Published online Jan 15, 2023. doi: 10.4251/wjgo.v15.i1.143
Peer-review started: November 14, 2022
First decision: November 30, 2022
Revised: December 6, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 15, 2023
Processing time: 57 Days and 8.6 Hours
Gastric cancer is one of the most common cancers worldwide, with a 5-year survival rate of only 20%. The age of onset of gastric cancer is in line with the general rule of cancer. Most of them occur after middle age, mostly between 40 and 60 years old, with an average age of about 50 years old, and only 5% of patients are under 30 years old. The incidence of male is higher than that of female.
To investigate the short-term efficacy and influencing factors of chemotherapy combined with irinotecan in patients with advanced gastric cancer.
Eighty patients with advanced gastric cancer who were treated in our hospital from January 2019 to January 2022 were selected. The patients were divided into an observation group (n = 40) and control group (n = 40) by the envelope method. The control group was given preoperative routine chemotherapy. The observation group was treated with irinotecan in addition to the chemotherapy given to the control group. The short-term efficacy of treatment in the two groups, as well as tumor marker levels and quality of life before and after treatment were evaluated.
The short-term treatment effect in the observation group was better than that in the control group (P < 0.05), and the total effective rate was 57.50%. The age and proportion of tumor node metastasis (TNM) stage IV patients with ineffective chemotherapy in the observation group were (65.12 ± 5.71) years and 52.94%, respectively, which were notably higher than those of patients with effective chemotherapy (P < 0.05), while the Karnofsky Performance Scale score was (67.70 ± 3.83) points, which was apparently lower than that of patients with effective chemotherapy (P < 0.05). After 3 mo of treatment, the SF-36 scale scores of physiological function, energy, emotional function, and mental health in the observation group were 65.12 ± 8.14, 54.76 ± 6.70, 47.58 ± 7.22, and 66.16 ± 8.11 points, respectively, which were considerably higher than those in the control group (P < 0.05). The incidence rates of grade III-IV diarrhea and grade III-IV thrombocytopenia in the observation group were 32.50% and 25.00%, respectively, which were markedly higher than those in the control group (P < 0.05).
Chemotherapy combined with irinotecan in patients with advanced gastric cancer has a good short-term efficacy and can significantly reduce serum tumor markers and improve the quality of life of patients. The efficacy may be affected by the age and TNM stage of the patients, and its long-term efficacy needs further study.
Core Tip: Gastric cancer is a malignant tumor of gastric mucosal epithelial cells with early symptoms not obvious, and most patients are advanced at the stage of diagnosis. Surgery, radiotherapy, and chemotherapy are often used in clinical treatment. Although chemotherapy is one of the main treatment options, there is no unified or standardized treatment method; therefore, it is particularly important to determine the effective treatment options for patients with advanced gastric cancer. This study explores the short-term efficacy and influencing factors of irinotecan combined with oxaliplatin and fluorouracil in patients with advanced gastric cancer.
- Citation: Wang JP, Du JL, Li YY. Short-term efficacy and influencing factors of conventional chemotherapy combined with irinotecan in patients with advanced gastric cancer. World J Gastrointest Oncol 2023; 15(1): 143-154
- URL: https://www.wjgnet.com/1948-5204/full/v15/i1/143.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i1.143
Gastric cancer is a malignant tumor of the gastric mucosal epithelium. Due to the insidious onset of gastric cancer, the early symptoms are not obvious. The main manifestations are abdominal pain and weight loss, and most patients are already in the advanced stage at the time of diagnosis. At that stage, cancer tissue can infiltrate into the submucosa or muscular layer and even pass through the muscular layer to the serosa. Surgery, radiotherapy, and chemotherapy are often used for clinical treatment[1,2]. Although chemotherapy is one of the main treatment options, there is no unified or standardized treatment; therefore, the identification of effective treatment options for patients with advanced gastric cancer is particularly important[3,4]. The conventional chemotherapy regimen consists of oxaliplatin combined with fluorouracil, where oxaliplatin is an anticancer drug with cytotoxic effects. Fluorouracil is a component of ribonucleic acid, which can play an anti-metabolite role[5,6]. However, studies have shown that treatment with only oxaliplatin combined with fluorouracil is not very effective in advanced gastric cancer[7]. Irinotecan is a semi-synthetic derivative of camptothecin and an S-phase cell cycle-specific antitumor drug that inhibits cancer cell proliferation[8]. Therefore, this study investigated the short-term efficacy and influencing factors of irinotecan combined with the conventional chemotherapy regimen of oxaliplatin and fluorouracil in advanced gastric cancer patients.
Eighty patients with advanced gastric cancer treated in our hospital from January 2019 to January 2022 were selected. Inclusion criteria[9]: (1) Diagnosed as gastric cancer by histopathology; (2) tumor node metastasis (TNM) stage ≥ IIIb; (3) received preoperative adjuvant chemotherapy; (4) lesions with objective measurements; (5) no anti-tumor treatment given before admission; and (6) patients and their families provided informed consent. Exclusion criteria: (1) Coexisting liver and kidney dysfunction, hematopoietic system diseases, autoimmune diseases, and other serious diseases; (2) patients getting retreatment; (3) history of mental illness; (4) poor compliance, cannot cooperate with follow-up treatment; and (5) Karnofsky Performance Scale (KPS) < 60 points. The patients were divided into the observation group (n = 40) and control group (n = 40) by the envelope method. The clinical data of the two groups are compared in Table 1, and were found to be comparable. This study was approved by a hospital ethics committee.
| Clinical general data | Observation group (n = 40) | Control group (n = 40) | t/χ2 value | P value |
| Gender, n (%) | 0.050 | 0.823 | ||
| Male | 22 (55.00) | 21 (52.50) | ||
| Female | 18 (45.00) | 19 (47.50) | ||
| Age (yr) | 62.21 ± 7.78 | 61.10 ± 8.43 | 0.612 | 0.542 |
| Body mass index (kg/m2) | 22.40 ± 2.05 | 22.16 ± 1.95 | 0.536 | 0.593 |
| KPS score (points) | 72.21 ± 5.54 | 71.19 ± 5.80 | 0.804 | 0.424 |
| TNM stage, n (%) | 0.238 | 0.626 | ||
| IIIb | 27 (67.50) | 29 (72.50) | ||
| IV | 13 (32.50) | 11 (27.50) |
The control group was treated with conventional chemotherapy consisting of oxaliplatin combined with fluorouracil: intravenous infusion of 180 mg/m2 fluorouracil for injection (Qilu Pharmaceutical Co., Ltd., batch number: Sinopharm H20094528) and intravenous infusion of 70 mg/m2 oxaliplatin for injection (Jiangsu Hengrui Pharmaceutical Co., Ltd., production batch number: Sinopharm H20000337). The experimental group was given irinotecan in addition to conventional chemotherapy (Shenyang Pharmaceutical Pharmaceutical Co., Ltd., SFDA Approval No. H20090659) on the first day of chemotherapy according to the body surface area at a dose of 180 mg/m2 intravenously over 90 min, along with careful monitoring for adverse reactions. Patients in both groups were treated for 4 wk as a treatment cycle, and the levels of tumor markers were measured on every Monday. The follow-up deadline was October 2022.
Fasting fresh blood samples (5 mL) were collected from the patients in the morning and centrifuged at 1000 r/min for 20 min with a centrifugal radius of 10 cm. The levels of tumor markers carbohydrate antigen 199 (CA199), carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), and epidermal growth factor receptor (EGFR) were detected by enzyme-linked immunosorbent assay kit (Suzhou Boyuan Medical Technology Co., Ltd.).
The short-term efficacy was evaluated by the World Health Organization (WHO) solid tumor treatment efficacy standards[10]. Complete remission (CR) was defined as the disappearance of the tumor lesion, which lasted for more than 4 wk, while partial remission (PR) was defined as a reduction in the lesion by more than 30%, which lasted for more than 4 wk. Stable disease (SD) was defined as a reduction of 25% in the lesion or new lesions. CR + PR was considered effective treatment response.
The side effects of chemotherapy were divided into grade 0, grade I, grade II, grade III, and grade IV according to the WHO chemotherapy toxicity grading standards[11], which can also be defined as no adverse reactions, mild adverse reactions, moderate adverse reactions but tolerable, moderate adverse reactions and intolerable, severe adverse reactions, respectively.
The quality of life was evaluated by the SF-36 scale[12]. The scale has eight aspects: physiological function, physiological function, physical pain, general health status, energy, social function, emotional function, and mental health. The higher the score, the better the quality of life of patients.
The data were analyzed by SPSS22.0 software. The measurement data included age, body mass index, tumor markers, etc. The data were expressed as mean ± SD, and the t test was used to analyze the differences between groups. Count data included sex, TNM stage, short-term efficacy, etc. The data were expressed as n (%), and χ2 test or rank sum test analysis index were used to assess the differences between groups. Survival was analyzed by the Kaplan–Meier method.
The short-term efficacy of the observation group was better than that of the control group (P < 0.05). The CR and PR accounted for 5.00% and 52.50% respectively, and the total effective rate was 57.50% (Table 2).
| Group | Cases | CR | PR | SD | PD | Z value | P value |
| Observation group | 40 | 2 (5.00) | 21 (52.50) | 12 (30.00) | 5 (12.50) | -2.799 | 0.005 |
| Control group | 40 | 0 (0.00) | 13 (32.50) | 12 (30.00) | 15 (37.50) |
The gender and body mass index of patients with effective and ineffective chemotherapy in the observation group were compared (P > 0.05). The age and TNM stage IV ratio of patients with ineffective chemotherapy in the observation group were apparently higher than those of patients with effective chemotherapy (P < 0.05), while the KPS score was significantly lower than that of patients with effective chemotherapy (P < 0.05, Table 3).
| Clinical data | Chemotherapy effective (n = 23) | Chemotherapy ineffective (n = 17) | t/χ2 value | P value |
| Gender, n (%) | 0.051 | 0.822 | ||
| Male | 13 (56.62) | 9 (52.94) | ||
| Female | 10 (43.48) | 8 (47.06) | ||
| Age (yr) | 60.60 ± 5.56 | 65.12 ± 5.71 | -3.587 | 0.001 |
| Body mass index (kg/m2) | 22.14 ± 2.03 | 22.75 ± 2.12 | -1.314 | 0.193 |
| KPS score (points) | 75.54 ± 4.32 | 67.70 ± 3.83 | 8.589 | 0.000 |
| TNM stages, n (%) | 5.631 | 0.018 | ||
| IIIb | 19 (82.61) | 8 (47.06) | ||
| IV | 4 (17.39) | 9 (52.94) |
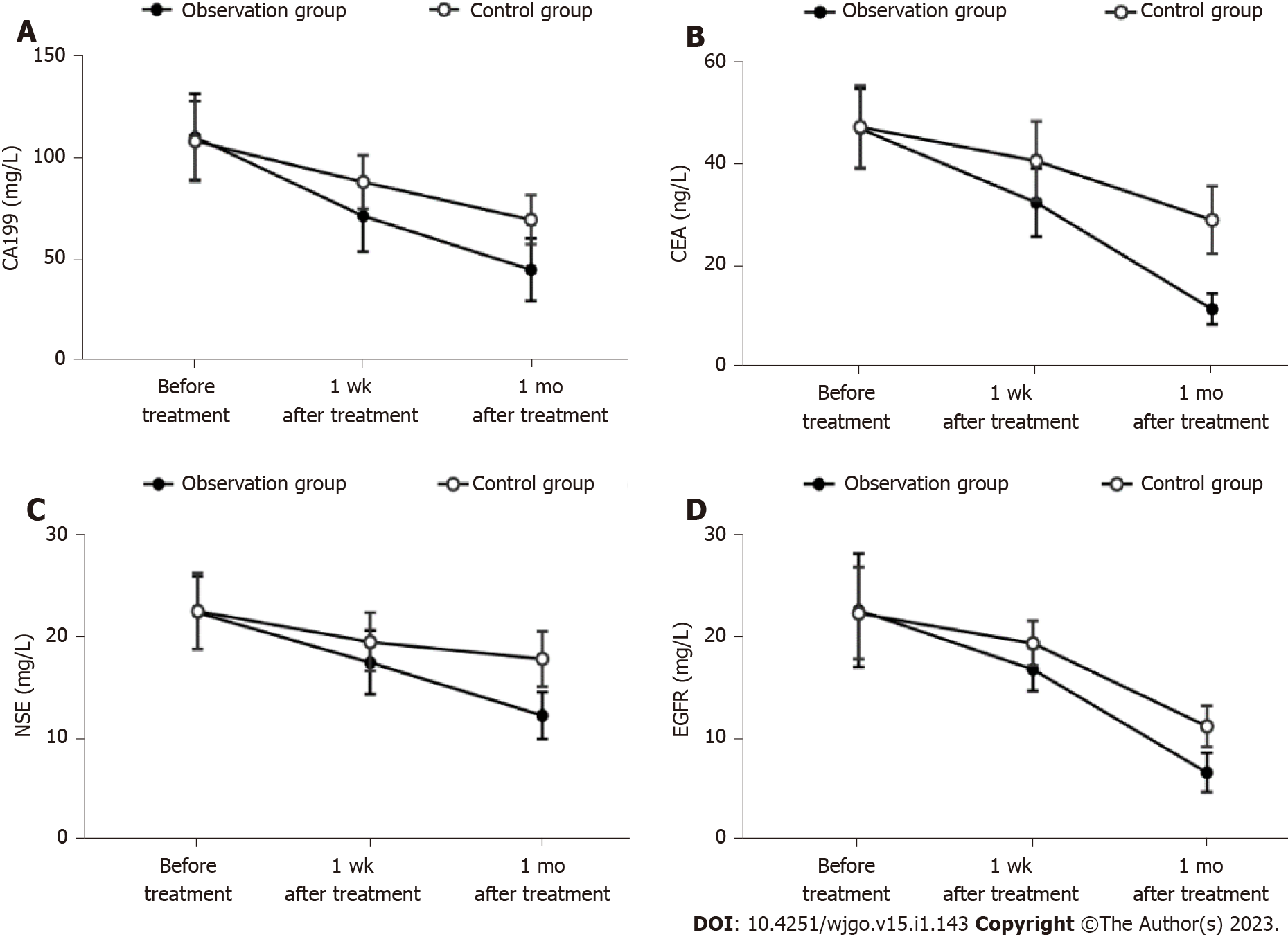
After treatment, CA199, CEA, NSE and EGFR in the observation group and the control group were markedly lower than those before treatment (P < 0.05). CA199, CEA, NSE and EGFR in the observation group were notably lower than those in the control group at 1 wk and 1 mo after treatment (P < 0.05) (Table 4 and Figure 1).
| Index | Observation group (n = 40) | Control group (n = 40) | t value | P value |
| CA199 (mg/L) | ||||
| Before treatment | 110.43 ± 21.32 | 108.28 ± 19.82 | 0.467 | 0.642 |
| 1 wk after treatment | 71.22 ± 17.28a | 88.01 ± 13.34a | -4.864 | 0.000 |
| 1 mo after treatment | 44.49 ± 15.52a,d | 69.32 ± 12.10a,d | -7.980 | 0.000 |
| CEA (ng/L) | ||||
| Before treatment | 46.69 ± 7.80 | 47.05 ± 8.00 | -0.204 | 0.839 |
| 1 wk after treatment | 32.21 ± 6.65a | 40.40 ± 7.71a | -5.087 | 0.000 |
| 1 mo after treatment | 11.38 ± 3.03a,d | 28.83 ± 6.62a,d | -15.159 | 0.000 |
| NSE (mg/L) | ||||
| Before treatment | 22.23 ± 3.54 | 22.40 ± 3.70 | -0.210 | 0.834 |
| 1 wk after treatment | 17.39 ± 3.11a | 19.40 ± 2.83a | -3.023 | 0.003 |
| 1 mo after treatment | 12.23 ± 2.29a,d | 17.73 ± 2.69a,d | -9.847 | 0.000 |
| EGFR (mg/L) | ||||
| Before treatment | 22.43 ± 5.54 | 22.15 ± 4.48 | 0.249 | 0.804 |
| 1 wk after treatment | 16.67 ± 2.11a | 19.22 ± 2.17a | -5.328 | 0.000 |
| 1 mo after treatment | 6.60 ± 1.92a,d | 11.11 ± 2.01a,d | -10.262 | 0.000 |
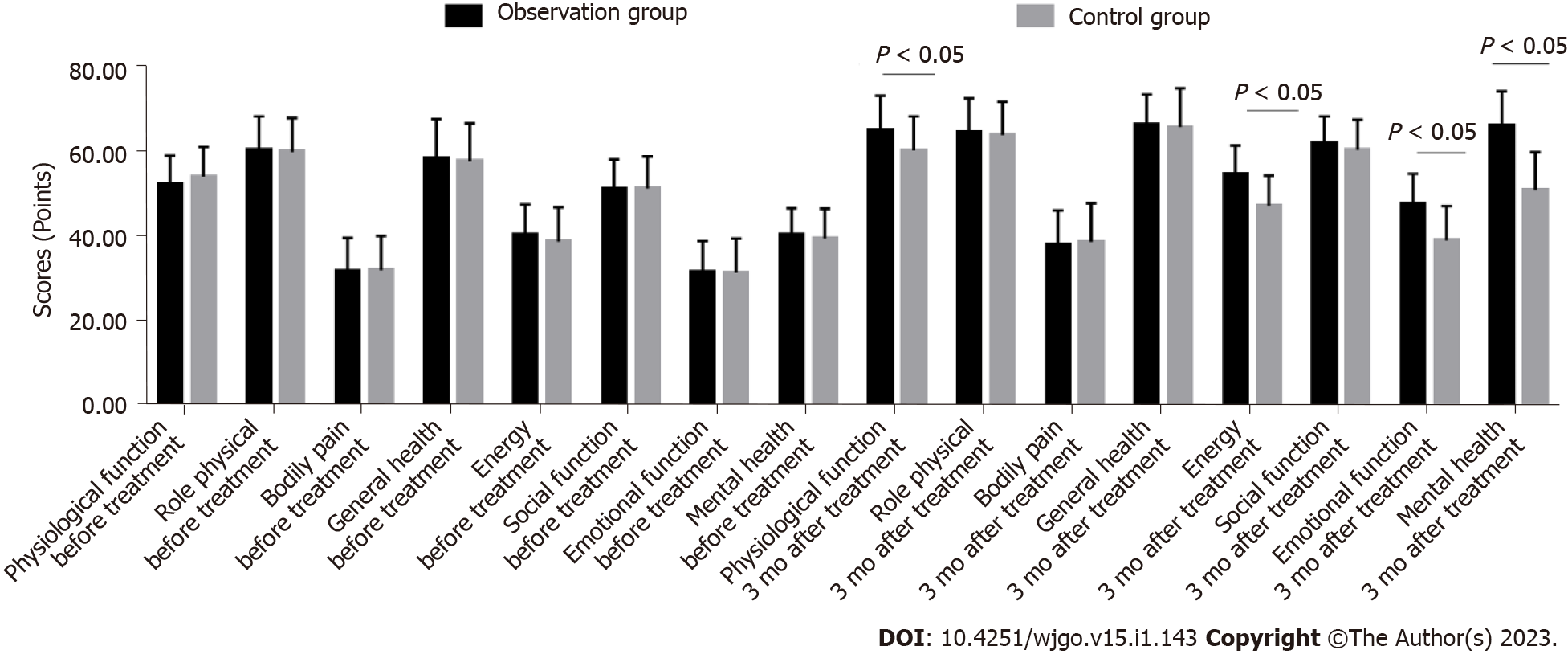
The SF-36 scale scores of the observation group and the control group before treatment were compared (P > 0.05). The items of SF-36 scale in the observation group and the control group were improved after treatment (P < 0.05). The physiological function, energy, emotional function and mental health of SF-36 in the observation group were significantly higher than those in the control group (P < 0.05) (Table 5 and Figure 2).
| Index | Observation group (n = 40) | Control group (n = 40) | t value | P value |
| Physiological function | ||||
| Before treatment | 52.34 ± 6.89 | 54.11 ± 7.04 | -1.136 | 0.259 |
| 3 mo after treatment | 65.12 ± 8.14a | 60.32 ± 8.04a | 2.653 | 0.010 |
| Role-physical | ||||
| Before treatment | 60.43 ± 7.92 | 59.90 ± 8.04 | 0.297 | 0.767 |
| 3 mo after treatment | 64.54 ± 8.09a | 63.93 ± 7.90a | 0.341 | 0.734 |
| Bodily pain | ||||
| Before treatment | 31.88 ± 7.78 | 32.01 ± 8.09 | -0.073 | 0.942 |
| 3 mo after treatment | 38.00 ± 8.12a | 38.70 ± 9.17a | -0.361 | 0.719 |
| General health | ||||
| Before treatment | 58.56 ± 9.15 | 57.74 ± 9.03 | 0.403 | 0.688 |
| 3 mo after treatment | 66.43 ± 7.12a | 65.80 ± 9.22a | 0.342 | 0.733 |
| Energy | ||||
| Before treatment | 40.32 ± 7.22 | 38.75 ± 8.11 | 0.914 | 0.363 |
| 3 mo after treatment | 54.76 ± 6.70a | 47.21 ± 7.14a | 4.877 | 0.000 |
| Social function | ||||
| Before treatment | 51.21 ± 6.98 | 51.33 ± 7.54 | -0.074 | 0.941 |
| 3 mo after treatment | 61.89 ± 6.45a | 60.40 ± 7.16a | 0.978 | 0.331 |
| Emotional function | ||||
| Before treatment | 31.65 ± 7.21 | 31.44 ± 8.01 | 0.123 | 0.902 |
| 3 mo after treatment | 47.58 ± 7.22a | 39.03 ± 8.12a | 4.977 | 0.000 |
| Mental health | ||||
| Before treatment | 40.34 ± 6.31 | 39.45 ± 7.08 | 0.594 | 0.555 |
| 3 mo after treatment | 66.16 ± 8.11a | 50.91 ± 9.03a | 7.947 | 0.000 |
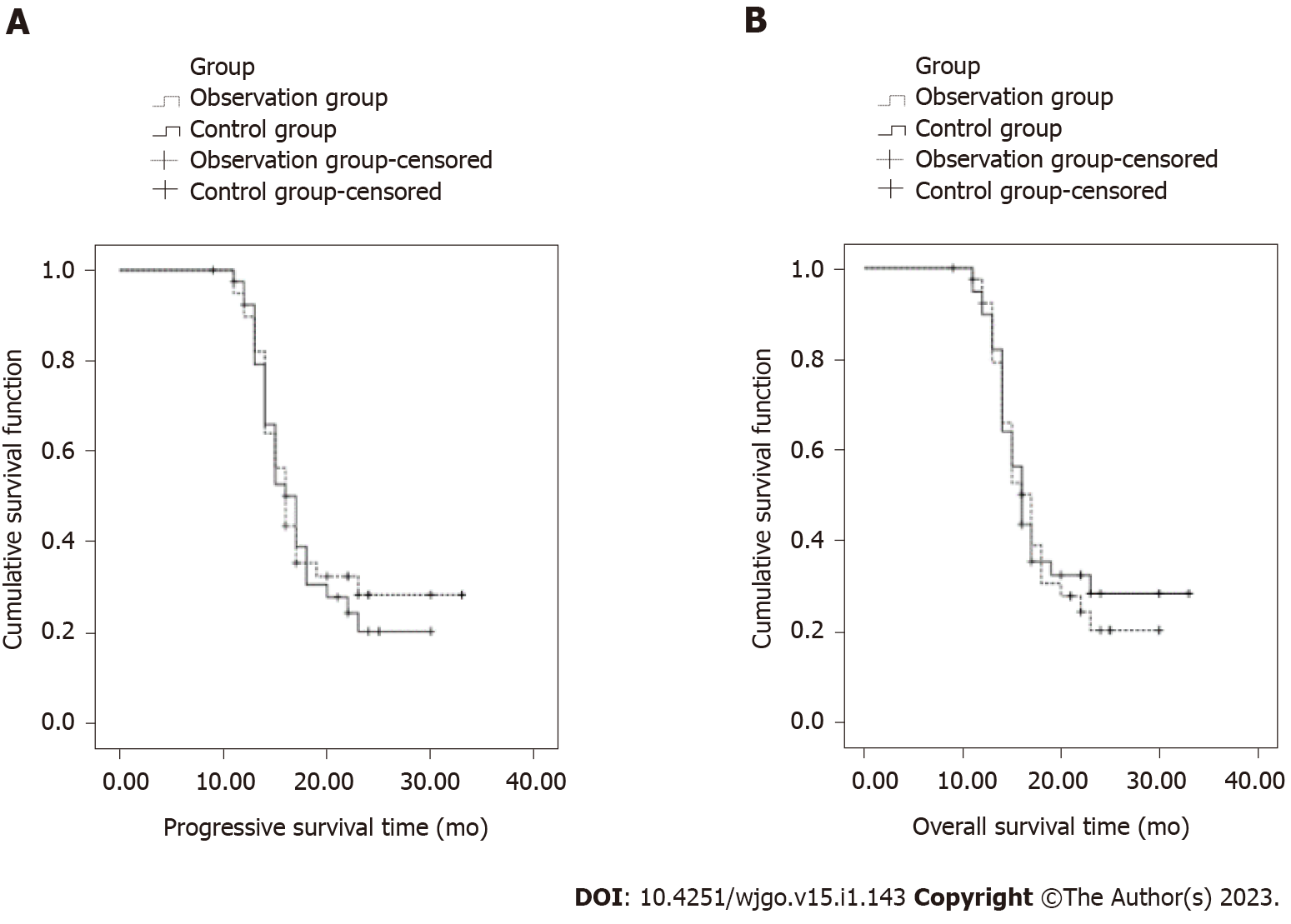
The median progression-free survival time of the observation group and the control group was 16 mo (95%CI: 14.79-17.21) and 17 mo (95%CI: 15.26-18.74), respectively, and the difference was not statistically significant (χ2 = 0.115, P = 0.734 > 0.05). The median overall survival of the observation group and the control group was 23 mo (95%CI: 22.05-23.94) and 17 mo (95%CI: 21.08-22.92), respectively, and the difference was not statistically significant (χ2 =0.643, P = 0.423 > 0.05, Figure 3).
The incidence of grade III-IV diarrhea and grade III-IV thrombocytopenia in the observation group was apparently higher than that in the control group (P < 0.05, Table 6).
| Group | Number of cases | III-IV grade leukopenia | III-IV grade nausea and vomiting | III-IV grade diarrhea | III-IV grade mouth ulcer | III-IV grade abnormal liver and kidney function | III-IV grade thrombocytopenia |
| Observation group | 40 | 8 (20.00) | 11 (27.50) | 13 (32.50) | 9 (22.50) | 15 (38.50) | 10 (25.00) |
| Control group | 40 | 5 (12.50) | 7 (17.50) | 4 (10.00) | 8 (20.00) | 12 (30.00) | 3 (7.50) |
| t/χ2 value | 0.827 | 1.147 | 6.050 | 0.075 | 0.503 | 4.501 | |
| P value | 0.363 | 0.284 | 0.014 | 0.785 | 0.478 | 0.034 |
Advanced gastric cancer invades the muscular layer and submucosa of the gastric mucosa due to the enlargement of the cancer lesion. Simple surgery cannot cure it, and postoperative chemotherapy, radiotherapy, targeted therapy, and immunotherapy are required[13]. Conventional chemotherapy comprising oxaliplatin and fluorouracil is generally used. Oxaliplatin and fluorouracil can improve the gastric environment and effectively inhibit the growth and spread of the cancer cells. However, normal cells are also inhibited and hematopoietic function is suppressed. Therefore, this study analyzed the combination of conventional chemotherapy with irinotecan to treat patients with gastric cancer[14].
This study also compared the short-term efficacy of the treatments in the two groups of patients, and the results revealed that the short-term efficacy was better in the observation group than in the control group, indicating that the treatment effect in the observation group was better. In the control group, fluorouracil first forms two intermediate products, deoxyfluorocytidine and deoxyfluorouridine, through the action of carboxylesterase and cytidine deaminase in the liver and tumor tissues, and finally transforms into 5-fluorouracil (5-FU) in the tumor cells through the catalysis of thymidine phosphorylase, which exerts a selective local anti-cancer effect[15]. Oxaliplatin cross-links with DNA to form adducts and increases the anti-tumor activity of 5-FU in advanced gastric cancer. At the same time, irinotecan is a derivative of semi-synthetic camptothecin. By interfering with the helix and non-helix of replication DNA, the synthesis of nucleic acids is inhibited, causing DNA single strand breaks, thereby inhibiting DNA replication and RNA synthesis, which leads to tumor cell atypia and death. Studies have shown that irinotecan monotherapy is effective in up to 18%-23% cancer patients[16]. Therefore, the therapeutic effect of oral irinotecan may be synergistic with fluorouracil and oxaliplatin, which maximizes the anti-cancer effect and controls disease progression, thereby improving the therapeutic effect.
In this study, the clinical data of effective and ineffective patients in the observation group were compared. The results revealed that the age and TNM stage IV ratio of patients with ineffective chemotherapy in the observation group were significantly higher than those of patients with effective chemotherapy, while the KPS score was evidently lower than that of patients with effective chemotherapy, indicating that the efficacy of treatment in the observation group was affected by the age and TNM stage of the patients. Because most patients were elderly, they had underlying diseases and decreased immune capacity, giving rise to increased severity of the cancer, resulting in reduced therapeutic effect[17]. The TNM stage reflects the severity of malignant tumors, and the higher the stage, the more serious the disease. Therefore, when the patient's cancer is severe, the tumor cells proliferate and differentiate, and the TNM stage increases, resulting in a decrease in the efficacy of chemotherapy. Therefore, the efficacy of treatment in the observation group was affected by the patient's age and TNM stage.
In this study, the serum tumor marker levels in the two groups before and after treatment were compared. The results showed that CA199, CEA, NSE, and EGFR were significantly decreased in the observation group at 1 wk and 1 mo after treatment, indicating that the treatment had a better inhibitory effect on tumor proliferation in the observation group. Among the tumor markers, CA199 is a oligosaccharide tumor-associated antigen and considered a new tumor marker. CEA exists on the surface of cancer cells differentiated from endoderm cells and is a structural protein of the cell membrane[18]. NSE is one of the enolases involved in the glycolysis pathway, which exists in the nerve tissue and neuroendocrine tissue. EGFR plays an important regulatory role in cellular physiological processes[19]. CA199, CEA, NSE, and EGFR levels can reflect tumor growth, which can be used to assess the condition of advanced gastric cancer[20]. When fluorouracil is given to patients with advanced gastric cancer, it accumulates in a large amount near the cancer cells. It has an effect on deoxyribonucleic acid, preventing thymidylate conversion to produce a large number of thymidines, and interferes with the synthesis of deoxyribonucleic acid and ribonucleic acid, while oxaliplatin can potentiate the effect of fluorouracil[21,22]. Combination of irinotecan with conventional chemotherapy can inhibit the proliferation and differentiation of gastric cancer cells, accelerate apoptosis and killing of cancer cells, block the binding and signal transduction between tumor factors and receptors, so as to minimize and reduce the expression of CA199, CEA, NSE, and EGFR[23,24].
In this study, the SF-36 scale scores of the two groups before and after treatment were compared. The results revealed that the SF-36 scale scores for physiological function, energy, emotional function, and mental health in the observation group were markedly higher than those in the control group 3 mo after treatment, indicating that the treatment given to the observation group could improve the SF-36 scale score and improve the quality of life of patients. Therefore, the treatment effect of the observation group was better, and the treatment could inhibit the proliferation of tumor cells and improve the condition of patients in the observation group. Studies have shown that irinotecan can improve immunity[25]. There is evidence that the addition of irinotecan can improve the immune function, promote metabolism, accelerate protein synthesis, regulate gastrointestinal function, and improve the physical condition of the patients[26]. At the same time, patients with gastric cancer may have fear of long-term chemotherapy, and the improvement of the treatment effect in the observation group can enhance the patients' confidence, eliminate fear of gastric cancer, promote their mental health, and thus improve the SF-36 scale score[27].
In this study, the toxic effects of treatment in the two groups were compared. The results showed that the rates of grade III-IV diarrhea and grade III-IV thrombocytopenia in the observation group were significantly higher than those in the control group, illustrating that the observation group experienced more toxic effects. Irinotecan is a DNA topoisomerase I inhibitor. Topoisomerase I-DNA-irinotecan (or SN-38) can form a triple complex, which interacts with each other, causing DNA double-strand breaks, resulting in cytotoxicity. While killing tumor cells, it can also cause damage to normal cells, leading to complications such as nausea and vomiting, diarrhea, and thrombocytopenia[28,29]. Studies have shown that the side effects of irinotecan in combination with other chemotherapy drugs are more obvious[30]. Therefore, the side effects in the observation group were more than those in the control group.
In summary, the standard chemotherapy regimen combined with irinotecan in patients with advanced gastric cancer demonstrated good short-term efficacy, which could notably reduce serum tumor marker levels and improve the quality of life of patients. However, its efficacy may be affected by patient age and TNM stage, and the long-term efficacy needs further investigation. In addition, there are still some shortcomings in this study. Based on the existing research, further in-depth hierarchical analysis should be carried out to make the research results more complete and convincing.
Surgery, radiotherapy, and chemotherapy are often used for the treatment of advanced gastric cancer. Although chemotherapy is one of the main treatment options, there is no unified or standardized treatment; therefore, the identification of effective treatment options for patients with advanced gastric cancer is particularly important.
The conventional chemotherapy regimen for advanced gastric cancer consists of oxaliplatin combined with fluorouracil. However, studies have shown that treatment with only oxaliplatin combined with fluorouracil is not very effective in advanced gastric cancer.
This study is designed to investigate the short-term efficacy and influencing factors of irinotecan combined with the conventional chemotherapy regimen of oxaliplatin and fluorouracil in advanced gastric cancer patients. The results can provide valuable reference for clinical treatment and further study.
Eighty patients with advanced gastric cancer were divided into two groups. The control group was given preoperative routine chemotherapy. The observation group was treated with irinotecan in addition to the chemotherapy given to the control group. The short-term efficacy of treatment in the two groups, as well as tumor marker levels and quality of life before and after treatment were evaluated.
The short-term treatment effect in the observation group was better than that in the control group. The median progression-free survival and overall survival were similar between two groups. The incidence rates of grade III-IV diarrhea and grade III-IV thrombocytopenia in the observation group were markedly higher than those in the control group. The age and proportion of tumor node metastasis (TNM) stage IV patients were notably higher, and the Karnofsky Performance Scale score was apparently lower in patients with ineffective chemotherapy.
Chemotherapy combined with irinotecan in patients with advanced gastric cancer has a good short-term efficacy and can significantly reduce serum tumor markers and improve the quality of life of patients. The efficacy may be affected by the age and TNM stage of the patients.
The long-term efficacy of chemotherapy combined with irinotecan need to be further studied.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Muro K, Japan; Tanaka T, Japan; Toyota S, Japan S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Xu D, Zhang Z, Zhang S, Fang X, Wang L, Li Q. Efficacy of trastuzumab combined with SOX or IP chemotherapy regimen in the treatment of advanced gastric cancer. J BUON. 2021;26:932-939. [PubMed] |
| 2. | Xie BW, Zang L, Ma JJ, Sun J, Yang X, Wang ML, Lu AG, Hu WG, Zheng MH. [Safety and effectiveness of oxaliplatin combined with capecitabine or oxaliplatin combined with S-1 neoadjuvant chemotherapy in the treatment of advanced gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Moore JL, Kumar S, Santaolalla A, Patel PH, Kapiris M, Van Hemelrijck M, Maisey N, Hill M, Lagergren J, Gossage JA, Kelly M, Chaudry A, Allum WH, Baker CR, Cunningham D, Davies AR; Guy's and St Thomas' Oesophago-gastric Research Group including the following co-authors. Effect of peri-operative chemotherapy regimen on survival in the treatment of locally advanced oesophago-gastric adenocarcinoma - A comparison of the FLOT and 'MAGIC' regimens. Eur J Cancer. 2022;163:180-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Kakuta T, Yabusaki H, Bamba T, Aizawa M, Nogami H, Nomura T, Matsuki A, Maruyama S, Takii Y, Nakagawa S. Efficacy and safety of ramucirumab plus paclitaxel therapy for advanced gastric cancer patients treated previously with docetaxel-containing chemotherapy. Int J Clin Oncol. 2021;26:684-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Zhang F, Yin Y, Ni T, Zhang M, Zhou Z, Sun X, Kuang W, Li P. Treatment effect of apatinib combined chemotherapy as second-line or above therapy in patients with advanced gastric cancer or adenocarcinoma of the gastroesophageal junction. Pharmazie. 2020;75:389-394. [PubMed] |
| 6. | Pourghasemian M, Danandeh Mehr A, Molaei M, Habibzadeh A. Outcome of FOLFOX and Modified DCF Chemotherapy Regimen in Patients with Advanced Gastric Adenocarcinoma. Asian Pac J Cancer Prev. 2020;21:2337-2341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Bao YD, Zhang H, Dong L, Jiang KW, Ye YJ, Wang S, Zhou J. [Safety and efficacy of adjuvant chemotherapy with oxaliplatin and S-1 for patients with locally advanced gastric cancer after D2 lymph nodes dissection]. Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Chen GD, Cao BX, Shi Y, Lv JM, Wang DH, Shi LB. Comparisons of effects of SOX and mFOLFOX6 chemotherapy regimens on patients with locally advanced gastric cancer. J Chemother. 2022;34:117-122. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Peng Z, Wei J, Wang F, Ying J, Deng Y, Gu K, Cheng Y, Yuan X, Xiao J, Tai Y, Wang L, Zou J, Zhang Y, Shen L. Camrelizumab Combined with Chemotherapy Followed by Camrelizumab plus Apatinib as First-line Therapy for Advanced Gastric or Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res. 2021;27:3069-3078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 10. | Ogata T, Narita Y, Kumanishi R, Nakazawa T, Matsubara Y, Kato K, Nozawa K, Honda K, Masuishi T, Bando H, Kadowaki S, Ando M, Tajika M, Muro K. Clinical Impact of Oral Intake in Second-line or Third-line Chemotherapy for 589 Patients With Advanced Gastric Cancer: A Retrospective Cohort Study. Am J Clin Oncol. 2021;44:388-394. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 482] [Article Influence: 160.7] [Reference Citation Analysis (0)] |
| 12. | Narita Y, Shoji H, Kawai S, Mizukami T, Nakamura M, Moriwaki T, Yamanaka T, Sunakawa Y, Kawakami H, Nishina T, Misumi T, Muro K. REVIVE study: a prospective observational study in chemotherapy after nivolumab therapy for advanced gastric cancer. Future Oncol. 2021;17:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1887] [Article Influence: 471.8] [Reference Citation Analysis (1)] |
| 14. | Hall PS, Swinson D, Cairns DA, Waters JS, Petty R, Allmark C, Ruddock S, Falk S, Wadsley J, Roy R, Tillett T, Nicoll J, Cummins S, Mano J, Grumett S, Stokes Z, Kamposioras KV, Chatterjee A, Garcia A, Waddell T, Guptal K, Maisey N, Khan M, Dent J, Lord S, Crossley A, Katona E, Marshall H, Grabsch HI, Velikova G, Ow PL, Handforth C, Howard H, Seymour MT; GO2 Trial Investigators. Efficacy of Reduced-Intensity Chemotherapy With Oxaliplatin and Capecitabine on Quality of Life and Cancer Control Among Older and Frail Patients With Advanced Gastroesophageal Cancer: The GO2 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021;7:869-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 15. | Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, Castro HR, Mansoor W, Braghiroli MI, Karaseva N, Caglevic C, Villanueva L, Goekkurt E, Satake H, Enzinger P, Alsina M, Benson A, Chao J, Ko AH, Wainberg ZA, Kher U, Shah S, Kang SP, Tabernero J. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:1571-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 864] [Article Influence: 172.8] [Reference Citation Analysis (1)] |
| 16. | Akin Telli T, Bregni G, Camera S, Deleporte A, Hendlisz A, Sclafani F. PD-1 and PD-L1 inhibitors in oesophago-gastric cancers. Cancer Lett. 2020;469:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Kawazoe A, Fukuoka S, Nakamura Y, Kuboki Y, Wakabayashi M, Nomura S, Mikamoto Y, Shima H, Fujishiro N, Higuchi T, Sato A, Kuwata T, Shitara K. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first-line or second-line setting (EPOC1706): an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1057-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 18. | Park S, Nam CM, Kim SG, Mun JE, Rha SY, Chung HC. Comparative efficacy and tolerability of third-line treatments for advanced gastric cancer: A systematic review with Bayesian network meta-analysis. Eur J Cancer. 2021;144:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Nishiyama T, Chen LT, Kang YK. Nivolumab in previously treated advanced gastric cancer (ATTRACTION-2): 3-year update and outcome of treatment beyond progression with nivolumab. Gastric Cancer. 2021;24:946-958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 20. | Kawazoe A, Ando T, Hosaka H, Fujita J, Koeda K, Nishikawa K, Amagai K, Fujitani K, Ogata K, Watanabe K, Yamamoto Y, Shitara K. Safety and activity of trifluridine/tipiracil and ramucirumab in previously treated advanced gastric cancer: an open-label, single-arm, phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Catenacci DVT, Kang YK, Park H, Uronis HE, Lee KW, Ng MCH, Enzinger PC, Park SH, Gold PJ, Lacy J, Hochster HS, Oh SC, Kim YH, Marrone KA, Kelly RJ, Juergens RA, Kim JG, Bendell JC, Alcindor T, Sym SJ, Song EK, Chee CE, Chao Y, Kim S, Lockhart AC, Knutson KL, Yen J, Franovic A, Nordstrom JL, Li D, Wigginton J, Davidson-Moncada JK, Rosales MK, Bang YJ; CP-MGAH22-5 Study Group. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22-05): a single-arm, phase 1b-2 trial. Lancet Oncol. 2020;21:1066-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 22. | Bang YJ, Golan T, Dahan L, Fu S, Moreno V, Park K, Geva R, De Braud F, Wainberg ZA, Reck M, Goff L, Laing N, Mi G, Oliveira JM, Wasserstrom H, Lin CC. Ramucirumab and durvalumab for previously treated, advanced non-small-cell lung cancer, gastric/gastro-oesophageal junction adenocarcinoma, or hepatocellular carcinoma: An open-label, phase Ia/b study (JVDJ). Eur J Cancer. 2020;137:272-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 23. | Olnes MJ, Martinson HA. Recent advances in immune therapies for gastric cancer. Cancer Gene Ther. 2021;28:924-934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Tabernero J, Bang YJ, Van Cutsem E, Fuchs CS, Janjigian YY, Bhagia P, Li K, Adelberg D, Qin SK. KEYNOTE-859: a Phase III study of pembrolizumab plus chemotherapy in gastric/gastroesophageal junction adenocarcinoma. Future Oncol. 2021;17:2847-2855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Komatsu Y, Hironaka S, Tanizawa Y, Cai Z, Piao Y, Boku N. Treatment Pattern for Advanced Gastric Cancer in Japan and Factors Associated with Sequential Treatment: A Retrospective Administrative Claims Database Study. Adv Ther. 2022;39:296-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 26. | Hofheinz RD, Hegewisch-Becker S, Kunzmann V, Thuss-Patience P, Fuchs M, Homann N, Graeven U, Schulte N, Merx K, Pohl M, Held S, Keller R, Tannapfel A, Al-Batran SE. Trastuzumab in combination with 5-fluorouracil, leucovorin, oxaliplatin and docetaxel as perioperative treatment for patients with human epidermal growth factor receptor 2-positive locally advanced esophagogastric adenocarcinoma: A phase II trial of the Arbeitsgemeinschaft Internistische Onkologie Gastric Cancer Study Group. Int J Cancer. 2021;149:1322-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 27. | Marino E, Graziosi L, Donini A. Neoadjuvant Chemotherapy for Locally Advanced Gastric Cancer: Where we Stand; An Italian Single Center Perspective. In Vivo. 2021;35:3459-3466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Onodera K, Ichiyanagi A, Ueno A, Tani M, Sato S, Shimizu H, Kaneto H. [Case Report of Four Patients Received Ramucirumab Monotherapy as Second-Line Chemotherapy for Advanced Gastric Cancer]. Gan To Kagaku Ryoho. 2021;48:1169-1171. [PubMed] |
| 29. | Liang H. [Progress in conversion therapy for stage IV gastric cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | Arai H, Kawahira M, Yasui H, Masuishi T, Muro K, Nakajima TE. Second-line chemotherapy using taxane in patients with advanced gastric cancer who presented with severe peritoneal metastasis: a multicenter retrospective study. Int J Clin Oncol. 2021;26:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |