Published online Oct 15, 2023. doi: 10.4251/wjgo.v15.i10.1829
Peer-review started: July 21, 2023
First decision: August 23, 2023
Revised: September 6, 2023
Accepted: September 18, 2023
Article in press: September 18, 2023
Published online: October 15, 2023
Processing time: 80 Days and 11.6 Hours
Although common in lung cancer, somatic epidermal growth factor receptor
A 72-year old woman with a past medical history of post-polio syndrome confined to a wheelchair, scoliosis and hypothyroidism presented with metastatic sigmoid colon adenocarcinoma with hepatic metastases. Next generation sequ
This case shows the benefit of multi-gene sequencing assays to identify potential therapeutic options in patients with refractory disease.
Core Tip: Somatic epidermal growth factor receptor (EGFR) mutations are rarely found in colorectal cancer. Treatment with anti-EGFR antibodies is commonplace, but EGFR tyrosine kinase inhibitors are not standard in colorectal cancer. Here we report a case of sustained response to osimertinib in a metastatic colorectal cancer patient with an EGFR T790M mutation detected with cell-free DNA. She progressed on three lines of treatment, and received fourth-line treatment with off-label osimertinib, with clinical response. She received treatment with osimertinib for seven months. This case shows the benefit of multi-gene sequencing assays to identify potential therapeutic options in patients with refractory disease.
- Citation: Buzard B, Douglass L, Gustafson B, Buckley J, Roth M, Kujtan L, Bansal D. Response to osimertinib in a colorectal cancer patient with an EGFR T790M mutation: A case report. World J Gastrointest Oncol 2023; 15(10): 1829-1834
- URL: https://www.wjgnet.com/1948-5204/full/v15/i10/1829.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i10.1829
Although common in lung cancer, somatic epidermal growth factor receptor (EGFR) mutations are rarely found in colorectal cancer, occurring in approximately 3% of cases[1]. Treatment with anti-EGFR antibodies is commonplace, but EGFR tyrosine kinase (TK) inhibitors (TKIs) are not standard treatments in colorectal cancer. Here we report a case of sustained response to osimertinib in a colorectal cancer patient with an EGFR T790M mutation on cell-free DNA analysis.
Our patient is a 72-year-old white female who presented with a chief complaint of right upper quadrant pain.
She was diagnosed with metastatic sigmoid colon adenocarcinoma with liver involvement in November 2020 (Table 1).
| Date | Event |
| November 2020 | Diagnosed with metastatic colon adenocarcinoma and started on panitumumab, 5-fluorouracil and leucovorin |
| January 2021 | Switched to trifluridine/tipiracil |
| November 2021 | Disease progression on CT imaging |
| December 2021 | Switched to regorafenib |
| February 2022 | Switched to osimertinib |
| November 2023 | Died |
She was a former light smoker who quit 10 years before the diagnosis of colon cancer.
The patient had a past medical history of post-polio syndrome confined to a wheelchair, scoliosis, and hypothyroidism.
Physical examination findings were significant for mild tenderness to palpation in the right upper quadrant without abdominal distention as well as chronic muscle wasting and decreased muscle tone secondary to post-polio syndrome. Her Eastern Cooperative Oncology Group (ECOG) performance status was 3.
Next-generation sequencing (NGS) covering over 600 genes was performed on the liver biopsy and revealed a RAS/RAF wild-type, microsatellite stable, PD-L1 negative malignancy. In addition, TP53 and APC mutations and EGFR amplification with C-terminal deletion in exons 27-28 were discovered. Cell-free DNA analysis revealed an EGFR p.T790M exon 20 somatic mutation with a variant allele frequency (VAF) of 12.3%.
Computed tomography (CT) imaging revealed hepatic metastases at diagnosis.
She was diagnosed with metastatic sigmoid colon adenocarcinoma with liver involvement in November 2020 (Table 1).
She desired to preserve her quality of life and minimize side effects and trips to the cancer center. With these goals in mind, she declined standard frontline treatment options, including FOLFOX and FOLFIRI. This patient was discussed at our molecular tumor board in December 2020. At that time, anti-EGFR antibody therapy was recommended as EGFR amplification was thought to be secondary to the EGFR exon 27-28 deletion and truncation of the C-terminal domain leading to a paradoxical, ligand-independent downstream activation of the MAPK pathway[2]. She initially received panitumumab, 5-fluorouracil, and leucovorin. However, shortly after receiving panitumumab, the patient complained of post-nasal drainage and difficulty swallowing. She declined further treatment with this regimen. Pursuant to her goals of minimizing time spent at the cancer center and using the least toxic regimen, she was transitioned to treatment with trifluridine/tipiracil plus bevacizumab in January 2021[3]. Imaging revealed treatment response with subsequent progression in November 2021, eleven months after initiation of treatment.
Subsequently, she was initiated on third-line treatment with regorafenib in December 2021. The patient experienced multiple treatment interruptions due to poor tolerability (primarily grade 3 hypertension), and the decision was made to stop regorafenib. Her case was re-presented at the molecular tumor board in January 2022. Recommendations at that time were to pursue clinical trial options for anti-EGFR therapy or off-label EGFR TKI therapy. An ECOG performance status of 3 precluded enrollment in local therapeutic clinical trials, and the patient expressed that she did not wish to travel. The decision was made to initiate off-label osimertinib.
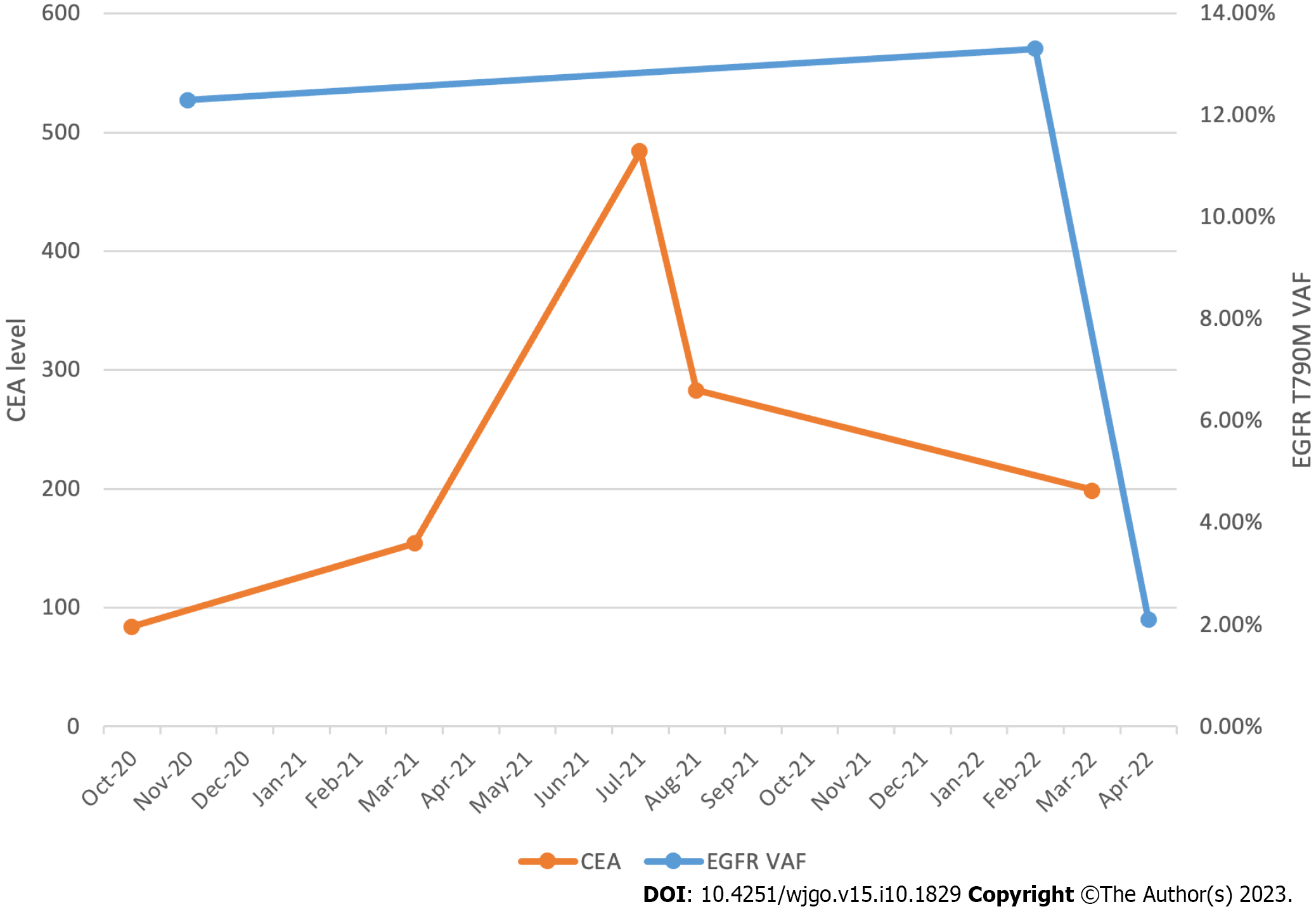
She started osimertinib 80 mg daily in February 2022. The VAF of the EGFR T790M mutation was 13.3%. Two weeks after initiating osimertinib, the patient developed an acneiform rash on both cheeks. Oral minocycline was prescribed, and the rash improved within two weeks. Worsening fatigue and an elevated total bilirubin of 2.1 mg/dL were noted within the first month of therapy. Her fatigue improved, and bilirubin normalized by the start of cycle two without dose modifications. Between March and April of 2022, the patient developed grade 2 anemia and grade 2 thrombocytopenia, both of which were monitored. A CT chest, abdomen, and pelvis with contrast was obtained five weeks after initiating osimertinib and revealed a decrease in the size of liver metastases and an unchanged appearance of the primary sigmoid colon malignancy. The VAF of the EGFR T790M mutation was then 2.1%, which correlated with the response seen on imaging (Figure 1).
In June 2022, a CT scan revealed portal vein thrombosis, and apixaban was initiated. In addition, the osimertinib dose was reduced from 80 mg to 40 mg daily due to the aforementioned hematologic toxicities.
This same CT scan obtained five months after the initiation of osimertinib revealed further improvement in hepatic metastases with a decrease in the size of the dominant hepatic mass from 11.9 cm at the time of initiation of therapy to 8.4 cm (Figure 2). An echocardiogram revealed a decrease in the size of a pericardial effusion, which was present at the time of initiation of osimertinib. Subsequent imaging seven months after initiation of treatment revealed progression of hepatic metastases and new onset large volume ascites and peritoneal carcinomatosis. She decided to pursue hospice at this juncture and passed away two weeks later.
Our patient presented with metastatic colorectal cancer, which became refractory to treatment with trifluridine/tipiracil and bevacizumab. In addition, as described above, she had an intolerance to panitumumab and regorafenib. Cell-free DNA analysis revealed an EGFR T790M mutation. The patient's case was referred to our molecular tumor board, and the recommendation was to consider a trial of osimertinib. The patient had a response for over six months. While there is preclinical evidence for utilizing osimertinib in colon cancer, we could find only one clinical case report of using osimertinib in colon cancer[4,5].
The EGFR gene is located on chromosome 7p12-13 and encodes a transmembrane receptor composed of extracellular ligand binding and intracellular TK domains[6]. EGFR regulation is tightly controlled, and variations within the EGFR signaling pathway play a key role in solid tumor oncogenesis. Commercially available EGFR antagonists include the monoclonal antibodies (mAbs) panitumumab and cetuximab and act by preventing epidermal growth factor ligand binding to the external EGFR domain. EGFR mAbs are considered a standard of care in treating patients with metastatic colorectal cancer lacking activating mutations in KRAS/NRAS downstream of EGFR[7].
In contrast to these mAbs, EGFR TKIs block intracellular signaling cascades through competition with adenine triphosphate. While both approaches lead to the inhibition of EGFR autophosphorylation, TKI efficacy is restricted to cancers that carry EGFR mutations in the TK domain (exons 18-21). Still, these mutations are rarely seen in colorectal cancer[8]. Available TKIs against EGFR TK mutations are approved for non-small cell lung cancer and include first-generation agents gefitinib and erlotinib and second-generation agents afatinib and dacomitinib[9]. Osimertinib is the only approved third-generation EGFR TKI and has efficacy against EGFR T790M, a mutation resistant to first- and second-generation EGFR TKIs[10].
There are currently no guidelines to direct therapy selection in EGFR-mutated colorectal cancer. However, case reports exist describing the efficacy of erlotinib in EGFR mutant colorectal cancer[11,12]. More recently, a 50-year-old Japanese woman with an EGFR T790M lung lesion from a colorectal primary responded to osimertinib for 95 days[5]. The patient was noted positive for RAS mutant G13D at diagnosis, which is downstream of EGFR. Mutations of this pathway are established as strong negative predictive markers, and may preclude efficacy of these therapies. This patient also had an uncommon EGFR L861Q mutation compounded with the EGFR T790M at the time of osimertinib initiation. It is speculated by the authors the patient originally only had the EGFR L861Q mutation and the T790M was acquired during the clinical course prior to starting osimertinib. With one mutation acquired during the clinical course, it is possible another resistance mechanism was acquired after starting osimertinib. This hypothesis along with the RAS mutation are potential explanations for the short response time noted compared to our patient. However, such cases highlight the additional options afforded to these patients by utilizing multi-gene sequencing panels.
The use of NGS in metastatic colorectal cancers is becoming standard in an effort to identify additional therapeutic options in the refractory setting. However, guidelines currently recommend testing for only a limited set of genes, including NRAS, KRAS, BRAF V600E, and mismatch repair/ microsatellite instability, with consideration to test for HER2 amplifications and NTRK fusions in the refractory disease setting[9]. A retrospective review of 23 US-based oncology practices demonstrates that even these limited gene panels are underutilized; only 40% of patients underwent guideline-recommended genomic testing for any of these genomic markers, a rate that has not increased since 2013[13,14].
Cell-free DNA plays an increasingly pivotal role in minimal residual disease monitoring for individuals with colon cancer[15]. Both tumor-informed and tumor-agnostic approaches are being investigated[16]. The clinical case described in this paper demonstrates the prospect of using cell-free DNA for response assessment, in addition to standard tumor markers such as carcinoembryonic antigen, as illustrated in Figure 1.
Our patient had a response to osimertinib for over six months of therapy. The previously under-recognized factor of the time-related burden that patients undergoing oncologic treatments experience is coming to the forefront with recent research[17]. We customized her treatment to meet her goals of minimizing toxicity and time spent traveling to the cancer center. In alignment with these goals, most of her visits were performed virtually.
This case shows the benefit of large panel multi-gene sequencing assays to identify potential therapeutic options in patients with refractory disease. Molecular tumor boards are integral in identifying patients appropriate for a targeted therapy approach and procuring these much-needed therapies. As demonstrated in this case, precision medicine holds promise to tailor patient treatments to align with their goals and expectations.
This case shows the benefit of large panel multi-gene sequencing assays to identify potential therapeutic options in patients with refractory disease. Molecular tumor boards are integral in identifying patients appropriate for a targeted therapy approach and procuring these much-needed therapies. As demonstrated in this case, precision medicine holds promise to tailor patient treatments to align with their goals and expectations.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ji DYX, China; Lin Q, China S-Editor: Lin C L-Editor: A P-Editor: Zhang XD
| 1. | Kim N, Cho D, Kim H, Kim S, Cha YJ, Greulich H, Bass A, Cho HS, Cho J. Colorectal adenocarcinoma-derived EGFR mutants are oncogenic and sensitive to EGFR-targeted monoclonal antibodies, cetuximab and panitumumab. Int J Cancer. 2020;146:2194-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Park AK, Francis JM, Park WY, Park JO, Cho J. Constitutive asymmetric dimerization drives oncogenic activation of epidermal growth factor receptor carboxyl-terminal deletion mutants. Oncotarget. 2015;6:8839-8850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Van Cutsem E, Danielewicz I, Saunders MP, Pfeiffer P, Argilés G, Borg C, Glynne-Jones R, Punt CJA, Van de Wouw AJ, Fedyanin M, Stroyakovskiy D, Kroening H, Garcia-Alfonso P, Wasan H, Falcone A, Fougeray R, Egorov A, Amellal N, Moiseyenko V. First-line trifluridine/tipiracil + bevacizumab in patients with unresectable metastatic colorectal cancer: final survival analysis in the TASCO1 study. Br J Cancer. 2022;126:1548-1554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Guo L, Huang S, Wang X. PUMA mediates the anti-cancer effect of osimertinib in colon cancer cells. Onco Targets Ther. 2017;10:5281-5288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Yanagisawa A, Kinehara Y, Kijima R, Tanaka M, Ninomiya R, Jokoji R, Tachibana I. Metastatic Lung Tumors from Colorectal Cancer with EGFR Mutations That Responded to Osimertinib. Intern Med. 2023;62:769-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Mitsudomi T, Yatabe Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010;277:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 418] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 7. | Cai WQ, Zeng LS, Wang LF, Wang YY, Cheng JT, Zhang Y, Han ZW, Zhou Y, Huang SL, Wang XW, Peng XC, Xiang Y, Ma Z, Cui SZ, Xin HW. The Latest Battles Between EGFR Monoclonal Antibodies and Resistant Tumor Cells. Front Oncol. 2020;10:1249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 8. | Altunel E, Aljamal AA, Mantyh J, Deak K, Glover W, McCall SJ, Datto M, Strickler J, Hsu DS. Characterization of the Epidermal Growth Factor Receptor T790M Mutation in Colorectal Cancer. JCO Precis Oncol. 2018;2:1-7. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Marin-Acevedo JA, Pellini B, Kimbrough EO, Hicks JK, Chiappori A. Treatment Strategies for Non-Small Cell Lung Cancer with Common EGFR Mutations: A Review of the History of EGFR TKIs Approval and Emerging Data. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Remon J, Steuer CE, Ramalingam SS, Felip E. Osimertinib and other third-generation EGFR TKI in EGFR-mutant NSCLC patients. Ann Oncol. 2018;29:i20-i27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 196] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 11. | Yarom N, Jonker DJ. The role of the epidermal growth factor receptor in the mechanism and treatment of colorectal cancer. Discov Med. 2011;11:95-105. [PubMed] |
| 12. | Li Y, Zhang HB, Chen X, Yang X, Ye Y, Bekaii-Saab T, Zheng Y, Zhang Y. A Rare EGFR-SEPT14 Fusion in a Patient with Colorectal Adenocarcinoma Responding to Erlotinib. Oncologist. 2020;25:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Gutierrez ME, Price KS, Lanman RB, Nagy RJ, Shah I, Mathura S, Mulcahy M, Norden AD, Goldberg SL. Genomic Profiling for KRAS, NRAS, BRAF, Microsatellite Instability, and Mismatch Repair Deficiency Among Patients With Metastatic Colon Cancer. JCO Precis Oncol. 2019;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Iyer P, Deng M, Handorf EA, Nakhoda S, Dotan E. Assessing Oncologists' Adoption of Biomarker Testing in Metastatic Colorectal Cancer Using Real-World Data. JNCI Cancer Spectr. 2022;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Venook AP. Colorectal Cancer Surveillance With Circulating Tumor DNA Assay. JAMA Netw Open. 2022;5:e221100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Gong J, Hendifar A, Gangi A, Zaghiyan K, Atkins K, Nasseri Y, Murrell Z, Figueiredo JC, Salvy S, Haile R, Hitchins M. Clinical Applications of Minimal Residual Disease Assessments by Tumor-Informed and Tumor-Uninformed Circulating Tumor DNA in Colorectal Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Gupta A, Jensen EH, Virnig BA, Beg MS. Time-Related Burdens of Cancer Care. JCO Oncol Pract. 2022;18:245-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |