Published online Oct 15, 2023. doi: 10.4251/wjgo.v15.i10.1739
Peer-review started: April 6, 2023
First decision: April 19, 2023
Revised: May 23, 2023
Accepted: July 19, 2023
Article in press: July 19, 2023
Published online: October 15, 2023
Processing time: 186 Days and 22.9 Hours
As an active ingredient derived from Dioscorea zingiberensis C.H. Wright, deltonin has been reported to show anti-cancer effects in a variety of malignancies.
To investigate the role and mechanism of action of deltonin in promoting gastric carcinoma (GC) cell apoptosis and chemosensitivity to cisplatin.
The GC cell lines AGS, HGC-27, and MKN-45 were treated with deltonin and then subjected to flow cytometry and 3-(4,5-dimethylthiazol-2-yl)-3,5-diphenyltetrazolium bromide assays for cell apoptosis and viability determination. Western blot analysis was conducted to examine alterations in the expression of apoptosis-related proteins (Bax, Bid, Bad, and Fas), DNA repair-associated proteins (Rad51 and MDM2), and phosphatidylinositol 3-kinase/protein kinase B/mammalian target of the rapamycin (PI3K/AKT/mTOR) and p38-mitogen-activated protein kinase (MAPK) axis proteins. Additionally, the influence of deltonin on GC cell chemosensitivity to cisplatin was evaluated both in vitro and in vivo.
Deltonin treatment weakened viability, enhanced apoptosis, and dampened DNA repair in GC cell lines in a dose-dependent pattern. Furthermore, deltonin mitigated PI3K, AKT, mTOR, and p38-MAPK phosphorylation. HS-173, an inhibitor of PI3K, attenuated GC cell viability and abolished deltonin inhibition of GC cell viability and PI3K/AKT/mTOR and p38-MAPK pathway activation. Deltonin also promoted the chemosensitivity of GC cells to cisplatin via repressing GC cell proliferation and growth and accelerating apoptosis.
Deltonin can boost the chemosensitivity of GC cells to cisplatin via inactivating p38-MAPK and PI3K/AKT/mTOR signaling.
Core Tip: Chemoradiotherapy is currently the mainstay of clinical treatment for advanced gastric carcinoma (GC). However, chemoradiotherapy is difficult to achieve the desired results due to the challenges of early diagnosis of GC and the characteristics of distant metastasis and drug resistance. This study attempted to enhance the efficacy of GC clinical treatment from a pharmacological mechanism perspective.
- Citation: Yang L, Liu YN, Gu Y, Guo Q. Deltonin enhances gastric carcinoma cell apoptosis and chemosensitivity to cisplatin via inhibiting PI3K/AKT/mTOR and MAPK signaling. World J Gastrointest Oncol 2023; 15(10): 1739-1755
- URL: https://www.wjgnet.com/1948-5204/full/v15/i10/1739.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i10.1739
Gastric carcinoma (GC) is a digestive tract malignancy prevalent worldwide, ranking second in cancer-related deaths[1]. Currently, it is still associated with a high incidence and mortality rate in developing countries[2]. There are several risk factors for GC, including diet patterns, smoking and drinking, family/genetic history, and Helicobacter pylori infection[3-5]. At present, chemoradiotherapy is the main clinical treatment for advanced GC. However, owing to the challenges in the early diagnosis of GC and the features of distant metastasis and drug resistance in the advanced stage, it is difficult for radiotherapy to achieve the expected results[6]. This experiment attempted to enhance the efficacy of GC clinical treatment from the perspective of drug mechanism.
Deltonin, an active ingredient in traditional Chinese medicine, is derived from Dioscorea zingiberensis C.H. Wright, and shows anti-cancer effects on many malignancies like colon cancer and breast cancer[7]. For instance, deltonin activates autophagy through the protein kinase B/mammalian target of the rapamycin (AKT/mTOR) axis and prevents FaDu, a head and neck squamous cell carcinoma cell line, from proliferating through cell cycle arrest and apoptosis induction, thus boosting cell apoptosis[8]. Moreover, through reactive oxygen species (ROS)-mediated mitochondrial disorders and extracellular signal-regulated kinase/AKT axis, deltonin restrains human breast carcinoma cell proliferation and promotes cell apoptosis[9]. Although previous studies have demonstrated that deltonin functions in most cancers, there are few studies on its role in GC cells and the relevant mechanisms.
The phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR signaling pathway is activated in multiple tumors and regulates various processes such as tumor cell growth, apoptosis, migration, invasiveness, autophagy, and survival[10]. Currently, this signaling pathway is deemed to be a crucial therapeutic target for tumors. Some studies have verified that apigenin inhibits the PI3K/AKT/mTOR axis to suppress liver cancer cell proliferation, thus eliciting autophagy in liver cancer cells and facilitating cell apoptosis[11]. Diallyl disulfide inhibits the PI3K/AKT/mTOR signaling pathway to elicit G2/M phase arrest of human osteosarcoma cells, as well as their apoptosis and autophagic death[12]. p38 mitogen-activated protein kinases (p38-MAPK), as a type of serine/threonine MAPK, participate in the signaling cascades of cytokines and stress cell responses and influence the occurrence, metastasis, and drug resistance of tumor cells[13,14]. For instance, diosgenin suppresses ovarian cancer cell activity by modulating the PI3K/AKT/p38-MAPK axis-associated protein profiles[15]. Another example is inotilone, which inhibits lung carcinoma cell migration and invasiveness through the ROS-mediated PI3K/AKT/p38-MAPK axis[16]. Thus, both p38-MAPK and PI3K/AKT/mTOR signals play essential regulatory roles in multiple malignancies. Nevertheless, whether deltonin influences drug resistance and disease progression in GC via the two signaling pathways still needs further investigation.
This study aimed at investigating the underlying anti-tumor function of deltonin in GC cells. Our experiments revealed that deltonin boosted cell apoptosis and improved the chemosensitivity of GC cells to cisplatin. Furthermore, deltonin inhibited PI3K/AKT/mTOR and p38-MAPK signaling pathway activation. Thus, our work provides a new therapeutic avenue to explore novel drugs for patients with GC undergoing end-stage chemotherapy.
The culture medium of GC (AGS, HGC-27, and MKN-45) and human gastric epithelial (GES-1) cell lines, all from the Chinese Academy of Sciences, Shanghai, China, was RPMI1640 medium (Thermo Fisher Scientific, MA, United States) + 1% penicillin/streptomycin (Thermo Fisher Scientific) + 10% fetal bovine serum (FBS; Invitrogen, CA, United States), and the culture condition was 37 °C and 5% CO2. Cells in logarithmic growth phase were trypsinized using 0.25% trypsin (Thermo Fisher HyClone, United States) and then harvested through centrifugation at 170 g for 5 min.
The three GC cell lines were treated with cisplatin (Cat. No. 15663-27-1, Sigma-Aldrich, United States; 5 μg/mL)[17], deltonin (Cat. No. HYN2283, MedChemExpress; 0, 0.625, 1.25, 2.5, 5, 10, and 20 μM)[9,18], and/or HS-173 (a PI3K inhibitor; Cat. No. HY-15868, MedChemExpress; 1 μM)[19], or 740 Y-P (a PI3K activator; Cat. No. HY-P0175, Med
The three GC cell lines in logarithmic growth phase were inoculated into 96-well plates (4 × 103 cells/well, 100 μL) and incubated for 24 h under conditions of 100% humidity, 37 °C, and 5% CO2 in air. They were then treated with cisplatin, deltonin, and/or the PI3K inhibitor HS-173; the control group was treated with phosphate buffered saline (PBS) of the same volume. Each group contained five replicates. Cells were immersed in 50 μL of 3-(4,5-dimethylthiazol-2-yl)-3,5-diphenyltetrazolium bromide (MTT) (5 g/L) (Beyotime Biotechnology, Shanghai, China) after 24-h culture, and the supernatant was aspirated following 4-h incubation at 37 °C. The cells were treated with DMSO at 150 μL per well, and then placed on a plate shaker. Ultimately, a microplate reader was used to examine each well’s OD value at 450 nm at 24, 48, and 72 h.
After cell treatment mentioned in section 2.2 and cultivation in 6-well plates, the cells were subjected to two PBS washes and 30 min of lysis in 200 μL RIPA (Beyotime Biotechnology, Shanghai, China). Thereafter, the lysates were collected for a 15-min centrifugation at 14000 rpm to obtain total protein. Protein concentrations were measured using Bradford dye (Bio-Rad). Following 2 h of separation on a polyacrylamide gel by electrophoresis at a voltage maintained at 100 V, the protein samples were electroblotted onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, United States). They were then blocked with 5% nonfat-dried milk for 1 h at room temperature (RT), followed by three 10-min Tris-buffered saline with 0.1% Tween® 20 detergent (TBST) rinses and overnight incubation at 4 °C with primary antibodies at 1:1000 dilution that were procured from Abcam (MA, United States): Anti-Bax (ab32503), anti-Bid (ab32060), anti-Bak (ab32371), anti-Fas (ab133619), anti-Rad51 (ab133534), anti-MDM2 (ab16895), anti-PI3K (ab32089), anti-mTOR (ab134903), anti-p-mTOR (ab137133), anti-p-PI3K (ab182651), anti-AKT (ab8805), anti-p-AKT (ab38449), anti-p38-MAPK (ab170099), anti-p-p38-MAPK (ab178867), and anti-β-actin (ab115777). Following TBST washes, the membranes were subjected to 1 h of RT incubation with horseradish peroxidase-labeled anti-rabbit secondary antibody (1:300 dilution). Thereafter, TBST was used to rinse the membranes again thrice (10-min rinses). Eventually, the membranes were imaged and the staining intensity was assessed using BeyoECL Plus (Beyotime Biotechnology, Shanghai, China) and ImageJ, respectively.
The human GC cell lines in logarithmic growth phase were harvested and prepared as single-cell suspensions for inoculation in a 25 cm2 culture flask. Following adherent culture overnight, the original medium was replaced with fresh medium containing 0.3% FBS for the experimental group and a comparable volume of PBS medium for the control group, followed by 24 h of incubation with 5% CO2 at 37 °C and cell supernatant collection. Thereafter, the cells were subjected to cold PBS flushing for 3 times, trypsinization using EDTA-free trypsin, and harvesting. Then, the cells were treated as instructed in the Annexin V-PI Apoptosis Detection Kit (Yeasen Biotech Co., Ltd.) protocol. Subsequently, flow cytometry was performed within 1 h for analyzing cell apoptosis.
We acquired 12 female athymic BALB/c nude mice (6 wk old with a weight of 22-24 g) from Shandong University Experimental Animal Center (Jinan, China) and reared them under normal specific pathogen-free conditions (24 °C, 12-h/12-h light/dark regime, and free access to food and water). Then, AGS cells were administered hypodermically at 2 × 106 cells/0.1 mL PBS into mouse right back according to a previous study[21]. Seven days later, the animals were randomly distributed to one of the following groups: Sham (treated with normal saline via intraperitoneal injection), cisplatin (once every 3 d at 3 mg/kg, for 3 times)[22,23], deltonin (once every 3 d at 50 mg/kg, for 3 times)[8], and cisplatin (once every 3 d at 1.5 mg/kg, for 3 times) + deltonin (once every 3 d at 25 mg/kg, for 3 times). During the following 28 d after drug treatment, a caliper was used for measuring the tumor volume (0.5 × length × width2) weekly. Four weeks later, the nude mice were sacrificed using 30 mg/kg phenobarbital sodium, and the tumor was resected and weighed. The animal experiments were approved by the Ethics Review Committee of the Second Affiliated Hospital of Soochow University (approval No. SZSH-2020-042), and were implemented strictly following the Declaration of Helsinki and the Regulations of the People’s Republic of China on the Management of Laboratory Animals issued on October 31, 2017.
Tumor tissue specimens were treated with 4% paraformaldehyde and then paraffin-embedded. Tumor sections were prepared (4 μm in thickness), dewaxed using gradient alcohol, and rehydrated. Following RT sealing with bovine serum albumin (5%) for half an hour, the sections were incubated with anti-p-PI3K/AKT/mTOR/p38 MAPK antibodies (ab191606, ab131443, ab109268, and ab38238) at RT for 1 h. After washing with PBS, they were incubated with the Cy3- (ab98416) or fluorescein isothiocyanate-labelled goat anti-rabbit secondary antibody (ab6717) for 60 min at RT. All antibodies were procured from Abcam. Following nuclei labeling with 4',6-diamidino-2-phenylindole (Beyotime Technology, Shanghai, China), a confocal immunofluorescence microscope (Leica LSM 800, Wetzlar, Germany) was used to visualize the images.
SPSS16.0 from SPSS Inc. (Chicago, IL, United States) was used for performing all statistical analyses, and P < 0.05 indicated statistical significance. Between-group differences were analyzed by unpaired, two-sided Student’s t-tests, and multi-group differences were determined by one-way ANOVA followed by Tukey’s post-hoc tests. All data are described as the mean ± SD.
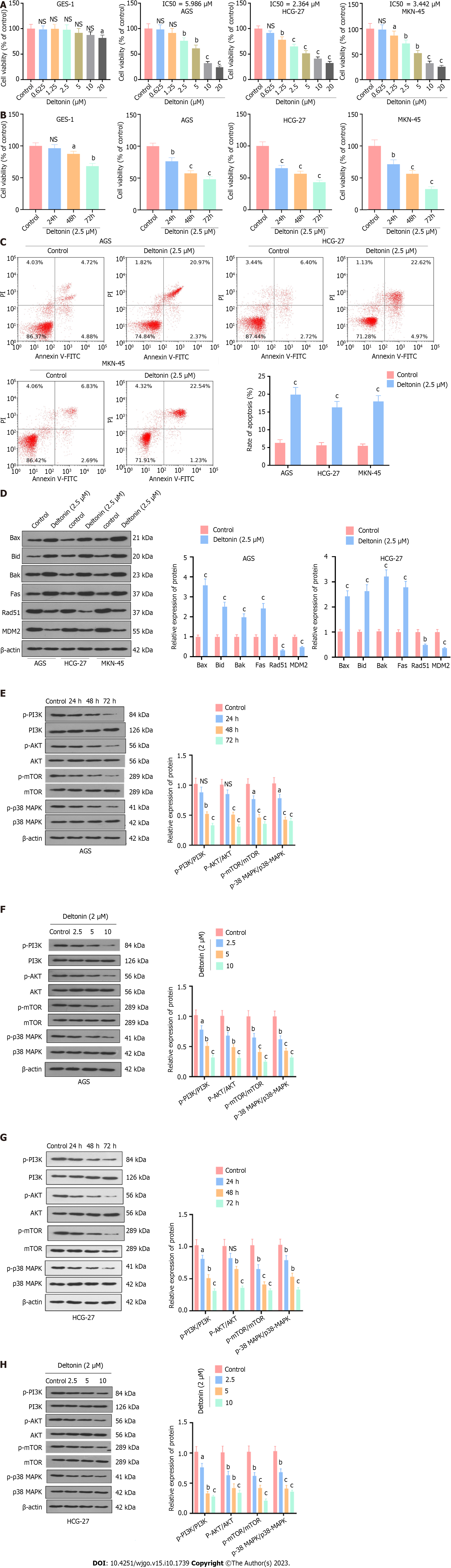
GES-1, AGS, HGC-27, and MKN-45 cells were all treated with 0-20 μM of deltonin for 24 h, after which their viability was examined using MTT assays. GC cell viability was observed to significantly decrease when the dose of deltonin exceeded 2.5 μM, while only 20 μM of deltonin exerted an inhibitory effect on GES-1 viability (P < 0.05 vs control, Figure 1A). The IC50 values were gauged for AGS, HGC-27, and MKN-45 cells following treatment with deltonin at different concentrations; the IC50 values were 3.487, 2.343, and 2.78 for AGS, HGC-27, and MKN-45 cells, respectively (Figure 1B). The GC cells were treated with 2.5 μM deltonin and then subjected to the MTT assay to examine cell viability at different time points. Deltonin inhibited GC cell viability in a time-dependent manner (P < 0.05 vs control, Figure 1C). Flow cytometry analysis revealed that deltonin treatment promoted cell apoptosis (P < 0.05 vs control, Figure 1D). And as indicated by Western blot analysis, deltonin treatment enhanced the protein levels of pro-apoptotic markers Bax, Bak, Bid, and Fas but reduced those of Rad51 and MDM2, which are associated with DNA repair processes (P < 0.05 vs control, Figure 1E). Western blot assays also indicated that deltonin (2.5 μM) treatment markedly lowered PI3K/AKT/mTOR and p38-MAPK protein levels in GC cells (including AGS and HGC-27), with the expression gradually decreasing with time (0, 24, 48, and 72 h) (P < 0.05 vs control, Figure 1F and G). Additionally, these proteins presented decreased expression in GC cells in a deltonin concentration-dependent manner (0, 2.5, 5, and 10 μM) (P < 0.05 vs control, Figure 1H and I). The above results demonstrated the ability of deltonin to exert an inhibitory effect on GC cell growth and enhance apoptosis while inactivating p38-MAPK and PI3K/AKT/mTOR axes in these cells.
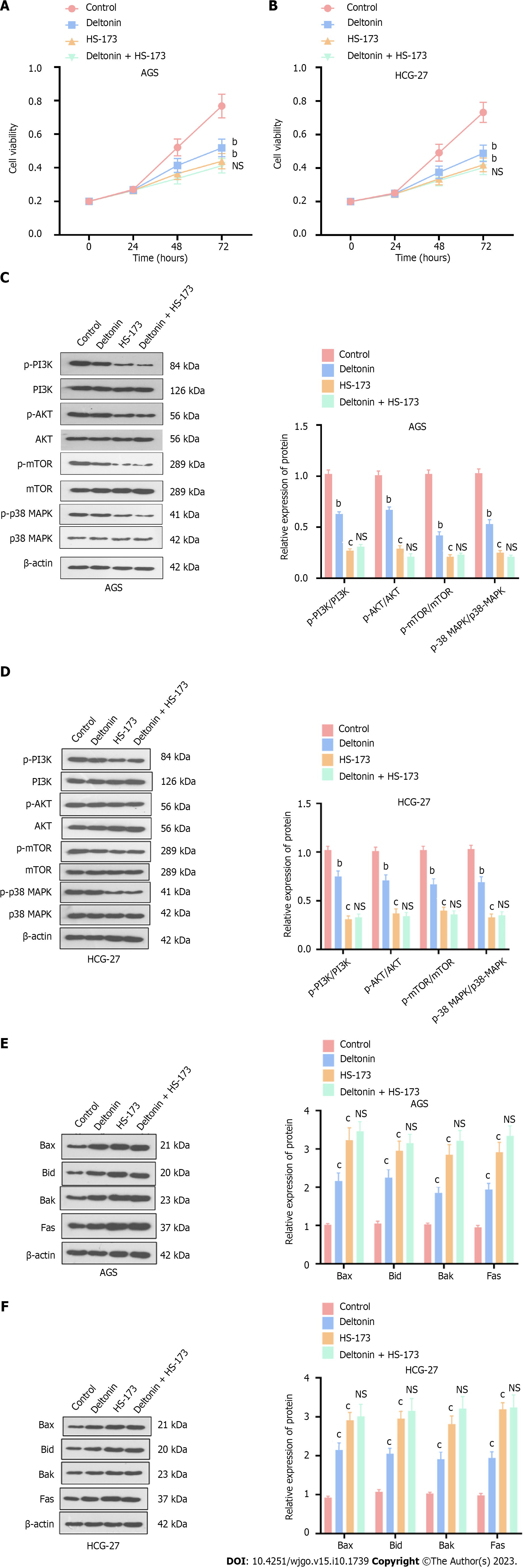
GC cells treated with deltonin (2.5 μM) and HS-173 (0.8 nM) showed remarkably lower viability compared to the control (P < 0.05, Figure 2A and B). Nevertheless, deltonin + HS-173 exerted no additional influence on cell viability compared to the HS-173 alone group (P > 0.05, Figure 2A and B). The determination of apoptosis-related protein profiles also determined that deltonin and HS-173 individually increased the expression of Bax, Bak, Bid, and Fas, whereas co-treatment with HS-173 and deltonin barely influenced their expression levels (P < 0.05, Figure 2C and D). Western blot analysis also showed that phosphorylated PI3K/AKT/mTOR and p38-MAPK protein levels were substantially reduced with deltonin or HS-173 treatment, whereas the administration of deltonin and HS-173 exerted no inhibitory effect on p38-MAPK and PI3K/AKT/mTOR axes (vs HS-173 group alone, P > 0.05, Figure 2E and F). Therefore, deltonin may repress GC cell viability by suppressing p38-MAPK and PI3K/AKT/mTOR signaling.
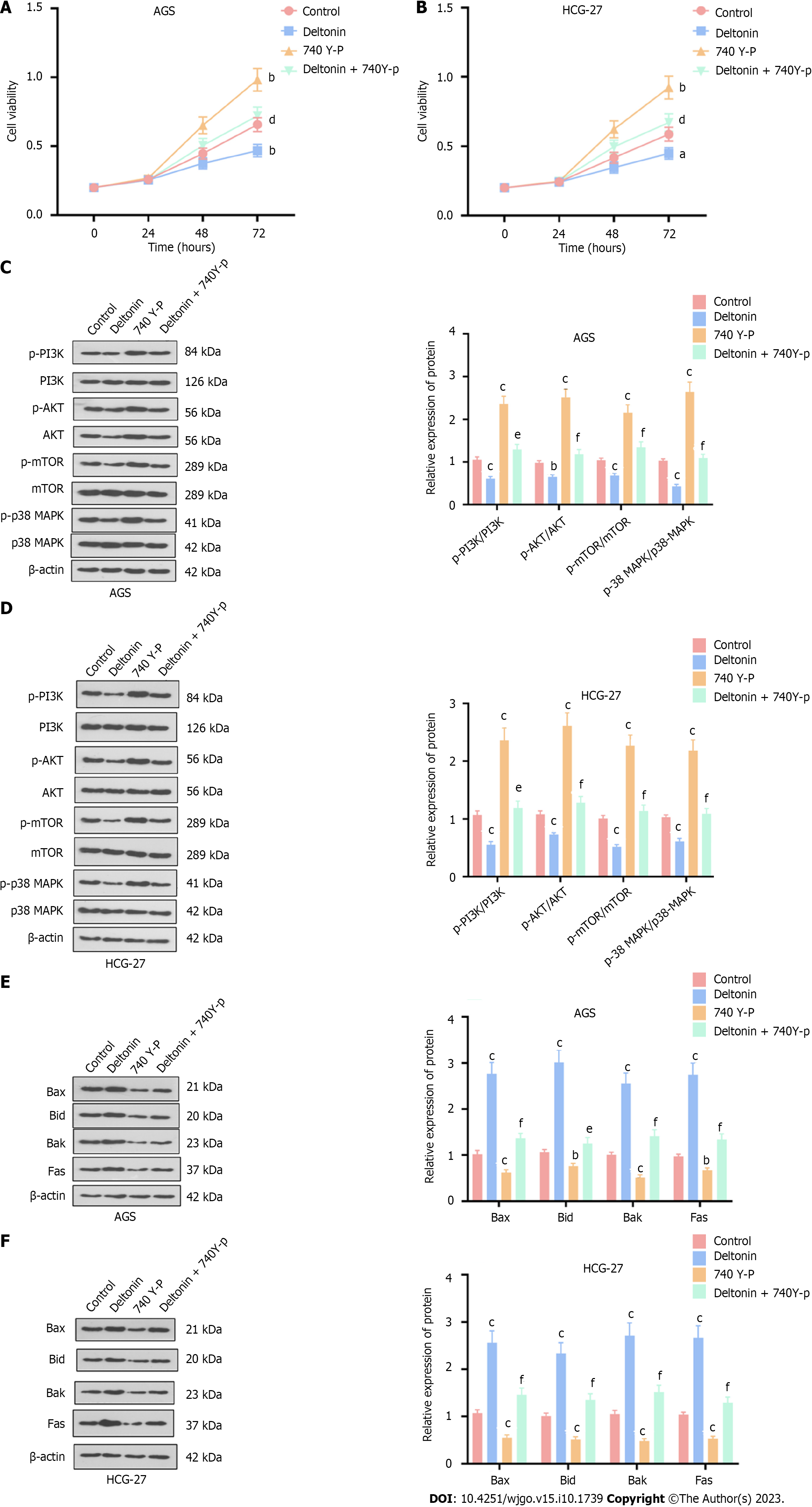
Next, we treated GC cells (AGS and HGC-27) with deltonin (2.5 μM) and the PI3K activator 740 Y-P (20 μM), and found that deltonin notably enhanced cell viability vs the control, wherein cell viability was inhibited by the addition of deltonin (P < 0.05, Figure 3A and B). Furthermore, Western blot analysis showed reduced expression of apoptosis-related proteins (Bax, Bak, Bid, and Fas) in the 740 Y-P group, while the deltonin + 740 Y-P group showed increased expression of these proteins in comparison to 740 Y-P alone treatment (P < 0.05, Figure 3C and D). Western blot analysis also indicated augmented PI3K, AKT, mTOR, and p38-MAPK phosphorylation in AGS and HGC-27 cells in the 740 Y-P group, whereas deltonin co-treatment suppressed such increased phosphorylation (P < 0.05 vs 740 Y-P group, Figure 3E and F). Together, these results suggest that activating PI3K/AKT/mTOR and p38-MAPK signaling may facilitate cell proliferation and weaken the anti-cancer effects of deltonin.
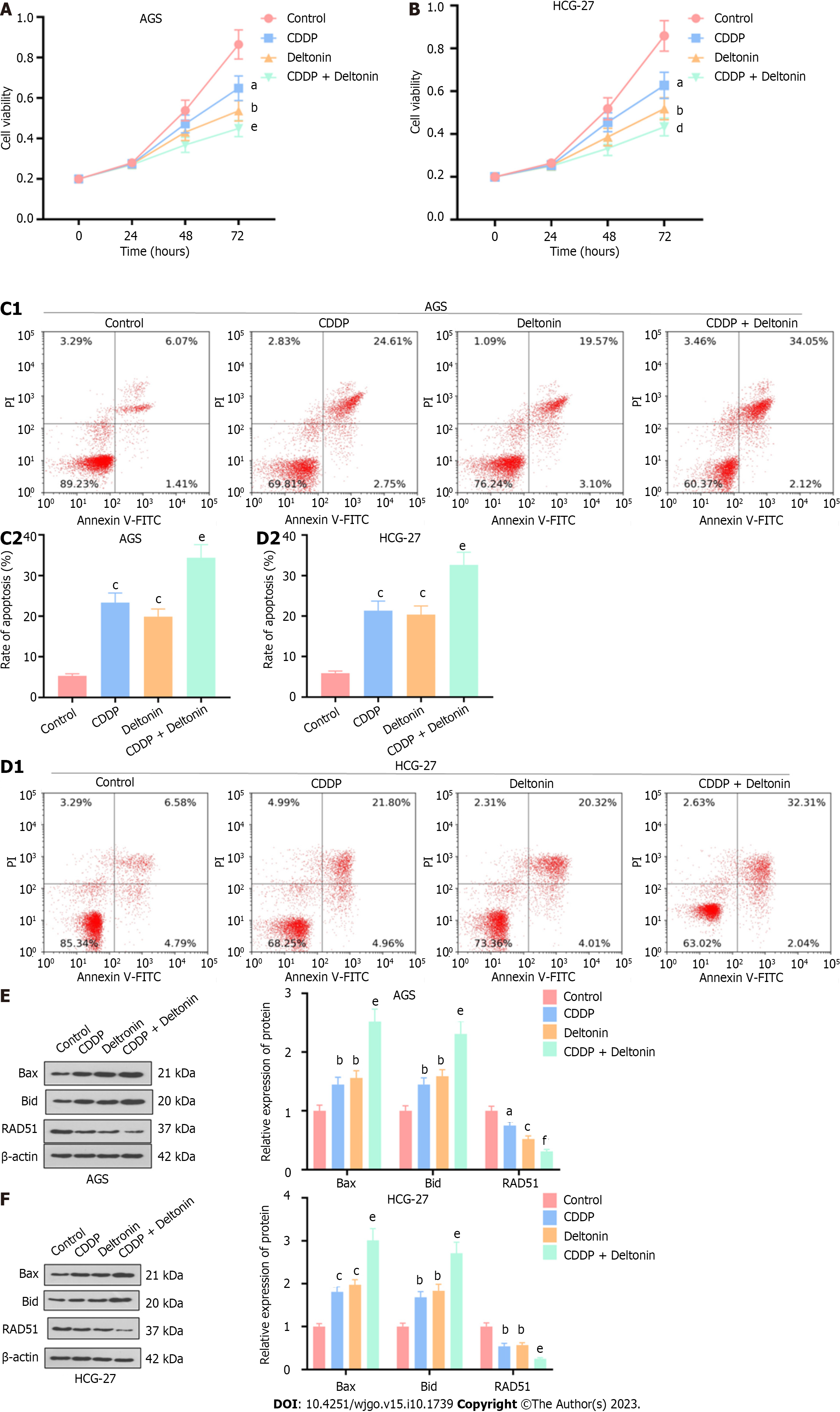
AGS and HGC-27 cells were treated with 2.5 μM of deltonin or 5 μg/mL of cisplatin or cisplatin (2.5 μg/mL) + deltonin (1.25 μM). Treatment with cisplatin or deltonin considerably attenuated cell viability, whereas cisplatin + deltonin co-treatment reduced cell viability compared to the cisplatin alone group (P < 0.05, Figure 4A and B). According to flow cytometry analysis, the apoptosis of cisplatin- or deltonin-treated cells was dramatically increased compared to the control (P < 0.05, Figure 4C and D), and it was further enhanced in the cisplatin + deltonin group (P < 0.05, Figure 4C and D, vs cisplatin group). Western blot analysis also showed elevated Bax and Bid and reduced Rad51 protein expression in cisplatin- or deltonin-treated cells vs the control. Moreover, Bax and Bid protein expression in the cisplatin + deltonin group was further increased, while Rad51 expression was considerably reduced in comparison to the expression levels in the cisplatin alone group (P < 0.05, Figure 4E and F). Based on the above findings, deltonin may exert a pro-apoptotic effect and promote the chemosensitivity of GC cells to cisplatin.
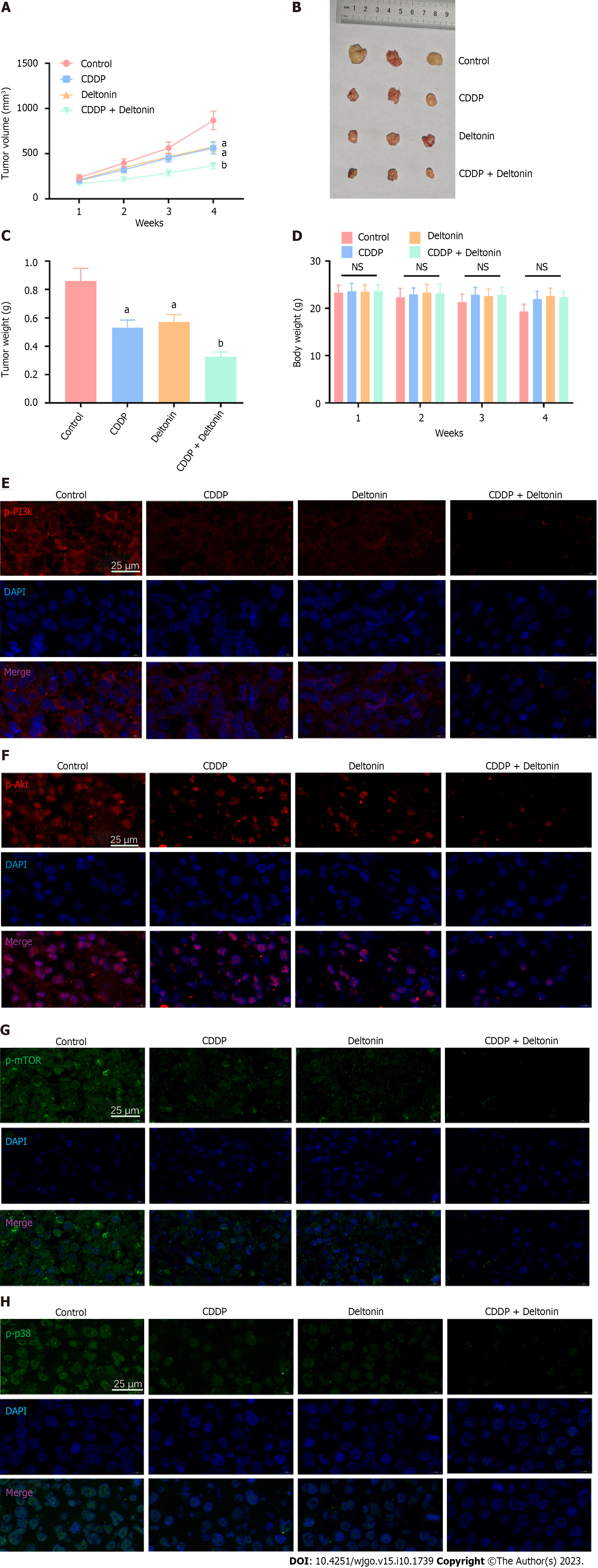
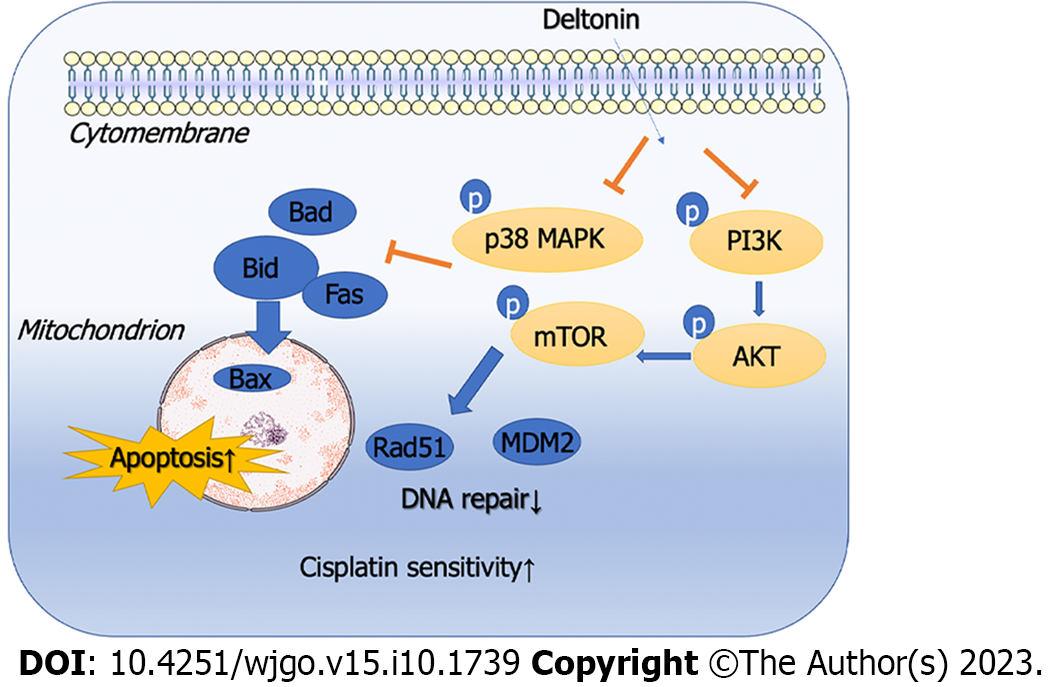
To further verify the function and mechanism of deltonin in chemosensitivity of GC cells to cisplatin, we conducted in vivo experiments in nude mice. The tumor-bearing mice were intervened with saline, deltonin (50 mg/kg), cisplatin (3 mg/kg), or deltonin (25 mg/kg) + cisplatin (1.5 mg/kg). Treatment with deltonin or cisplatin both reduced tumor volume and weight compared to the sham group (P < 0.05, Figure 5A-C), but failed to reduce mouse body weight (P > 0.05, Figure 5D). Interestingly, the joint application of deltonin + cisplatin further mitigated the mouse tumor volume and weight in comparison to cisplatin treatment alone (P < 0.01, Figure 5A-C), but barely altered the body weight (P > 0.05, Figure 5D). We then carried out immunofluorescence assays to determine PI3K/AKT/mTOR and p38-MAPK phosphorylation levels in the tumor tissues. Both deltonin and cisplatin reduced the levels of phosphorylated p38-MAPK and PI3K/AKT/mTOR, and their combination further reduced the levels compared to the cisplatin alone group (Figure 5E-H). These findings suggest that deltonin enhances chemosensitivity of GC cells to cisplatin by suppressing p38-MAPK and PI3K/AKT/mTOR signaling activation (Figure 6).
GC is a prevalent internal gastrointestinal malignancy with a high clinical fatality rate[24]. The current methods are ineffective for early GC diagnosis, owing to which GC is often diagnosed at the end stage when it is accompanied by distant metastasis and chemotherapy resistance. Moreover, surgical treatment and drug chemotherapy display poor efficacy[25]. Cisplatin is a frequently used chemotherapy drug for many malignant tumor diseases and is also extensively adopted in the context of GC[26,27]. Regarding the primary mechanism of cisplatin in cancer treatment, it triggers DNA damage in tumor cells. Unfortunately, cisplatin treatment can easily contribute to the drug resistance of tumor cells and influence the function of chemotherapy[28]. Hence, probing the drug action mechanisms in GC has great clinical implications for its treatment. Here, we discovered that deltonin hinders p38-MAPK and PI3K/AKT/mTOR signaling activation to boost GC cell apoptosis and promote their chemosensitivity to cisplatin.
Deltonin is known as an anti-tumor drug that curbs tumor cell angiogenesis to restrain tumor growth and facilitate apoptosis[29]. Deltonin inhibits AKT and p38-MAPK signaling pathway activation to further inhibit mouse colon cancer cell proliferation and bolster tumor cell apoptosis[18]. Furthermore, the intake of deltonin significantly suppresses colon cancer C26 cell proliferation in tumor-bearing mice, restricts tumor angiogenesis, and elicits cell apoptosis, thus prolonging the life cycle of the mice[30]. All the above studies confirm that deltonin enhances cancer cell apoptosis and represses cancer in a multitude of tumor diseases, which aligns with the observations in this study. Here, we demonstrated that deltonin considerably inhibits proliferation, boosts apoptosis, and dampens DNA repair in GC cells.
Chemotherapy is a prevailing method for GC, effectively extending patients’ life. Cisplatin is a typical drug used in GC chemotherapy. Nonetheless, GC resistance is a leading contributor to chemotherapy failure[31,32]. Many studies have evaluated drug resistance in GC, including the most complicated molecular and drug mechanisms[33]. For instance, ten-eleven translocation-2 (TET2), a DNA demethylase, modulates interleukin (IL)-6 levels in the tumor microenvironment via histone acetylation, thus influencing cell resistance, and TET2 overexpression notably mitigates cisplatin resistance in GC cells[34]. Curcumin also augments the sensitivity of GC cells to adriamycin and other chemotherapy drugs by down-regulating the nuclear factor-kappaB (NF-κB) axis in human GC SGC-7901 cells and a downstream anti-apoptotic target gene of NF-κB[35]. Most of the prior studies have investigated the tolerance of chemotherapeutic drugs in GC from the aspect of molecular and drug mechanisms. Here, we unveiled that deltonin efficaciously augmented the chemosensitivity of GC cells to cisplatin and thereby boosted the anti-tumor function of cisplatin via eliciting apoptosis and DNA damage.
PI3K/AKT/mTOR and p38-MAPK signals were initially considered as factors that could regulate inflammation and immune response and affect inflammatory reactions, cell proliferation, differentiation, apoptosis, and other cellular processes[36,37]. Recent evidence has also demonstrated the pro-oncogenic functions of p38-MAPK and PI3K/AKT/mTOR in several tumors[38,39]. For instance, an in vitro experiment on GC cells has revealed that blocking PI3K/AKT/mTOR signaling activation augments the resistance of GC cells to paclitaxel and promotes their apoptosis[40]. Afatinib dampens p38-MAPK and PI3K/AKT/mTOR signaling activation, thereby eliciting GC cell apoptosis and bolstering their resistance to chemotherapy[41]. All these conclusions align with our current study findings. Here, we discovered that deltonin significantly hinders p38-MAPK and PI3K/AKT/mTOR signaling activation, thereby bolstering GC cell apoptosis and attenuating the resistance of GC cells to cisplatin.
In summary, through a series of experiments, we uncovered that treating GC cells (AGS, HGC-27, and MKN-45) with deltonin results in reduced proliferation ability and increased apoptosis rate; of these, HGC-27 cells exhibited the best proliferation capability and the lowest apoptosis rate. Therefore, we exploited AGS and HGC-27 cells for further experiments and analyses. Our experiments demonstrated the ability of deltonin to promote GC cell apoptosis and chemosensitivity to cisplatin by lowering PI3K/AKT/mTOR and p38-MAPK-associated protein levels, offering novel insights into the mechanism of drug action. Nevertheless, further investigations are required to understand how deltonin represses these two axes, and in vivo experiments should be conducted using both male and female nude mice and other GC cell lines.
Despite being the main clinical treatment modality for advanced gastric cancer (GC), chemoradiotherapy is still difficult to achieve the expected effect due to the early diagnosis of GC and the characteristics of distant metastasis and drug resistance. Deltonin, an active ingredient in traditional Chinese medicine, shows anti-cancer effects on many malignancies.
This study attempted to optimize the treatment strategies for advanced GC and enhance the therapeutic effect on patients from a pharmacological mechanism perspective.
Here, we investigated the role and mechanism of action of deltonin in promoting GC cell apoptosis and chemosensitivity to cisplatin.
In this study, gastric cancer cell lines (AGS, HGC-27, and MKN-45 cells) were treated with deltonin. Then, apoptosis was observed, and the expression of apoptosis-related proteins (Bax, Bid, Bad, and Fas), DNA repair-related proteins (Rad51 and MDM2), and phosphatidylinositol 3-kinase/protein kinase B/mammalian target of the rapamycin (PI3K/AKT/mTOR-MAPK) proteins was detected by Western blot analysis. In addition to this, the effect of deltonin on the chemosensitivity of GC cells to cisplatin was evaluated by in vivo and in vitro experiments
Treating GC cells (AGS, HGC-27, and MKN-45) with deltonin resulted in reduced proliferation ability and increased apoptosis rate; of these, HGC-27 cells exhibited the best proliferation capability and the lowest apoptosis rate. Our experiments demonstrated the ability of deltonin to promote GC cell apoptosis and chemosensitivity to cisplatin by lowering PI3K/AKT/mTOR and p38-MAPK-associated protein levels, offering novel insights into the mechanism of drug action.
Deltonin enhances the chemosensitivity of GC cells to cisplatin by inhibiting the p38-MAPK and PI3K/AKT/mTOR signaling pathways.
This study has verified that deltonin is able to regulate GC cell apoptosis as well as chemosensitivity to cisplatin through the PI3K/AKT/mTOR and p38-AMPK signaling pathways by in vivo and in vitro experiments. Such results provide a new direction for drug therapy of gastric cancer. However, the study of the regulatory role of the pathways in this study was limited and could not fully elucidate its mechanism of action. Therefore, further analysis as well as nude mouse experiments and more cellular experiments are needed to excavate the mechanism.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Delko T, Switzerland; Thakur U, India S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Zhang XD
| 1. | Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 1088] [Article Influence: 272.0] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1754] [Cited by in RCA: 1883] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 3. | Freedman ND, Abnet CC, Leitzmann MF, Mouw T, Subar AF, Hollenbeck AR, Schatzkin A. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol. 2007;165:1424-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 269] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 4. | Yaghoobi M, Bijarchi R, Narod SA. Family history and the risk of gastric cancer. Br J Cancer. 2010;102:237-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Kamangar F, Sheikhattari P, Mohebtash M. Helicobacter pylori and its effects on human health and disease. Arch Iran Med. 2011;14:192-199. [PubMed] |
| 6. | Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39:1010428317714626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 644] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, Tian Z, Wan H, Liu W, Kong F, Ma G. Deltonin Ameliorates Cerebral Ischemia/Reperfusion Injury in Correlation with Modulation of Autophagy and Inflammation. Neuropsychiatr Dis Treat. 2020;16:871-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Xie YL, Fan M, Jiang RM, Wang ZL, Li Y. Deltonin induced both apoptosis and autophagy in head and neck squamous carcinoma FaDu cell. Neoplasma. 2015;62:419-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Zhang S, He Y, Tong Q, Chen Q, Wu X, Huang W. Deltonin induces apoptosis in MDAMB231 human breast cancer cells via reactive oxygen speciesmediated mitochondrial dysfunction and ERK/AKT signaling pathways. Mol Med Rep. 2013;7:1038-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Aoki M, Fujishita T. Oncogenic Roles of the PI3K/AKT/mTOR Axis. Curr Top Microbiol Immunol. 2017;407:153-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 11. | Yang J, Pi C, Wang G. Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed Pharmacother. 2018;103:699-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 299] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 12. | Yue Z, Guan X, Chao R, Huang C, Li D, Yang P, Liu S, Hasegawa T, Guo J, Li M. Diallyl Disulfide Induces Apoptosis and Autophagy in Human Osteosarcoma MG-63 Cells through the PI3K/Akt/mTOR Pathway. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 13. | Coulthard LR, White DE, Jones DL, McDermott MF, Burchill SA. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol Med. 2009;15:369-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 527] [Cited by in RCA: 505] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 14. | Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1064] [Cited by in RCA: 1289] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 15. | Guo X, Ding X. Dioscin suppresses the viability of ovarian cancer cells by regulating the VEGFR2 and PI3K/AKT/MAPK signaling pathways. Oncol Lett. 2018;15:9537-9542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Chao W, Deng JS, Li PY, Kuo YH, Huang GJ. Inotilone from Inonotus linteus suppresses lung cancer metastasis in vitro and in vivo through ROS-mediated PI3K/AKT/MAPK signaling pathways. Sci Rep. 2019;9:2344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Xue M, Liu X, Cheng B, Rui X, Wu M, Lv J. Epigallocatechin Gallate Enhances Inhibition Effect of DDP on the Proliferation of Gastric Cancer BGC-823 Cells by Regulating p19Arf-p53-p21Cip1 Signaling Pathway. Asian Pac J Cancer Prev. 2021;22:1263-1270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Shu D, Qing Y, Tong Q, He Y, Xing Z, Zhao Y, Li Y, Wei Y, Huang W, Wu X. Deltonin isolated from Dioscorea zingiberensis inhibits cancer cell growth through inducing mitochondrial apoptosis and suppressing Akt and mitogen activated protein kinase signals. Biol Pharm Bull. 2011;34:1231-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Son MK, Ryu YL, Jung KH, Lee H, Lee HS, Yan HH, Park HJ, Ryu JK, Suh JK, Hong S, Hong SS. HS-173, a novel PI3K inhibitor, attenuates the activation of hepatic stellate cells in liver fibrosis. Sci Rep. 2013;3:3470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Feng X, Chen L, Guo W, Zhang Y, Lai X, Shao L, Li Y. Graphene oxide induces p62/SQSTM-dependent apoptosis through the impairment of autophagic flux and lysosomal dysfunction in PC12 cells. Acta Biomater. 2018;81:278-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Wang J, Liu R, Mo H, Xiao X, Xu Q, Zhao W. Deubiquitinase PSMD7 promotes the proliferation, invasion, and cisplatin resistance of gastric cancer cells by stabilizing RAD23B. Int J Biol Sci. 2021;17:3331-3342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 22. | Li H, Xu W, Liu X, Ye J, Li P, Shang F, Yu X. Curcumin Alleviates the Side Effects of Cisplatin on Gastric Emptying of Mice by Inhibiting the Signal Changes of Acetylcholine and Interstitial Cells of Cajal. J Med Food. 2020;23:920-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Ando K, Takagi K, Tsubone H. Enhanced gastric retention of solid resin beads as a marker for emetic potential of agents in rats. J Toxicol Sci. 2012;37:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Digklia A, Wagner AD. Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol. 2016;22:2403-2414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 359] [Cited by in RCA: 405] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 25. | Choi AH, Kim J, Chao J. Perioperative chemotherapy for resectable gastric cancer: MAGIC and beyond. World J Gastroenterol. 2015;21:7343-7348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Hayashi N, Kataoka H, Yano S, Kikuchi JI, Tanaka M, Nishie H, Kinoshita Y, Hatano M, Nomoto A, Ogawa A, Inoue M, Mizoshita T, Shimura T, Mori Y, Kubota E, Tanida S, Joh T. Anticancer Effects of a New Aminosugar-conjugated Platinum Complex Agent Against Cisplatin-resistant Gastric Cancer. Anticancer Res. 2016;36:6005-6009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2728] [Cited by in RCA: 2936] [Article Influence: 146.8] [Reference Citation Analysis (0)] |
| 28. | Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1561] [Cited by in RCA: 2016] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 29. | Tong Q, Zhao Q, Qing Y, Hu X, Jiang L, Wu X. Deltonin inhibits angiogenesis by regulating VEGFR2 and subsequent signaling pathways in endothelial cells. Steroids. 2015;96:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Tong QY, Qing Y, Shu D, He Y, Zhao YL, Li Y, Wang ZL, Zhang SY, Xing ZH, Xu C, Wei YQ, Huang W, Wu XH. Deltonin, a steroidal saponin, inhibits colon cancer cell growth in vitro and tumor growth in vivo via induction of apoptosis and antiangiogenesis. Cell Physiol Biochem. 2011;27:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Archie V, Kauh J, Jones DV Jr, Cruz V, Karpeh MS Jr, Thomas CR Jr. Gastric cancer: standards for the 21st century. Crit Rev Oncol Hematol. 2006;57:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Zhang XL, Shi HJ, Wang JP, Tang HS, Cui SZ. MiR-218 inhibits multidrug resistance (MDR) of gastric cancer cells by targeting Hedgehog/smoothened. Int J Clin Exp Pathol. 2015;8:6397-6406. [PubMed] |
| 33. | Chen C, Tang X, Liu Y, Zhu J, Liu J. Induction/reversal of drug resistance in gastric cancer by non-coding RNAs (Review). Int J Oncol. 2019;54:1511-1524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Zhou K, Guo H, Zhang J, Zhao D, Zhou Y, Zheng Z, Xu Y, Li Y, Wang D. Potential role of TET2 in gastric cancer cisplatin resistance. Pathol Res Pract. 2019;215:152637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Yu LL, Wu JG, Dai N, Yu HG, Si JM. Curcumin reverses chemoresistance of human gastric cancer cells by downregulating the NF-κB transcription factor. Oncol Rep. 2011;26:1197-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1191] [Cited by in RCA: 1244] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 37. | Polivka J Jr, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther. 2014;142:164-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 613] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 38. | Cai C, Dang W, Liu S, Huang L, Li Y, Li G, Yan S, Jiang C, Song X, Hu Y, Gu J. Anthrax toxin receptor 1/tumor endothelial marker 8 promotes gastric cancer progression through activation of the PI3K/AKT/mTOR signaling pathway. Cancer Sci. 2020;111:1132-1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Du F, Sun L, Chu Y, Li T, Lei C, Wang X, Jiang M, Min Y, Lu Y, Zhao X, Nie Y, Fan D. DDIT4 promotes gastric cancer proliferation and tumorigenesis through the p53 and MAPK pathways. Cancer Commun (Lond). 2018;38:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 40. | Chen D, Lin X, Zhang C, Liu Z, Chen Z, Li Z, Wang J, Li B, Hu Y, Dong B, Shen L, Ji J, Gao J, Zhang X. Dual PI3K/mTOR inhibitor BEZ235 as a promising therapeutic strategy against paclitaxel-resistant gastric cancer via targeting PI3K/Akt/mTOR pathway. Cell Death Dis. 2018;9:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 41. | Chen Z, Liu Z, Zhang M, Huang W, Li Z, Wang S, Zhang C, Dong B, Gao J, Shen L. EPHA2 blockade reverses acquired resistance to afatinib induced by EPHA2-mediated MAPK pathway activation in gastric cancer cells and avatar mice. Int J Cancer. 2019;145:2440-2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |