Published online Jan 15, 2023. doi: 10.4251/wjgo.v15.i1.155
Peer-review started: November 14, 2022
First decision: November 24, 2022
Revised: November 27, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 15, 2023
Processing time: 57 Days and 4 Hours
Older patients represent a unique subgroup of the cancer patient population, for which the role of cancer therapy requires special consideration. However, the outcomes of radiation therapy (RT) in elderly patients with pancreatic ductal ad
To explore the use and effectiveness of RT in the treatment of elderly patients wi
Data from patients with PDAC aged ≥ 65 years between 2004 and 2018 were collected from the Surveillance, Epidemiology, and End Results database. Multivariate logistic regression analysis was performed to determine factors ass
A total of 12245 patients met the inclusion criteria, of whom 2551 (20.8%) were treated with RT and 9694 (79.2%) were not. The odds of receiving RT increased with younger age, diagnosis in an earlier period, primary site in the head, loc
RT improved the outcome of elderly patients with PDAC, particularly those aged 65 to 80 years, in regional and distant stages, with no surgery, and who received chemotherapy. Further prospective studies are warranted to validate our results.
Core Tip: Older patients represent a unique subgroup of the cancer patient population, for which the role of cancer therapy requires special consideration. The effects of radiation therapy (RT) on the outcomes of elderly patients with pancreatic ductal adenocarcinoma (PDAC) are not well-defined in the literature. Herein, data were extracted from the Surveillance, Epidemiology, and End Results database to identify factors associated with RT administration and explore the impact of RT on survival in elderly patients with PDAC. This study highlights the survival benefit of RT in elderly patients with PDAC on a larger population scale and proposes possible obstacles to accessing treatment for elderly patients with PDAC.
- Citation: Cao BY, Wang QQ, Zhang LT, Wu CC, Tong F, Yang W, Wang J. Survival benefits and disparities in radiation therapy for elderly patients with pancreatic ductal adenocarcinoma. World J Gastrointest Oncol 2023; 15(1): 155-170
- URL: https://www.wjgnet.com/1948-5204/full/v15/i1/155.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i1.155
Pancreatic ductal adenocarcinoma (PDAC) is the 12th most common malignancy and the 7th most common cause of cancer-related deaths worldwide[1]. The incidence of PDAC increases with age and is more common in older adults. According to the World Health Organization (WHO), the number of elderly individuals with PDAC will increase as life expectancy increases[2]. Approximately 70% of cancers, including PDAC, are projected to be diagnosed in adults over 65 years of age by 2030[3].
Surgery is the only treatment option for this disease; however, only 10%–20% of patients are eligible for surgical resection[4]. Elderly patients with PDAC are frequently debilitated at baseline because of poor nutrition, pain, and jaundice, along with other symptoms; consequently, the majority of this population is medically inoperable or refuses to undergo surgery. Furthermore, intensive chemotherapy cannot be tolerated by some elderly patients due to their poor performance status; for these individuals, radiation therapy (RT) is considered a treatment option with a variety of goals (definitive, neoadjuvant, adjuvant, or palliative)[5]. Recent advances in computational modeling and medical imaging have enabled more precise treatment administration and decreased toxicity[6]. These advances are expected to continue to accelerate following a "double-exponential” growth pattern in the coming years. Recently, RT has become an increasingly popular nonsurgical treatment for multiple types of cancer in the elderly[7-9].
Elderly patients, defined by the WHO as those ≥ 65 years of age, represent a unique patient population for whom anticancer treatment requires special consideration. However, this population is under-represented in clinical trials because patients often have comorbid illnesses that exclude them from participation[10,11]. The management of these conditions remains largely unknown and is primarily extrapolated from retrospective studies including younger patients with small sample sizes. To date, no randomized studies on the utilization of RT in elderly patients with PDAC have been published, and the survival impact of RT in these patients has not yet been clarified.
To further investigate the survival benefits and disparities in RT for elderly patients with PDAC, data from the Surveillance, Epidemiology, and End Results (SEER) database were used to identify factors associated with RT administration and to explore the impact of RT on survival in PDAC patients over 65 years of age. This study highlights the survival benefit of RT in elderly patients with PDAC on a larger population scale and proposes potential obstacles to accessing treatment for elderly patients with PDAC.
PDAC cases were identified as those pathologically diagnosed between 2004 and 2018 as having primary malignant tumors of the pancreas using the International Classification of Diseases for Oncology, Third Edition histology codes 8140 and 8500 from the SEER program of the National Cancer Institute. The inclusion criteria were as follows: (1) Age ≥ 65 years; (2) Pathologic diagnosis of PDAC; and (3) Diagnosis of PDAC between 2004 and 2018. Samples were excluded based on the following criteria: (1) Diagnosis at autopsy or via death certificates; (2) Missing cancer-specific death classification; (3) Unknown survival time or survival < 1 mo; (4) Non-primary tumor or more than one primary tumor present; (5) Unknown radiation receipt information; and (6) Unknown follow-up information or incomplete demographic or clinical characteristics information. The detailed patient selection process is shown in Figure 1. All patient data in this study were collected from the SEER database using SEER*Stat v8.3.8 software (seer.cancer.gov/seerstat). The SEER Research Plus Data Agreement was signed and a license to analyze the study data was obtained in November 2021 (username: 15159-Nov2020).
Demographic, clinicopathological, and therapeutic information was extracted along with survival information. Demographic characteristics included age at diagnosis, sex, race, year of diagnosis, and marital status. Clinicopathological features included the primary tumor site, SEER stage, tumor size, node status, and histological grade. Treatments included surgery at the primary site, radiotherapy, and chemotherapy. The survival information included survival months, survival months flag, vital status, and cancer-specific death classification. Patients were classified by age at diagnosis into two groups (65-80 years or > 80 years) based on classifications used in previous studies[12,13] and our clinical practice. Tumor size was divided into three groups according to the T classification of the American Joint Committee on Cancer 8th edition (< 2 cm, 2-4 cm, or > 4 cm). Diagnoses between 2004 and 2010 were considered the earlier period, and those from 2010 to 2018 were defined as the latter period. SEER stages were classified as localized-, regional-, or distant-stage diseases.
The primary endpoint was overall survival (OS), and the secondary endpoint was cancer-specific survival (CSS). OS was defined as the time interval from the first PDAC diagnosis to death for any reason or the last follow-up. CSS was defined as the time interval from the diagnosis of PDAC to PDAC-related death or the last follow-up. Follow-up was initiated at the time of diagnosis, and all enrolled patients were effectively followed up. The final follow-up period ended on November 31, 2018.
The patients were divided into RT and non-RT groups. All categorical variables were represented as frequencies with percentages (%) and compared using the chi-square test. Propensity score matching (PSM) was performed to minimize the effects of potential confounding factors. To maximally inform the propensity of the dependent variable, all baseline characteristics except for the use of RT were included in the propensity score model. The variables included age at diagnosis, sex, race, year of diagnosis, marital status, primary site, SEER stage, tumor size, node status, histology grade, surgery to the primary site, and chemotherapy. Propensity scores were estimated using logistic regression modeling, with the receipt of RT as the dependent variable. In a nearest-neighbor matching algorithm without replacement, patients who received RT and those who did not were matched 1:1 using a caliper size of 0.05 times the standard deviation of the propensity. PSM was performed using the “Matchit” package in R software (version 4.0.1, The R Foundation for Statistical Computing).
Multivariate logistic regression analyses were performed to identify factors associated with RT administration. Univariate and multivariate analyses of OS and CSS were conducted using Cox proportional hazard regression before and after PSM. The covariates that had a P value < 0.05 in the univariate analysis were selected for further multivariate analysis. Survival curves were estimated using the Kaplan–Meier method and compared using the log-rank test. Subgroup analyses were performed using univariate Cox regression. All statistical analyses were conducted using R software (version 4.0.1; http://www.r-project.org). A two-tailed P value of < 0.05 was considered statistically significant.
Between January 2004 and December 2018, 20690 patients aged ≥ 65 years with PDAC were identified in the SEER database. In total, 12245 patients met the inclusion and exclusion criteria for the study. The patients were separated into two groups based on whether they underwent RT. Before PSM, there were 2551 cases of RT and 9694 cases of non-RT; after PSM, there were 2250 cases in each group. Table 1 summarizes the demographic and clinical features of patients before and after PSM. Before PSM, the RT group contained more younger, female, and married patients, had a higher proportion of patients with a primary site in the head, a higher proportion of patients with well or moderately differentiated tumors, a higher percentage of patients with positive node status and larger tumors (2–4 cm), and patients were more likely to undergo surgery and chemotherapy than the non-RT group. After PSM, all baseline covariates were well-balanced between the RT and non-RT groups (Table 1).
| Variables | Subgroups | Before propensity score matching, n (%) of patients (n = 12245) | After propensity score matching, n (%) of patients (n = 4500) | ||||
| RT | Non-RT | P value | RT | Non-RT | P value | ||
| All parents | 9694 (79.2) | 2551 (20.8) | 2250 (50) | 2250 (50) | |||
| Age (yr) | 65-80 | 2160 (84.7) | 7423 (76.6) | < 0.001 | 1884 (83.7) | 1857 (82.5) | 0.301 |
| > 80 | 391 (15.3) | 2271 (23.4) | 366 (16.3) | 393 (17.5) | |||
| Year of diagnosis | 2004-2010 | 1329 (52.1) | 5195 (53.6) | 0.186 | 1185 (52.7) | 1184 (52.6) | 1.00 |
| 2011-2018 | 1222 (47.9) | 4499 (46.4) | 1065 (47.3) | 1066 (47.4) | |||
| Sex | Female | 1166 (45.7) | 3203 (33.0) | < 0.001 | 898 (39.9) | 875 (38.9) | 0.502 |
| Male | 1385 (54.3) | 6491 (67.0) | 1352 (60.1) | 1375 (61.1) | |||
| Race | White | 2021 (79.2) | 7687 (79.3) | 0.713 | 1784 (79.3) | 1789 (79.5) | 0.916 |
| Black | 249 (9.8) | 983 (10.1) | 218 (9.7) | 210 (9.3) | |||
| Other | 281 (11.0) | 1024 (10.6) | 248 (11.0) | 251 (11.2) | |||
| Marital status | Married | 1586 (62.2) | 5384 (55.5) | < 0.001 | 1377 (61.2) | 1359 (60.4) | 0.604 |
| Unmarried | 965 (37.8) | 4310 (44.5) | 873 (38.8) | 891 (39.6) | |||
| Primary site | Head | 1737 (68.1) | 5414 (55.8) | < 0.001 | 1509 (67.1) | 1536 (68.3) | 0.635 |
| Body/tail | 503 (19.7) | 2739 (28.3) | 452 (20.1) | 428 (19.0) | |||
| Other | 311 (12.2) | 1541 (15.9) | 289 (12.8) | 286 (12.7) | |||
| Histology grade | Grade I-II | 692 (27.1) | 1882 (19.4) | < 0.001 | 579 (25.7) | 578 (25.7) | 0.952 |
| Grade III-IV | 472 (18.5) | 1344 (13.9) | 406 (18.0) | 414 (18.4) | |||
| Unknow | 1387 (54.4) | 6468 (66.7) | 1265 (56.2) | 1258 (55.9) | |||
| Node status | Negative | 1457 (57.1) | 6047 (62.4) | < 0.001 | 1295 (57.6) | 1328 (59.0) | 0.333 |
| Positive | 1094 (42.9) | 3647 (37.6) | 955 (42.4) | 922 (41.0) | |||
| Tumor size (cm) | < 2 | 229 (9.0) | 909 (9.4) | < 0.001 | 195 (8.7) | 207 (9.2) | 0.821 |
| 2-4 | 1491 (58.4) | 5076 (52.4) | 1317 (58.5) | 1310 (58.2) | |||
| > 4 | 831 (32.6) | 3709 (38.3) | 738 (32.8) | 733 (32.6) | |||
| SEER stage | Localized | 320 (12.5) | 1019 (10.5) | < 0.001 | 285 (12.7) | 306 (13.6) | 0.606 |
| Regional | 1813 (71.1) | 3726 (38.4) | 1547 (68.8) | 1540 (68.4) | |||
| Distant | 418 (16.4%) | 4949 (51.1) | 418 (18.6) | 404 (18.0) | |||
| Surgery | No | 1592 (62.4) | 7622 (78.6) | < 0.001 | 1439 (64.0) | 1466 (65.2) | 0.418 |
| Yes | 959 (37.6) | 2072 (21.4) | 811 (36.0) | 784 (34.8) | |||
| Chemotherapy | No | 297 (11.6) | 4304 (44.4) | < 0.001 | 297 (13.2) | 310 (13.8) | 0.601 |
| Yes | 2254 (88.4) | 5390 (55.6) | 1953 (86.8) | 1940 (86.2) | |||
In the multivariate logistic regression model evaluating the factors associated with RT receipt (Table 2), greater tumor size and receiving chemotherapy were associated with RT administration, while older age, latter period of diagnosis, a primary site in the body or tail, and distant disease were associated with RT not being administered. Patients aged > 80 had a significantly lower likelihood of receiving RT [odds ratio (OR): 0.84, 95% confidence interval (CI): 0.74–0.97; P = 0.014]. Patients diagnosed between 2011 and 2018 had a lower likelihood of receiving RT (OR: 0.47, 95%CI: 0.42–0.52; P < 0.001), as did patients with a primary site in the body or tail (OR: 0.87, 95%CI: 0.77–0.99; P = 0.032). RT was less likely to be administered to patients with distant metastasis (OR: 0.17, 95%CI: 0.14-0.21; P < 0.001). Patients who had received chemotherapy were more likely to receive RT (OR: 7.05, 95%CI: 6.14–8.10; P < 0.001). The likelihood of receiving RT did not differ according to sex, race, marital status, histological grade, node status, or surgery.
| Variables | Subgroups | Odds ratio (95%CI) | P value |
| Age (yr) | 65-80 | Reference | |
| > 80 | 0.84 (0.74-0.97) | 0.014 | |
| Year of diagnosis | 2004-2010 | Reference | |
| 2011-2018 | 0.47 (0.42-0.52) | < 0.001 | |
| Sex | Female | Reference | |
| Male | 1.01 (0.91-1.11) | 0.909 | |
| Race | White | Reference | |
| Black | 1.1(0.93-1.3) | 0.25 | |
| Other | 0.90 (0.77-1.05) | 0.19 | |
| Marital status | Married | Reference | |
| Unmarried | 0.92 (0.83-1.03) | 0.144 | |
| Primary site | Head | Reference | |
| Body/tail | 0.87 (0.77-0.99) | 0.032 | |
| Other | 0.85 (0.73-0.98) | 0.031 | |
| Histology grade | Grade I-II | Reference | |
| Grade III-IV | 0.95(0.82-1.11) | 0.55 | |
| Unknow | 1(0.87-1.16) | 0.948 | |
| Node status | Negative | Reference | |
| Positive | 0.99 (0.89-1.11) | 0.864 | |
| Tumor size (cm) | < 2 | Reference | |
| 2-4 | 1.24 (1.04-1.48) | 0.016 | |
| > 4 | 1.23 (1.02-1.48) | 0.033 | |
| SEER stage | Localized | Reference | |
| Regional | 1.01 (0.86-1.19) | 0.891 | |
| Distant | 0.17 (0.14-0.21) | < 0.001 | |
| Surgery | No | Reference | |
| Yes | 1.02 (0.88-1.17) | 0.804 | |
| Chemotherapy | No | Reference | |
| Yes | 7.05 (6.14-8.1) | < 0.001 |
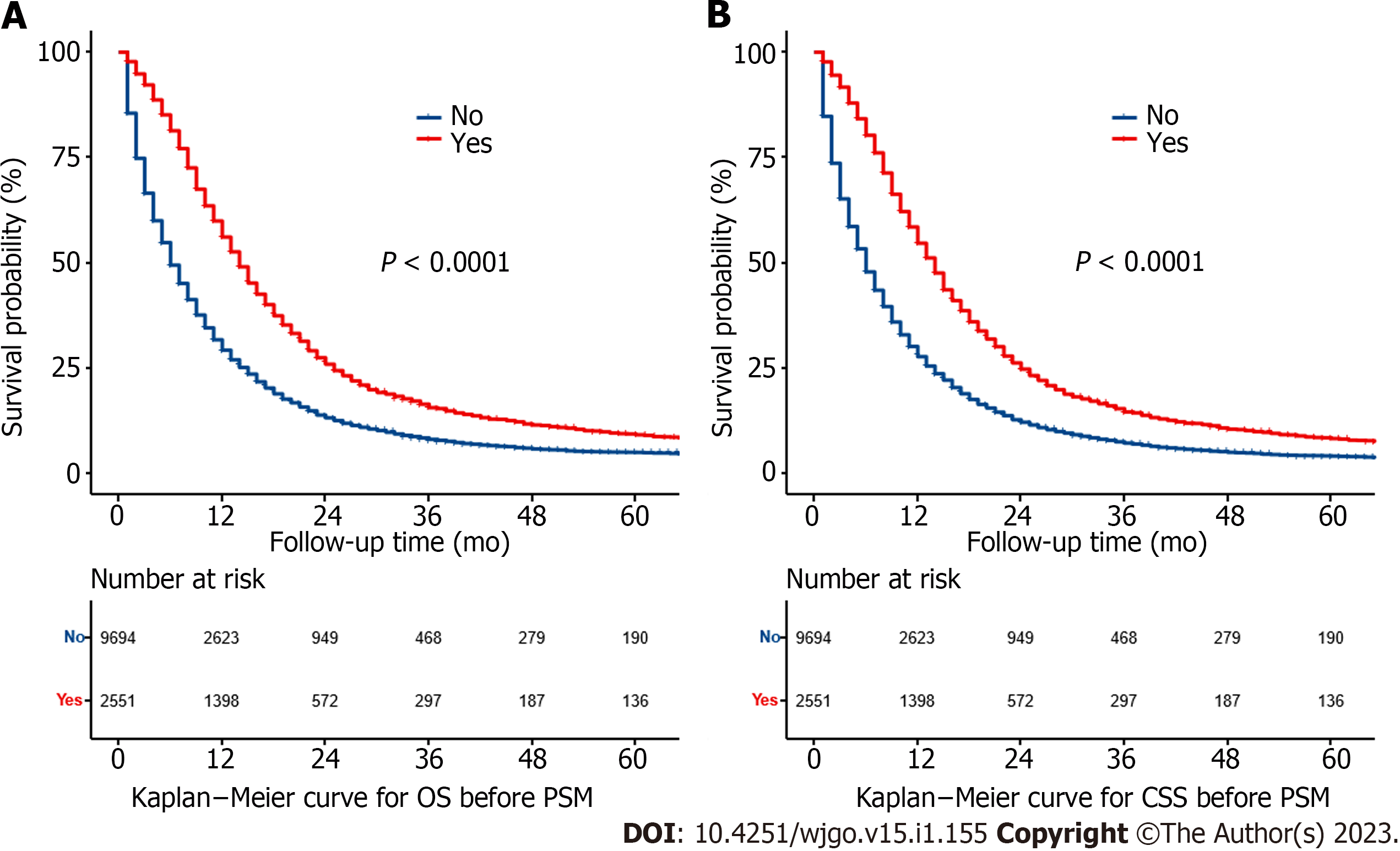
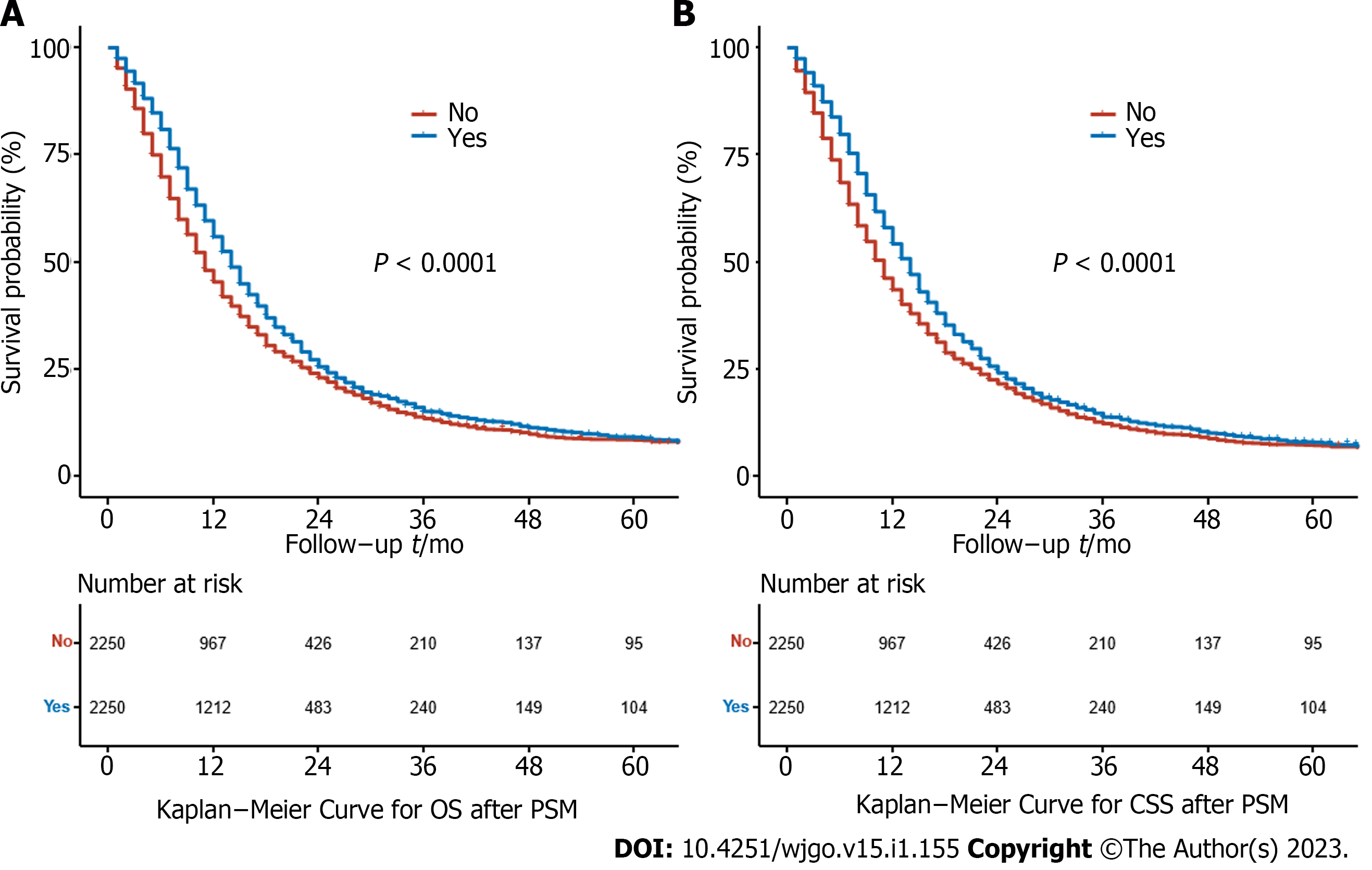
Before PSM, the median OS was 14.0 mo (95%CI: 13.5–14.5 mo) in the RT group and 6.0 mo (95%CI: 5.8–6.2 mo) in the non-RT group. The 5-year OS rates were 8.34% (95%CI: 7.20%–9.67%) and 4.16% (95%CI: 3.70%–4.68%) for the RT and non-RT groups, respectively (P < 0.0001) (Figure 2A). The median CSS was 14.0 mo (95%CI: 13.4–14.6 mo) in the RT group and 6.0 mo (95%CI: 5.8–6.2 mo) in the non-RT group. The 5-year CSS rates were 9.26% (95%CI: 8.02%–10.70%) and 5.01% (95%CI: 4.48%–5.60%) in the RT and non-RT groups, respectively (P < 0.0001) (Figure 2B). RT significantly improved survival even after PSM. The median OS for the two groups was 14.0 mo (95%CI: 13.4–14.6 mo) and 11.0 mo (95%CI: 10.4–11.6 mo), respectively, and the 5-year OS rates were 8.1% (95%CI: 31.9%–51.4%) and 7.3% (95%CI: 14.5%–30.5%) for the RT and non-RT groups, respectively (P < 0.0001) (Figure 3A). The median CSS was 14.0 mo (95%CI: 13.4–14.6 mo) and 11.0 mo (95%CI: 10.4–11.6 mo), respectively, and the 5-year CSS rates were 9.10% (95%CI: 7.76%–10.70%) and 8.62% (95%CI: 7.33%–10.10%) for the RT and non-RT groups, respectively (P < 0.0001) (Figure 3B).
After PSM, univariate analysis revealed that RT, age, year of diagnosis, marital status, primary site, histological grade, SEER stage, node status, tumor size, surgery at the primary site, and chemotherapy were all significantly associated with OS and CSS. Other variables had no effect on OS or CSS in elderly patients with PDAC. In the multivariable analysis, RT, histological grade, SEER stage, node status, tumor size, surgery at the primary site, and chemotherapy were all statistically significant. RT was found to be an independent predictor of both OS [hazard ratio (HR): 0.818, 95%CI: 0.768–0.872, P < 0.001] and CSS (HR: 0.816, 95%CI: 0.765–0.871, P < 0.001) (Table 3). The results of univariate and multivariate analyses based on unmatched data were consistent with those predicted from matched data, except for race, which was identified as an independent prognostic factor in both OS and CSS (Supplementary Table 1).
| Variables | Subgroups | Overall survival | Cancer-specific survival | ||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | ||
| Age (yr) | 65-80 | Reference | Reference | Reference | Reference | ||||
| > 80 | 1.486 (1.367-1.615) | < 0.001 | 0.966 (0.852-1.095) | 0.588 | 1.473 (1.352-1.605) | < 0.001 | 0.953 (0.838-1.084) | 0.466 | |
| Year of diagnosis | 2004-2010 | Reference | Reference | Reference | Reference | ||||
| 2011-2018 | 0.825 (0.774-0.880) | < 0.001 | 1.008 (0.894-1.137) | 0.893 | 0.833 (0.780-0.889) | < 0.001 | 1.024 (0.905-1.159) | 0.704 | |
| Sex | Female | Reference | Reference | ||||||
| Male | 0.997 (0.936-1.063) | 0.932 | 0.986 (0.924-1.052) | 0.67 | |||||
| Race | White | Reference | Reference | ||||||
| Black | 1.101 (0.988-1.227) | 0.082 | 1.073 (0.959-1.201) | 0.219 | |||||
| Other | 1.005 (0.907-1.114) | 0.919 | 1.006 (0.905-1.117) | 0.916 | |||||
| Marital status | Married | Reference | Reference | Reference | Reference | ||||
| Unmarried | 1.217 (1.141-1.299) | < 0.001 | 1.061 (0.993-1.133) | 0.08 | 1.226 (1.147-1.310) | < 0.001 | 1.065 (0.995-1.139) | 0.068 | |
| Primary site | Head | Reference | Reference | Reference | Reference | ||||
| Body/tail | 1.092 (1.006-1.185) | 0.034 | 1.008 (0.927-1.096) | 0.849 | 1.101 (1.013-1.197) | 0.024 | 1.012 (0.929-1.103) | 0.78 | |
| Other | 1.216 (1.106-1.336) | < 0.001 | 1.015 (0.922-1.118) | 0.756 | 1.233 (1.120-1.358) | < 0.001 | 1.024 (0.929-1.130) | 0.632 | |
| Histology grade | Grade I-II | Reference | Reference | Reference | Reference | ||||
| Grade III-IV | 1.344 (1.219-1.482) | < 0.001 | 1.367 (1.240-1.508) | < 0.001 | 1.332 (1.204-1.473) | < 0.001 | 1.355 (1.224-1.500) | < 0.001 | |
| Unknow | 1.965 (1.818-2.125) | < 0.001 | 1.100 (1.005-1.204) | 0.039 | 2.006 (1.851-2.173) | < 0.001 | 1.096 (0.999-1.203) | 0.052 | |
| Node status | Negative | Reference | Reference | Reference | Reference | ||||
| Positive | 0.835 (0.783-0.891) | < 0.001 | 1.209 (1.125-1.300) | < 0.001 | 0.837 (0.783-0.894) | < 0.001 | 1.227 (1.140-1.321) | < 0.001 | |
| Tumor size (cm) | < 2 | Reference | Reference | Reference | |||||
| 2-4 | 1.482 (1.317-1.668) | < 0.001 | 1.400 (1.242-1.578) | < 0.001 | 1.497 (1.325-1.692) | < 0.001 | 1.403 (1.239-1.587) | < 0.001 | |
| > 4 | 1.854 (1.639-2.098) | < 0.001 | 1.505 (1.322-1.712) | < 0.001 | 1.875 (1.651-2.130) | < 0.001 | 1.492 (1.306-1.705) | < 0.001 | |
| SEER stage | Localized | Reference | Reference | Reference | Reference | ||||
| Regional | 0.948 (0.860-1.045) | 0.28 | 1.124 (1.009-1.252) | 0.033 | 0.967 (0.874-1.070) | 0.512 | 1.147 (1.026-1.282) | 0.016 | |
| Distant | 2.058 (1.836-2.308) | < 0.001 | 1.723 (1.522-1.951) | < 0.001 | 2.154 (1.914-2.423) | < 0.001 | 1.782 (1.568-2.025) | < 0.001 | |
| Surgery | No | Reference | Reference | Reference | Reference | ||||
| Yes | 0.337 (0.314-0.363) | < 0.001 | 0.352 (0.320-0.387) | < 0.001 | 0.322 (0.299-0.347) | < 0.001 | 0.334 (0.303-0.368) | < 0.001 | |
| Radiation | No | Reference | Reference | Reference | Reference | ||||
| Yes | 0.850 (0.798-0.906) | < 0.001 | 0.818 (0.768-0.872) | < 0.001 | 0.851 (0.797-0.908) | < 0.001 | 0.816 (0.765-0.871) | < 0.001 | |
| Chemotherapy | No | Reference | Reference | Reference | Reference | ||||
| Yes | 0.469 (0.428-0.513) | < 0.001 | 0.534 (0.485-0.589) | < 0.001 | 0.467 (0.425-0.512) | < 0.001 | 0.530 (0.480-0.586) | < 0.001 | |
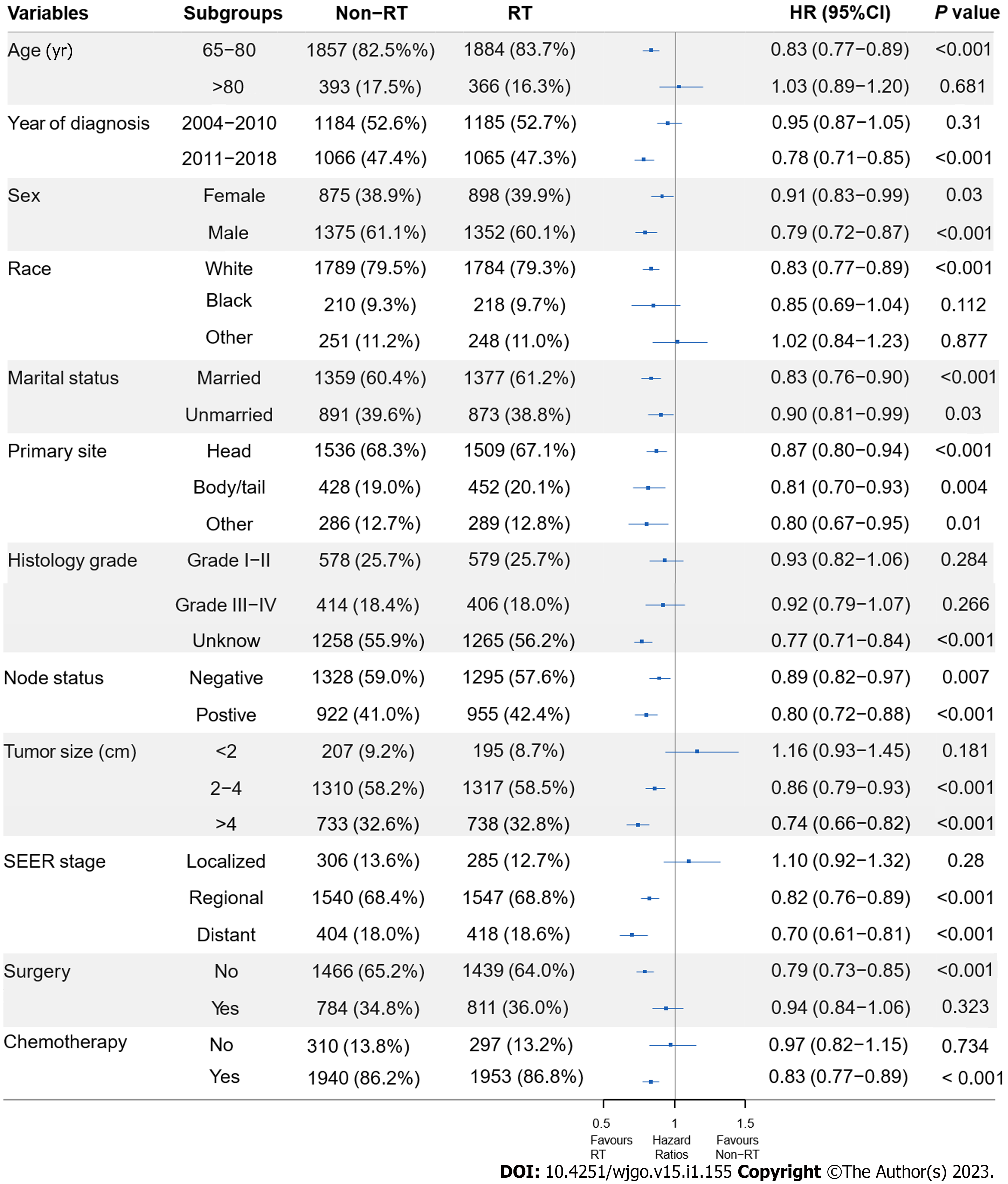
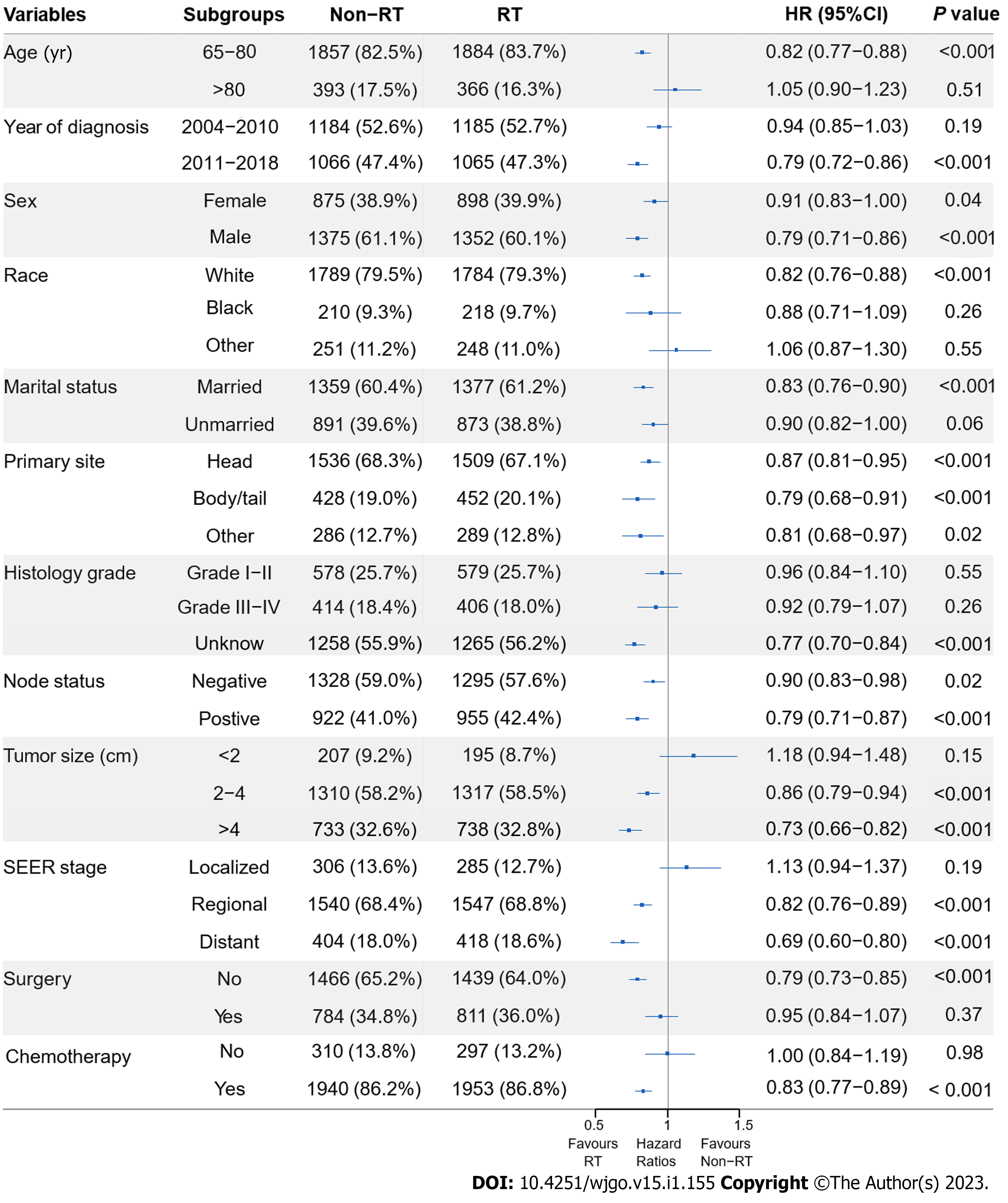
To assess the effect of RT in the subpopulations, subgroup analyses before and after PSM were performed. Before PSM, RT benefited patients in all subgroups, except RT failed to significantly improve OS (P = 0.092) and CSS (P = 0.215) in patients with localized disease. The HRs and associated CIs from all subgroup analyses are graphically summarized in forest plots in Supplemen
Using data from a relatively large sample size of patients from the SEER database, we found a survival benefit in elderly patients who received RT for PDAC. We showed that elderly patients with PDAC who were treated with RT had longer median OS and CSS outcomes than those who did not receive RT. Before PSM, the RT group had superior OS (median OS: 14.0 vs 6.0 mo, P < 0.0001) and CSS (median CSS: 14.0 vs 6.0 mo, P < 0.0001) with an 8-mo median survival advantage over the non-RT group. After PSM, the RT group still outperformed the non-RT group in terms of OS (median OS: 14.0 vs 11.0 mo, P < 0.0001) and CSS (median CSS: 14.0 vs 11.0 mo, P < 0.0001). Even after PSM, RT resulted in a 3-mo increase in median survival time. Furthermore, we performed multivariate Cox regression analysis to determine the independent impact of RT on survival. Before PSM, the RT group was found to have a superior OS (HR: 0.862; 95%CI: 0.819–0.908, P < 0.001) and CSS (HR: 0.867; 95%CI: 0.823–0.914, P < 0.001) with a lower mortality rate than the non-RT group. After PSM, the RT group consistently performed better than the non-RT group in terms of OS (HR: 0.818; 95%CI: 0.768–0.872, P < 0.001) and CSS (HR: 0.816; 95%CI: 0.765–0.871, P < 0.001). After excluding potential confounding factors, the above multivariate analysis results indicated that elderly PDAC patients have improved survival after receiving RT. Studies evaluating the use of RT in PDAC have been reviewed and discussed in the context of treating older patients[14-23]; Table 4 summarizes the characteristics and outcomes of these findings. The results of these studies indicate that RT appears to be tolerable in older patients and can be considered a viable treatment option for PDAC in this population, further confirming the conclusions of our study.
| Ref. | No. of patients | Median age (range), years | Clinical stage | Median dose (range), Gy | Median follow-up (months) | Technique | Treatment modality | OS1 | Toxicities (%) |
| Davila et al[14], 2009 | 553 | > 65 | I-III | NR | NR | NR | Surg: 51% | NR | NR |
| Surg + CRT: 31% | |||||||||
| Surg + CT: 9.0% | |||||||||
| Surg + RT: 9.0% | |||||||||
| Miyamoto et al[17], 2010 | 42 | 78 (75–90) | I-III | 48.1 (38.9–57.3) | 11.7 (2.9–41.7) | 3D-CRT/IMRT | CRT: 57.1% | mOS: 8.6 mo2 | Acute (G ≥ 3): 49.0 |
| Surg + CRT: 40.5% | mOS: 20.6 mo3 | Late (G ≥ 2): NR | |||||||
| CRT + Surg: 2.4% | |||||||||
| Horowitz et al[16], 2011 | 49 | 79 (75–90) | I-II | 50.0 | 19 (2.6–57.4) | NR | Surg + CRT: 29.5% | mOS: 22.6 mo | NR |
| Surg: 70.5% | 5 yr-OS: 49.7% | ||||||||
| Kim et al[19], 2013 | 26 | 86 (80–91) | I-IV | 24 (22–36) | 11.6 (3.5–24.6) | SBRT | RT ± CT: 100% | LC: 11.5 mo | Acute (G ≥ 3): 0.0 |
| MFS: 8.4 mo | Late (G ≥ 2): 0.0 | ||||||||
| mOS: 7.6 mo | |||||||||
| 2 yr-OS: 6.6% | |||||||||
| Herman et al[20], 2015 | 33 | > 65 | NR | 33 | 13.9 (3.9–45.2) | SBRT | CT+ RT + CT ± Surg: 89.8% | mOS: 11.0 mo | NR |
| RT + CT ± Surg: 10.2% | 2 yr-OS: 20% | ||||||||
| Hayman et al[15], 2015 | 53 | 77 (> 70) | NR | 50 (43.2–63.0) | 36 | NR | Surg: 30.4% | mOS: 21.1 mo | NR |
| Surg + CT: 22.3% | |||||||||
| Surg + CRT: 47.3% | |||||||||
| Yechieli et al[21], 2017 | 20 | 83.2 (77-90) | I-II | 35 (30–36) | 5.3 (2.3–26.2) | SBRT | RT: 100% | mOS: 6.4 mo | Acute (G ≥ 3): 0.0 |
| 2 yr-OS: 7.7% | Late (G ≥ 2): 15.0 | ||||||||
| Zhu et al[22], 2017 | 417 | 73 (65–90) | II-IV | NR (30–46.8) | 11 (4–28) | SBRT | CT + RT: 11.2% | LC: 10.0 mo | Acute (G ≥ 3): 0.5 |
| Surg + RT: 7.7% | PFS: 8.0 mo | Late (G ≥ 3): 0.0 | |||||||
| RT: 81.1% | MFS: 9.5 mo | ||||||||
| mOS: 10.0 mo | |||||||||
| 1 yr-OS: 35.5% | |||||||||
| Frakes et al[18], 2017 | 555 | 75 (70–88) | I-III | NR | NR | NR | Surg + RT: 100% | mOS: 19.0 mo | NR |
| Sutera et al[23], 2018 | 145 | 79 (70.1-90.3) | I-III | 36 Gy/3f or 24 Gy/f | 12.3 (6.0–23.3) | SBRT | Surg + RT: 30.3% | mOS: 40.0 mo | |
| CT + RT: 53.8% | 1 yr-LC: 72% | Acute (G ≥ 3): 0.7 | |||||||
| 2 yr-LC: 63% | Acute (G ≥ 2): 4.1 | ||||||||
| RT: 15.9% | 1 yr-MFS: 62% | Late (G ≥ 3): 1 | |||||||
| 2 yr-MFS: 47% | Late (G ≥ 2): 2 |
In addition, lower histological grade, early SEER stage, negative node status, smaller tumor size, surgery at the primary site, and receiving chemotherapy were identified as independent favorable prognostic predictors for OS and CSS in elderly PDAC patients in the matched population. Histological grade, tumor stage, node status and tumor size are histological and biological characteristics of malignant neoplasms, which have been confirmed as prognostic features for OS in multiple malignancies, including PDAC[24-26]. Our results were consistent with those of previous studies[12,23,27], which have suggested that older patients are less likely than younger patients to accept or be prescribed surgical treatment that might extend their lives. Our study demonstrated that resection of the primary tumor could prolong survival in elderly patients with PDAC. A previous SEER database analysis revealed that elderly patients with PDAC who underwent cancer-directed surgical procedures had a higher survival rate than those who did not undergo surgery[28]. In recent years, with more methods to optimize pancreatic cancer surgery using Warshaw technology and minimally invasive surgery, cancer-directed surgical treatment options have been recommended for carefully selected elderly patients with PDAC[4]. Chemotherapy is the most common form of treatment for PDAC, including PDAC in elderly patients. Resection with or without subsequent chemotherapy is the sta
We performed subgroup analysis and discovered that most subgroups could benefit from RT, including those aged 65 to 80 years, diagnosed in the latter period, with regional or distant disease, tumor size of 2 to 4 cm or greater than 4cm, no surgery, and receiving chemotherapy. Subgroup analysis stratified by age at diagnosis revealed that RT may be beneficial for older patients, particularly those aged 65 to 80 years. This finding could be because patients aged 65 to 80 years have a higher tolerance for RT and are in better general physical condition for consequential treatment than those older than 80 years[33]. Patients with PDAC are frequently incapacitated at the outset owing to malnutrition, discomfort, jaundice, or other symptoms. Only 20% of patients with surgically resectable disease receive radical surgery for cure[34]. Elderly individuals are more likely to require inpatient nursing home care after surgery and have a higher surgical mortality rate[35], even though high-volume facilities and less invasive procedures can enhance outcomes. A subset of older individuals who are deemed ineligible for surgery or refuse surgery due to poor performance status or comorbidities is treated with definitive chemoradiation in clinical settings. Thus, in the current study, patients who did not have surgery or who received chemotherapy were more likely to benefit from RT. Similarly, patients with regional disease were more prone to have involvement or direct extension invasion of peripheral organs and major vessels, as well as a lower chance of undergoing curative surgery, resulting in a greater benefit from RT in subgroups of regional disease. For patients with metastatic disease, the role of RT in achieving local control and symptom relief has been established by current guidelines, including the management of obstruction, analgesic-resistant pain, and bleeding[5]. Furthermore, we discovered that patients with larger tumors benefited more from RT than those with smaller tumors did. One probable explanation for this benefit is that patients with larger tumors were more likely to present with involvement or direct extension invasion to peripheral organs and major arteries, and were more likely to receive definitive radiation or chemoradiotherapy. Patients diagnosed in the latter period benefited significantly more from RT than those diagnosed in the earlier period. This benefit could be attributed to the growing implementation of modern radiation treatments, thus improving the protection of organs at risk and decreasing side effects[6].
The elderly population is a distinct subgroup of cancer patients, for whom the role of RT requires special consideration. To our knowledge, this is the first population-based analysis utilizing PSM to assess the survival benefits of RT in older patients with PDAC. In this study, both multivariate regression and propensity score-matched analyses showed that RT could improve survival in patients with PDAC over 65 years of age. The favorable effect on survival found in the comprehensive SEER database highlights the necessity of RT in the treatment of elderly patients with PDAC. Based on these findings, older individuals should not reject RT on the sole basis of clinical concerns for toxicity, as RT may be a viable therapeutic choice for PDAC even in elderly patients. Further research and trials with samples of older patients are required to determine the risk/benefit ratio of various therapeutic scenarios.
This study had several limitations. First, even when using the PSM approach to balance the baseline, selection bias in the retrospective analysis may not be eliminated due to unmeasured confounders, as with any other observational study. Second, in the SEER database, treatment is defined as being used during the first course of cancer-directed therapy without detailed data about chemotherapy and the dose or duration of RT; thus, the current study aimed to qualitatively and quantitatively describe the role of RT in elderly patients with PDAC. Incorporating additional information on chemotherapy, RT, and treatment modalities would be advantageous for investigating the effects of RT on survival in elderly patients with PDAC. Investigation of the impact of the type, dose, timing, intent, or method used in RT for elderly patients with PDAC may also be aided by data from randomized clinical trials.
In summary, the current study demonstrated that elderly patients with PDAC who were treated with RT had improved OS and CSS compared with patients who were not treated with RT. This study adds to the growing literature on retrospective studies of the role on RT in elderly patients with PDAC and highlights the need for a large multicenter randomized trial to further understand this subject.
Pancreatic ductal adenocarcinoma (PDAC) is primarily a disease of the elderly, with a median age at diagnosis of 70 years. Elderly patients represent a unique subgroup of the cancer patient population, for which the role of cancer therapy requires special consideration.
Radiation therapy (RT) plays an evolving and pivotal role in providing optimal care for patients with PDAC. However, studies evaluating the use and effectiveness of RT for treating PDAC in older patients are scarce.
To explore the use and effectiveness of RT in the treatment of elderly patients with PDAC in clinical practice.
Data from patients with PDAC aged ≥ 65 years between 2004 and 2018 were collected from the Surveillance, Epidemiology, and End Results database. Multivariate logistic regression analysis was performed to determine factors associated with RT administration. Overall survival (OS) and cancer-specific survival (CSS) were evaluated using the Kaplan-Meier method with the log-rank test. Univariate and multivariate analyses with the Cox proportional hazards model were used to identify prognostic factors for OS. Propensity score matching (PSM) was applied to balance the baseline characteristics between the RT and non-RT groups. Subgroup analyses were performed based on clinical characteristics.
A total of 12245 patients met the inclusion criteria, with 2551 (20.8%) patients who were treated with RT and 9694 (79.2%) who were not. The odds of receiving RT increased with younger age, diagnosis in the earlier period, primary site in the head, localized disease, greater tumor size, and receiving chemotherapy (all P < 0.05). Before PSM, the RT group had better outcomes than did the non-RT group [median OS: 14.0 vs 6.0 mo; hazard ratio (HR) for OS: 0.862, 95% confidence interval (CI): 0.819–0.908, P < 0.001; and HR for CSS: 0.867, 95%CI: 0.823–0.914, P < 0.001]. After PSM, the survival benefit associated with RT remained comparable (median OS: 14.0 vs 11.0 mo; HR for OS: 0.818, 95%CI: 0.768–0.872, P < 0.001; and HR for CSS: 0.816, 95%CI: 0.765–0.871, P < 0.001). Subgroup analysis revealed that the survival benefits (OS and CSS) of RT were more significant in patients aged 65 to 80 years, in regional and distant stages, with no surgery, and receiving chemotherapy.
The current study demonstrated that elderly PDAC patients who were treated with RT had improved OS and CSS when compared to those patients who were not treated with RT.
This study adds to the growing literature on retrospective studies on the role of RT in elderly patients with PDAC and highlights the need for a large multicenter randomized trial to further understand this subject.
We sincerely appreciate the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in establishing the SEER database.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maehira H, Japan; Ott G, Germany; Radhakrishnan VS, India S-Editor: Fan JR L-Editor: A P-Editor: Cai YX
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64628] [Article Influence: 16157.0] [Reference Citation Analysis (176)] |
| 2. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20512] [Article Influence: 2051.2] [Reference Citation Analysis (20)] |
| 3. | Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758-2765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1195] [Cited by in RCA: 1318] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 4. | Strobel O, Neoptolemos J, Jäger D, Büchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol. 2019;16:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 603] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 5. | Tempero MA, Behrman S, Ben-Josef E, Benson AB 3rd, Cameron JL, Casper ES, Hoffman JP, Karl RC, Kim P, Koh WJ, Kuvshinoff BW 2nd, Melvin WS, Muscarella P 2nd, Sasson AR, Shibata S, Shrieve DC, Talamonti MS, Tyler DS, Vickers SM, Warren RS, Willett C, Wolff RA; National Comprehensive Cancer Network. Pancreatic adenocarcinoma: Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2005;3:598-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Hall WA, Kamgar M, Erickson BA, Ponce SB, Tsai S, Nevalainen MT, Christians KK, George B, Dua KS, Khan AH, Evans DB, Azmi AS. Updates and new directions in the use of radiation therapy for the treatment of pancreatic adenocarcinoma: dose, sensitization, and novel technology. Cancer Metastasis Rev. 2021;40:879-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 7. | Kunkler IH, Audisio R, Belkacemi Y, Betz M, Gore E, Hoffe S, Kirova Y, Koper P, Lagrange JL, Markouizou A, Pfeffer R, Villa S; SIOG Radiotherapy Task Force. Review of current best practice and priorities for research in radiation oncology for elderly patients with cancer: the International Society of Geriatric Oncology (SIOG) task force. Ann Oncol. 2014;25:2134-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Quinn MA, Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, Heintz AP, Ngan HY, Pecorelli S. Carcinoma of the cervix uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95 Suppl 1:S43-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 443] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 9. | Sharma C, Deutsch I, Horowitz DP, Hershman DL, Lewin SN, Lu YS, Neugut AI, Herzog TJ, Chao CK, Wright JD. Patterns of care and treatment outcomes for elderly women with cervical cancer. Cancer. 2012;118:3618-3626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626-4631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 543] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 11. | Scher KS, Hurria A. Under-representation of older adults in cancer registration trials: known problem, little progress. J Clin Oncol. 2012;30:2036-2038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 328] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 12. | Lianyuan T, Deyu L, Haibo Y, Yadong D, Guanjing T. Clinical features and prognostic factors of elderly patients with metastatic pancreatic cancer: a population-based study. Aging (Albany NY). 2021;13:7133-7146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Mehtsun WT, McCleary NJ, Maduekwe UN, Wolpin BM, Schrag D, Wang J. Patterns of Adjuvant Chemotherapy Use and Association With Survival in Adults 80 Years and Older With Pancreatic Adenocarcinoma. JAMA Oncol. 2022;8:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Davila JA, Chiao EY, Hasche JC, Petersen NJ, McGlynn KA, Shaib YH. Utilization and determinants of adjuvant therapy among older patients who receive curative surgery for pancreatic cancer. Pancreas. 2009;38:e18-e25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Hayman TJ, Strom T, Springett GM, Balducci L, Hoffe SE, Meredith KL, Hodul P, Malafa M, Shridhar R. Outcomes of resected pancreatic cancer in patients age ≥70. J Gastrointest Oncol. 2015;6:498-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Horowitz DP, Hsu CC, Wang J, Makary MA, Winter JM, Robinson R, Schulick RD, Cameron JL, Pawlik TM, Herman JM. Adjuvant chemoradiation therapy after pancreaticoduodenectomy in elderly patients with pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2011;80:1391-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Miyamoto DT, Mamon HJ, Ryan DP, Willett CG, Ancukiewicz M, Kobayashi WK, Blaszkowsky L, Fernandez-del Castillo C, Hong TS. Outcomes and tolerability of chemoradiation therapy for pancreatic cancer patients aged 75 years or older. Int J Radiat Oncol Biol Phys. 2010;77:1171-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Frakes J, Mellon EA, Springett GM, Hodul P, Malafa MP, Fulp WJ, Zhao X, Hoffe SE, Shridhar R, Meredith KL. Outcomes of adjuvant radiotherapy and lymph node resection in elderly patients with pancreatic cancer treated with surgery and chemotherapy. J Gastrointest Oncol. 2017;8:758-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Kim CH, Ling DC, Wegner RE, Flickinger JC, Heron DE, Zeh H, Moser AJ, Burton SA. Stereotactic body radiotherapy in the treatment of pancreatic adenocarcinoma in elderly patients. Radiat Oncol. 2013;8:240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Herman JM, Chang DT, Goodman KA, Dholakia AS, Raman SP, Hacker-Prietz A, Iacobuzio-Donahue CA, Griffith ME, Pawlik TM, Pai JS, O'Reilly E, Fisher GA, Wild AT, Rosati LM, Zheng L, Wolfgang CL, Laheru DA, Columbo LA, Sugar EA, Koong AC. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128-1137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 367] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 21. | Yechieli RL, Robbins JR, Mahan M, Siddiqui F, Ajlouni M. Stereotactic Body Radiotherapy for Elderly Patients With Medically Inoperable Pancreatic Cancer. Am J Clin Oncol. 2017;40:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Zhu X, Li F, Ju X, Cao F, Cao Y, Fang F, Qing S, Shen Y, Jia Z, Zhang H. Prognostic role of stereotactic body radiation therapy for elderly patients with advanced and medically inoperable pancreatic cancer. Cancer Med. 2017;6:2263-2270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Sutera PA, Bernard ME, Wang H, Heron DE. Prognostic Factors for Elderly Patients Treated With Stereotactic Body Radiation Therapy for Pancreatic Adenocarcinoma. Front Oncol. 2018;8:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | He C, Cai Z, Zhang Y, Lin X. Prognostic Model to Predict Cancer-Specific Survival for Patients With Gallbladder Carcinoma After Surgery: A Population-Based Analysis. Front Oncol. 2019;9:1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Jiang Y, Zhang Q, Hu Y, Li T, Yu J, Zhao L, Ye G, Deng H, Mou T, Cai S, Zhou Z, Liu H, Chen G, Li G, Qi X. ImmunoScore Signature: A Prognostic and Predictive Tool in Gastric Cancer. Ann Surg. 2018;267:504-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 342] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 26. | Chen KH, Jiang YT, Zhao R, Sun YC, Zhu XD. Development and validation of prognostic nomograms in patients with ascending type of nasopharyngeal carcinoma: Retrospective study based on SEER database. Head Neck. 2022;44:2649-2659. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Kim SS, Lee S, Lee HS, Bang S, Park MS. Prognostic factors in patients with locally advanced or borderline resectable pancreatic ductal adenocarcinoma: chemotherapy vs. chemoradiotherapy. Abdom Radiol (NY). 2021;46:655-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Xie D, Qian B, Yang J, Peng X, Li Y, Hu T, Lu S, Chen X, Han Y. Can Elderly Patients With Pancreatic Cancer Gain Survival Advantages Through More Radical Surgeries? Front Oncol. 2020;10:598048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 29. | Gervaso L, Lordick F, Fazio N. Adjuvant Chemotherapy for Stage I Pancreatic Ductal Adenocarcinoma-Is It Based on Evidence or Clinical Wisdom? JAMA Oncol. 2021;7:1759-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Katz MHG, Shi Q, Meyers J, Herman JM, Chuong M, Wolpin BM, Ahmad S, Marsh R, Schwartz L, Behr S, Frankel WL, Collisson E, Leenstra J, Williams TM, Vaccaro G, Venook A, Meyerhardt JA, O'Reilly EM. Efficacy of Preoperative mFOLFIRINOX vs mFOLFIRINOX Plus Hypofractionated Radiotherapy for Borderline Resectable Adenocarcinoma of the Pancreas: The A021501 Phase 2 Randomized Clinical Trial. JAMA Oncol. 2022;8:1263-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 183] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 31. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5637] [Article Influence: 402.6] [Reference Citation Analysis (1)] |
| 32. | Heinemann V, Haas M, Boeck S. Systemic treatment of advanced pancreatic cancer. Cancer Treat Rev. 2012;38:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 33. | Higuera O, Ghanem I, Nasimi R, Prieto I, Koren L, Feliu J. Management of pancreatic cancer in the elderly. World J Gastroenterol. 2016;22:764-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (2)] |
| 34. | Grossberg AJ, Chu LC, Deig CR, Fishman EK, Hwang WL, Maitra A, Marks DL, Mehta A, Nabavizadeh N, Simeone DM, Weekes CD, Thomas CR Jr. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J Clin. 2020;70:375-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 319] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 35. | Frakes JM, Strom T, Springett GM, Hoffe SE, Balducci L, Hodul P, Malafa MP, Shridhar R. Resected pancreatic cancer outcomes in the elderly. J Geriatr Oncol. 2015;6:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |