Published online Sep 15, 2022. doi: 10.4251/wjgo.v14.i9.1711
Peer-review started: April 29, 2022
First decision: July 6, 2022
Revised: July 14, 2022
Accepted: August 9, 2022
Article in press: August 9, 2022
Published online: September 15, 2022
Processing time: 133 Days and 5.9 Hours
The effects of consolidation chemotherapy (CC) in neoadjuvant therapy in locally advanced rectal cancer (LARC) have been explored. However, the optimal neo
To evaluate the effects of one to two cycles of CC with capecitabine on high-risk patients with LARC without extending NCRT and surgery interval.
We retrospectively evaluated high-risk patients with LARC, who were defined as having at least one of the following factors by magnetic resonance imaging: depth of invasion beyond the muscularis propria of more than 5 mm (cT3c-cT3d), T4, meso-rectal fascia or extramural vascular invasion positive, and treatment date between January 2015 and July 2019 in our center. Patients were divided into the CC and non-CC group according to whether they received CC (capecitabine 1000 mg/m2 twice daily from days 1 to 14 every 21 d) after NCRT. Propensity score matching (PSM) and inverse probability of treatment weight (IPTW) were used to balance the differences between the two groups. The main outcome was the complete response (CR) rate.
A total of 265 patients were enrolled: 136 patients in the CC group and 129 patients in the non-CC group. The median interval was 70 d (range, 37-168). The CR rate was 24.3% and 16.3% (P = 0.107) in the CC and non-CC groups’ original samples, respectively. After PSM and IPTW, the CR rate in the CC group was higher than that in non-CC group (27.6% vs 16.2%, P = 0.045; 25.9% vs 16.3%, P = 0.045). The median follow-up was 39.8 mo (range, 2.9-74.8), and there were no differences in 3-year non-regrowth disease-free survival nor overall survival in the original samples (73.2% vs 71.9%, P = 0.913; 92.3% vs 86.7%, P = 0.294), PSM (73.2% vs 73.5%, P = 0.865; 92.5% vs 89.3%, P = 0.612), and IPTW (73.8% vs 72.1%, P = 0.913; 92.4% vs 87.4%, P = 0.294). There was also no difference in grade 2 or higher acute toxicity during neoadjuvant therapy in the two groups (49.3% vs 53.5%, P = 0.492).
One to two cycles of CC with capecitabine after NCRT was safe and increased the CR rate in high-risk LARC but failed to improve the long-term outcomes.
Core Tip: This is the first study to explore the effects of one to two cycles of consolidation chemotherapy with capecitabine after neoadjuvant chemoradiotherapy (NCRT) in magnetic resonance imaging-defined high-risk patients with locally advanced rectal cancer without extending NCRT and surgery interval. After propensity score-matching and inverse probability of treatment weighting, the complete response rate increased. Although it showed no significant difference in long-term results, this relatively low-toxicity program deserves further exploration.
- Citation: Sheng XQ, Wang HZ, Li S, Zhang YZ, Geng JH, Zhu XG, Quan JZ, Li YH, Cai Y, Wang WH. Consolidation chemotherapy with capecitabine after neoadjuvant chemoradiotherapy in high-risk patients with locally advanced rectal cancer: Propensity score study. World J Gastrointest Oncol 2022; 14(9): 1711-1726
- URL: https://www.wjgnet.com/1948-5204/full/v14/i9/1711.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i9.1711
Neoadjuvant chemoradiotherapy (NCRT) followed by total mesorectal excision (TME) was the standard treatment for patients with locally advanced rectal cancer (LARC)[1,2]. After NCRT, approximately 50% to 60% of LARC patients were downstaged, and nearly 20% achieved pathologic complete response (pCR)[3,4]. Patients with pCR had better prognosis than those with worse regression[4-6]. In addition, the “watch-and-wait” approach was feasible for patients who achieved clinical complete response (cCR) after neoadjuvant therapy, which significantly improved their quality of life[7-10].
Accurate staging before treatment is extremely important, and magnetic resonance imaging (MRI) has unique advantages compared with other radiology methods for rectal cancers[11]. Although the current American Joint Committee on Cancer (AJCC) tumor node metastasis staging system stratifies patients with rectal cancer, some rectal MRI-based parameters, such as the extramuscular invasion distance, mesorectal fascia (MRF), and extramural venous invasion (EMVI) statuses are strongly related to the prognosis[12]. On the basis of the MERCURY series study[13], the European Society for Medical Oncology (ESMO) clinical practice guidelines recommend treatments after stratifying rectal cancer by using pelvic MRI[11]. Previous studies showed that the complete response (CR) rate after NCRT of low-risk patients with rectal cancer was more than 30%[14-16]. However, that of high-risk patients with rectal cancer were approximately 10%-20%[5,17]. Increasing the CR rate, especially in high-risk patients, is a current research target for neoadjuvant therapy in LARC.
Several studies have explored the effects of additional induction or consolidation chemotherapy (CC)[18-22] in neoadjuvant therapy in LARC. However, the optimal timing, regimen, and number of cycles in chemotherapy remained unknown. Compared with induction chemotherapy, CC seemed to improve CR rate, but the increase in CR rate might also be related to the prolonged interval between NCRT and TME surgery[23-27]. The extended time could also aggravate pelvic fibrosis, thus making surgery more difficult[28] and potentially offsetting the tumor reduction benefit. In addition, most of the regimens in neoadjuvant chemotherapy consisted of double or triple drugs that increased the toxicity induced by treatment[21,22]. The additional oxaliplatin in concurrent chemotherapy not only increased toxicity but also failed to improve the efficacy[29-31]. Previous studies have also explored CC with capecitabine monotherapy in LARC[32,33]. However, patients in these studies were not stratified by pelvic MRI before treatment. This retrospective study explored the effects of one to two cycles of CC with capecitabine after NCRT in high-risk LARC patients without extending the time between the end of NCRT and surgery by considering the efficacy and low toxicity of capecitabine in the treatment of rectal cancer and the convenience of oral therapy.
From January 2015 to July 2019, all patients with histologically confirmed, newly diagnosed locally advanced rectal adenocarcinoma with tumors within 15 cm of the anal verge were included in the screening. The inclusion criteria included: (1) High-risk patients with LARC defined by MRI, including at least one of the following high-risk factors: depth of invasion beyond the muscularis propria of more than 5 mm (cT3c-T3d), T4, EMVI (+), or MRF (+); (2) patients who had not received induction chemotherapy; (3) patients who achieved cCR or underwent surgery after NCRT in our center; (4) patients older than 18 years old; and (5) patients with an Eastern Cooperative Oncology Group score of ≤ 2 points and with no medical comorbidities or other tumors with a poor prognosis. Patients were divided into two groups, namely, the CC and non-CC groups, on the basis of CC administration during the interval between NCRT and surgery.
A high-resolution, diagnostic, or simulation 3D T2-weighted sequence MRI was performed before NCRT. The scanning layer thickness was 3-5 mm, with mandatory axial scanning perpendicular to the long axis of the rectal tumor[34,35]. The tumor stage, T3 substage, lymph node metastases, EMVI, MRF, and tumor length and thickness were evaluated in primary MRI on the basis of the ESMO and the European Society of Gastrointestinal and Abdominal Radiology consensus meeting guidelines[11,35]. Evaluating tumor regression by MRI is still strongly recommended after NCRT, especially to diagnose cCR.
Computed tomography (CT) simulations were performed with a thermoplastic film with patients in the supine position by using contrast-enhanced CT with a 5 mm slice thickness. An empty rectum and a filled bladder were required to ensure consistency in the rectal tumor positioning and protect the intestine from radiation. MRI simulation was mandatory to obtain a more accurate tumor location. The target contour details were described previously[36]. The Simultaneous Integrated Boost-Intensity Modulated Radiation Therapy was delivered during radiotherapy. The prescription doses for the planning gross tumor volume and planning target volume were 50-50.6 Gy and 41.8-45 Gy, respectively, in 22-25 fractions. Chemotherapy with capecitabine at 825 mg/m2 was administered orally twice daily and concomitantly with radiotherapy. One to two weeks after NCRT, one to two cycles of capecitabine (1000 mg/m2 twice daily, d1-d14/q21d) were administered.
Patients underwent detailed and comprehensive restaging, including tumor marker, digital rectal examination, rectal endoscopy, and pelvic MRI six to eight weeks after NCRT. CT scans of the chest and abdomen were also performed to assess distant metastases. All patients received a multi-disciplinary team evaluation to develop a further treatment strategy. For patients who achieved cCR, a non-operative “watch-and-wait” strategy with rigorous and meticulous follow-up was feasible. The cCR diagnostic criteria included the following: (1) The absence of a viable tumor on MRI; (2) negative biopsies from the scar; (3) normal carcinoembryonic antigen (CEA) levels (< 5 ng/mL); and (4) no signs of distant metastasis. Patients who did not achieve cCR were highly recommended with surgery based on the TME principles. The pathology reports were based on the AJCC/College of American Pathologists standards[37]. R0 resection was defined as a longitudinal margin and circumferential resection margin of no more than 1 mm.
Adjuvant CapeOX chemotherapy (oxaliplatin 130 mg/m2, d1; capecitabine 1000 mg/m2 twice daily, d1-d14/q21d) was recommended for every patient, and capecitabine monotherapy was the alternative. Full-dose adjuvant chemotherapy was defined as capecitabine for six months or CapeOX for more than six cycles.
Toxicities during neoadjuvant treatment were evaluated on the basis of the Common Terminology Criteria for Adverse Events (version 3.0). After completing primary treatment, the patients were followed up at three-month intervals for the first two years, six-month intervals until five years, and annually thereafter by evaluating the symptoms, tumor markers, chest and abdominal CT, pelvic CT or MRI, and physical examination results.
The primary outcome was CR rate, including the pCR and cCR rate. Other outcomes included pCR, TRG classification, non-regrowth disease-free survival (NR-DFS), overall survival (OS), and acute toxicity during neoadjuvant treatment. TRG classification was based on the NCCN standard. NR-DFS was measured from the first day of NCRT to any type of recurrence or death for any reason. OS was calculated from the first day of NCRT to death for any reason.
Data were collected and analyzed using the Statistical Package for the Social Sciences (IBM Corp. SPSS Statistics for Windows, version 22.0, Armonk, NY, United States) and R statistical software package (R Project for Statistical Computing, version 4.1.2, Vienna, Austria). The chi-square test and independent sample t-test/Wilcoxon test were used to compare the differences in the two groups. Propensity score (PS) analysis, including PS matching (PSM) and inverse probability of treatment weighting (IPTW), were applied to balance the baseline characteristics of the two groups. The PS was developed with a logistic regression model, and variables including gender, age, tumor location, pathology, CEA, T stage, tumor length, thickness, MRF, EMVI, and interval were included. Patients in CC and non-CC groups were randomly matched 1:1 on the basis of PS by using the nearest neighbor method (maximum caliper distance, 0.2). IPTW was then calculated with PS by using IPTWs, and the number of observations is the sum of the weights[38]. The CR rates of the two groups in the original samples after PSM and IPTW were compared. The proportions of pCR, TRG, pT0-2, and pN0 were compared in the original samples and after PSM. The Kaplan-Meier method was used to plot NG-DFS and OS and was compared with the log-rank test. After PSM, subgroup analysis and interaction were conducted to assess the heterogeneity of treatment effects. A P value < 0.05 was considered statistically significant.
During the study period, 265 patients who met the screening criteria were included in the analysis. The median age was 59 years (range, 25-82). In total, 183 (69.1%) were males, 130 (49.1%) were categorized as a low location LARC, and 130 (49.4%) had normal CEA levels. There were 168 (63.4%) patients with stage > T3b disease, 206 (77.7%) patients who were MRF positive, and 170 (64.2%) patients with clinical EMVI positivity. Overall, 136 patients (51.3%) received CC after NCRT (CC group), of whom 79 (56.8%) received 1 cycle of capecitabine, and the remaining 129 patients were classified as the non-CC group.
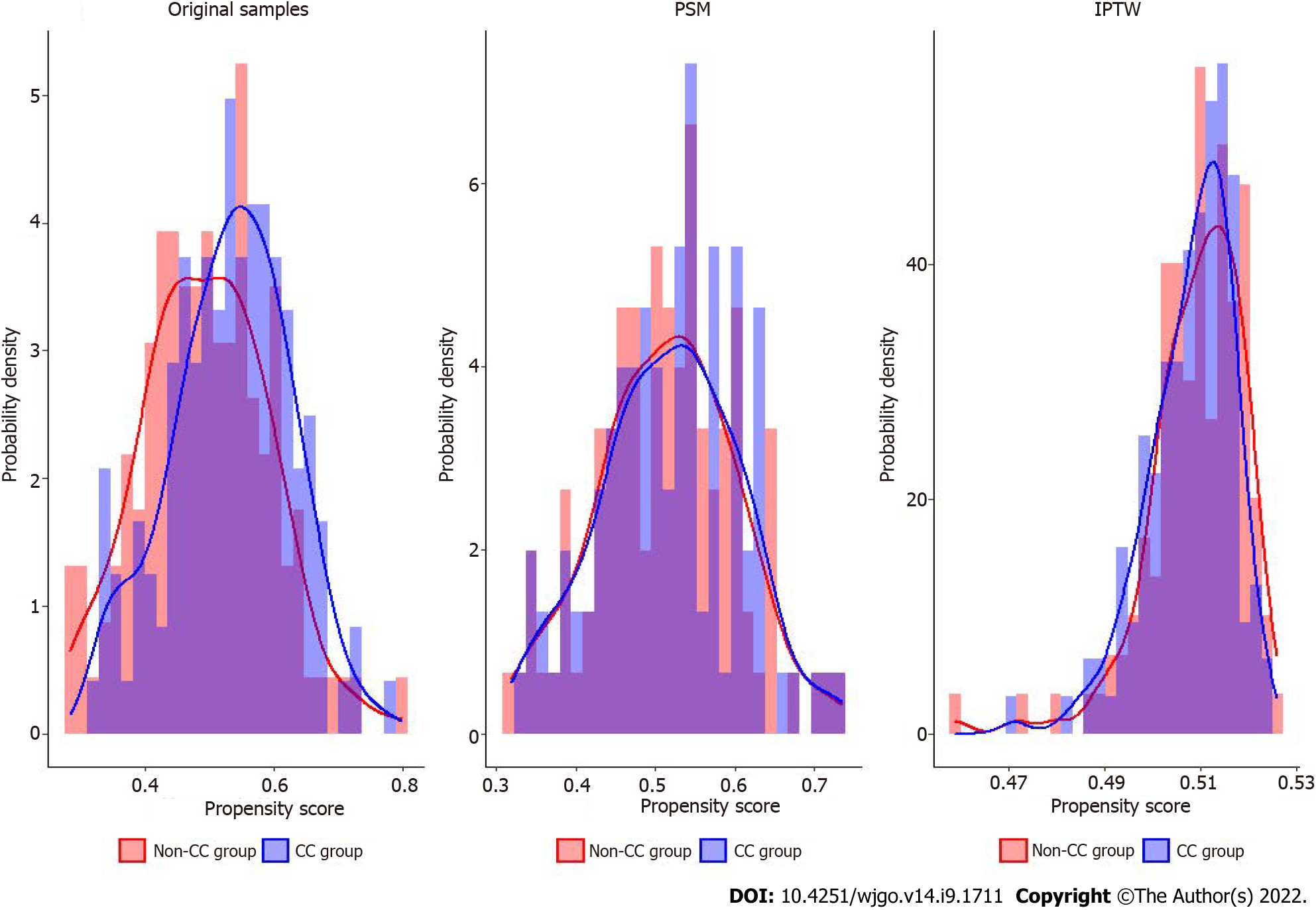
Patients in the CC group had a longer interval between the end of NCRT and surgery (or the time of diagnosis of distant metastasis or cCR) than those in the non-CC group (P = 0.04). All other factors did not differ between the two groups (Table 1). PS analysis with PSM and IPTW achieved balance for all variables between the two groups (Table 2). Histograms and density graphs description comparisons of the original, PSM, and IPTW distributions of each group are shown in Figure 1.
| CC group (n = 129) | non-CC group (n = 136) | P value | |
| Gender, n (%) | 0.177 | ||
| Male | 84 (65.1) | 99 (72.8) | |
| Female | 45 (34.9) | 37 (27.2) | |
| Age, yr | 0.446 | ||
| mean (SD) | 57.5 (11.4) | 58.5 (9.8) | |
| Primary location, n (%) | 0.812 | ||
| Up | 4 (3.1) | 6 (4.4) | |
| Middle | 60 (46.5) | 65 (47.8) | |
| Low | 65 (50.4) | 65 (47.8) | |
| Pathology, n (%) | 0.996 | ||
| Well differentiated | 6 (4.7) | 6 (4.4) | |
| Moderately differentiated | 95 (73.6) | 102 (75.0) | |
| Poorly differentiated | 16 (12.4) | 16 (11.8) | |
| Others | 12 (9.3) | 12 (8.8) | |
| CEA, n (%) | 0.307 | ||
| Normal | 67 (51.9) | 64 (47.1) | |
| Unnormal | 49 (38.0) | 63 (46.3) | |
| Unidentified | 13 (10.1) | 9 (6.6) | |
| T stage, n (%) | 0.650 | ||
| < T3c | 49 (38.0) | 48 (35.3) | |
| > T3b | 80 (62.0) | 88 (64.7) | |
| N stage, n (%) | 0.190 | ||
| N0 | 12 (9.3) | 7 (5.1) | |
| N+ | 117 (90.7) | 129 (94.9) | |
| Tumor length (mm) | 0.916 | ||
| mean (SD) | 49.0 (12.7) | 49.1 (13.7) | |
| Tumor thickness (mm) | 0.838 | ||
| mean (SD) | 16.4 (5.0) | 16.5 (7.2) | |
| MRF, n (%) | 0.501 | ||
| Negative | 31 (24.0) | 28 (20.6) | |
| Positive | 98 (76.0) | 108 (79.4) | |
| EMVI, n (%) | 0.565 | ||
| Negative | 44 (34.1) | 51 (37.5) | |
| Positive | 85 (65.9) | 85 (62.5) | |
| Numbers of high-risk factor, n (%) | 0.557 | ||
| 1 | 38 (29.5) | 34 (25.0) | |
| 2 | 48 (37.2) | 59 (43.4) | |
| 3 | 43 (33.3) | 43 (31.6) | |
| Interval time (d) | 0.040 | ||
| mean (SD) | 71.7 (21.7) | 76.8 (18.5) |
| PSM | IPTW | |||||
| non-CC group (n = 105) | CC-group (n = 105) | P value | non-CC group (n = 130) | CC-group (n = 135) | P value | |
| Gender, n (%) | 0.762 | 0.970 | ||||
| Male | 75 (71.4) | 73 (69.5) | 89.4 (68.5) | 92.6 (68.7) | ||
| Female | 30 (28.6) | 32 (30.5) | 41.1 (31.5) | 42.2 (31.3) | ||
| Age | 0.692 | 0.993 | ||||
| mean (SD) | 57.7 (11.8) | 58.3 (9.7) | 58.2 (11.2) | 58.2 (9.7) | ||
| Primary location, n (%) | 0.849 | 0.996 | ||||
| Up | 3 (2.9) | 2 (1.9) | 4.9 (3.8) | 4.9 (3.7) | ||
| Middle | 52 (49.5) | 50 (47.6) | 61.0 (46.7) | 63.8 (47.3) | ||
| Low | 50 (47.6) | 53 (50.5) | 64.6 (49.5) | 66.1 (49.0) | ||
| Pathology, n (%) | 0.903 | 0.999 | ||||
| Well-differentiated | 5 (4.8) | 5 (4.8) | 6.3 (4.9) | 6.6 (4.9) | ||
| Moderately-differentiated | 79 (75.2) | 75 (71.4) | 94.0 (72.0) | 97.9 (72.6) | ||
| Poorly-differentiated | 12 (11.4) | 13 (12.4) | 16.6 (12.7) | 17.0 (12.6) | ||
| Others | 9 (8.6) | 12 (11.4) | 13.6 (10.4) | 13.3 (9.9) | ||
| CEA, n (%) | 0.428 | 0.997 | ||||
| Normal | 51 (48.6) | 58 (55.2) | 64.1 (49.1) | 66.8 (49.5) | ||
| Unnormal | 45 (42.9) | 42 (40.0) | 55.2 (42.3) | 56.7 (42.1) | ||
| unidentified | 9 (8.6) | 5 (4.8) | 11.2 (8.6) | 11.3 (8.4) | ||
| T stage, n (%) | 0.568 | 0.992 | ||||
| < T3c | 41 (39.0) | 37 (35.2) | 48.0 (36.8) | 49.7 (36.9) | ||
| > T3b | 64 (61.0) | 68 (64.8) | 82.5 (63.2) | 85.1 (63.1) | ||
| N stage, n (%) | 0.097 | 0.176 | ||||
| N0 | 10 (9.5) | 4 (3.8) | 12.1 (9.3) | 6.7 (5.0) | ||
| N+ | 95 (90.5) | 101 (96.2) | 118.4 (90.7) | 128.1 (95.0) | ||
| Tumor length (mm) | 0.916 | 0.983 | ||||
| mean (SD) | 48.6 (13.0) | 48.4 (13.2) | 48.9 (12.5) | 48.9 (13.5) | ||
| Tumor thickness (mm) | 0.484 | 0.999 | ||||
| mean (SD) | 16.6 (5.0) | 16.0 (7.0) | 16.4 (4.9) | 16.4 (7.2) | ||
| MRF, n (%) | > 0.99 | 0.865 | ||||
| Negative | 23 (21.9) | 23 (21.9) | 29.7 (22.8) | 29.5 (21.9) | ||
| Positive | 82 (78.1) | 82 (78.1) | 100.7 (77.2) | 105.3 (78.1) | ||
| EMVI, n (%) | 0.771 | 0.998 | ||||
| Negative | 35 (33.3) | 37 (35.2) | 46.4 (35.6) | 48.0 (35.6) | ||
| Positive | 70 (66.7) | 68 (64.8) | 84.0 (64.4) | 86.8 (64.4) | ||
| Numbers of high-risk factor, n (%) | 0.510 | 0.883 | ||||
| 1 | 31 (29.5) | 26 (24.8) | 36.5 (28.0) | 35.1 (26.0) | ||
| 2 | 37 (35.2) | 45 (42.9) | 51.2 (39.2) | 56.9 (42.2) | ||
| 3 | 37 (35.2) | 34 (32.4) | 42.8 (32.8) | 42.8 (31.7) | ||
| Interval time (d) | 0.659 | 0.819 | ||||
| mean (SD) | 74.4 (20.0) | 75.6 (18.4) | 75.5 (25.1) | 74.8 (17.7) | ||
In the original samples before matching, 6 patients (2.3%) developed distant metastasis, 9 (3.4%) achieved cCR and received the “watch-and-wait” approach, and the remaining 250 (94.3%) underwent surgery after neoadjuvant therapy. Among patients who received surgery, 126 were in the CC group, and 124 were in the non-CC group. The mean interval in the CC and non-CC groups were 77.9 and 71.7 days (P = 0.015). The rates of pCR and TRG0 were 21.4% vs 14.5% (P = 0.155) and 24.6% vs 16.9% (P = 0.123) in the CC and non-CC group, respectively. The proportion of pN0 and pT0-2N0 was 78.6% vs 72.6% (P = 0.541) and 52.4% vs 46.0% (P = 0.311).
After PSM, each group had 105 patients: 6 (2.9%) developed distant metastasis, 8 (3.8%) achieved cCR, and the remaining 196 (93.3%) underwent surgery after neoadjuvant therapy. Among patients who received surgery, 96 were in the CC group, and 100 were in the non-CC group. The mean interval in the CC and non-CC groups were 76.8 and 74.5 days (P = 0.410). The rate of TRG 0 in the CC group was higher than that in the non-CC group (29.1% vs 17.0%, P = 0.015). The pCR rate was 25.0% (24/96) in the CC group, and 14.0% (14/100) in the non-CC group (P = 0.051). The proportions of pT0-2N0 and ypN0 in CC and non-CC groups were 59.4% vs 46.0% (P = 0.061) and 77.1% vs 72.0% (P = 0.712), respectively. Table 3 shows the details of surgery and pathology in the two groups in the original samples before matching and after PSM.
| Original samples | PSM | |||||
| non-CC group (n = 124) | CC group (n = 126) | P value | non-CC group (n = 100) | CC group (n = 96) | P value | |
| Interval time (d) | 0.015 | 0.410 | ||||
| mean (SD) | 71.7 (21.9) | 77.9 (18.6) | 74.5 (20.1) | 76.8 (18.7) | ||
| Surgical method, n (%) | 0.232 | 0.990 | ||||
| APR | 42 (33.9) | 31 (24.6) | 30 (30.0) | 29 (30.2) | ||
| LAR | 77 (62.1) | 91 (72.2) | 66 (66.0) | 63 (65.6) | ||
| Hartmann | 5 (4.0) | 4 (3.2) | 4 (4.0) | 4 (4.2) | ||
| Surgery time (h) | 0.684 | 0.953 | ||||
| mean (SD) | 3.0 (1.3) | 3.1 (1.4) | 3.0 (1.3) | 3.0 (1.4) | ||
| Blood loss (mL) | 0.345 | 0.407 | ||||
| mean (SD) | 75.4 (51.4) | 105.4 (145.5) | 74.5 (47.8) | 99.3 (105.0) | ||
| R0, n (%) | 123 (99.2) | 124 (98.4) | 0.571 | 99 (99.0) | 94 (97.9) | 0.537 |
| Numbers of dissected lymph nodes | 0.194 | 0.502 | ||||
| mean (SD) | 9.1 (4.9) | 8.3 (5.0) | 9 (4.8) | 8.54 (5.0) | ||
| pT satge, n (%) | 0.400 | 0.136 | ||||
| T0 | 21 (16.9) | 31 (24.6) | 17 (17.0) | 28 (29.2) | ||
| T1 | 6 (4.8) | 10 (7.0) | 5 (5.0) | 9 (9.4) | ||
| T2 | 41 (33.1) | 34 (27.0) | 32 (32.0) | 28 (29.2) | ||
| T3 | 54 (43.5) | 50 (39.7) | 44 (44.0) | 30 (31.2) | ||
| T4 | 2 (1.6) | 1 (0.8) | 2 (2.0) | 1 (1.0) | ||
| pN stage, n (%) | 0.541 | 0.712 | ||||
| N0 | 90 (72.6) | 99 (78.6) | 72 (72.0) | 74 (77.1) | ||
| N1 | 26 (21.0) | 21 (16.7) | 22 (22.0) | 17 (17.7) | ||
| N2 | 8 (6.5) | 6 (4.8) | 6 (6.0) | 5 (5.2) | ||
| TRG, n (%) | 0.123 | 0.015 | ||||
| 0 | 21 (16.9) | 31 (24.6) | 17 (17.0) | 28 (29.1) | ||
| 1 | 43 (34.7) | 51 (40.5) | 33 (33.0) | 41 (42.7) | ||
| 2 | 59 (47.6) | 42 (33.3) | 49 (49.0) | 26 (27.1) | 0.176 | |
| 3 | 1 (0.8) | 2 (1.6) | 1 (1.0) | 1 (1.1) | ||
| pT0-2N0, n (%) | 57 (46.0) | 66 (52.4) | 0.311 | 46 (46.0) | 57 (59.4) | 0.061 |
| pCR, n (%) | 18 (14.5) | 27 (21.4) | 0.155 | 14 (14.0) | 24 (25.0) | 0.051 |
In the original samples before matching, there were 24.3% (33/136, 6 cCR and 27 pCR) of patients in the CC group, and 16.3% (21/129, 3 cCR and 18 pCR) of patients in the non-CC group obtained CR (P = 0.107). After PSM, 5 and 24 patients achieved cCR and pCR in the CC group, respectively, and 3 and 14 patients achieved cCR and pCR in the non-CC group, respectively. The CR rate in the CC group was higher than that in the non-CC group (27.6% vs 16.2%, P = 0.045). After IPTW, the CR rate in the CC group and the non-CC group was 25.9% (35/135) and 16.2% (21/130), respectively (P = 0.045). Table 4 shows the CR rates and univariate regression of CC in the original samples before matching and after PSM and IPTW.
| CR | Univariate regression | ||||
| non-CC group, n (%) | CC group, n (%) | P value | OR (95%CI) | P value | |
| Original samples | 21 (16.3) | 33 (24.3) | 0.107 | 1.648 (0.895-3.033) | 0.109 |
| PSM | 17 (16.2) | 29 (27.6) | 0.045 | 1.975 (1.008-3.871) | 0.047 |
| IPTW | 21 (16.3) | 35 (25.9) | 0.045 | 1.185 (1.008-3.395) | 0.047 |
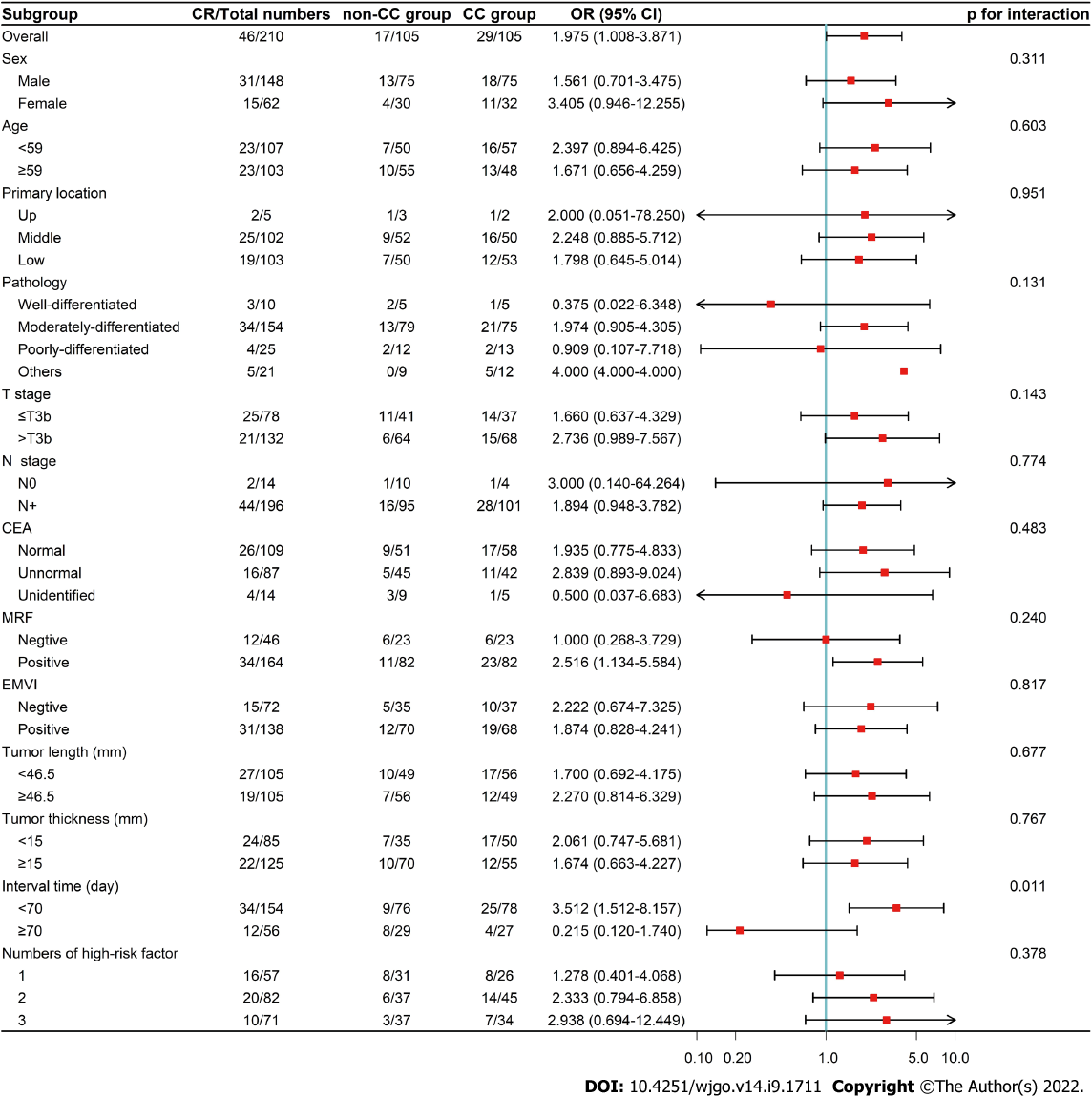
In the exploratory subgroup analysis of the PSM cohort, the median of continuous variables was used for grouping. The results showed that CC could improve the CR rate in patients with MRF positive and intervals < 70 d. After the interaction test, the heterogeneity of the CC effect remained in the subgroup with interval (Figure 2).
Adjuvant chemotherapy was collected for patients who underwent surgery. In the original samples before matching, 146 patients (58.4%) received adjuvant chemotherapy: 73 (57.9%) in the CC group, and 73 (58.9%) in the non-CC group (P = 0.881). Among them, 38 (30.2%) patients in the CC group and 34 (27.4%) patients in the non-CC group completed the full dose of adjuvant chemotherapy (P = 0.632). After PSM, 117 patients (59.7%) received adjuvant chemotherapy: 56 (58.3%) in the CC group, and 61 (61.0%) in the non-CC group (P = 0.704). A total of 28 patients (29.2%) in the CC group and 27 (27.0%) patients in the non-CC group completed the full dose of adjuvant chemotherapy (P = 0.736).
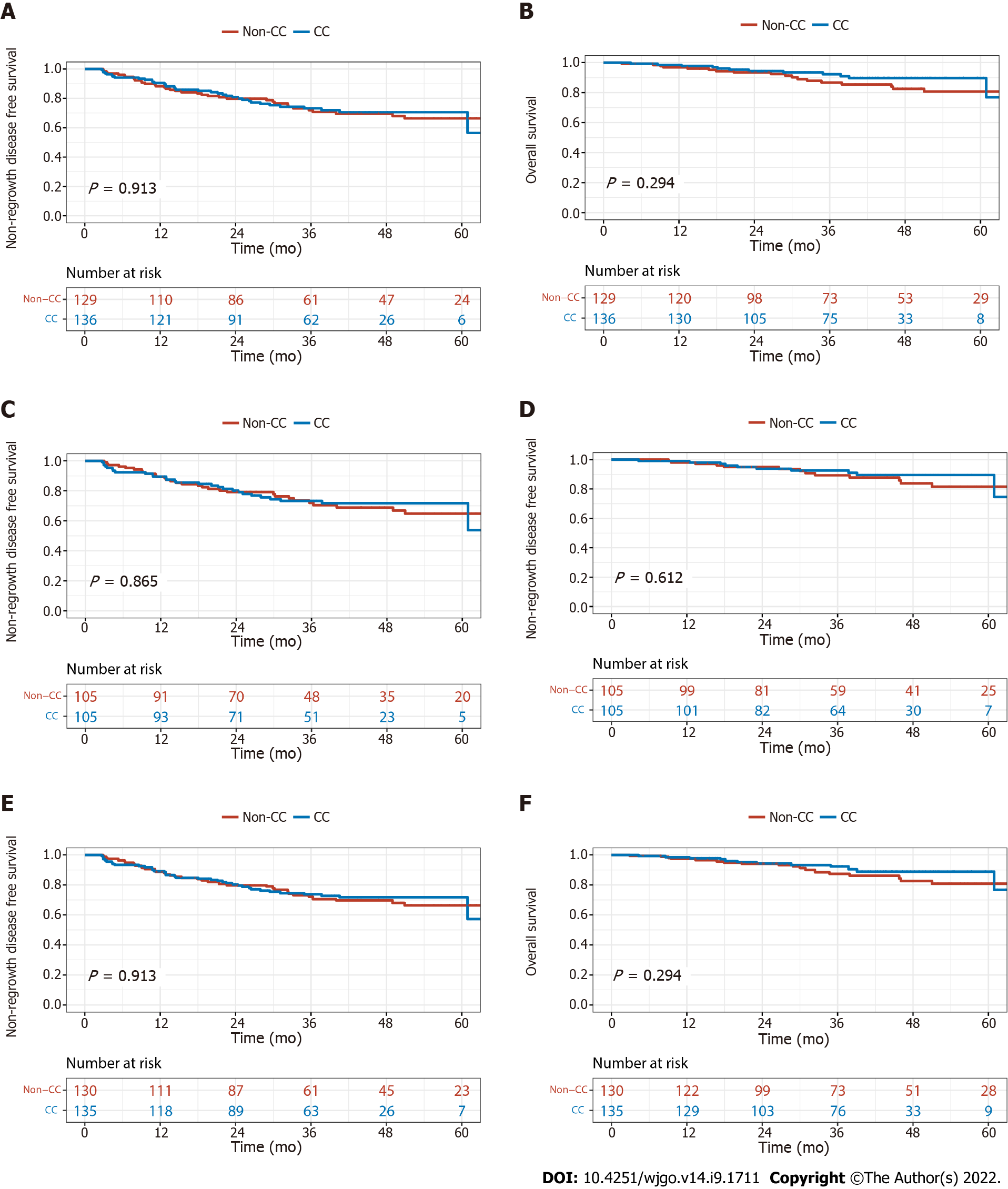
The median follow-up time was 39.8 mo (range, 2.9-74.8). In the original samples before matching, three (33.3%) of nine cCR patients developed local regrowth: two patients within one year and one patient after two years; all three patients received radical surgery. Furthermore, one (11.11%) of the nine patients developed distant metastasis after one year. The three-year NR-DFS and OS were 73.2% vs 71.9% (P = 0.913) and 92.3% vs 86.7% (P = 0.294) in the CC and non-CC groups, respectively. After PSM, the three-years NR-DFS and OS were 73.2% vs 73.5% (P = 0.865) and 92.5% vs 89.3% (P = 0.612). After IPTW, the three-year NR-DFS and OS in the CC group and non-CC groups were 73.8% vs 72.1% (P = 0.913) and 92.4% vs 87.4% (P = 0.294), respectively (Figure 3).
Treatment-related toxicity during neoadjuvant treatment was collected for all 265 patients. In total, 136 (51.3%) patients showed grade ≥ 2 toxicity; 67 (49.3%) patients were in the CC group, and 69 (53.5%) patients were in the non-CC group (P = 0.492). Proctitis/diarrhea (28.3%) was the most common grade ≥ 2 acute toxicity, followed by leukopenia (21.9%). Nine (3.4%) patients developed grade 3 acute toxicity; 4 (2.9%) patients were in the CC group, and 5 (3.9%) patients were in the non-CC group. There was no grade 4 toxicity, as well as toxicity-related deaths, in the two groups (Table 5).
| non-CC group (n = 129), n (%) | CC group (n = 136), n (%) | |||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4-5 | Grade 1 | Grade 2 | Grade 3 | Grade 4-5 | |
| Total | 59 (45.7) | 64 (49.6) | 5 (3.9) | 0 | 66 (48.5) | 63 (46.3) | 4 (2.9) | 0 |
| Leukopenia | 47 (36.4) | 29 (22.5) | 2 (1.6) | 0 | 51 (37.5) | 27 (19.9) | 0 | 0 |
| Neutropenia | 22 (17.1) | 9 (7.0) | 0 | 0 | 22 (16.2) | 9 (6.6) | 0 | 0 |
| Anemia | 5 (3.9) | 6 (4.7) | 2 (1.6) | 0 | 14 (10.3) | 5 (3.7) | 0 | 0 |
| Thrombocytopenia | 9 (7.0) | 0 | 1 (0.8) | 0 | 5 (3.7) | 0 | 0 | 0 |
| Aminotransferase increased | 0 (0.0) | 1 (0.8) | 0 | 0 | 6 (4.4) | 0 | 0 | 0 |
| Bilirubin increased | 19 (14.7) | 2 (3.1) | 0 | 0 | 18 (13.2) | 2 (1.5) | 1 (0.7) | 0 |
| Nausea | 39 (30.2) | 0 | 0 | 0 | 30 (22.1) | 1 (0.7) | 0 | 0 |
| Fatigue | 58 (45.0) | 3 (2.3) | 0 | 0 | 66 (44.9) | 2 (1.5) | 0 | 0 |
| Proctitis/diarrhea | 66 (51.2) | 36 (27.9) | 1 (0.8) | 0 | 66 (48.5) | 39 (28.7) | 2 (1.5) | 0 |
| Cystitis | 38 (29.5) | 0 | 0 | 0 | 42 (30.9) | 0 | 0 | 0 |
| Radiodermatitis | 75 (58.1) | 6 (4.7) | 0 | 0 | 70 (51.5) | 3 (2.2) | 0 | 0 |
To our knowledge, this is the first study to explore the effects of one to two cycles of CC with capecitabine after NCRT for high-risk LARC patients. The results showed that without extending the interval between the end of NCRT and surgery, this regimen increased CR rates, but did not improve the three-year NR-DFS and OS.
Pelvic MRI has been widely used to evaluate rectal cancer. It could evaluate the primary tumor and pelvic lymph node stage and accurately determine the depth of invasion beyond the muscularis propria, MRF, and EMVI status that affected the prognosis of patients. In 2001, Merkel et al[39] analyzed the postoperative pathology of 853 patients with rectal cancer and found that patients with tumor invasion distance ≤ 5 mm had a better 5-year local recurrence rate and tumor-specific survival than those with > 5 mm (10.4% vs 26.3%, P < 0.0001; 85.4% vs 54.1%, P < 0.0001). In the MERCURY study, patients who were MRF negative had better three-year DFS and OS than those who were MRF positive (47.3% vs 67.2%, P < 0.05; 42.2% vs 62.2%, P < 0.01)[40]. A meta-analysis that included 6 studies of 1262 rectal cancer found that patients with EMVI-positive were 3.91 times more likely to develop distant metastases than EMVI-negative patients[41]. According to the depth of invasion beyond muscularis propria, MRF, EMVI status and other factors, ESMO guidelines stratified the risk groups in rectal cancer and recommended treatment options within the risk category[11]. For patients with high-risk rectal cancer, neoadjuvant chemoradiotherapy was still the standard treatment[11].
After neoadjuvant treatment, patients with pCR had good long-term prognosis[4,5], and patients with cCR could receive the “‘watch-and-wait” strategy, which improved the quality of life[7-10]. Maas et al[5] analyzed 3105 LARC, and the results showed that patients with pCR had significantly better five-year DFS (83.3% vs 65.6%, P < 0.0001), local recurrence (2.8% vs 9.7%, P < 0.0001), and distant metastases (11.2% vs 25.1%, P < 0.0001) rates than those who did not achieve pCR. The International Watch and Wait Database and OnCoRe project showed that cCR patients had stable biological behavior and good prognosis with a local regrowth rate of 20%-25.2%, distant metastasis of 7%-9%, and a five-year OS of 73%-97%[7-10]. In our study, 33.3% (3/9) patients had local tumor growth, and 11.1% (1/9) had distant metastasis; these findings were higher than those in published data. This might be related to the small size of the cCR patients, and all patients enrolled in the study were at high-risk with LARC. Therefore, this result deserved further exploration.
Although patients with pCR had good prognosis, the pCR rate after NCRT was approximately 20%, and it was even lower in patients with high-risk LARC[5,17]. To increase the CR rate, some studies explored the effect of CC. Garcia-Aguilar et al[20] analyzed zero, two, four, and six cycles of FOLFOX after NCRT in LARC, and the pCR rates increased (18% for zero cycles, 25% for two cycles, 30% for four cycles, and 38% for six cycles). The CAO/ARO/AIO-12 study analyzed three additional chemotherapy cycles before and after NCRT in MRI-defined high-risk LARC. The results demonstrated that the pCR rate in the CC group was better than that in the induction chemotherapy group (25% vs 17%)[19]. However, increasing the cycles of CC also prolonged the interval between NCRT and surgery, and current research indicates that the extended intervals increase the pCR rate[28,42]. When the time was 10-11 wk, the pCR rate was the highest[23]. In the original samples before matching, the interval in the CC group was longer than that in the non-CC group. After PSM and IPTW, the interval was balanced in the 2 groups with a median of 70 days, and the CR rate in the CC group was higher than that in the non-CC group. The subgroup analysis showed that the CR rates increased when the interval was < 70 d. This may be because all the patients enrolled in this study were at high-risk with LARC, and the standard dose of NCRT was not enough to get the best regression. When the interval was < 70 d, both low-intensity CC and extending time could increase the tumor regression.
Several studies have also explored the effect of CC with capecitabine after NCRT. Zampino et al[32] evaluated the effect of NCRT followed by 2 cycles of capecitabine in 51 patients. The interval between the end of NCRT and surgery was less than eight weeks. The results showed that the pCR rate was 18%, and the five-year DFS was 85.4%, with no increase in acute toxicity or postoperative complications. The OIGIT-01 trial was designed with 1 cycle of induction chemotherapy with capecitabine followed by NCRT and 2 cycles of CC with capecitabine in 66 patients. The median interval was eight weeks, and this regimen was well-tolerated. The pCR rate was 17.5%, and the 5-year DFS was 64%[33]. However, these two studies were single-arm studies with a small sample size, and the patients were not stratified by pelvic MRI before treatment. In a previous study, we analyzed the efficacy of one to two cycles of CC with capecitabine in low-risk patients with LARC, which did not improve the CR rate and three-year NR-DFS[16]. In the current study, we included high-risk patients with LARC. After PSM and IPTW, the CR rate in the CC group was higher than that in the non-CC group. Data after PSM also showed that the CC increased the rate of TRG 0. In addition, subgroup analysis after PSM showed that MRF-positive patients were more likely to benefit from CC. These results suggest that one to two cycles of CC with capecitabine can increase tumor regression in high-risk patients with LARC, thus providing new evidence for the individualized treatment of patients with LARC.
The PRODIGE 23 trial explored the intensification of chemotherapy by using triple drugs before NCRT, and the results showed that it significantly improved three-year DFS (76% vs 69%, P = 0.034) compared with NCRT in patients with LARC[22]. In the CAO/ARO/AIO-12 study, there were no difference in the three-year DFS of patients in the induction chemotherapy and CC groups (73% vs 73%, P = 0.82)[43]. In the current study, one to two cycles of CC with capecitabine did not increase the three-year NR-DFS in high-risk patients with LARC (73.2% vs 71.9%, P = 0.913). Intensified systemic therapy should be implemented to improve long-term outcomes.
As a single-center retrospective study, this study had some inherent limitations. First, despite applying the PSM and IPTW analysis to balance differences between the two groups, bias might still exist in the study. Second, the sample size was small, and the follow-up time was short. Prospective studies with more participants and a longer follow-up period need to be performed to confirm these findings.
Without extending the interval between the end of NCRT and surgery, one to two cycles of CC with capecitabine after NCRT was safe and increased the CR rate in high-risk patients with LARC. However, it failed to improve long-term outcomes. This study provides a powerful rationale for further exploration in phase 3, multicenter, randomized trails.
Patients with locally advanced rectal cancer (LARC) who achieved complete response (CR) after neoadjuvant therapy had a better prognosis, but the optimal neoadjuvant therapy regimen remained unclear.
Several studies have suggested that consolidation chemotherapy (CC) after neoadjuvant chemoradiotherapy (NCRT) seemed to improve CR rate, however it also prolonged interval between NCRT and surgery, making surgery more difficult. Besides, in the concurrent chemotherapy, the additional oxaliplatin not only increased toxicity but also failed to improve the efficacy. Further, high-risk patients with LARC were less likely to achieve CR, and had worse prognosis than patients in low-risk. Considering the efficacy and low toxicity of capecitabine in the treatment of rectal cancer and the convenience of oral therapy, we designed this retrospective study.
To evaluate the effects of one to two cycles of CC with capecitabine in high-risk patients with LARC without extending NCRT and surgery interval.
From January 2015 to July 2019, high-risk patients with LARC were divided into the CC and non-CC group according to whether they received CC after NCRT. Propensity score matching (PSM) and inverse probability of treatment weight (IPTW) were used to balance the differences between the two groups.
After PSM and IPTW, the CR rate in the CC group was higher than that in the non-CC group. The median follow-up was over three years, and there were no differences in 3-year non-regrowth disease-free survival nor overall survival in the two groups. There was also no increase in acute toxicity in the CC group.
Our study first confirmed without extending the interval between the end of NCRT and surgery, one to two cycles of CC with capecitabine after NCRT was safe and increased the CR rate in high-risk patients with LARC. However, it failed to improve long-term outcomes.
Further studies with more participants and a longer follow-up period need to be investigated to confirm these findings.
We thank all the patients and staff of Peking University Cancer Hospital who participated in this study for their valuable contributions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bairwa DBL, India; Cabezuelo AS, Spain; Preziosi F, Italy S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R; German Rectal Cancer Study Group. Preoperative vs postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4460] [Article Influence: 212.4] [Reference Citation Analysis (1)] |
| 2. | Kitz J, Fokas E, Beissbarth T, Ströbel P, Wittekind C, Hartmann A, Rüschoff J, Papadopoulos T, Rösler E, Ortloff-Kittredge P, Kania U, Schlitt H, Link KH, Bechstein W, Raab HR, Staib L, Germer CT, Liersch T, Sauer R, Rödel C, Ghadimi M, Hohenberger W; German Rectal Cancer Study Group. Association of Plane of Total Mesorectal Excision With Prognosis of Rectal Cancer: Secondary Analysis of the CAO/ARO/AIO-04 Phase 3 Randomized Clinical Trial. JAMA Surg. 2018;153:e181607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Das P, Skibber JM, Rodriguez-Bigas MA, Feig BW, Chang GJ, Wolff RA, Eng C, Krishnan S, Janjan NA, Crane CH. Predictors of tumor response and downstaging in patients who receive preoperative chemoradiation for rectal cancer. Cancer. 2007;109:1750-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez-Bigas MA, Eng C, Feig BW, Das P, Krishnan S, Crane CH, Hu CY, Chang GJ. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 382] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 5. | Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K, Mohiuddin M, Pucciarelli S, Small W Jr, Suárez J, Theodoropoulos G, Biondo S, Beets-Tan RG, Beets GL. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1459] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 6. | Jäger T, Neureiter D, Urbas R, Klieser E, Hitzl W, Emmanuel K, Dinnewitzer A. Applicability of American Joint Committee on Cancer and College of American Pathologists Regression Grading System in Rectal Cancer. Dis Colon Rectum. 2017;60:815-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Chadi SA, Malcomson L, Ensor J, Riley RD, Vaccaro CA, Rossi GL, Daniels IR, Smart NJ, Osborne ME, Beets GL, Maas M, Bitterman DS, Du K, Gollins S, Sun Myint A, Smith FM, Saunders MP, Scott N, O'Dwyer ST, de Castro Araujo RO, Valadao M, Lopes A, Hsiao CW, Lai CL, Smith RK, Paulson EC, Appelt A, Jakobsen A, Wexner SD, Habr-Gama A, Sao Julião G, Perez R, Renehan AG. Factors affecting local regrowth after watch and wait for patients with a clinical complete response following chemoradiotherapy in rectal cancer (InterCoRe consortium): an individual participant data meta-analysis. Lancet Gastroenterol Hepatol. 2018;3:825-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 8. | van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, Habr-Gama A, Perez RO, Renehan AG, van de Velde CJH; IWWD Consortium. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391:2537-2545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 733] [Article Influence: 104.7] [Reference Citation Analysis (0)] |
| 9. | Smith JJ, Strombom P, Chow OS, Roxburgh CS, Lynn P, Eaton A, Widmar M, Ganesh K, Yaeger R, Cercek A, Weiser MR, Nash GM, Guillem JG, Temple LKF, Chalasani SB, Fuqua JL, Petkovska I, Wu AJ, Reyngold M, Vakiani E, Shia J, Segal NH, Smith JD, Crane C, Gollub MJ, Gonen M, Saltz LB, Garcia-Aguilar J, Paty PB. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA Oncol. 2019;5:e185896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 411] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 10. | Renehan AG, Malcomson L, Emsley R, Gollins S, Maw A, Myint AS, Rooney PS, Susnerwala S, Blower A, Saunders MP, Wilson MS, Scott N, O'Dwyer ST. Watch-and-wait approach vs surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17:174-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 559] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 11. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22-iv40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1195] [Article Influence: 149.4] [Reference Citation Analysis (0)] |
| 12. | Shepherd NA, Baxter KJ, Love SB. Influence of local peritoneal involvement on pelvic recurrence and prognosis in rectal cancer. J Clin Pathol. 1995;48:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, Sebag-Montefiore DJ, Tekkis P, Brown G; MERCURY study group. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 465] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 14. | Garcia-Aguilar J, Renfro LA, Chow OS, Shi Q, Carrero XW, Lynn PB, Thomas CR Jr, Chan E, Cataldo PA, Marcet JE, Medich DS, Johnson CS, Oommen SC, Wolff BG, Pigazzi A, McNevin SM, Pons RK, Bleday R. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol. 2015;16:1537-1546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 296] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 15. | Wilkins S, Haydon A, Porter I, Oliva K, Staples M, Carne P, McMurrick P, Bell S. Complete Pathological Response After Neoadjuvant Long-Course Chemoradiotherapy for Rectal Cancer and Its Relationship to the Degree of T3 Mesorectal Invasion. Dis Colon Rectum. 2016;59:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Sheng X, Li S, Zhang Y, Geng J, Wang H, Zhu X, Quan J, Li Y, Cai Y, Wang W. One to Two Cycles of Consolidation Chemotherapy With Capecitabine After Neoadjuvant Chemoradiotherapy Does Not Benefit Low-Risk Patients With Locally Advanced Middle-Low Rectal Cancer. Front Oncol. 2021;11:695726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Chua YJ, Barbachano Y, Cunningham D, Oates JR, Brown G, Wotherspoon A, Tait D, Massey A, Tebbutt NC, Chau I. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 18. | Fernandez-Martos C, Garcia-Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C, Safont MJ, Salud A, Vera R, Massuti B, Escudero P, Alonso V, Bosch C, Martin M, Minsky BD. Chemoradiation, surgery and adjuvant chemotherapy vs induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial†. Ann Oncol. 2015;26:1722-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 205] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 19. | Fokas E, Allgäuer M, Polat B, Klautke G, Grabenbauer GG, Fietkau R, Kuhnt T, Staib L, Brunner T, Grosu AL, Schmiegel W, Jacobasch L, Weitz J, Folprecht G, Schlenska-Lange A, Flentje M, Germer CT, Grützmann R, Schwarzbach M, Paolucci V, Bechstein WO, Friede T, Ghadimi M, Hofheinz RD, Rödel C; German Rectal Cancer Study Group. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J Clin Oncol. 2019;37:3212-3222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 354] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 20. | Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, Kumar AS, Oommen S, Coutsoftides T, Hunt SR, Stamos MJ, Ternent CA, Herzig DO, Fichera A, Polite BN, Dietz DW, Patil S, Avila K; Timing of Rectal Cancer Response to Chemoradiation Consortium. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16:957-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 515] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 21. | Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, Roodvoets AGH, Nagtegaal ID, Beets-Tan RGH, Blomqvist LK, Fokstuen T, Ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes A, Nilsson PJ, Glimelius B, van de Velde CJH, Hospers GAP; RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) vs preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 956] [Article Influence: 239.0] [Reference Citation Analysis (0)] |
| 22. | Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, Vendrely V, Artignan X, Bouché O, Gargot D, Boige V, Bonichon-Lamichhane N, Louvet C, Morand C, de la Fouchardière C, Lamfichekh N, Juzyna B, Jouffroy-Zeller C, Rullier E, Marchal F, Gourgou S, Castan F, Borg C; Unicancer Gastrointestinal Group and Partenariat de Recherche en Oncologie Digestive (PRODIGE) Group. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:702-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 732] [Article Influence: 183.0] [Reference Citation Analysis (0)] |
| 23. | Probst CP, Becerra AZ, Aquina CT, Tejani MA, Wexner SD, Garcia-Aguilar J, Remzi FH, Dietz DW, Monson JR, Fleming FJ; Consortium for Optimizing the Surgical Treatment of Rectal Cancer (OSTRiCh). Extended Intervals after Neoadjuvant Therapy in Locally Advanced Rectal Cancer: The Key to Improved Tumor Response and Potential Organ Preservation. J Am Coll Surg. 2015;221:430-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 24. | Evans J, Tait D, Swift I, Pennert K, Tekkis P, Wotherspoon A, Chau I, Cunningham D, Brown G. Timing of surgery following preoperative therapy in rectal cancer: the need for a prospective randomized trial? Dis Colon Rectum. 2011;54:1251-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Bujko K. Timing of surgery following preoperative therapy in rectal cancer: there is no need for a prospective randomized trial. Dis Colon Rectum. 2012;55:e31; author reply e31-e31; author reply e32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Petrelli F, Coinu A, Lonati V, Barni S. A systematic review and meta-analysis of adjuvant chemotherapy after neoadjuvant treatment and surgery for rectal cancer. Int J Colorectal Dis. 2015;30:447-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, Souquet JC, Adeleine P, Gerard JP. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 558] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 28. | Huntington CR, Boselli D, Symanowski J, Hill JS, Crimaldi A, Salo JC. Optimal Timing of Surgical Resection After Radiation in Locally Advanced Rectal Adenocarcinoma: An Analysis of the National Cancer Database. Ann Surg Oncol. 2016;23:877-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Rödel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T, Graeven U, Arnold D, Lang-Welzenbach M, Raab HR, Sülberg H, Wittekind C, Potapov S, Staib L, Hess C, Weigang-Köhler K, Grabenbauer GG, Hoffmanns H, Lindemann F, Schlenska-Lange A, Folprecht G, Sauer R; German Rectal Cancer Study Group. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin vs fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 504] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 30. | Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, Chen D, Cao J, Wei H, Peng X, Huang Z, Cai G, Zhao R, Xu L, Zhou H, Wei Y, Zhang H, Zheng J, Huang Y, Zhou Z, Cai Y, Kang L, Huang M, Wu X, Peng J, Ren D, Wang J. Neoadjuvant Modified FOLFOX6 With or Without Radiation Versus Fluorouracil Plus Radiation for Locally Advanced Rectal Cancer: Final Results of the Chinese FOWARC Trial. J Clin Oncol. 2019;37:3223-3233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 31. | Gérard JP, Azria D, Gourgou-Bourgade S, Martel-Lafay I, Hennequin C, Etienne PL, Vendrely V, François E, de La Roche G, Bouché O, Mirabel X, Denis B, Mineur L, Berdah JF, Mahé MA, Bécouarn Y, Dupuis O, Lledo G, Seitz JF, Bedenne L, Juzyna B, Conroy T. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012;30:4558-4565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 308] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 32. | Zampino MG, Magni E, Leonardi MC, Petazzi E, Santoro L, Luca F, Chiappa A, Petralia G, Trovato C, Fazio N, Orecchia R, Nolè F, de Braud F. Capecitabine initially concomitant to radiotherapy then perioperatively administered in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2009;75:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Golo D, But-Hadzic J, Anderluh F, Brecelj E, Edhemovic I, Jeromen A, Omejc M, Oblak I, Secerov-Ermenc A, Velenik V. Induction chemotherapy, chemoradiotherapy and consolidation chemotherapy in preoperative treatment of rectal cancer - long-term results of phase II OIGIT-01 Trial. Radiol Oncol. 2018;52:267-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Brown G, Daniels IR, Richardson C, Revell P, Peppercorn D, Bourne M. Techniques and trouble-shooting in high spatial resolution thin slice MRI for rectal cancer. Br J Radiol. 2005;78:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S, Halligan S, Hoeffel C, Kim SH, Laghi A, Maier A, Rafaelsen SR, Stoker J, Taylor SA, Torkzad MR, Blomqvist L. Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2018;28:1465-1475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 605] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 36. | Li JL, Ji JF, Cai Y, Li XF, Li YH, Wu H, Xu B, Dou FY, Li ZY, Bu ZD, Wu AW, Tham IW. Preoperative concomitant boost intensity-modulated radiotherapy with oral capecitabine in locally advanced mid-low rectal cancer: a phase II trial. Radiother Oncol. 2012;102:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6460] [Article Influence: 430.7] [Reference Citation Analysis (0)] |
| 38. | Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13:273-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 577] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 39. | Merkel S, Mansmann U, Siassi M, Papadopoulos T, Hohenberger W, Hermanek P. The prognostic inhomogeneity in pT3 rectal carcinomas. Int J Colorectal Dis. 2001;16:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 211] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, Sebag-Montefiore D, Tekkis P, Brown G; Magnetic Resonance Imaging in Rectal Cancer European Equivalence Study Study Group. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 441] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 41. | Siddiqui MRS, Simillis C, Hunter C, Chand M, Bhoday J, Garant A, Vuong T, Artho G, Rasheed S, Tekkis P, Abulafi AM, Brown G. A meta-analysis comparing the risk of metastases in patients with rectal cancer and MRI-detected extramural vascular invasion (mrEMVI) vs mrEMVI-negative cases. Br J Cancer. 2017;116:1513-1519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 42. | Rombouts AJM, Hugen N, Elferink MAG, Nagtegaal ID, de Wilt JHW. Treatment Interval between Neoadjuvant Chemoradiotherapy and Surgery in Rectal Cancer Patients: A Population-Based Study. Ann Surg Oncol. 2016;23:3593-3601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 43. | Fokas E, Schlenska-Lange A, Polat B, Klautke G, Grabenbauer GG, Fietkau R, Kuhnt T, Staib L, Brunner T, Grosu AL, Kirste S, Jacobasch L, Allgäuer M, Flentje M, Germer CT, Grützmann R, Hildebrandt G, Schwarzbach M, Bechstein WO, Sülberg H, Friede T, Gaedcke J, Ghadimi M, Hofheinz RD, Rödel C; German Rectal Cancer Study Group. Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Patients With Locally Advanced Rectal Cancer: Long-term Results of the CAO/ARO/AIO-12 Randomized Clinical Trial. JAMA Oncol. 2022;8:e215445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 193] [Article Influence: 48.3] [Reference Citation Analysis (0)] |