Published online Sep 15, 2022. doi: 10.4251/wjgo.v14.i9.1808
Peer-review started: February 28, 2022
First decision: April 17, 2022
Revised: April 21, 2022
Accepted: July 31, 2022
Article in press: July 31, 2022
Published online: September 15, 2022
Processing time: 192 Days and 13.4 Hours
Gastric cancer (GC) is one of the most common malignancies in China with a high morbidity and mortality.
To determine whether interleukin (IL)-31, IL-32, and IL-33 can be used as biomarkers for the detection of GC, via evaluating the correlations between their expression and clinicopathological parameters of GC patients.
Tissue array (n = 180) gastric specimens were utilised. IL-31, IL-32, and IL-33 expression in GC and non-GC tissues was detected immunohistochemically. The correlations between IL-31, IL-32, and IL-33 expression in GC and severity of clinicopathological parameters were evaluated. Survival curves were plotted using the Kaplan-Meier method/Cox regression. Circulating IL-31, IL-32, and IL-33 were detected by ELISA.
We found that the expression levels of IL-31, IL-32, and IL-33 were all lower in GC than in adjacent non-GC gastric tissues (P < 0.05). IL-33 in peripheral blood of GC patients was significantly lower than that of healthy individuals (1.50 ± 1.11 vs 9.61 ± 8.00 ng/mL, P <0.05). Decreased IL-31, IL-32, and IL-33 in GC were observed in younger patients (< 60 years), and IL-32 and IL-33 were lower in female patients (P < 0.05). Higher IL-32 correlated with a longer survival in two GC subgroups: T4 invasion depth and TNM I-II stage. Univariate/multivariate analysis revealed that IL-32 was an independent prognostic factor for GC in the T4 stage subgroup. Circulating IL-33 was significantly lower in GC patients at TNM stage IV than in healthy people (P < 0.05).
Our findings may provide new insights into the roles of IL-31, IL-32, and IL-33 in the carcinogenesis of GC and demonstrate their relative usefulness as prognostic markers for GC. The underlying mechanism of IL-31, IL-32, and IL-33 actions in GC should be further explored.
Core Tip: Gastric cancer (GC) is one of the most common malignancies in China with a high morbidity and mortality. This study aimed to determine whether interleukin (IL)-31, IL-32, and IL-33 can be used as biomarkers for the detection of GC, via evaluating the correlations between their expression and clinicopathological parameters of GC patients. IL-31, IL-32, and IL-33 expression in GC was correlated with the severity of clinicopathological parameters. Circulating IL-33 was significantly low in GC patients. Our findings may provide new insights into the roles of IL-31, IL-32, and IL-33 in the carcinogenesis of GC.
- Citation: Liu QH, Zhang JW, Xia L, Wise SG, Hambly BD, Tao K, Bao SS. Clinical implications of interleukins-31, 32, and 33 in gastric cancer. World J Gastrointest Oncol 2022; 14(9): 1808-1822
- URL: https://www.wjgnet.com/1948-5204/full/v14/i9/1808.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i9.1808
Gastric cancer (GC) is one of the most common malignancies in China with a high morbidity (approximately 24%) and mortality (approximately 17%)[1] and is ranked third amongst malignant tumours[2]. Despite the more widespread use of recently developed diagnostic techniques, including endoscopic examination, many GC patients are diagnosed at advanced stages, resulting in a poor 5-year survival rate (< 20%). This emphasises the critical need for development of a reliable biomarker(s)[3] with high specificity and sensitivity, to improve the prediction of prognosis for more successful outcomes for GC patients. Endoscopic examination provides a useful approach in the early detection of GC and in reducing cancer-related mortality.
Immunity is critically important in inhibiting the development of malignancy[4], but the precise underlying mechanism concerning how host defence is involved in the oncogenesis of GC remains to be explored[5]. The role of pro-inflammatory and anti-inflammatory responses during the development of malignancy has been well established to be able to either stimulate or inhibit the growth of a cancer[4,6]. The actions of the immune checkpoint molecules PD-1 and CTLA-4 have been elegantly demon
Helicobacter pylori (H. pylori), a spiral Gram-negative rod that infects and colonizes the human stomach in 50% of the human population, is a definite human oncogenic agent[11]. In addition, it has been suggested that H. pylori contributes to > 60% of all GCs, although the precise underlying mechanisms are complex[12]. It has been well illustrated by the Nobel laureate Barry Marshal that chronic gastric ulceration is caused by H. pylori infection, which can be eliminated by a cocktail of antibiotics[13]. It has been reported that the constitutive levels of interleukin 32 (IL-32) in both the gastric mucosa and GC tissue is upregulated in H. pylori infection[14]. Thus, it is reasonable to speculate that host immunity plays a critical role during the development of GC.
The cell-mediated immune response is extremely important in defence against tumour development, since compromised host immunity is known to contribute to the establishment, proliferation, and metastasis of malignant tumours[15]. Although high host inflammatory status has been reported in the tumour microenvironment, an incompetent inflammatory/immune response will lead to tumour progression[16].
IL-31, an immunoregulatory cytokine secreted mainly by activated Th2 cells, plays a major role in the process of chronic inflammation[17]. However, the involvement of IL-31 in the pathogenesis of cancer is unclear. Recent studies have shown that malignant Tcells produce IL-31, with an associated increase in serum levels of IL-31[18]. Additionally, in the advanced stages of cutaneous T cell lymphoma, improved pruritus in patients correlates with lower levels of IL-31[19].
IL-32, a pro-inflammatory cytokine, is highly produced in several autoimmune diseases, e.g., rheumatoid arthritis, inflammatory bowel disease, and atopic dermatitis[20,21]. However, by contrast with autoimmune and inflammatory diseases, the role of IL-32 appears to differ amongst different forms of cancer, e.g., IL-32 exhibits anti-tumour effects in human colon cancer and leukaemia[22,23], however, it promotes tumorigenesis in human pancreatic cancers[24]. The role of IL-32 in GC is controversial, i.e., one study found that IL-32 expression is elevated in GC compared with normal stomach tissue[14], while another study reported that there is no significant difference between GC and normal stomach tissue[25]. The precise role of IL-32 in tumorigenesis of GC and other malignancies remains to be fully explored. An additional controversial finding, however, has also reported that there is substantially reduced IL32 expression in the GC tissue of patients with the diffuse type of GC[26]. These divergent observations concerning IL-32 expression in GC may be due to different races and/or different tumour microenvironments.
IL-33, a member of the IL-1 family, regulates innate and adaptive immunity as a potent inducer of pro-inflammatory cytokines. The involvement of IL-33 in non-small cell lung cancer is controversial, i.e., high IL-33 has been found to be of diagnostic and prognostic value[27], but another group has reported no significant associations[28]. The possible role of IL-33 in GC remains to be explored. IL-33 promotes GC invasion and migration via stimulating production of MMP-3 and IL-6 in vitro, using the ST2ERK1/2 pathway[29], which has been confirmed in a GC animal model by ablation of the cognate IL-33 receptor ST2[30]. IL-33 mRNA expression is significantly higher in GC tissue compared to that of non-cancer tissue[31], suggesting that IL-33 promotes the development of GC. However, another controversial report failed to demonstrate an association between IL-33 and the overall 5-year survival rate[32].
In this study, we specifically assessed the relationships among IL-31, IL-32, and IL-33 in GC utilising the same cohort of patients. We aimed to identify the expression of IL-31, IL-32, and IL-33 in GC and assess their inter-correlations and clinical significance. Our data may provide useful information for both basic understanding of tumour immunology and/or therapeutic targets for GC patients.
GC tissues and adjacent histologically normal gastric tissues (control) were obtained from 180 GC patients undergoing subtotal gastrectomy at the Affiliated Hospital, Xuzhou Medical University, China between 2015 and 2020. None of these patients had a total gastrectomy. These GC patients were comprised of 140 males and 40 females, aged from 23 to 85 years. No chemotherapy was administered to these patients prior to subtotal gastrectomy. There were no cases of local recurrences within the stomach after subtotal gastrectomy among the 180 GC patients included in the study. Non-cancer tissues were also collected (n = 159), but did not include cases without a mucosal layer present under microscopic examination (n = 21). This study was approved by the Human Ethical Committee, the Institutional Review Boards of Affiliated Hospitals of Xuzhou Medical University.
Sections (5 µm) from tissue microarray blocks were labelled with three antibodies, as described previously[33]. The antibodies used are: Rabbit anti-IL-31 polyclonal antibody (22859-1-AP, Proteintech, China), rabbit anti-IL-32 polyclonal antibody (11079-1-AP, Proteintech), and rabbit anti-IL-33 polyclonal antibody (12372-1-AP, Proteintech, China). The dilution for all three antibodies was 1:100. A horseradish peroxidase-conjugated secondary antibody (12127A07, Beijing Sequoia Jinqiao Biological Technology Co., Ltd.) was used. The specific target(s) were visualized with a DAB detection kit (Beijing Sequoia Jinqiao Biological Technology Co., Ltd.) and counterstained with hematoxylin.
Photomicrographs from each of the tissue arrays were taken with a fixed exposure time and colour balance to ensure consistency. IL-31, IL-32, and IL-33 production was quantified using ImagePro Plus9.1 (Media Cybernetic, Silver Spring, MD, United States), as described previously[34].
To determine if there was a correlation between GC and circulating IL-31, IL-32, and IL-33, we enrolled prospectively ten GC patients prior to preoperative chemotherapy in the Affiliated Hospital, Xuzhou Medical University, China. Blood from ten healthy age and sex matched persons presenting for a routine health check-up were collected as controls. Consent was obtained from both GC patients and healthy controls. The circulating cytokine study was also approved by the Human Ethical Committee, the Institutional Review Boards of the Affiliated Hospitals of Xuzhou Medical University. Plasma samples were collected from subjects and stored at -80 °C until analysis. The concentrations of IL-31, IL-32, and IL-33 were determined using an ELISA instrument (Bio-Rad 550, United States) at 450 nm, following the manufacturers’ instructions for human IL-31 (KGEHC141, KeyGEN BioTECH, Nanjing, Jiangsu Province, China), IL-32 (SEB802Hu, Cloud-Clone Corp, Wuhan, Hubei Province, China) and IL-33 (KGEHC151, KeyGEN BioTECH). All samples were tested in duplicate.
GraphPad Prism 6.0 and SPSS 16.0 statistical software packages were used for the statistical analysis of the results of immunohistochemistry and ELISA. Comparison between two groups was performed via the Mann-Whitney U-test. Comparisons among multi-groups were performed via the Kruskal-Wallis test. Low and high cut-off values for cytokine expression were defined by receiver operating characteristic (ROC) curve analysis. Survival curves were plotted by the Kaplan-Meier method and compared by the log-rank test. Cox proportional hazards model was used to identify the prognostic factors that influenced survival. P < 0.05 was considered statistically significant[35].
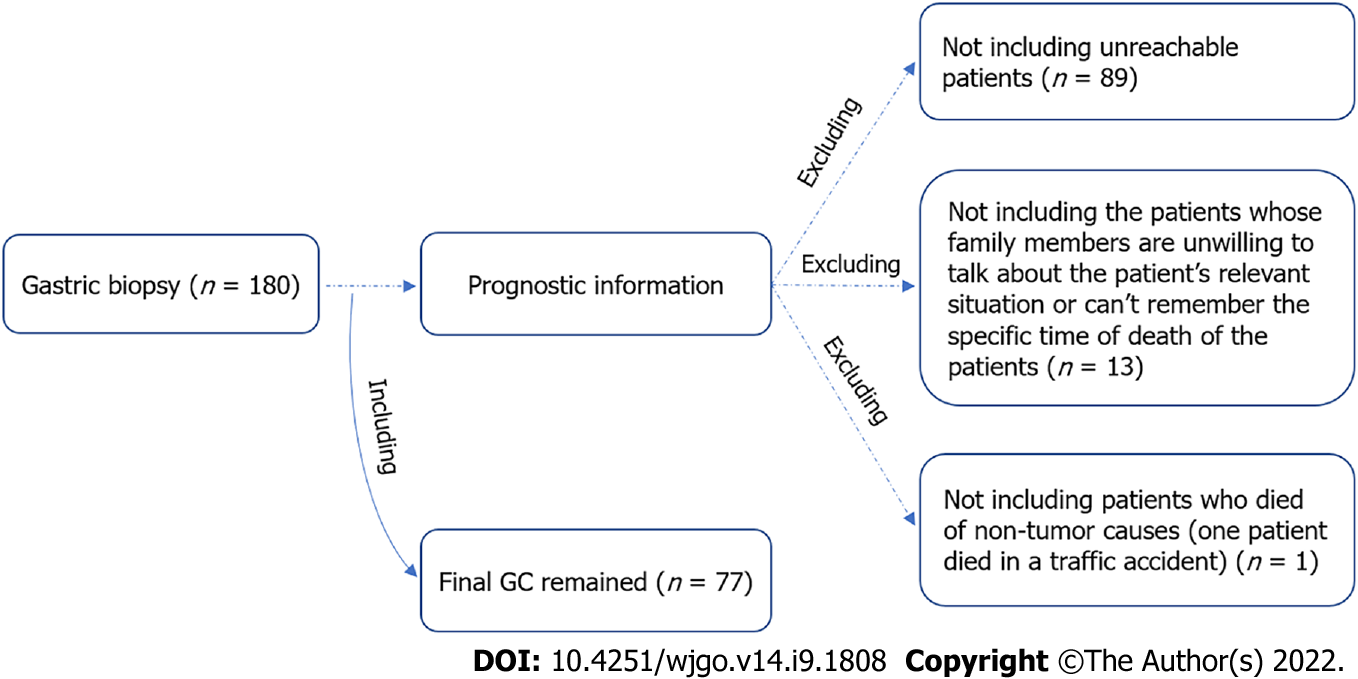
The detailed patients’ information is presented in Table 1. Notably, there were four early GC patients, specifically stage T1 patients, among the 180 GC patients involved (Table 1). The management of patients after gastric resection uniformly followed the 2018 Chinese guidelines for diagnosis and treatment of GC, the National Health Commission of The People's Republic of China[36]. All patients had complete clinical information. Among them, 77 had follow-up until their death or until their most recent contact. The other patients were lost to follow-up (Figure 1). There were 42 cancerrelated deaths among the 77 patients (54.5%). Thus, amongst the 77 cases, 6 were stage I, and 32 were stage II.
| Characteristic | Patient number | IL-31 median | P value | IL-32 median | P value | IL-33 median | P value |
| All cancer | 180 | 1.333 × 106 | 99245 | 125998 | |||
| Noncancer (non) | 159 | 1.472 × 106 | 0.043 | 138164 | 0.001 | 173818 | < 0.0001 |
| Gender | |||||||
| Male | 140 | 1.344 × 106 | 106075 | 143830 | |||
| Female | 40 | 1.208 × 106 | 0.329 | 81009 | 0.040 | 89697 | 0.029 |
| Age | |||||||
| ≤ 60 | 79 | 1.082 × 106 | 74098 | 106857 | |||
| > 60 | 101 | 1.404 × 106 | 0.007 | 122682 | 0.001 | 148615 | 0.026 |
| Tumour size (diameter) | |||||||
| < 5 cm | 87 | 1.325 × 106 | 98583 | 122572 | |||
| ≥ 5 cm | 93 | 1.335 × 106 | > 0.999 | 101583 | > 0.999 | 126415 | > 0.999 |
| Lymph node metastasis | |||||||
| No | 75 | 1.404 × 106 | 106075 | 143359 | |||
| Yes | 105 | 1.267 × 106 | 0.284 | 93196 | 0.671 | 113657 | 0.3 |
| Differentiation | |||||||
| High | 14 | 1.609 × 106 | H/M > 1 | 114379 | H/M > 1 | 171038 | H/M: > 0.999 |
| Moderate | 78 | 1.393 × 106 | H/L: 0.6 | 113024 | H/L> 1 | 142850 | H/L: 0.2 |
| Low | 88 | 1.146 × 106 | M/L: 0.3 | 91551 | M/L: 0.4 | 104570 | M/L: 0.1 |
| Invasion depth | |||||||
| T1 | 4 | 2.072 × 106 | 218529 | T1/T3: 0.5, T1/T4: 0.6 | 156096 | ||
| T2 | 27 | 1.600 × 106 | 110353 | 143582 | |||
| T3 | 75 | 1.208 × 106 | 98367 | 116081 | |||
| T4 | 74 | 1.318 × 106 | All > 1 | 96542 | T1/T2, T2/T3, T2/T4, T3/T4, all > 1 | 125941 | All > 1 |
| TNM | |||||||
| I | 12 | 1.355 × 106 | 98583 | 142117 | I/IV: 0.8 | ||
| II | 70 | 1.414 × 106 | 113560 | II/ IV: 0.6 | 147031 | II/III: 0.3, II/IV: 0.1 | |
| III | 92 | 1.288 × 106 | 87667 | 107919 | III/IV: 0.7 | ||
| IV | 6 | 0.950 × 106 | All > 1 | 54851 | I/II, I/III, I/IV, II/ III, III/IV, all > 1 | 52195 | I/II, I/III>1 |
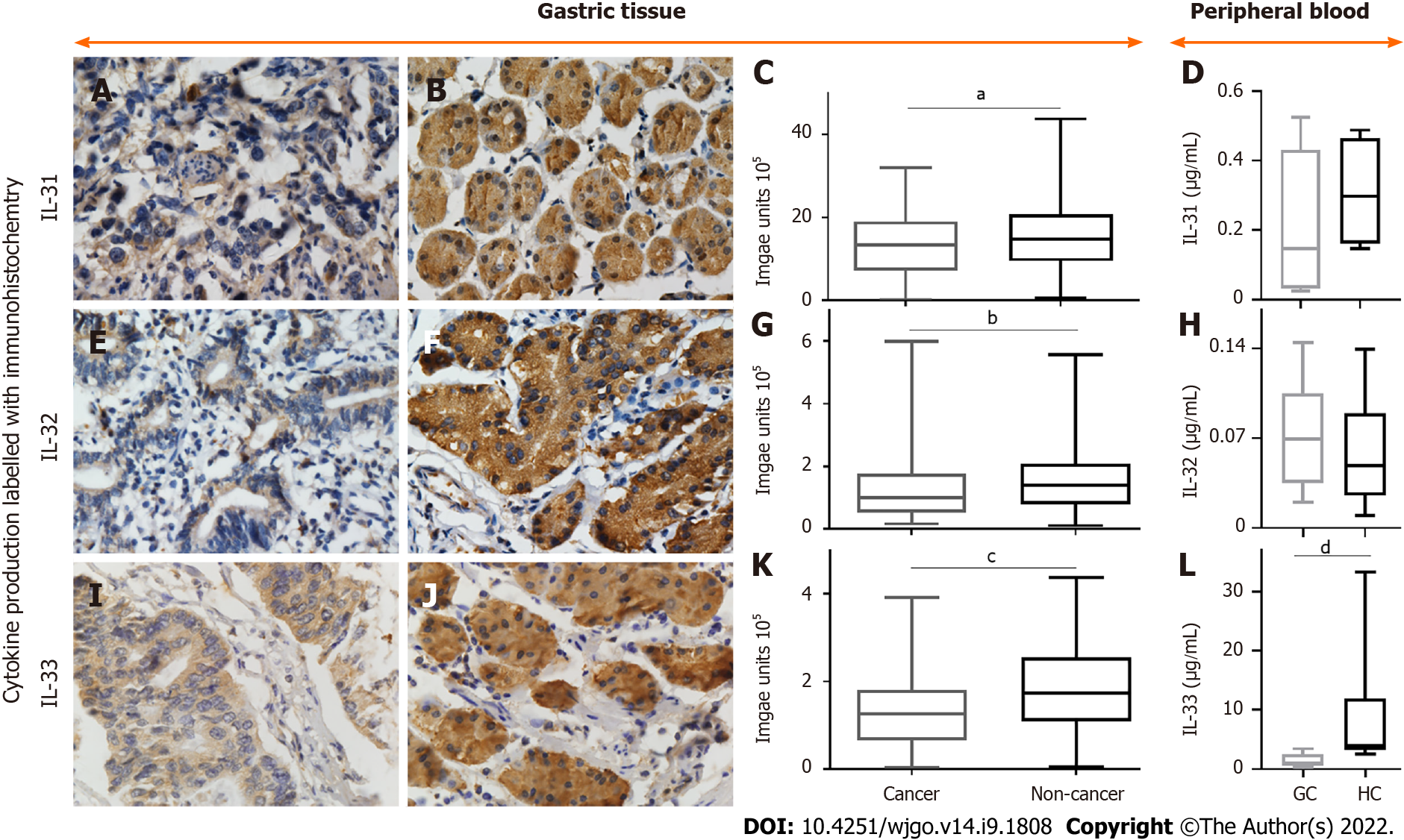
The expression levels of IL-31 (Figure 2A and B), IL-32 (Figure 2E and F), and IL-33 (Figure 1I and J) in GC tissue were investigated using immunohistochemistry. The densities of IL31 (Figure 1C), IL-32 (Figure 2G), and IL-33 (Figure 2K) are presented as box plots, including medians and 25th and 75th percentiles. IL-31, IL-32, and IL-33 were decreased by 9.4%, 28.2% and 27.5%, respectively, in GC compared to histologically normal adjacent gastric tissues (P < 0.05).
There was no significant difference in IL-31 (Figure 2D) or IL-32 (Figure 2H) concentration in the peripheral blood between GC patients and heathy controls. However, the mean value for IL-33 levels in peripheral blood of GC patients was 1.50 ± 1.11 ng/mL, which was significantly lower than that of healthy individuals (9.61 ± 8.00 ng/mL; P < 0.05) (Figure 2L).
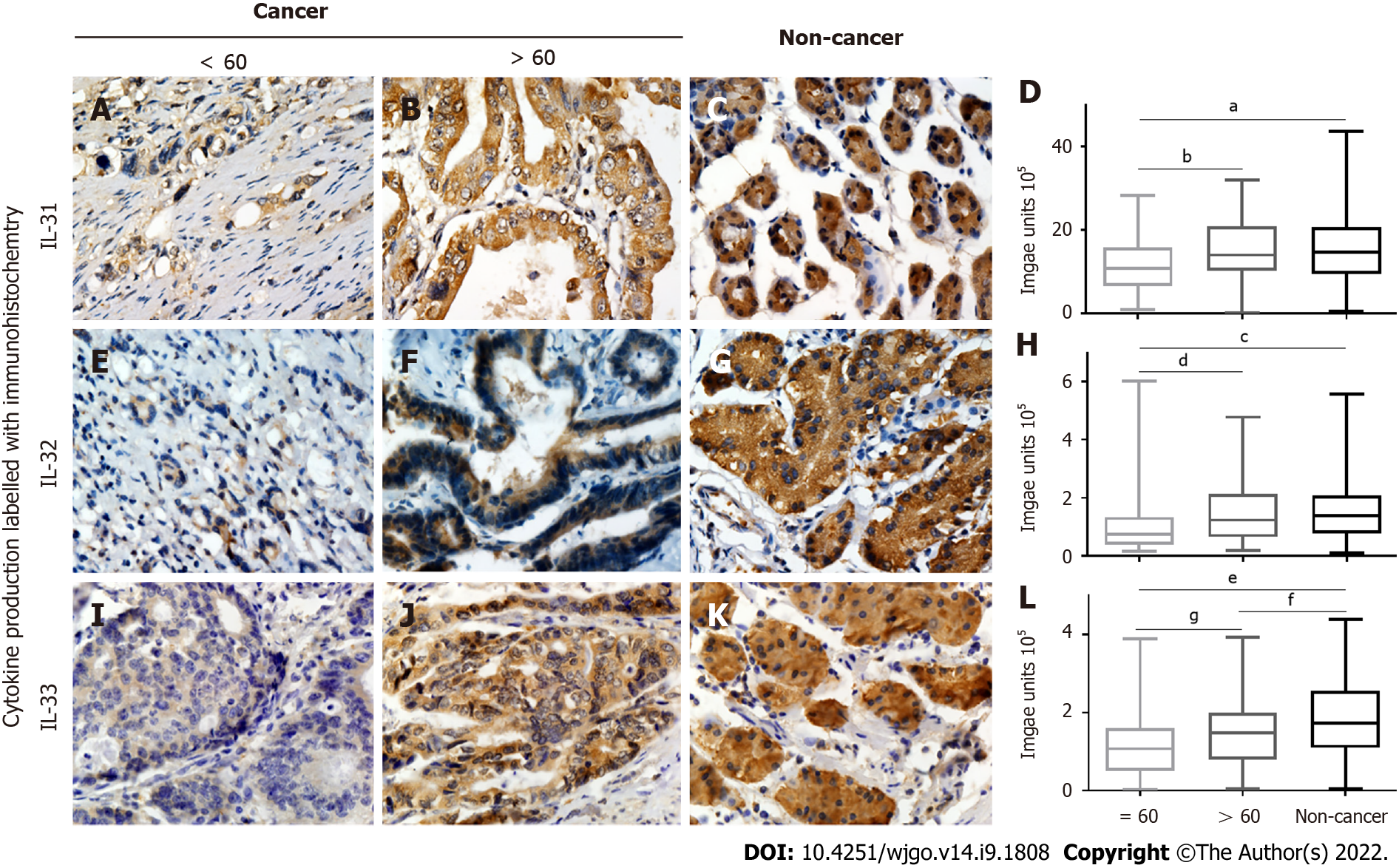
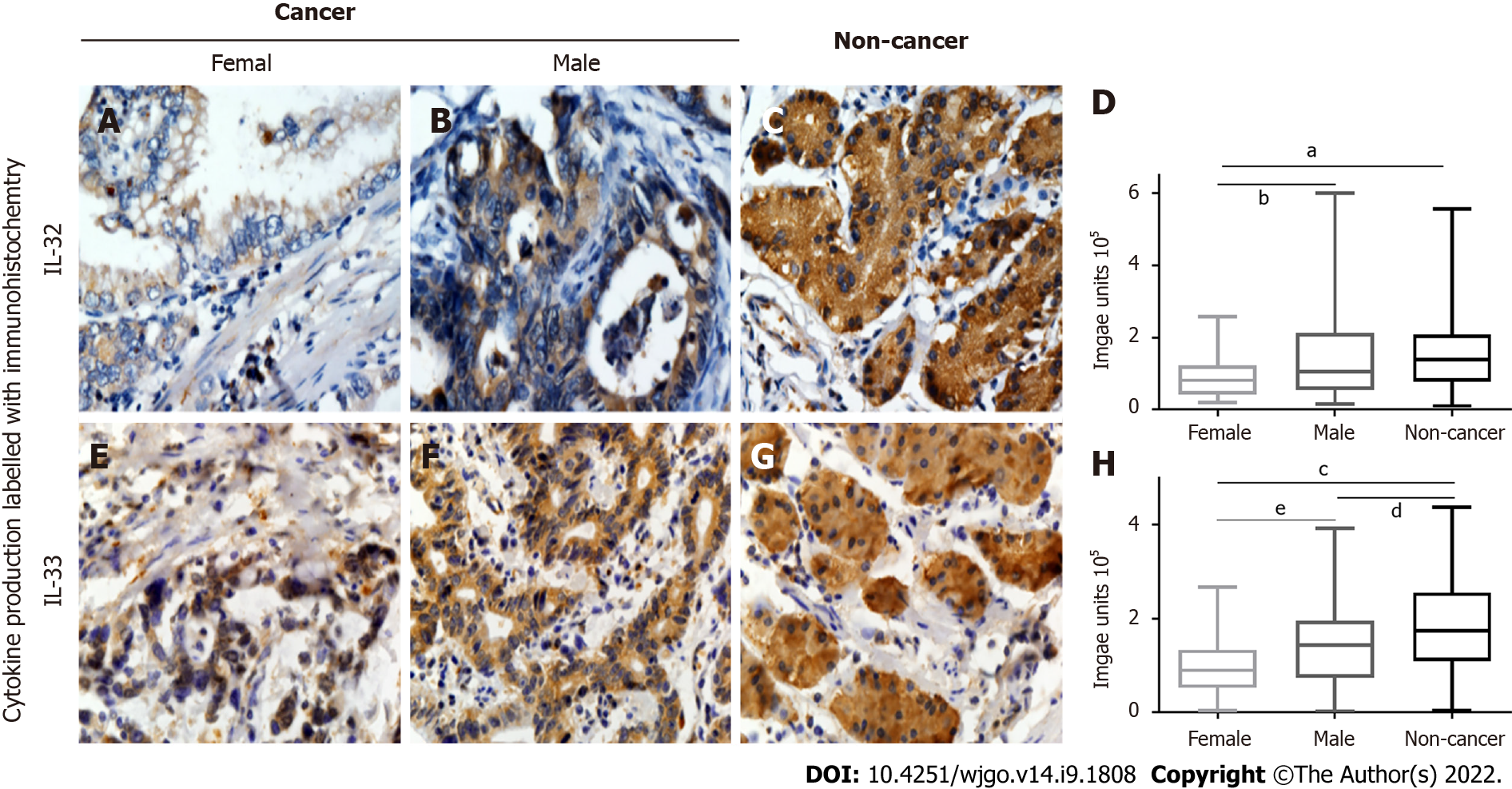
Associations between clinicopathological parameters and IL-31, IL-32, and IL-33 expression are listed in Table 1, Figures 3 and 4, and Supplementary Figures 1 and 2. All three ILs were associated with the age of GC patients (Figure 3A-D, IL-31; Figure 3E-H, IL-32; Figure 3I-L, IL-33). There was significantly lower expression of IL-31, IL-32, and IL-33 in the group of GC patients aged ≤ 60 years compared to the patients aged > 60 (P < 0.05). Significantly lower IL-32 (Figure 4A-D) and IL-33 (Figure 4E-H) expression was also observed in female GC patients compared to male GC patients (P < 0.05). However, no significant difference was observed in IL-31 expression when GC patients were stratified by sex (Supplementary Figure 2). Additionally, there were no correlations observed among IL31, IL-32, and IL-33 and other parameters, such as tumour size, lymph node metastasis, tumour differentiation, tumour invasion depth (Supplementary Figure 1), and TNM stage (Supplementary Figure 2) of GC.
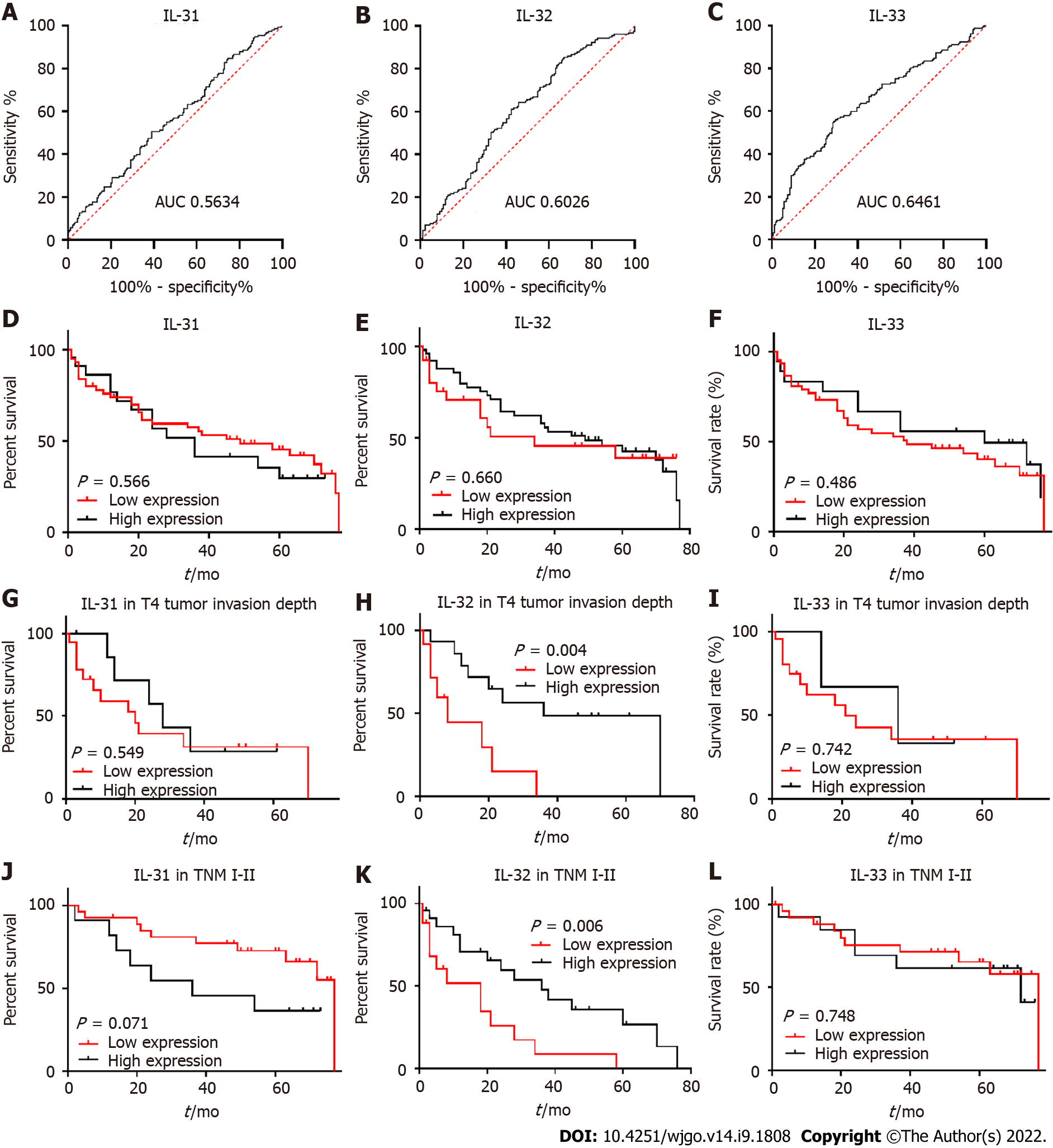
To evaluate whether decreased IL-31, IL-32, and IL-33 correlate with survival of GC patients, low and high cut-off points for IL-31 (Figure 5A), IL-32 (Figure 5B), and IL-33 (Figure 5C) were defined by ROC curve analysis (Figure 5). The cut-off values for the three ILs were determined to be: IL-31, 1486000 AU; IL-32, 64893 AU; IL-33, 166291 AU. Kaplan-Meier survival curves were constructed to compare the survival of GC patient with high and low expression of IL-31 (Figure 5D), IL-32 (Figure 5E), and IL33 (Figure 5F). The data revealed that there were no correlations between IL-31, IL-32, and IL-33 expression and the prognosis of GC patients (Figure 4). However, Kaplan-Meier analysis was applied to further compare overall survival according to IL-31 (Figure 5G), IL-32 (Figure 5H), and IL33 (Figure 5I) expression in different subgroups of GC (Figure 5). Figure 4 shows that decreased IL-32 staining correlated with a significantly worse survival of patients in the TNM I-II stage subgroup (P = 0.006) (Figure 5K) and in the tumour invasion depth T4 subgroup (P = 0.004). There were no significant differences in the other clinicopathological subgroups of GC for IL-31, IL-32, and IL-33 (Supple
Univariate and multivariate Cox regression analyses were used to examine whether IL-32 is an independent prognostic marker for subgroups of GC patients, including IL32 expression level, age, sex, tumour differentiation, lymph node invasion, tumour size, depth of tumour invasion, and TNM stage.
Data from patients within the T4 stage subgroup, analysed by univariate analysis, exhibited a correlation between the survival of GC patients and IL-32 expression and TNM stage. In multivariate analysis, IL-32 expression and TNM stage remained as significant independent prognostic factors for survival of GC patients (Table 2).
| Variables analysis | Univariate HR (95%CI) | P value | Multivariate HR (95%CI) | P value |
| IL-32 (low/high) | 4.338 (1.450-12.980) | 0.009 | 3.287 (1.024-10.555) | 0.046 |
| Tumour differentiation (low/moderate) | 0.710 (0.225-2.237) | 0.559 | ||
| TNM | 0.008 | 0.037 | ||
| IV (reference) | 1 | 1 | ||
| II | 0.034 (0.003-0.423) | 0.008 | 0.069 (0.005-0.946) | 0.045 |
| III | 0.203 (0.018-0.464) | 0.004 | 0.127 (0.025-0.646) | 0.013 |
| Lymph node metastasis (no/yes) | 0.441 (0.098-1.982) | 0.285 | ||
| Diameter (< 5/≥ 5, cm) | 0.475 (0.161-1.404) | 0.178 | ||
| Female/male | 0.912 (0.323-2.573) | 0.862 | ||
| Age (≤ 60/> 60) | 1.950 (0.688-5.529) | 0.209 |
Furthermore, decreased survival of GC patients in the TNM I-II stage subgroup was found to correlate with lymph node metastasis and tumour size on univariate analysis, but not on multivariate analysis. However, both univariate and multivariate analyses revealed no significant correlations between decreased IL-32 expression and survival of GC patients in the TNM I-II stage subgroup of GC patients (Table 3).
| Variable | Univariate HR (95%CI) | P value | Multivariate HR (95%CI) | P value |
| IL-32 (low/high) | 0.180 (0.024-1.370) | 0.098 | ||
| Tumour differentiation | 0.947 | |||
| Low (reference) | 1 | |||
| High | 1.259 (0.258-6.133) | 0.776 | ||
| Moderate | 0.964 (0.324-2.871) | 0.947 | ||
| Tumour invasion depth | 0.546 | |||
| T4 (reference) | 1 | |||
| T2 | 0.567 (0.059-5.491) | 0.624 | ||
| T3 | 1.460 (0.187-11.379) | 0.718 | ||
| Lymph node metastasis (no/yes) | 0.307 (0.108-0.868) | 0.026 | 0.490 (0.152-1.578) | 0.232 |
| Diameter (< 5/≥ 5, cm) | 0.259 (0.092-731) | 0.011 | 0.368 (0.112-1.165) | 0.088 |
| Female/male | 0.522 (0.116-2.340) | 0.396 | ||
| Age (≤ 60/> 60) | 0.562 (0.192-1.646) | 0.293 |
The current study demonstrated that the levels of expression of IL-31, IL-32, and IL-33 were all decreased in GC tissue compared to adjacent non-cancer gastric tissue and that the extent of these reductions in expression was higher in younger patients below the age of 60 years. Additionally, in the case of IL-32 and IL-33, their expression was found to be lower in females compared to males. However, the levels of expression of all three ILs amongst all the GC patients as a group did not correlate with a survival benefit, although subgroup analysis did reveal a survival benefit associated with higher levels of expression of IL-32 in the T4 stage and the TNM I-II stage subgroups.
H. pylori, a spiral Gram-negative rod that infects the human stomach in 50% of humans, is a definite human oncogenic agent[11], consistent with the previous finding that H. pylori contributed to > 60% of all GCs[12]. It has been clearly demonstrated by the Nobel laureate Barry Marshal that chronic gastric ulceration is caused by H. pylori infection[13]. The constitutive level of IL-32 is upregulated in both the gastric mucosa and GC tissue infected with H. pylori[14]. The cell-mediated immune response is extremely important in defence against tumour development, since compromised host immunity contributes to the establishment, proliferation, and metastasis of malignant tumours[15], a concept that is further supported by others who have shown that incompetent inflammation/immunity leads to tumour progression[16].
IL-31, an immunoregulatory cytokine secreted mainly by activated Th2 cells, plays a major role in the process of chronic inflammation[17]. However, the involvement of IL31 in the pathogenesis of cancer is unclear. Malignant Tcells produce IL-31, consistent with increased circulating IL-31[18]. Additionally, in the advanced stages of cutaneous T cell lymphoma, improved pruritus in patients correlates with lower levels of IL-31[19].
We found decreased IL-31 in GC patients, particularly in younger patients. Our data are consistent with other studies that have shown that younger patients are more likely to have more poorly differentiated tumours compared to older patients with GC, suggesting that younger GC patients have more malignant types of GC[37]. The activity of IL-31 is mediated through the IL31 receptor A (IL-31RA) and the oncostatin M receptor[38,39]. The two different isoforms of the IL-31RA consist of either long (745 residues) or short (560 residues) isoforms which may induce contrary functions[40]. Proliferation of follicular lymphoma is enhanced via the long IL-31RA isoform, whereas germinal centre-derived B-cell malignancy is inhibited via the short IL-31RA isoform[41]. There is no direct evidence available that identifies which isoform/s of IL-31RA are activated in GC via the IL-31 signalling pathway. However, our data are consistent with the hypothesis that IL-31 mediates an anti-cancer role in GC through the short IL-31RA isoform.
The involvement of IL-33 in non-small cell lung cancer is controversial, i.e., high IL33 has been found to be of diagnostic and prognostic value[27], but another report shows no significant associations[28] between IL-33 and the overall 5-year survival rate[32]. IL-33 promotes GC invasion/migration via stimulating MMP-3 and IL6 in vitro[29], which has been confirmed in a GC animal model by ablation of the cognate IL-33 receptor ST2[30]. IL-33 mRNA is significantly higher in GC tissue compared to that of non-cancer tissue[31], suggesting that IL-33 promotes the development of GC.
We observed similar levels of expression of IL-31 and IL-33 in GC, with decreased IL33 in both younger GC patients and in female GC patients, which is consistent with data from others, who have shown that female sex is a significant factor for predicting a higher likelihood of lymph node metastasis in mucosa-confined, poorly differentiated GC[42]. IL-33 is a multifunctional cytokine that can bind to the IL-33 receptor (ST2), to regulate immunity via activating Th1 cells, Th2 cells, CD8+ T cells, and NK cells[43,44]. There are two forms of ST2: The transmembrane form ST2L that when bound to IL-33, is able to activate target cells[45], and the soluble, secreted form of ST2 (sST2) that acts as a decoy receptor and negatively regulates IL-33 signalling[46]. The possible role of IL33 in carcinogenesis has been demonstrated in an IL-33 transgenic mouse metastasis model, demonstrating inhibition of the growth and metastasis of B16 melanoma and Lewis lung carcinoma cells, via activating CD8+ T cells and NK cells[47]. Thus, these data may be useful for future therapeutic design, utilising the anti-cancer role of IL-33 in GC.
IL-32, a proinflammatory cytokine, is highly expressed in several autoimmune diseases, e.g., rheumatoid arthritis, inflammatory bowel disease, and atopic dermatitis[20,21]. However, the role of IL-32 appears to vary amongst different forms of cancer, e.g., IL32 has been reported to inhibit colon cancer and leukaemia[22,23], but promotes pancreatic cancer[24]. The role of IL-32 in GC is also controversial, i.e., IL-32 is elevated in GC compared with normal stomach tissue[14], but other groups have found either substantially reduced IL32 expression in GC for the diffuse type of GC[26], or no significant difference has been observed between GC and normal stomach tissue[25]. These divergent observations concerning IL32 expression in GC may be due to different races and/or different tumour micro-environments.
We found that the expression of IL-32 was decreased in both younger patients and in female patients with GC, consistent with more severe forms of GC in younger and female patients, suggesting that IL-32 may mediate host defence against the development of GC. Furthermore, we found that high IL-32 expression correlated with a longer survival of GC patients, in the T4 stage and TNM I-II stage subgroups and that IL32 was an independent prognostic factor for survival in the T4 stage subgroup. Interestingly, the IL-32 positive rate in GC (12%) has been reported to be much lower than the rate in oesophageal squamous cell carcinoma (60%), but no comparison to non-cancerous tissues has been made[48,49]. Thus, we propose a hypothesis for the possible mechanism of IL-32 involvement in carcinogenesis as follows: Because IL-32 contributes to the host defence via enhancing differentiation of monocytes into macrophages[50], decreased IL-32 in GC tissue, seen particularly amongst the younger or female patients, may compromise host innate immunity, and subsequently contribute to poorly controlled development of cancer. Notably, macrophages are classified as either classical M1 macrophages that promote the inflammatory response against microorganism invasion and are thought to inhibit carcinogenesis, or as M2 macrophages that regulate host immunity and are thought to promote carcinogenesis[51]. It remains to be clarified whether tumourassociated macrophages in GC are derived from one subset or the other, which either promote the development of cancer (M2) or suppress cancer growth (M1), which is perhaps dependent on the tumour microenvironment[52]. For example, IL-32 can induce cell death in thyroid cancer cells through the induction of IL-8 and caspase-8[53], subsequently up-regulating the proinflammatory response.
IL-32 may also be able to inhibit tumour growth indirectly, hence it may be efficacious as a clinical anti-cancer therapy[54]. For example, the application of siRNA to inhibit IL-32 enhances angiogenesis in HUVECs[55] via up-regulation of VEGF and PDGF. Our current findings showed an inverse correlation between IL-32 and the development of GC, suggesting that IL-32 inhibits the development of cancer directly and/or indirectly, which will be further investigated in future experiments.
Finally, the levels of circulating IL-31, IL-32, and IL-33 were found to be consistent with their respective expression levels in GC tissue, further supporting the relevance of the potential role for these cytokines in mediating tumour-related immunity. However, we hypothesise that the host systemic and/or local inflammatory/immune response may be insufficient to inhibit the development of GC, among the GC cohorts studied, leading to tumour progression[16].
Unfortunately, no correlation with survival of GC patients was observed among any combination of IL-31, IL-32, and IL-33 expression, a similar result that we have reported previously for the relationship with IL-34 in GC[35]. The current observations are consistent with others, showing that there is no significant correlation between IL-33 expression and overall survival[32]. However, the advantage of our current data is the analysis for the combined IL-31, IL-32, and IL-33 data, to determine the correlation with GC patients from the same cohort. It remains to be explored why there is a discrepancy among IL-31, IL-32, IL-33, and IL-34 during the development of GC, which may be due to different receptors and/or signalling pathways, which will be clarified in the conditioning knockout mice in future studies.
There are some limitations for the current study. First, the number of GC patients and normal individuals who were sampled was rather small for the evaluation of circulating cytokines, using ELISA. However, this pilot study was undertaken to simply provide proof of concept that a systemic response is involved compared to only local cytokine expression in the affected gastric tissues, as well as to support our immunohistochemistry findings. A study with a larger sample size and a range of different backgrounds will be performed in the future.
Second, the stomach tissue of normal healthy people would be the ideal control for GC for comparison, and would offer more convincing evidence. However, we were unable to collect any normal healthy stomach tissue due to ethical issues. We are applying for human ethics approval for the collection of normal healthy stomach tissue from organ donors in the future.
The GC patient cohort recruited for this study was initially set at a reasonable size, i.e., 180 in total, to establish sufficient power to detect clinically relevant differences in the expression levels of the ILs that we examined. Regrettably, more than half of the patients were lost to follow-up during the course of the study, and only 77 GC patients had complete followup data (Figure 5). The data in relation to expression levels were based on all 180 patient samples that were initially recruited to ensure that the study was sufficiently powered to detect the potential role of IL31, IL32, and IL33 during the development of GC. If we had only selected the 77 GC patients with complete follow-up data for all aspects of this study, we would be highly likely to lose some important information and/or statistical power in exploring the correlation of these cytokines with clinical presentations. However, the survival analysis could only be performed on the adequately followed sub-cohort of 77 patients. We are currently collecting more samples with a full history and complete follow-up data in collaboration with other institutes, i.e., a larger number of samples for more convincing information for our future studies.
Because there was no local recurrence of GC within the current cohort, we cannot explore the potential role of these cytokines in the prediction of local recurrence of GC. We are currently searching for both primary and recurrent GC cases for future study.
In summary, our data demonstrate that IL-31, IL-32, and IL-33 expression in GC is all decreased, which correlates with younger age of the GC patients. IL-32 and IL-33 also correlate with the sex of the GC patients. Decreased IL-32 correlates with a poorer survival of GC patients in the T4 stage and TNM I-II stage subgroups. Downregulation of IL-32 is an independent prognostic factor for survival of T4 GC patients. Finally, low IL-33 in peripheral blood may be considered as an objective predictive marker for the development of GC. However, further studies are required to investigate the mechanism of action of these ILs in GC.
Gastric cancer (GC) is one of the most common malignancies in China with a high morbidity and mortality. Despite the more widespread use of recent diagnostic techniques, including endoscopic examination, many GC patients are diagnosed at advanced stages, resulting in a poor 5-year survival rate, emphasizing the critical need for development of a reliable biomarker(s) with high specificity and sensitivity to improve the prediction of prognosis for more successful outcomes for GC patients. Endoscopic examination provides a useful approach in the early detection of GC, and in reducing cancer-related mortality.
The cell-mediated immune response is extremely important in defence against tumour development, since compromised host immunity is known to contribute to the establishment, proliferation, and metastasis of malignant tumours. Although high host inflammatory status has been reported in the tumour microenvironment, an incompetent inflammatory/immune response will lead to tumour progression.
We aimed to identify the expression of interleukin (IL)-31, IL-32, and IL-33 in GC and assess their inter-correlation and clinical significance.
GC tissues were obtained from patients without local recurrences for immunohistochemistry to determine the expression of IL-31, IL-32, and IL-33. Additionally, circulating levels of IL-31, 32, 33 were determined using ELISA. The Mann-Whitney U test or the Kruskal-Wallis test was used for statistical analysis.
IL-31, IL-32, and IL-33 expression was all lower in GC than in adjacent non-cancer gastric tissues (P < 0.05). IL-33 level in peripheral blood of GC patients was significantly lower than that of healthy individuals (1.50 ± 1.11 vs 9.61 ± 8.00 ng/mL, (P < 0.05). Decreased IL-31, IL-32, and IL-33 expression in GC was observed in younger patients (< 60 years), and IL-32 and IL-33 expression was lower in female patients (P < 0.05). Higher IL-32 expression correlated with a longer survival in two GC subgroups: T4 invasion depth and TNM stage I-II. Univariate/multivariate analysis revealed that IL-32 was an independent prognostic factor for GC in the T4 stage subgroup. Circulating IL-33 was significantly lower in GC patients at TNM stage IV than in healthy people (P < 0.05).
IL-31, IL-32, and IL-33 expression in GC is all decreased, which correlates with younger age of the GC patients. IL-32 and IL-33 expression also correlates with the sex of the GC patients. Decreased IL-32 correlates with a poorer survival of GC patients in the T4 stage and TNM stage I-II subgroups. Down-regulation of IL-32 is an independent prognostic factor for survival of T4 GC patients. Finally, low IL-33 in peripheral blood may be considered as an objective predictive marker for the development of GC.
Further studies are required to investigate the mechanism of action of these ILs in GC.
We acknowledge the staff from the Department of Pathology, Xuzhou Medical University for their support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kawabata H, Japan; Yakar M, Turkey S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Li X
| 1. | Zheng R, Zeng H, Zhang S, Chen W. Estimates of cancer incidence and mortality in China, 2013. Chin J Cancer. 2017;36:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 2. | Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 306] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 3. | Yoon H, Kim N. Diagnosis and management of high risk group for gastric cancer. Gut Liver. 2015;9:5-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 184] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 4. | Calì B, Molon B, Viola A. Tuning cancer fate: the unremitting role of host immunity. Open Biol. 2017;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Kwak Y, Seo AN, Lee HE, Lee HS. Tumor immune response and immunotherapy in gastric cancer. J Pathol Transl Med. 2020;54:20-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Mager LF, Wasmer MH, Rau TT, Krebs P. Cytokine-Induced Modulation of Colorectal Cancer. Front Oncol. 2016;6:96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 167] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 7. | Zang X. 2018 Nobel Prize in medicine awarded to cancer immunotherapy: Immune checkpoint blockade - A personal account. Genes Dis. 2018;5:302-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 2068] [Article Influence: 188.0] [Reference Citation Analysis (0)] |
| 9. | Tan P, Yeoh KG. Genetics and Molecular Pathogenesis of Gastric Adenocarcinoma. Gastroenterology. 2015;149:1153-1162.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 360] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 10. | Li L, Wang X. Identification of gastric cancer subtypes based on pathway clustering. NPJ Precis Oncol. 2021;5:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | IARC working group on the evaluation of carcinogenic risks to humans: some industrial chemicals. Lyon, 15-22 February 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;60:1-560. [PubMed] |
| 12. | Holmes L Jr, Rios J, Berice B, Benson J, Bafford N, Parson K, Halloran D. Predictive Effect of Helicobacter pylori in Gastric Carcinoma Development: Systematic Review and Quantitative Evidence Synthesis. Medicines (Basel). 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3302] [Cited by in RCA: 3257] [Article Influence: 79.4] [Reference Citation Analysis (1)] |
| 14. | Sakitani K, Hirata Y, Hayakawa Y, Serizawa T, Nakata W, Takahashi R, Kinoshita H, Sakamoto K, Nakagawa H, Akanuma M, Yoshida H, Maeda S, Koike K. Role of interleukin-32 in Helicobacter pylori-induced gastric inflammation. Infect Immun. 2012;80:3795-3803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1633] [Cited by in RCA: 1640] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 16. | Viers BR, Boorjian SA, Frank I, Tarrell RF, Thapa P, Karnes RJ, Thompson RH, Tollefson MK. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol. 2014;66:1157-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 17. | Di Salvo E, Ventura-Spagnolo E, Casciaro M, Navarra M, Gangemi S. IL-33/IL-31 Axis: A Potential Inflammatory Pathway. Mediators Inflamm. 2018;2018:3858032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Singer EM, Shin DB, Nattkemper LA, Benoit BM, Klein RS, Didigu CA, Loren AW, Dentchev T, Wysocka M, Yosipovitch G, Rook AH. IL-31 is produced by the malignant T-cell population in cutaneous T-Cell lymphoma and correlates with CTCL pruritus. J Invest Dermatol. 2013;133:2783-2785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Cedeno-Laurent F, Singer EM, Wysocka M, Benoit BM, Vittorio CC, Kim EJ, Yosipovitch G, Rook AH. Improved pruritus correlates with lower levels of IL-31 in CTCL patients under different therapeutic modalities. Clin Immunol. 2015;158:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Joosten LA, Netea MG, Kim SH, Yoon DY, Oppers-Walgreen B, Radstake TR, Barrera P, van de Loo FA, Dinarello CA, van den Berg WB. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2006;103:3298-3303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 270] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 21. | Heinhuis B, Koenders MI, van Riel PL, van de Loo FA, Dinarello CA, Netea MG, van den Berg WB, Joosten LA. Tumour necrosis factor alpha-driven IL-32 expression in rheumatoid arthritis synovial tissue amplifies an inflammatory cascade. Ann Rheum Dis. 2011;70:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Marcondes AM, Mhyre AJ, Stirewalt DL, Kim SH, Dinarello CA, Deeg HJ. Dysregulation of IL-32 in myelodysplastic syndrome and chronic myelomonocytic leukemia modulates apoptosis and impairs NK function. Proc Natl Acad Sci U S A. 2008;105:2865-2870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Oh JH, Cho MC, Kim JH, Lee SY, Kim HJ, Park ES, Ban JO, Kang JW, Lee DH, Shim JH, Han SB, Moon DC, Park YH, Yu DY, Kim JM, Kim SH, Yoon DY, Hong JT. IL-32γ inhibits cancer cell growth through inactivation of NF-κB and STAT3 signals. Oncogene. 2011;30:3345-3359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Nishida A, Andoh A, Inatomi O, Fujiyama Y. Interleukin-32 expression in the pancreas. J Biol Chem. 2009;284:17868-17876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Gonzalez-Hormazabal P, Musleh M, Bustamante M, Stambuk J, Escandar S, Valladares H, Lanzarini E, Chiong H, Rojas J, Castro VG, Rubio-Reyes C, Jara L, Berger Z. Role of cytokine gene polymorphisms in gastric cancer risk in Chile. Anticancer Res. 2014;34:3523-3530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Pavlovic M, Gajovic N, Jurisevic M, Mitrovic S, Radosavljevic G, Pantic J, Arsenijevic N, Jovanovic I. Diverse Expression of IL-32 in Diffuse and Intestinal Types of Gastric Cancer. Gastroenterol Res Pract. 2018;2018:6578273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Hu LA, Fu Y, Zhang DN, Zhang J. Serum IL-33 as a diagnostic and prognostic marker in non- small cell lung cancer. Asian Pac J Cancer Prev. 2013;14:2563-2566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 28. | Naumnik W, Naumnik B, Niewiarowska K, Ossolinska M, Chyczewska E. Novel cytokines: IL-27, IL-29, IL-31 and IL-33. Can they be useful in clinical practice at the time diagnosis of lung cancer? Exp Oncol. 2012;34:348-353. [PubMed] |
| 29. | Yu XX, Hu Z, Shen X, Dong LY, Zhou WZ, Hu WH. IL-33 Promotes Gastric Cancer Cell Invasion and Migration Via ST2-ERK1/2 Pathway. Dig Dis Sci. 2015;60:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 30. | Eissmann MF, Dijkstra C, Jarnicki A, Phesse T, Brunnberg J, Poh AR, Etemadi N, Tsantikos E, Thiem S, Huntington ND, Hibbs ML, Boussioutas A, Grimbaldeston MA, Buchert M, O'Donoghue RJJ, Masson F, Ernst M. IL-33-mediated mast cell activation promotes gastric cancer through macrophage mobilization. Nat Commun. 2019;10:2735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 156] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 31. | Deng K, Wang H, Shan T, Chen Y, Zhou H, Zhao Q, Xia J. Tristetraprolin inhibits gastric cancer progression through suppression of IL-33. Sci Rep. 2016;6:24505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Hu W, Li X, Li Q, Tan Y, Xu B, Xie Q, Deng X, Lu B, Jiang J, Wu C. Interleukin-33 Expression does not Correlate with Survival of Gastric Cancer Patients. Pathol Oncol Res. 2017;23:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Liu QH, Shi ML, Bai J, Zheng JN. Identification of ANXA1 as a lymphatic metastasis and poor prognostic factor in pancreatic ductal adenocarcinoma. Asian Pac J Cancer Prev. 2015;16:2719-2724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Chen F, Qu M, Zhang F, Tan Z, Xia Q, Hambly BD, Bao S, Tao K. IL-36 s in the colorectal cancer: is interleukin 36 good or bad for the development of colorectal cancer? BMC Cancer. 2020;20:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Liu Q, Zhang Y, Zhang J, Tao K, Hambly BD, Bao S. Inverse correlation between Interleukin-34 and gastric cancer, a potential biomarker for prognosis. Cell Biosci. 2020;10:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | National Health Commission Of The People's Republic Of China. Chinese guidelines for diagnosis and treatment of gastric cancer 2018 (English version). Chin J Cancer Res. 2019;31:707-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 37. | Nakamura T, Yao T, Niho Y, Tsuneyoshi M. A clinicopathological study in young patients with gastric carcinoma. J Surg Oncol. 1999;71:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 38. | Diveu C, Lelièvre E, Perret D, Lak-Hal AH, Froger J, Guillet C, Chevalier S, Rousseau F, Wesa A, Preisser L, Chabbert M, Gauchat JF, Galy A, Gascan H, Morel A. GPL, a novel cytokine receptor related to GP130 and leukemia inhibitory factor receptor. J Biol Chem. 2003;278:49850-49859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Zhang Q, Putheti P, Zhou Q, Liu Q, Gao W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008;19:347-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 40. | Diveu C, Lak-Hal AH, Froger J, Ravon E, Grimaud L, Barbier F, Hermann J, Gascan H, Chevalier S. Predominant expression of the long isoform of GP130-like (GPL) receptor is required for interleukin-31 signaling. Eur Cytokine Netw. 2004;15:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Ferretti E, Tripodo C, Pagnan G, Guarnotta C, Marimpietri D, Corrias MV, Ribatti D, Zupo S, Fraternali-Orcioni G, Ravetti JL, Pistoia V, Corcione A. The interleukin (IL)-31/IL-31R axis contributes to tumor growth in human follicular lymphoma. Leukemia. 2015;29:958-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Pyo JH, Lee H, Min BH, Lee JH, Choi MG, Sohn TS, Bae JM, Kim KM, Ahn HS, Jung SH, Kim S, Kim JJ. A Risk Prediction Model Based on Lymph-Node Metastasis in Poorly Differentiated-Type Intramucosal Gastric Cancer. PLoS One. 2016;11:e0156207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Bonilla WV, Fröhlich A, Senn K, Kallert S, Fernandez M, Johnson S, Kreutzfeldt M, Hegazy AN, Schrick C, Fallon PG, Klemenz R, Nakae S, Adler H, Merkler D, Löhning M, Pinschewer DD. The alarmin interleukin-33 drives protective antiviral CD8⁺ T cell responses. Science. 2012;335:984-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 345] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 44. | Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 788] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 45. | Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179:2551-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 416] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 46. | Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. 2007;282:26369-26380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 440] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 47. | Gao K, Li X, Zhang L, Bai L, Dong W, Gao K, Shi G, Xia X, Wu L. Transgenic expression of IL-33 activates CD8(+) T cells and NK cells and inhibits tumor growth and metastasis in mice. Cancer Lett. 2013;335:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 48. | Ishigami S, Arigami T, Uchikado Y, Setoyama T, Kita Y, Sasaki K, Okumura H, Kurahara H, Kijima Y, Harada A, Ueno S, Natsugoe S. IL-32 expression is an independent prognostic marker for gastric cancer. Med Oncol. 2013;30:472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Nabeki B, Ishigami S, Uchikado Y, Sasaki K, Kita Y, Okumura H, Arigami T, Kijima Y, Kurahara H, Maemura K, Natsugoe S. Interleukin-32 expression and Treg infiltration in esophageal squamous cell carcinoma. Anticancer Res. 2015;35:2941-2947. [PubMed] |
| 50. | Netea MG, Lewis EC, Azam T, Joosten LA, Jaekal J, Bae SY, Dinarello CA, Kim SH. Interleukin-32 induces the differentiation of monocytes into macrophage-like cells. Proc Natl Acad Sci U S A. 2008;105:3515-3520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 51. | Ley K. M1 Means Kill; M2 Means Heal. J Immunol. 2017;199:2191-2193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 201] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 52. | Bao S, Hu R, Hambly BD. IL-34, IL-36 and IL-38 in colorectal cancer-key immunoregulators of carcinogenesis. Biophys Rev. 2020;12:925-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Heinhuis B, Plantinga TS, Semango G, Küsters B, Netea MG, Dinarello CA, Smit JWA, Netea-Maier RT, Joosten LAB. Alternatively spliced isoforms of IL-32 differentially influence cell death pathways in cancer cell lines. Carcinogenesis. 2016;37:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 54. | Yun HM, Oh JH, Shim JH, Ban JO, Park KR, Kim JH, Lee DH, Kang JW, Park YH, Yu D, Kim Y, Han SB, Yoon DY, Hong JT. Antitumor activity of IL-32β through the activation of lymphocytes, and the inactivation of NF-κB and STAT3 signals. Cell Death Dis. 2013;4:e640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 55. | Meyer N, Christoph J, Makrinioti H, Indermitte P, Rhyner C, Soyka M, Eiwegger T, Chalubinski M, Wanke K, Fujita H, Wawrzyniak P, Bürgler S, Zhang S, Akdis M, Menz G, Akdis C. Inhibition of angiogenesis by IL-32: possible role in asthma. J Allergy Clin Immunol. 2012;129:964-73.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |