Published online Aug 15, 2022. doi: 10.4251/wjgo.v14.i8.1552
Peer-review started: November 27, 2021
First decision: January 23, 2022
Revised: January 19, 2022
Accepted: July 26, 2022
Article in press: July 26, 2022
Published online: August 15, 2022
Processing time: 256 Days and 7.7 Hours
Duodenal-type follicular lymphoma (D-FL) has been recognized as a rare entity that accounts for approximately 4% of primary gastrointestinal lymphomas. D-FL follows an indolent clinical course compared with common nodal FL and is generally considered to have a better prognosis. Therefore, the “watch and wait” approach is frequently adopted as the treatment method. Alternatively, there is an option to actively intervene in D-FL. However, the long-term outcomes of such cases are poorly understood.
To clarify the clinical outcomes after long-term follow-up in cases of D-FL with treatment intervention.
We retrospectively analyzed patients who met the following criteria: the lesion was confirmed by endoscopy, the diagnosis of D-FL was confirmed histopathologically, and the patient was followed-up for more than 10 years after the intervention at our center.
We identified 5 cases of D-FL. Two patients showed a small amount of bone marrow involvement (Stage IV). Rituximab was used as a treatment for remission in all 5 patients. It was also used in combination with chemotherapy in 2 Stage IV patients as well as for maintenance treatment. Radiation therapy was performed in 2 cases, which was followed by complete remission (CR). Eventually, all 5 patients achieved CR and survived for more than 10 years. However, 3 patients experienced recurrence. One patient achieved a second CR by retreatment, and in another case, the lesion showed spontaneous disappearance. The remaining patient had systemic widespread recurrence 13 years after the first CR. Biopsy results suggested that the FL lesions were transformed into diffuse large B-cell lymphoma. The patient died 4 years later despite receiving various chemotherapies.
In this study, the treatment for patients of D-FL in Stage IV was successful. In the future, criteria for how to treat “advanced” D-FL should be established based on additional cases. This study of patients with D-FL indicates that whole-body follow-up examinations should continue for a long time due to a fatal recurrence 13 years after reaching CR.
Core Tip: Since duodenal-type follicular lymphoma (D-FL) progresses more indolently than common nodal FL, the “watch and wait” approach is frequently used without intervention. To elucidate the clinical assessments of long-term follow-up in cases of D-FL with treatment intervention, we retrospectively examined 5 D-FL patients for more than 10 years after treatment at our center. All 5 patients eventually achieved complete remission and survived for a long period. However, 3 patients experienced recurrence, and 1 patient died of the primary disease 21 years after first onset. In the future, it will be necessary to establish criteria for how to treat Stage IV “advanced” D-FL.
- Citation: Saito M, Mori A, Tsukamoto S, Ishio T, Yokoyama E, Izumiyama K, Morioka M, Kondo T, Sugino H. Duodenal-type follicular lymphoma more than 10 years after treatment intervention: A retrospective single-center analysis. World J Gastrointest Oncol 2022; 14(8): 1552-1561
- URL: https://www.wjgnet.com/1948-5204/full/v14/i8/1552.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i8.1552
Duodenal-type follicular lymphoma (D-FL) is an entity that was newly classified as a variant of FL in the 2017 World Health Organization classification[1]. Most D-FLs are asymptomatic and are often incidentally found by esophagogastroduodenoscopy (EGD). Many large-scale clinical analyses of D-FL have been conducted in Japan[2,3], where endoscopic screening for gastric cancer is frequently performed. This is because the incidence of gastric cancer is higher in Japan than in Western countries due to genetic and dietary factors[4]. The most common endoscopic findings are “white granular or multiple nodular, polypoid lesions” in the descending portion of the duodenum[5]. In addition, 85% of D-FL cases have been shown to have jejunal or ileal lesions, which were detected by capsule or double-balloon enteroscopy[6].
D-FL follows an indolent progression compared with common nodal FL and has a generally better prognosis. Gene expression profiling of D-FL has yielded results similar to those obtained for mucosa-associated lymphoid tissue (MALT) lymphoma[7,8]. Sufficient consensus has not yet been reached as to whether therapeutic treatment should be administered to patients with D-FL, and the “watch and wait” strategy is currently frequently performed[9,10]. Long-term observations after intervention for D-FL have not been reported, and the long-term outcome of therapeutic treatment is not well understood. To clarify the clinical outcomes after long-term follow-up in cases of D-FL with treatment, we analyzed patients with D-FL who were followed-up for more than 10 years after intervention at our center.
This was a retrospective, observational study at our center.
We included D-FL patients who were diagnosed endoscopically and histopathologically and followed clinically for more than 10 years after starting treatment intervention at our center between January 1998 and December 2009.
FL was predominantly diagnosed by a pathological diagnosis, although the detection of IgH-BCL2 by fluorescence in situ hybridization (FISH) was also respected to the same extent. In addition to EGD, patients were examined by colonoscopy, contrast computed tomography (CT), positron emission tomography (PET)-CT, bone marrow aspiration/biopsy, and wherever possible enteroscopy of the distal small intestine. Patients with a predominant systemic spread of nodal lesions were excluded. However, not all patients with swelling of the lymph nodes were excluded, as mentioned below in the “Results” section. Characteristics of the patients were assessed by performance status, histological grading, and follicular lymphoma international prognostic index. Staging followed the Lugano classification for gastrointestinal lymphoma.
This study was carried out as part of standard care in daily clinical practice under Japanese health insurance, and no treatments specific to this study were performed. Because bendamustine had not been approved by health insurance in Japan by 2010, we did not use it at the time of initial onset for applicable patients. Since 2008, for patients with newly developed nodal FL that progressed to Ann Arbor Stage III or higher, our center has provided maintenance treatment with rituximab bimonthly after reaching complete remission (CR).
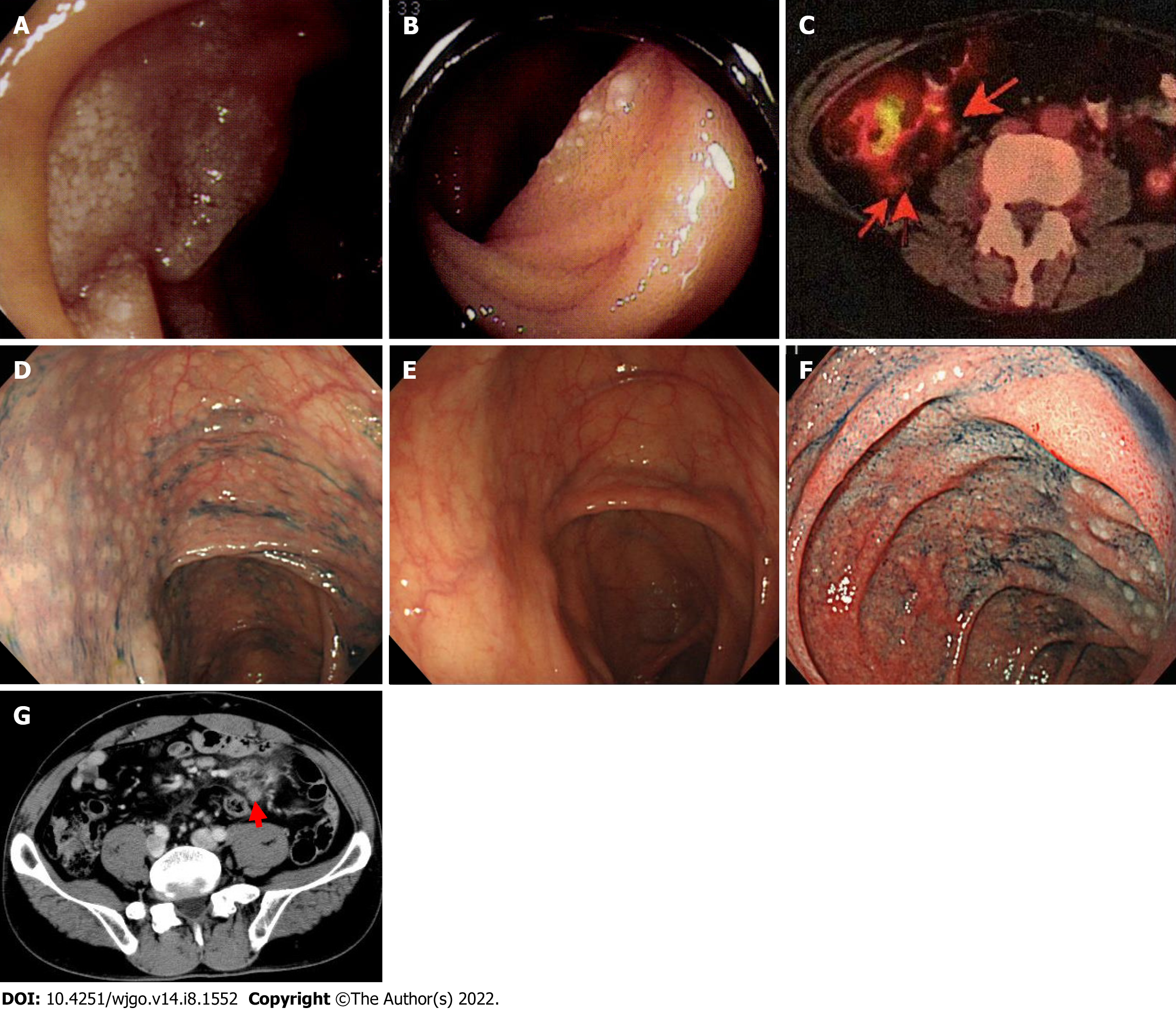
Five patients were included in this study. Of these patients, 4 were referred by Hokkaido University Hospital (Cases 1-4), including 1 recurrent patient (Case 1), and we were requested to continuously follow these patients. Table 1 shows the clinical features of these patients. Three patients were males in their 40 s, and two patients were females in their 60 s. None of the 5 patients had subjective symptoms, and the trigger for the diagnosis was discovered incidentally by EGD. In all patients, performance status was 0, and the histopathological grade was 1. Enteroscopy of the small bowel was performed in 3 patients, and distal intestinal lesions were observed in 2 patients (Figure 1A and B). Two patients showed a small amount of bone marrow involvement and were evaluated as Stage IV. Based on the follicular lymphoma international prognostic index, 4 patients were low risk, and 1 patient was intermediate risk.
| Case | Age | Sex | Trigger to be found | PS | Grade | Distal intestinal lesion | Extra-duodenal lesion | Initial stage | FLIPI |
| 1 | 65 | F | Screening EGD | 0 | 1 | Jejunum | (-) | I | Low |
| 2 | 63 | F | Follow-up for GERD | 0 | 1 | Jejunum | Bone marrow | IV | Int |
| 3 | 40 | M | Screening EGD | 0 | 1 | Not tested | Bone marrow, mesenteric LN | IV | Low |
| 4 | 42 | M | Screening EGD | 0 | 1 | (-) | (-) | I | Low |
| 5 | 42 | M | Screening EGD | 0 | 1 | Not tested | (-) | I | Low |
In all 5 patients, therapeutic intervention was performed with the goal of remission, and the treatment details and outcomes are shown in Table 2. Two patients were treated because of an advanced stage (Stage IV), and the other 2 patients requested treatment.
| Case | Treatment motive | Initial treatment | Effect | Rituximab maintenance | Relapse lesion/re-Stage (from the end of treatment) | 2nd treatment | Outcome (from the 1st onset) |
| 1 | Previously followed doctor’s judgment | RTX | 1st CR | (-) | Duodenum + cervical LN/Stage IV (1 yr and 3 mo) | R-THP-COP | 2nd CR (18 years) |
| 2 | In stage IV | R-CVP + R-F | 1st CR | (+) | Colon + mesenteric LN/Stage II1 (1 yr and 7 mo) | Watch | 2nd CR (12 yr) |
| 3 | In stage IV | R-CHOP | 1st CR | (+) | (-) | - | 1st CR (13 yr) |
| 4 | Patient’s request | Radiation + RTX | 1st CR | (-) | (-) | - | 1st CR (16 yr) |
| 5 | Patient’s request | CHOP/RTX/radiation | 1st CR | (-) | Lung + systemic LN/Stage IV (13 yr) | B-R/R-BAC/CHOEP/ONTZ | Death due to primary disease (21 yr) |
Rituximab was used as a treatment for remission in all 5 patients. In 2 patients, it was used as a single agent, and in 2 Stage IV patients it was used in combination with chemotherapy. Additionally, it was used for maintenance treatment. Radiation therapy was performed in 2 cases, followed by CR. Eventually, all 5 patients achieved CR and survived for more than 10 years. However, 3 patients experienced recurrence but not within 10 years. One patient achieved a second CR by retreatment, and for another patient, the lymphoma lesion disappeared spontaneously. The remaining patient had systemic widespread recurrence 13 years after the first CR. Later, the biopsy results suggested that the FL lesions had transformed into diffuse large B-cell lymphoma (DLBCL). The patient died 4 years later despite receiving various anticancer drugs.
Case 1: Rituximab monotherapy (375 mg/m2 × 4 times) resulted in a first CR at Hokkaido University Hospital. However, 1 year and 3 mo later, D-FL recurred not only locally in the duodenum but also in the cervical lymph nodes (Lugano Stage IV). Six cycles of rituximab, cyclophosphamide, pirarubicin, vincristine, and prednisone chemotherapy [a regimen in which doxorubicin in cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) was changed to pirarubicin, which is less cardiotoxic; rituximab 375 mg/m2 + cyclophosphamide 750 mg/m2, pirarubicin 50 mg/m2, and vincristine 1.4 mg/m2, all on day 1, and prednisone 60 mg/body on days 1-5, every 3 wk] were administered, and this patient achieved a second CR.
Case 2: In addition to the duodenum, FL lesions spread to the jejunum and a slight extent to the bone marrow. IgH-BCL2 was detected in barely 1% of the nucleated cells by FISH. The lesion was in Stage IV and presented an intermediate risk in the follicular lymphoma international prognostic index. The patient’s first CR, including bone marrow findings (IgH-BCL2, 0.0%), was reached after administration of 3 cycles of rituximab + cyclophosphamide, vincristine, and prednisone (rituximab + cyclophosphamide 750 mg/m2 and vincristine 1.4 mg/m2, all on day 1, and prednisone 60 mg/body on days 1-5 every 3 wk) and 5 cycles of rituximab + oral fludarabine (40 mg/m2 on days 1-5 every 4 wk). Maintenance treatment with rituximab monotherapy was performed; however, 1 year and 7 mo later, PET-CT indicated lesions of the intestinal tract and nearby mesenteric lymph nodes in the ileocecal region (Figure 1C). Although no abnormalities were found in the duodenum or jejunum by endoscopy, multiple lymphomatous polyposis-like lesions were found in the ascending colon to the cecum (Figure 1D) and the rectum. IgH-BCL2 positivity was found in 78.0% of the cells in biopsy tissue by FISH, suggesting recurrence (Lugano Stage II1). After follow-up with no treatment, the lesion disappeared spontaneously approximately 1 year later (Figure 1E).
Case 3: FL lesions were observed not only in the duodenum (Figure 1F) but also in the mesenteric small lymph nodes (Figure 1G, diagnosed by biopsy) and bone marrow. IgH-BCL2 positivity was observed in 5.8% of the nucleated cells by FISH (Stage IV). The distal small intestine was not searched by enteroscopy, and it cannot be ruled out completely that an extraduodenal primary lesion was present. The first CR was achieved after 8 cycles of R-CHOP (rituximab + cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, and vincristine 1.4 mg/m2, all on day 1, and prednisone 60 mg/m2 on days 1-5 every 3 wk), and maintenance treatment with rituximab was administered. The patient has not relapsed and still maintains the first CR.
Case 4: Double-balloon enteroscopy revealed no abnormal lesions in the distal small intestine. Radiation therapy (30 Gy) was performed, and rituximab (375 mg/m2) was administered twice during this treatment. This treatment strategy resulted in CR. The patient’s D-FL did not recur without additional treatment.
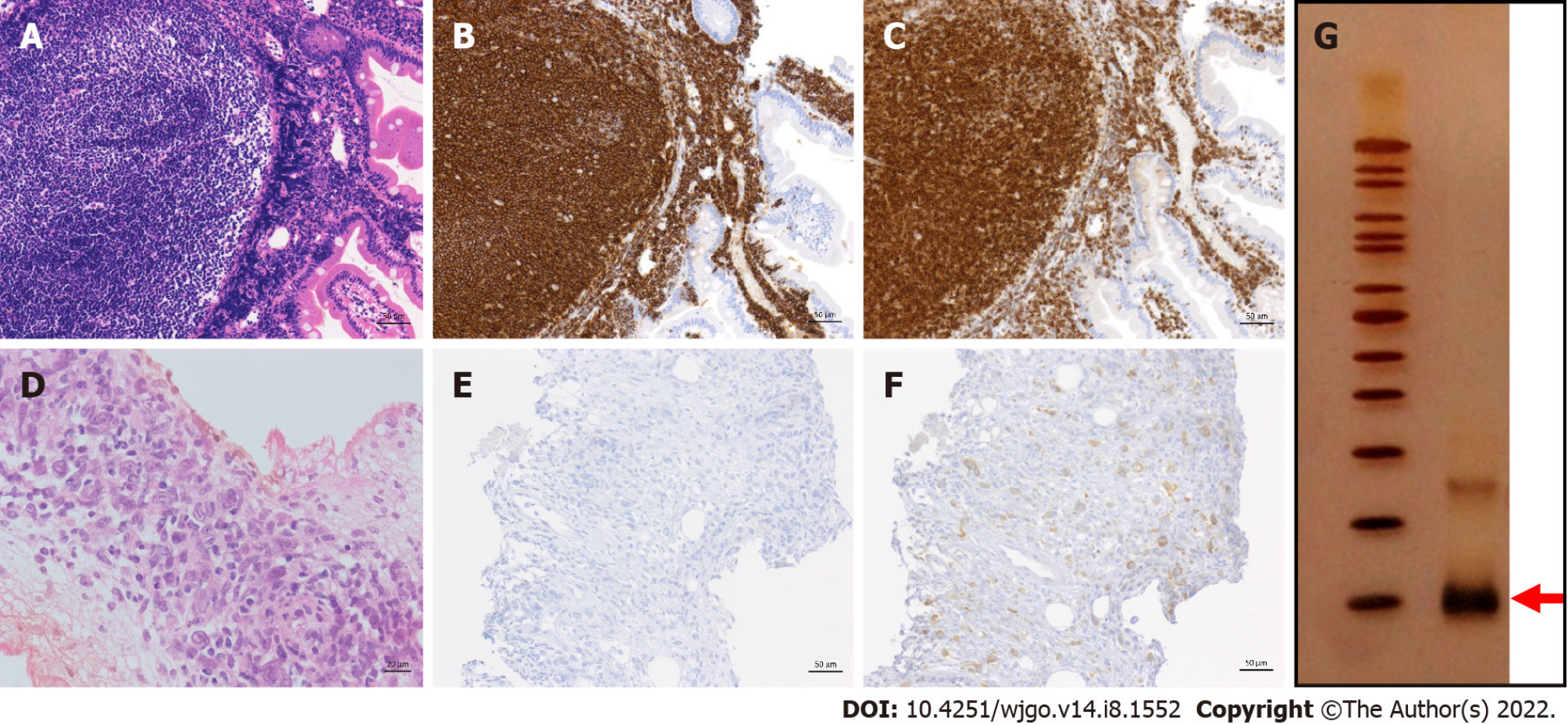
Case 5: A 42-year-old male was incidentally found to have an FL lesion in the descending portion of the duodenum by EGD screening at our hospital, and he was pathologically diagnosed with D-FL (Figure 2A-C). Although small bowel enteroscopy had not been performed, the tumor indicated Lugano Stage I. Thus, we continued the ‘watch and wait’ approach for a year. However, the patient and his family requested treatment. He underwent chemotherapy with CHOP × 2 cycles, followed by oral therapy with etoposide (50 mg) for 2 mo. The extent of the duodenal lesion was slightly decreased (minimal response), and the patient was followed-up without treatment. Rituximab, which had at that time just been approved for use in Japan, was then administered as a single agent (once weekly, 4 times), and the lesion regressed steadily (partial response). Seven months later, radiation (40 Gy) was administered, and the patient’s first CR was finally achieved 3 years after the intervention.
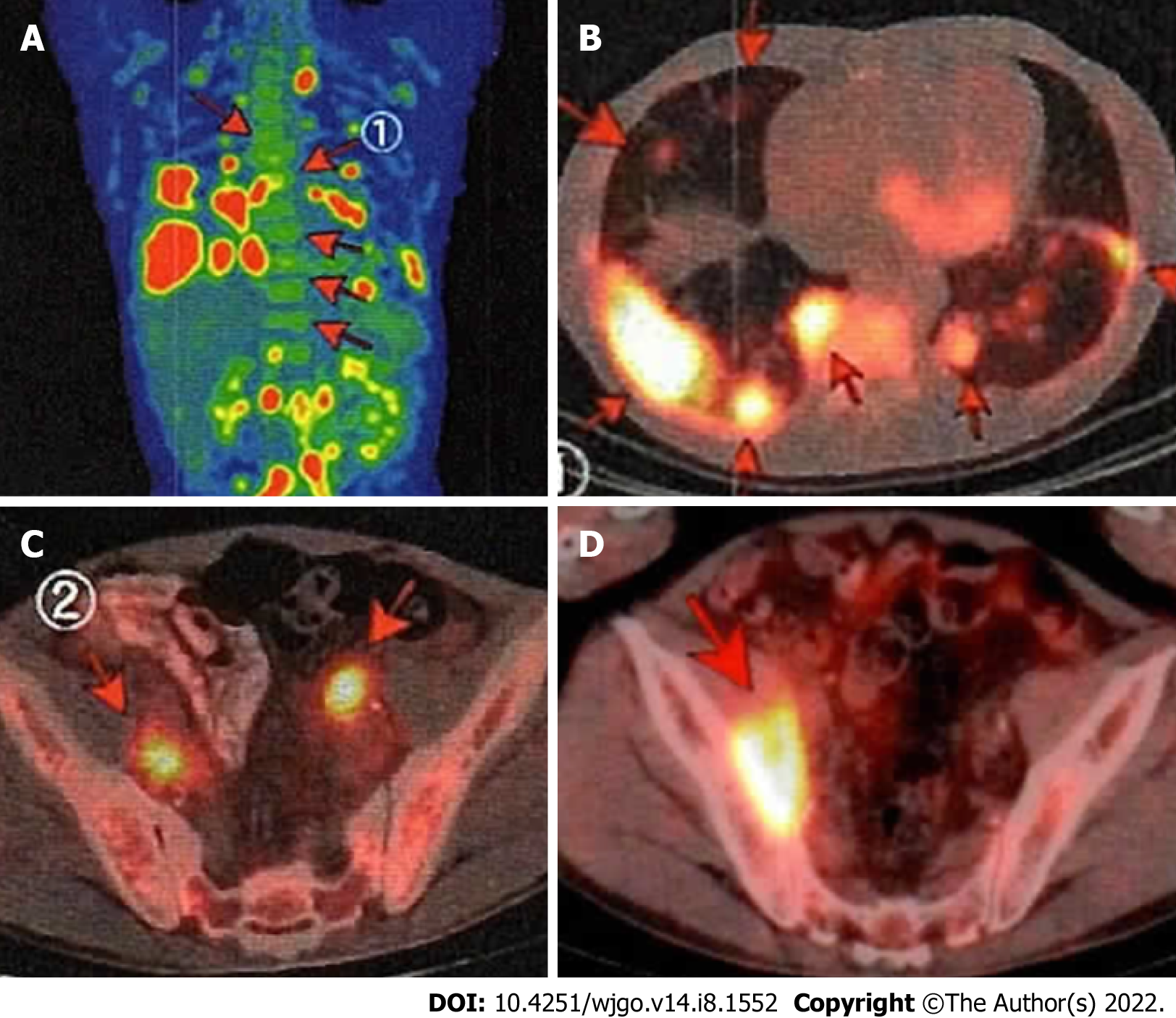
Treatment-free follow-up continued nearly 13 years after achieving the first CR, and then the patient noted swelling in his neck. Despite having a lymph node biopsy, a pathological diagnosis could not be made. PET-CT showed clear uptake in the lungs and lymph nodes throughout the whole body. The maximum standardized uptake value ranged from 3 to 15, which is consistent with the recurrence of FL (Stage IV) (Figure 3A-C). The duodenal lesion had maintained CR. After R-CHOP × 1 (stable disease), the patient underwent six cycles of the rituximab + bendamustine (B-R) regimen (90 mg/m2 days 1-2, every 4 wk) and achieved metabolic CR according to PET-CT.
He then received maintenance treatment with rituximab every 2-3 mo. However, the lesions of the lung and pelvic lymph nodes recurred. After 3 cycles of chemotherapy with rituximab + bendamustine 70 mg/m2 days 1-2, cytarabine 800 mg/m2 days 1-3, every 4 wk, the lung lesions disappeared. However, the nodal lesions in the pelvic cavity had progressed on PET-CT (Figure 3D). He was then treated with 2 cycles of cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2 all day 1, etoposide 100 mg/body days 1-3, and prednisone 60 mg/m2 days 1-5, every 3 wk and with various other drugs, including obinutuzumab. He remained non-CR.
Biopsy of the pelvic lesion showed that the tumor cells were CD20-negative (Figure 2D and E). However, they were positive for BCL2 (Figure 2F), and B-cell clonality was demonstrated by the PCR-single strand conformation polymorphism method (Figure 2G). The pathological diagnosis was DLBCL transformed from FL. The patient died approximately 4 years after recurrence and 21 years from the first onset.
D-FL is a unique subtype of FL that is classified in the WHO 2017 classification of FLs[1]. It is usually localized to the intestinal tract and does not spread to the lymph nodes[11]. Two clinical studies conducted by Takata et al[6] and Schmatz et al[11] reported that of a total of 162 patients with D-FL, no patients had a Lugano stage higher than Stage II. Epidemiologically, D-FL has been recognized as a rare entity that accounts for approximately 4% of primary gastrointestinal lymphomas[9]. Bende et al[12] identified the expression of surface IgA, which is not found in nodal FL, in the mucosal immune system as a feature of D-FL cells in the intestinal mucosa and the expression of α4β7 integrin, which is thought to mediate “mucosal homing.” In addition, the gene expression profile of D-FL has been shown to be similar to that of MALT lymphoma[8]. D-FL is almost asymptomatic and has an indolent clinical course, suggesting that it is biologically more similar to MALT lymphoma than to nodal FL[7]. Therefore, follow-up with a “watch and wait” approach without immediate intervention after diagnosis is frequently applied in cases of D-FL[9,10]. In Case 2, FL lesions recurred in the ileocecal region and the rectum but spontaneously regressed. It was previously reported that D-FL disappeared spontaneously in 3%-30% of patients[6,11]; as a result, it may have been possible to address this patient even if “watch and wait” was initiated.
Radiation is a representative treatment for D-FL, and there are several reports on its effectiveness[11,13,14]. However, Takata et al[6] reported that 46 of 54 patients (85%) with D-FL in the descending portion of the duodenum also had lesions in the distal small intestine, primarily the jejunum; thus, it is necessary to reliably determine the extent of the lesion. In Case 4, enteroscopy was performed, and the lesion did not extend to the distal small intestine. Because the lesion was localized to the duodenum, local irradiation was considered to be the most reasonable treatment.
Anticancer drug treatments for D-FL are based on the administration of rituximab ± chemotherapy, such as R-CHOP/rituximab + cyclophosphamide, vincristine, and prednisone, and B-R. D-FL is a low-grade malignancy and rarely requires chemotherapy, except in cases that exhibit histological transformation[11,15]. In indolent non-Hodgkin B-cell lymphoma, it has been reported that B-R was significantly better in progression-free survival than R-CHOP[16], and there is also a case report of D-FL for which B-R was effective[17]. However, as mentioned above, bendamustine was not yet available in Japan until 2010. In this case series, two patients had Lugano Stage IV disease (Case 2 and Case 3), and therapeutic intervention was performed using R-CHOP/Rituximab + cyclophosphamide, vincristine, and prednisone (change to fludarabine during the treatment course in Case 2). CR was reached in both cases, and maintenance treatment with rituximab monotherapy was performed. Rituximab monotherapy is effective for patients with high tumor-burden nodal FL[18] and has been used as a treatment at our center. The efficacy and safety of chemotherapy in Stage IV “advanced” D-FL cases without histological transformation and the importance of subsequent rituximab maintenance therapy should be investigated in a large number of cases in the future.
In Case 5, this patient was treated with a long nontreatment interval and reached his first CR over 3 years after the intervention. Considering this result, it might have been possible to achieve CR earlier by radiation-centered treatment from the beginning, as in Case 4. Unfortunately, the patient relapsed nearly 13 years after reaching his first CR. Although he was treated with various anticancer drugs, he died of the primary disease approximately 4 years after recurrence. The PET-CT findings at the time of recurrence were consistent with FL. Lesions showing maximum standardized uptake value of 15 (> 13.55, mean value of FL-grade 3b/DLBCL[19]) were also detected, suggesting that they contained partial DLBCL component. Therefore, we clinically speculated that the initial D-FL in this patient underwent clonal evolution to become the final DLBCL.
The incidence of histological transformation of D-FL into DLBCL was found to be 3.8%[20] in 5 retrospective studies[2,14,15,21,22] and 1 prospective study[3]. This incidence is lower than the incidence of nodal FL, which has an incidence of histological transformation of 10.7% over 5 years at a rate of 2% per year[23]. This transformation incidence is close to that of gastric MALT lymphoma, which is almost 3%[24]. In past cases, D-FL patients with histological transformation to DLBCL did not receive systemic chemotherapy at the time of onset but instead underwent the “watch and wait” approach[15,25-27]. Even in that situation, since the lymphoma was in remission due to R-CHOP chemotherapy, D-FL did not require aggressive treatment in the absence of histological transformation. Thus, the “watch and wait” follow-up approach was approved. However, in recent years, several patients with D-FL with histological transformation refractory to R-CHOP chemotherapy have been reported[20,28].
The reason our patient became refractory was likely histological transformation in addition to a change of the immunophenotype of the tumor to CD20-negative[29]. Furthermore, the therapeutic response to the anticancer drugs was not good at the first onset. Fatal histological transformation occurred even after a long period of more than 17 years from the first onset; thus, patients with D-FL require lifelong follow-up.
In this study, 5 patients with D-FL who received treatment intervention regardless of clinical stage were evaluated with respect to the therapeutic effects. The treatment of 3 Stage IV cases was successful, and in the future, criteria for how to treat “advanced” D-FL should be established based on additional cases. This study indicates that it is necessary to continue to follow-up with whole body examinations while paying careful attention to the possibility of recurrence in D-FL because fatal recurrence can occur even 13 years after a patient achieves CR.
Duodenal-type follicular lymphoma (D-FL) has been recognized as a rare primary gastrointestinal lymphoma. Because D-FL follows an indolent clinical course compared to nodal FL, the “watch and wait” approach is currently the general follow-up policy.
There is still insufficient consensus regarding the appropriate treatment of D-FL, and an option to actively treat D-FL is available. The long-term outcomes following the active treatment of D-FL are poorly understood.
This study aimed to clarify the clinical outcomes through long-term follow-up in cases of D-FL with treatment intervention.
We retrospectively examined 5 D-FL patients who underwent therapeutic intervention at our center from January 1998 to December 2009 and followed the clinical course of these patients for more than 10 years.
As a result of therapeutic intervention, all 5 cases reached complete remission (CR) and survived for more than 10 years. However, 3 of these cases experienced recurrence. One patient achieved a second CR after retreatment, and in the other case, the lesion spontaneously disappeared. The remaining patient experienced widespread systemic recurrence 13 years after the first CR. This patient died 4 years later despite treatment with various anticancer chemotherapies.
Five patients with D-FL who received treatment interventions regardless of clinical stage were evaluated with respect to the therapeutic effects of the treatment. Because fatal recurrence was found to occur even 13 years after the first CR, it is necessary to continue whole-body follow-up examinations for individuals diagnosed with D-FL.
Only 5 cases were examined in this study. By including more D-FL patients and evaluating their treatment, criteria for how to treat Stage IV “advanced” cases can be explored.
We would like to thank Dr. Fumio Tokuchi, the director of the Tokuchi Internal Medicine Clinic, for continuing to follow-up on Case 4 and providing a description of his current condition.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dong XD, China; Zhao CF, China S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YL
| 1. | Swerdlow SH, Campo E, Seto M, Müller-Hermelink HK. Mantle cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, editors. WHO classification of tumours of haematopietic and lymphoid tissues. Revised 4th ed. Lyon, France: IARC press, 2017: 285-290. |
| 2. | Tari A, Asaoku H, Takata K, Fujimori S, Tanaka S, Fujihara M, Koga T, Yoshino T. The role of "watch and wait" in intestinal follicular lymphoma in rituximab era. Scand J Gastroenterol. 2016;51:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Tari A, Kitadai Y, Mouri R, Takigawa H, Asaoku H, Mihara K, Takata K, Fujihara M, Yoshino T, Koga T, Fujimori S, Tanaka S, Chayama K. Watch-and-wait policy versus rituximab-combined chemotherapy in Japanese patients with intestinal follicular lymphoma. J Gastroenterol Hepatol. 2018;33:1461-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Naylor GM, Gotoda T, Dixon M, Shimoda T, Gatta L, Owen R, Tompkins D, Axon A. Why does Japan have a high incidence of gastric cancer? Gut. 2006;55:1545-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Maeshima AM, Taniguchi H, Suzuki T, Yuda S, Toyoda K, Yamauchi N, Makita S, Fukuhara S, Munakata W, Maruyama D, Kobayashi Y, Saito Y, Tobinai K. Comparison of clinicopathologic characteristics of gastric follicular lymphomas and duodenal follicular lymphomas. Hum Pathol. 2017;65:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Takata K, Okada H, Ohmiya N, Nakamura S, Kitadai Y, Tari A, Akamatsu T, Kawai H, Tanaka S, Araki H, Yoshida T, Okumura H, Nishisaki H, Sagawa T, Watanabe N, Arima N, Takatsu N, Nakamura M, Yanai S, Kaya H, Morito T, Sato Y, Moriwaki H, Sakamoto C, Niwa Y, Goto H, Chiba T, Matsumoto T, Ennishi D, Kinoshita T, Yoshino T. Primary gastrointestinal follicular lymphoma involving the duodenal second portion is a distinct entity: a multicenter, retrospective analysis in Japan. Cancer Sci. 2011;102:1532-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Sato Y, Ichimura K, Tanaka T, Takata K, Morito T, Sato H, Kondo E, Yanai H, Ohara N, Oka T, Yoshino T. Duodenal follicular lymphomas share common characteristics with mucosa-associated lymphoid tissue lymphomas. J Clin Pathol. 2008;61:377-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Takata K, Tanino M, Ennishi D, Tari A, Sato Y, Okada H, Maeda Y, Goto N, Araki H, Harada M, Ando M, Iwamuro M, Tanimoto M, Yamamoto K, Gascoyne RD, Yoshino T. Duodenal follicular lymphoma: comprehensive gene expression analysis with insights into pathogenesis. Cancer Sci. 2014;105:608-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Marks E, Shi Y. Duodenal-Type Follicular Lymphoma: A Clinicopathologic Review. Arch Pathol Lab Med. 2018;142:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Duffles Amarante G, Collins G, Rocha V. What do we know about duodenal-type follicular lymphoma? Br J Haematol. 2020;188:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Schmatz AI, Streubel B, Kretschmer-Chott E, Püspök A, Jäger U, Mannhalter C, Tiemann M, Ott G, Fischbach W, Herzog P, Seitz G, Stolte M, Raderer M, Chott A. Primary follicular lymphoma of the duodenum is a distinct mucosal/submucosal variant of follicular lymphoma: a retrospective study of 63 cases. J Clin Oncol. 2011;29:1445-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Bende RJ, Smit LA, Bossenbroek JG, Aarts WM, Spaargaren M, de Leval L, Boeckxstaens GE, Pals ST, van Noesel CJ. Primary follicular lymphoma of the small intestine: alpha4beta7 expression and immunoglobulin configuration suggest an origin from local antigen-experienced B cells. Am J Pathol. 2003;162:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 13. | Harada A, Oguchi M, Terui Y, Takeuchi K, Igarashi M, Kozuka T, Harada K, Uno T, Hatake K. Radiation therapy for localized duodenal low-grade follicular lymphoma. J Radiat Res. 2016;57:412-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Lee H, Oh D, Yang K, Ko YH, Ahn YC, Kim WS, Kim SJ. Radiation Therapy Outcome and Clinical Features of Duodenal-Type Follicular Lymphoma. Cancer Res Treat. 2019;51:547-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Mori M, Kobayashi Y, Maeshima AM, Gotoda T, Oda I, Kagami Y, Bennett S, Nomoto J, Azuma T, Yokoyama H, Maruyama D, Kim SW, Watanabe T, Matsuno Y, Tobinai K. The indolent course and high incidence of t(14;18) in primary duodenal follicular lymphoma. Ann Oncol. 2010;21:1500-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grünhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M, Balser C, Kaiser U, Weidmann E, Dürk H, Ballo H, Stauch M, Roller F, Barth J, Hoelzer D, Hinke A, Brugger W; Study group indolent Lymphomas (StiL). Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 1106] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 17. | Cencini E, Fabbri A, Mecacci B, Bocchia M. Is bendamustine plus rituximab a suitable option for rituximab-refractory duodenal-type follicular lymphoma? Acta Gastroenterol Belg. 2020;83:493. [PubMed] |
| 18. | Salles G, Seymour JF, Offner F, López-Guillermo A, Belada D, Xerri L, Feugier P, Bouabdallah R, Catalano JV, Brice P, Caballero D, Haioun C, Pedersen LM, Delmer A, Simpson D, Leppa S, Soubeyran P, Hagenbeek A, Casasnovas O, Intragumtornchai T, Fermé C, da Silva MG, Sebban C, Lister A, Estell JA, Milone G, Sonet A, Mendila M, Coiffier B, Tilly H. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 804] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 19. | Novelli S, Briones J, Flotats A, Sierra J. PET/CT Assessment of Follicular Lymphoma and High Grade B Cell Lymphoma - Good Correlation with Clinical and Histological Features at Diagnosis. Adv Clin Exp Med. 2015;24:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Saburi M, Kondo Y, Ogata M, Soga Y, Abe M, Takano K, Kohno K, Nagai T, Nakayama T. Development of diffuse large B-cell lymphoma from duodenal type follicular lymphoma: a retrospective study of 23 cases. Int J Hematol. 2020;112:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Sentani K, Maeshima AM, Nomoto J, Maruyama D, Kim SW, Watanabe T, Kobayashi Y, Tobinai K, Matsuno Y. Follicular lymphoma of the duodenum: a clinicopathologic analysis of 26 cases. Jpn J Clin Oncol. 2008;38:547-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Shia J, Teruya-Feldstein J, Pan D, Hegde A, Klimstra DS, Chaganti RS, Qin J, Portlock CS, Filippa DA. Primary follicular lymphoma of the gastrointestinal tract: a clinical and pathologic study of 26 cases. Am J Surg Pathol. 2002;26:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Link BK, Maurer MJ, Nowakowski GS, Ansell SM, Macon WR, Syrbu SI, Slager SL, Thompson CA, Inwards DJ, Johnston PB, Colgan JP, Witzig TE, Habermann TM, Cerhan JR. Rates and outcomes of follicular lymphoma transformation in the immunochemotherapy era: a report from the University of Iowa/MayoClinic Specialized Program of Research Excellence Molecular Epidemiology Resource. J Clin Oncol. 2013;31:3272-3278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 252] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 24. | Tamura N, Maeda H, Nishikori M, Fujita H, Hishizawa M, Haga H, Takaori-Kondo A. Histologic transformation of t(11;18)-positive MALT lymphoma presented with aberrant T-cell marker expression. Int J Hematol. 2020;111:724-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Hangai S, Nakamura F, Kamikubo Y, Ichikawa M, Suzuki H, Yoshida S, Yamada A, Takazawa Y, Fukayama M, Koike K, Kurokawa M. Primary gastrointestinal follicular lymphoma with histological transformation. Ann Hematol. 2013;92:993-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Akiyama S, Izutsu K, Ota Y, Imamura T, Ogawa O, Wake A, Takeuchi K. A case report of the histologic transformation of primary follicular lymphoma of the duodenum. Medicine (Baltimore). 2014;93:e165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Kitabatake H, Nagaya T, Tanaka N, Ota H, Sano K, Asano N, Suga T, Nakamura Y, Akamatsu T, Tanaka E. Development of diffuse large B-cell lymphoma from follicular lymphoma of the duodenum: changes in endoscopic findings during a 6-year follow-up. Clin J Gastroenterol. 2017;10:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Tanigawa T, Abe R, Kato J, Hosoe N, Ogata H, Kameyama K, Okamoto S, Mori T. Histological transformation in duodenal-type follicular lymphoma: a case report and review of the literature. Oncotarget. 2019;10:3424-3429. [PubMed] |
| 29. | Rasheed AA, Samad A, Raheem A, Hirani SI, Shabbir- Moosajee M. Cd20 Expression and Effects on Outcome of Relapsed/ Refractory Diffuse Large B Cell Lymphoma after Treatment with Rituximab. Asian Pac J Cancer Prev. 2018;19:331-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |