Published online Aug 15, 2022. doi: 10.4251/wjgo.v14.i8.1540
Peer-review started: May 6, 2022
First decision: June 12, 2022
Revised: June 19, 2022
Accepted: July 22, 2022
Article in press: July 22, 2022
Published online: August 15, 2022
Processing time: 96 Days and 2.6 Hours
For Siewert type II/III adenocarcinoma of gastroesophageal junction (AGE), the efficacy of adjuvant chemoradiotherapy (CRT) after D2/R0 resection remains uncertain.
To determine whether CRT was superior to chemotherapy (CT) alone after D2/R0 resection for locally advanced Siewert type II/III AGE.
We identified 316 locally advanced Siewert type II/III AGE patients who were treated with D2/R0 resection at National Cancer Center from 2011 to 2018. 57 patients received adjuvant CRT and 259 patients received adjuvant CT. We followed patients for overall survival (OS), relapse-free survival, and recurrence pattern.
Five-year OS rates of the CRT group and the CT group for all patients were 66.7% and 41.9% (P = 0.010). Five-year OS rates of the CRT group and the CT group for Siewert type III AGE patients were 65.7% and 43.9% (P = 0.006). Among the 195 patients whose recurrence information could be obtained, 18 cases (34.6%) and 61 cases (42.7%) were diagnosed as recurrence in the CRT group and CT group, respectively. The local and regional recurrence rates in the CRT group were lower than that in the CT group (22.2% vs 24.6%, 27.8% vs 39.3%). Multivariable cox regression analysis showed that vascular invasion, nerve invasion, and adjuvant CRT were important prognostic factors for Siewert type III AGE.
For locally advanced Siewert type III AGE, adjuvant CRT may prolong OS and reduce the regional recurrence rate.
Core Tip: This is a retrospective study to investigate the value of adjuvant chemoradiotherapy (CRT) in locally advanced Siewert type II/III adenocarcinoma of gastroesophageal junction. We identified 316 such patients and followed their overall survival (OS), relapse-free survival, and recurrence pattern. Our study found that for locally advanced Siewert type III gastroesophageal junction, adjuvant CRT may prolong OS and reduce the regional recurrence rate.
- Citation: Kang WZ, Shi JM, Wang BZ, Xiong JP, Shao XX, Hu HT, Jin J, Tian YT. Adjuvant chemoradiotherapy vs adjuvant chemotherapy in locally advanced Siewert type II/III adenocarcinoma of gastroesophageal junction after D2/R0 resection. World J Gastrointest Oncol 2022; 14(8): 1540-1551
- URL: https://www.wjgnet.com/1948-5204/full/v14/i8/1540.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i8.1540
In recent years, the incidence of adenocarcinoma of the gastroesophageal junction (AGE) has been increasing[1-3]. The Siewert type II/III type is the most common type of AGE in Asia[4-6]. At present, treatment for this type of AGE is based on the principle of gastric cancer. However, due to the special anatomical site of Siewert type II/III AGE, it differs from middle-distal gastric cancer in terms of its biological characteristics and prognosis. An increasing number of researchers have recognized AGE as an independent tumor entity[7,8]. For locally advanced AGE, the local and regional recurrence and distant metastasis rates remain high after D2/R0 resection, leading to poor prognosis[9-11]. As an important local treatment, radiotherapy can reduce the local recurrence rate and prolong survival time[12]. Phase III clinical studies of adjuvant chemoradiotherapy after D2/R0 resection. For resectable gastric cancer in the East and West have reached different conclusions[13,14]. Postoperative radiotherapy may be beneficial for patients who fail to achieve D2/R0 resection for various reasons, as well as in those with high-risk factors for local recurrence (high rate of lymph node metastasis, insufficient safe resection distance, etc.)[15]. Although the INT-0116 trial confirmed the survival benefit of postoperative radiotherapy in patients with resectable gastric cancer, the majority of patients in this study underwent D0 or D1 gastrectomy[16]. The efficacy of adjuvant radiotherapy in patients with gastric cancer undergoing D2/R0 resection is controversial[17]. Neoadjuvant CRT + D2/R0 resection + adjuvant chemotherapy (CT) has been successful in the study of AGE. Long-term follow-up results from the POET study showed that preoperative CRT had the advantage of reducing local and regional recurrence and was prone to improving overall survival (OS) compared to preoperative CT[18]. However, the significance of postoperative adjuvant radiotherapy for locally advanced Siewert type II/III AGE is unclear. In this study, we reviewed 316 patients with locally advanced Siewert type II/III AGE patients to determine whether CRT was superior to CT alone after D2/R0 resection, comparing the OS, relapse-free survival (RFS), and recurrence modes between the CRT and CT groups.
We identified 316 patients with locally advanced Siewert type II/III AGE who were admitted to the Department of Pancreatic and Gastric Surgery, National Cancer Center, between January 2011 and May 2018. All patients underwent D2/R0 resection and did not receive neoadjuvant CT or radiotherapy. Patients were divided into a CT group and a CRT group according to whether they received postoperative adjuvant radiotherapy. Patients were followed-up by telephone, which was completed on April 30, 2020. The median follow-up time was 62.7 mo.
Pathologists determined the classification of the Siewert type. The pathological staging (pTNM) criteria were based on the 8th edition of the American Joint Committee on Cancer guidelines for esophageal adenocarcinoma (all Siewert type II adenocarcinomas invade the dentate line) and gastric cancer (Siewert type III). Patients who were lost to follow-up or were unwilling to cooperate were excluded. Patients with Siewert type I AGE were not included in this study. Patients younger than 18 years or older than 80 years, those who received neoadjuvant CT or radiotherapy, and those with less than 1 mo of postoperative survival were excluded from the study.
Patients in the CRT group received a total dose of 45–50.4 Gy in 25–28 fractions. Intensity-modulated radiation therapy or volumetric modulated arc therapy were used. For tumor margins ≤ 3 cm, the anastomosis site was included in the clinical target volume (CTV). For the T4b stage, the tumor bed should also be included. Regional draining lymph nodes according to the Japanese Gastric Cancer Association (JGCA)[19] were included in the CTV. Based on the International Commission Radiological Units report No. 83[20], the planning target volume should consider not only the setup error but also the breath elements and tumor movement. Concurrent CT regimens included FU-based drugs such as 5-FU, capecitabine, or tegafur (S-1) on radiotherapy days. Patients in the CRT group received 4-6 cycles of CT, followed by CRT.
In the CT group, the main CT regimens included oxaliplatin + 5-FU (SOX, XELOX), cisplatin + 5-FU (PF, XP, SP), albumin-bound paclitaxel + S-1, and single-drug CT regimen (S-1, docetaxel, and irino
Specific imaging or pathological diagnosis is needed when defining tumor recurrence. In this study, we divided recurrence into local recurrence, regional recurrence, and metastatic recurrence according to the first site of recurrence during the follow-up period.
Recurrence in the anastomotic stoma, tumor bed, and remnant stomach was defined as a local recurrence. Recurrence in the lymphatic drainage area, according to the JGCA guidelines[19] for gastric cancer and the Japan Esophageal Cancer Research Association guidelines[21] for the esophagus, was defined as a regional recurrence. The diagnosis of recurrent lymph nodes on imaging should satisfy one of the following criteria: (1) Short diameter of lymph nodes > 8 mm; (2) Ratio of the long diameter to the short diameter of lymph nodes is close to one; (3) Central lymph nodes are necrotic; (4) Multiple lymph nodes are aggregated; and (5) Lymph nodes are significantly enhanced on computed tomography. Distant recurrence was defined as distant lymph node, bone, peritoneal or pleural, and solid organ metastases such as liver, lung, and ovarian metastases.
Table 1 summarizes the clinicopathological characteristics of the patients enrolled in this study. There were 57 patients in the CRT group and 295 patients in the CT group. The number and proportion of patients with Siewert type II and III AGE were 148 (46.8%) and 168 (53.2%), respectively.
| Variable | Overall | Adjuvant chemoradiotherapy group | Adjuvant chemotherapy group |
| 316 | 57 | 259 | |
| Age, n (%) | |||
| < 40 yr | 6 (1.9) | 2 (3.5) | 4 (1.5) |
| ≥ 40 yr | 310 (98.1) | 55 (96.5) | 255 (98.5) |
| Sex, n (%) | |||
| Male | 268 (84.8) | 46 (80.7) | 222 (85.7) |
| Female | 48 (15.2) | 11 (19.3) | 37 (14.3) |
| BMI, n (%) | |||
| < 18.5 or > 23.9 | 169 (53.5) | 27 (47.4) | 142 (54.8) |
| 18.5-23.9 | 147 (46.5) | 30 (52.6) | 117 (45.2) |
| The degree of differentiation, n (%) | |||
| Poorly differentiated | 233 (73.7) | 45 (78.9) | 188 (72.6) |
| Moderately-highly differentiated | 83 (26.3) | 12 (21.1) | 71 (27.4) |
| Nerve invasion, n (%) | |||
| Yes | 269 (85.1) | 43 (75.4) | 226 (87.3) |
| No | 47 (14.9) | 14 (24.6) | 33 (12.7) |
| Vascular invasion, n (%) | |||
| Yes | 263 (83.2) | 40 (70.2) | 223 (86.1) |
| No | 53 (16.8) | 17 (29.8) | 36 (13.9) |
| Pathologic T stage, n (%) | |||
| pT1-3 | 179 (56.6) | 34 (59.6) | 145 (56.0) |
| pT4a-4b | 137 (43.4) | 23 (40.4) | 114 (44.0) |
| Pathologic N stage, n (%) | |||
| pN1-2 | 73 (23.1) | 10 (17.5) | 63 (24.3) |
| PN3 | 243 (76.9) | 47 (82.5) | 196 (75.7) |
| Pathological stage, Siewert type II | 148 (46.8) | 25 (43.9) | 123 (47.5) |
| IIIB stage | 30 (9.5) | 6 (10.5) | 24 (9.3) |
| IVA stage | 118 (37.3) | 19 (33.3) | 99 (38.2) |
| Pathological stage, Siewert type III | 168 (53.2) | 32 (56.1) | 136 (52.5) |
| IIIA | 32 (10.1) | 1 (1.8) | 31 (12.0) |
| IIIB | 80 (25.3) | 22 (38.6) | 58 (22.4) |
| IIIC | 56 (17.7) | 9 (15.8) | 47 (18.1) |
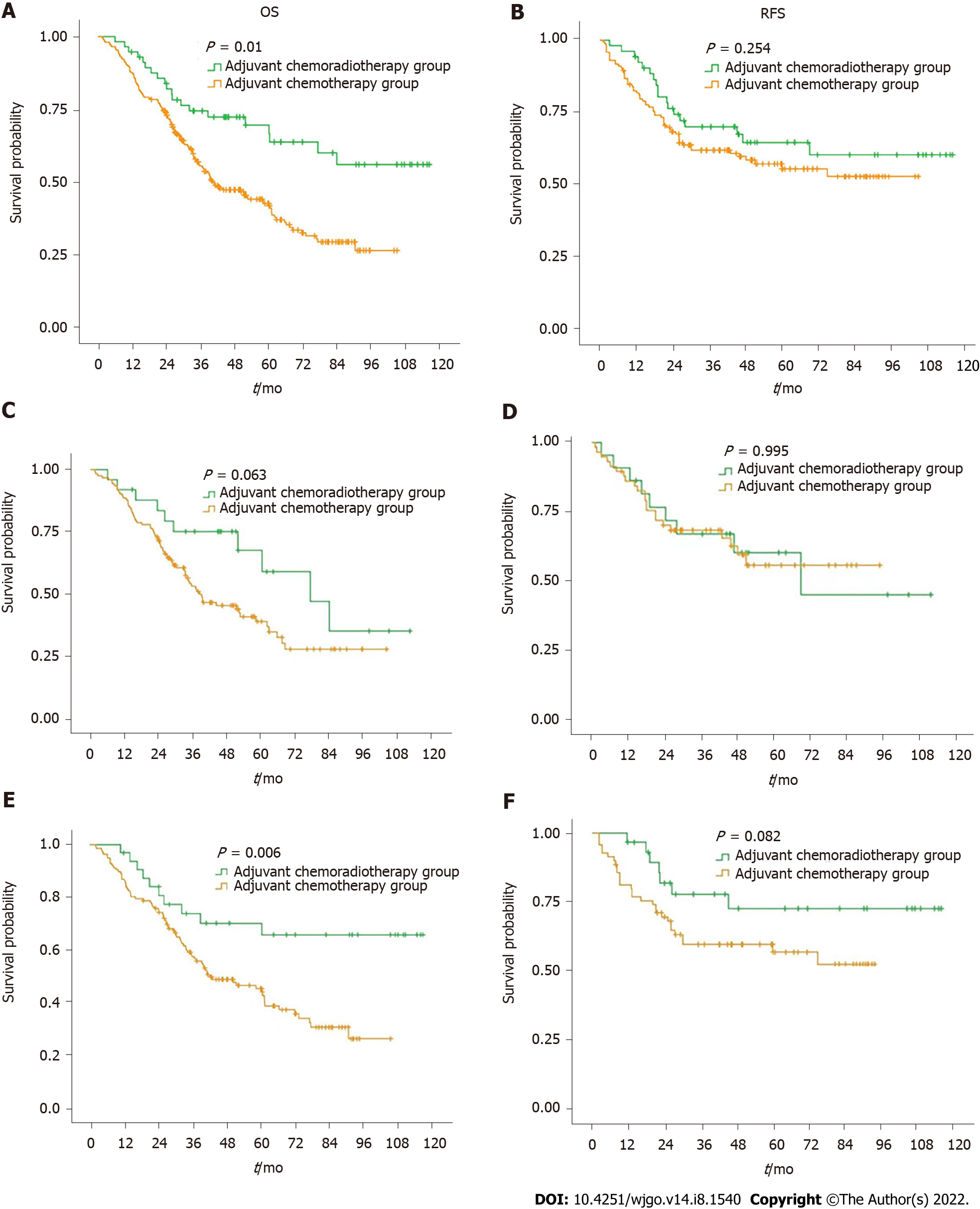
Figure 1A and B summarize the OS and RFS curves of the CRT and CT groups, respectively, for all patients. Figure 1C and D summarize the OS and RFS curves of the CRT and CT groups for patients with Siewert type II AGE, respectively. Figure 1E and F summarize the OS and RFS curves of the CRT and CT groups for patients with Siewert type III AGE, respectively.
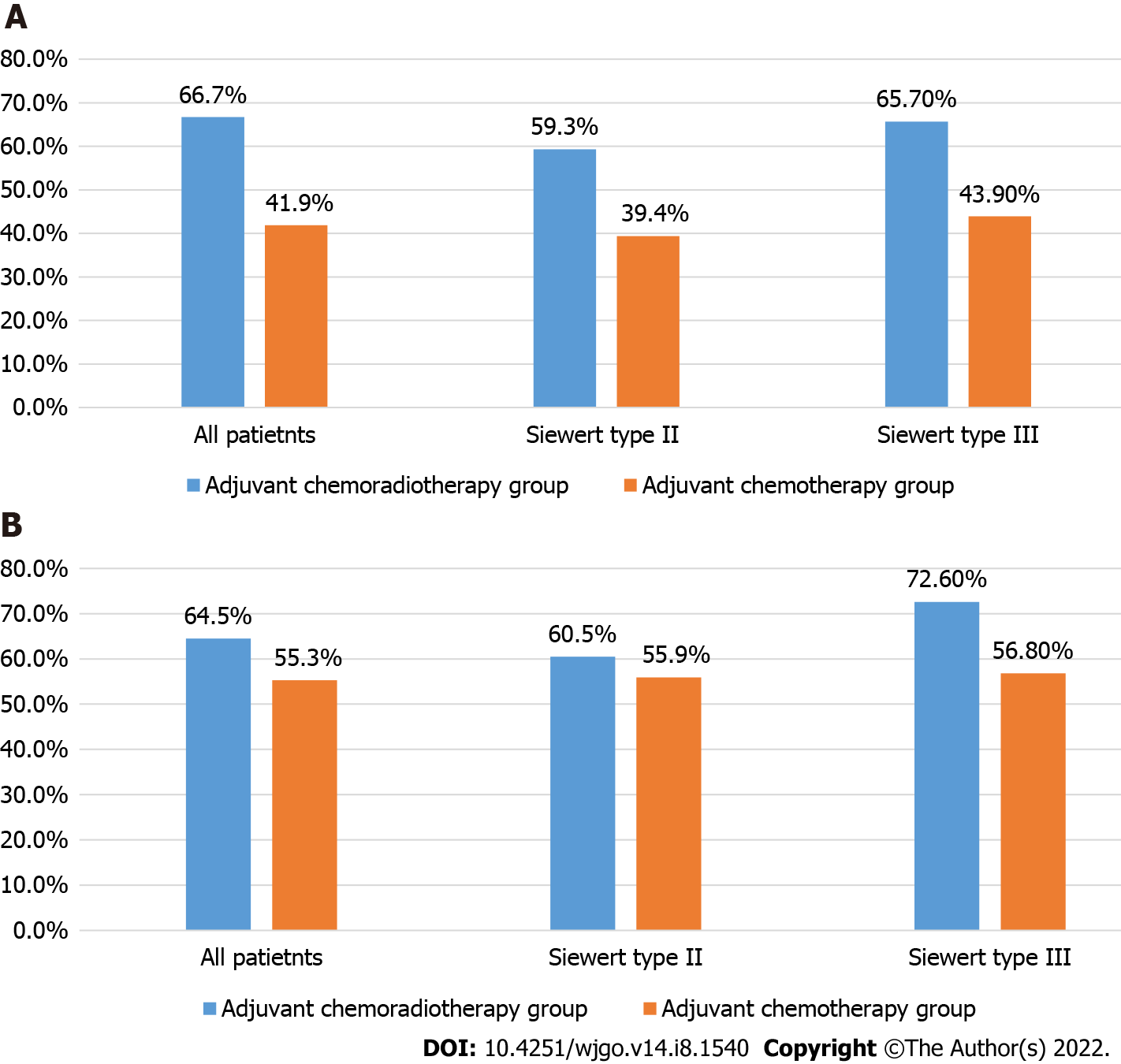
Five-year OS rates of the CRT and CT groups for all patients were 66.7% and 41.9%, respectively (P = 0.010). Five-year OS rates of the CRT and CT groups for Siewert type II AGE patients were 59.3% and 39.4%, respectively (P = 0.063). Five-year OS rates of the CRT and CT groups for Siewert type III AGE patients were 65.7% and 43.9%, respectively (P = 0.006).
Five-year recurrence-free survival rates of the CRT and CT groups for all patients were 64.5% and 55.3%, respectively (P = 0.254). Five-year recurrence-free survival rates of the CRT and CT groups for Siewert type II AGE patients were 60.5% and 55.9%, respectively (P = 0.995). Five-year recurrence-free survival rates of the CRT and CT groups for Siewert type III AGE patients were 72.6% and 56.8%, respectively (P = 0.082) (Table 2 and Figure 2).
| Group | N | 5-yr survival rate (%) | Log rank test | |
| OS | All patietnts | P = 0.010 | ||
| Adjuvant chemoradiotherapy group | 57 | 66.7 | ||
| Adjuvant chemotherapy group | 295 | 41.9 | ||
| RFS | All patietnts | P = 0.254 | ||
| Adjuvant chemoradiotherapy group | 52 | 64.5 | ||
| Adjuvant chemotherapy group | 128 | 55.3 | ||
| OS | Siewert type II | P = 0.063 | ||
| Adjuvant chemoradiotherapy group | 25 | 59.3 | ||
| Adjuvant chemotherapy group | 123 | 39.4 | ||
| RFS | Siewert type II | P = 0.995 | ||
| Adjuvant chemoradiotherapy group | 22 | 60.5 | ||
| Adjuvant chemotherapy group | 58 | 55.9 | ||
| OS | Siewert type III | P = 0.006 | ||
| Adjuvant chemoradiotherapy group | 32 | 65.7 | ||
| Adjuvant chemotherapy group | 136 | 43.9 | ||
| RFS | Siewert type III | P = 0.082 | ||
| Adjuvant chemoradiotherapy group | 30 | 72.6 | ||
| Adjuvant chemotherapy group | 70 | 56.8 |
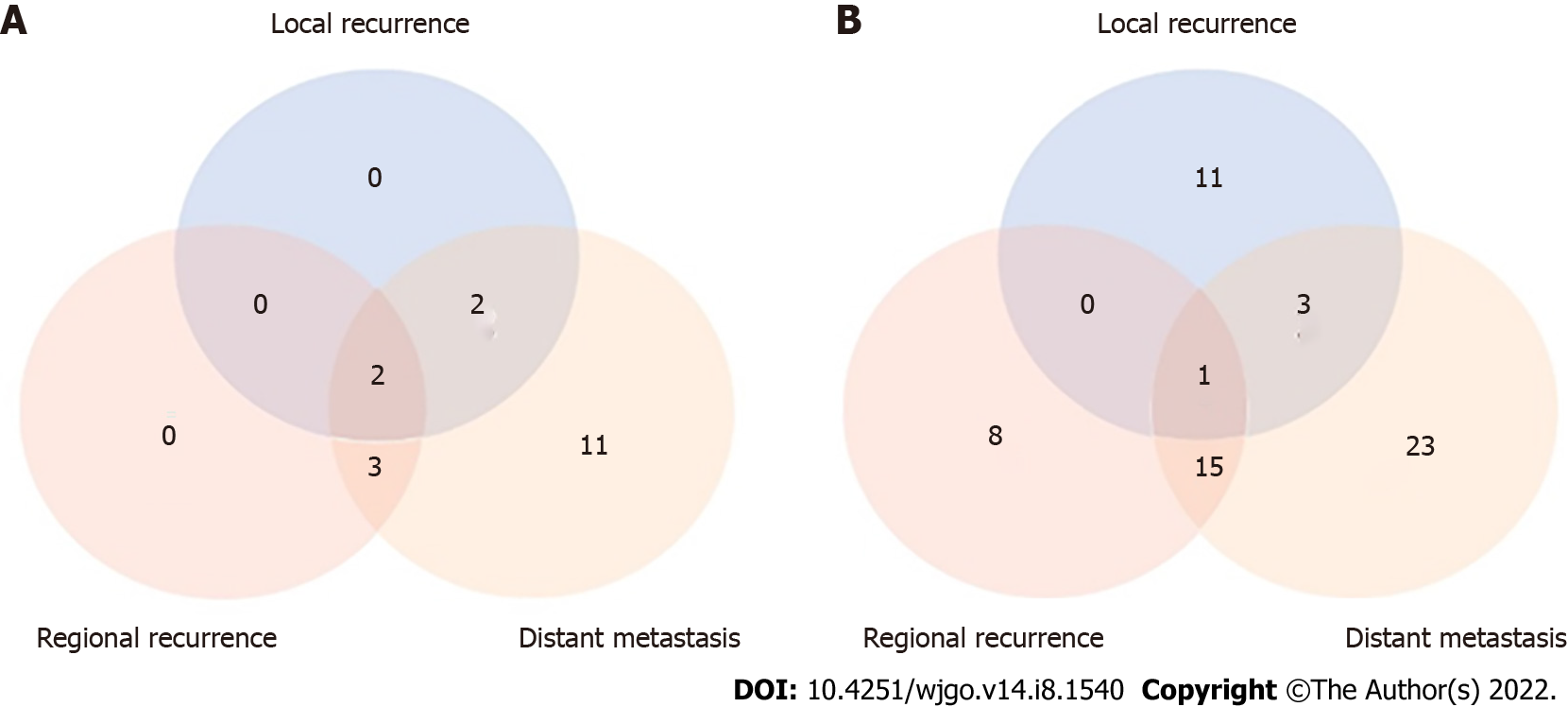
Among the 195 patients for whom recurrence information could be obtained, 18 (34.6%) and 61 (42.7%) were diagnosed with recurrence in the CRT and CT groups, respectively. The local and regional recurrence rates were lower in the CRT group than in the CT group (22.2% vs 24.6% and 27.8% vs 39.3%, respectively). The distant recurrence rate was higher in the CRT group than in the CT group (100% vs 68.9%) (Table 3 and Figure 3).
| Recurrence site | Adjuvant chemoradiotherapy group | Adjuvant chemotherapy group | ||
| No. of patients | % of recurrence patients (n = 18) | No. of patients | % of recurrence patients (n = 61) | |
| Local recurrence, n (%) | ||||
| Remnant stomach | 1 | 5.6 | 1 | 1.6 |
| Anastomosis site | 3 | 16.7 | 14 | 23.0 |
| Regional recurrence | 5 | 27.8 | 24 | 39.3 |
| Distant metastasis, n (%) | ||||
| One site | ||||
| Peritoneum | 5 | 27.8 | 8 | 13.1 |
| Pleura | 2 | 11.1 | 4 | 6.6 |
| Solid organ | 4 | 22.2 | 16 | 26.2 |
| Distant LNs | 2 | 11.1 | 5 | 8.2 |
| Bone metastases | 2 | 11.1 | 3 | 4.9 |
| ≥ 2 sites | ||||
| Peritoneum + solid organ | 2 | 11.1 | 3 | 4.9 |
| Solid organs | 1 | 5.6 | 2 | 3.3 |
| Peritoneum + distant LNs | 0 | 0 | 1 | 1.6 |
| Solid organs + distant LNs | 0 | 0 | 1 | 1.6 |
For Siewert type II AGE, multivariate Cox regression analysis showed that vascular invasion was an important prognostic factor (Table 4). For Siewert type III AGE, multivariate Cox regression analysis showed that vascular invasion, nerve invasion, and adjuvant CRT were important prognostic factors (Table 5).
| Clinicopathological features | Univariate analysis | Multivariable analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex | ||||
| Male | Ref. | |||
| Female | 0.683 (0.389-1.199) | P = 0.185 | ||
| BMI | ||||
| 18.5-23.9 | Ref. | |||
| < 18.5 or > 23.9 | 1.019 (0.657-1.581) | P = 0.931 | ||
| The degree of differentiation | ||||
| Poorly differentiated | Ref. | |||
| Moderately-highly differentiated | 0.733 (0.437-1.227) | P = 0.237 | ||
| Nerve invasion | ||||
| Yes | Ref. | Ref. | ||
| No | 0.083 (0.020-0.339) | P = 0.001 | 0.117 (0.028-0.500) | P = 0.004 |
| Vascular invasion | ||||
| Yes | Ref. | Ref. | ||
| No | 0.215 (0.093-0.496) | P < 0.001 | 0.425 (0.178-1.014) | P = 0.054 |
| Pathologic T stage | ||||
| Group 1-3 | Ref. | Ref. | ||
| Group 4a-4b | 1.404 (0.899-2.194) | P = 0.136 | 1.271 (0.777-2.080) | P = 0.340 |
| Pathologic N stage | ||||
| Group 1-2 | Ref. | Ref. | ||
| Group 3a-b | 2.089 (1.137-3.721) | P = 0.012 | 1.027 (0.385-2.739) | P = 0.958 |
| Pathologic TNM stage | ||||
| IIIB stage | Ref. | Ref. | ||
| IVA stage | 2.540 (1.267-5.091) | P = 0.009 | 2.081 (0.629-6.885) | P = 0.230 |
| Adjuvant chemoradiotherapy | ||||
| Yes | Ref. | Ref. | ||
| No | 1.859 (0.956-3.614) | P = 0.068 | 1.877 (0.943-3.738) | P = 0.073 |
| Clinicopathological features | Univariate analysis | Multivariable analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex | ||||
| Male | Ref. | |||
| Female | 0.573 (0.277-1.186) | P = 0.134 | ||
| BMI | ||||
| 18.5-23.9 | Ref. | |||
| < 18.5 or > 23.9 | 1.142 (0.755-1.728) | P = 0.530 | ||
| The degree of differentiation | ||||
| Poorly differentiated | Ref. | |||
| Moderately-highly differentiated | 0.945 (0.592-1.509) | P = 0.813 | ||
| Nerve invasion | ||||
| Yes | Ref. | Ref. | ||
| No | 0.086 (0.021-0.349) | P = 0.001 | 0.169 (0.037-0.774) | P = 0.022 |
| Vascular invasion | ||||
| Yes | Ref. | Ref. | ||
| No | 0.041 (0.006-0.295) | P = 0.002 | 0.092 (0.012-0.689) | P = 0.020 |
| Pathologic T stage | ||||
| Group 1-3 | Ref. | Ref. | ||
| Group 4a-4b | 1.479 (0.975-2.242) | P = 0.065 | 1.151 (0.751-1.763) | P = 0.518 |
| Pathologic N stage | ||||
| Group 1-2 | Ref. | Ref. | ||
| Group 3 | 1.983 (1.099-3.580) | P = 0.023 | 3.621 (0.380-34.469) | P = 0.263 |
| Pathologic TNM stage | ||||
| IIIA stage | Ref. | Ref. | ||
| IIIB stage | 1.420 (0.759-2.656) | P = 0.273 | 1.801 (0.180-18.037) | P = 0.617 |
| IIIC stage | 2.783 (1.472-5.261) | P = 0.002 | 0.681 (0.434-1.069) | P = 0.095 |
| Adjuvant chemoradiotherapy | ||||
| Yes | Ref. | Ref. | ||
| No | 2.465 (1.270-4.782) | P = 0.008 | 2.258 (1.145-4.453) | P = 0.019 |
In the past, adjuvant treatment for AGE was based on the experience of esophageal adenocarcinoma or gastric adenocarcinoma. However, a growing number of researchers believe that AGE is a separate tumor[22]. There have been very few randomized controlled studies on the significance of adjuvant CRT for AGE[23]. In this study, we found that postoperative adjuvant CT combined with regional radio
The role of adjuvant radiotherapy after D2/R0 resection in gastric cancer remains controversial. The Korean ARTIST 1 study[17] suggested that patients with lymph node metastases may benefit from postoperative irradiation. However, the ARTIST 2 study[24] showed that SOX CT combined with radiotherapy did not improve outcomes in patients with lymph node metastasis after D2/R0 resection. In addition, the majority of patients enrolled in these clinical trials had distal gastric cancer, while fewer had proximal gastric cancer. Owing to the specificity of the anatomic site, the benefits of adjuvant radiotherapy in AGE may not be consistent with those in gastric cancer. Therefore, we designed this retrospective clinical study to evaluate the value of adjuvant CRT in locally advanced AGE.
Because of the special location of AGE, cancer cells can metastasize to the mediastinum and abdominal cavity along the lymphatic vessels. However, for Siewert type II/III AGE, abdominal lymph node metastasis is the main direction of metastasis[25]. The JCOG9502 study showed that for Siewert type II/III AGE that underwent D2/R0 lymph node dissection, the positive rate of lymph nodes at station 16 was 15.2%, and the postoperative lymph node recurrence rate was 17.4%[26]. These results provide evidence for the delineation of radiotherapy targets for locally advanced AGE.
Our study found that adjuvant radiotherapy did not significantly improve RFS in all patients or OS in patients with Siewert type II AGE. However, we observed a significant extension of OS in patients with Siewert type III AGE after adjuvant CRT. A possible explanation is that adjuvant radiotherapy reduces local and regional recurrence rates. The overall recurrence rate in the CRT group was lower than that in the CT group. Compared with the CT group, more attention should be paid to distant metastasis during postoperative reexamination in the CRT group. We found that patients with Siewert type III AGE were more sensitive to adjuvant radiotherapy. The difference between Siewert type II and III AGE in adjuvant CRT requires further study. Multivariable Cox regression analysis showed that adjuvant CRT was an important factor affecting the prognosis of patients with Siewert type III AGE, further verifying the necessity of adjuvant CRT.
Our study has several limitations. First, this was a single-center retrospective study, and thus inherently has a lower level of evidence than a multicenter prospective clinical trial. Furthermore, did not compare surgical approaches for different types of AGE. In addition, the toxic effects of radiotherapy were not investigated in this study.
For locally advanced Siewert type III AGE, adjuvant CRT following D2/R0 resection may prolong OS and reduce the local and regional recurrence rate. Postoperative radiotherapy may be feasible for Siewert type III AGE patients. Multicenter prospective clinical trials should be conducted to investigate the significance of adjuvant CRT in AGE.
The role of adjuvant chemoradiotherapy (CRT) in adenocarcinoma of gastroesophageal junction (AGE) is unclear.
Radiotherapy may reduce the local recurrence rate and prolong survival time for locally advanced Siewert II/III type adenocarcinoma of AGE.
To evaluate the effect of adjuvant CRT vs adjuvant chemotherapy (CT) on overall survival (OS), relapse-free survival (RFS), and recurrence pattern in locally advanced Siewert II/III type adenocarcinoma of AGE patients undergoing D2/R0 resection.
We compared the OS, RFS, and recurrence modes between the adjuvant CRT and adjuvant CT groups.
Adjuvant CRT improves the 5-year survival rate of adenocarcinoma of AGE, especially Siewert type III adenocarcinoma of AGE, and reduces local and regional recurrence rates.
Adjuvant CRT may be appropriate for adenocarcinoma of AGE, especially for Siewert type III adenocarcinoma of AGE.
Multicenter prospective clinical trials should be conducted to investigate the significance of adjuvant CRT in adenocarcinoma of AGE.
We sincerely thank all the researchers of Department of Pancreatic and Gastric Surgery, Department of Radiation Oncology, and Department of Pathology in National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College. We are also grateful to the patients enrolled in the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Katagiri R, Japan; Politis C, Belgium; Yashiro M, Japan S-Editor: Fan JR L-Editor: A P-Editor: Wu RR
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64636] [Article Influence: 16159.0] [Reference Citation Analysis (176)] |
| 2. | Liu K, Yang K, Zhang W, Chen X, Zhang B, Chen Z, Chen J, Zhao Y, Zhou Z, Chen L, Hu J. Changes of Esophagogastric Junctional Adenocarcinoma and Gastroesophageal Reflux Disease Among Surgical Patients During 1988-2012: A Single-institution, High-volume Experience in China. Ann Surg. 2016;263:88-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 3. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2857] [Article Influence: 571.4] [Reference Citation Analysis (5)] |
| 4. | Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T. Adenocarcinoma of the gastroesophageal junction in Japan: relevance of Siewert's classification applied to 177 cases resected at a single institution. J Am Coll Surg. 1999;189:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13213] [Article Influence: 1468.1] [Reference Citation Analysis (3)] |
| 6. | Shen L, Shan YS, Hu HM, Price TJ, Sirohi B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X, Park SR, Fujii M, Kang YK, Chen LT. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol. 2013;14:e535-e547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 382] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 7. | Goetze TO, Al-Batran SE, Berlth F, Hoelscher AH. Multimodal Treatment Strategies in Esophagogastric Junction Cancer: a Western Perspective. J Gastric Cancer. 2019;19:148-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Zhang S, Orita H, Fukunaga T. Current surgical treatment of esophagogastric junction adenocarcinoma. World J Gastrointest Oncol. 2019;11:567-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Chang JS, Kim KH, Yoon HI, Hyung WJ, Rha SY, Kim HS, Lee YC, Lim JS, Noh SH, Koom WS. Locoregional relapse after gastrectomy with D2 lymphadenectomy for gastric cancer. Br J Surg. 2017;104:877-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Chang JS, Kim KH, Keum KC, Noh SH, Lim JS, Kim HS, Rha SY, Lee YC, Hyung WJ, Koom WS. Recursive partition analysis of peritoneal and systemic recurrence in patients with gastric cancer who underwent D2 gastrectomy: Implications for neoadjuvant therapy consideration. J Surg Oncol. 2016;114:859-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Wang SB, Qi WX, Chen JY, Xu C, Kirova YM, Cao WG, Cai R, Cao L, Yan M, Cai G. Competing risk nomogram predicting initial loco-regional recurrence in gastric cancer patients after D2 gastrectomy. Radiat Oncol. 2019;14:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten Kate FJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A; CROSS study group. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1292] [Cited by in RCA: 1825] [Article Influence: 182.5] [Reference Citation Analysis (0)] |
| 13. | Lee J, Lim DH, Kim S, Park SH, Park JO, Park YS, Lim HY, Choi MG, Sohn TS, Noh JH, Bae JM, Ahn YC, Sohn I, Jung SH, Park CK, Kim KM, Kang WK. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol. 2012;30:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 580] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 14. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1089] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 15. | Stiekema J, Trip AK, Jansen EP, Boot H, Cats A, Ponz OB, Verheij M, van Sandick JW. The prognostic significance of an R1 resection in gastric cancer patients treated with adjuvant chemoradiotherapy. Ann Surg Oncol. 2014;21:1107-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2465] [Cited by in RCA: 2437] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 17. | Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim KM, Sohn I, Jung SH, Choi MG, Lee JH, Bae JM, Kim S, Kim ST, Park JO, Park YS, Lim HY, Kang WK. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J Clin Oncol. 2015;33:3130-3136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 300] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 18. | Stahl M, Walz MK, Stuschke M, Lehmann N, Meyer HJ, Riera-Knorrenschild J, Langer P, Engenhart-Cabillic R, Bitzer M, Königsrainer A, Budach W, Wilke H. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol. 2009;27:851-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 695] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 19. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2872] [Article Influence: 205.1] [Reference Citation Analysis (0)] |
| 20. | Grégoire V, Mackie TR. State of the art on dose prescription, reporting and recording in Intensity-Modulated Radiation Therapy (ICRU report No. 83). Cancer Radiother. 2011;15:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 21. | Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus. 2017;14:1-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 479] [Cited by in RCA: 708] [Article Influence: 88.5] [Reference Citation Analysis (1)] |
| 22. | Shen J, Zhu X, Du Y, Zhu Y, Yu P, Yang L, Xu Z, Huang L, Zhang Y, Liu L, Cheng X. Adjuvant SOX chemotherapy versus concurrent chemoradiotherapy after D2 radical resection of locally advanced esophagogastric junction (EGJ) adenocarcinoma: study protocol for a randomized phase III trial (ARTEG). Trials. 2021;22:753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, Wang C, Qiu MZ, Cai MY, Wu Q, Liu H, Guan WL, Zhou AP, Zhang YJ, Liu TS, Bi F, Yuan XL, Rao SX, Xin Y, Sheng WQ, Xu HM, Li GX, Ji JF, Zhou ZW, Liang H, Zhang YQ, Jin J, Shen L, Li J, Xu RH. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond). 2021;41:747-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 463] [Article Influence: 115.8] [Reference Citation Analysis (1)] |
| 24. | Park SH, Lim DH, Sohn TS, Lee J, Zang DY, Kim ST, Kang JH, Oh SY, Hwang IG, Ji JH, Shin DB, Yu JI, Kim KM, An JY, Choi MG, Lee JH, Kim S, Hong JY, Park JO, Park YS, Lim HY, Bae JM, Kang WK; ARTIST 2 investigators. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial☆. Ann Oncol. 2021;32:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 25. | Rüdiger Siewert J, Feith M, Werner M, Stein HJ. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 529] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 26. | Sasako M, Sano T, Yamamoto S, Sairenji M, Arai K, Kinoshita T, Nashimoto A, Hiratsuka M; Japan Clinical Oncology Group (JCOG9502). Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol. 2006;7:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 303] [Article Influence: 15.9] [Reference Citation Analysis (0)] |