Published online Oct 15, 2023. doi: 10.4251/wjgo.v15.i10.1771
Peer-review started: March 12, 2023
First decision: April 19, 2023
Revised: July 1, 2023
Accepted: August 15, 2023
Article in press: August 15, 2023
Published online: October 15, 2023
Processing time: 211 Days and 22.7 Hours
Modified albumin-bilirubin (mALBI) grade has been established as a survival determinant in hepatocellular carcinoma (HCC) patients who receive locoregional and targeted therapies.
To investigate whether mALBI could predict survival in unresectable HCC (uHCC) patients who were treated with atezolizumab plus bevacizumab (AB).
A single-center, retrospective cohort study enrolled uHCC patients who received AB treatment between September 2020 and April 2023 and were followed up until June 2023. An association between mALBI and patient survival was determined using Cox proportional hazards analysis.
Of the 83 patients, 67 patients (80.7%) were male with the mean age of 60.6 years. Among them, 22 patients (26.5%) were classified as Barcelona Clinic Liver Cancer B, and 61 patients (73.5%) were classified as Barcelona Clinic Liver Cancer C. Cirrhosis was present in 76 patients (91.6%), with 58 patients classified as Child-Turcotte-Pugh (CTP) A and 18 as CTP B. The median overall survival (OS) and progression-free survival were 13.0 mo [95% confidence interval (CI): 5.2-20.8] and 9.0 mo (95%CI: 5.0-13.0), respectively. The patients were divided into two groups based on mALBI grades: 42 patients (50.6%) in the mALBI 1 + 2a group; and 41 patients (49.4%) in the mALBI 2b + 3 group. During the median follow-up period of 7.0 mo, the mALBI 1 + 2a group exhibited significantly better survival compared to the mALBI 2b + 3 group, with a median OS that was not reached vs 3.0 mo (95%CI: 0.1-6.0, P < 0.001). In a subgroup of patients with CTP A, the mALBI 1 + 2a group also showed significantly longer survival compared to the mALBI 2b + 3 group, with a median OS that was not reached vs 6.0 mo (95%CI: 3.4-8.6, P < 0.001). In the multivariate analysis, both CTP class and mALBI grade were independently associated with survival, with adjusted hazard ratios (95%CI) of 2.63 (1.19-5.78, P = 0.020) and 3.90 (1.71-8.90, P = 0.001), respectively.
mALBI grades can determine survival of uHCC patients receiving AB treatment, particularly those who have mildly impaired liver function. This highlights the importance of assessing mALBI before initiating AB treatment to optimize therapeutic efficacy in clinical practice.
Core Tip: The modified albumin-bilirubin (mALBI) grade has been shown to determine survival in hepatocellular carcinoma patients receiving locoregional and targeted therapies. This study demonstrated that mALBI can also predict survival in unresectable hepatocellular carcinoma patients receiving atezolizumab plus bevacizumab. To improve the therapeutic outcome of atezolizumab plus bevacizumab treatment in clinical practice, mALBI assessment before initiating treatment can help in identifying suitable candidates for immunotherapy.
- Citation: Navadurong H, Prasoppokakorn T, Siriwong N, Phathong C, Teeyapun N, Tanasanvimon S, Thanapirom K, Komolmit P, Tangkijvanich P, Treeprasertsuk S, Chaiteerakij R. Modified albumin-bilirubin predicted survival of unresectable hepatocellular carcinoma patients treated with immunotherapy. World J Gastrointest Oncol 2023; 15(10): 1771-1783
- URL: https://www.wjgnet.com/1948-5204/full/v15/i10/1771.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i10.1771
Hepatocellular carcinoma (HCC) represents a growing global health concern due to its increasing incidence and poor prognosis[1]. The survival of HCC patients is primarily influenced by tumor burden, liver functional reserve, and patient performance status[2]. The Child-Turcotte-Pugh (CTP) score has long been utilized to assess liver functional reserve in patients with cirrhosis. The score was initially designed to evaluate the prognosis of patients with cirrhosis undergoing shunt surgery for variceal bleeding[3]. It has several limitations including the subjectivity of certain parameters, such as grade of ascites and encephalopathy, which reduce its accuracy. Additionally, the CTP score is incapable of distinguishing between CTP class C patients with higher serum bilirubin levels and more severe coagulopathy from those with lower bilirubin levels and less severe coagulopathy. Notably, some HCC patients do not have cirrhosis, and therefore the CTP score may not accurately reflect their liver functional reserve.
To address the limitations of the CTP classification, the albumin-bilirubin (ALBI) grade was developed to specifically evaluate the liver functional reserve of HCC patients. The ALBI grade is calculated using albumin and bilirubin levels, making it more objective than the CTP classification. Overall, the ALBI grade has performed similarly to CTP classification in predicting the survival of HCC patients treated with various modalities, including resection, transplantation, radiofrequency ablation, microwave ablation, transarterial chemoembolization (TACE), transarterial radioembolization, external beam radiotherapy (EBRT), and targeted therapy[4,5]. Moreover, the ALBI grade has outperformed the CTP class since it can differentiate between patients with good and poor prognoses within the same CTP class. For instance, when CTP A patients were separated into two groups based on ALBI grade, there was a 10-mo difference in survival between those with ALBI grade 1 and those with ALBI grade 2[4].
Although ALBI has been proposed as an index for evaluating liver functional reserve and prognosis in HCC patients[2], its performance remains suboptimal. Despite the greater granularity of the ALBI compared to the CTP score, the distribution of HCC patients across the ALBI grades remains uneven, with 52%-65% of patients classified as ALBI grade 2 and very few classified as ALBI grade 3[4]. Accordingly, some ALBI grade 2 patients had survival comparable to ALBI grade 1 patients, while others had survival similar to ALBI grade 3 patients. To overcome the limitation of the original ALBI score, a modified ALBI (mALBI) grading system was recently developed. The mALBI score showed better predictive performance than the original ALBI grade in assessing liver functional reserve and predicting prognosis in HCC patients. The mALBI score divides the ALBI grade 2 into 2a and 2b, resulting in a more balanced distribution of patients across all grades and better performance in stratifying patients into different groups with different outcomes[6]. The mALBI score also demonstrated superior stratification performance than the original ALBI score in patients treated with resection, radiofrequency ablation, microwave ablation, TACE, and targeted therapy[7].
The current first-line treatment for unresectable HCC (uHCC) is atezolizumab plus bevacizumab (AB)[2], which has demonstrated a significant prolongation of overall survival (OS) and progression-free survival (PFS)[8]. However, few studies have explored the relationship between mALBI grade and prognosis in uHCC patients treated with AB[9,10]. Previous research has suggested that patients with mALBI 1 or 2a experienced significantly longer PFS and a higher objective response rate (ORR) than those with mALBI 2b or 3[10]. Nonetheless, the predictive value of mALBI grade for predicting outcomes of HCC patients receiving AB treatment has yet to be fully investigated. In this study, we aimed to investigate the association between mALBI grade and survival in uHCC patients treated with AB as the first-line, second-line, or subsequent line of treatment after locoregional and systemic therapies.
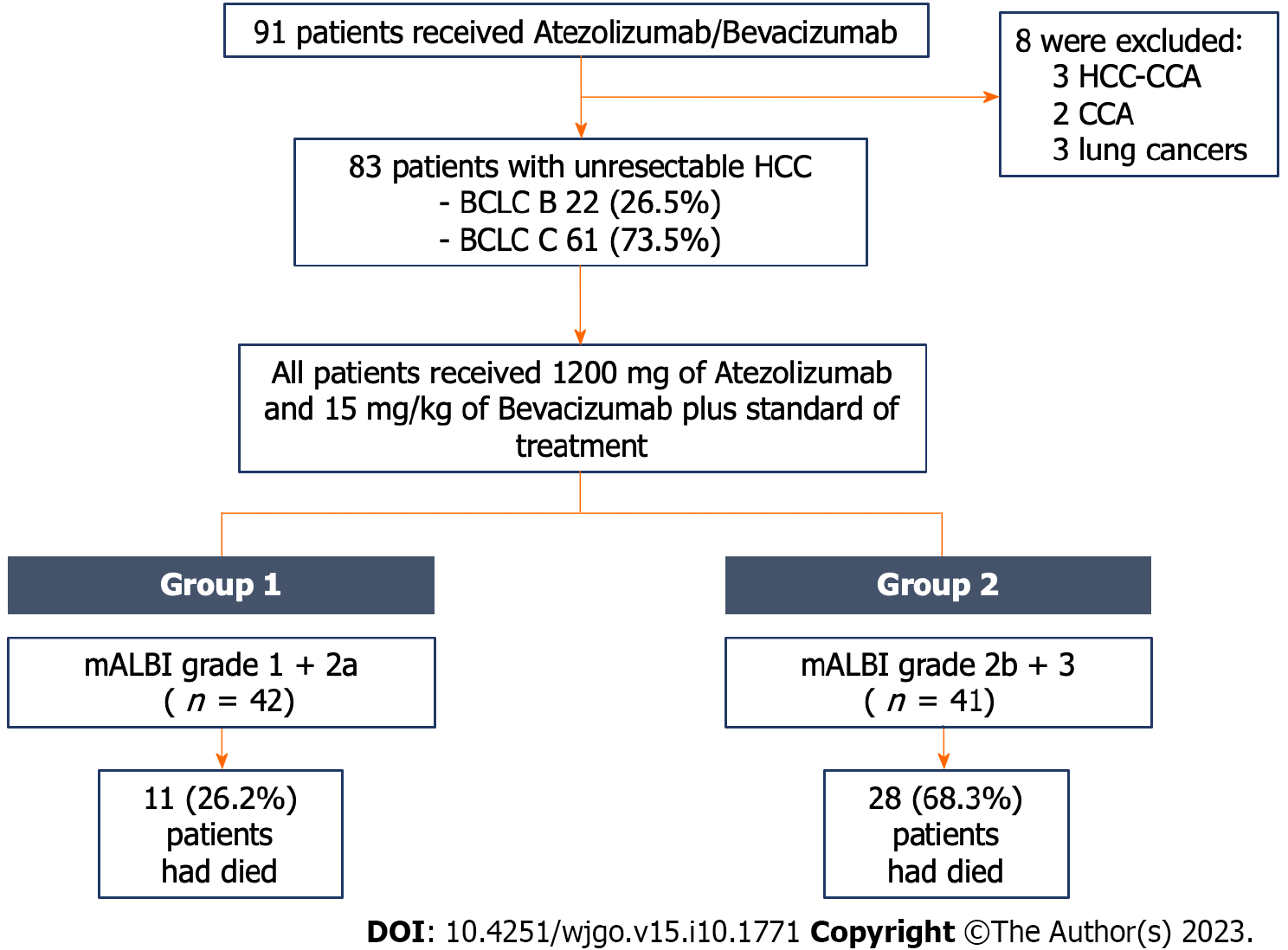
A single-center, retrospective cohort study was conducted at the King Chulalongkorn Memorial Hospital in Bangkok, Thailand. Patients were enrolled between September 2020 and April 2023. The inclusion criteria were patients aged ≥ 18 years who received AB and were diagnosed with HCC by either pathologically or typical radiologically via contrast-enhanced magnetic resonance imaging or dynamic computed tomography[11]. The exclusion criteria were patients with other malignancies and mixed hepatocholangiocarcinoma (Figure 1).
Patient baseline characteristics were collected including performance status, tumor burden, underlying chronic liver disease, presence of cirrhosis, liver functional reserve, and alpha fetoprotein level. Patient performance status was evaluated using the Eastern Cooperative Oncology Group Performance Status (ECOG-PS) score[12]. The ECOG-PS scores were defined as follows: 0 (fully active); 1 (restricted in physically strenuous activity but able to perform light work); 2 (unable to perform any work activities > 50% of waking hours); 3 (confined to bed or chair > 50% of waking hours); and 4 (totally confined to bed).
Tumor burden was assessed using the 2022 Barcelona Clinic Liver Cancer (BCLC) staging system[2], which classified HCC into 5 stages as BCLC stage 0 (very early stage) for solitary nodule ≤ 2 cm, BCLC stage A (early stage) for multifocal HCCs up to three nodules with size ≤ 3 cm, BCLC stage B (intermediate stage) for multifocal HCCs exceeding the stage A criteria, BCLC stage C (advanced stage) for the presence of vascular invasion or extrahepatic spread, and BCLC stage D (end stage) for patients with ECOG-PS > 2 and/or impaired liver function who are not transplant candidates. Tumor size was determined by measuring the maximum diameter of the largest intrahepatic lesion.
The liver functional reserve was evaluated using several measures, including the CTP score, ALBI, and mALBI grades. The ALBI and mALBI grades were calculated using the following equation: (log10 bilirubin in µmol/L 0.66) + [albumin in g/L (-0.085)]. The cutoff points for ALBI grades 1, 2, and 3 were ≤ -2.60, > -2.60 to ≤ -1.39, and >-1.39, respectively. For mALBI grades, the cutoff points were ≤ -2.60, > -2.60 to ≤ -2.27, and > -2.27 to ≤ -1.39, and > -1.39 for grades 1, 2a, 2b, and 3, respectively[4,7].
All patients received 1200 mg of atezolizumab and 15 mg/kg of bevacizumab intravenously every 3 wk. The administration of AB was discontinued when the disease progressed by radiologic evidence or by the patient’s preference. If any adverse events grade 3 or 4 occurred as defined by the Common Terminology Criteria for Adverse Events version 5.0, AB was temporarily withheld and resumed when the adverse event improved to a milder grade.
The response to AB treatment was evaluated every three cycles using dynamic computed tomography or magnetic resonance imaging and classified according to the Modified Response Evaluation Criteria in Solid Tumors. A complete response was defined as the absence of intratumoral arterial enhancement in all lesions, while a partial response was defined as at least a 30% decrease in the sum of diameters of viable lesions. Progressive disease was defined as at least 20% increase in the sum of the diameters of viable lesions, while stable disease was defined as not in the criteria of either partial response or progressive disease[13]. Regardless of AB treatment, all patients received optimal treatment decided by a multidisciplinary team including hepatologists, surgeons, interventionists, and oncologists. All patients were followed for disease progression and OS.
Continuous variables with normal distribution were presented as mean and standard deviation, while variables with non-normal distribution were presented as median and interquartile range (IQR). They were compared using independent t tests or the Mann–Whitney U, as appropriate. Categorical variables were presented as numbers and percentages and compared using Fisher’s exact test or χ2 test, as appropriate. Patients were divided into two groups based on their mALBI grades: group 1 (mALBI grades 1 and 2a) and group 2 (mALBI grades 2b and 3). The patient’s survival was calculated from the enrollment date until death or the last follow-up date, which was on June 8, 2023. OS and PFS were estimated using the Kaplan-Meier survival method and compared using the log-rank test. An association between mALBI and patient survival was determined using Cox proportional hazards analysis. Other factors associated with patient survival were also determined using the univariate Cox proportional hazards model. Age, sex, and other factors with a P value of < 0.05 in the univariate model were included in the multivariate model. A P value of < 0.05 was considered statistically significant. Statistical analyses were performed using the SPSS package version 22.0.0 (SPSS Inc., Chicago, IL, United States). The study protocol was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University (IRB No.892/63), and was in accordance with the Helsinki Declaration of 1983.
A total of 91 patients received AB in our hospital. Of those, 83 patients were uHCC and included in the study. Eight patients were excluded due to other cancer diagnoses (three hepatocholangiocarcinomas, two cholangiocarcinomas, and three lung cancers). Among the 83 uHCC patients, 67 patients (80.7%) were male with a mean age of 60.6 ± 12.8 years. Baseline characteristics of the entire cohort are displayed in Table 1. Chronic liver disease was primarily caused by viral hepatitis B/C infection (53 patients, 63.9%), followed by nonalcoholic steatohepatitis (23, 27.7%), alcohol liver disease (7, 8.4%), and autoimmune hepatitis (1, 1.2%). Cirrhosis was present in 76 patients (91.6%), with 58 patients (69.9%) classified as CTP class A and 18 patients (21.7%) classified as CTP class B. The baseline median alpha fetoprotein level in the entire cohort was 339.0 ng/mL (IQR 10.7, 6300.0).
| Variables | Total, n = 83 | mALBI 1+2a, n = 42 | mALBI 2b+3, n = 41 | P value |
| Age in yr, mean ± SD | 60.6 ± 12.8 | 59.8 ± 14.0 | 61.5 ± 11.5 | 0.560 |
| Male | 67 (80.7) | 34 (81.0) | 33 (80.5) | 0.960 |
| ECOG-PS | 0.680 | |||
| 0 | 78 (94.0) | 40 (95.2) | 38 (92.7) | |
| 1 | 5 (6.0) | 2 (4.8) | 3 (7.3) | |
| Presence of cirrhosis | 76 (91.6) | 35 (83.3) | 41 (100) | < 0.001 |
| CTP A | 58 (69.9) | 35 (83.3) | 23 (56.1) | |
| CTP B | 18 (21.7) | 0 (0) | 18 (43.9) | |
| Etiology of disease | ||||
| Viral hepatitis | 53 (63.9) | 27 (64.3) | 26 (63.4) | 0.930 |
| NASH | 23 (27.7) | 11 (26.2) | 12 (29.3) | 0.750 |
| Alcohol related | 7 (8.4) | 1 (2.4) | 6 (14.6) | 0.060 |
| Others | 1 (1.2) | 1 (2.4) | 0 (0) | 1.000 |
| BCLC staging | ||||
| BCLC B | 22 (26.5) | 6 (14.3) | 16 (39.0) | 0.010 |
| BCLC C | 61 (73.5) | 36 (85.7) | 25 (61.0) | |
| Macrovascular invasion | 30 (36.1) | 15 (35.7) | 15 (36.6) | 0.930 |
| Extrahepatic metastasis | 40 (48.2) | 25 (59.5) | 15 (36.6) | 0.040 |
| AFP in ng/mL, median (IQR) | 339.0 (10.7, 6300.0) | 581.5 (11.5, 6869.5) | 339.0 (10.5, 9144.5) | 0.880 |
| Tumor size in cm, median (IQR) | 5.3 (1.7, 12.0) | 4.8 (1.7, 11.0) | 5.6 (1.7, 14.3) | 0.730 |
| Portal vein invasion grade of 2/3/4 | 8 (26.7)/13 (43.3)/9 (30.0) | 4 (26.7)/7 (46.7)/4 (26.7) | 4 (26.7)/6 (40.0)/5 (33.3) | 1.000 |
| EHM bone/lymph node/lung/peritoneum | 11 (27.5)/14 (35.0)/21 (52.5)/9 (22.5) | 8 (32.0)/7 (28.0)/13 (52.0)/3 (12.0) | 3 (20.0)/7 (46.7)/8 (53.3)/6 (40.0) | 0.350 |
| ALBI score, median (IQR) | -2.270 (-2.628 to -1.826) | -2.622 (-2.836 to -2.398) | -1.826 (-2.067 to -1.434) | < 0.001 |
| mALBI grade | < 0.001 | |||
| 1: ≤ -2.60 | 23 (27.7) | 23 (54.8) | 0 (0) | |
| 2a: > -2.60 to ≤ -2.27 | 19 (22.9) | 19 (45.2) | 0 (0) | |
| 2b: > -2.27 to ≤ -1.39 | 32 (38.6) | 0 (0) | 32 (78.0) | |
| 3: > -1.39 | 9 (10.8) | 0 (0) | 9 (22.0) | |
| Prior local therapy for HCC | 62 (74.7) | 33 (78.6) | 29 (70.7) | 0.410 |
| Resection | 22 (35.5) | 17 (51.5) | 5 (17.2) | 0.005 |
| Ablation | 17 (27.4) | 9 (27.3) | 8 (27.6) | 0.980 |
| TACE | 48 (77.4) | 23 (69.7) | 25 (86.2) | 0.120 |
| TARE | 9 (14.5) | 5 (15.2) | 4 (13.8) | 1.000 |
| EBRT | 19 (30.6) | 11 (33.3) | 8 (27.6) | 0.620 |
| Prior systemic therapy for HCC | 10 (12.0) | 4 (9.5) | 6 (14.6) | 0.460 |
| Sorafenib | 5 (50.0) | 1 (25.0) | 4 (66.7) | 0.530 |
| Lenvatinib | 4 (40.0) | 2 (50.0) | 2 (33.3) | 1.000 |
| > 2 lines of systemic therapy | 2 (20.0) | 1 (25.0) | 1 (16.7) | 1.000 |
| AB as first-line treatment | 20 (24.1) | 9 (21.4) | 11 (26.8) | 0.570 |
| Combination of AB and other local therapy as first-line treatment | 3 (3.6) | 3 (7.1) | 0 (0) | 0.240 |
| Resection/TACE/TARE/EBRT | 0 (0)/0 (0)/0 (0)/3 (100) | 0 (0)/0 (0)/0 (0)/3 (100.0) | 0 (0)/0 (0)/0 (0)/0 (0) | 0.240 |
| Number of AB cycle, median (IQR) | 4.0 (2.0, 9.0) | 5.0 (4.0, 11.3) | 3.0 (1.0, 4.5) | < 0.001 |
At the time of AB initiation, 22 patients (26.5%) were classified as BCLC stage B with extensive tumor involvement, while 61 patients (73.5%) were classified as BCLC stage C. Among them, 30 patients (36.1%) had portal vein invasion, and 40 patients (48.2%) had extrahepatic metastasis. AB was utilized as first-line therapy in 20 patients (24.1%), with 17 patients (20.5%) receiving AB monotherapy and 3 patients (3.6%) receiving AB in combination with EBRT. Sixty-three patients (75.9%) received AB as a second or subsequent line of treatment. Among them, 62 patients (74.7%) had received prior locoregional treatment (48 TACE, 22 resections, 19 EBRT, 17 ablations, and 9 transarterial radioembolization), and 10 patients (12.0%) had received prior systemic therapies (5 sorafenib, 4 lenvatinib, and 2 had at least two systemic therapy regimens).
Regarding the mALBI classification, there were 23 patients (27.7%), 19 patients (22.9%), 32 patients (38.9%), and 9 patients (10.8%) classified as grade 1, 2a, 2b, and 3, respectively. This distribution resulted in 42 patients (50.6%) being categorized to group 1 (mALBI 1 + 2a), and 41 patients (49.4%) being categorized to group 2 (mALBI 2b + 3).
Regarding liver functional reserve, patients in group 1 with a lower mALBI grade showed a significantly higher percentage of CTP A compared to those in group 2 with a higher mALBI grade [35 patients (83.3%) vs 23 patients (56.1%), P < 0.001]. As for tumor burden, patients in group 1 had a higher percentage of BCLC C compared to those in group 2 [36 patients (85.7%) vs 25 patients (61.0%), P = 0.010]. When considering patients with BCLC C, there was no significant difference in the number of patients with macrovascular invasion between the two groups [15 patients (35.7%) vs 15 patients (36.6%), P = 0.930]. We did find a significantly higher number of patients with extrahepatic metastasis in group 1 compared to group 2 [25 patients (59.5%) vs 15 patients (36.6%), P = 0.040] (Table 1).
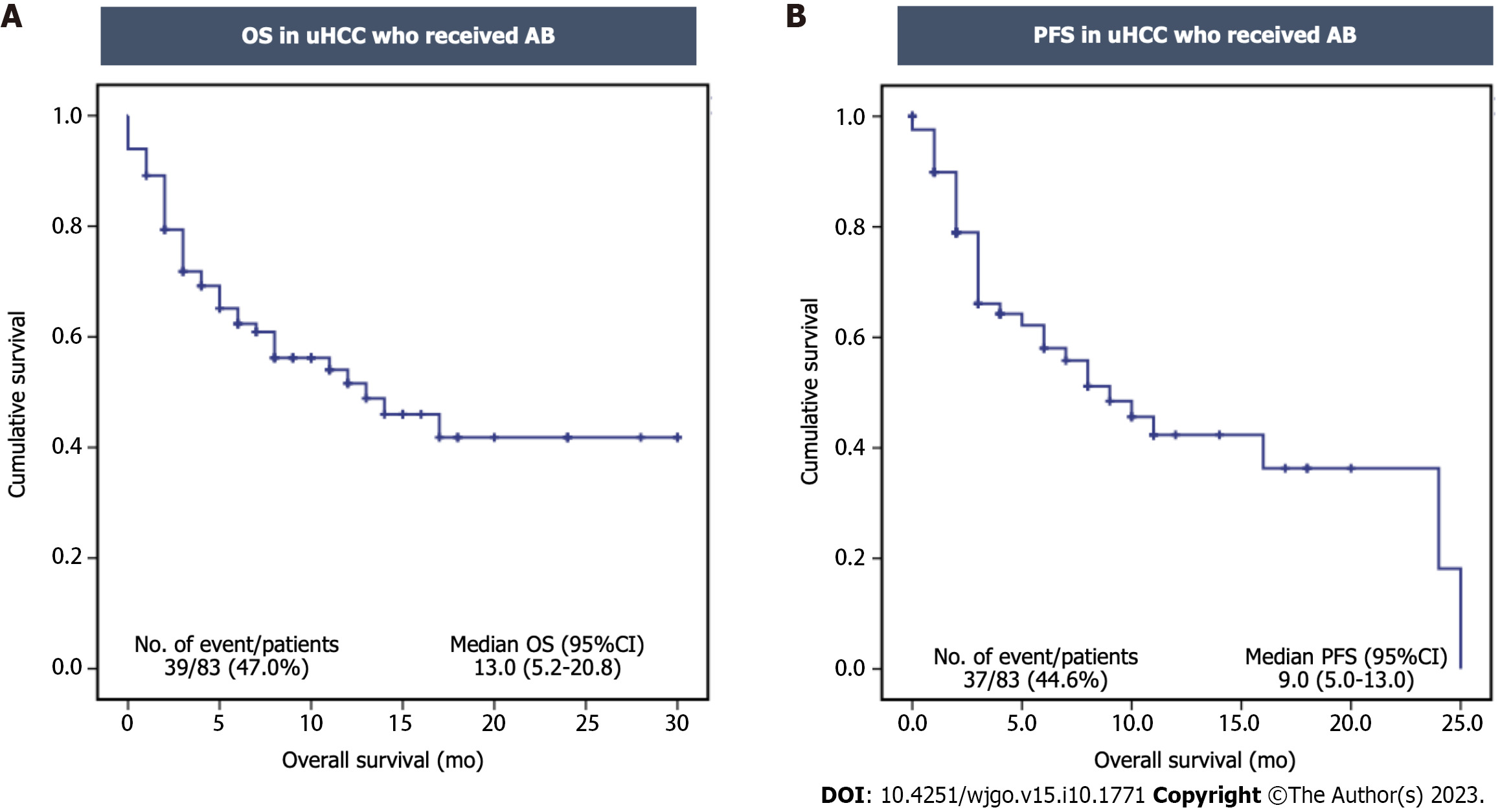
During the median follow-up period of 7 mo (range 0.75-30.0 mo), 39 patients (47.0%) had died. The median OS and PFS were 13.0 mo [95% confidence interval (CI): 5.2-20.8] and 9.0 mo (95%CI: 5.0-13.0), respectively (Figure 2A and B). Of those, 61 patients were evaluated for treatment responses. The disease control rate was 62.3% (n = 38), with a complete response rate of 6.6% (n = 4), a partial response rate of 21.3% (n = 13), and a stable disease rate of 34.4% (n = 21). The disease progression rate was 37.7% (n = 23). However, there were no statistically significant differences in the ORR and disease control rate between the two groups (Table 2).
| Variables | Total, n = 61 | mALBI 1 + 2a, n = 39 | mALBI 2b + 3, n = 22 | P value |
| Complete response | 4 (6.6) | 3 (7.7) | 1 (4.5) | 1.000 |
| Partial response | 13 (21.3) | 11 (28.2) | 2 (9.1) | 0.110 |
| Stable disease | 21 (34.4) | 12 (30.8) | 9 (40.9) | 0.420 |
| Objective response rate | 17 (27.9) | 14 (35.9) | 3 (13.6) | 0.060 |
| Disease control rate | 38 (62.3) | 26 (66.7) | 12 (54.5) | 0.350 |
| Progressive disease | 23 (37.7) | 13 (33.3) | 10 (45.5) | 0.350 |
We further performed subgroup analyses based on CTP, tumor size, and tumor stages. We found that patients with CTP class A had a significantly longer median OS compared to those with CTP class B, with values of 17 mo (95%CI: not estimated) and 2.0 mo (95%CI: 1.1-2.9), respectively (P < 0.001) (Table 3).
| Variables | No. of events/No. of patients | Median OS (95%CI), mo | P value | No. of events/No. of patients | Median OS (95%CI), mo | P value |
| A. Entire cohort, n = 83 | ||||||
| CTP class | < 0.001 | NE | ||||
| CTP A | 25/65 | 17 (-) | 34/65 | 9.0 (5.1-12.9) | ||
| CTP B | 14/18 | 2.0 (1.1-2.9) | 3/18 | NE | ||
| Tumor size in cm | 0.790 | 0.130 | ||||
| ≤ 5.0 | 21/41 | 12.0 (4.8-19.2) | 21/41 | 7.0 (2.8-11.2) | ||
| > 5.0 | 18/42 | NE | 16/42 | 16.0 (6.5-25.5) | ||
| B. BCLC C group, n = 61 | 27/61 | 14.0 (-) | 28/61 | 10.0 (0.5-19.5) | ||
| CTP class | < 0.001 | 0.810 | ||||
| CTP A | 18/49 | NE | 26/49 | 10.0 (0.3-19.7) | ||
| CTP B | 9/12 | 1.0 (0.1-2.5) | 2/12 | NE | ||
| Tumor size in cm | 0.850 | 0.060 | ||||
| ≤ 5.0 | 13/26 | 12.0 (3.4-20.6) | 15/26 | 6.0 (2.5-9.5) | ||
| > 5.0 | 14/35 | NE | 13/35 | 24.0 (9.4-38.6) | ||
| With PV invasion, n = 30 | 0.140 | 0.030 | ||||
| No | 12/31 | NE | 20/31 | 6.0 (3.2-8.8) | ||
| Yes | 15/30 | 13.0 (2.0-24.0) | 8/30 | 24.0 (-) | ||
| With EHM metastasis, n = 40 | 0.540 | 0.170 | ||||
| No | 10/21 | 13.0 (0.1-27.3) | 7/21 | 24 (-) | ||
| Yes | 17/40 | NE | 21/40 | 6.0 (1.1-10.9) | ||
| C. BCLC B group, n = 22 | 12/22 | 11.0 (2.7-19.3) | 9/22 | 8.0 (1.3-14.7) | ||
| CTP class | 0.020 | 0.660 | ||||
| CTP A | 7/16 | 11.0 (5.6-16.4) | 8/16 | 8.0 (1.5-14.5) | ||
| CTP B | 5/6 | 2.0 (0.8-3.2) | 1/6 | 5.0 (-) | ||
| Tumor size in cm | 0.490 | 0.140 | ||||
| ≤ 5.0 | 8/15 | 11.0 (0.1-24.1) | 6/15 | 9.0 (6.9-11.1) | ||
| > 5.0 | 4/7 | 7.0 (0.1-17.3) | 3/7 | 5.0 (-) | ||
Regarding tumor characteristics, there was no significant difference in survival among the following groups: tumor size less than or equal 5.0 cm vs 5.0 cm or more; BCLC stage C with and without portal vein invasion; and BCLC stage C with and without extrahepatic metastasis. However, the PFS in BCLC stage C with portal vein invasion was significantly longer compared to those without portal vein invasion, with values of 24.0 mo (95%CI: Not estimated) vs 6.0 mo (95%CI: 3.2-8.8), respectively (P = 0.030) (Table 3).
The median number of AB cycles administered in the cohort was 4.0 cycles (IQR: 2.0, 9.0). Patients in group 1 received a significantly higher number of AB cycles than those in group 2 [5.0 cycles (4.0, 11.3) vs 3.0 cycles (1.0, 4.5), P < 0.001] (Table 1).
Of the 83 study patients, 37 patients experienced disease progression after AB treatment. Among them, 32 patients (86.5%) received additional systemic therapies (19 received lenvatinib, 3 received chemotherapy, 1 received sorafenib, and 9 received two or more consecutive systemic therapies). Other treatment options included EBRT (8 patients, 21.6%), TACE (4 patients, 10.8%), and best supportive care (4 patients, 10.8%). In the cohort, 6 patients (16.2%) were treated with a combination of systemic treatment and EBRT, 3 patients (8.1%) received a combination of systemic treatment and TACE, and 1 patient (2.7%) received a combination of systemic treatment, TACE, and EBRT (Table 4).
| Treatment | Total, n = 37 | mALBI 1 + 2a, n = 24 | mALBI 2b + 3, n = 13 | P value |
| Systemic therapy | 32 (86.5) | 22 (91.7) | 10 (76.9) | 0.320 |
| TACE | 4 (10.8) | 4 (16.7) | 0 (0) | 0.280 |
| EBRT | 8 (21.6) | 6 (25.0) | 2 (15.4) | 0.690 |
| Best supportive care | 4 (10.8) | 1 (4.2) | 3 (23.1) | 0.120 |
Among patients who experienced disease progression after AB treatment, those who received subsequent therapeutic interventions had longer survival than those who received the best supportive care. However, the survival difference did not reach statistical significance. The median survival time was not reached vs 5.0 mo (95%CI: 1.8-8.2, P = 0.050).
In the univariate analysis, mALBI grade 2b + 3 showed a significant association with survival, with a hazard ratio (HR) of 5.20 (95%CI: 2.52-10.76, P < 0.001). Similarly, CTP class B was also significantly associated with survival, with an HR of 5.38 (95%CI: 2.66-10.89, P < 0.001). In the multivariate analysis adjusted for age and sex, both mALBI grade 2b + 3 and CTP class B remained independently associated with worse survival, with adjusted HRs of 3.90 (95%CI: 1.71-8.90, P = 0.001) and 2.63 (95%CI: 1.19-5.78, P = 0.020), respectively (Table 5).
| Variables | Univariate | Multivariate | ||
| Hazard ratio (95%CI) | P value | Adjusted hazard ratio (95%CI) | P value | |
| Age | 1.00 (0.97-1.02) | 0.970 | 0.99 (0.96-1.02) | 0.540 |
| Male sex | 1.15 (0.51-2.60) | 0.740 | 0.97 (0.41-2.28) | 0.940 |
| ECOG-PS | ||||
| 0 | 1.00 (Reference) | |||
| 1 | 2.03 (0.62-6.66) | 0.240 | ||
| Presence of cirrhosis | ||||
| No | 1.00 (Reference) | |||
| Yes | 2.19 (0.53-9.13) | 0.280 | ||
| Etiology of disease | ||||
| NASH | 0.70 (0.32-1.52) | 0.360 | ||
| Viral hepatitis | 1.48 (0.73-2.97) | 0.280 | ||
| Alcohol | 2.09 (0.81-5.39) | 0.130 | ||
| BCLC stage | ||||
| B | 1.00 (Reference) | |||
| C | 0.66 (0.33-1.31) | 0.230 | ||
| Extrahepatic metastasis | ||||
| No | 1.00 (Reference) | |||
| Yes | 0.70 (0.37-1.32) | 0.270 | ||
| Portal invasion | ||||
| No | 1.00 (Reference) | |||
| Yes | 1.29 (0.68-2.47) | 0.440 | ||
| AFP > 500 ng/mL | 1.26 (0.67-2.36) | 0.480 | ||
| CTP class | ||||
| A | 1.00 (Reference) | |||
| B | 5.38 (2.66-10.89) | < 0.001 | 2.63 (1.19-5.78) | 0.020 |
| mALBI grade | ||||
| 1 + 2a | 1.00 (Reference) | |||
| 2b + 3 | 5.20 (2.52-10.76) | < 0.001 | 3.90 (1.71-8.90) | 0.001 |
| Prior treatment for HCC | ||||
| No | 1.00 (Reference) | |||
| Yes | 0.87 (0.42-1.78) | 0.700 | ||
| Prior local therapy for HCC | ||||
| No | 1.00 (Reference) | |||
| Yes | 0.79 (0.39-1.58) | 0.500 | ||
| Prior systemic therapy for HCC | ||||
| No | 1.00 (Reference) | |||
| Yes | 1.80 (0.79-4.11) | 0.160 | ||
| Combination of AB and local treatment as first-line treatment | ||||
| No | 1.00 (Reference) | |||
| Yes | 0.05 (0.01-27.12) | 0.340 | ||
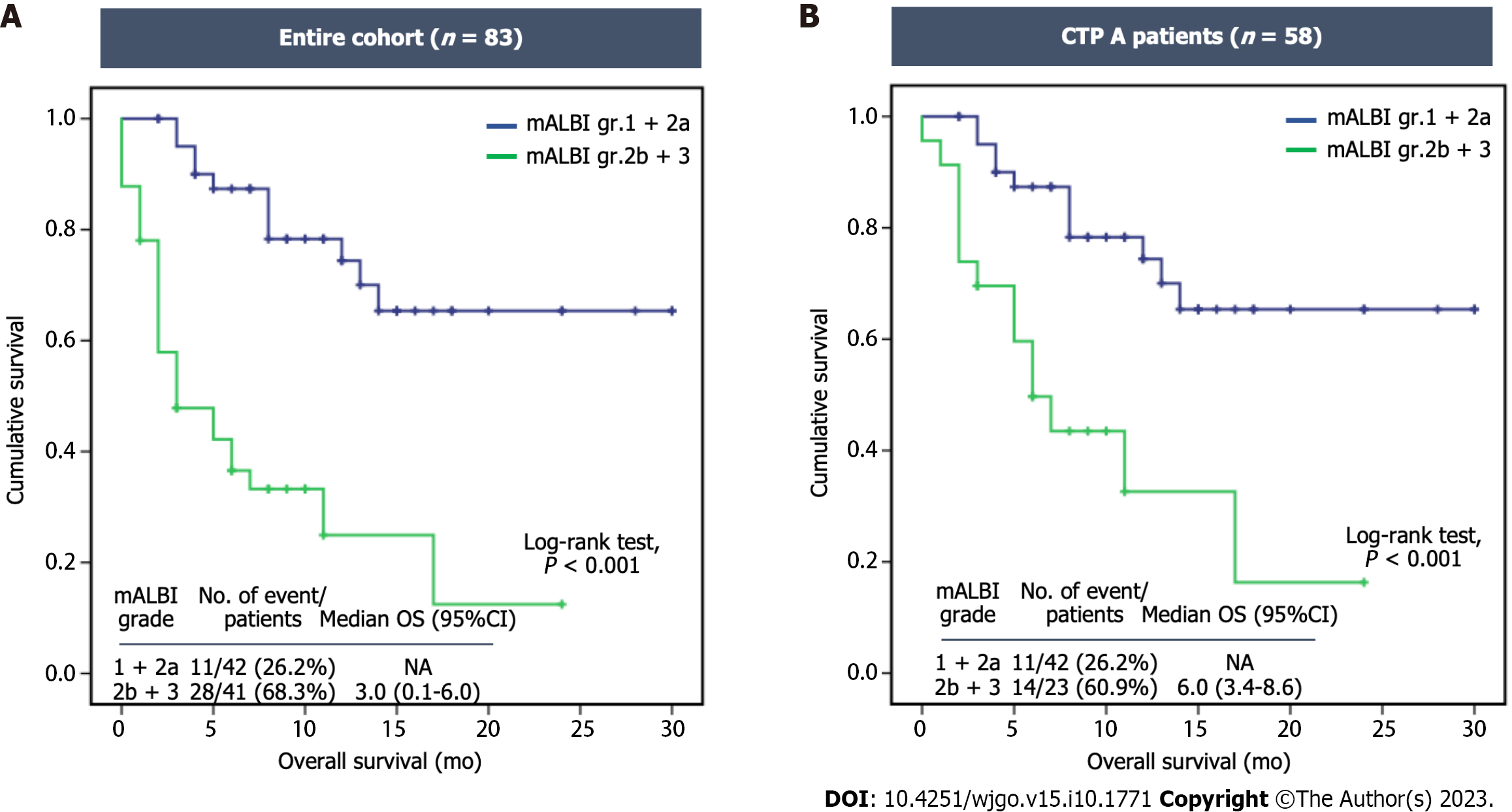
Patients in group 1 (lower mALBI grade) exhibited a significantly longer survival than those in group 2 (higher mALBI grade), i.e. not reached vs 3.0 mo (95%CI: 0.1–6.0, P < 0.001) (Figure 3A). When considering the classification based on CTP class, the OS of patients with CTP class A was 17 mo (95%CI: Not estimated), while it was only 2 mo (95%CI: 1.1-2.9) for those with CTP class B (P < 0.001). Among CTP class A patients, the median survival for those in group 1 remained significantly longer than for those in group 2, i.e. not reached vs 6.0 mo (95%CI: 3.4–8.6, P < 0.001) (Figure 3B).
Furthermore, among patients with CTP class A and a CTP score of 5 (n = 46), group 1 patients exhibited a significantly longer survival than group 2 patients [not reached vs 6.0 mo (95%CI: 3.4-8.6), P < 0.001], suggesting that mALBI grades performed better than CTP score and CTP classification in predicting survival. In contrast, among patients with CTP class A and a CTP score of 6 (n = 19), there was no significant difference in survival between group 2 and group 1 [11.0 mo (95%CI: 2.5-19.5) vs 3 mo (95%CI: not estimated), P = 0.830]. Likewise, the median PFS of group 1 and group 2 showed no significant difference [8.0 mo (95%CI: 3.2-12.8) vs 9.0 mo (95%CI: 4.5–13.5), respectively, P = 0.920].
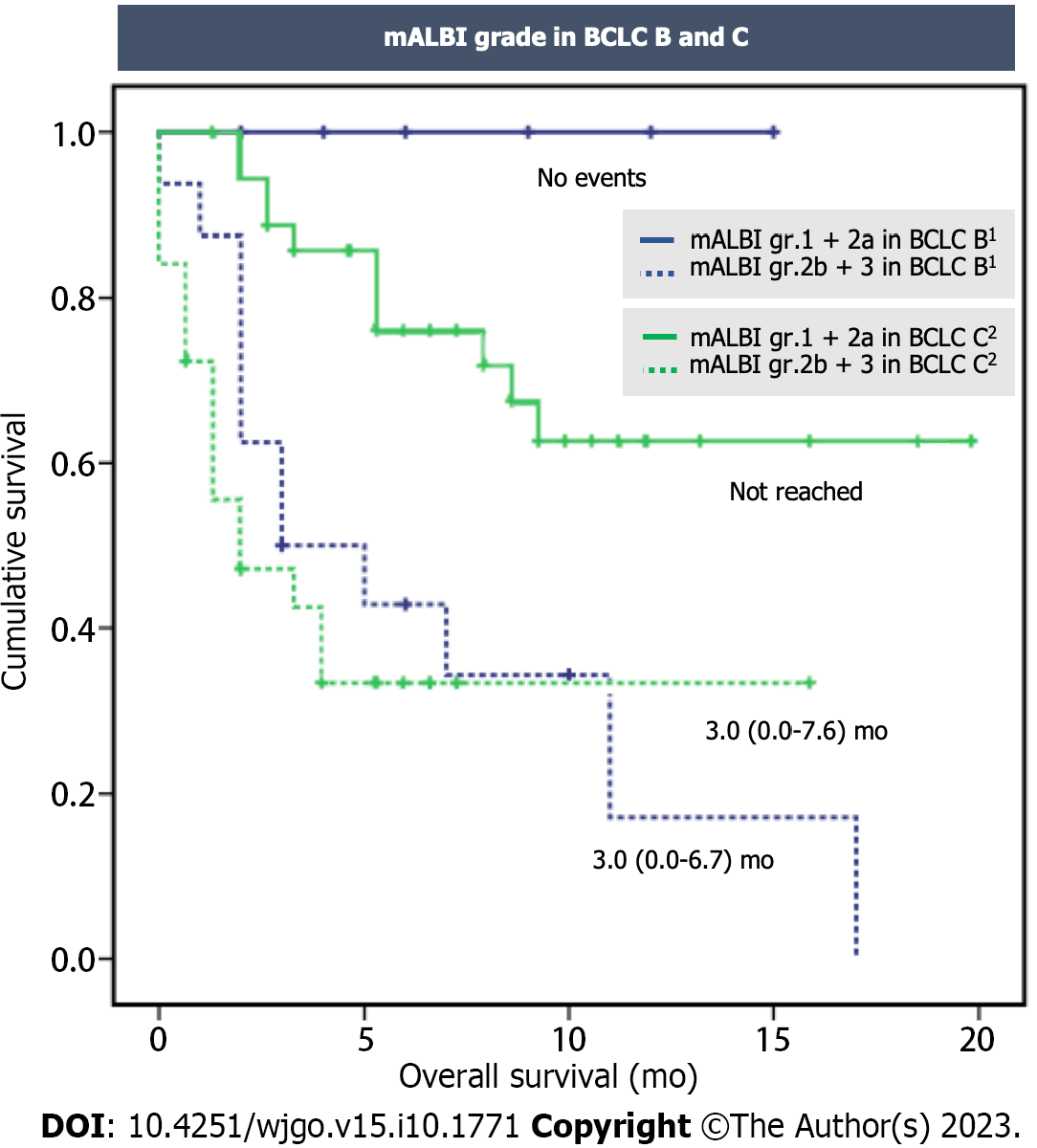
Similar findings were observed when considering the classification based on BCLC stages. In the BCLC C subgroup, patients in group 1 had an estimated median survival that was not reached, while those in group 2 had a median survival of 3.0 mo (95%CI: 0.0-7.6, P < 0.001). In the BCLC B subgroup, death was not reported in group 1, while those in group 2 had a median survival of 3.0 mo (95%CI: 0.0-6.7, P = 0.014) (Figure 4).
This study presented the efficacy of AB treatment for uHCC and highlighted the significance of liver functional reserve as assessed by mALBI grades in relation to patient survival. The findings of the study suggested that mALBI grades offer a more reliable prognostic ability compared to CTP classification because mALBI grades can distinguish between patients who share the same CTP score or classification but exhibit different outcomes.
The landmark phase III clinical trial for AB treatment in uHCC showed prolonged patient survival of 19.2 mo and PFS of 6.9 mo over a follow-up period of 15.6 mo. However, in real-world cohorts, patients receiving AB treatment had shorter survival, ranging from 10.6-15.0 mo, but similar PFS, ranging from 5.1-6.9 mo[14-16]. Our cohort demonstrated an OS of 13 mo and a PFS of 9 mo. The shorter survival observed in our study compared to the landmark study was likely explained by differences in patient characteristics, particularly the liver functional reserve. The AB combination was tested in a phase III clinical trial for its efficacy in CTP class A patients. In practice, however, AB was given not only to CTP class A patients but also to CTP class B patients. The OS and PFS of patients in our study were relatively similar to a real-world cohort. Focusing on a subgroup of CTP A patients, our study found a survival rate of 17 mo, which was similar to the clinical trial results. These findings underscore the significance of liver functional reserve in determining outcomes for patients receiving AB treatment.
The mALBI grades exhibited a significant correlation with survival in uHCC patients who underwent systemic therapy, where patients with mALBI grades 2a had a survival rate of 11 mo compared to 7 mo for those with mALBI grades 2b who received lenvatinib or ramucirumab[6]. Our study consistently demonstrated that mALBI grades are reliable predictors of OS in patients with uHCC undergoing AB treatment. Patients with mALBI grades 1 or 2a had significantly longer survival compared to those with mALBI grades 2b or 3, with a median survival that was not reached compared to 3.0 mo, respectively. The more precise scoring system of mALBI grade may account for its superior predictive performance, as it breaks down ALBI grade 2 into 2a and 2b using a cutoff value of 30% indocyanine green retention rate at 15 min (ICG-R15). ICG-R15 was initially developed to evaluate liver functional reserve in patients undergoing hepatic resection; those with an ICG-R15 of ≤ 30% were eligible for segmentectomy[17]. The mALBI grades demonstrated superior performance compared to the original ALBI in identifying patients with favorable or unfavorable survival outcomes, especially among those with a CTP score of 5. Our study consistently observed this trend[7].
The observed PFS in our cohort was 9 mo. In a retrospective study involving 71 Japanese uHCC patients who received AB treatment, the PFS was significantly longer in the mALBI 1 + 2a group compared to the mALBI 2b + 3 group (10.5 mo vs 3.0 mo, P < 0.010)[10]. This suggests that AB therapy was more effective in patients with mALBI 1 + 2a. However, our study found that the mALBI 1 + 2a and 2b + 3 groups had similar PFS durations of 8-9 mo. This could be attributed to the higher proportion of BCLC stage C patients in the mALBI 1 + 2a group compared to the mALBI 2b + 3 group. Advanced stages of HCC are associated with reduced efficacy of AB therapy, as indicated in a previous study involving BCLC stage C patients, which reported a significantly lower ORR compared to BCLC stage B patients (32% vs 62%, P < 0.050)[10]. In our study, we found that the ORR in the mALBI grade 1 + 2a group was not significantly higher than in the mALBI grade 2b group (35.9% vs 13.6%, P= 0.060). Similarly, another retrospective study involving 115 uHCC patients treated with AB showed no significant difference in ORR between the mALBI grade 1 + 2a and mALBI grade 2b groups (21.9% vs 12.9%, P = 0.460), which is consistent with our findings[9].
In patients with uHCC, the treatment outcome is more dependent on liver functional reserve rather than tumor burden. Within our cohort, we observed that patients with a low mALBI grade of 1 + 2a had a longer survival compared to those with a high mALBI grade of 2b + 3, specifically within the BCLC B and C subgroups. Among patients with a low mALBI grade in the BCLC B subgroup, there were no deaths by the end of the study period. Conversely, patients with a high mALBI grade in the BCLC C subgroup experienced poor survival. In our study, patients with BCLC B and C HCCs exhibited comparable survival of 11 mo and 14 mo, respectively. This finding was consistent with a previous study that reported survival of 25.8 mo and 24.6 mo in BCLC stage B and C patients, respectively[18]. These findings support the notion that the effectiveness of AB therapy is primarily influenced by the liver’s functional reserve rather than the stage of the tumor.
Among patients with progressive disease, we observed that those who received subsequent treatment, including additional systemic therapies or locoregional therapies, had slightly longer survival compared to those who received the best supportive care, although the difference did not reach statistical significance. We believe it remains worth considering the continuation of treatment with alternative options if feasible, as it has the potential to extend the survival of uHCC patients who face disease progression after AB treatment.
Our study had several strengths. First, we included diverse uHCC patients who received real-world AB treatment, including those with liver functional reserve in CTP B, which extends beyond the recommended guidelines. This reflects the practical treatment approach in the Asian population, where various treatment options are commonly used, deviating from recommended guidelines. Second, we were able to track post-AB treatment and disease progression, providing real-life survival outcomes. Despite these strengths, our study also had some limitations due to its retrospective nature, relatively short follow-up period, and a small number of patients in the BCLC B subgroup. A large, multicenter prospective cohort study with an extended follow-up duration is necessary to gain a deeper understanding of the efficacy of AB therapy in HCC.
In uHCC patients, liver functional reserve plays a significant role as a prognostic factor and is essential for maximizing the effectiveness of AB therapy in clinical practice. Our study demonstrated that mALBI grades are a reliable prognostic factor, particularly for distinguishing patients with CTP A. The assessment of liver functional reserve using mALBI before initiating AB treatment can assist in identifying appropriate candidates for this therapy.
Modified albumin-bilirubin (mALBI) grade has been established as a survival determinant in hepatocellular carcinoma (HCC) patients who receive locoregional and targeted therapies.
The predictive value of mALBI grade for predicting outcomes of HCC patients receiving atezolizumab plus bevacizumab (AB) treatment has yet to be fully investigated.
To assess whether mALBI could predict survival in unresectable HCC patients who were treated with AB.
A single-center, retrospective cohort study enrolled unresectable HCC patients who received AB treatment between September 2020 and April 2023 and were followed up until June 2023. An association between mALBI and patient survival was determined using Cox proportional hazards analysis.
Of the 83 patients, the median overall survival (OS) was 13.0 mo [95% confidence interval (CI): 5.2-20.8]. The median progression-free survival was 9.0 mo (95%CI: 5.0-13.0). The patients were divided into two groups based on mALBI grades: 42 patients (50.6%) in the mALBI 1 + 2a group and 41 patients (49.4%) in the mALBI 2b + 3 group. The mALBI 1 + 2a group exhibited significantly better survival compared to the mALBI 2b + 3 group, with a median OS that was not reached vs 3.0 mo (95%CI: 0.1-6.0) (P < 0.001). In a subgroup of patients with Child-Turcotte-Pugh (CTP) A, the mALBI 1 + 2a group also showed significantly longer survival compared to the mALBI 2b + 3 group, with a median OS that was not reached vs 6.0 mo (95%CI: 3.4-8.6, P < 0.001).
Our study demonstrated that mALBI grades are a more reliable prognostic factor than CTP classification, particularly for distinguishing outcomes of patients within the CTP A class.
The assessment of liver functional reserve using mALBI before initiating AB treatment can assist in identifying appropriate candidates for this therapy.
This work was supported by the Division of Gastroenterology, Faculty of Medicine Foundation, Chulalongkorn University.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bi JF, China; Liang HL, Taiwan; Maher H, China S-Editor: Lin C L-Editor: Filipodia P-Editor: Zhang XD
| 1. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 3994] [Article Influence: 570.6] [Reference Citation Analysis (6)] |
| 2. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2394] [Article Influence: 798.0] [Reference Citation Analysis (58)] |
| 3. | Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1-85. [PubMed] |
| 4. | Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1962] [Cited by in RCA: 1954] [Article Influence: 195.4] [Reference Citation Analysis (0)] |
| 5. | Toyoda H, Johnson PJ. The ALBI score: From liver function in patients with HCC to a general measure of liver function. JHEP Rep. 2022;4:100557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 67] [Reference Citation Analysis (1)] |
| 6. | Kudo M. Newly Developed Modified ALBI Grade Shows Better Prognostic and Predictive Value for Hepatocellular Carcinoma. Liver Cancer. 2022;11:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Hiraoka A, Kumada T, Tsuji K, Takaguchi K, Itobayashi E, Kariyama K, Ochi H, Tajiri K, Hirooka M, Shimada N, Ishikawa T, Tachi Y, Tada T, Toyoda H, Nouso K, Joko K, Hiasa Y, Michitaka K, Kudo M. Validation of Modified ALBI Grade for More Detailed Assessment of Hepatic Function in Hepatocellular Carcinoma Patients: A Multicenter Analysis. Liver Cancer. 2019;8:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 181] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 8. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4467] [Article Influence: 893.4] [Reference Citation Analysis (2)] |
| 9. | Sho T, Suda G, Yamamoto Y, Furuya K, Baba M, Ogawa K, Kubo A, Tokuchi Y, Fu Q, Yang Z, Kimura M, Kitagataya T, Maehara O, Ohnishi S, Nakamura A, Yamada R, Ohara M, Kawagishi N, Natsuizaka M, Nakai M, Suzuki K, Izumi T, Meguro T, Terashita K, Takagi T, Ito J, Kobayashi T, Miyagishima T, Sakamoto N. Efficacy and Effect on Liver Functional Reserve of Atezolizumab and Bevacizumab for Unresectable Hepatocellular Carcinoma in Patients Who Do Not Meet Eligibility Criteria of IMbrave150. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 10. | Tomonari T, Tani J, Sato Y, Tanaka H, Tanaka T, Taniguchi T, Asahiro M, Okamoto K, Sogabe M, Miyamoto H, Muguruma N, Masaki T, Takayama T. Initial therapeutic results of atezolizumab plus bevacizumab for unresectable advanced hepatocellular carcinoma and the importance of hepatic functional reserve. Cancer Med. 2023;12:2646-2657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, Waljee AK, Singal AG. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018;154:1706-1718.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 774] [Article Influence: 110.6] [Reference Citation Analysis (0)] |
| 12. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. [PubMed] |
| 13. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2583] [Cited by in RCA: 3235] [Article Influence: 215.7] [Reference Citation Analysis (36)] |
| 14. | de Castro T, Jochheim LS, Bathon M, Welland S, Scheiner B, Shmanko K, Roessler D, Ben Khaled N, Jeschke M, Ludwig JM, Marquardt JU, Weinmann A, Pinter M, Lange CM, Vogel A, Saborowski A. Atezolizumab and bevacizumab in patients with advanced hepatocellular carcinoma with impaired liver function and prior systemic therapy: a real-world experience. Ther Adv Med Oncol. 2022;14:17588359221080298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 15. | Fulgenzi CAM, Cheon J, D'Alessio A, Nishida N, Ang C, Marron TU, Wu L, Saeed A, Wietharn B, Cammarota A, Pressiani T, Personeni N, Pinter M, Scheiner B, Balcar L, Napolitano A, Huang YH, Phen S, Naqash AR, Vivaldi C, Salani F, Masi G, Bettinger D, Vogel A, Schönlein M, von Felden J, Schulze K, Wege H, Galle PR, Kudo M, Rimassa L, Singal AG, Sharma R, Cortellini A, Gaillard VE, Chon HJ, Pinato DJ. Reproducible safety and efficacy of atezolizumab plus bevacizumab for HCC in clinical practice: Results of the AB-real study. Eur J Cancer. 2022;175:204-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 54] [Reference Citation Analysis (0)] |
| 16. | Himmelsbach V, Pinter M, Scheiner B, Venerito M, Sinner F, Zimpel C, Marquardt JU, Trojan J, Waidmann O, Finkelmeier F. Efficacy and Safety of Atezolizumab and Bevacizumab in the Real-World Treatment of Advanced Hepatocellular Carcinoma: Experience from Four Tertiary Centers. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 17. | Miyagawa S, Makuuchi M, Kawasaki S, Kakazu T. Criteria for safe hepatic resection. Am J Surg. 1995;169:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 252] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Kudo M. A New Era in Systemic Therapy for Hepatocellular Carcinoma: Atezolizumab plus Bevacizumab Combination Therapy. Liver Cancer. 2020;9:119-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |