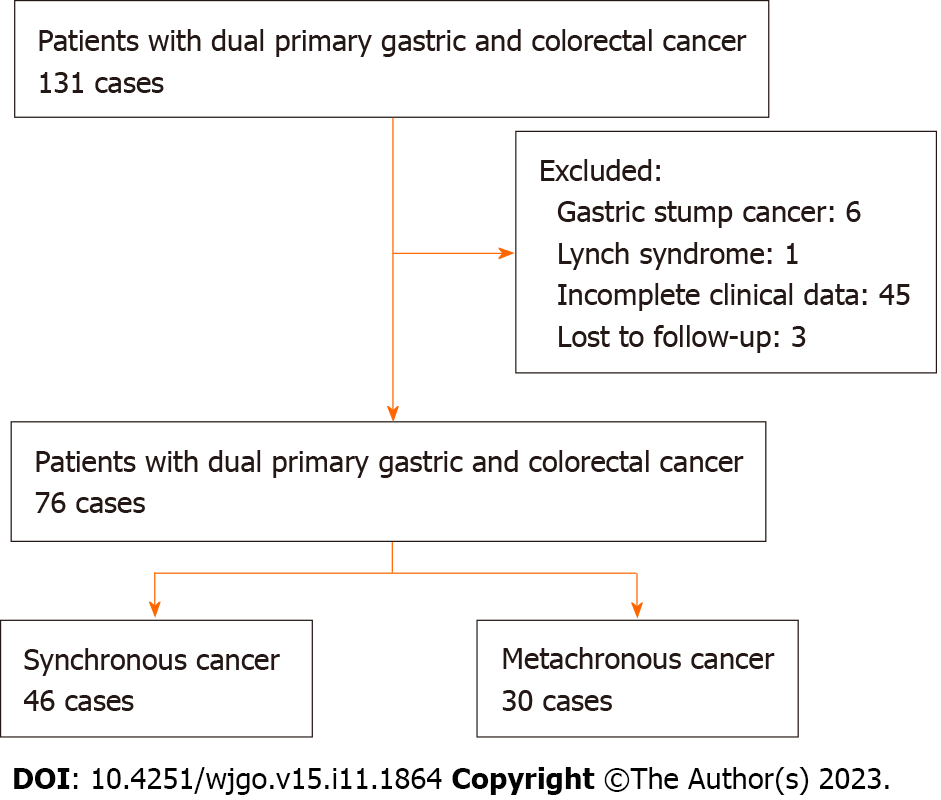
DPGCC was diagnosed according to the criteria of Warren and Gates[5]. The inclusion criteria were as follows: (1) Patients were pathologically diagnosed with DPGCC; and (2) All clinical data for patients were available. Patients were excluded if any of the following were present: (1) Incomplete clinical data and follow-up data; (2) Gastric stump cancer; and (3) Lynch syndrome, familial adenomatous polyposis, or hereditary nonpolyposis CRC. Figure 1 shows the flowchart of the patient selection process.
The clinical data of this study included age, sex, body mass index, tumor location, stage, and the time of diagnosis of the tumors, which were collected from the cancer database of the Sixth Affiliated Hospital of Sun Yat-Sen University. Follow-up data were obtained from the hospital’s follow-up office.
The location of the GC was divided into 3 areas, namely, the upper, middle, and lower regions. The location of CRC was categorized as the right hemicolon (cecum, ascending, and transverse colon), left hemicolon (descending colon), sigmoid colon, and rectum. Synchronous cancer was defined as the second primary cancer diagnosed within 6 months before or after the first primary cancer detection, and metachronous cancer was defined as the second primary cancer detected more than 6 months before or after the first primary cancer[6]. The cTNM stage or (y)pTNM stage was determined by the eighth edition of the American Joint Committee on Cancer Staging Manual[7]. The primary study outcome was overall survival (OS). In this study, OS was defined as the time from the date of diagnosis of the first tumor to death from any cause.
Statistical evaluation was performed using R software (version 4.1.2, http://www.r-project.org). Continuous variables with a normal distribution are presented as the mean ± SD. Continuous variables that were not normally distributed are presented as medians (interquartile ranges). To test the associations between categorical variables either Pearson’s chi-squared test or Fisher’s exact test was used. Time-dependent survival probabilities were calculated using the Kaplan-Meier method, and the log-rank significance test was used to estimate the survival differences among various subgroups. Univariate and multivariate analyses of various variables were performed using Cox’s proportional hazards model to identify the independent prognostic factors for OS. Clinical covariates with P < 0.05 in the univariate analysis were used in the multivariate Cox regression. All statistical tests were two-sided, and P < 0.05 was considered statistically significant.
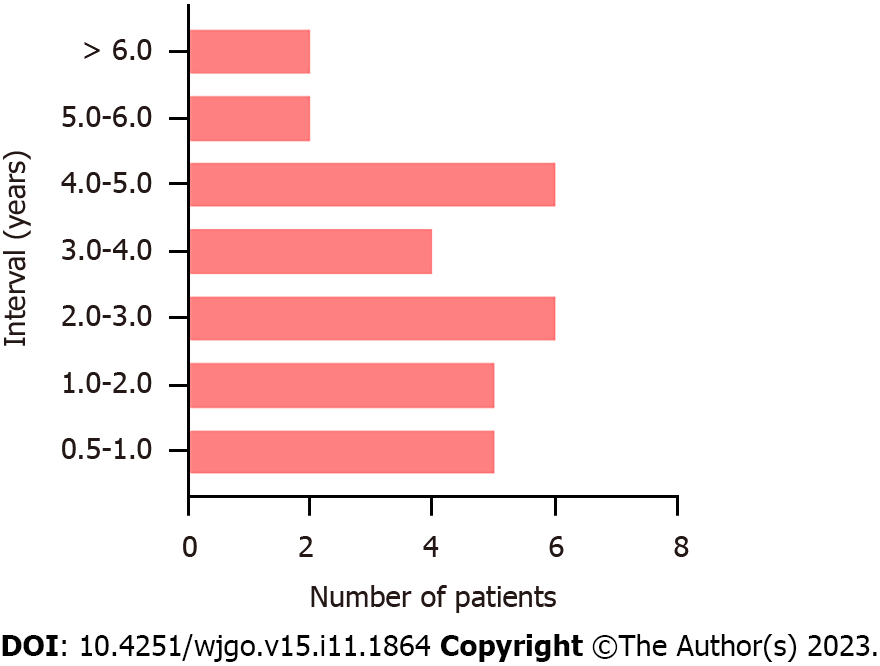
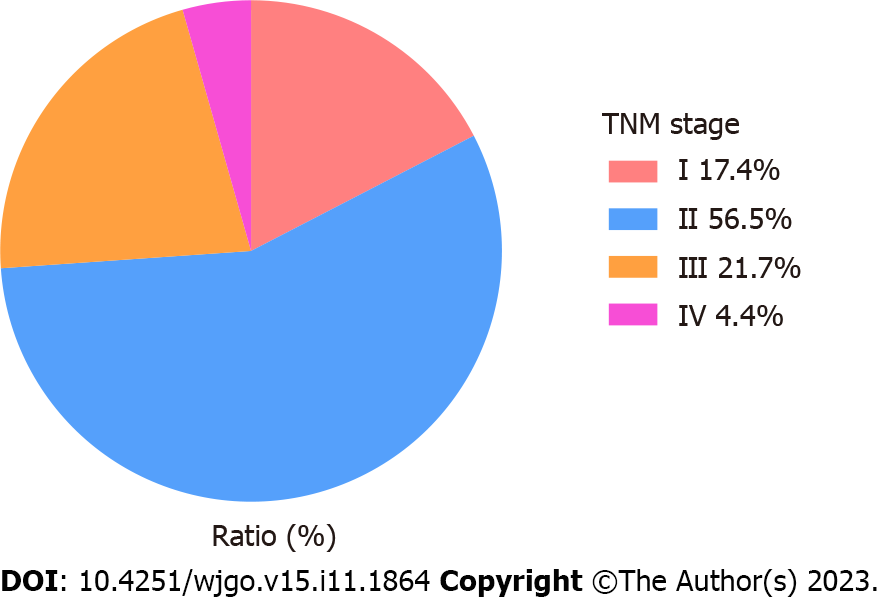
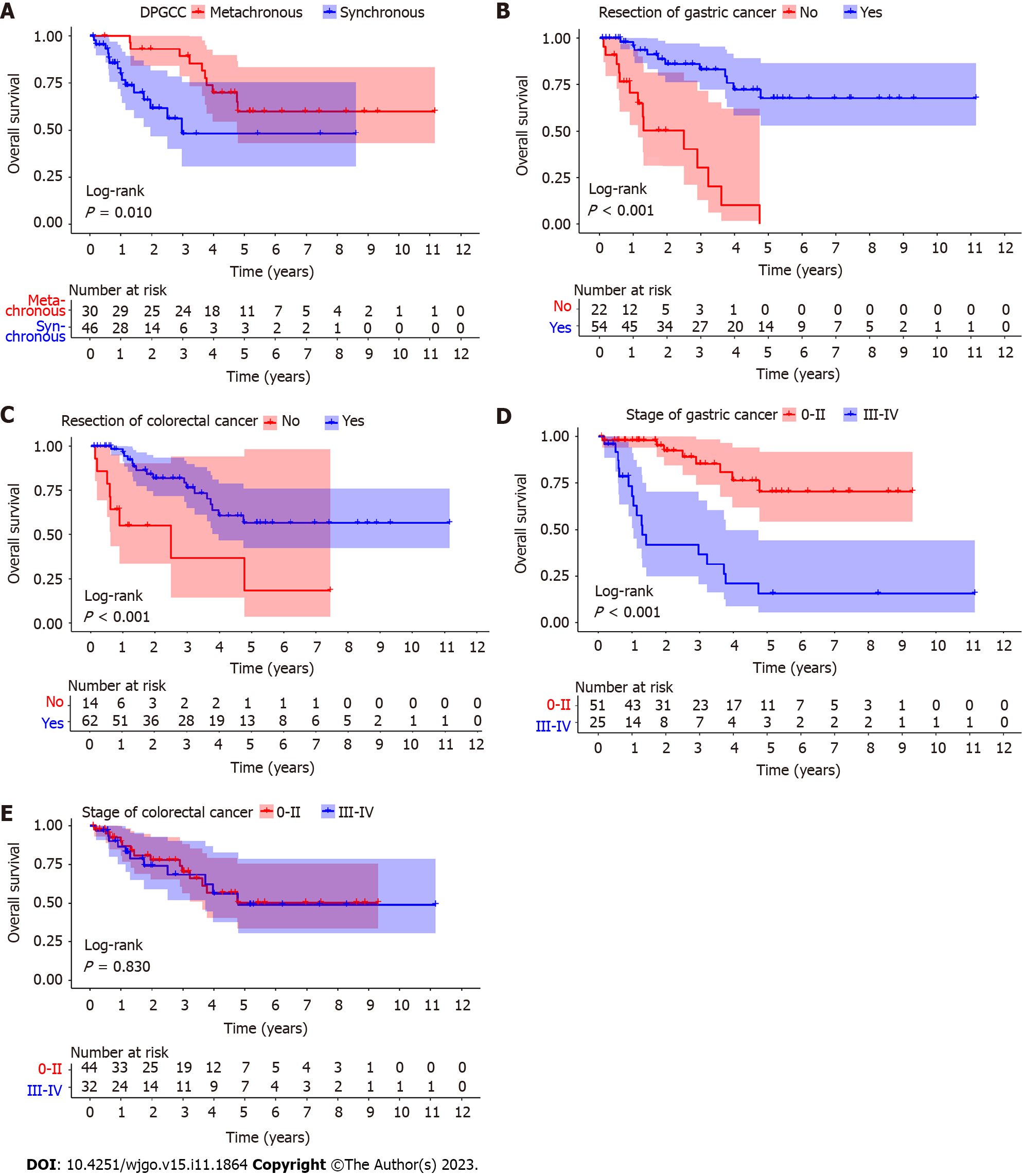
A total of 76 patients with DPGCC were finally included in the study. Of the 76 patients, 46 had synchronous cancers and 30 had metachronous cancers. There were 52 males (68.4%) and 24 females (31.6%). The average age was 66.3 ± 12.3 years (range 22-88 years). For metachronous cancers, the interval time from the first primary cancer to the second primary cancer is shown in Figure 2. The majority of the second primary cancers had occurred within 5 years. Among the 30 patients with metachronous cancers, the first primary cancer was CRC in 23 patients, while the second primary cancer was GC in 7 patients. Consequently, we focused on the stage of the second primary cancer. For the patients with DPGCC whose first primary cancer was CRC, the majority of the second primary cancer (GC) was stage I-II (73.9%) (Figure 3). The proportion of unresectable CRC in patients with synchronous cancers was higher than that in patients with metachronous cancers (28.3% vs 3.3%, P = 0.015). There were no significant differences in the distributions of the other variables (Table 1). Figure 4 illustrates the Kaplan-Meier survival analysis. The patients with metachronous cancer had a better prognosis than that of patients with synchronous cancer (P = 0.010) (Figure 4A). The patients with stage 0- II GC had a better prognosis than that of patients with stage III-IV GC (P < 0.001) (Figure 4D). The patients who had undergone gastrectomy (P < 0.001) or CRC resection (P < 0.001) had a better prognosis than that of patients who had not (Figure 4B and C). Nonetheless, no significant divergence in the survival rates was observed between stage 0-II and stage III-IV CRC (Figure 4E). The median follow-up duration was 3.4 years. The 1-, 3-, and 5-year OS rates in the cohort were 88.4%, 69.6%, and 49.7%, respectively.
| Characteristics | Overall, n = 76 | Metachronous, n = 30 | Synchronous, n = 46 | P value | |
| Age (yr) (%) | ≤ 65 | 35 (46.1) | 15 (50.0) | 20 (43.5) | 0.747 |
| > 65 | 41 (53.9) | 15 (50.0) | 26 (56.5) | ||
| Sex (%) | Female | 24 (31.6) | 9 (30.0) | 15 (32.6) | 1.000 |
| Male | 52 (68.4) | 21 (70.0) | 31 (67.4) | ||
| Location of gastric cancer (%) | Lower | 40 (52.6) | 17 (56.7) | 23 (50.0) | 0.850 |
| Middle | 11 (14.5) | 4 (13.3) | 7 (15.2) | ||
| Upper | 25 (32.9) | 9 (30.0) | 16 (34.8) | ||
| Stage of gastric cancer (%) | 0-II | 51 (67.1) | 21 (70.0) | 30 (65.2) | 0.854 |
| III-IV | 25 (32.9) | 9 (30.0) | 16 (34.8) | ||
| Resection of gastric cancer (%) | No | 22 (28.9) | 7 (23.3) | 15 (32.6) | 0.540 |
| Yes | 54 (71.1) | 23 (76.7) | 31 (67.4) | ||
| Location of colorectal cancer (%) | Left hemicolon | 6 (7.9) | 2 (6.7) | 4 (8.7) | 0.121 |
| Rectum | 27 (35.5) | 14 (46.7) | 13 (28.3) | ||
| Right hemicolon | 24 (31.6) | 5 (16.7) | 19 (41.3) | ||
| Sigmoid colon | 19 (25.0) | 9 (30.0) | 10 (21.7) | ||
| Stage of colorectal cancer (%) | 0-II | 44 (57.9) | 16 (53.3) | 28 (60.9) | 0.680 |
| III-IV | 32 (42.1) | 14 (46.7) | 18 (39.1) | ||
| Resection of colorectal cancer (%) | No | 14 (18.4) | 1 (3.3) | 13 (28.3) | 0.0151 |
| Yes | 62 (81.6) | 29 (96.7) | 33 (71.7) | ||
To evaluate the prognostic factors of DPGCC, univariate and multivariate Cox regression analyses were performed. According to the univariate analysis, synchronous/metachronous cancer [hazard ratio (HR) = 3.0, 95% confidence interval (95%CI): 1.3-7.2, P = 0.013)], stage of GC (HR = 6.7, 95%CI: 2.9-15.6, P < 0.001), resection of GC (HR = 0.1, 95%CI: 0.1-0.3, P < 0.001), and resection of CRC (HR = 0.3, 95%CI: 0.1-0.6, P = 0.002) were associated with the prognosis of DPGCC (Table 2). In the multivariate analysis, synchronous cancer (HR = 6.8, 95%CI: 2.0-22.7, P = 0.002) and stage III-IV of GC (HR = 10.0, 95%CI: 3.4-29.5, P < 0.001) were independently associated with a worse prognosis. Patients who underwent gastrectomy (HR = 0.2, 95%CI: 0.1-0.6, P = 0.002) had a better OS rate.
| Characteristics | Univariate | Multivariate | |||
| HR (95%CI) | P value | HR (95%CI) | P value | ||
| Age | ≤ 65 | Reference | |||
| > 65 | 1.8 (0.8-4.1) | 0.162 | |||
| Sex | Female | Reference | |||
| Male | 0.9 (0.4-2.0) | 0.719 | |||
| Location of gastric cancer | Lower | Reference | |||
| Middle | 1.9 (0.6-6.2) | 0.262 | |||
| Upper | 1.8 (0.7-4.1) | 0.200 | |||
| Stage of gastric cancer | 0-II | Reference | |||
| III-IV | 6.7 (2.9-15.6) | < 0.001 | 10.0 (3.4-29.5) | < 0.0011 | |
| Resection of gastric cancer | No | Reference | |||
| Yes | 0.1 (0.1-0.3) | < 0.001 | 0.2 (0.1-0.6) | 0.0021 | |
| Location of colorectal cancer | Right hemicolon | Reference | |||
| Left hemicolon | 1.1 (0.2-5.3) | 0.909 | |||
| Sigmoid colon | 1.1 (0.4-3.1) | 0.882 | |||
| Rectum | 0.9 (0.3-2.5) | 0.863 | |||
| Stage of colorectal cancer | 0-II | Reference | |||
| III-IV | 1.1 (0.5-2.4) | 0.831 | |||
| Resection of colorectal cancer | No | Reference | |||
| Yes | 0.3 (0.1-0.6) | 0.002 | 0.4 (0.1-1.5) | 0.202 | |
| DPGCC | Metachronous | Reference | |||
| Synchronous | 3.0 (1.3-7.2) | 0.013 | 6.8 (2.0-22.7) | 0.0021 | |
In this retrospective study, we collected cases of DPGCC in a tertiary hospital and analyzed the clinical features and prognoses between synchronous and metachronous cancer. The study showed that patients with metachronous cancer had a better prognosis than that of patients with synchronous cancer. Synchronous cancer and stage III-IV GC were independent risk prognostic factors for OS, while resection of GC was an independent protective prognostic factor.
The theory of the etiologic field effect has often been applied to explain the predisposition and progression of multiple primary cancers[8]. The theory is also applicable to the initiation and progression of DPGCC. These patients' gastrointestinal tracts are exposed to a combination of genetic and environmental factors, which contribute to the development of the disease. As integral components of the continuous mucosal epithelium lining the gastrointestinal tract, the epithelium of the stomach and colorectum is influenced by these factors, which predispose to synchronous or metachronous carcinogenesis. Furthermore, the second primary cancer has a certain correlation with the first primary cancer in patients with multiple primary cancers[9]. In this study, we focused on DPGCC because both are digestive tract cancers and have a specific correlation. CRC is one of the most common tumors with multiple primary cancers, with an incidence of 11.4%[9]. In addition, GC can also develop into a second primary cancer[10]. The incidence of second primary cancer in GC patients ranges from 1% to 4.2%[2,11,12]. CRC is the most common second primary cancer in patients with GC. A study suggested that the incidence of CRC in patients with GC was 1.3% (42/3291)[13]. In addition, GC is also the most common second primary cancer in patient with CRC. Another study showed that the incidence of GC in patients with CRC was 2%-2.4%[14,15]. With the advancement of examination techniques and treatment methods, the survival of patients with cancer will be prolonged, and the rate of detection and incidence of multiple primary cancers will gradually increase. Therefore, for patients with DPGCC, regardless of which tumor is diagnosed first, checking for the presence of another tumor should be done[16,17].
In the present study, we found that patients with synchronous cancer had a worse prognosis than patients with metachronous cancer. We defined survival time as the time from the date of diagnosis of the first tumor to death from any cause. These patients with metachronous cancer had a better treatment response for the first primary cancer, resulting in having enough time to develop a second primary cancer. Therefore, patients with synchronous cancer were associated with a poor prognosis, which is consistent with previous findings[18]. In addition, another study defined survival time as the time from the date of surgery or chemotherapy for the second GC or CRC[3]. They also found that patients with synchronous cancer had a worse prognosis than patients with metachronous cancer. Taken together, these results suggest that synchronous cancer may be associated with poor prognosis in patients with DPGCC.
The treatment strategy for synchronous cancer is worth further study, because of the worse prognosis of synchronous cancer. In this cohort of synchronous cancer, approximately 70% of patients had a chance to receive resection. Moreover, the resection of GC was a prognostic protective factor. We believe that for synchronous cancer with a definitive diagnosis, radical surgery should be performed for all tumors. Even if radical resection is not possible, palliative resection should be attempted to achieve the best therapeutic outcome. A previous study found that the majority of second primary cancers develop within 3 years after the first primary cancer[19].
In this study, we found that most of the second primary cancers occurred within 5 years. The second primary cancer was detected by gastrointestinal endoscopy in long-term follow-up with surveillance. Therefore, it is essential and valuable for patients to undergo regular follow-up and surveillance. For patients with GC or CRC, we believe that postoperative surveillance of cancer should cover the whole digestive tract, rather than focusing on the primary site. Typically, the first primary cancer is considered cured 5 years after radical resection, and the risk of second primary cancer then significantly decreases. However, a study reported that second primary cancer occurred 10 years after the treatment of the first primary cancer in some cases with GC[20]. Therefore, the author proposed that patients with GC who underwent radical resection may need a longer time for regular and comprehensive surveillance. The current strategy for the follow-up of cancer focuses on surveillance within 5 years after surgery, but there is insufficient attention to longer follow-up. Considering economic factors, more evidence is needed to determine a more cost-effective surveillance strategy.
This type of multiple primary cancer has also been reported in other countries[3,18,21-23]. Studies have assessed the clinical characteristics of patients with GC who develop tumors at other locations, such as CRC and thyroid cancer[21]. Our study exclusively focused on the cases of DPGCC due to their significant interrelation. Unlike other studies that primarily examined the risk factors for developing secondary tumors[22], we placed greater emphasis on the prognostic factors of patients diagnosed with DPGCC. In addition to demographic characteristics and pathological factors, therapeutic factors also play a crucial role in predicting the prognosis of DPGCC. Our results revealed that the resection of GC was an independent predictor of OS. This finding will aid in the development of more effective treatment and follow-up strategies. However, unlike the study conducted by Bok et al[23], this study did not compare DPGCC with simple GC or CRC.
This study has some limitations. First, this is a retrospective study. Second, this was a single-center study with a small sample size. To overcome these limitations, a multicenter and large sample size study is needed to validate these results.
In this study, the unresectable rate of synchronous CRC was higher than that of metachronous CRC. Early diagnosis and surgical resection are the key factors in improving the outcome of patients with DPGCC. The prognosis appears to be influenced by the stage of GC rather than the stage of CRC. Patients with synchronous cancer had worse OS, so radical resection should be tried, and a better treatment strategy is worth further investigation.
Colorectal cancer (CRC) and gastric cancer (GC) show high morbidity in China. The incidence of synchronous and metachronous dual primary gastric and CRC (DPGCC) has increased. The studies evaluating the characteristics of DPGCC are limited.
Currently, there are limited clinical investigations regarding the prognosis of DPGCC. The current treatment strategy primarily comprises the treatment approach utilized for colorectal or GC. However, DPGCC may represent a unique tumor type with distinct histological, clinical, and molecular features. Therefore, it is crucial to meticulously analyze the clinical characteristics and prognosis of DPGCC to aid in clinical diagnosis and treatment.
The present study analyses the clinicopathologic characteristics and prognosis in patients with DPGCC. These data will provide important information to further our understanding of the diagnosis and treatment for DPGCC.
Seventy-six patients with DPGCC at the Sixth Affiliated Hospital of Sun Yat-Sen University from October 2010 to August 2021 were included in the study cohort. The patients with DPGCC were divided into two groups synchronous and metachronous. We compared overall survival (OS) between groups using Kaplan-Meier survival methods. Univariate and multivariate analyses were performed using Cox's proportional hazards model to identify the independent prognostic factors for OS.
Of the 76 patients with DPGCC, 46 and 30 were synchronous and metachronous cancers, respectively. The proportion of unresectable CRC in patients with synchronous cancers was higher than that in patients with metachronous cancers (28.3% vs 3.3%, P = 0.015). The majority of the second primary cancers had happened within 5 years. Kaplan-Meier survival analysis showed that the patients with metachronous cancers had a better prognosis than patients with synchronous cancers (P = 0.010). The patients who had undergone gastrectomy (P < 0.001) or CRC resection (P < 0.001) had a better prognosis than those who had not. In the multivariate analysis, synchronous cancer [hazard ratio (HR) = 6.8, 95% confidence interval (95%CI): 2.0-22.7, P = 0.002] and the stage III-IV of GC (HR = 10.0, 95%CI: 3.4-29.5, P < 0.001) were a risk prognostic factor for OS, while patients who underwent gastrectomy was a protective prognostic factor for OS (HR = 0.2, 95%CI: 0.1-0.6, P = 0.002).
In this study, early diagnosis and surgical resection are the key factors in improving the outcome of patients with DPGCC. The prognosis appears to be influenced by the stage of GC rather than the stage of CRC. The patients with synchronous cancer had worse OS, so radical resection should be tried and the better treatment strategy is worth further investigation.
To further validate our findings and provide a more comprehensive understanding of the prognostic factors of DPGCC, an additional retrospective and prospective study with a larger sample size and more extensive prognostic information is warranted.
We appreciated the cancer database of the Sixth Affiliated Hospital of Sun Yat-Sen University for helping to collect data.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jeong KY, South Korea; Ogino S, United States S-Editor: Lin C L-Editor: A P-Editor: Wu RR
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64104] [Article Influence: 16026.0] [Reference Citation Analysis (174)] |
| 2. | Ikeda Y, Saku M, Kawanaka H, Nonaka M, Yoshida K. Features of second primary cancer in patients with gastric cancer. Oncology. 2003;65:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Watanabe M, Kochi M, Fujii M, Kaiga T, Mihara Y, Funada T, Tamegai H, Shimizu H, Takayama T. Dual primary gastric and colorectal cancer: is the prognosis better for synchronous or metachronous? Am J Clin Oncol. 2012;35:407-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Xie Y, Shi L, He X, Luo Y. Gastrointestinal cancers in China, the USA, and Europe. Gastroenterol Rep (Oxf). 2021;9:91-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 127] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 5. | Warren S. Multiple primary malignant tumors: a survey of the literature and statistical study. Am J cancer. 1932;51:1358-1414. |
| 6. | Lee SH, Ahn BK, Baek SU. Multiple primary cancers in extracolonic sites with colorectal cancer. Int J Colorectal Dis. 2009;24:301-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC cancer staging manual. 9th ed. Cham: Springer, 2017. |
| 8. | Lochhead P, Chan AT, Nishihara R, Fuchs CS, Beck AH, Giovannucci E, Ogino S. Etiologic field effect: reappraisal of the field effect concept in cancer predisposition and progression. Mod Pathol. 2015;28:14-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 9. | Tanjak P, Suktitipat B, Vorasan N, Juengwiwattanakitti P, Thiengtrong B, Songjang C, Therasakvichya S, Laiteerapong S, Chinswangwatanakul V. Risks and cancer associations of metachronous and synchronous multiple primary cancers: a 25-year retrospective study. BMC Cancer. 2021;21:1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (1)] |
| 10. | Buyukasik O, Hasdemir AO, Gulnerman Y, Col C, Ikiz O. Second primary cancers in patients with gastric cancer. Radiol Oncol. 2010;44:239-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Ha TK, An JY, Youn HG, Noh JH, Sohn TS, Kim S. Surgical outcome of synchronous second primary cancer in patients with gastric cancer. Yonsei Med J. 2007;48:981-987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Eom BW, Lee HJ, Yoo MW, Cho JJ, Kim WH, Yang HK, Lee KU. Synchronous and metachronous cancers in patients with gastric cancer. J Surg Oncol. 2008;98:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Lee JH, Bae JS, Ryu KW, Lee JS, Park SR, Kim CG, Kook MC, Choi IJ, Kim YW, Park JG, Bae JM. Gastric cancer patients at high-risk of having synchronous cancer. World J Gastroenterol. 2006;12:2588-2592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Lim SB, Jeong SY, Choi HS, Sohn DK, Hong CW, Jung KH, Chang HJ, Park JG, Choi IJ, Kim CG. Synchronous gastric cancer in primary sporadic colorectal cancer patients in Korea. Int J Colorectal Dis. 2008;23:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Yoon SN, Oh ST, Lim SB, Kim TW, Kim JH, Yu CS, Kim JC. Clinicopathologic characteristics of colorectal cancer patients with synchronous and metachronous gastric cancer. World J Surg. 2010;34:2168-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Kim HO, Hwang SI, Yoo CH, Kim H. Preoperative colonoscopy for patients with gastric adenocarcinoma. J Gastroenterol Hepatol. 2009;24:1740-1744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Park DI, Park SH, Yoo TW, Kim HS, Yang SK, Byeon JS, Koh BM, Kim JO, Shim KN, Jeen YT, Lee BI, Choi KY, Lee HL, Han DS, Baek I, Park CH, Park SJ. The prevalence of colorectal neoplasia in patients with gastric cancer: a Korean Association for the Study of Intestinal Disease (KASID) Study. J Clin Gastroenterol. 2010;44:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Park JH, Baek JH, Yang JY, Lee WS, Lee WK. Clinicopathologic characteristics and survival rate in patients with synchronous or metachronous double primary colorectal and gastric cancer. Korean J Clin Oncol. 2018;14:83-88. [DOI] [Full Text] |
| 19. | Ueno M, Muto T, Oya M, Ota H, Azekura K, Yamaguchi T. Multiple primary cancer: an experience at the Cancer Institute Hospital with special reference to colorectal cancer. Int J Clin Oncol. 2003;8:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Kim JY, Jang WY, Heo MH, Lee KK, Do YR, Park KU, Song HS, Kim YN. Metachronous double primary cancer after diagnosis of gastric cancer. Cancer Res Treat. 2012;44:173-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Kim JH, Rha SY, Kim C, Kim GM, Yoon SH, Kim KH, Kim MJ, Ahn JB, Chung HC, Roh JK, Kim HS. Clinicopathologic features of metachronous or synchronous gastric cancer patients with three or more primary sites. Cancer Res Treat. 2010;42:217-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Kim HJ, Kim N, Choi YJ, Yoon H, Shin CM, Park YS, Lee HS, Ahn SH, Park do J, Kim HH, Son IT, Kang SB, Lee DH. Clinicopathologic features of gastric cancer with synchronous and metachronous colorectal cancer in Korea: are microsatellite instability and p53 overexpression useful markers for predicting colorectal cancer in gastric cancer patients? Gastric Cancer. 2016;19:798-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Bok HJ, Lee JH, Shin JK, Jeon SM, Park JJ, Moon CM, Hong SP, Cheon JH, Kim TI, Kim WH. [Clinicopathologic features of colorectal cancer combined with synchronous and metachronous gastric cancer]. Korean J Gastroenterol. 2013;62:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |