Published online Jan 15, 2023. doi: 10.4251/wjgo.v15.i1.195
Peer-review started: October 20, 2022
First decision: November 15, 2022
Revised: December 11, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 15, 2023
Processing time: 81 Days and 21.5 Hours
Intestinal natural killer/T-cell lymphoma (NKTCL) is a rare and aggressive non-Hodgkin’s lymphoma, and its occurrence is closely related to Epstein-Barr virus infection. In addition, the clinical symptoms of NKTCL are not obvious, and the specific pathogenesis is still uncertain. While NKTCL may occur in any segment of the intestinal tract, its distinct location in the periampullary region, which leads clinicians to consider mimics of a pancreatic head mass, should also be addressed. Therefore, there remain huge challenges in the diagnosis and treatment of intestinal NKTCL.
In this case, we introduce a male who presented to the clinic with edema of both lower limbs, accompanied by diarrhea, and abdominal pain. Endoscopic ultra
Clinicians should enhance their understanding of NKTCL. Some key factors, including EUS characteristics, the right choice of FNB needle, and combination with MFCI, are crucial for improving the diagnostic rate and reducing the misdiagnosis rate.
Core Tip: Intestinal natural killer/T-cell lymphoma (NKTCL) is a rare form of lymphoma with a low diagnosis rate. The patient presented in this case was eventually diagnosed with intestinal NKTCL. The patient initially improved after receiving chemotherapy with the steroid (dexamethasone), methotrexate, ifosfamide, L-asparaginase, and etoposide regimen but died two days after being discharged from the hospital. This case tells us that endoscopic ultrasound may be very helpful in the diagnosis of NKTCL. A 19 gauge or 22 gauge fine needle biopsy needle combined with multicolor flow cytometry immunophenotyping may be a good choice for diagnosing and subtyping lymphoma.
- Citation: Wang YN, Zhu YM, Lei XJ, Chen Y, Ni WM, Fu ZW, Pan WS. Intestinal natural killer/T-cell lymphoma presenting as a pancreatic head space-occupying lesion: A case report. World J Gastrointest Oncol 2023; 15(1): 195-204
- URL: https://www.wjgnet.com/1948-5204/full/v15/i1/195.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i1.195
Intestinal natural killer/T-cell lymphoma (NKTCL) is a rare and aggressive lymphoma[1]. NKTCL accounts for approximately 8%-15% of all gastrointestinal (GI) lymphomas[2]. Today, Asian populations are more likely to develop Epstein-Barr virus (EBV)-associated subtypes of non-Hodgkin's lymphoma, a situation that appears to be related to geographic location[3]. Presently, conventional colonoscopy biopsy has difficulty obtaining a deep enough depth to diagnose the tissue, and the diagnostic rate is low. Here, we present the case of a 44-year-old male patient who presented clinically with edema of both lower limbs associated with diarrhea and abdominal pain. Under the guidance of endoscopic ultrasound (EUS), fine needle biopsy (FNB) with 19 gauge (19 G) or 22 gauge (22 G) needles combined with multicolor flow cytometry immunophenotyping (MFCI) may play a decisive role in the final diagnosis of intestinal NKTCL.
A 44-year-old Chinese male was admitted to the hospital with persistent diarrhea, abdominal pain, lower limb edema and weight loss for more than one year that had been aggravated for one month.
The patient had loose yellow stools 4 to 5 times a day, and the stool weighed more than 500 g total, without bloody stools or melena. He suffered from this condition and lower limb edema for one year while also experiencing periumbilical abdominal pain, which was moderately paroxysmal colic pain. The patient visited several hospitals and was diagnosed with possible “protein-losing enteropathy” according to “hypoproteinemia” in laboratory tests and “edema and thickening of the small bowel wall” on contrast-enhanced computed tomography (CT). However, the gastrointestinal endoscopy reported a normal view. Probiotics, antibiotics, and digestive enzymes were prescribed to the patient, but his symptoms did not improve. One month before admission, symptoms of abdominal distention, mild abdominal pain in the right upper quadrant, and obvious weight loss (8 kg one month) had developed, without fever or jaundice.
The patient reported no remarkable history of past illness.
The patient drank 500 mL of alcohol and smoked 10 cigarettes a day for 30 years. He reported no remarkable family history.
On physical examination, the patient’s vital signs were stable, with a body temperature of 36.8 °C on admission. No pale or jaundice was observed, and there was no superficial lymphadenopathy. A mass of approximately 4 cm in diameter was palpable in the right part of the distend abdomen. Shifting dullness was positive upon examination. There was no tenderness, no abnormal bowel sounds or rate, and no palpable hepatosplenomegaly or Murphy’s sign. Moderate symmetric edema of both lower limbs was observed.
Laboratory findings on admission indicated mild anemia (hemoglobin, 116 g/L) and hypoproteinemia (albumin, 18.5 g/L). Liver function was mildly elevated (alkaline phosphatase, 290 U/L; gamma-glutamyl transpeptidase (γ-GT) 87 U/L). Leukocyte, platelet, bilirubin, lactate dehydrogenase (LDH), thyroid function and serum tumor markers were within normal limits, except elevated carbohydrate antigen 125 (517 U/mL). Tests for autoantibody, stool culture, antibody against tuberculosis, cytomegalovirus immunoglobulin M antibody, EBV DNA, and Clostridium difficile toxin detection were negative. In addition, the vitamin B3 Level was 17.1 ng/mL, which was within the normal range. The reticulocyte count was 45.50 × 109/L, and the reticulocyte ratio was 1.12%, and these results were negative. The total bilirubin level was 12.0 μmol/L, the direct bilirubin level was 4.9 μmol/L, the indirect bilirubin level was 7.1 μmol/L, the creatinine level was 63.1 μmol/L, and the glomerular filtration rate was 134.76 mL/min/1.73 m2), which were all within the normal range.
A diagnostic paracentesis was performed, which revealed bloody ascites with increased red blood cells (81500), nucleated cells (657), and protein concentrations (1790 mg/dL). The adenosine deaminase, amylase, LDH, and carcinoembryonic antigen levels in ascites were normal. The smear for acid-fast bacilli and exfoliative cytology were negative.
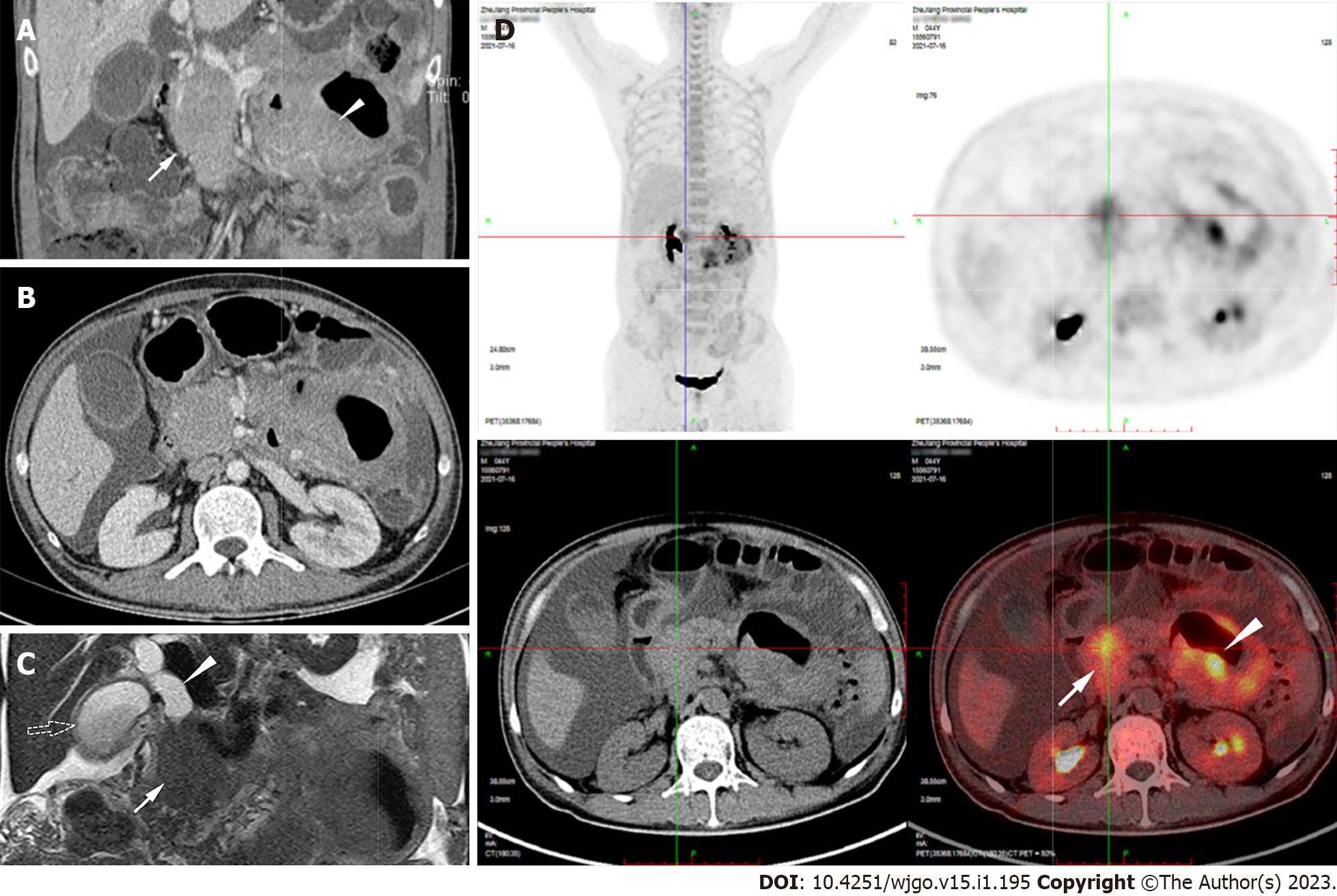
Esophagogastroduodenoscopy in a previous hospital revealed chronic gastritis, while colonoscopy found several small polyps, which were confirmed as tubular adenomas according to pathology. Capsule endoscopy showed segmental hyperemia, edema, and partial atrophy of villi in the jejunum. On the computed tomography enterograph, there was a circular, solid-occupying lesion in the pancreatic head that was 88 mm × 47 mm × 52 mm in size. The jejunal wall was thickened to 34 mm but did not cause intestinal stenosis or obstructive signs. These two lesions had uniform CT density and moderate enhancement (Figure 1A and B). Magnetic resonance (MR) cholangiopancreatography revealed that the pancreatic head mass, together with the biliary duct, was dilated to 18 mm and the gallbladder was enlarged (Figure 1C). 18F-Fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) detected intense FDG uptake within the pancreatic head lesion and jejunal wall thickening (SUVmax = 7.2 and SUVmax = 10.3, respectively) (Figure 1D).
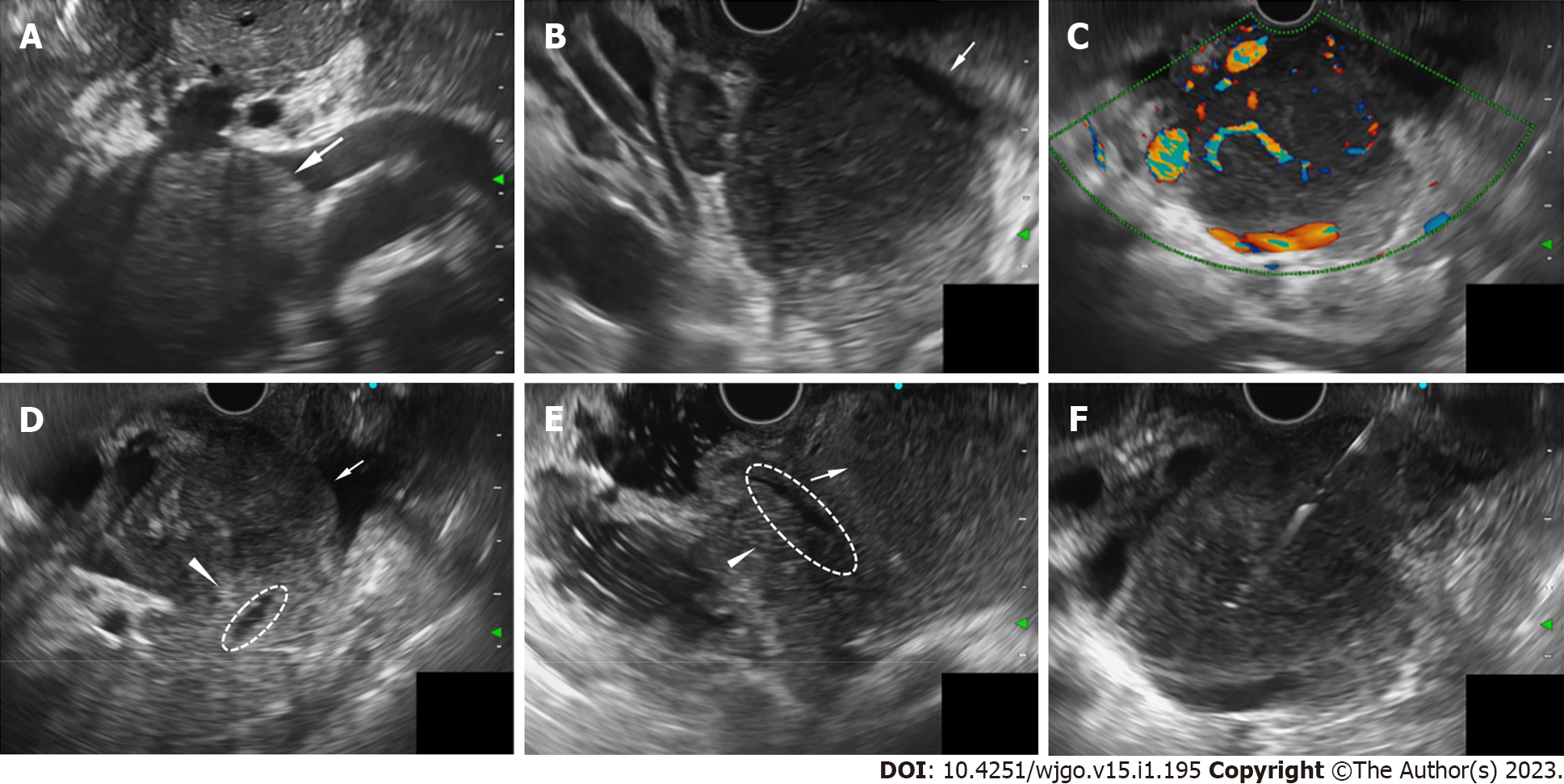
During hospitalization, the patient developed a high fever of 38.8 °C and jaundice. The direct bilirubin level increased to 110.3 μmol/L, ALP 432 U/L, and γ-GT 281 U/L. We administered moxifloxacin as an anti-infective treatment and performed EUS-FNB for further diagnosis. EUS (OLYMPUS EUS EU-ME2, GIF-UCT260, Olympus Corporation, Tokyo, Japan) revealed a 51.0 mm × 45.8 mm well-defined homogenously hypoechoic lesion in the pancreatic head region. Color and power Doppler ultrasound showed hypervascularity. The pancreatic head parenchyma was adjacent to the mass, but the boundaries were preserved. The main pancreatic duct ran behind the lesion naturally, without any signs of infiltration or dilatation (Figure 2).
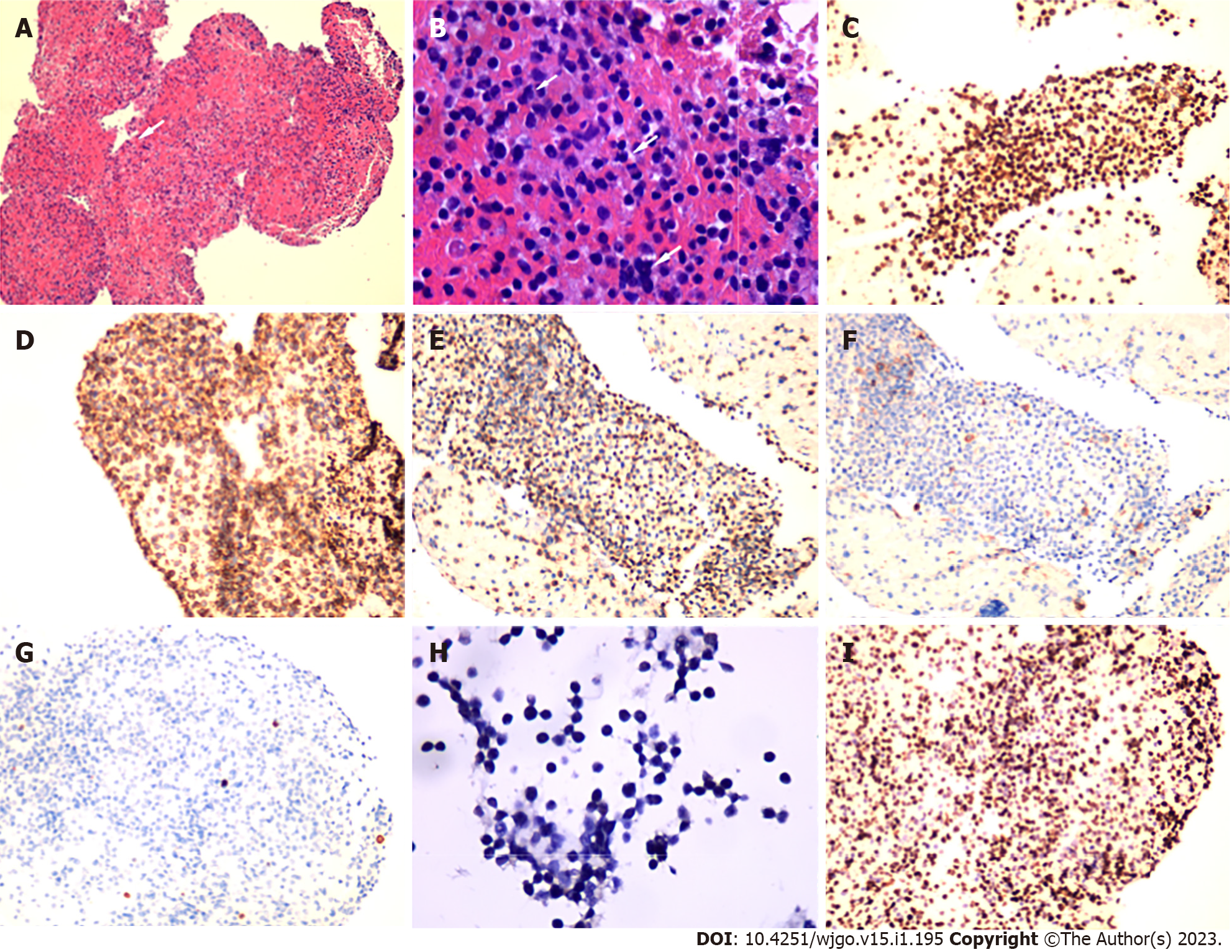
A 22 G needle (EchoTip ProCore®, Cook Medical, Limerick, Ireland) was applied for FNB using a transpyloric approach, with suction applied using a 20 mL syringe (Figure 2F). Biopsy specimens were partially fixed in formalin, and embedded in paraffin for pathology and partially preserved in saline solution for MFCI. Microscopic examination revealed small round cells arranged in diffuse sheets. On immunohistochemistry, the cells were positive for CD3, CD56, CD45RO, and CD8 and were scattered positive for T-cell intracellular antigen and granzyme B. CD20, chromogranin A, synaptophysin, and Epstein-Barr virus-encoded RNA were negatively expressed in the tumor cells. The Ki-67 proliferation index was 80% (Figure 3).
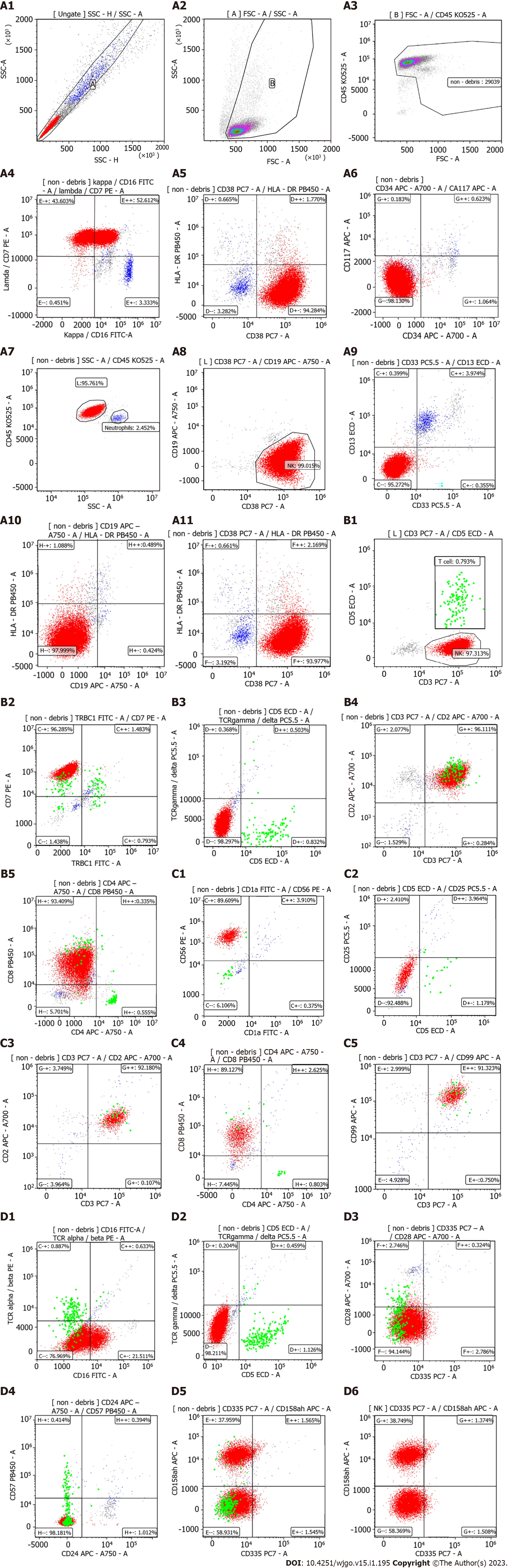
In MFCI, a predominantly heterotypic (90.953%) NK lymphocyte cell population was identified. These cells were TRBC1-, CD5-, TCRγδ-, CD2++, CD3++, CD4-, CD8+, CD56++, CD25-, CD5-, CD99++, TCRαβ-, CD335-, CD28- , and CD57-. CD158a, h expression in abnormal NK cells was 40.123% (Figure 4).
Based on the above findings and advice from consulted hematologists, we proposed a diagnosis of intestinal NKTCL with retroperitoneal lymph node involvement (pancreatic head lesion).
Endoscopic retrograde cholangiopancreatography was performed for biliary drainage with fully covered self-expanding metal stents inserted into the common bile duct to relieve biliary obstruction. The patient's temperature and jaundice gradually stabilized. Subsequently, he was transferred to the hematology department. Further bone marrow (BM) biopsy and flow cytometry indicated that no heterotypic NK lymphocytes were observed, and the NK cells in BM were in a normal proportion as well as phenotype. Therefore, the patient was prescribed the SMILE chemotherapy regimen.
The first SMILE regimen was performed from August 1, 2021 to August 4, 2021. On the first day of chemotherapy, the patient had diarrhea and developed hematochezia once, approximately 50 g. However, the symptoms of diarrhea, abdominal pain, and hematochezia were relived on the second day of chemotherapy. The patients stayed in the hospital until August 10, 2021, when he improved uneventfully without leukocytopenia (white blood cell count, 5.05 × 109/L). Unfortunately, two days after discharge, he died from leukopenia and severe septic shock in a local hospital.
We introduced a case of intestinal NKTCL with retroperitoneal lymph node involvement, presenting as a pancreatic head space-occupying lesion. The patient was efficiently diagnosed through EUS-FNB combined with multicolor flow cytometry immunophenotyping.
Intestinal NKTCL, as a category of extranodal lymphoma, has a low incidence, accounting for approximately 8%-15% of GI lymphomas, but has a poor prognosis[2,4]. Intestinal NKTCL is mostly associated with EBV infection, and its incidence is characterized by distinct ethnic groups and regional distributions; it is more prevalent in the East and in South America, but with a low overall incidence[5]. Abdominal lymphoma is often encountered in clinical practice, while primary pancreatic lymphoma (PPL) only accounts for 0.1% of malignant tumors[6,7]. Due to the nonspecific clinical symptoms and endoscopic findings, as well as the high negative rate of mucosal biopsy, it is difficult to distinguish intestinal NKTCL from inflammatory disease or malignant tumors of the pancreas, hepatobiliary tissue and other organs. For NKTCL, its diagnosis and prognosis have always been less satisfactory[8]. In a retrospective study published in 2014, the authors suggested that the average time required for a correct diagnosis was 12.8 mo. They noted that inflammatory cell infiltration is the most common factor that delays the diagnosis of NKTCL[9].
In the differential diagnosis process of NKTCL, PET-CT can not only provide standard images but also help accurately stage the disease[10-12]. At the same time, the accuracy of MR is also relatively high[13]. A study of 36 patients with nasal-type NKTCL reported that the accuracy of PET-CT vs MR was 99% vs 92.7%, respectively. However, the sensitivity of PET-CT compared with MR was 100% vs 81.4%, respectively, and MR examination of 16 Lesions showed 11 negative results[14]. With high resolution, EUS mainly provides real-time imaging and texture of the target object, providing a more detailed description of lesion characteristics and the relationship with the surrounding organs. To our knowledge, there is no literature summary about the EUS characteristics of NKTCL. According to our case and experience, the EUS features of lymphoma, as a mesenchymal tumor, are summarized as follows: it has well-defined borders, homogeneous internal echogenicity, and abundant blood flow signal lesions with a relatively large diameter. Even in large lesions, manifestations of compression and pushing are commonly be found, without signs of the invasion of blood vessels, bile ducts, pancreatic ducts, or parenchyma of surrounding organs, which are common in epithelial carcinoma.
In the case of suspected lymphoma, FNB should be applied in addition to the immunohistochemistry analysis for not only diagnosis but also subtyping. In a report of 240 patients with lymphoma, needle sizes of 19 G and 22 G were used. For the most part, the choice of the conventional 19 G needle was valid; however, it was ineffective in two patients with periduodenal lesions[15]. The results of a multicenter study of 109 patients with solid lesions indicated that the use of 19 G ProCore™ needles achieved a pathology assessment rate of 89% and an accuracy of 86% for solid lesions[16]. With the use of a small-gauge needle such as 22 G, the lymphoma subtyping rate of cases may range from 66.6% to 87.5%[15]. MFCI plays an integral role in the screening and diagnosis of lymphoma. In some cases, especially when observing relatively clear space-occupying lesions is difficult, MFCI is indispensable[17]. In this case, we used MFCI to help us make a correct diagnosis. A recent study showed that only 28% of patients were diagnosed with lymphoma, compared with 91% of patients with pancreatic adenocarcinoma. However, when MFCI was added, the diagnostic rate of primary PPL increased to 100%[18]. Presently, in the course of NKTCL treatment, asparaginase-containing chemotherapeutic schemes are the standard treatment regimen[19]. Based on previous experience, SMILE is the most popular regimen for the treatment of NKTCL[20]. In a study of 87 NKTCL patients treated with the SMILE regimen, for newly diagnosed stage III/IV patients, the complete response rate was 40%-54%, and the 5-year overall survival rate was 47%[21].
In this case, the patient presented with persistent diarrhea, abdominal pain, edema of the lower limbs, and weight loss. EUS showed a clear boundary, homogeneous internal echo, abundant blood flow signal lesions and compression signs. Intestinal NKTCL is a rare disease with a poor prognosis and high mortality. In light of the lack of understanding of the disease, diagnosis and treatment are particularly crucial. In this case, the combination of EUS-FNB and MFCI played a key role in the diagnosis of intestinal NKTCL.
We report a case of intestinal NKTCL presenting as a pancreatic head space-occupying lesion. The summarized EUS characteristics, including well-defined borders, homogeneous internal echogenicity, abundant blood flow signal lesions, and compression signs, might be helpful in differential diagnosis. EUS-FNB with a 19 G or 22 G biopsy needle combined with MFCI could be a good choice for diagnosis and for subtyping lymphoma.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ishida T, Japan; Soewondo W, Indonesia; Zhai J, China S-Editor: Wang LL L-Editor: A P-Editor: Yu HG
| 1. | Tang XF, Yang L, Duan S, Guo H, Guo QN. Intestinal T-cell and NK/T-cell lymphomas: A clinicopathological study of 27 Chinese patients. Ann Diagn Pathol. 2018;37:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Hue SS, Ng SB, Wang S, Tan SY. Cellular Origins and Pathogenesis of Gastrointestinal NK- and T-Cell Lymphoproliferative Disorders. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 3. | Küçük C, Wang J, Xiang Y, You H. Epigenetic aberrations in natural killer/T-cell lymphoma: diagnostic, prognostic and therapeutic implications. Ther Adv Med Oncol. 2020;12:1758835919900856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Li H, Lyu W. Intestinal NK/T cell lymphoma: A case report. World J Gastroenterol. 2020;26:3989-3997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Xiong J, Zhao WL. Advances in multiple omics of natural-killer/T cell lymphoma. J Hematol Oncol. 2018;11:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Yu RS, Zhang WM, Liu YQ. CT diagnosis of 52 patients with lymphoma in abdominal lymph nodes. World J Gastroenterol. 2006;12:7869-7873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Facchinelli D, Sina S, Boninsegna E, Borin A, Tisi MC, Piazza F, Scapinello G, Maiolo E, Hohaus S, Zamò A, Merli M, Stefani PM, Mellone F, Basso M, Sartori R, Rusconi C, Parisi A, Manfrin E, Krampera M, Ruggeri M, Visco C, Tecchio C. Primary pancreatic lymphoma: Clinical presentation, diagnosis, treatment, and outcome. Eur J Haematol. 2020;105:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Liu H, Liu M, You H, Li X. Oncogenic Network and Hub Genes for Natural Killer/T-Cell Lymphoma Utilizing WGCNA. Front Oncol. 2020;10:223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 9. | Yanagi H, Nakamura Y, Takagi D, Kubota K. Extranodal natural killer/T-cell lymphoma: a diagnostic dilemma. Rhinology. 2012;50:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Nakamura S, Matsumoto T. Gastrointestinal lymphoma: recent advances in diagnosis and treatment. Digestion. 2013;87:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 11. | Chan WK, Au WY, Wong CY, Liang R, Leung AY, Kwong YL, Khong PL. Metabolic activity measured by F-18 FDG PET in natural killer-cell lymphoma compared to aggressive B- and T-cell lymphomas. Clin Nucl Med. 2010;35:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Khong PL, Pang CB, Liang R, Kwong YL, Au WY. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Chen Y, Wang X, Li L, Li W, Xian J. Differential diagnosis of sinonasal extranodal NK/T cell lymphoma and diffuse large B cell lymphoma on MRI. Neuroradiology. 2020;62:1149-1155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Guo R, Xu P, Cheng S, Lin M, Zhong H, Li W, Huang H, Ouyang B, Yi H, Chen J, Lin X, Shi K, Zhao W, Li B. Comparison of Nasopharyngeal MR, (18) F-FDG PET/CT, and (18) F-FDG PET/MR for Local Detection of Natural Killer/T-Cell Lymphoma, Nasal Type. Front Oncol. 2020;10:576409. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Gimeno-García AZ, Elwassief A, Paquin SC, Sahai AV. Endoscopic ultrasound-guided fine needle aspiration cytology and biopsy in the evaluation of lymphoma. Endosc Ultrasound. 2012;1:17-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Iglesias-Garcia J, Poley JW, Larghi A, Giovannini M, Petrone MC, Abdulkader I, Monges G, Costamagna G, Arcidiacono P, Biermann K, Rindi G, Bories E, Dogloni C, Bruno M, Dominguez-Muñoz JE. Feasibility and yield of a new EUS histology needle: results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 17. | Zheng Y, Wan X, Gui X, Chen Y, Gao L, Zhang H, Wang Y. Value of multi-parameter flow cytometry immunophenotyping in T/NK-cell neoplasms in cytology specimens: A retrospective study in Chinese patients. Pathol Res Pract. 2020;216:152921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Mohamadnejad M, Khosravi P, Khani M, Nikmanesh A, Eloubeidi MA. Primary pancreatic Hodgkin's lymphoma diagnosed on EUS-guided FNA. Gastrointest Endosc. 2016;83:844-5; discussion 845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Tse E, Zhao WL, Xiong J, Kwong YL. How we treat NK/T-cell lymphomas. J Hematol Oncol. 2022;15:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 20. | Yamaguchi M, Kwong YL, Kim WS, Maeda Y, Hashimoto C, Suh C, Izutsu K, Ishida F, Isobe Y, Sueoka E, Suzumiya J, Kodama T, Kimura H, Hyo R, Nakamura S, Oshimi K, Suzuki R. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol. 2011;29:4410-4416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 463] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 21. | Kwong YL, Kim WS, Lim ST, Kim SJ, Tang T, Tse E, Leung AY, Chim CS. SMILE for natural killer/T-cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120:2973-2980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 333] [Article Influence: 25.6] [Reference Citation Analysis (0)] |