Published online Jan 15, 2023. doi: 10.4251/wjgo.v15.i1.186
Peer-review started: October 8, 2022
First decision: October 17, 2022
Revised: October 31, 2022
Accepted: December 6, 2022
Article in press: December 6, 2022
Published online: January 15, 2023
Processing time: 94 Days and 3.2 Hours
Mucinous adenocarcinoma of the colorectum is a rare histological subtype characterized by an abundant mucinous component. Mucinous tumors are frequently diagnosed at an advanced stage, which indicates an aggressive subtype. However, few case reports have been published, and little information is available concerning genetic alterations in mucinous adenocarcinoma.
A 76-year-old man underwent en bloc endoscopic submucosal dissection (ESD) for the management of a type 0-Is+IIa lesion. Histological examination revealed an intramucosal mucinous adenocarcinoma with signet-ring cell carcinoma and well-to-moderately differentiated tubular adenocarcinoma. Three years after the ESD, local recurrence was detected by an endoscopic examination, revealing a new 0-Is+IIa lesion with a phenotype similar to the previously resected lesion. Re-ESD was chosen for the management of the recurrent tumor, and the histological examination showed positive tumor infiltration at the vertical margin. Additional surgical resection was performed for the curative treatment. Genetic analysis showed pathogenic alterations in RNF43 and TP53 in the adenoma and an additional SMAD4 alteration in the carcinoma.
This mucinous mucosal adenocarcinoma case was suggested to have an aggressive phenotype and a careful and close follow-up are required.
Core Tip: Colorectal mucinous adenocarcinoma, as characterized by an abundant mucinous component, is a rare histological subtype frequently diagnosed at an advanced stage. Intramucosal mucinous adenocarcinoma was dissected by endoscopic submucosal dissection and local recurrence was detected three years after the treatment. Genetic analysis showed pathogenic alterations of RNF43, TP53, and SMAD4. This case of mucinous mucosal adenocarcinoma was suggested to have an aggressive phenotype based on the treatment course and advanced genotype identified by target sequencing. Careful and close follow-up should be performed, and additional surgery should be considered when managing patients with mucinous adenocarcinoma.
- Citation: Murakami Y, Tanabe H, Ono Y, Sugiyama Y, Kobayashi Y, Kunogi T, Sasaki T, Takahashi K, Ando K, Ueno N, Kashima S, Yuzawa S, Moriichi K, Mizukami Y, Fujiya M, Okumura T. Local recurrence after successful endoscopic submucosal dissection for rectal mucinous mucosal adenocarcinoma: A case report. World J Gastrointest Oncol 2023; 15(1): 186-194
- URL: https://www.wjgnet.com/1948-5204/full/v15/i1/186.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i1.186
The World Health Organization classifies colorectal cancers according to histology[1]. The most common type of colorectal cancer is adenocarcinoma, which is frequently heterogeneous and is composed of several histological variants: Mucinous adenocarcinoma, signet-ring cell carcinoma, neuroendocrine carcinoma, squamous cell carcinoma, medullary carcinoma, and undifferentiated carcinoma. The histological subtype presumably plays a role in tumor biology and patient prognosis. Approximately 10% of colorectal carcinomas are mucinous adenocarcinomas, which are characterized by an abundant mucinous component comprising more than 50% of the tumor volume[2]. The clinicopathological characteristics are reported include young age, proximal colon origin, and a larger tumor size[3,4]. Signet-ring cell carcinomas are observed in approximately 1% of colorectal carcinoma cases and are present as single cells with abundant cytoplasmic mucin. Mucinous adenocarcinomas with signet-ring cell carcinomas are distinctively different from adenocarcinoma in tumor biology, with an aggressive phenotype[5].
A multistep model of carcinogenesis has been proposed in colorectal cancer. Biallelic APC mutations are known to be an early event leading to adenoma, followed by activating mutations in KRAS in the advancing adenoma and TP53 mutations during the transition to malignancy in common colorectal cancer[6]. Gene expression-based subtyping in colorectal cancers has been widely accepted as a relevant source of disease stratification in colorectal cancer. The genetic pathway of mucinous adenocarcinoma has also been investigated, suggesting a separate genetic pathway with high mutations in K-RAS and TP53[7]. However, these mutation frequencies differ among reports, indicating that colorectal mucinous adenocarcinomas have heterogeneous phenotypes[3,7,8]. Detailed examinations are required for genetic analysis of mucinous adenocarcinoma in the colorectum.
Although the overall survival of mucinous carcinoma patients was shown to be poorer than that of adenocarcinoma patients in some reviews, the prognosis and treatment options for mucinous adenocarcinoma remain controversial[3,9,10]. As mucinous adenocarcinomas are frequently diagnosed as advanced stages, so patients with mucinous carcinoma have poor prognosis. In contrast, only a few cases of intramucosal mucinous colorectal carcinoma have been reported thus far, and the prognosis of patients with early mucinous adenocarcinoma treated by endoscopic resection remains unclear[11,12].
We herein report a case of early mucinous rectal adenocarcinoma that was removed by endoscopic submucosal dissection (ESD), with recurrence observed after three years.
A 76-year-old man with a history of hematochezia underwent colonoscopy at a hospital, and an elevated tumor was observed in the lower rectum. He was referred to our department.
The patient had a history of hematochezia, with no abdominal symptoms.
The patient had a history of appendectomy due to appendicitis and prostate cancer surgery.
The patient had no family history of malignant tumors.
Physical examination was unremarkable, and his abdomen was soft, nontender, and nondistended with no palpable mass.
Routine laboratory examination showed normal blood counts with a white blood cell count of 5930/μL, red blood cell count of 4920/μL, and platelet count of 24.2/μL. Tumor markers were within the normal range; carcinoembryonic antigen was 3.2 ng/mL, and carbohydrate antigen 19-9 was < 2 U/mL.
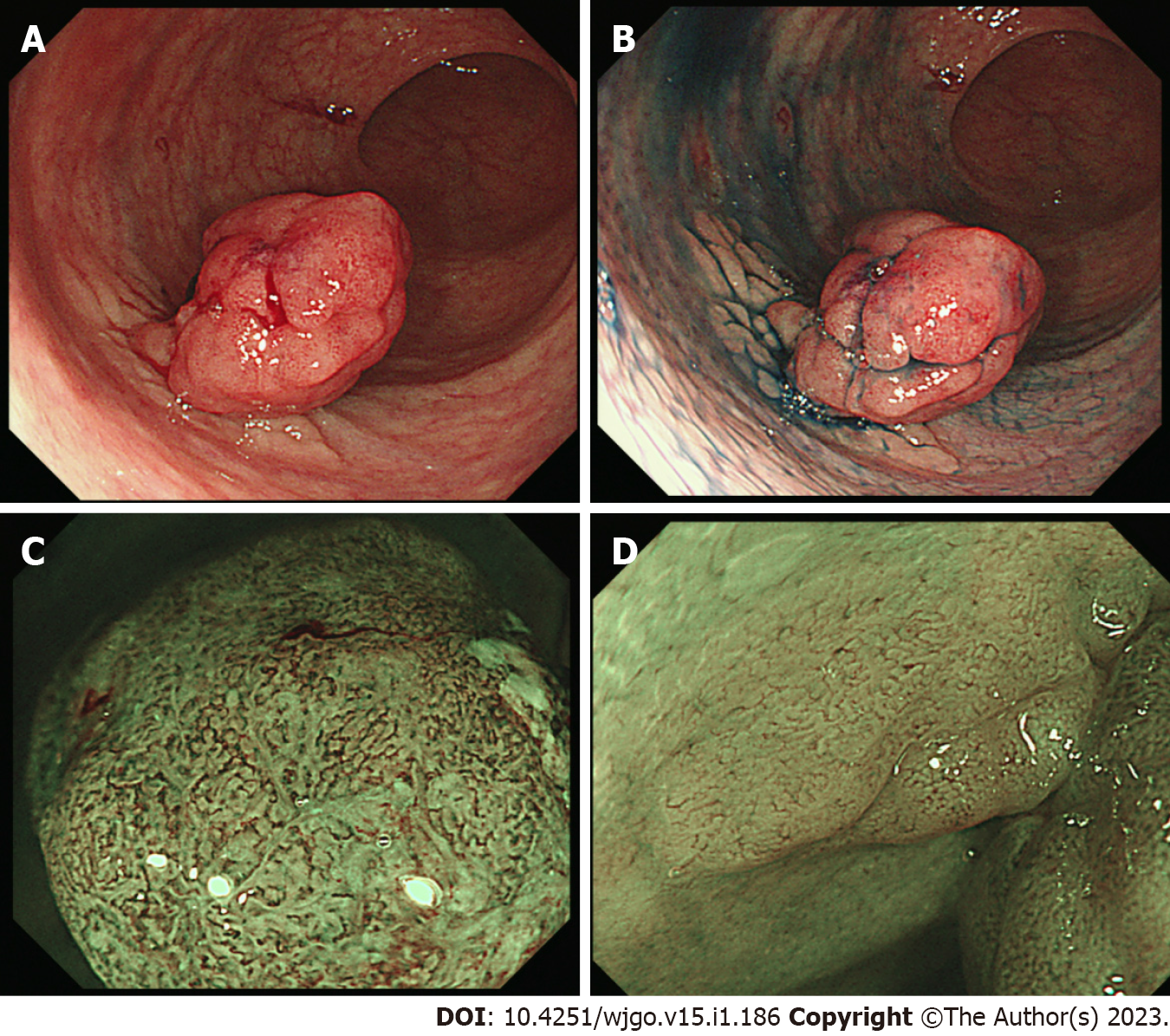
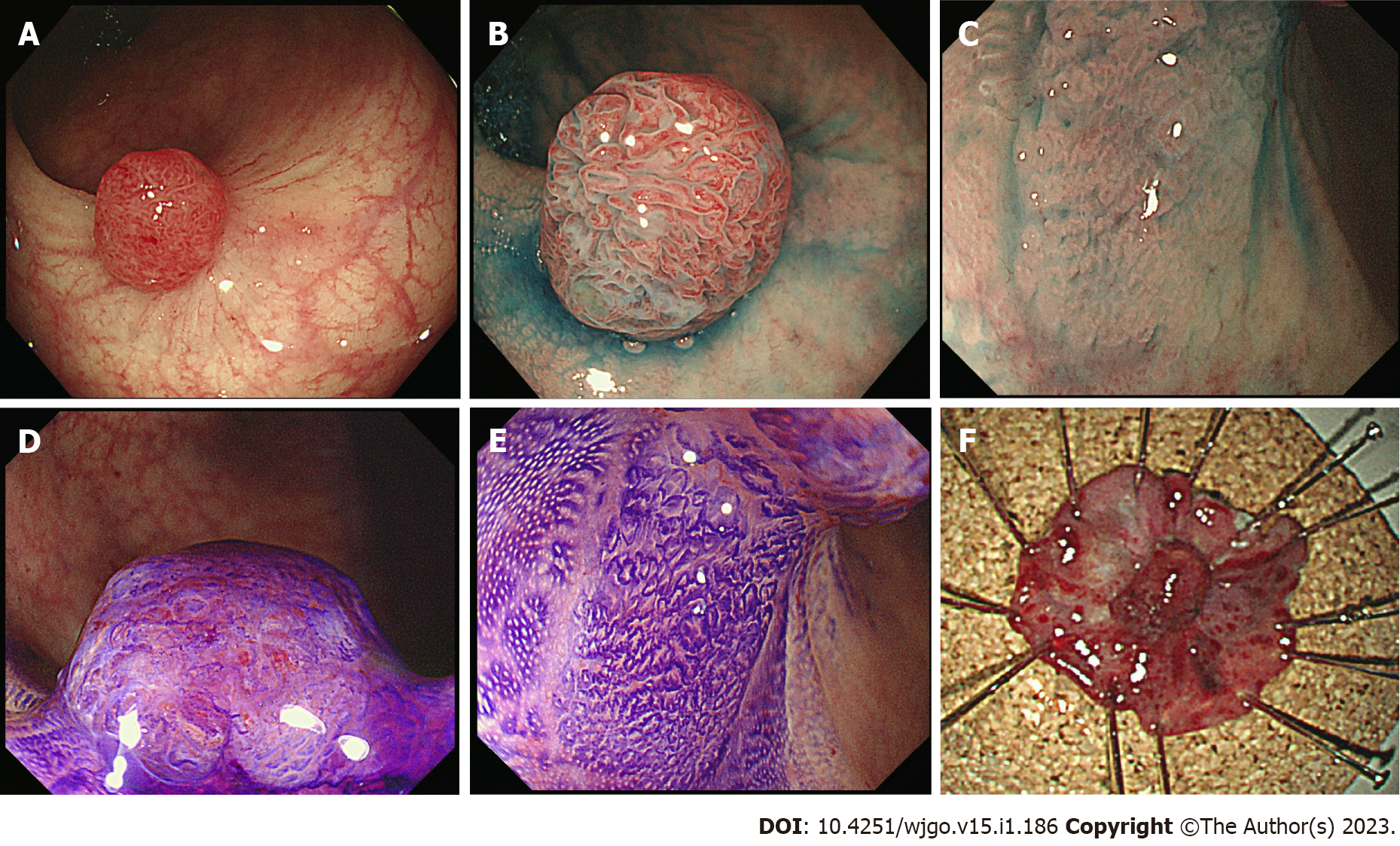
Colonoscopy revealed a 0-Is+Ⅱa lesion with slight bleeding (Figure 1A), chromoendoscopy emphasized a laterally spreading flat lesion (IIa) at the base of the protrusion (Is) (Figure 1B), and magnified endoscopy with narrow-band imaging revealed an intense irregular microvascular pattern in the Is lesion and regular caliber vascular pattern in the IIa lesion (Figure 1C and D). Computed tomography (CT) did not show any lymph node metastasis or distant metastasis.
We analyzed the genetic alterations of the lesions in a previous report[13]. In brief, genomic DNA was isolated from formalin-fixed paraffin embedded (FFPE) specimens using a GeneRead DNA FFPE kit (Qiagen, Hilden, Germany). The mutation profile was determined by target amplicon sequencing using a GeneStudio S5 system (Thermo Fisher Scientific, Carlsbad, CA). A colorectal cancer associated gene panel including ARID1A, MUTYH, NRAS, CTNNB1, PIK3CA, FBXW7, APC, BRAF, and KRAS[14,15] and a pancreatic ductal adenocarcinoma (PDA)-medium panel including KRAS, TP53, SMAD4, CDKN2A, GNAS, BRAF, PIK3CA, RNF43, STK11, and HRAS were designed using the Ampliseq Designer website (https://www.ampliseq.com). Variants were identified using the Variant Caller plugin (version 5.0.4.0). Genetic mutations of colorectal cancers reported in the Catalog of Somatic Mutations in Cancer (COSMIC) database (https://cancer.sanger.ac.uk/cosmic/) were identified.
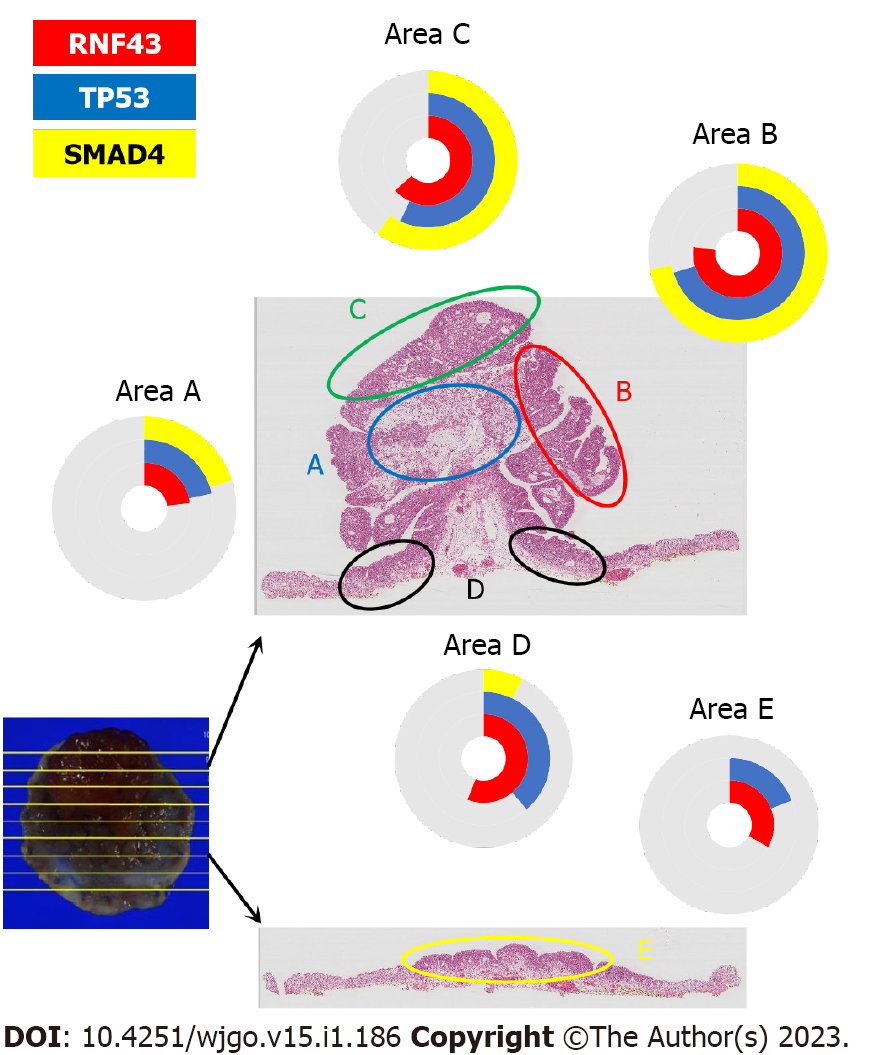
A total of four pathogenic mutations were found when using the PDA-medium panel for genetic analysis (Table 1). TP53p. R273H and RNF43 p. W159Afs*8 were found in the adenoma, and SMAD4 p. D351G, and PIK3CA p. T74N were additionally found in the adenocarcinoma (Figure 2). No mutations were detected in any other genes, including KRAS, GRAS, BRAF, STK11, and HRAS. The TP53 mutation in the tumor cells was confirmed by p53 immunohistochemistry. Mutations were not found when using the colorectal cancer-associated gene panel, indicating no somatic mutations in APC. The protocol of the genetic analysis was approved by Asahikawa Medical University Research Ethics Committee, and written informed consent was obtained from the patient.
| Areas | Pathological diagnosis | Somatic mutations | Qubit (ng/ul) | Library yield (pM) | Mapped Reads | Mean Depth | Uniformity | Target base coverage at 100x (%) | Target base coverage at 500x (%) | |||
| RNF43 p.W159Afs*8 | TP53 p.R273H | SMAD4p.D351G | PIC3CA p.T74N | |||||||||
| a | Mucinous and signet-cell adenocarcinoma | 23.1% | 21.5% | 20.5% | 0.0% | 26.8 | 3736.6 | 384372 | 3040 | 98% | 99% | 98% |
| b | Tubular adenocarcinoma | 77.3% | 70.3% | 72.1% | 0.0% | 55.0 | 2901.2 | 369702 | 2925 | 98% | 99% | 99% |
| c | Tubular adenocarcinoma | 63.2% | 56.8% | 59.6% | 0.0% | 75.6 | 2025.8 | 411829 | 3280 | 97% | 99% | 99% |
| d | Adenoma | 56.0% | 38.9% | 7.2% | 8.0% | 29.6 | 2832.3 | 421877 | 3376 | 99% | 99% | 99% |
| e | Adenoma | 33.3% | 19.1% | 0.0% | 0.0% | 66.6 | 1614.0 | 620398 | 4949 | 98% | 99% | 99% |
| f | Normal epithelia | 0.0% | 0.0% | 0.0% | 0.0% | 18.7 | 2280.3 | 645324 | 5114 | 98% | 99% | 99% |
The tumor was diagnosed as a mucosal (Tis) rectal adenocarcinoma.
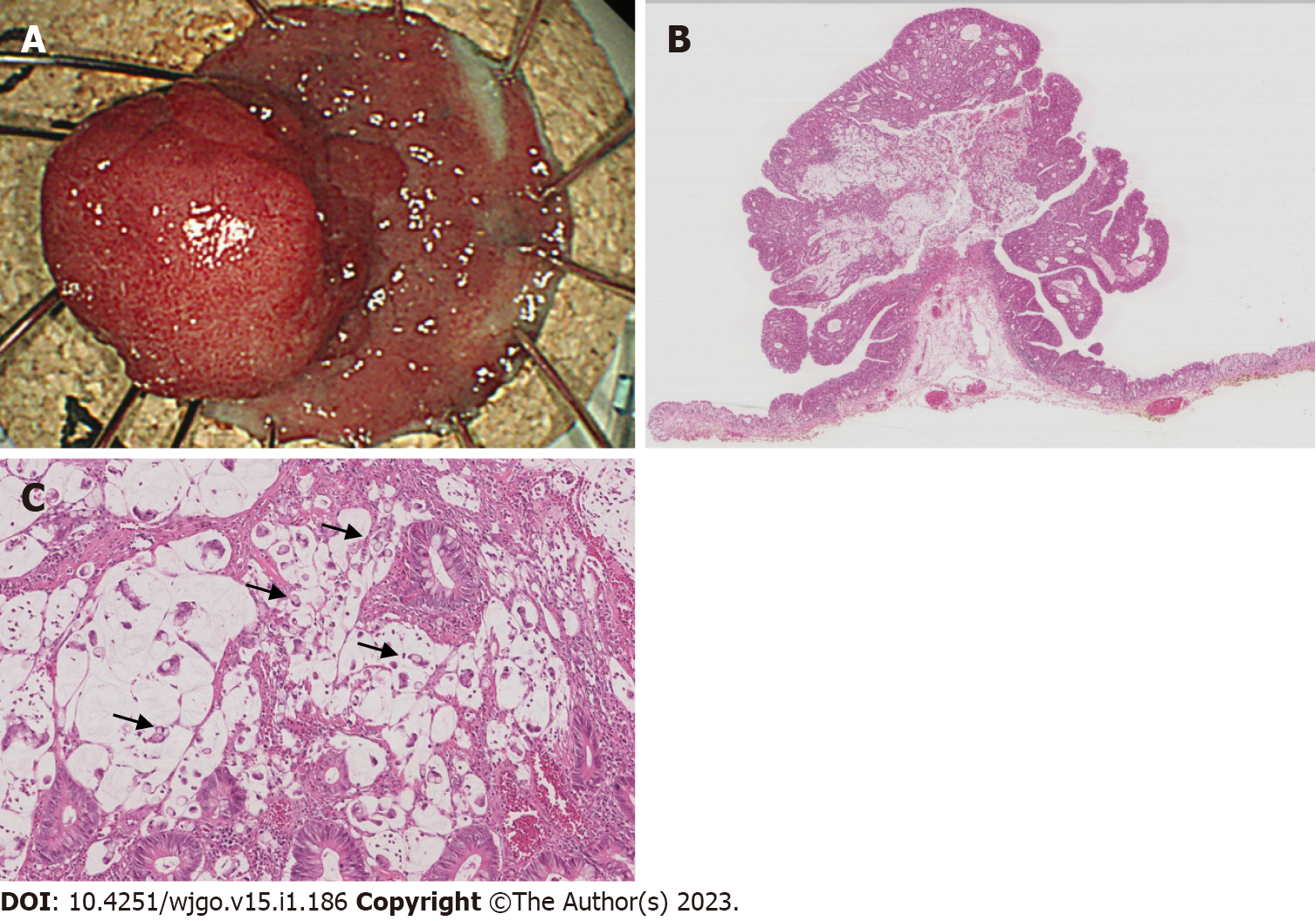
The tumor was removed by ESD. In the procedure, diluted hyaluronic acid solution was injected into the submucosa on the anal side of the tumor. Repeated local injections were needed while the submucosa was dissected just above the muscular layer toward the proximal side of the tumor. The en bloc resected tissue with the lateral normal epithelia measured 30 mm (Figure 3A).
A microscopic examination revealed an intramucosal mucinous adenocarcinoma with signet-ring cell carcinoma and well-to-moderately differentiated adenocarcinoma, with a negative margin in both the vertical and horizontal aspects (Figure 3B). A marked mucous lake was found in the center of the tumor, with signet-ring cells spreading in the mucin (Figure 3C). A basal spreading tumor 22 mm in diameter was composed of adenoma with mild to moderate dysplastic cells. The pathological diagnosis was Tis, ly0, v0, Stage 0.
Colonoscopy and CT were conducted every six months for one year, and the annual surveillance was conducted thereafter. An ESD scar was observed by the colonoscopy for two years (Supple
Based on these results, additional surgical resection was recommended, and Mile’s operation was performed (Supplementary Figure 2). The mucinous adenocarcinoma had infiltrated the muscle layer with lymphatic invasion, but there were no lymph node metastases. The eventual histological diagnosis was T2, N0, M0, Stage I. The postoperative course was normal, and the patient had no recurrence for one year.
A mucosal mucinous adenocarcinoma with signet-ring cell carcinoma in the rectum was en bloc resected by ESD, but recurrence occurred at the scar three years later. Heterogenous histology, whereby the mucinous and tubular adenocarcinoma were developed from adenoma, was observed, and a genetic analysis revealed that a single mutated pathway was associated with these tumors. Thus, the mucinous adenocarcinoma was deemed to have originated from a laterally spreading adenoma with initial mutations in the RNF43 and TP53 cancer-suppresser genes.
Mucinous colorectal adenocarcinomas account for approximately 10% of colorectal carcinomas, and signet-ring cell carcinoma is rare (approximately 1%) in the incidence[16]. Signet-ring cell carcinoma has not been well evaluated due to its low incidence, and the most cases are found at an advanced stage[17]. Interestingly, as some signet-ring cell carcinomas are found in combination with adenoma, the origin of the carcinoma is speculated to originate from the adenoma[18,19]. Our case supports this theory, as the elevated lesion (Is) with an irregular vascular surface pattern developed from the IIa lesion with a regular vascular pattern. The endoscopic findings indicated the malignant histology of the tumors, with Is and IIa lesions from the carcinoma and adenoma, respectively. A histological feature of this tumor was its heterogenicity, comprising mucinous, tubular adenocarcinoma, and adenoma components.
Overall, a genetic approach is expected to clarify the tumor progression pathway. Adenoma-carcinoma sequences are proposed in common adenocarcinoma, in which sequential mutations in APC, KRAS, TP53, and SMAD4 occur. However, a recent genetic examination of colorectal adenocarcinoma with a mucinous component indicated fewer TP53 mutations than in classical adenocarcinoma[20]. In contrast, our genetic analyses of these mucinous adenocarcinomas with its adenoma component showed TP53 p.R273H (c.818G>A, COSM10660), which is a frequent variant in common colorectal cancers. Mutations of APC and KRAS, which are frequently observed in colorectal cancers, are not observed in either adenocarcinoma or adenoma. RNF43 W159Afs*8 (c.474_476delCTGinsA, which is not in the COSMIC database) was found in all areas of the tumor, and SMAD4 D351G (c.1052A>G, COSM373800) was found in areas of the carcinoma. The mutation sequence in the progression from adenoma to adenocarcinoma was occurred from RNF43 and TP53, followed by SMAD4. The process was similar to that observed in colitis-associated cancers, in which somatic mutation of RNF43 is the driver genetic alteration linking chronic inflammation and cancer development in colitic cancers[21]. A lack of APC mutations in mucinous cancer supports similarity to the colitic cancer pathway. Coincidentally, one of the characteristics of colitis-associated cancer is a high proportion of mucinous or signet-ring cell carcinomas. The histological nature corresponds to the genetic characteristics.
The mucinous colorectal adenocarcinoma-mucosal type, which was endoscopically resected in this case, was first reported in 2010[22]. To date, no other mucinous adenocarcinoma treated endoscopically has been reported in the English literature. The tumor in this case was extremely rare, as it was a limited intramucosal lesion and removed endoscopically. The presence of mucosal carcinoma with adenoma and genetic analyses of the lesions indicated the initiation of mucinous carcinogenesis. In this case, genetic mutational analysis confirmed that the mucinous adenocarcinomas derived from adenoma, and the involvement of the RNF43 pathway in mucinous carcinogenesis was proposed. In addition, the mucinous adenocarcinoma in the initial lesion was limited to the mucosal layer without a negative margin in the vertical or horizontal aspects, at the primary treatment. However, the mucinous adenocarcinoma recurred three years later. These findings suggested an aggressive genotype for the mucinous adenocarcinoma; thus, even mucosal tumors should receive close follow-up. The Japan Gastroenterological Endoscopy Society guidelines for ESD/EMR note that Tis carcinomas generally do not metastasize to lymph nodes or other organs[23]. Additional surgical resection should be considered for T1 carcinoma with malignant indictors, including a poorly differentiated phenotype (e.g., mucinous adenocarcinoma). The European Society of Gastrointestinal Endoscopy Guidelines recommend additional surgical intervention “in the cases with massive submucosal invasion, undifferentiated adenocarcinoma, positive or nonvaluable vertical margins, and/or lymphovascular infiltration by cancer cells”[24]. Mucosal (Tis) carcinoma with mucinous component is included in these criteria. The treatment strategy for mucosal (Tis) mucinous adenocarcinoma remains to be addressed.
One limitation associated with the present study warrants mentioning. The patient ultimately underwent Mile’s operation after undergoing ESD twice throughout the clinical course. Endoscopic ultrasound is useful for evaluating the depth of submucosal invasive lesions in rectal tumors before endoscopic resection[25]. The second ESD procedure might have been avoided if a more accurate pretreatment diagnosis concerning the depth of the local recurrence had been available.
This case of mucinous mucosal adenocarcinoma was suggested to have an aggressive phenotype based on the treatment course and the advanced genotype detected by target sequencing. Careful and close follow-up should be performed when managing patients with mucinous adenocarcinoma.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vyshka G, Albania; Zhang JW, China S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system vol. 3 4th ed Lyon. Geneva: World Health Organization Classification of Tumours 2010.. |
| 2. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2441] [Article Influence: 488.2] [Reference Citation Analysis (3)] |
| 3. | Huang A, Yang Y, Shi JY, Li YK, Xu JX, Cheng Y, Gu J. Mucinous adenocarcinoma: A unique clinicopathological subtype in colorectal cancer. World J Gastrointest Surg. 2021;13:1567-1583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (2)] |
| 4. | Ahnen DJ, Wade SW, Jones WF, Sifri R, Mendoza Silveiras J, Greenamyer J, Guiffre S, Axilbund J, Spiegel A, You YN. The increasing incidence of young-onset colorectal cancer: a call to action. Mayo Clin Proc. 2014;89:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 343] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 5. | Fadel MG, Malietzis G, Constantinides V, Pellino G, Tekkis P, Kontovounisios C. Clinicopathological factors and survival outcomes of signet-ring cell and mucinous carcinoma versus adenocarcinoma of the colon and rectum: a systematic review and meta-analysis. Discov Oncol. 2021;12:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8087] [Cited by in RCA: 8006] [Article Influence: 228.7] [Reference Citation Analysis (1)] |
| 7. | Hanski C. Is mucinous carcinoma of the colorectum a distinct genetic entity? Br J Cancer. 1995;72:1350-1356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Lan YT, Chang SC, Lin PC, Lin CC, Lin HH, Huang SC, Lin CH, Liang WY, Chen WS, Jiang JK, Lin JK, Yang SH. Clinicopathological and Molecular Features of Colorectal Cancer Patients With Mucinous and Non-Mucinous Adenocarcinoma. Front Oncol. 2021;11:620146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | O'Connell E, Reynolds IS, McNamara DA, Burke JP, Prehn JHM. Resistance to Cell Death in Mucinous Colorectal Cancer-A Review. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Luo C, Cen S, Ding G, Wu W. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond). 2019;39:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 11. | Huang L, Luo S, Lai S, Liu Z, Hu H, Chen M, Kang L. Survival after curative resection for stage I colorectal mucinous adenocarcinoma. BMC Gastroenterol. 2022;22:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Yoshikane H, Mizuno K, Sakakibara A, Hidano H, Takahashi Y, Yokoi T. Endoscopic image of early mucinous carcinoma of the sigmoid colon. Endoscopy. 1998;30:S111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Tanabe H, Moriichi K, Takahashi K, Ono Y, Kobayashi Y, Murakami Y, Iwama T, Kunogi T, Sasaki T, Ando K, Ueno N, Kashima S, Takei H, Mizukami Y, Fujiya M, Okumura T. Genetic alteration of colorectal adenoma-carcinoma sequence among gastric adenocarcinoma and dysplastic lesions in a patient with attenuated familial adenomatous polyposis. Mol Genet Genomic Med. 2020;8:e1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Bai J, Gao J, Mao Z, Wang J, Li J, Li W, Lei Y, Li S, Wu Z, Tang C, Jones L, Ye H, Lou F, Liu Z, Dong Z, Guo B, Huang XF, Chen SY, Zhang E. Genetic mutations in human rectal cancers detected by targeted sequencing. J Hum Genet. 2015;60:589-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Del Vecchio F, Mastroiaco V, Di Marco A, Compagnoni C, Capece D, Zazzeroni F, Capalbo C, Alesse E, Tessitore A. Next-generation sequencing: recent applications to the analysis of colorectal cancer. J Transl Med. 2017;15:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Hyngstrom JR, Hu CY, Xing Y, You YN, Feig BW, Skibber JM, Rodriguez-Bigas MA, Cormier JN, Chang GJ. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol. 2012;19:2814-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 266] [Article Influence: 20.5] [Reference Citation Analysis (1)] |
| 17. | Hugen N, Verhoeven RH, Lemmens VE, van Aart CJ, Elferink MA, Radema SA, Nagtegaal ID, de Wilt JH. Colorectal signet-ring cell carcinoma: benefit from adjuvant chemotherapy but a poor prognostic factor. Int J Cancer. 2015;136:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Mai KT, Isotalo PA, Guindi M, Burns BF, Parks W. Intestinal epithelial lesions associated with signet ring cell carcinoma of the colon and small intestine. Pathology. 2002;34:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Tandon M, Sostek M, Klein MA. Focus of signet ring cell carcinoma in an adenoma of the sigmoid colon. Arch Pathol Lab Med. 1999;123:957-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Chen J, Zhou L, Gao J, Lu T, Wang J, Wu H, Liang Z. Clinicopathological Characteristics and Mutation Spectrum of Colorectal Adenocarcinoma With Mucinous Component in a Chinese Cohort: Comparison With Classical Adenocarcinoma. Front Oncol. 2020;10:917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Fujita M, Matsubara N, Matsuda I, Maejima K, Oosawa A, Yamano T, Fujimoto A, Furuta M, Nakano K, Oku-Sasaki A, Tanaka H, Shiraishi Y, Mateos RN, Nakai K, Miyano S, Tomita N, Hirota S, Ikeuchi H, Nakagawa H. Genomic landscape of colitis-associated cancer indicates the impact of chronic inflammation and its stratification by mutations in the Wnt signaling. Oncotarget. 2018;9:969-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Wolf EM, Vieth M, Watanabe H, Spuller E, Leskowschek H, Langner C. Early mucinous colorectal adenocarcinoma--mucosal type. Endoscopy. 2010;42 Suppl 2:E236-E237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Kudo SE, Tsuruta O, Sugihara KI, Watanabe T, Saitoh Y, Igarashi M, Toyonaga T, Ajioka Y, Ichinose M, Matsui T, Sugita A, Sugano K, Fujimoto K, Tajiri H. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 437] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 24. | Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 766] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 25. | Harada N, Hamada S, Kubo H, Oda S, Chijiiwa Y, Kabemura T, Maruoka A, Akahoshi K, Yao T, Nawata H. Preoperative evaluation of submucosal invasive colorectal cancer using a 15-MHz ultrasound miniprobe. Endoscopy. 2001;33:237-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |