Published online Sep 15, 2022. doi: 10.4251/wjgo.v14.i9.1785
Peer-review started: May 23, 2022
First decision: July 6, 2022
Revised: July 16, 2022
Accepted: August 15, 2022
Article in press: August 15, 2022
Published online: September 15, 2022
Processing time: 109 Days and 1.4 Hours
Multiple studies have demonstrated that neoadjuvant chemotherapy (NACT) can prolong the overall survival of pancreatic ductal adenocarcinoma (PDAC) patients. However, most studies have focused on open surgery following NACT.
To investigate the efficacy and safety of laparoscopic radical resection following NACT for PDAC.
We retrospectively analyzed the clinical data of 15 patients with pathologically confirmed PDAC who received NACT followed by laparoscopic radical surgery in our hospital from December 2019 to April 2022. All patients underwent abdo
All 15 patients with pancreatic cancer were successfully converted to surgical resection after NACT, including 8 patients with pancreatic head cancer and 7 patients with pancreatic body and tail cancer. Among them, 13 patients received the nab-paclitaxel plus gemcitabine regimen (gemcitabine 1000 mg/m2 plus nab-paclitaxel 125 mg/m2 on days 1, 8, and 15 every 4 wk) and 2 patients received the modified FOLFIRINOX regimen (intravenous oxaliplatin 68 mg/m2, irinotecan 135 mg/m2, and leucovorin 400 mg/m2 on day 1 and fluorouracil 400 mg/m2 on day 1, followed by 46-h continuous infusion of fluorouracil 2400 mg/m2). After each treatment cycle, abdominal CT, tumor markers, and circulating tumor cell counts were reviewed to evaluate the treatment efficacy. All 15 patients achieved partial remission. The surgical procedures included laparoscopic pancreaticoduodenectomy (LPD, n = 8) and laparoscopic radical antegrade modular pancreatosplenectomy (L-RAMPS, n = 7). None of them were converted to a laparotomy. One patient with pancreatic head carcinoma was found to have portal vein involvement during the operation, and LPD combined with vascular resection and reconstruction was performed. The amount of blood loss and operation times of L-RAMPS vs LPD were 435.71 ± 32.37 mL vs 343.75 ± 145.01 mL and 272.52 ± 49.14 min vs 444.38 ± 68.63 min, respectively. The number of dissected lymph nodes was 16.87 ± 4.10, and 3 patients had positive lymph nodes. One patient developed grade B postoperative pancreatic fistula (POPF) after L-RAMPS, and one patient experienced jaundice after LPD. None of the patients died after surgery. As of April 2022, progressive disease was noted in 4 patients, 2 patients had liver metastasis, and one had both liver metastasis and lymph node metastasis and died during the follow-up period.
Laparoscopic radical resection of PDAC after NACT is safe and effective if it is performed by a surgeon with rich experience in LPD and in a large center of pancreatic surgery.
Core Tip: We retrospectively analyzed the clinical data of 15 patients with pathologically confirmed pancreatic cancer who received neoadjuvant therapy followed by laparoscopic radical surgery in our hospital from December 2019 to April 2022. All patients underwent abdominal contrast-enhanced computed tomography (CT) and positron emission tomography-CT before surgery to accurately assess tumor stages and exclude distant metastasis. This retrospective study demonstrated that laparoscopic radical resection of pancreatic cancer after neoadjuvant therapy is safe and effective if it is performed by a surgeon with rich experience in laparoscopic pancreaticoduodenectomy in a large center of pancreatic surgery.
- Citation: He YG, Huang XB, Li YM, Li J, Peng XH, Huang W, Tang YC, Zheng L. Efficacy and safety of laparoscopic radical resection following neoadjuvant therapy for pancreatic ductal adenocarcinoma: A retrospective study. World J Gastrointest Oncol 2022; 14(9): 1785-1797
- URL: https://www.wjgnet.com/1948-5204/full/v14/i9/1785.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i9.1785
Pancreatic ductal adenocarcinoma (PDAC) is a highly malignant digestive system tumor with an extremely poor prognosis[1-4]. Surgical resection remains the only potentially curative therapy for pancreatic cancer, but only 10%-20% of PDAC patients are operable at diagnosis. Even among patients who have undergone surgery, the 5-year survival rate is below 20%[2-5]. Although surgery is still the main treatment to achieve long-term survival in PDAC patients, the cancer is often diagnosed in an advanced stage or a progressive stage, during which the large tumor size and increased number of nodules make surgical resection particularly risky and difficult to perform. Moreover, advances in surgical technology and increased surgical safety have not significantly improved the prognosis of PDAC patients, and surgical resection alone can no longer meet the comprehensive treatment needs of patients[6]. Therefore, the principle of the diagnosis and treatment of PDAC has gradually transitioned from “surgery first” to surgery-centered multidisciplinary modes to improve the overall outcomes of patients[7].
With the increased clinical application of neoadjuvant chemotherapy (NACT), many studies have indicated that by shrinking the primary tumor and reducing vascular invasion and micrometastatic lesions, NACT for PDAC can increase the resectability rate, lower the incidence of postoperative complications, and ultimately prolong survival and improve prognosis[8-10]. The 2021 National Comprehensive Cancer Network® (NCCN) guidelines recommend NACT for patients with high-risk resectable PDAC, borderline resectable PDAC, and locally advanced PDAC[11].
In recent years, with the development of surgical instruments and minimally invasive techniques, laparoscopic techniques have been increasingly applied in PDAC, and more studies have been performed in multicenter settings[12,13]. However, severe fibrosis of local tumor tissue may occur after NACT; in addition, most tumors are borderline resectable or advanced PDAC, with large tumor sizes and close relationships with blood vessels, making the surgical procedure more complicated and difficult[14]. Most reported patients with PDAC underwent open surgery after NACT[15-17]. On the basis of our experience in laparoscopic surgery and open surgery for PDAC[12,18], we performed laparoscopic radical resection of PDAC after NACT. This study aimed to investigate the efficacy and safety of laparoscopic radical resection following NACT for PDAC.
We retrospectively analyzed the clinical data of patients with PDAC in our hospital from December 2019 to April 2022. The patients were diagnosed with borderline resectable PDAC or locally advanced PDAC, which was confirmed by pathology and further assessed by medical imaging, and received NACT followed by laparoscopic surgery. All patients underwent abdominal contrast-enhanced computed tomography (CT) and positron emission tomography (PET)-CT before surgery to accurately assess tumor stage and exclude distant metastasis. This retrospective observational study was approved by the Medical Ethics Committee of our hospital and was conducted in accordance with the Declaration of Helsinki and the International Ethical Guidelines for Biomedical Research Involving Human Subjects (2022-r177-01). Written informed consent was obtained from all the patients.
Inclusion criteria were as follows: (1) PDAC was confirmed by pathology of endoscopic ultrasonography-guided fine-needle biopsy specimens; (2) NACT was recommended by a multidisciplinary team (MDT), as they believed that direct or immediate surgical resection was not feasible due to the inoperability of the tumor and/or other underlying diseases; and (3) Patients received at least 2 cycles of NACT. The exclusion criteria were as follows: (1) Distant metastasis found on preoperative PET-CT or other imaging examinations; (2) Received other antitumor treatments, such as radiotherapy or targeted therapy, before surgery; (3) Radical resection was not performed as scheduled during the operation; and (4) Presence of other malignant tumors.
NACT: First-line treatment regimens were adopted. Nab-paclitaxel plus gemcitabine regimen (AG regimen) [11] (n = 13, 86.7%): Gemcitabine 1000 mg/m2 plus nab-paclitaxel 125 mg/m2 on days 1, 8, and 15 every 4 wk; modified FOLFIRINOX regimen[19,20] (n = 2, 13.3%): Intravenous oxaliplatin 68 mg/m2, irinotecan 135 mg/m2, and leucovorin 400 mg/m2 on day 1 and fluorouracil 400 mg/m2 on day 1, followed by 46-h continuous infusion of fluorouracil 2400 mg/m2; 14 d as a cycle. Before each treatment, clinicians assessed the patient’s physical status and individual differences to adjust the drug dose and treatment cycle.
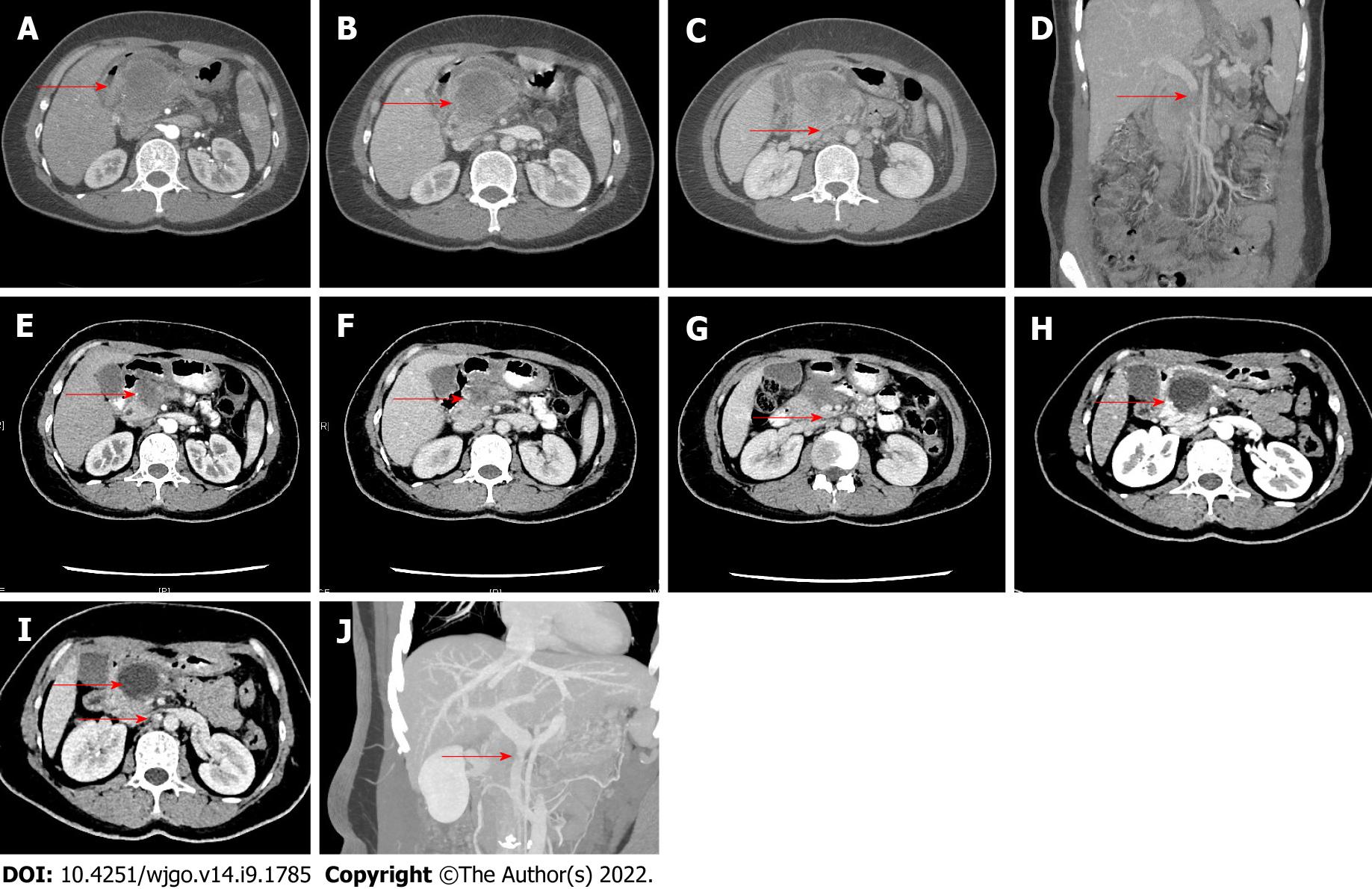
After each treatment cycle, abdominal CT, tumor markers, and circulating tumor cell (CTC) counts were reviewed to evaluate the treatment efficacy. The course of treatment consisted of 2-4 treatment cycles. After 2 treatment cycles, the treatment efficacy was assessed using the 2021 NCCN Guidelines and the Response Evaluation Criteria in Solid Tumors (version 1.1)[21]. NACT was judged as effective by a MDT if: (1) The tumor diameter was reduced; (2) The carbohydrate antigen 19-9 (CA19-9) level markedly decreased; (3) The CTC count significantly decreased; (4) The patient’s symptoms were obviously improved; and (5) There was no distant metastasis on PET-CT. After communicating with the patients and their families and obtaining written informed consent, we performed laparoscopic surgery. If the above criteria were not met, NACT might be continued. For borderline resectable or advanced PDAC, if the portal vein or superior mesenteric vein is partially involved or has a thrombus, resection should be considered only when appropriate vascular reconstruction at the distal and proximal ends is possible after vascular resection. The imaging findings before and after NACT are shown in Figure 1.
Surgical procedure: All patients laid in the prone position, with two legs apart. Under general anesthesia, the five-hole method was used to distribute the trocar position. The pneumoperitoneum pressure value was 12-14 mmHg, a 10-mm trocar was placed on the lower edge of the umbilicus to establish an observation port, two 12-mm trocars were placed on the left and right mid-clavicular lines parallel to the umbilicus, two 12-mm trocars were placed on the left and right anterior axillary lines, and one 5-12 mm trocar was placed on the costal edge to establish the main and auxiliary operating ports. The operator stood at the right side of the patient, the assistant stood at the left side of the patient, and the camera holder stood between the two legs of the patient.
The right-sided superior mesenteric artery (SMA) approach was used during laparoscopic pancreaticoduodenectomy (LPD), and the other surgical steps were the same as in the literature[18]. The lymph nodes on the right side of the SMA were dissected using a 180-degree arc incision. When the surgical maneuver was difficult, the “Easy First” approach was used instead, during which a vascular occlusion belt was placed, which made the difficult LPD safe and feasible[22]. After the operation, one abdominal drainage tube was placed ahead the pancreatic duct-jejunal anastomosis and one behind the bile duct-jejunal anastomosis, respectively.
Laparoscopic radical antegrade modular pancreatosplenectomy (L-RAMPS) was performed for pancreatic body and tail tumors. A retropancreatic tunnel was established in front of the superior mesenteric vein in the neck of the pancreas. After the pancreas was severed, the splenic artery and vein were isolated and then severed at their roots. The lymph nodes on the left side of the celiac trunk and SMA were dissected. Then, the pancreatic body and tail containing the tumor, the spleen, the left prerenal fascia, the left adrenal gland, and the left prerenal fat sac were removed en bloc from the back of the left prerenal fascia to the left along the surface of the left renal vein. Finally, the lymph nodes on the right side of the SMA were dissected, and the Heidelberg triangle (i.e., an anatomic triangle bordered by the SMA, celiac axis, and portal vein) was exposed (See Video).
Postoperative management: All patients received prophylactic antibiotics, proton-pump inhibitors, and parenteral nutrition during the perioperative period, and the nasogastric tube was removed on the 2nd postoperative day (POD 2). Patients started drinking water after feeling hungry, and a liquid diet was given after exhaustion. All patients were routinely tested for amylase levels in the drainage fluid on POD 3. When the amylase level in the drainage fluid was less than three times the normal upper limit of serum amylase and the risk of intra-abdominal hemorrhage was excluded, the abdominal drainage tube was removed (usually on day 5).
According to the International Study Group of Pancreatic Fistula[23], POPF was defined as a drainage amylase level of more than three times the normal serum amylase level on or after POD 3. The diagnosis of delayed gastric emptying (DGE) was based on the definition suggested by the International Study Group for Pancreatic Surgery in 2007[24]; i.e., a diagnosis of DGE was made if one of the following conditions occurred after excluding mechanical factors such as anastomotic obstruction by upper gastrointestinal barium study or gastroscopy: (1) The gastric tube needed to be indwelled for more than three days after surgery; (2) The gastric tube needed to be reinserted due to vomiting and other reasons after extubation; or (3) Solid food was still not allowed seven days after surgery. The diagnosis of surgical site infection was based on the criteria developed by the United States Nosocomial Infections Surveillance System, United States Centers for Disease Control[25]. The short-term postoperative complications were graded using the 2004 Clavien-Dindo system[26].
Statistical analysis was performed using the SPSS 26.0 software package (SPSS Inc., IBM, Armonk, NY, United States). The measurement data were first tested for normality. Normally distributed data are presented as the means ± SD; otherwise, medians (interquartile ranges) are used. The count data are expressed as the number of cases. The survival curve was plotted by the Kaplan-Meier method.
Fifteen patients with PDAC were included. After NACT, all patients were converted to resectable from borderline resectable or unresectable, including 8 patients (53.3%) with pancreatic head cancer and 7 patients (46.7%) with pancreatic body and tail cancer. Partial response to NACT was achieved in these 15 patients, and laparoscopic surgery was then performed. The demographic characteristics of all patients are shown in Table 1. There were 7 males (46.7%) and 8 females (53.3%) aged 55.53 ± 7.89 years. The average body mass index was 22.29 ± 2.94 kg/m2. Compared with the measurement/count values before NACT, the tumor diameter, CA19-9 level, and CTC count decreased by 28.40% ± 9.71%, 57.07% ± 32.07%, and 65.33% ± 12.09%, respectively, after NACT. After the tumors were assessed as resectable, all patients underwent PET-CT to rule out the possibility of distant metastases.
| Variables | |
| Sex, n (%) | |
| Male | 7 (46.7) |
| Female | 8 (53.3) |
| Age (yr) | 55.53 ± 7.89 |
| Body mass index, kg/m2 | 22.29 ± 2.94 |
| Resectability, n (%) | |
| Borderline resectable (n) | 7 (46.7) |
| Advanced pancreatic cancer (n) | 8 (53.3) |
| ASA grade, n (%) | |
| I | 13 (86.7) |
| II | 2 (13.3) |
| Chemotherapy regimen, n (%) | |
| AG | 13 (86.7) |
| Modified FOLFIRINOX | 2 (13.3) |
| ECOG score, n (%) | |
| 0 | 11 (73.3) |
| 1 | 2 (13.35) |
| 2 | 2 (13.35) |
| Chemotherapy cycle | 4 ± 1 |
| Response to chemotherapy | |
| PR | 15 (100%) |
| CR | 0 |
| Tumor diameter before chemotherapy (cm) | 4.17 ± 1.40 |
| Tumor diameter before surgery (cm) | 3.03 ± 1.13 |
| Tumor regression (%) | 28.40 ± 9.71 |
| CA19-9 level before chemotherapy (U/mL) | 736.25 (8.44-1200.00)1 |
| CA19-9 level before surgery (U/mL) | 51.85 (4.81-341.3)1 |
| Decrease in CA19-9 level (%) | 57.07 ± 32.07 |
| Total count of CTCs before chemotherapy (n) | 16 (13-26)1 |
| Total count of CTCs before surgery (n) | 7.13 ± 2.88 |
| Decrease in the total number of CTCs (%) | 65.33 ± 12.09 |
All surgeries were completed under laparoscopy, and none of them were converted to laparotomy. The surgical procedures included LPD (n = 8, 53.3%) and L-RAMPS (n = 7, 46.7%). One patient (6.67%) with pancreatic head carcinoma was found to have portal vein involvement during the operation, and LPD combined with vascular resection and reconstruction was performed. The L-RAMPS time was 272.52 ± 49.14 min, and the average intraoperative blood loss was 435.71 ± 32.37 mL; LPD lasted 444.38 ± 68.63 min, and the average intraoperative blood loss was 343.75 ± 145.01 mL. Intraoperative blood transfusion was administered in 4 patients (26.66%). The number of dissected lymph nodes was 16.87 ± 4.10. In one patient (6.67%) with pancreatic body and tail cancer, grade B POPF occurred after L-RAMPS and was improved after drainage, pancreatic enzyme replacement therapy, and nutritional counseling. One patient (6.67%) with pancreatic head cancer developed jaundice after LPD, in whom percutaneous transhepatic biliary drainage (PTBD) was performed after surgery, and the drainage catheter was removed two weeks later; the condition was improved after the placement of a biliary metal stent. None of the patients died after surgery. The average hospital stay was 13 (12-14) d (Table 2).
| Variables | |
| Tumor location, n (%) | |
| Head of the pancreas | 8 (53.3) |
| Pancreatic body and tail | 7 (46.7%) |
| Surgical procedure, n (%) | |
| L-RAMPS | 7 (46.7) |
| LPD | 8 (53.3) |
| Vascular resection and reconstruction, n (%) | 1 (6.67) |
| Operative time (min) | |
| L-RAMPS | 326.43 ± 49.14 |
| LPD | 444.38 ± 68.63 |
| Intraoperative blood loss (mL) | |
| L-RAMPS | 435.71 ± 262.54 |
| LPD | 343.75 ± 145.01 |
| Intraoperative blood transfusion, n (%) | |
| L-RAMPS | 2 (13.35) |
| LPD | 2 (13.35) |
| Conversion, n (%) | 0 (100) |
| Complications, n (%) | |
| Jaundice | 1 (6.67) |
| Grade B POPF | 1 (6.67) |
| Postoperative hospital stay (d) | 13 (12-14)1 |
| Follow-up duration (mo) | 7 (5-16)1 |
| Recurrence/metastasis, n (%) | |
| Liver metastasis | 3 (20) |
| Lymph node metastasis | 1 (6.67) |
| Mortality within the follow-up period, n (%) | 1 (6.67) |
R0 resection was achieved in all 15 patients. The postoperative pathology showed that all the tumors were PDAC, and residual cancer was detected by multipoint sampling in one patient. The tumors were moderately differentiated in 11 patients (73.33%), moderately to poorly differentiated in 3 patients (20%), and poorly differentiated in 1 patient (6.67%). According to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system, 4 patients (26.66%) were in stage IA, 7 (46.66%) were in stage IB, 1 (6.67%) was in stage IIB, 1 (6.67%) was in stage IIIA, 1 (6.67%) was in stage IIIB, and 1 (6.67%) was in stage IIIC. The number of dissected lymph nodes was 16.87 ± 4.10, and 3 patients (20%) had positive lymph nodes (Table 3).
| Variables | |
| Degree of differentiation, n (%) | |
| Moderately differentiated | 11 (73.33) |
| Moderately to poorly differentiated | 3 (20) |
| Poorly differentiated | 1 (6.67) |
| AJCC pathological stage, n (%) | |
| IA | 4 (26.66) |
| IB | 7 (46.66) |
| IIB | 1 (6.67) |
| IIIA | 1 (6.67) |
| IIIB | 1 (6.67) |
| IIIC | 1 (6.67) |
| R0 resection, n (%) | 15 (100) |
| Total number of lymph nodes dissected (n) | 16.87 ± 4.10 |
| Number of patients with positive lymph nodes (n) | 3 |
One patient (6.67%) developed a grade B POPF after surgery, which improved after drainage, pancreatic enzyme replacement therapy, and nutritional counseling. One patient (6.67%) had jaundice after LPD, and abdominal ultrasonography and magnetic resonance cholangiopancreatography showed anastomotic stenosis and dilated intrahepatic bile duct above the anastomosis. Thus, jaundice was considered to be caused by biliary-enteric anastomotic stenosis after cholangiojejunostomy. PTBD was then performed, and the drainage catheter was removed two weeks later. The biliary obstruction was alleviated after the placement of a biliary metal stent. We assumed that the patient had a small bile duct diameter, and anastomotic stenosis was caused by continuous suturing.
All patients who underwent surgery after NACT were evaluated for their physical status and nutritional status, and postoperative adjuvant therapy was scheduled if they could tolerate it. The adjuvant treatment regimen was selected according to the efficacy of NACT. After multidisciplinary discussions, 15 patients received 6 cycles of treatment after surgery. Generally, adjuvant therapy was started 6 to 8 wk after surgery and repeated every 3 wk. Routine blood tests and biochemical tests were performed before each chemotherapy session. Gastrointestinal tumor marker detection, CTC counts, and contrast-enhanced CT or magnetic resonance imaging (MRI) were performed every 3 cycles to determine whether the tumor had recurred or metastasized.
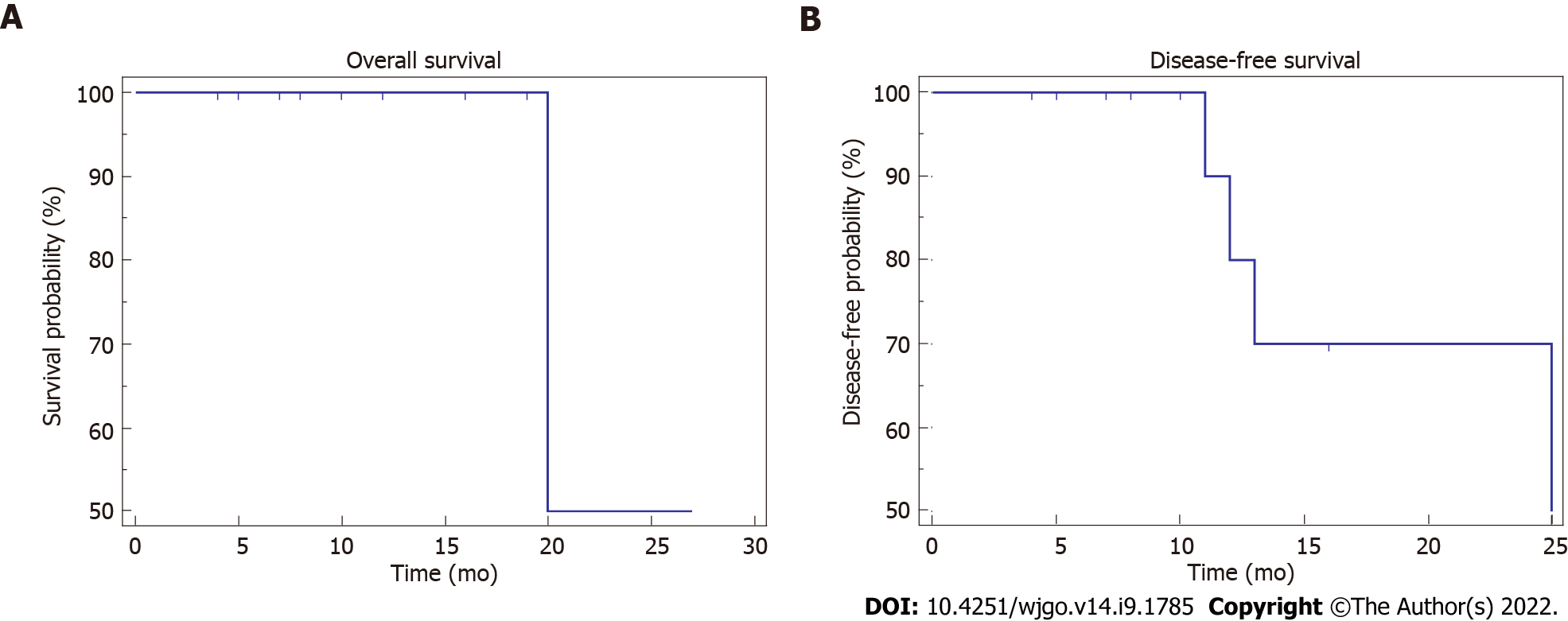
The patients were followed up every 3 mo after adjuvant chemotherapy was completed, during which routine blood tests, biochemical tests, gastrointestinal tumor marker detection, CTC counts, and contrast-enhanced CT or MRI examinations were performed. Follow-up was conducted by telephone, the WeChat app, and outpatient visits, and the date of the last follow-up visit was recorded. As of April 2022, all 15 patients had been followed up for 7 mo (range: 5-16 mo). Progressive disease was noted in 4 patients (26.66%), including liver metastases in 2 patients (13.3%), among whom one patient (6.67%) had both liver metastasis and lymph node metastasis. Adjuvant therapy was repeated when PD was detected during the follow-up period according to the opinions of the MDT. The patient with both liver metastasis and lymph node metastasis died due to tumor progression (Table 2). To date, the 1- and 2-year survival rates are both 50.00%, and the 1- and 2-year disease-free survival (DFS) rates are 60.00% and 40.00%, respectively (Table 2 and Figure 2).
Many studies have demonstrated that NACT can increase the R0 resection rate, prolong DFS, and increase the long-term survival rate in patients with borderline resectable PDAC[8-10]. Therefore, guidelines on PDAC treatment have included NACT as a recommended treatment option for resectable PDAC, borderline PDAC, and locally advanced PDAC with high-risk factors (high serum CA19-9 level, large primary tumor, extensive lymph node metastasis, significant weight loss, and extreme pain)[11]. Treatments for PDAC include chemotherapy, radiotherapy, and immunotherapy. Among them, NACT is the core treatment, resulting in notable efficacy when combined with other therapies[11].
The currently available NACT regimens for PDAC include FOLFIRINOX, modified FOLFIRINOX, AG regimen, and gemcitabine + S-1[11,27]. It has been reported that FOLFIRINOX and the AG regimen showed no significant difference in terms of the R0 resection rate and overall survival[28]. However, the AG regimen has many advantages: Acceptable toxicities; good tolerance, which leads to good compliance and a high rate of treatment completion; and good feasibility for the Chinese population[29]. Therefore, the AG regimen is used in most of the patients in our center, and modified FOLFIRINOX is also used in some patients. For patients with poor physical performance, gemcitabine + S-1 may be used to improve the quality of life and prolong the survival time. In the present study, the 15 patients whose diseases were successfully converted after NACT were all treated with the AG regimen, and these patients were in good general condition during the treatment and had no chemotherapy-induced myelosuppression.
Based on the Chinese and foreign guidelines[27,30], we used imaging findings (tumor diameter, relationship between tumor and adjacent blood vessels, and surrounding lymph nodes) before and after NACT, tumor markers [mainly CA19-9 but also carcinoembryonic antigen (CEA)], and improvement in clinical symptoms to evaluate the treatment cycle. For patients receiving NACT for PDAC, an abdominal contrast-enhanced CT examination was performed upon the completion of each cycle, CTA was performed every 2 cycles, and the levels of CA19-9, CEA, and other tumor markers were measured during each follow-up visit. Changes in clinical symptoms were monitored and recorded. CA19-9 can be easily influenced by various factors, such as inflammation and infection, and its potential as a biomarker for monitoring PDAC progression and recurrence has been compromised by false negative results before surgery. The use of microRNAs has been reported in the literature[31,32], but with limited clinical value. CTCs have been used as a predictor of long-term survival in patients with PDAC, and the overall survival and progression-free survival rates significantly decreased in patients with total CTCs ≥ 6[33,34]. In our study, we used the Canpatrol™ CTC assay (SurExam, Guangzhou, China) to detect CTCs before NACT and re-examined CTCs after each cycle of treatment. It was observed that the CTC count markedly increased in patients with elevated CA19-9 levels; in CA19-9-negative patients, the CTC count was also significantly higher than the normal value but gradually dropped with the application of NACT. Therefore, we speculate that CTCs may be one of the predictors of the resectability of PDAC. In the absence of standard evaluation criteria, monitoring changes in imaging features and tumor markers is currently an important method to evaluate the efficacy of NACT for PDAC[27].
Our criteria for the resectability of PDAC after NACT are as follows: (1) The diameter of the pan
R0 resection and lymph node negativity are key factors to ensure survival after PDAC surgery[35-37]. In addition to negative surgical margins, it is important to ensure a sufficient number of negative lymph nodes and negative vascular margins. After NACT for PDAC, the diameter of the primary lesion is decreased, along with a lowered rate of positive lymph nodes, which can reduce vascular invasion and micrometastases. According to the 8th edition of the AJCC guidelines, the number of lymph nodes to be dissected should be no less than 12. In the present study, the total number of lymph nodes dissected during surgery in the 15 patients was 16.87 ± 4.10, and the rate of positive lymph nodes was 2.1%, reaching the AJCC guideline-recommended requirement for lymph node dissection in pancreatic cancer. Another key factor in achieving R0 resection is a negative vascular margin. In a recent study, compared with laparotomy, LPD had similar short- and long-term prognoses, and LPD combined with venous resection and reconstruction was safe; notably, the laparoscopic technique was easier to perform.
Mokdad et al[15] reported on patients with resectable PDAC who received NACT followed by radical resection, and the study results showed that NACT significantly improved the overall survival of the patients (26 mo vs 21 mo, P < 0.01) and could reduce the positive rate of surgical margins (17% vs 24%, P < 0.01). The study by Reni et al[38] came to similar conclusions, with an overall survival time of 38.2 mo for patients with resectable PDAC who received NACT followed by surgery, compared with overall survival times of 20.4 mo and 26.2 mo for patients who underwent surgery followed by NACT. We have performed radical resection of PDAC following NACT since December 2019. Thus far, a total of 15 patients have achieved R0 resection, and the lymphadenectomy rate in these patients is high with a low positive rate, but during the follow-up process, 3 patients had liver metastasis, 1 patient had lymph node metastasis, and the rest were tumor-free. The 1-year survival rate and 2-year survival rate were 50.00%, the 1-year tumor-free survival rate was 60.00%, and the 2-year tumor-free survival rate was 40.00%. Most of our patients had been followed up for no more than 2 years (less than 1 year in most cases), which may explain the low 1- and 2-year survival rates.
We believe that resection is the challenging part of LPD after NACT for PDCA, and the difficulty of resection is the management of anatomical structure and vessels. Our experience is as follows: (1) Although more challenging, LPD after NACT can be performed by a surgeon with rich experience in LPD surgery; (2) It is very difficult to find a single approach that suitable for all cases. During the operation, we preferentially adopt the “early first” principle, and gradually separate and resect to complete. However, in some cases, we chose different arterial approaches according to the direction of tumor invasion; (3) Due to portal vein adhesion and tumor invasion after NACT in some pancreatic cases, procedures of the superior mesenteric vein behind the neck of the pancreas may cause bleeding, and the establishment of a retropanctreatic tunnel is more challenging. In these cases, the pancreas can be separated and resected from 2-3 cm to the left side of the superior mesenteric vein and the neck of the pancreas. The advantage of choosing here is that it is far away from the tumor, the tissue separation is easier than performing behind the neck of the pancreas, and the space between the splenic vein and the pancreas can be easily separated. It is safer to search the superior mesenteric vein after the resection of the pancreas and dissection of surrounding tissues from left to right; and (4) The digestive tract reconstruction was performed according to a routine procedure after lesion resection in pancreaticoduodenectomy and was barely affected by NACT.
Laparoscopic radical resection of PDAC after NACT is safe and feasible if it is performed by an operator with experience of at least 100 cases of relevant surgeries in a specialist pancreas center. However, since our study was a retrospective analysis with a small sample from a single center, the safety and feasibility of this technique need to be verified by prospective large-sample randomized controlled trials in multiple pancreatic centers.
Multiple studies have demonstrated that neoadjuvant chemotherapy (NACT) can prolong the overall survival of pancreatic ductal adenocarcinoma (PDAC) patients. However, most studies have focused on open surgery following NACT.
Despite the development of surgical instruments and minimally invasive techniques, laparoscopic techniques have been increasingly applied in pancreatic surgery. However, most reported cases of PDAC patients underwent open surgery after NACT. At present, we performed laparoscopic radical resection of PDAC after NACT.
Our aims were to investigate the efficacy and safety of laparoscopic radical resection following NACT for PDAC.
We retrospectively analyzed the clinical data of 15 patients with pathologically confirmed PDAC who received NACT followed by laparoscopic radical surgery in our hospital from December 2019 to April 2022. All patients underwent abdominal contrast-enhanced computed tomography (CT) and positron emission tomography-CT before surgery to accurately assess tumor stage and exclude distant metastasis.
All 15 patients with PDAC were successfully converted to surgical resection after NACT, including 8 patients with pancreatic head cancer and 7 patients with pancreatic body and tail cancer. Among them, 13 patients received the nab-paclitaxel plus gemcitabine regimen (gemcitabine 1000 mg/m2 plus nab-paclitaxel 125 mg/m2 on days 1, 8, and 15 every 4 wk), and 2 patients received the modified FOLFIRINOX regimen (intravenous oxaliplatin 68 mg/m2, irinotecan 135 mg/m2, and leucovorin 400 mg/m2 on day 1 and fluorouracil 400 mg/m2 on day 1, followed by a 46-h continuous infusion of fluorouracil 2400 mg/m2). After each treatment cycle, abdominal CT, tumor markers, and circulating tumor cell (CTC) counts were reviewed to evaluate the treatment efficacy. All 15 patients achieved partial remission. The surgical procedures included laparoscopic pancreaticoduodenectomy (LPD, n = 8) and laparoscopic radical antegrade modular pancreatosplenectomy (L-RAMPS, n = 7). One patient with pancreatic head carcinoma was found to have portal vein involvement during the operation, and LPD combined with vascular resection and reconstruction was performed. One patient developed grade B postoperative pancreatic fistula after L-RAMPS, and one patient experienced jaundice after LPD. None of the patients died after surgery.
Laparoscopic radical resection of PDAC after neoadjuvant therapy is safe and effective if it is performed by a surgeon with rich experience in LPD and L-RAMPS in a large center of pancreatic surgery.
With the increased clinical application of NACT, many studies have indicated that by shrinking the primary tumor and reducing vascular invasion and micrometastatic lesions, NACT for PDAC can increase the resectability rate, lower the incidence of postoperative complications, and ultimately prolong survival and improve prognosis. Most reported cases of pancreatic cancer patients underwent open surgery after NACT. LPD has certain advantages, such as less trauma, quick recovery, less bleeding, and a good postoperative quality of life. Therefore, laparoscopic surgery after NACT for PDAC has certain advantages.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Trna J, Czech Republic; Zimmitti G, Italy S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Schizas D, Charalampakis N, Kole C, Economopoulou P, Koustas E, Gkotsis E, Ziogas D, Psyrri A, Karamouzis MV. Immunotherapy for pancreatic cancer: A 2020 update. Cancer Treat Rev. 2020;86:102016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 295] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 2. | Carpenter E, Nelson S, Bednar F, Cho C, Nathan H, Sahai V, di Magliano MP, Frankel TL. Immunotherapy for pancreatic ductal adenocarcinoma. J Surg Oncol. 2021;123:751-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 3. | Balachandran VP, Beatty GL, Dougan SK. Broadening the Impact of Immunotherapy to Pancreatic Cancer: Challenges and Opportunities. Gastroenterology. 2019;156:2056-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 326] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 4. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1671] [Article Influence: 334.2] [Reference Citation Analysis (1)] |
| 5. | Ducreux M, Seufferlein T, Van Laethem JL, Laurent-Puig P, Smolenschi C, Malka D, Boige V, Hollebecque A, Conroy T. Systemic treatment of pancreatic cancer revisited. Semin Oncol. 2019;46:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 6. | Winter JM, Brennan MF, Tang LH, D'Angelica MI, Dematteo RP, Fong Y, Klimstra DS, Jarnagin WR, Allen PJ. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol. 2012;19:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 279] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 7. | Patkar V, Acosta D, Davidson T, Jones A, Fox J, Keshtgar M. Cancer multidisciplinary team meetings: evidence, challenges, and the role of clinical decision support technology. Int J Breast Cancer. 2011;2011:831605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Nagakawa Y, Sahara Y, Hosokawa Y, Murakami Y, Yamaue H, Satoi S, Unno M, Isaji S, Endo I, Sho M, Fujii T, Takishita C, Hijikata Y, Suzuki S, Kawachi S, Katsumata K, Ohta T, Nagakawa T, Tsuchida A. Clinical Impact of Neoadjuvant Chemotherapy and Chemoradiotherapy in Borderline Resectable Pancreatic Cancer: Analysis of 884 Patients at Facilities Specializing in Pancreatic Surgery. Ann Surg Oncol. 2019;26:1629-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 9. | Murphy JE, Wo JY, Ryan DP, Jiang W, Yeap BY, Drapek LC, Blaszkowsky LS, Kwak EL, Allen JN, Clark JW, Faris JE, Zhu AX, Goyal L, Lillemoe KD, DeLaney TF, Fernández-Del Castillo C, Ferrone CR, Hong TS. Total Neoadjuvant Therapy With FOLFIRINOX Followed by Individualized Chemoradiotherapy for Borderline Resectable Pancreatic Adenocarcinoma: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 434] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 10. | Wolff RA. Adjuvant or Neoadjuvant Therapy in the Treatment in Pancreatic Malignancies: Where Are We? Surg Clin North Am. 2018;98:95-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:439-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 694] [Article Influence: 173.5] [Reference Citation Analysis (0)] |
| 12. | Wang M, Li D, Chen R, Huang X, Li J, Liu Y, Liu J, Cheng W, Chen X, Zhao W, Tan Z, Huang H, Zhu F, Qin T, Ma J, Yu G, Zhou B, Zheng S, Tang Y, Han W, Meng L, Ke J, Feng F, Chen B, Yin X, Chen W, Ma H, Xu J, Lin R, Dong Y, Yu Y, Zhang H, Qin R; Minimally Invasive Treatment Group in the Pancreatic Disease Branch of China's International Exchange and Promotion Association for Medicine and Healthcare (MITG-P-CPAM). Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2021;6:438-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 166] [Article Influence: 41.5] [Reference Citation Analysis (1)] |
| 13. | Qin R, Kendrick ML, Wolfgang CL, Edil BH, Palanivelu C, Parks RW, Yang Y, He J, Zhang T, Mou Y, Yu X, Peng B, Senthilnathan P, Han HS, Lee JH, Unno M, Damink SWMO, Bansal VK, Chow P, Cheung TT, Choi N, Tien YW, Wang C, Fok M, Cai X, Zou S, Peng S, Zhao Y. International expert consensus on laparoscopic pancreaticoduodenectomy. Hepatobiliary Surg Nutr. 2020;9:464-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Barreto SG, Loveday B, Windsor JA, Pandanaboyana S. Detecting tumour response and predicting resectability after neoadjuvant therapy for borderline resectable and locally advanced pancreatic cancer. ANZ J Surg. 2019;89:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 15. | Mokdad AA, Minter RM, Zhu H, Augustine MM, Porembka MR, Wang SC, Yopp AC, Mansour JC, Choti MA, Polanco PM. Neoadjuvant Therapy Followed by Resection Versus Upfront Resection for Resectable Pancreatic Cancer: A Propensity Score Matched Analysis. J Clin Oncol. 2017;35:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 307] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 16. | Ren X, Wei X, Ding Y, Qi F, Zhang Y, Hu X, Qin C, Li X. Comparison of neoadjuvant therapy and upfront surgery in resectable pancreatic cancer: a meta-analysis and systematic review. Onco Targets Ther. 2019;12:733-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Rangelova E, Wefer A, Persson S, Valente R, Tanaka K, Orsini N, Segersvärd R, Arnelo U, Del Chiaro M. Surgery Improves Survival After Neoadjuvant Therapy for Borderline and Locally Advanced Pancreatic Cancer: A Single Institution Experience. Ann Surg. 2021;273:579-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 18. | Tang YC, Liu QQ, He YG, Li J, Huang XB. Laparoscopic pancreaticoduodenectomy: a retrospective study of 200 cases and the optimization of the single-center learning curve. Transl Cancer Res. 2021;10:3436-3447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Mahaseth H, Brutcher E, Kauh J, Hawk N, Kim S, Chen Z, Kooby DA, Maithel SK, Landry J, El-Rayes BF. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas. 2013;42:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 20. | Bai X, Su R, Ma T, Shen S, Li G, Lou J, Gao S, Que R, Yuan Y, Yu R, Wei Q, Liang T. [Modified FOLFIRINOX for advanced pancreatic cancer: a tertiary center experience from China]. Zhonghua Wai Ke Za Zhi. 2016;54:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 21. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21611] [Article Influence: 1350.7] [Reference Citation Analysis (1)] |
| 22. | Jin WW, Ajoodhea H, Mou YP, Zhang RC, Lu C, Xu X-WJ. Tips of laparoscopic pancreaticoduodenectomy for borderline resectable pancreatic cancer: “easy first” approach. Transl Cancer Res. 2016;5:613-617. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 2957] [Article Influence: 369.6] [Reference Citation Analysis (35)] |
| 24. | Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Büchler MW. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 2327] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 25. | Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control. 1992;20:271-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 565] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 26. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24829] [Article Influence: 1182.3] [Reference Citation Analysis (0)] |
| 27. | Qiu JD, Zhu RZ, Chen H, Zhang TP. [Interpretation of the guidelines for neoadjuvant therapy of pancreatic cancer in China(2020 edition)]. Zhonghua Wai Ke Za Zhi. 2021;59:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Dhir M, Zenati MS, Hamad A, Singhi AD, Bahary N, Hogg ME, Zeh HJ 3rd, Zureikat AH. FOLFIRINOX Versus Gemcitabine/Nab-Paclitaxel for Neoadjuvant Treatment of Resectable and Borderline Resectable Pancreatic Head Adenocarcinoma. Ann Surg Oncol. 2018;25:1896-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 29. | Miyasaka Y, Ohtsuka T, Kimura R, Matsuda R, Mori Y, Nakata K, Kakihara D, Fujimori N, Ohno T, Oda Y, Nakamura M. Neoadjuvant Chemotherapy with Gemcitabine Plus Nab-Paclitaxel for Borderline Resectable Pancreatic Cancer Potentially Improves Survival and Facilitates Surgery. Ann Surg Oncol. 2019;26:1528-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Michelakos T, Pergolini I, Castillo CF, Honselmann KC, Cai L, Deshpande V, Wo JY, Ryan DP, Allen JN, Blaszkowsky LS, Clark JW, Murphy JE, Nipp RD, Parikh A, Qadan M, Warshaw AL, Hong TS, Lillemoe KD, Ferrone CR. Predictors of Resectability and Survival in Patients With Borderline and Locally Advanced Pancreatic Cancer who Underwent Neoadjuvant Treatment With FOLFIRINOX. Ann Surg. 2019;269:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 242] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 31. | Kawaguchi T, Komatsu S, Ichikawa D, Morimura R, Tsujiura M, Konishi H, Takeshita H, Nagata H, Arita T, Hirajima S, Shiozaki A, Ikoma H, Okamoto K, Ochiai T, Taniguchi H, Otsuji E. Clinical impact of circulating miR-221 in plasma of patients with pancreatic cancer. Br J Cancer. 2013;108:361-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 32. | Funamizu N, Lacy CR, Kamada M, Yanaga K, Manome Y. MicroRNA-200b and -301 are associated with gemcitabine response as biomarkers in pancreatic carcinoma cells. Int J Oncol. 2019;54:991-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Zhao XH, Wang ZR, Chen CL, Di L, Bi ZF, Li ZH, Liu YM. Molecular detection of epithelial-mesenchymal transition markers in circulating tumor cells from pancreatic cancer patients: Potential role in clinical practice. World J Gastroenterol. 2019;25:138-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 34. | Pan Y, Li D, Yang J, Wang N, Xiao E, Tao L, Ding X, Sun P. Portal Venous Circulating Tumor Cells Undergoing Epithelial-Mesenchymal Transition Exhibit Distinct Clinical Significance in Pancreatic Ductal Adenocarcinoma. Front Oncol. 2021;11:757307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Morales-Oyarvide V, Rubinson DA, Dunne RF, Kozak MM, Bui JL, Yuan C, Qian ZR, Babic A, Da Silva A, Nowak JA, Khalaf N, Brais LK, Welch MW, Zellers CL, Ng K, Chang DT, Miksad RA, Bullock AJ, Tseng JF, Swanson RS, Clancy TE, Linehan DC, Findeis-Hosey JJ, Doyle LA, Hornick JL, Ogino S, Fuchs CS, Hezel AF, Koong AC, Wolpin BM. Lymph node metastases in resected pancreatic ductal adenocarcinoma: predictors of disease recurrence and survival. Br J Cancer. 2017;117:1874-1882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 36. | Chopra A, Zenati M, Hogg ME, Zeh HJ 3rd, Bartlett DL, Bahary N, Zureikat AH, Beane JD. Impact of Neoadjuvant Therapy on Survival Following Margin-Positive Resection for Pancreatic Cancer. Ann Surg Oncol. 2021;28:7759-7769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Marchegiani G, Andrianello S, Nessi C, Sandini M, Maggino L, Malleo G, Paiella S, Polati E, Bassi C, Salvia R. Neoadjuvant Therapy Versus Upfront Resection for Pancreatic Cancer: The Actual Spectrum and Clinical Burden of Postoperative Complications. Ann Surg Oncol. 2018;25:626-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 38. | Reni M, Balzano G, Zanon S, Zerbi A, Rimassa L, Castoldi R, Pinelli D, Mosconi S, Doglioni C, Chiaravalli M, Pircher C, Arcidiacono PG, Torri V, Maggiora P, Ceraulo D, Falconi M, Gianni L. Safety and efficacy of preoperative or postoperative chemotherapy for resectable pancreatic adenocarcinoma (PACT-15): a randomised, open-label, phase 2-3 trial. Lancet Gastroenterol Hepatol. 2018;3:413-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |