Published online Sep 15, 2022. doi: 10.4251/wjgo.v14.i9.1771
Peer-review started: April 6, 2022
First decision: June 12, 2022
Revised: June 19, 2022
Accepted: July 27, 2022
Article in press: July 27, 2022
Published online: September 15, 2022
Processing time: 156 Days and 1.9 Hours
There were few studies on the prognosis of tumor patients with sepsis after gastrointestinal surgery and there was no relevant nomogram for predicting the prognosis of these patients.
To establish a nomogram for predicting the prognosis of tumor patients with sepsis after gastrointestinal surgery in the intensive care unit (ICU).
A total of 303 septic patients after gastrointestinal tumor surgery admitted to the ICU at Peking University Cancer Hospital from January 1, 2013 to December 31, 2020 were analysed retrospectively. The model for predicting the prognosis of septic patients was established by the R software package.
The most common infection site of sepsis after gastrointestinal surgery in the ICU was abdominal infection. The 90-d all-cause mortality rate was 10.2% in our study group. In multiple analyses, we found that there were statistically significant differences in tumor type, septic shock, the number of lymphocytes after ICU admission, serum creatinine and total operation times among tumor patients with sepsis after gastrointestinal surgery (P < 0.05). These five variables could be used to establish a nomogram for predicting the prognosis of these septic patients. The nomogram was verified, and the initial C-index was 0.861. After 1000 internal validations of the model, the C-index was 0.876, and the discrimination was good. The correction curve indicated that the actual value was in good agreement with the predicted value.
The nomogram based on these five factors (tumor type, septic shock, number of lymphocytes, serum creatinine, and total operation times) could accurately predict the prognosis of tumor patients with sepsis after gastrointestinal surgery.
Core Tip: There were few studies on the prognosis of tumor patients with sepsis after gastrointestinal surgery and there was no relevant nomogram for predicting the prognosis of these patients. The aim of the study was to establish a nomogram for predicting the prognosis of tumor patients with sepsis after gastrointestinal surgery in the intensive care unit (ICU).The most common infection site of sepsis was abdominal infection and the 90-d all-cause mortality rate was 10.2% in our study group. In multiple analyses, we found that there were statistically significant differences in tumor type, septic shock, the number of lymphocytes after ICU admission, serum creatinine and total operation times among tumor patients with sepsis after gastrointestinal surgery (P < 0.05). These five variables could be used to establish a nomogram for predicting the prognosis of these septic patients. The nomogram was verified, and the initial C-index was 0.861. After 1000 internal validations of the model, the C-index was 0.876, and the discrimination was good. The correction curve indicated that the actual value was in good agreement with the predicted value.
- Citation: Chen RX, Wu ZQ, Li ZY, Wang HZ, Ji JF. Nomogram for predicting the prognosis of tumor patients with sepsis after gastrointestinal surgery. World J Gastrointest Oncol 2022; 14(9): 1771-1784
- URL: https://www.wjgnet.com/1948-5204/full/v14/i9/1771.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i9.1771
Since the definition of sepsis in version 1.0 (infection + systemic inflammatory response syndrome) was too sensitive and the specificity was poor and the definition of sepsis in version 2.0 was too cumbersome, the new definition of sepsis was life-threatening organ dysfunction caused by the im
The incidence rate of sepsis was notably high and mortality was especially high. It is estimated that tens of millions of septic patients die worldwide every year[2,3]. Sepsis not only increased the hospitalization expenses of patients but also prolonged the hospitalization time of patients. According to statistics, the total hospitalization cost of sepsis has jumped first in the United States, with an annual cost of approximately 38.2 billion United States dollars[4]. Therefore, we should pay more attention to the prevention and treatment of sepsis.
The predisposing factors of sepsis include community infection and nosocomial infection, and the mortality of septic patients induced by nosocomial infection is often higher[5,6]. Early identification of infection, infection source control, appropriate application of antibiotics and aggressive volume resuscitation of critically ill patients were the cornerstones of septic patient management[7-10]. These factors had a major influence on the prognosis of septic patients. It is well known that many factors could affect the prognosis of septic patients. However, there were few factors widely used to predict the prognosis of septic patients and there was no relevant nomogram for predicting the prognosis of these patients. In this study, we first retrospectively analysed 303 septic patients after gastrointestinal tumor surgery, collected some factors, analysed their relationship with prognosis, and then established a model for predicting the prognosis of these septic patients.
The study which was registered at the Chinese Clinical Trial Registry (registration ID: ChiCTR2100051826) was conducted according to the Declaration of Helsinki (as revised in 2013). Ethical approval for the study was obtained from the Medical Ethical Committee of Peking University Cancer Hospital (ethics approval number 2020KT33) and informed consent from all the septic patients or their relatives. Inclusion criteria: From January 1, 2013 to December 31, 2020, a total of 4731 patients were admitted to the intensive care unit (ICU) at Peking University Cancer Hospital, of which 2448 patients were transferred to the ICU for various reasons (complicated with chronic medical diseases, sepsis, bleeding, acute myocardial infarction, acute heart failure, acute pulmonary embolism, acute cerebral infarction, pneumothorax, etc.) after gastrointestinal tumor surgery in the gastrointestinal tumor center. According to the new definition of sepsis, 303 septic patients were included in our study. Exclusion criteria: Those patients without surgery; postoperative patients with non-gastrointestinal tumors; patients without sepsis. The flow diagram is shown in Supplementary Figure 1.
The treatment of septic patients before 2016 mainly referred to the 2012 version of sepsis/septic shock guidelines[11], while the treatment of septic patients after 2016 mainly referred to the 2016 version of sepsis/septic shock guidelines[7]. For patients whose final culture results were negative, at least two experts would finally determine the most likely infection source of the patients after discussion.
For abdominal sepsis, we controlled the source of infection actively through minimally invasive drainage or surgical debridement by a multidisciplinary team.
Clinical data and some laboratory tests of septic patients after gastrointestinal surgery were collected. Baseline information included age, body mass index (BMI), basic diseases, chronic diseases, Charlson score and tumor type. Clinical diagnosis and treatment data included whether the first operation was an emergency operation, laparoscopic or open in the first operation, the length of the first operation, drug sensitivity test results, antibiotics used, septic shock, number of blood leukocytes, number of lymphocytes, percentage of lymphocytes, percentage of neutrophils, activated partial thromboplastin time (APTT), albumin, serum creatinine, cardiac troponin I (cTnI), procalcitonin (PCT), blood lactic acid, oxygenation index (PaO2/FiO2), SOFA within 24 h after ICU admission, whether had gastrointestinal fistula or perforation and total operation times. Except for specially specified data, other data were the first data collected in the ICU.
The survival time of septic patients was calculated from entering the ICU and followed up to 90 d. If the patient's death occurred before 90 d, he was followed up to the day of death. Follow-up was carried out through an inpatient electronic case system or telephone and patient's survival status was recorded.
The data were processed by the R3.6.3 software package. Continuous variables were statistically described as the means ± SD and discontinuous variables were described by medians (Q1, Q3). The enumeration data were expressed as numerical values (percentages).
Univariate analysis was performed by the log rank test. Those factors with P < 0.05 in the univariate analysis were included in the multiple analysis and Cox regression analysis was used. Statistically significant factors in multiple survival analysis were used to establish a nomogram for predicting the prognosis of septic patients with the R3.6.3 software package. The performance of the model was evaluated by the C-index and calibration curve. The bootstrap method was used for the internal ve
According to the new definition, 303 tumor patients were diagnosed with sepsis after gastrointestinal surgery, including 119 patients who needed vasopressors who were diagnosed with septic shock. The median age of these septic patients was 66 years. The most common complication was hypertension, followed by diabetes. According to the classification of tumor types, there were 138 patients with gastric cancer, 148 patients with colorectal cancer and 17 patients with other abdominal and pelvic tumors (5 cases of gastrointestinal stromal tumors, 4 cases of lymphoma, 2 cases of melanoma, 2 cases of implanted intestinal wall of ovarian cancer, 1 case of cervical cancer with postoperative intestinal perforation, 1 case of ileal metastasis of renal cancer, 1 case of abdominal fibromatosis and 1 case of colonic adenoma).
Among these septic patients, 35 underwent emergency surgery and 268 underwent limited surgery for the first operation. The median time of the first operation was 180 minutes. In the course of treatment, 24 patients with sepsis were complicated with abdominal bleeding or gastrointestinal hemorrhage, 28 patients with venous thrombosis (including 9 cases of acute pulmonary embolism), 2 patients with acute cerebral infarction, 2 patients with acute myocardial infarction and 1 patient with cerebral hemorrhage. A total of 12 patients needed continuous renal replacement therapy due to renal failure, 149 patients received ventilator treatment and 1 patient received extracorporeal membrane oxygenation. The baseline information is shown in Table 1.
| Baseline characteristics | Number (%) |
| Age, median (Q1, Q3) | 66 (59,73) |
| Sex | |
| Male | 235 (77.6) |
| Female | 68 (22.4) |
| BMI, Mean (SD), kg/m2 | 23.7 (4.0) |
| Tumor type | |
| Gastric cancer | 138 (45.5) |
| Colorectal cancer | 148 (48.8) |
| Other abdominal tumors | 17 (5.6) |
| Coexisting disease1 | |
| Hypertension | 106 (35.0) |
| Diabetes | 55 (18.2) |
| Coronary heart disease | 32 (10.6) |
| Chronic obstructive pulmonary disease | 15 (5.0) |
| Arrhythmia | 22 (7.3) |
| Chronic renal insufficiency | 4 (1.3) |
| Location of infection2 | |
| Abdominal infection | 229 (75.6) |
| Pneumonia | 58 (19.1) |
| Intrathoracic infection | 19 (6.3) |
| Enterogenous infection | 16 (5.3) |
| Surgical wound infection | 7 (2.3) |
| Skin and soft tissue infection | 6 (2.0) |
| Central line-associated bloodstream infection | 4 (1.3) |
| Urinary tract infection | 4 (1.3) |
| Biliary infection | 2 (0.7) |
| First surgery | |
| Laparoscopic | 76 (25.1) |
| Open | 227 (74.9) |
The most common infection site of sepsis after gastrointestinal surgery was abdominal infection, followed by pneumonia. Pathogenic microorganisms could be isolated in 255 cases (84.2%) of these septic patients, however 48 cases (15.8%) could not. Gram-negative bacilli (197 cases) were the most common pathogenic microorganisms, followed by gram-positive cocci (100 cases), fungi (28 cases) and gram-positive bacilli (2 cases). See Supplementary Table 1 for the microorganisms in each infection site.
The common isolated pathogens were as follows (≥ 5 cases): Ninety-seven cases of Escherichia coli (E. coli), 50 cases of Pseudomonas aeruginosa, 40 cases of Klebsiella pneumoniae, 30 cases of Enterococcus faecalis, 22 cases of Candida albicans, 20 cases of Enterococcus faecium, 12 cases of Staphylococcus aureus, 11 cases of Acinetobacter baumannii, 11 cases of Stenotrophomonas maltophilia, 10 cases of Streptococcus pharyngitis, 9 cases of Enterococcus avium and 8 cases of Staphylococcus epidermidis, 7 cases of hemolytic Staphylococcus, 7 cases of Klebsiella aerogenes and 6 cases of Enterobacter cloacae.
The distribution of common drug-resistant bacteria isolated was as follows: Seventy cases of E. coli producing extended spectrum β-lactamase (ESBL) and 7 cases of Klebsiella pneumoniae producing ESBL; 11 cases of carbapenem resistant Pseudomonas aeruginosa, 3 cases of carbapenem resistant Acinetobacter baumannii, and 2 cases of carbapenem resistant Enterobacteriaceae; 7 cases of methicillin resistant Staphylococcus epidermidis, 6 cases of methicillin resistant Staphylococcus aureus and 5 cases of methicillin resistant hemolytic Staphylococcus; 2 cases of vancomycin resistant enterococci.
Three hundred and three septic patients were followed up for 90 d. A total of 31 patients died (27 patients died of multiple organ failure caused by septic shock, 2 patients died of hemorrhagic shock, 1 patient died of intracerebral hemorrhage and 1 patient died of respiratory failure). The 90 d all-cause mortality was 10.2%. Since there were slight differences in the sepsis/septic shock guidelines for the treatment of septic patients before and after 2016, we first performed a comparative analysis of the survival rate of septic patients before and after 2016. There was no significant difference in the 90-d survival rate among septic patients before and after January 1, 2016 (P = 0.415).
The univariate survival analysis showed that there were statistically significant differences in BMI, tumor type, empirical anti-infection evaluation, septic shock, number of lymphocytes after entering the ICU, the activated prothrombin time after entering the ICU, blood creatinine, PCT, blood lactic acid, oxygenation index, SOFA score within 24 h after entering the ICU and total operation times (P < 0.05). See Table 2 for the results of univariate analysis of these septic patients.
| Parameters | Number (%) | Survival rate at 90-d | P value |
| Age, yr | 0.405 | ||
| ≤ 65 | 149 (49.2) | 0.913 | |
| > 65 | 154 (50.8.0) | 0.883 | |
| Sex | 0.190 | ||
| Male | 235 (77.6) | 0.885 | |
| Female | 68 (22.4) | 0.941 | |
| BMI, kg/m2 | 0.013 | ||
| ≤ 20 | 59 (19.5) | 0.797 | |
| 20 < BMI ≤ 30 | 225 (74.3) | 0.924 | |
| > 30 | 19 (6.3) | 0.895 | |
| Charlson score | 0.298 | ||
| ≤ 3 | 229 (75.6) | 0.908 | |
| > 3 | 74 (24.4) | 0.865 | |
| Tumor type | 0.026 | ||
| Gastric cancer | 138 (45.5) | 0.848 | |
| Colorectal cancer | 148 (48.8) | 0.946 | |
| Other abdominal tumors | 17 (5.6) | 0.882 | |
| The first operation was emergency | 0.725 | ||
| No | 268 (88.4) | 0.896 | |
| Yes | 35 (11.6) | 0.914 | |
| First surgery | 0.575 | ||
| Laparoscopic | 76 (25.1) | 0.882 | |
| Open | 227 (74.9) | 0.903 | |
| Length of first operation, min | 0.526 | ||
| ≤ 240 | 220 (72.6) | 0.905 | |
| > 240 | 83 (27.4) | 0.880 | |
| Empirical anti infection evaluation | 0.001 | ||
| Sensitive | 229 (75.6) | 0.917 | |
| Resistance | 26 (8.6) | 0.692 | |
| No pathogen detected | 48(17.1) | 0.917 | |
| Septic shock | 0.001 | ||
| No | 184 (60.7) | 0.978 | |
| Yes | 119 (39.3) | 0.773 | |
| Leukocyte count, 109/L | 0.143 | ||
| ≤ 4 | 49 (16.2) | 0.837 | |
| 4 < WBC ≤ 12 | 142 (46.9) | 0.930 | |
| > 12 | 112 (37.0) | 0.884 | |
| Number of lymphocytes, 109/L | 0.004 | ||
| ≤ 0.2 | 28 (9.2) | 0.750 | |
| > 0.2 | 275 (90.8) | 0.913 | |
| Neutrophil to lymphocyte ratio | 0.883 | ||
| ≤ 20 | 218 (71.9) | 0.899 | |
| > 20 | 85 (28.1) | 0.894 | |
| APTT, S | 0.003 | ||
| ≤ 50 | 244 (80.5) | 0.922 | |
| > 50 | 59 (19.5) | 0.797 | |
| Albumin, g/L | 0.279 | ||
| ≤ 30 | 168 (55.4) | 0.881 | |
| > 30 | 135 (44.6) | 0.919 | |
| Serum creatinine, umol/L | 0.001 | ||
| ≤ 120 | 256 (84.5) | 0.926 | |
| > 120 | 47 (15.5) | 0.745 | |
| Cardiac troponin I, ng/mL | 0.130 | ||
| ≤ 0.05 | 253 (83.5) | 0.909 | |
| > 0.05 | 50 (16.5) | 0.840 | |
| Procalcitonin, ng/mL | 0.034 | ||
| ≤ 10 | 214 (70.6) | 0.921 | |
| > 10 | 89 (29.4) | 0.843 | |
| Lactic acid, mmol/L | 0.001 | ||
| ≤ 3 | 227 (74.9) | 0.934 | |
| > 3 | 76 (25.1) | 0.789 | |
| Oxygenation index, mmHg | 0.001 | ||
| ≤ 200 | 146 (48.2) | 0.836 | |
| > 200 | 157 (51.8) | 0.955 | |
| SOFA score | 0.001 | ||
| ≤ 6 | 175 (57.8) | 0.983 | |
| > 6 | 128 (42.2) | 0.781 | |
| Gastrointestinal fistula or perforation | 0.364 | ||
| No | 183 (60.4) | 0.885 | |
| Yes | 120 (39.6) | 0.917 | |
| Operation times | 0.001 | ||
| 1 | 174 (57.4) | 0.885 | |
| 2 | 123 (40.6) | 0.943 | |
| 3 | 6 (2.0) | 0.333 |
The twelve factors with P < 0.05 in univariate analysis were included in multiple analyses. The results showed that there were significant differences in tumor type, whether there was septic shock, number of lymphocytes after entering the ICU, serum creatinine and total operation times on the prognosis of these septic patients (P < 0.05). The areas under the receiver operating characteristic (ROC) curve of these five factors predicting the prognosis of postoperative sepsis of gastrointestinal tumors were 0.614, 0.766, 0.574, 0.629, and 0.513, respectively. The results of multiple analyses of tumor patients with sepsis after gastrointestinal surgery are shown in Table 3.
| Factors | B | HR | 95% interval | P value | |
| Lower | Upper | ||||
| Tumor type (Ref: Gastric cancer) | 0.007 | ||||
| Colorectal cancer | -1.254 | 0.286 | 0.125 | 0.657 | 0.003 |
| Other abdominal tumors | -0.249 | 0.780 | 0.180 | 3.370 | 0.739 |
| Septic shock | 2.204 | 7.569 | 2.539 | 22.557 | 0.001 |
| Number of lymphocytes | -1.209 | 0.298 | 0.120 | 0.742 | 0.009 |
| Serum creatinine | 1.163 | 3.199 | 1.463 | 6.992 | 0.004 |
| Operation times (Ref: 1) | 0.006 | ||||
| 2 | -0.704 | 0.485 | 0.202 | 1.162 | 0.105 |
| 3 | 1.609 | 4998 | 1.613 | 15.490 | 0.005 |
The 90-d survival rate of patients with postoperative sepsis of gastric cancer was worse than that of patients with postoperative sepsis of colorectal cancer (P = 0.003). However, there was no statistically significant difference in the survival rate between patients with postoperative sepsis of gastric cancer and patients with postoperative sepsis of other abdominal and pelvic tumors (P = 0.739). The 90-d survival rate of patients with postoperative sepsis of gastrointestinal tumors who underwent three operations was lower than that of patients who underwent only one operation (P = 0.005). However, there was no significant difference in the survival rate between patients with postoperative sepsis of gastrointestinal tumors who underwent two operations and patients who underwent only one operation (P = 0.105).
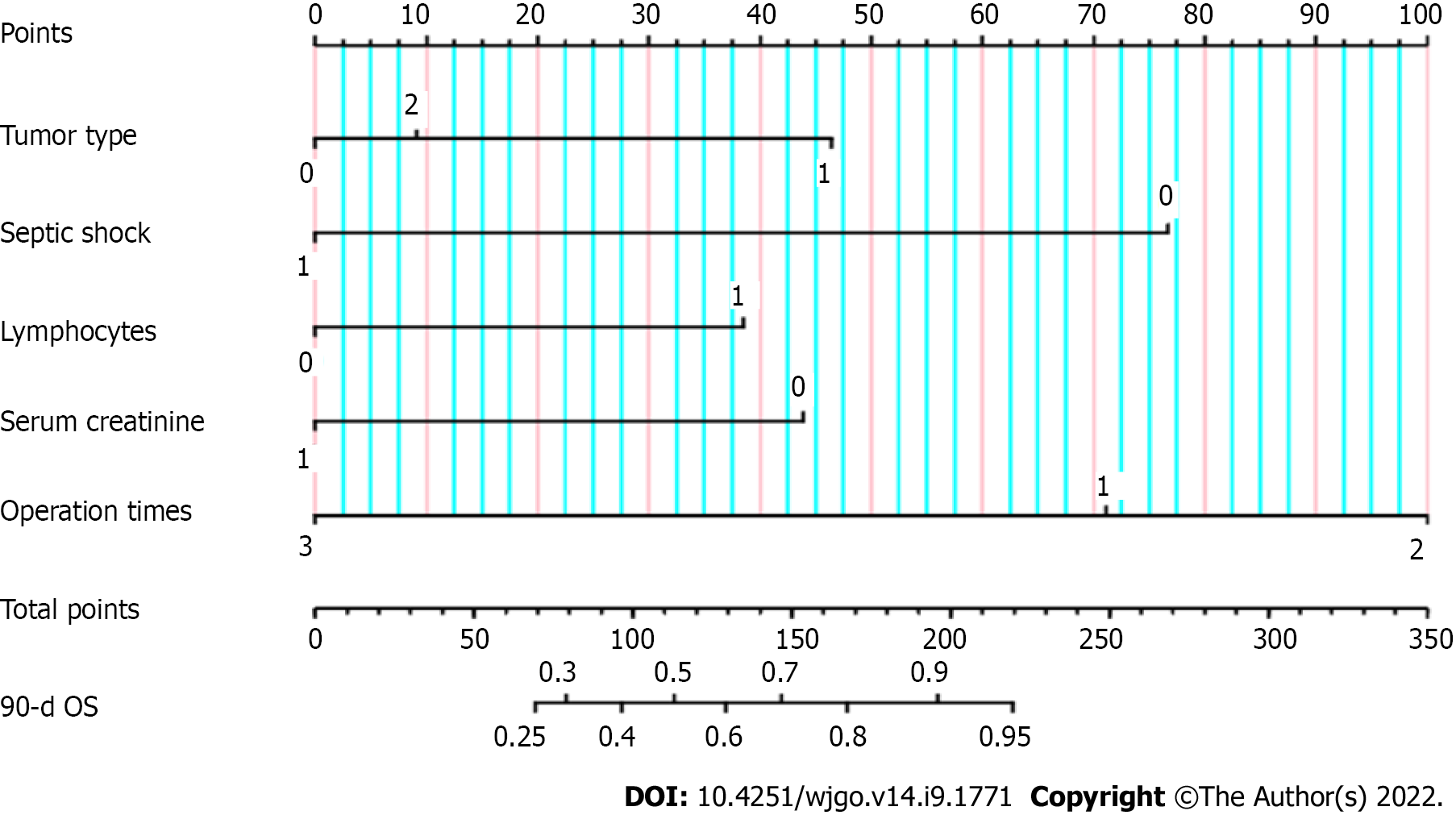
The five factors affecting the 90-d survival rate of patients with postoperative sepsis of gastrointestinal tumors screened by Cox regression analysis were included in the prediction of the prognosis model. A nomogram for predicting the prognosis of patients with postoperative sepsis of gastrointestinal tumors was established and output by R statistical software (Figure 1). In clinical application, we found the corresponding value of each predictor in the nomogram and added the scores of each predictor to the total score. Finally, the total score was read on the axis of 90-d overall survival rate, which was the 90-d survival probability of the patient.
We used the C-index to evaluate the differentiation of a nomogram for predicting the prognosis in septic patients after gastrointestinal surgery. The initial C-index of the nomogram was 0.861 and the 95%CI was 0.809-0.913, indicating that the nomogram for predicting the prognosis in septic patient after gastrointestinal tumor surgery had good discrimination.
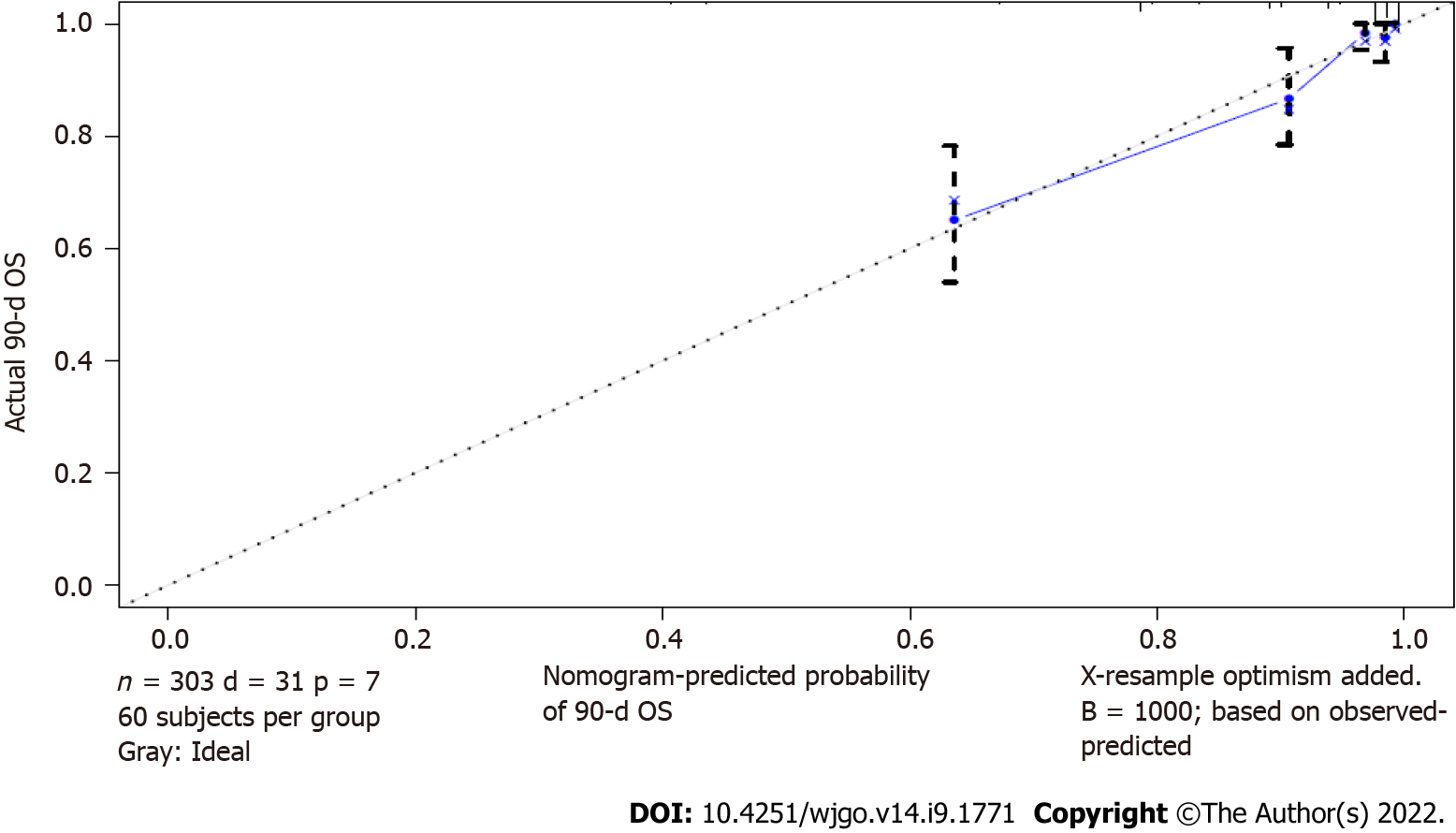
The calibration of the nomogram for predicting the prognosis in septic patients after gastrointestinal surgery was carried out through the correction curve. The correction curve revealed that the observed value was consistent with the predicted value (Figure 2). The above results showed that the nomogram for predicting the prognosis in septic patients after gastrointestinal surgery could accurately predict the 90-d survival rate.
We used the bootstrap method to internally verify the nomogram for predicting the prognosis in septic patients after gastrointestinal surgery. After 1000 internal verifications using the R software package and a repeated bootstrap self sampling method, the C-index was 0.876 (Supplementary Figure 2). This result was consistent with the initial C-index of the nomogram, indicating that the nomogram had good discrimination.
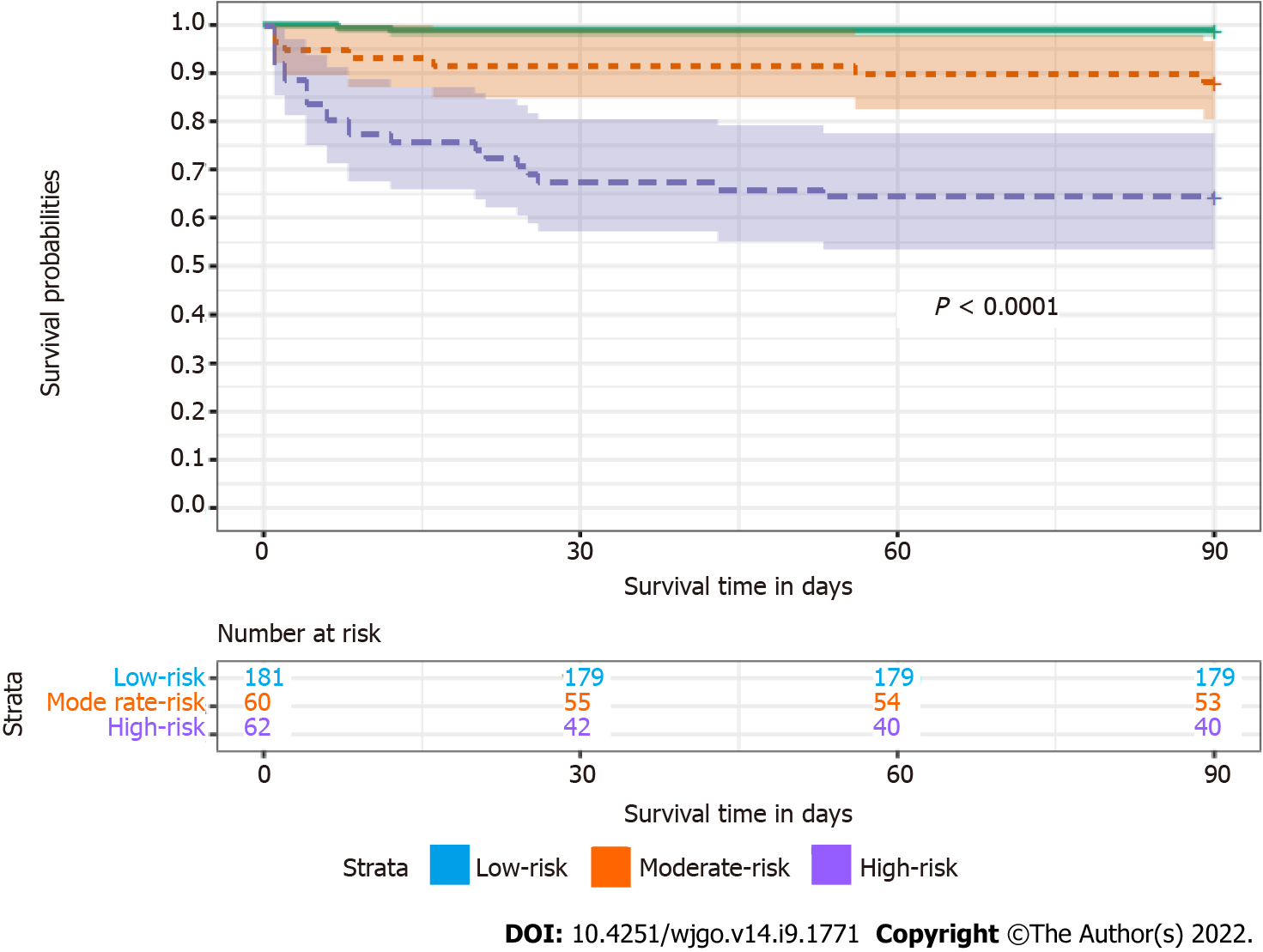
The total score was calculated according to the nomogram for predicting the prognosis of tumor patients with sepsis after gastrointestinal surgery, with a median of 233 points. According to the nomogram, 303 postoperative septic patients were divided into three subgroups: High-risk group (total score < 233), moderate-risk group (192 ≤ total score < 233), and low-risk group (total score ≥ 233).
The survival curves of postoperative septic patients are shown in Figure 3. The 90-d overall survival rates of postoperative septic patients in the high-risk group, moderate-risk group and low-risk group were 0.645, 0.883, and 0.989, respectively. There was a statistically significant difference in the 90-d survival rate among the three groups (P < 0.0001).
The nomogram was a graphical representation of complex mathematical formulas. Medical nomograms mainly use biological and clinical data to describe statistical prediction models. As a graphical statistical prediction model, a nomogram could provide clinicians with a personalized prediction to quantitatively evaluate the prognosis of patients. This study established an effective nomogram for the first time, that could accurately predict the 90-d survival rate of septic patients after gastrointestinal tumor surgery. The calibration curve showed that the nomogram was highly reliable. At the same time, we used the bootstrap method for internal verification, which showed that the prediction ability of the model was very good. In addition, the nomogram could divide individuals into high-risk, moderate-risk and low-risk groups, which indicated that it might be a good tool for predicting the prognosis of tumor patients with sepsis after gastrointestinal surgery.
The main purpose of analysing the prognostic factors of tumor patients with sepsis and establishing a prognostic prediction model was to identify high-risk patients with sepsis as soon as possible and improve the prognosis of these patients. At the same time, it also provided a reference for follow-up clinical research. How to quantify clinical features to achieve individualized prediction of prognosis in septic patients is still a great challenge. The nomogram listed each variable separately by graph and allocated a corresponding number of points for a given variable size. Then, the cumulative score of all variables was matched with the result scale to obtain the corresponding probability. Many studies have confirmed that the nomograms can predict the prognosis of clinical diseases[12-14]. Our study was also based on the nomogram established by the corresponding prognostic factors in septic patients, and we conducted internal validation.
Based on the nomogram for predicting the prognosis of the septic patients, the total score of patients could be calculated and patients could be divided into a high-risk group, moderate-risk group and low-risk group. According to the survival curve based on the nomogram to evaluate the prognosis of sepsis, we found a significant difference in the 90-d survival rate among the three groups, which might warn us to take early intervention in patients with sepsis. For an individual, we could score the patients according to the nomogram, and the corresponding scores could be preliminarily divided into risk groups, which could provide a basis for clinicians to explain the condition to patients and their families and reduce some doctor-patient contradictions and disputes. Of course, whether the nomogram could be widely popularized remains to be verified.
Sepsis was one of the most common causes of mortality in the ICU. Due to its complex etiology and high heterogeneity, there were great differences in the mortality reported in various studies. At present, only a few studies on sepsis have been aimed at postoperative patients with gastrointestinal tumor[15-17]. The object of the study was septic patients after gastrointestinal tumor surgery, and the mortality was lower than that of septic patients reported in some literature[2,18], which might be closely related to the fact that most of the infection sources of septic patients in this study were abdominal infections that could be actively treated at an early stage by multidisciplinary cooperation in our hospital. In this study, 303 septic patients after gastrointestinal surgery were analysed retrospectively. Multiple survival analysis showed that there were statistically significant differences in tumor type, whether there was septic shock, number of lymphocytes after entering the ICU, serum creatinine and total operation times on the prognosis of these septic patients. Among these factors, except whether there was septic shock, which had a medium ability to predict the prognosis of these septic patients alone, the other factors had a low ability to predict the prognosis of these septic patients. The predictive ability of the nomogram established by combining the five factors was significantly higher than that of individual factors. In the following, we analysed some prognostic factors.
Data published in recent years by the National Cancer Center show that gastric cancer and colorectal cancer incidence rates were the second and third respectively[19]. It is well known that there are differences in the long-term survival rates between patients with gastric cancer and colorectal cancer. On the basis of the estimation of the World Health Organization's Global Cancer Observatory, the 1-year and 5-year survival rates of gastric cancer patients in the United Kingdom from 2010 to 2014 were 46.8% and 20.8% respectively; while in colorectal cancer they were 79.3% and 60% respectively[20]. However, there have been few studies on the prognostic difference between septic patients after gastric cancer surgery and septic patients after colorectal cancer surgery. A prospective, multicenter study in Finland showed that the inpatient mortality of sepsis after gastrointestinal surgery was 30.5%, but the study included fewer tumor patients and did not report the impact of tumor type on prognosis[16]. Our study was the first direct comparative analysis of the prognosis of septic patients after gastric cancer surgery and septic patients after colorectal cancer surgery. Because of the difference in prognosis between the two groups of septic patients, we considered that it was related to pathogenic microorganisms, the difficulty of infection source control and the stronger corrosiveness of digestive fluid in the upper gastrointestinal tract. In this study, the stratified analysis revealed that the pathogenic microorganisms isolated from septic patients after gastric cancer surgery included 83 cases of G-bacilli, 43 cases of G + cocci and 21 cases of fungi; pathogenic microorganisms isolated from patients with postoperative sepsis of colorectal cancer included 106 cases of G-bacilli, 53 cases of G + cocci and 6 cases of fungi. There was a significant difference in the pathogens isolated from these two groups.
Patients with sepsis often experience severe immunosuppression and have a poor prognosis[21]. The immunosuppression of sepsis included innate and acquired immunosuppression. Human leukocyte antigen HLA-DR on the surface of monocytes, dendritic cell count and NK cell count were mainly used to monitor congenital immunosuppression in patients with sepsis, while acquired immunosuppression could be monitored by the number of lymphocytes. Studies have shown that the decrease in lym
The kidney is one of the vulnerable organs in septic patients, and acute kidney injury (AKI) can even be as high as 50% in septic patients[24]. With the aggravation of sepsis, the probability of acute kidney injury increases accordingly[25]. The pathogenesis of acute kidney injury in sepsis is complex. Current evidence suggests that acute kidney injury might be functional rather than structural for at least the first 48 h. For example, septic AKI lacked histopathological changes but had microvascular abnormalities and tubular stress changes. In this case, renal medullary hypoxia caused by the redistribution of intrarenal perfusion was becoming a key factor in acute kidney injury in sepsis. Risk factors for acute kidney injury in septic patients included advanced age, chronic renal insufficiency, diabetes, heart failure and cancer, etc. Septic patients complicated with acute kidney injury significantly worsen the prognosis[26,27]. At present, the diagnosis of sepsis related AKI followed the criteria of acute kidney injury issued by the global working group on improving the prognosis of kidney diseases (KDIGO) in 2012[28]. The treatment of AKI in sepsis mainly included volume resuscitation, antibiotics and renal replacement therapy. In our study, a serum creatinine level of 120 μmol/L was the cut-off value, and the patients were divided into two groups. There was a statistically significant difference in the 90-d survival rate between the two groups.
After gastrointestinal surgery, some patients with abdominal sepsis needed more than one operation to control the source of infection, which often suggested that the patient was in poor condition. The incidence of unplanned reoperation varies among hospitals due to the technical level of doctors[29]. As an important component of medical safety and quality management, unplanned reoperation is often used to assess the technical level of surgery. Therefore, we selected the number of operations to predict the prognosis of patients with sepsis. In our study, we found that there was a significant difference in the 90-d survival rate between septic patients after three operations and septic patients after one operation or two operations, although there was no statistically significant difference in the 90-d survival rate between septic patients after two operations and septic patients after only one operation. We considered that the mortality of patients with indirect operation-related infections (including pulmonary infection, urinary infection and central venous catheter-related infection) was higher than that of patients with direct operation-related infections (including thoracic and abdominal infection, intestinal infection, wound infection, skin and soft tissue infection and biliary tract infection). Among the patients who underwent only one operation, the proportion of indirect operation-related infections was higher. According to the stratified analysis of direct and indirect infections related to gastrointestinal surgery, the 90-d survival rate of patients in the group with two operations was slightly higher than that in the group with one operation, however the difference was not statistically significant. This suggested that we might need more active surgical intervention for the treatment of sepsis caused by infection directly related to gastrointestinal tumor surgery. Of course, it needs to be verified by subsequent randomized controlled trials.
Some limitations of this study should be stated. First, this study was a single-center study, and the sample size was limited, so the results of this study might have some bias. Second, although the nomogram was established to predict the prognosis of these septic patients, it was not externally verified due to the limited sample size. Since there might be differences in patients with sepsis in different research centers, multicenter studies and external validation should be considered in the follow-up. Third, the population in our study was septic patients after gastrointestinal surgery in the ICU. Whether the results could be extended to all septic populations remains to be confirmed. Fourth, new biomarkers were not included in the prognostic factors selected in this study. These factors will be considered for further research to elaborate on the value of these new biomarkers. Fifth, this study spanned a long time, but since there was no significant difference in the 90-d survival rate of septic patients after gastrointestinal surgery before and after January 1, 2016, we believed that this study was highly feasible. Finally, with the progress of technology and treatment, the survival rate of patients with sepsis may be improved. Therefore, the accuracy of predicting prognosis by nomogram may be affected, which needs our attention.
The nomogram based on these five factors (tumor type, septic shock, number of lymphocytes, serum creatinine, and total operation times) could accurately predict the prognosis of tumor patients with sepsis after gastrointestinal surgery.
There were few studies on the prognosis of tumor patients with sepsis after gastrointestinal surgery and there was no relevant nomogram for predicting the prognosis of these patients.
To explore the prognostic predictors in patients with sepsis after gastrointestinal tumor surgery.
To establish a nomogram for predicting the prognosis of tumor patients with sepsis after gastro
A total of 303 septic patients after gastrointestinal tumor surgery admitted to the ICU at Peking University Cancer Hospital from January 1, 2013 to December 31, 2020 were analysed retrospectively. The model for predicting the prognosis of these septic patients was established by the R software package.
The most common infection site of sepsis after gastrointestinal surgery in the ICU was abdominal infection. The 90-d all-cause mortality rate was 10.2% in our study group. In multiple analyses, we found that there were statistically significant differences in tumor type, septic shock, the number of lymphocytes after ICU admission, serum creatinine and total operation times among tumor patients with sepsis after gastrointestinal surgery (P < 0.05). These five variables could be used to establish a nomogram for predicting the prognosis of these septic patients. The nomogram was verified, and the initial C-index was 0.861. After 1000 internal validations of the model, the C-index was 0.876, and the discrimination was good. The correction curve indicated that the actual value was in good agreement with the predicted value.
The nomogram based on these five factors (tumor type, septic shock, number of lymphocytes, serum creatinine and total operation times) could accurately predict the prognosis of tumor patients with sepsis after gastrointestinal surgery.
Need external validation in the future to verify the results.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmed SK, Iraq; Saito R, Japan; Totoki T, Japan S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17171] [Article Influence: 1907.9] [Reference Citation Analysis (2)] |
| 2. | Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K; International Forum of Acute Care Trialists. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016;193:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1639] [Cited by in RCA: 2312] [Article Influence: 256.9] [Reference Citation Analysis (0)] |
| 3. | Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, Fleischmann-Struzek C, Machado FR, Reinhart KK, Rowan K, Seymour CW, Watson RS, West TE, Marinho F, Hay SI, Lozano R, Lopez AD, Angus DC, Murray CJL, Naghavi M. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2870] [Cited by in RCA: 4113] [Article Influence: 822.6] [Reference Citation Analysis (4)] |
| 4. | Liang L, Moore B, Soni A. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2017. 2020 Jul 14. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006 Feb-. [PubMed] |
| 5. | Page DB, Donnelly JP, Wang HE. Community-, Healthcare-, and Hospital-Acquired Severe Sepsis Hospitalizations in the University HealthSystem Consortium. Crit Care Med. 2015;43:1945-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 6. | Rhee C, Wang R, Zhang Z, Fram D, Kadri SS, Klompas M; CDC Prevention Epicenters Program. Epidemiology of Hospital-Onset Versus Community-Onset Sepsis in U.S. Hospitals and Association With Mortality: A Retrospective Analysis Using Electronic Clinical Data. Crit Care Med. 2019;47:1169-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 7. | Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45:486-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1784] [Cited by in RCA: 1986] [Article Influence: 248.3] [Reference Citation Analysis (1)] |
| 8. | Levy MM, Evans LE, Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018;44:925-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 687] [Article Influence: 98.1] [Reference Citation Analysis (0)] |
| 9. | Sartelli M, Chichom-Mefire A, Labricciosa FM, Hardcastle T, Abu-Zidan FM, Adesunkanmi AK, Ansaloni L, Bala M, Balogh ZJ, Beltrán MA, Ben-Ishay O, Biffl WL, Birindelli A, Cainzos MA, Catalini G, Ceresoli M, Che Jusoh A, Chiara O, Coccolini F, Coimbra R, Cortese F, Demetrashvili Z, Di Saverio S, Diaz JJ, Egiev VN, Ferrada P, Fraga GP, Ghnnam WM, Lee JG, Gomes CA, Hecker A, Herzog T, Kim JI, Inaba K, Isik A, Karamarkovic A, Kashuk J, Khokha V, Kirkpatrick AW, Kluger Y, Koike K, Kong VY, Leppaniemi A, Machain GM, Maier RV, Marwah S, McFarlane ME, Montori G, Moore EE, Negoi I, Olaoye I, Omari AH, Ordonez CA, Pereira BM, Pereira Júnior GA, Pupelis G, Reis T, Sakakhushev B, Sato N, Segovia Lohse HA, Shelat VG, Søreide K, Uhl W, Ulrych J, Van Goor H, Velmahos GC, Yuan KC, Wani I, Weber DG, Zachariah SK, Catena F. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;12:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 251] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 10. | Sawyer RG, Claridge JA, Nathens AB, Rotstein OD, Duane TM, Evans HL, Cook CH, O'Neill PJ, Mazuski JE, Askari R, Wilson MA, Napolitano LM, Namias N, Miller PR, Dellinger EP, Watson CM, Coimbra R, Dent DL, Lowry SF, Cocanour CS, West MA, Banton KL, Cheadle WG, Lipsett PA, Guidry CA, Popovsky K; STOP-IT Trial Investigators. Trial of short-course antimicrobial therapy for intraabdominal infection. N Engl J Med. 2015;372:1996-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 486] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 11. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R; Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4031] [Cited by in RCA: 3979] [Article Influence: 331.6] [Reference Citation Analysis (0)] |
| 12. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 2392] [Article Influence: 239.2] [Reference Citation Analysis (0)] |
| 13. | Dong YM, Sun J, Li YX, Chen Q, Liu QQ, Sun Z, Pang R, Chen F, Xu BY, Manyande A, Clark TG, Li JP, Orhan IE, Tian YK, Wang T, Wu W, Ye DW. Development and Validation of a Nomogram for Assessing Survival in Patients With COVID-19 Pneumonia. Clin Infect Dis. 2021;72:652-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 14. | Morici N, Viola G, Antolini L, Alicandro G, Dal Martello M, Sacco A, Bottiroli M, Pappalardo F, Villanova L, De Ponti L, La Vecchia C, Frigerio M, Oliva F, Fried J, Colombo P, Garan AR. Predicting survival in patients with acute decompensated heart failure complicated by cardiogenic shock. Int J Cardiol Heart Vasc. 2021;34:100809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Blair LJ, Huntington CR, Cox TC, Prasad T, Lincourt AE, Gersin KS, Heniford BT, Augenstein VA. Risk factors for postoperative sepsis in laparoscopic gastric bypass. Surg Endosc. 2016;30:1287-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Ukkonen M, Karlsson S, Laukkarinen J, Rantanen T, Paajanen H; Finnsepsis Study Group. Severe Sepsis in Elderly Patients Undergoing Gastrointestinal Surgery-a Prospective Multicenter Follow-up Study of Finnish Intensive Care Units. J Gastrointest Surg. 2016;20:1028-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Xu X, Dong HC, Yao Z, Zhao YZ. Risk factors for postoperative sepsis in patients with gastrointestinal perforation. World J Clin Cases. 2020;8:670-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Herrán-Monge R, Muriel-Bombín A, García-García MM, Merino-García PA, Martínez-Barrios M, Andaluz D, Ballesteros JC, Domínguez-Berrot AM, Moradillo-Gonzalez S, Macías S, Álvarez-Martínez B, Fernández-Calavia MJ, Tarancón C, Villar J, Blanco J. Epidemiology and Changes in Mortality of Sepsis After the Implementation of Surviving Sepsis Campaign Guidelines. J Intensive Care Med. 2019;34:740-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Chen W, Sun K, Zheng R, Zeng H, Zhang S, Xia C, Yang Z, Li H, Zou X, He J. Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 704] [Article Influence: 100.6] [Reference Citation Analysis (2)] |
| 20. | IARC. The Global Cancer Observatory New England source: Globocan. 2021. [cited 3 April 2022]. Available from: https://gco.iarc.fr/. |
| 21. | Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol. 2018;14:121-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 585] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 22. | Drewry AM, Samra N, Skrupky LP, Fuller BM, Compton SM, Hotchkiss RS. Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock. 2014;42:383-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 337] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 23. | Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am J Emerg Med. 2020;38:641-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 310] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 24. | Ma S, Evans RG, Iguchi N, Tare M, Parkington HC, Bellomo R, May CN, Lankadeva YR. Sepsis-induced acute kidney injury: A disease of the microcirculation. Microcirculation. 2019;26:e12483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 129] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 25. | Lopes JA, Jorge S, Resina C, Santos C, Pereira A, Neves J, Antunes F, Prata MM. Acute kidney injury in patients with sepsis: a contemporary analysis. Int J Infect Dis. 2009;13:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Kellum JA, Wen X, de Caestecker MP, Hukriede NA. Sepsis-Associated Acute Kidney Injury: A Problem Deserving of New Solutions. Nephron. 2019;143:174-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Bellomo R, Kellum JA, Ronco C, Wald R, Martensson J, Maiden M, Bagshaw SM, Glassford NJ, Lankadeva Y, Vaara ST, Schneider A. Acute kidney injury in sepsis. Intensive Care Med. 2017;43:816-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 502] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 28. | Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-c184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 3332] [Article Influence: 256.3] [Reference Citation Analysis (0)] |
| 29. | Sah BK, Chen MM, Yan M, Zhu ZG. Reoperation for early postoperative complications after gastric cancer surgery in a Chinese hospital. World J Gastroenterol. 2010;16:98-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |