Published online Oct 15, 2023. doi: 10.4251/wjgo.v15.i10.1756
Peer-review started: May 21, 2023
First decision: June 1, 2023
Revised: July 5, 2023
Accepted: August 31, 2023
Article in press: August 31, 2023
Published online: October 15, 2023
Processing time: 142 Days and 5.9 Hours
Colon cancer remains a leading cause of death globally. Pomolic acid (PA) can be separated from the ethyl acetate fraction of achyrocline satureioides.
To determine the effects of PA and its glucopyranose ester, pomolic acid-28-O-β-D-glucopyranosyl ester (PAO), on colon cancer HT-29 cells.
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide assay was used to measure cell viability. Apoptosis was detected via hoechst 33342 staining. PI single staining was identified by flow cytometry to determine the cycle and scratch assay was used to observe the migration of HT-29 cells. The levels of mRNA and proteins were evaluated by q polymerase chain reaction and western blotting, respectively.
PA and PAO considerably inhibited the growth of the HT-29 cell line in a time and dose-dependent manner. After the administration of PA and PAO for 24 and 48 h, cell apoptosis was significantly promoted and HT-29 cells were arrested in the G0/G1 stage. The Bax/Bcl2 ratio was also increased, which activated cysteinyl aspartate specific proteinase 3, leading to apoptosis; it also increased the expression of light chain 3 II/I and Beclin1, which activated autophagy and caused cell death. This in turn increased the expression of p62 to promote cell apoptosis, inhibiting the levels of signal transducer and activator of transcription 3 (STAT3) and p-STAT3, suppressing the level of Bcl2, and promoting cell.
Both PA and PAO provide novel therapeutic strategies for treating colorectal cancer.
Core Tip: Compounds pomolic acid (PA) and pomolic acid-28-O-β-D-glucopyranosyl ester (PAO) exhibited a considerable growth inhibitory effect against HT-29 cell lines in a time-dose-dependent manner. PA and PAO promote apoptosis through autophagy in HT-29 colon tumor cells. Both PA and PAO provide novel therapeutic strategy for colorectal cancers treatment.
- Citation: Liu LY, Yu TH, Liao TS, Xu P, Wang Y, Shi M, Li B. Pomolic acid and its glucopyranose ester promote apoptosis through autophagy in HT-29 colon cancer cells. World J Gastrointest Oncol 2023; 15(10): 1756-1770
- URL: https://www.wjgnet.com/1948-5204/full/v15/i10/1756.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i10.1756
Colon cancer remains a leading cause of death globally[1], while colorectal cancer has become the third most common tumor with the highest incidence[2]. Surgical treatment is generally the best choice for early-stage colon cancer patients, but unfortunately many patients are diagnosed at an advanced stage. Surgery-based postoperative adjuvant chemotherapy is currently the most important method for treating colon cancer. However, resistance and toxicity of chemotherapy have severely hampered the implementation of chemotherapy regimens. There is thus a need for new therapeutic options for patients at an advanced stage of the disease, so the search for new drugs and targets has become a key component of efforts to treat colon cancer.
There are abundant active substances in nature, especially in plants of medicine food homology. Achyrocline satureioides is a plant from the achyrocline genus brassica, which is a medicinal herb widely used in Latin America for gastrointestinal diseases, bacterial infections, anti-inflammatory effects, pain relief, and for treating other diseases[3-7]. We previously isolated many compounds from the flowers of A. satureioides, including triterpenics, anthraquinones, and flavonoids. Research has shown that pomolic acid (PA) and its glucopyranose ester have effects against breast cancer[8-10], prostate cancer[11], leukemia[12-15], and other malignant tumors. Because of its high safety, these agents have been increasingly used in the treatment of cancer. However, there has been little research on the use of PA and its glucopyranose ester in treating colon cancer, or on the mechanisms behind their effects. We thus investigated the influence of PA and its glucopyranose ester on colon cancer cells. In this study, PA and its glucopyranose ester showed good inhibitory effects on colon cancer cells and have potential as new drugs for future use in a clinical context.
PA was separated and purified from A. satureioides (purity > 98%). Pomolic acid-28-O-β-D-glucopyranosyl ester (PAO) was obtained from Chengdu Alpha Biological Co., Ltd. (cas: 83725-25-0), with purity exceeding 94%. Oxaliplatin was obtained from Hengrui Medicine (China). Annexin-V-fluorescein isothiocyanate and propidium iodide (BD Biosciences, Franklin Lake, NJ, United States), McCoys’ 5A (Modified; Gibco, United States), and [fetal bovine serum (FBS); EXCEll, China] were also obtained. Bcl2, anti-sequestosome-1 (p62), anti-light chain 3 (LC3) A/B, Bax, Beclin-1, anti-janus kinase (JAK), anti-p-signal transducer and activator of transcription 3 (STAT3), and anti-STAT3 antibodies were obtained from cell signaling technology (United States). Anti-β-actin was purchased Protech (China). 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) and dimethyl sulfoxide (DMSO) were obtained from Solarbio (China). The HT-29 cell line was purchased from National Collection of Authenticated Cell Cultures. Finally, the laser confocal microscope leica DMI3000B was used (Leica, Germany).
Preparation of PA:A. Satureioides was identified by Professor Peng HS of Anhui University of Chinese Medicine. Nine kilograms of dried A. satureioides was pulverized mechanically and extracted five times with the amount of 95% ethanol. It was then heated and refluxed two times for 1 h each, and subsequently heated and refluxed three times with the amount of 50% ethanol two times for 1 h each. Then, the ethanol extracts were combined and concentrated under reduced pressure to give a brown solid material (1.6 kg), which was extracted using ethyl acetate. Next, this ethyl acetate fraction (950 g) was subjected to silica gel column chromatography (petroleum ether: Acetone 100: 0, 50: 1, 20: 1, 5: 1, and 0: 100) to obtain six fractions (Fr. 1-6). Among them, Fr. 2 (110 g) was eluted by silica gel column chromatography (petroleum ether: Acetone 50: 1–1: 50) to obtain 10 fractions. Among these, Fr. 2-10 were subjected to medium-pressure preparative chromatography (methanol: Water 30: 70-100: 0) gradient elution to obtain five fractions. Fr. 2-10-3-5 was then subjected to gel column chromatography (CH2Cl2/MeOH gradient elution system), silica gel column chromatography (petroleum ether/acetone gradient elution system), and preparative thin-layer chromatography (petroleum ether/acetone gradient elution system) to afford the compound PA (116.6 mg). The concentrations of PA and PAO in this study were determined based on previous publications[16,17] and our preliminary experiment.
Nuclear magnetic resonance (NMR) assay: DMSO-d6 was used to dissolve the compound PA. An NMR spectrometer (Bruker Corporation, Solna, Sweden) was used to record C-NMR (125 MHz) and H-NMR (500 MHz) spectra. All chemical shifts were reported in δ (ppm) relative to tetramethylsilane.
Cell culture and proliferation assay: Cells were cultured in McCoy’s 5A (modified) medium containing 50 U/mL penicillin, 50 mg/mL streptomycin, and 10% FBS under conditions of 5% CO2 at 37 ℃. The medium was replaced with serum-free medium 24 h before the different treatments.
The MTT method was used to detect cell proliferation. Cells (1 × 104) were seeded in a 96-well plate. After 12 h, the cells were treated with different concentrations of PA (5, 6.25, 7.5, 10, 12.5, 15, and 20 μg/mL equivalent to 10.59, 13.24, 15.89, 21.18, 26.48, 31.77, and 42.36 μM, respectively) or PAO (10, 20, 40, 60, 80, and 100 μM, respectively) medium with 0.1% DMSO. After different durations of incubation (24, 48, and 72 h), MTT reagents were used to incubate cells for 3 h. Then, the OD value was detected with an enzyme-linked immunosorbent assay reader (Thermo Fisher Scientific, United States) at a wavelength of 490 nm. The IC50 values and inhibition rate were calculated.
Hoechst 33342 staining: Cells (2 × 103/well) were seeded in a 24-well plate and cell slides were added in per well. After being synchronized, the cells were treated with the medium or PA for 24 and 48 h. The cells were then washed in phosphate buffer saline (PBS) three times, while Hoechst 33342 (10 μg/mL) was added to each well for 30 min. The cell slides were taken out, placed on a glass slide, and observed under a laser scanning confocal microscope.
Acridine orange/ethidium bromide (AO/EB) double staining: Cells (2 × 103/well) were seeded in a 24-well plate and cell slides were added in per well. After being synchronized, the cells were treated with the medium or PA for 24 and 48 h. The cells were washed in PBS three times, while 10 μL of AO/EB solution was added for incubation (5 min). Then, the cell slides were taken out and observed under a confocal microscope.
Cell apoptosis analysis: Cells (4 × 105/well) were seeded in a six-well plate. After the administration of drugs for 24 and 48 h, the cells were collected and washed with PBS three times. Annexin V-FITC and PI were used for staining, and the cells were analyzed with a FACS verse instrument (BD Biosciences, San Jose, CA, United States).
Cell cycle analysis: Cells (4 × 105/well) were seeded in a six-well plate. After the administration of drugs for 24 and 48 h, the cells were collected and washed with PBS three times. The cells were then fixed in ice-cold 70% ethanol overnight. They were then stained with 500 μL of a PI RNase solution for 15 min and analyzed by flow cytometry (FACS verse; BD Biosciences, United States). FlowJo version 10 software (BD Biosciences, United States) was used for cell phase analysis.
Scratch motility assay: Cells (4 × 105/well) were seeded in a six-well plate and cultured under conditions of 37 ℃ and 5% CO2. A 10 μL sterilized pipette tip was then used to scrape the cell monolayer. The particular drug was added in the form of serum-free medium containing different drug concentrations, while the vehicle group was treated with 0.1% DMSO serum-free medium. The distance of cell movement was measured every 24 h until 48 h. The migration area was measured by ImageJ[18].
Reverse-transcription and real-time polymerase chain reaction (RT-PCR): HT-29 cells were exposed to the drugs for 48 h. TRIzol reagent was used for RNA extraction. Reverse-transcription PCR was performed using an RT-PCR Kit (TransGen Biotech, China). Real-time PCR was performed with TransStart® Top Green qPCR SuperMix (TransGen Biotech, China). The 2-ΔΔt method was used for gene expression analysis. The primers used are listed in Supple
Western blotting: After the administration of drugs for 24 and 48 h, cell lysates were collected. Protein samples (30 μg) were separated by 10% SDS-PAGE and transferred to PVDF membranes. These PVDF membranes were incubated with related primary antibodies overnight. These membranes were then incubated with secondary antibodies for 4 h. An enhanced chemiluminescence kit (Transgen, Beijing, China) was used to detect immunolabeling. Grayscale values were measured using ImageJ.
Statistical analysis: All data are presented here as mean ± SD from at least three independent experiments. In the figures, data representative of the experiments are presented. The statistical significance of differences was assessed by one-way analysis of variance. P < 0.05 was considered to reflect statistical significance.
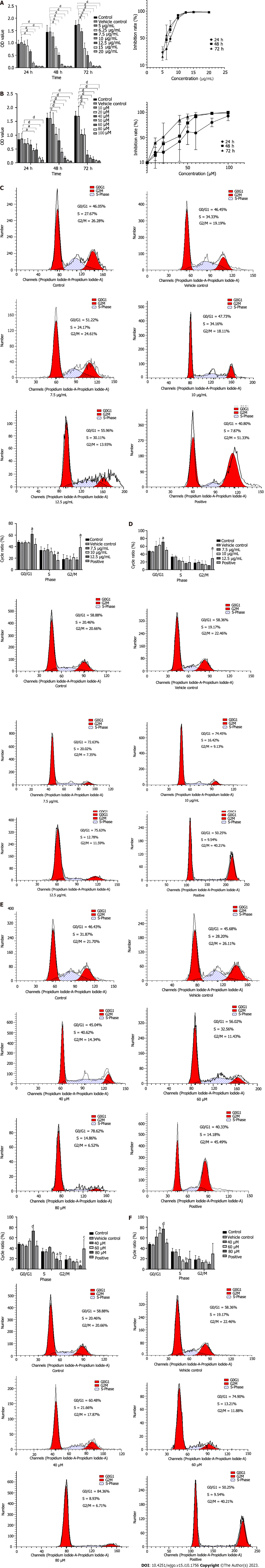
To investigate the effects of PA and PAO on colon cancer cells, the MTT assay was performed. PA treatment at 6.25, 7.5, 10, 12.5, 15, and 20 μg/mL exerted significant inhibitory effects. IC50 values for the treatments lasting 24, 48, and 72 h were 9.7, 7.6, and 8.8 μg/mL, respectively (Figure 1A). Meanwhile, PAO treatments at 10, 20, 40, 60, 80, and 100 μM also exerted significant inhibitory effects in a concentration- and time-dependent manner (P < 0.05). Here the IC50 values for the treatments lasting 24, 48, and 72 h were 50.4 (79.4 μg/mL), 24.3 μM (38.3 μg/mL), and 11.96 μM (18.8 μg/mL), respectively (Figure 1B). Compared with that in the vehicle control group, the cell cycle distribution was changed and the cells were arrested at the G0/G1 phase in the groups treated with PA (Figure 1C and D) and PAO (Figure 1E and F) for 24 (Figure 1C and E) and 48 h (Figure 1D and F).
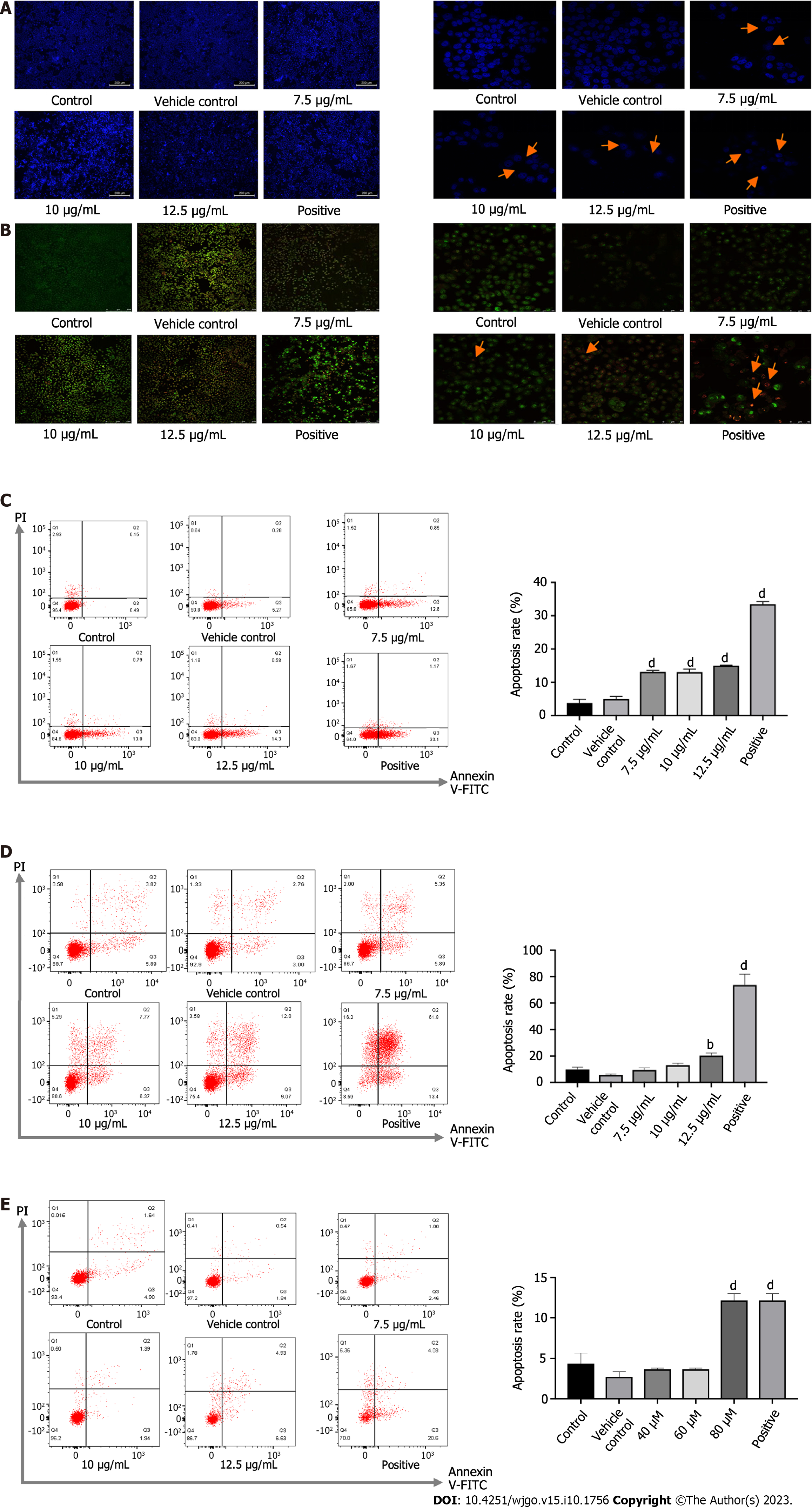
After drug administration for 24 or 48 h, the cells were stained with Hoechst 33342. In this approach, live cells with an intact cellular structure could be distinguished from dead cells with an incomplete structure in which the nucleus was stained. The drug-administered group, especially the high-dose group and the positive group, showed more dead cells, as indicated in Figure 2A and B. Morphologically, the live cells were normal, with the nucleus being uniformly fluorescent green, while the early apoptotic cells were condensed into a hanging bead, with a green or yellow-green color or fragmented coloration. The late apoptotic cells were orange in color and the chromatin was concentrated. Meanwhile, the necrotic cells were round or elliptical, in which the nucleus was dyed orange, and the sizes were relatively small. Among PA-treated cells, there were increases in apoptotic cells compared with the rate of 2.67% in control cells to 12.07%, 14.14%, and 15.11% in groups treated with 7.5, 10, and 12.5 μg/mL for 24 h (Figure 2C) and from 4.36% to 7.02%-21.45% in groups treated with 7.5, 10, and 12.5 μg/mL for 48 h (Figure 2D). After PAO treatment, the apoptosis rate in the 80 μM and positive group was markedly higher than in the control group (Figure 2E).
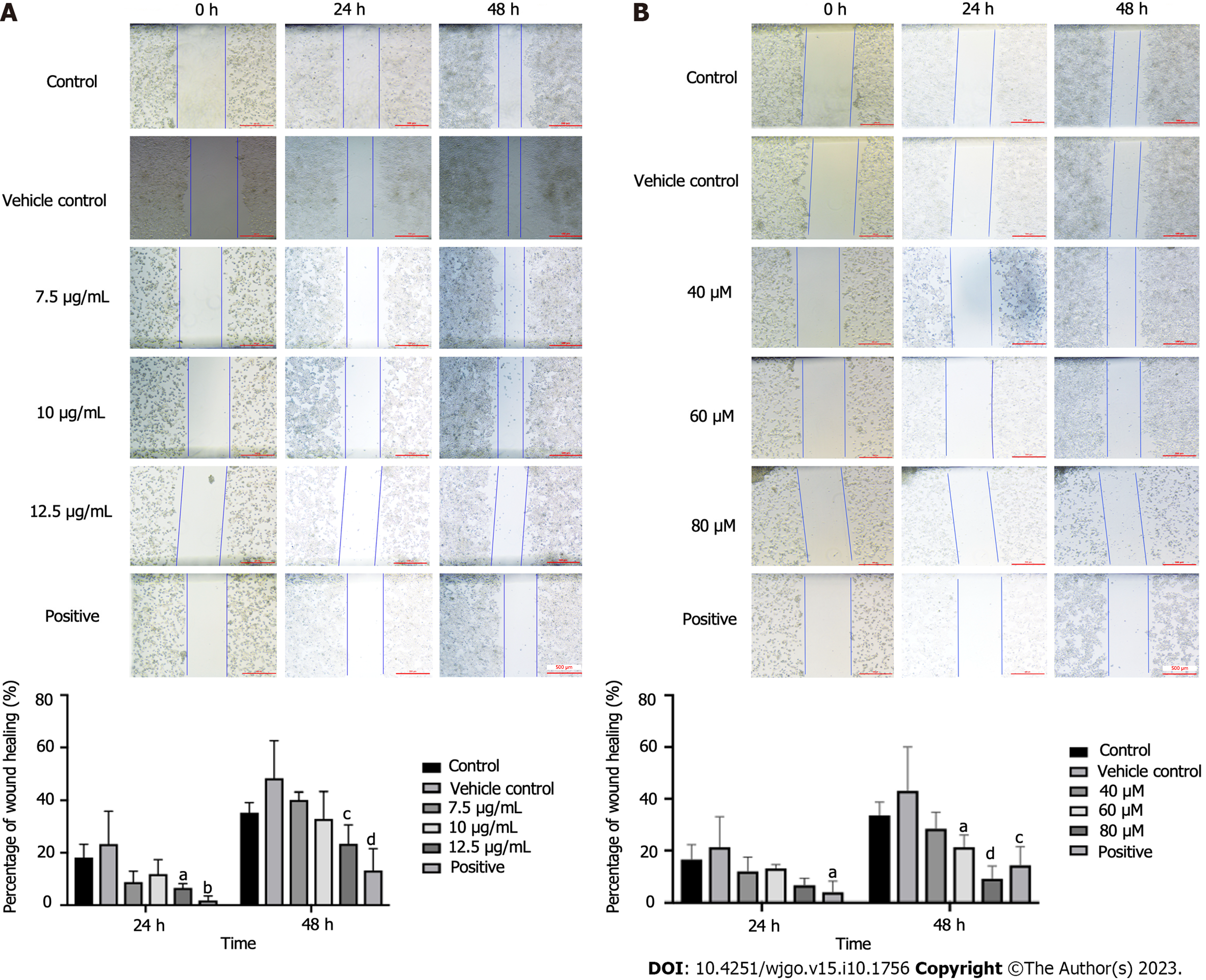
After the administration of drugs, the speed and extent of scratch healing in the drug group were lower than those in the vehicle group. A concentration of 12.5 μg/mL could significantly reduce the scratch healing rate (P < 0.05 and P < 0.001 at 24 and 48 h, respectively) (Figure 3A). There was no significant difference at 24 h, but the PAO concentrations of 60 and 80 μM significantly reduced the healing rate of scratches at 48 h (P < 0.05 and P < 0.0001, Figure 3B).
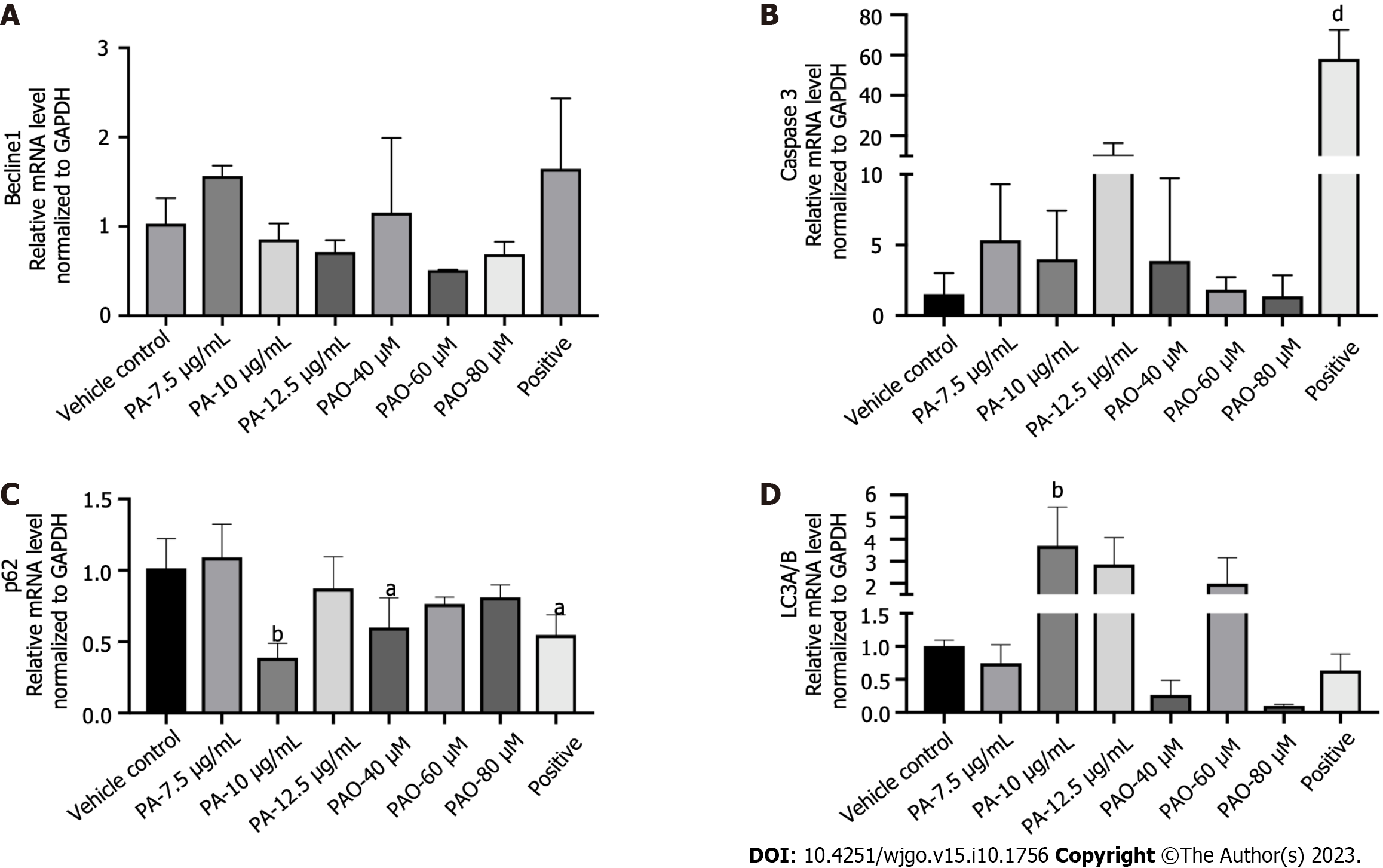
To expand these findings, the mechanisms behind the anti-colon cancer effects of PA and PAO were explored further. More protein levels were determined. We found that the levels of Bax/Bcl2, cysteinyl aspartate specific proteinase (Caspase) 3, LC3II/I, Beclin1, and p62 proteins were markedly enhanced in HT-29 cells under PA or PAO treatment and the expression of JAK STAT3 or p-STAT3 was downregulated (Figure 4). Meanwhile, no effect on the expression of Beclin1 was noted in the PA or PAO group (Figure 5A). Notably, the mRNA expression of Caspase3 and LC3II/I was upregulated while p62 was downregulated after treatment with PA or PAO (Figure 5B-D). These results are basically consistent with the results of phenotypic research mentioned above.
Colon cancer has the highest morbidity and mortality among gastrointestinal tumors, making it a major threat to health and a particular focus for researchers[19]. Owing to the serious side effects of chemotherapy and the high cost of targeted drugs, patient compliance and overall survival are poor. Combination therapy with fluorouracil, oxaliplatin, and calcium folinate is a common method for treating colon cancer. However, severe side effects including gastrointestinal reactions, bone marrow suppression, liver damage, and individual differences in drug sensitivity limit its application[20]. Natural products with strong biological activity are optional drugs for clinical application. A. satureioides, an edible dual-use plant, has been used to cure a variety of diseases in Brazilian folk medicine. In this study, we searched for the active component, in the form of PA, from the plant for its anti-colon cancer effects, high safety, and strong medicinal properties. We also clarified its mechanism of action against colon cancer. PA has the particular advantage of having minimal side effects.
Our study showed that PA can inhibit HT-29 cell proliferation in a time- and dose-dependent manner and promote HT-29 cell apoptosis, as well as changing the distribution of HT-29 cells among the phases of the cell cycle. Specifically, HT-29 cells were arrested at the G0/G1 phase and their rate of migration was significantly reduced. The results also showed that, after the administration of PA or PAO, the levels of Bax/Bcl2, Caspase3, LC3II/I, Beclin1, and p62 in HT-29 cells were markedly elevated.
In the process of tumor development, apoptosis is usually downregulated. Therefore, reduced apoptosis is considered to be a sign of cancer[21-24]. Members of the Bcl2 family play key roles in regulating cell apoptosis[25]. Bax and Bak (known as multi-domain pro-apoptotic proteins) can promote apoptosis by forming oligomers on the mitochondrial membrane. There, they directly induce apoptosis after receiving the death signal, resulting in the release of cytochrome c, and apoptotic protease activator-activating factor 1 and Caspase activation[26]. Our research has shown that PA and PAO can significantly reduce the Bcl2/Bax ratio, which is basically consistent with the findings in the above literature.
In cancer, autophagy plays two roles of restricting the occurrence of tumors in the early stage but also promoting tumor development in cancers that have already become established. When autophagy is activated, LC3 is catalyzed and cleaved by the corresponding protease, so that the C-terminal glycine residue of LC3 is exposed to form LC3I, which is then processed by ubiquitination. This in turn upregulates autophagy. Beclin1 is a homolog of mammalian ATG6, which is encoded by the only confirmed mammalian “autophagy gene”. It is an executor of autophagy and plays an important role in autophagy. It has been reported that Beclin1 monoallelic deletion can promote cancer development and progression[27]. Beclin1 can form a complex with type III phosphatidylinositol-3 kinase, which can recruit autophagy-related protein LC3 to regulate the maturation and formation of autophagosomes, leading to autophagy. Defects in autophagy can lead to the accumulation of p62, which is an autophagy substrate protein and also a ubiquitin-binding protein. The sustained expression of p62 can change the regulatory expression of NF-κB and promote tumorigenesis[28-30]. The involvement of Bcl2 in the process of autophagy is mainly related to Beclin 1, which binds to and is inhibited by Bcl-2 or the Bcl-2 homolog Bcl-XL under physiological conditions. Our study showed that PA significantly increased the LC3II/I ratio and upregulated Beclin1. Interestingly, our experimental results revealed that PA can increase the level of p62 protein after PA administration for 24 and 48 h, but the positive drugs showed a decrease. This might be linked to the fact that, in addition to acting as a marker of autophagy activation, p62 can also serve as an important bridge for Caspase8-dependent cell activation, promoting the accumulation of Caspase8 and leading to apoptosis[31,32]. Our study showed that, after PA or PAO treatment, the level of Bcl2 decreased while the level of Beclin1 increased, which may have resulted in autophagy activation.
PA can promote the apoptosis of colon cancer cells, possibly through upregulating the expression of LC3II/I and Beclin1 and then activating autophagy, while upregulating the expression of p62, Bax/Bcl2, and Caspase3. These results indicate the PA is a potential anticancer agent.
Colon cancer remains as a high death leading cause in the world. Pomolic acid (PA) is separated from the ethyl acetate fraction of achyrocline satureioides.
We want to explore a novel, safe, effective agent for the treatment of colon cancer.
We aimed to examine the effects of PA and its glucopyranose ester, pomolic acid-28-O-β-D-glucopyranosyl ester (PAO) on colon cancer HT-29 cells.
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide assay was used to measure cell viability. Apoptosis was detected via Hoechst 33342 Staining. PI single staining by flow cytometry was determine the cycle and scratch assay was used to observe the migration of HT-29 cells. The levels of mRNA and proteins were evaluated with the q-polymerase chain reaction and western blot assay, respectively.
Compounds PA and PAO exhibited a considerable growth inhibitory effect against HT-29 cell lines in a time-dose-dependent manner. After administration of drugs for 24h and 48h, it showed that PA and PAO could significantly promote the cell apoptosis, and arrested HT-29 cells at G0/G1 stage; the ratio of Bax/Bcl2 was increased and activated the cysteinyl aspartate specific proteinase 3 which leading to an apoptosis, and the expression of anti-light chain 3 II/I and Beclin1 activate autophagy and cause cell death, increasing the expression of p62 promotes a cell apoptosis, inhibiting the level of signal transducer and activator of transcription 3 (STAT3) and p-STAT3 can suppress the level of Bcl2 and promote cell.
Both PA and PAO provide novel therapeutic strategy for colorectal cancers treatment.
The inhibitions of colon cancer by PA and PAO were validated with HT-29 cells.
The authors thank Professor Peng HS from Anhui University of Chinese Medicine for identifying achyrocline satureioides.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kim K, South Korea; Rau B, Germany S-Editor: Qu XL L-Editor: A P-Editor: Zhang XD
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15322] [Article Influence: 3064.4] [Reference Citation Analysis (4)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55854] [Article Influence: 7979.1] [Reference Citation Analysis (132)] |
| 3. | Retta D, Dellacassa E, Villamil J, Suárez SA, Bandoni AL. Marcela, a promising medicinal and aromatic plant from Latin America: A review. Industrial Crops & Products. 2012;38:27-38. [DOI] [Full Text] |
| 4. | Pereira CG, Gualtieri IP, Meireles MAA. Effect of Different Extraction Processes on the Recovery of Extracts from Achyrocline satureioides D.C.: An Evaluation of Antioxidant Activity. Separation Science & Technology. 2008;43:1549-1563. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Puhlmann J, Knaus U, Tubaro L, Schaefer W, Wagner H. Immunologically active metallic ion-containing polysaccharides of Achyrocline satureioides. Phytochemistry. 1992;31:2617-2621. [PubMed] [DOI] [Full Text] |
| 6. | Sabini MC, Escobar FM, Tonn CE, Zanon SM, Contigiani MS, Sabini LI. Evaluation of antiviral activity of aqueous extracts from Achyrocline satureioides against Western equine encephalitis virus. Nat Prod Res. 2012;26:405-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Baldissera MD, Oliveira CB, Rech VC, Rezer JF, Sagrillo MR, Alves MP, da Silva AP, Leal DB, Boligon AA, Athayde ML, Da Silva AS, Mendes RE, Monteiro SG. Treatment with essential oil of Achyrocline satureioides in rats infected with Trypanosoma evansi: relationship between protective effect and tissue damage. Pathol Res Pract. 2014;210:1068-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Kim B, Kim JH, Park B. Pomolic acid inhibits invasion of breast cancer cells through the suppression of CXC chemokine receptor type 4 expression. J Cell Biochem. 2016;117:1296-1307. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Kim B, Kim YC, Park B. Pomolic acid inhibits metastasis of HER2 overexpressing breast cancer cells through inactivation of the ERK pathway. Int J Oncol. 2016;49:744-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Park JH, Yoon J, Park B. Pomolic acid suppresses HIF1α/VEGF-mediated angiogenesis by targeting p38-MAPK and mTOR signaling cascades. Phytomedicine. 2016;23:1716-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Martins CA, Rocha GDG, Gattass CR, Takiya CM. Pomolic acid exhibits anticancer potential against a docetaxel‑resistant PC3 prostate cell line. Oncol Rep. 2019;42:328-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Pereira MXG, Hammes ASO, Vasconcelos FC, Pozzo AR, Pereira TH, Caffarena ER, Gattass CR, Maia RC. Antitumor Effect of Pomolic Acid in Acute Myeloid Leukemia Cells Involves Cell Death, Decreased Cell Growth and Topoisomerases Inhibition. Anticancer Agents Med Chem. 2018;18:1457-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Kuete V, Sandjo LP, Seukep JA, Zeino M, Mbaveng AT, Ngadjui B, Efferth T. Cytotoxic compounds from the fruits of Uapaca togoensis towards multifactorial drug-resistant cancer cells. Planta Med. 2015;81:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Fernandes J, Weinlich R, Castilho RO, Kaplan MA, Amarante-Mendes GP, Gattass CR. Pomolic acid triggers mitochondria-dependent apoptotic cell death in leukemia cell line. Cancer Lett. 2005;219:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Vasconcelos FC, Gattass CR, Rumjanek VM, Maia RC. Pomolic acid-induced apoptosis in cells from patients with chronic myeloid leukemia exhibiting different drug resistance profile. Invest New Drugs. 2007;25:525-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Guimarães LPTP, Rocha GDG, Queiroz RM, Martins CA, Takiya CM, Gattass CR. Pomolic acid induces apoptosis and inhibits multidrug resistance protein MRP1 and migration in glioblastoma cells. Oncol Rep. 2017;38:2525-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Martins CA, Rocha GDG, Gattass CR, Takiya CM. Pomolic acid exhibits anticancer potential against a docetaxelresistant PC3 prostate cell line. Oncol Rep 2019; 42: 328-338 . [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Chung DJ, Wang CJ, Yeh CW, Tseng TH. Inhibition of the Proliferation and Invasion of C6 Glioma Cells by Tricin via the Upregulation of Focal-Adhesion-Kinase-Targeting MicroRNA-7. J Agric Food Chem. 2018;66:6708-6716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15478] [Article Influence: 2579.7] [Reference Citation Analysis (2)] |
| 20. | Gosavi R, Chia C, Michael M, Heriot AG, Warrier SK, Kong JC. Neoadjuvant chemotherapy in locally advanced colon cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2021;36:2063-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 21. | Dai H, Meng WX, Kaufmann SH. BCL2 Family, Mitochondrial Apoptosis, and Beyond. Cancer Transl Med. 2016;2. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Thandapani P, Aifantis I. Apoptosis, Up the Ante. Cancer Cell. 2017;32:402-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Pullarkat VA, Newman EM. BCL2 Inhibition by Venetoclax: Targeting the Achilles' Heel of the Acute Myeloid Leukemia Stem Cell? Cancer Discov. 2016;6:1082-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Hata AN, Engelman JA, Faber AC. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer Discov. 2015;5:475-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 470] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 25. | Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 832] [Cited by in RCA: 834] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 26. | Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2959] [Cited by in RCA: 3076] [Article Influence: 128.2] [Reference Citation Analysis (0)] |
| 27. | Peng Y, Miao H, Wu S, Yang W, Zhang Y, Xie G, Xie X, Li J, Shi C, Ye L, Sun W, Wang L, Liang H, Ou J. ABHD5 interacts with BECN1 to regulate autophagy and tumorigenesis of colon cancer independent of PNPLA2. Autophagy. 2016;12:2167-2182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Øvervatn A, Bjørkøy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131-24145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3082] [Cited by in RCA: 3632] [Article Influence: 201.8] [Reference Citation Analysis (0)] |
| 29. | Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, Dipaola RS, Karantza-Wadsworth V, White E. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1442] [Cited by in RCA: 1420] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 30. | Ichimura Y, Kumanomidou T, Sou YS, Mizushima T, Ezaki J, Ueno T, Kominami E, Yamane T, Tanaka K, Komatsu M. Structural basis for sorting mechanism of p62 in selective autophagy. J Biol Chem. 2008;283:22847-22857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 642] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 31. | Zhang YB, Gong JL, Xing TY, Zheng SP, Ding W. Autophagy protein p62/SQSTM1 is involved in HAMLET-induced cell death by modulating apotosis in U87MG cells. Cell Death Dis. 2013;4:e550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 32. | Jin Z, Li Y, Pitti R, Lawrence D, Pham VC, Lill JR, Ashkenazi A. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 501] [Article Influence: 31.3] [Reference Citation Analysis (0)] |