Published online Aug 15, 2022. doi: 10.4251/wjgo.v14.i8.1510
Peer-review started: March 21, 2022
First decision: April 25, 2022
Revised: May 8, 2022
Accepted: July 16, 2022
Article in press: July 16, 2022
Published online: August 15, 2022
Processing time: 142 Days and 12.7 Hours
Starting a second-line systemic treatment for hepatocellular carcinoma (HCC) is a common situation. The only therapeutic options in France are two broad-spectrum tyrosine kinase inhibitors (TKIs), regorafenib (REG) and cabozantinib (CBZ), but no comparative real-life studies are available.
To evaluate the progression-free survival (PFS) of patients treated with REG or CBZ, we investigated the disease control rate (DCR), overall survival (OS), and safety of both drugs. To identify the variables associated with disease progression over time.
A retrospective multicenter study was performed on the clinical data of patients attending one of three referral centers (Avignon, Marseille, and Nice) between January 2017 and March 2021 using propensity score matching. PFS and OS were assessed using the Kaplan-Meier method. Multivariate analysis (MA) of progression risk factors over time was performed in matched-pair groups.
Fifty-eight patients 68 (62-74) years old with HCC, Barcelona clinic liver cancer (BCLC) B/C (86%), Child-Pugh (CP)-A/B (24%) received REG for 3.4 (1.4-10.5) mo as second-line therapy. Twenty-eight patients 68 (60-73) years, BCLC B/C (75%), CP-A/B (25%) received CBZ for 3.7 (1.8-4.9) mo after first-line treatment with sorafenib [3 (2-4) (CBZ) vs 4 (2.9-11.8) mo (REG), P = 0.0226]. Twenty percent of patients received third-line therapy. After matching, PFS and DCR were not significantly different after a median follow-up of 6.2 (2.7-11.7) mo (REG) vs 5.2 (4-7.2) mo (CBZ), P = 0.6925. There was no difference in grade 3/4 toxicities, dose reductions, or interruptions. The OS of CP-A patients was 8.3 (5.2-24.8) vs 4.9 (1.6-11.7) mo (CP-B), P = 0.0468. The MA of risk factors for progression over time identified C-reactive protein (CRP) > 10 mg/L, neutrophil-to-lymphocyte ratio (NLR) > 3, and aspartate aminotransferase (AST) > 45 IU as predictive factors.
This multicenter indirect comparative study found no significant difference in PFS between REG and CBZ as second-line therapy for advanced HCC. Elevated levels of inflammatory markers (CRP and NLR) and AST were associated with non-control of TKIs over time. A 2-mo online progression risk calculation is proposed.
Core Tip: One limited population of advanced hepatocellular carcinoma patients has sustained disease control using tyrosine kinase inhibitors (TKIs) as first-line systemic therapy. Patients with preserved liver function and performance status progress to second-line systemic therapy. Only two broad-spectrum TKIs are approved for this indication in France, and no direct comparative studies are available. Immune checkpoint inhibitors are currently the standard of care as first-line therapy in combination with an anti-angiogenic agent and will most likely change the treatment strategy of second-line therapy. No biomarkers are available to guide treatment, but serum inflammation-related factors may provide additional support.
- Citation: Adhoute X, De Matharel M, Mineur L, Pénaranda G, Ouizeman D, Toullec C, Tran A, Castellani P, Rollet A, Oules V, Perrier H, Si Ahmed SN, Bourliere M, Anty R. Second-line therapy for advanced hepatocellular carcinoma with regorafenib or cabozantinib: Multicenter French clinical experience in real-life after matching. World J Gastrointest Oncol 2022; 14(8): 1510-1527
- URL: https://www.wjgnet.com/1948-5204/full/v14/i8/1510.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i8.1510
Most patients with advanced hepatocellular carcinoma (HCC) do not have sustained disease control with first-line systemic therapy, particularly after tyrosine kinase inhibitors (TKIs)[1], due to failure, secondary progression and/or intolerance to therapy. Switching to a second line of systemic therapy has become a common situation. During the last decade, several phase II/III trials evaluated different protein kinase inhibitors after sorafenib[2,3], including one targeting an overexpressed oncogene (tivantinib, a MET pathway inhibitor)[4], and all of these trials were negative. However, there have been important therapeutic advances in the treatment of HCC over the past four years with various anti-cancer agents, multi-kinase inhibitors[5,6], monoclonal antibodies targeting vascular endothelial growth factor receptor 2 (VEGF-R2)[7], and antibodies directed against the immune checkpoint molecules human cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed death receptor-1 (PD-1)[8,9] and its ligand PD-L1, after progression ± intolerance to sorafenib, similar to other cancer types. The most significant results were achieved with the combination of a monoclonal antibody against PD-L1 and an anti-angiogenic agent targeting VEGF-A[10]. Angiogenesis contributes to immunosuppression via a direct effect of the VEGF-VEGFR interaction or from the tumor microenvironment[11]. Combination therapies have become the standard of care in first-line systemic treatment of HCC. Therefore, the therapeutic landscape in second-line treatment is expected to change in the future. All of these advances should not omit HCC specificity, which is generally linked to a chronic liver disease with cirrhosis of various etiologies. The strict selection criteria of clinical trials have resulted in a lack of data for a large number of patients in routine practice. Two multi-targeted TKIs regorafenib (REG) and cabozantinib (CBZ) are the only treatment options available in France based on phase III trials after sorafenib. Notably, no controlled trials of second-line treatment were performed after first-line treatment with atezolizumab-bevacizumab (lenvatinib[12] is not approved for this indication in France). There are also no direct comparison studies between the "approved" second-line molecules or any predictive biomarker correlated with treatment activity[13]. Elevated pre-treatment inflammation-related factors, such as C-reactive protein (CRP) and neutrophil-to-lymphocyte ratio (NLR) are clearly associated with poor survival outcomes in various tumor types and across all stages[14,15]. Inflammation supports tumor development and metastasis[16] because inflammatory cells [particularly macrophages, mast cells, neutrophils, myeloid-derived suppressive cells (MDSCs) and selected lymphocytes] release various mediators, such as growth factors, pro-inflammatory cytokines and metalloproteinases, which result in stromal remodeling and tumor growth and spread.
The present study (1) Evaluated the survival of advanced HCC patients treated with second-line systemic therapy in a real-life cohort; (2) Evaluated the progression-free survival (PFS) of patients treated with REG or CBZ, the disease control rate (DCR), overall survival (OS) and the safety of both drugs after matching; and (3) Identified factors associated with disease progression over time, with a focus on inflammatory markers recorded at baseline and longitudinally during treatment.
This study was a retrospective multicenter study in three institutions from southern France (Nice, Marseille, and Avignon). All patients with advanced HCC (radiologically proven according to the European Association for the Study of the Liver[17]/Association for the Study of Liver Diseases[18] criteria or with histology) who received a second-line treatment with REG or CBZ from January 2017-March 2021 were included. Eligible patients included patients with prior first-line systemic sorafenib treatment that was discontinued after failure and/or intolerability. Patients who received REG or CBZ in combination with other therapies were excluded. The decision of second-line treatment for HCC in all three centers followed a multidisciplinary team discussion. Selected patients were Barcelona clinic liver cancer (BCLC) HCC stage B or C, without curative options: Evolutive multinodular HCC, refractory transarterial chemoembolization patients, or patients with vascular invasion and/or metastatic disease, Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) 0/1/2, Child–Pugh (CP)-A or B grade. Baseline and follow-up demographic, clinical, and biological characteristics, including full blood count (neutrophils, lymphocytes, hemoglobin and platelets), biochemical blood tests [particularly alpha-fetoprotein (AFP) and CRP levels] and radiological features, were collected prospectively and analyzed retrospectively following a similar process in all three centers. Only patients with full available data were included. The local institutional review board in each center approved the study protocol. Informed consent from patients was waived by the IRBs because of the retrospective nature of this study.
Procedure and assessments: Before starting TKI treatment, patients were informed of potential adverse events (AEs) and useful prophylactic measures to prevent or reduce these events[19]. Monitoring included clinical evaluation twice monthly during the first two cycles then at each treatment cycle, focusing on TKI treatment tolerance. Radiological assessment included initial cross-sectional imaging (computed tomography and/or magnetic resonance imaging) 8 to 12 wk after the initiation of therapy then every 2 to 3 mo, using Response Evaluation Criteria in Solid Tumors version 1.1 for the grading of tumor responses. Patients with controlled disease included patients with radiological response and stable disease as the best response. Liver function or AFP serum levels and inflammation-related factors (NLR and CRP) were also assessed at each treatment cycle.
Treatment schemes: REG: Patients received 160 mg once daily during the first 3 wk of each 4-wk cycle. REG was continued until progression or intolerable AE occurrence. Interruptions and dose reductions were based on the severity and nature of AEs, which were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4. REG was first started at 80 mg/d in the setting of ECOG PS 2 or CP-B cirrhosis, with subsequent dose escalation in cases of adequate safety.
Cabometyx: Patients received 60 mg once daily continuously until progression or intolerable toxicities. Interruptions and dose reductions were also determined by the severity and nature of AEs graded according to NCI CTCAE version 4. Treatment was started at 40 mg/d in the setting of ECOG PS 2 or CP-B cirrhosis, with subsequent dose escalation when the treatment was well tolerated.
Dose reductions were used for grade 2 toxicity that was not controlled by symptomatic treatment. Treatment interruption was used for any grade ≥ 3 toxicity until recovery to ≤ grade 1 severity.
Quantitative data are reported using medians and interquartile ranges. Qualitative data are reported using frequencies and percentages. Crude comparisons between CBZ and REG were performed using the nonparametric Wilcoxon test for median comparisons and the chi-squared test or Fisher’s test for frequency comparisons. The Mantel-Haenszel chi-squared test was performed to compare ordinal scale data.
OS was defined as the time interval between the initiation of CBZ or REG and death or the time of last follow-up for patients who were still alive. PFS was defined as the time interval between the initiation of CBZ or REG and the time until first progression or the time of last follow-up for patients with no progression. Survival between groups was compared using the log-rank test.
Paired analysis between CBZ and REG was performed using propensity score matching (PSM) on BCLC staging, CP grade, vascular invasion, metastasis, and AFP. Risk factors for tumor progression were analyzed using univariate logistic regression analysis followed by multivariate logistic regression analysis. Factors with significant results in univariate analysis were included in multivariate model analysis. All P values were considered significant at α-level = 0.05. All calculations were performed using SAS V9.1 statistical software (SAS Institute Inc., Cary, NC).
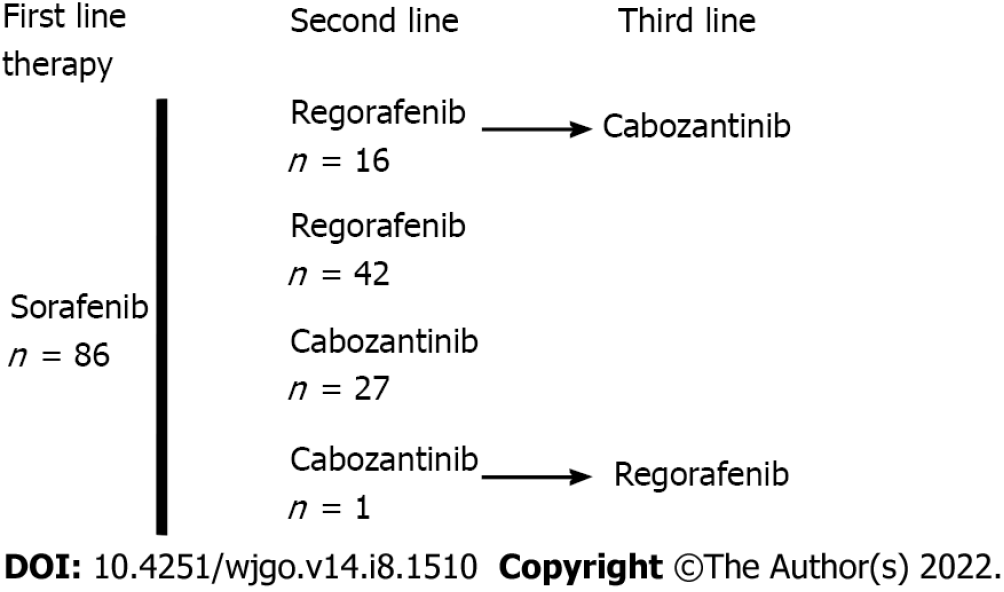
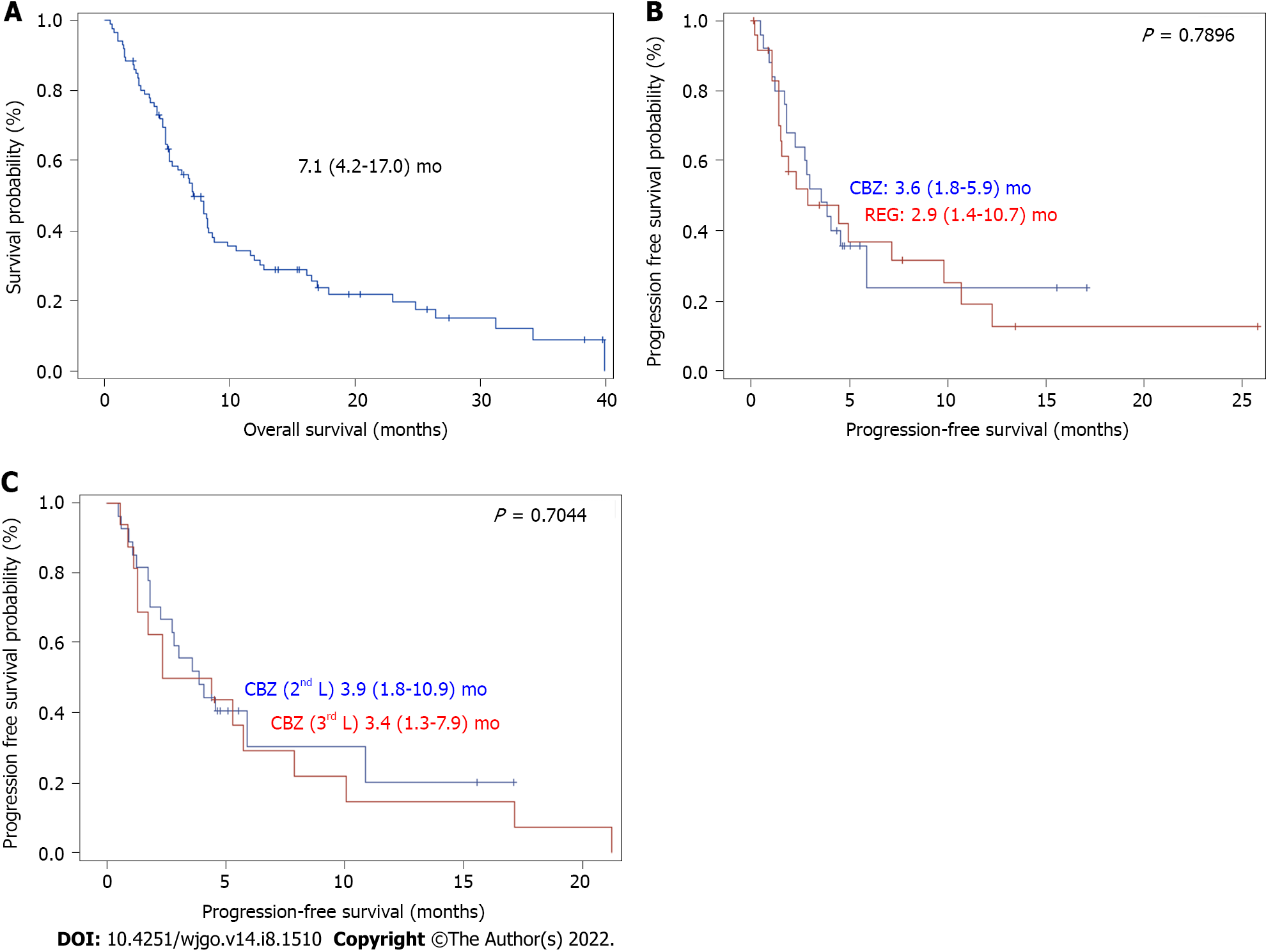
Table 1 shows the patients’ characteristics. There were 86 patients with a median age of 68 (60-74) years at the start of treatment, mostly men, with an ECOG PS of 0/1 (78%) or 2 (22%). Patients had cirrhosis due to viral, alcoholic or metabolic etiology in most cases and CP grade A or B liver function. The tumors were classified as stage B or C (83%) according to the BCLC system. Macroscopic vascular invasion was present in 48% of cases, and metastasis was present in 43% of cases. AFP elevation ≥ 400 ng/mL was found in 48% of cases. The largest tumor diameter was 69 (40-100) mm. The baseline CRP serum level was 22 (8-51) mg/L, and 53% of patients had an NLR > 3. The median duration of prior treatment with sorafenib was 3.5 (2.7-9.2) mo Fifty-eight patients received REG as second-line therapy, and 28 patients received CBZ as second-line therapy (Figure 1). The median second-line treatment duration was 3.5 (1.6, 8.3) mo. Twenty percent of patients received third-line therapy. After a median follow-up of 6.9 (4.0-13.7) mo, 79% of patients died, and the median OS was 7.1 (4.2, 17.0) mo (Figure 2A). PFS was 3.6 (1.6, 10.9) mo and the DCR was 37% at the end of follow-up.
| Characteristics at baseline | n = 86 |
| Age–median (Q1Q3), yr | 68.0 (60-74) |
| Gender, n (%) | |
| Male | 77 (90) |
| Female | 9 (10) |
| Etiology of HCC, n (%) | |
| Alcohol use | 30 (35) |
| Virus/Virus + Alcohol | 26 (30)/8 (9) |
| Non-alcoholic steatohepatitis | 13 (15) |
| Other | 9 (10) |
| ECOG performance status, n (%) | |
| 0 | 33 (38) |
| 1 | 34 (40) |
| 2 | 19 (22) |
| Esophageal varices1, n (%) | 36 (45) |
| Macrovascular invasion, n (%) | 41 (48) |
| Extrahepatic disease, n (%) | 37 (43) |
| Child-Pugh class, n (%) | |
| A | 65 (76) |
| B2 | 21 (24) |
| BCLC stage, n (%) | |
| B | 15 (17) |
| C | 71 (83) |
| AFP, ng/mL, n (%) | |
| < 400 | 45 (52) |
| ≥ 400 | 41 (48) |
| HCC morphology3, n (%) | |
| Diffuse | 15 (18) |
| Mass forming | 24 (29) |
| Multinodular | 44 (53) |
| Maximal tumor diameter, mm–median (Q1Q3) | 69 (40-100) |
| Hemoglobin, g/dL–median (Q1Q3) | 13 (12-14) |
| Platelet’s count (× 100/L)–median (Q1Q3) | 153 (95-213) |
| Neutrophil count/L–median (Q1Q3) | 3675 (2700-4600) |
| Lymphocyte count/L–median (Q1Q3) | 1118 (810-1650) |
| Neutrophil-to-lymphocyte ratio, n (%) | |
| ≤ 3 | 40 (47) |
| > 3 | 46 (53) |
| CRP, mg/L–median (Q1Q3) | 22 (8-51) |
| AST, IU/L–median (Q1Q3) | 62 (46-117) |
| ALT, IU/L–median (Q1Q3) | 40 (28-64) |
| GGT, IU/L–median (Q1Q3) | 187 (112-360) |
| ALP, IU/L–median (Q1Q3) | 166 (128-267) |
| Total bilirubin, μmol/L–median (Q1Q3) | 17 (12-27) |
| Albumin, g/L–median (Q1Q3) | 35 (29-39) |
| Creatinine, μmol/L–median (Q1Q3) | 70 (57-85) |
| Prothrombin time, %–median (Q1Q3) | 79 (68-93) |
| Duration of prior Sorafenib treatment, months–median (Q1Q3) | 3.5 (2.7-9.2) |
After matching CP grade, BCLC staging, vascular invasion, metastasis and AFP level < ≥ 400 ng/mL, the median OS of HCC patients classified CP-A was 8.3 (5.2-24.8) mo vs 4.9 (1.6-11.7) mo for CP-B (P = 0.0468). The median OS of HCC patients without vascular invasion was 12.0 (5.2-24.8) mo vs 6.3 (3.4-23.0) mo for patients with vascular invasion (P = 0.3471). The survival time of patients with and without metastases was 8.3 (4.2-24.8) vs 8.2 (4.9-17.0) mo, respectively (P = 0.8902).
Fifty-eight patients, who were 68 (62-74) years old, with BCLC stage B/C (86%) HCC and CP-A/B (24%) received REG for 3.4 (1.4-10.5) mo as second-line therapy (Figure 1). Twenty-eight patients, who were 68 (60-73) years old, with BCLC stage B/C (75%) HCC and CP-A/B (25%) received CBZ for 3.7 (1.8-4.9) mo as second-line therapy. The median time on sorafenib was 3 (2-4) mo in the CBZ group and 4 (2.9-11.8) mo in the REG group (P = 0.0226). The median PFS was not significantly different [3.6 (1.4-11.7) mo REG vs 4.0 (1.8-10.9) mo CBZ, P = 0.7495], and the DCR was not different [24% (REG) vs 32% (CBZ), P = 0.4466] after a median follow-up period of 7.8 (3.6-14.1) mo (REG) vs 5.2 (4.1-9.4) mo (CBZ) (P = 0.3049) (Table 2).
| Characteristics at baseline | Cabozantinib (n = 28) | Regorafenib (n = 58) | P value |
| Age–median (Q1Q3), yr | 68 (60-73) | 68 (62.74) | 0.6828 |
| Gender, n (%) | 0.4645 | ||
| Male | 24 (86) | 53 (91) | |
| Female | 4 (14) | 5 (9) | |
| Etiology of HCC, n (%) | 0.4219 | ||
| Alcohol use | 6 (21) | 24 (41) | |
| Virus/virus + alcohol | 10 (36)/4 (14) | 16 (28)/4 (7) | |
| NASH | 5 (18) | 8 (14) | |
| Other | 3 (11) | 6 (10) | |
| PS, n (%) | 0.6286 | ||
| 0 | 12 (44) | 21 (38) | |
| 1 | 9 (32) | 25 (43) | |
| 2 | 7 (24) | 12 (20) | |
| Esophageal varices1, n (%) | 12 (44) | 24 (45) | 0.9432 |
| Macrovascular invasion, n (%) | 13 (46) | 28 (51) | 0.6995 |
| Extrahepatic disease, n (%) | 10 (36) | 27 (50) | 0.2177 |
| Child-Pugh class, n (%) | 1.0000 | ||
| A | 21 (75) | 44 (76) | |
| B | 7 (25) | 14 (24) | |
| BCLC, n (%) | 0.2375 | ||
| B | 7 (25) | 8 (14) | |
| C | 21 (75) | 50 (86) | |
| AFP, ng/mL, n (%) | 0.4468 | ||
| < 400 | 13 (46) | 32 (55) | |
| ≥ 400 | 15 (54) | 26 (45) | |
| Morphology2, n (%) | 0.0830 | ||
| Diffuse | 7 (26) | 8 (14) | |
| Mass forming | 4 (15) | 20 (36) | |
| Multinodular | 16 (59) | 28 (50) | |
| Maximal tumor diameter, mm–median (Q1Q3) | 69.5 (37.5-118.5) | 68.5 (40-100) | 0.7495 |
| Hemoglobin, g/dL–median (Q1Q3) | 13.3 (12-14) | 13 (11-14.7) | 0.9730 |
| Platelet’s count (× 100/L)–median (Q1Q3) | 136 (94-197) | 173 (97-215) | 0.2582 |
| Neutrophil count/L-median (Q1Q3) | 3118 (2120-3720) | 4081 (3000-5668) | 0.0042 |
| Lymphocyte count/L–median (Q1Q3) | 1130 (820-1675) | 1105 (810-1643) | 0.7723 |
| Neutrophil-to- lymphocyte ratio, n (%) | 0.0217 | ||
| ≤ 3 | 18 (64) | 22 (38) | |
| > 3 | 10 (36) | 36 (62) | |
| CRP, mg/L–median (Q1Q3) | 14.5 (6.7-36.2) | 29.7 (8.3-58) | 0.1665 |
| AST, IU/L–median (Q1Q3) | 73 (52-132) | 59 (41-95) | 0.0681 |
| ALT, IU/L–median (Q1Q3) | 47 (33-73) | 37 (26-51) | 0.0970 |
| GGT, IU/L–median (Q1Q3) | 150 (100-350) | 194 (117-362) | 0.3538 |
| ALP, IU/L–median (Q1Q3) | 159 (137-231) | 182 (122-269) | 0.6232 |
| Total bilirubin, μmol/L–median (Q1Q3) | 21 (14-29) | 17 (11-25) | 0.1135 |
| Albumin, g/L–median (Q1Q3) | 36 (31-39) | 34 (29-40) | 0.7476 |
| Creatinine, μmol/L–median (Q1Q3) | 67 (55-87) | 71 (57-84) | 0.6051 |
| Prothrombin time, %–median (Q1Q3) | 81 (68-99) | 78 (68-88) | 0.1878 |
| Duration of prior Sorafenib treatment, months–median (Q1Q3) | 3 (2-4) | 4 (2.9-11.8) | 0.0226 |
Only patients with two lines of systemic TKI therapy were considered (Figure 1). A total of 42 patients received REG as second-line therapy, and 27 patients received CBZ without subsequent treatment (Figure 1). After PSM, there were 25 patients in each group. The main characteristics of these patients are shown in Table 3. There was no significant difference between the two groups in PS, liver function, cirrhosis etiology, tumor burden, CRP level, or the number of patients with AFP ≥ 400 ng or NLR > 3. The median duration of prior sorafenib treatment was 3.2 (2.7-10.9) mo (REG) vs 3 (1.7-4.1) mo (CBZ) (P = 0.1865). After a median follow-up period of 6.2 (2.7-11.7) mo (REG) vs 5.2 (4-7.2) mo (CBZ) (P = 0.6925), 92% of patients receiving REG died compared to 64% of patients receiving CBZ (P = 0.0374). PFS was not significantly different [2.9 (1.4-10.7) mo (REG) vs 3.6 (1.8-5.9) mo (CBZ), P = 0.7896] (Figure 2B), and the DCR was not different [28% (REG) vs 32% (CBZ), P = 1.0000] (Table 3).
| Characteristics at baseline | Cabozantinib (n = 25) | Regorafenib (n = 25) | P value |
| Age–median (Q1Q3), yr | 69 (60-74) | 68 (58-72) | 0.7870 |
| Gender, n (%) | 1.0000 | ||
| Male | 23 (92) | 22 (88) | |
| Female | 2 (8) | 3 (12) | |
| Etiology of HCC, n (%) | 0.6370 | ||
| Alcohol | 6 (24) | 10 (40) | |
| Virus/virus + alcohol | 8 (32)/4 (16) | 7 (28)/2 (8) | |
| NASH | 4 (16) | 2 (8) | |
| Other | 3 (12) | 4 (16) | |
| PS, n (%) | 0.4591 | ||
| 0 | 9 (36) | 8 (29) | |
| 1 | 9 (36) | 10 (42) | |
| 2 | 7 (28) | 7 (29) | |
| Esophageal varices1, n (%) | 10 (42) | 10 (45) | 0.7957 |
| Macrovascular invasion, n (%) | 12 (48) | 14 (58) | 0.4687 |
| Extrahepatic disease, n (%) | 10 (40) | 13 (54) | 0.3206 |
| Child-Pugh class, n (%) | 0.5512 | ||
| A | 18 (72) | 15 (60) | |
| B | 7 (28) | 10 (40) | |
| BCLC, n (%) | 0.7585 | ||
| B | 5 (20) | 5 (20) | |
| C | 20 (80) | 20 (80) | |
| AFP, ng/mL, n (%) | 0.5713 | ||
| < 400 | 12 (48) | 14 (56) | |
| ≥ 400 | 13 (52) | 11 (44) | |
| Morphology2, n (%) | 0.2393 | ||
| Diffuse | 7 (29) | 4 (14) | |
| Mass | 4 (17) | 9 (36) | |
| Multinodular | 13 (54) | 12 (50) | |
| Maximal tumor diameter, mm–median (Q1Q3) | 74 (38-130) | 70 (40-94) | 0.6067 |
| Hemoglobin g/dL–median (Q1Q3) | 13 (12-13.9) | 12.5 (10-13.7) | 0.2875 |
| Platelet’s count (× 100/L)–median (Q1Q3) | 148 (95-193) | 152 (97-206) | 0.6229 |
| Neutrophil count/L–median (Q1Q3) | 3150 (1970-3760) | 4100 (3000-5676) | 0.0276 |
| Lymphocyte count/L–median (Q1Q3) | 1140 (810-1700) | 940 (739-1600) | 0.5828 |
| Neutrophil-to-lymphocyteratio, n (%) | 0.1564 | ||
| ≤ 3 | 16 (64) | 10 (40) | |
| > 3 | 9 (36) | 15 (60) | |
| CRP, mg/L–median (Q1Q3) | 14.5 (7.2-41.1) | 32 (8-65) | 0.2900 |
| AST, IU/L–median (Q1Q3) | 75 (56-134) | 64 (4 6-79) | 0.0940 |
| ALT, IU/L–median (Q1Q3) | 48 (33-77) | 30 (26-47) | 0.0556 |
| GGT, IU/L–median (Q1Q3) | 179 (99-360) | 187 (112-322) | 0.7925 |
| ALP, IU/L–median (Q1Q3) | 162 (138-252) | 203 (122-269) | 0.6094 |
| Total bilirubin, μmol/L–median (Q1Q3) | 17.5 (14-29) | 15.6 (12-27) | 0.5123 |
| Albumin, g/L–median (Q1Q3) | 36 (29-39) | 31.6 (28-35) | 0.1772 |
| Creatinine, μmol/L–median (Q1Q3) | 69 (57-89) | 72 (58-91) | 0.4996 |
| Prothrombin time, %–median (Q1Q3) | 80 (68-100) | 71 (61-78) | 0.0792 |
| Duration of prior Sorafenib treatment, months–median (Q1Q3) | 3 (1.7-4.1) | 3.2 (2.7-10.9) | 0.1865 |
Sixteen patients received CBZ as third-line systemic therapy. The median age was 68 (64-75) years at the start of treatment, and HCCs were classified as BCLC stage B/C (81%). Vascular invasion was present in 38% of cases, and metastasis was present in 37% of cases. Fifty percent of the patients had an AFP ≥ 400 ng/mL. After a median follow-up of 5.2 (3.1-16.6) mo, 63% of patients died, and the median OS was 8.1 (3.8-24.3) mo. There was no significant difference in PFS between patients who received CBZ as second-line or third-line therapy (P = 0.7044) (Figure 2C) after a comparable follow-up period [5.2 (4.0-8.2) mo vs 5.2 (3.1-16.6) mo, respectively, P = 0.8907] (Table 4).
| Characteristics at baseline | Cabozantinib (n = 16) |
| Age–median (Q1Q3), yr | 68 (64-75) |
| Gender, n (%) | |
| Male | 15 (94) |
| Female | 1 (6) |
| PS, n (%) | |
| 0 | 8 (50) |
| 1 | 7 (44) |
| 2 | 1 (6) |
| Macrovascular invasion, n (%) | 6 (38) |
| Extrahepatic disease, n (%) | 10 (62) |
| Child-Pugh class, n (%) | |
| A | 10 (62) |
| B | 6 (38) |
| BCLC, n (%) | |
| B | 3 (19) |
| C | 13 (81) |
| AFP, ng/mL, n (%) | |
| < 400 | 8 (50) |
| ≥ 400 | 8 (50) |
| Maximal tumor diameter, mm–median (Q1Q3) | 60 (32-106) |
| Hemoglobin, g/dL–median (Q1Q3) | 13.7 (11.4-14.7) |
| Platelet’s count (× 100/L)–median (Q1Q3) | 159 (107-246) |
| Neutrophil count/L–median (Q1Q3) | 4690 (3128-7463) |
| Lymphocyte count/L–median (Q1Q3) | 1063 (783-1359) |
| Neutrophil–lymphocyte ratio, n (%) | |
| ≤ 3 | 3 (19) |
| > 3 | 13 (81) |
| CRP, mg/L–median (Q1Q3) | 33 (7-78) |
| AST, IU/L–median (Q1Q3) | 51 (33-76) |
| ALT, IU/L–median (Q1Q3) | 29 (21-53) |
| GGT, IU/L–median (Q1Q3) | 188 (111-323) |
| ALP, IU/L–median (Q1Q3) | 162 (120-253) |
| Total bilirubin, μmol/L–median (Q1Q3) | 15.9 (11.1-25.6) |
| Albumin, g/L–median (Q1Q3) | 33.5 (27.9-39.2) |
| Creatinine, μmol/L–median (Q1Q3) | 82 (56-91) |
| Prothrombin time, %–median (Q1Q3) | 81 (70-92) |
Adverse events, such as fatigue, anorexia and weight loss, were observed in both treatment groups with no significant difference between groups, primarily grades 1 and 2 toxicities. There was no significant difference in other common adverse events associated with TKIs, such as diarrhea, hand-foot skin reaction, increased blood bilirubin, increased Aspartate aminotransferase (AST)/Alanine aminotransferase, or hypertension. Drug-related AEs leading to interruptions or dose reduction were reported in greater than 40% of cases without a significant difference between groups (Table 5).
| Adverse event | Cabozantinib (n = 28) | Regorafenib (n = 58) | P value |
| Fatigue and/or decreased appetite and/or weight loss, n (%) | 22 (79) | 46 (79) | 1.0000 |
| Grade 1-2/3-4 | 20 (89)/2 (11) | 40 (85)/7 (15) | 1.0000 |
| Hand-foot skin, n (%) | 9 (32) | 16 (28) | 0.8005 |
| Grade 1-2/3-4 | 8 (89)/1 (11) | 11 (69)/5 (31) | 0.3644 |
| Diarrhea, n (%) | 11 (39) | 13 (22) | 0.1021 |
| Grade 1-2/3-4 | 11 (100)/0 | 13 (100)/0 | 1.0000 |
| Increased blood Bilirubin and/or AST and/or ALT, n (%) | 9 (32) | 17 (29) | 0.8063 |
| Grade 1-2/3-4 | 4 (44)/5 (56) | 11 (65)/6 (35) | 0.4185 |
| Hypertension, n (%) | 6 (21) | 12 (21) | 1.0000 |
| Grade 1-2/3-4 | 5 (83)/1 (17) | 11 (92)/1 (8) | 1.0000 |
| Other disorders1, n (%) | 14 (50) | 19 (33) | 0.1234 |
| Grade 1-2/3-4 | 12 (86)/2 (14) | 18 (95)/1 (5) | 0.5612 |
| Interruptions, n (%) | 12 (43) | 31 (53) | 0.3573 |
| Dose reduction, n (%) | 23 (82) | 49 (84) | 0.7646 |
Univariate analysis of risk factors for tumor progression over time identified the following baseline variables: Bilirubin > 17 μmol, increased AST > 45 IU, increased CRP > 10 mg/L, and NLR > 3 (Table 6).
| Variables | Univariate analysis, P value | Multivariate analysis, P value |
| Treatment with CBZ vs REG | 0.8851 | - |
| NLR ≤ 3 vs > 3 | 0.00061 | 0.0006 |
| CRP (mg/L) > 10 vs ≤ 10 | 0.03641 | 0.0624 |
| ALP (IU) > 200 vs ≤ 200 | 0.5545 | - |
| Bilirubin total (μmol/L) > 17 vs ≤ 17 | 0.02701 | 0.3262 |
| Albumin (g/L) > 36 vs ≤ 36 | 0.3026 | - |
| PT (%) > 70 vs ≤ 70 | 0.0534 | - |
| AST (IU) > 45 vs ≤ 45 | 0.00481 | 0.0132 |
| AFP (ng/mL) > 400 vs ≤ 400 | 0.0634 | - |
Multivariate analysis (MA) of risk factors for progression identified NLR > 3, increased CRP > 10 mg/L, and increased AST > 45 IU as independent variables over time (Table 6).
Based on these results, we defined a progression risk score at two months that was calculated at T0 before REG or CBZ: Score 2 M = - 0.1849 + 0.1943 × (1 if NLR ratio > 3, and 0 if < 3) + 0.3053 × (1 if CRP > 10, and 0 if < 10) + 0.4962 × (1 if AST > 45, and 0 if < 45). Scores approaching 1 indicate a higher the risk of progression (i.e., score > 0.50 indicates increased risk of progression), and scores approaching 0, indicate a low risk of progression (i.e., score < 0.50 indicates low risk).
To simplify the calculation, we used the following online application: https://jscalc.io/calc/3nzmguiJK5QIn8eQ#%7B%221%22:null,%222%22:null,%223%22:null%7D.
The present real-life multicenter cohort studied the use of second-line therapy with TKIs for advanced HCC and found PFS of 3.6 (1.6-10.9) mo, which was similar to phase III studies with TKI[5] and anti-PD-1 monotherapy[8]. The median OS of 7.1 (4.2, 17.0) mo was naturally lower, despite an equivalent duration of treatment [3.5 (1.6-8.3) mo] as the phase III studies with TKIs[5,6], which is consistent with our cohort's features, including PS 2 patients or patients classified as CP-B grade, but inconsistent with the RESORCE[5], CELESTIAL[6], REACH-2[7] trials. These randomized controlled studies included CP-A patients, PS 0/1, mostly with viral disease, except for the KEYNOTE-240[8] study. Vascular invasion (recognized as a significant aggressive feature) was found in 13% to 36% of all patients in these studies[5-8], as opposed to one of two patients in our cohort. The median OS of HCC patients without vascular invasion in our cohort at 12 mo was comparable to real-life studies with REG[20] or CBZ[21]. The Korean (n = 440) and Italian (n = 96) cohorts included CP-A patients, and the Refine[22] study (n = 498) included 11% CP-B patients. These three studies reported a high proportion of metastatic patients (> 60%) with lower vascular invasion in 30%-35% of patients. Other evidence of cohort differences is that the prior duration of sorafenib treatment in our study was reduced compared to phase III studies, except for REACH-2[7] (4 mo), which included only patients with AFP levels ≥ 400 ng/mL. The median treatment duration was 5.0 mo in the CELESTIAL[6] study (with 43% of patients receiving more than 6 mo of sorafenib[23]). It was also 5 mo in the KEYNOTE-240[8] study and 7.8 mo in the RESORCE[5] study. Yoo et al[20] found that the time to progression on prior sorafenib < median was an independent outcome factor that adversely affected survival. Another indirect comparison study with CBZ and REG in real life that arose from the CELESTIAL study reported that the OS of patients on REG was 6.5 mo (IQR: 4.7-10.9) for a prior duration of sorafenib treatment < 3 mo[24].
Clinicians are dealing with populations that do not fit the phase III trials, and moving phase III trial results to real-life patients in clinical practice is challenging. This difficulty highlights the importance of real-life cohorts. Patients with preserved liver function in our cohort had better OS than CP-B patients. Consistent with other studies of TKIs[25,26], the OS of CP-B patients was low at less than 5 mo. Kim et al[26] did not find any difference in PFS or OS between CP-B7 patients and CP-B 8/9 patients. Our study included too few patients to make this distinction. CP-B liver function was an independent prognostic variable in MA that adversely affected PFS and OS in Kim et al[26] Therefore, preserved liver function is an essential criterion for first- or second-line systemic TKI therapy eligibility. Real-life studies with immune checkpoint inhibitors also suggest caution[27]. A multicenter retrospective cohort study assessing antibodies targeting the immune checkpoint molecule PD-1 in advanced HCC patients with or without prior systemic therapy found a comparable rate of side effects but a significant difference in survival between patients classified as CP-A and CP-B [16.7 (8.2-25.2) mo vs 8.6 (4.8-12.4) mo, respectively, P = 0.065].
Switching to a second-line systemic therapy is now a common situation, although it occurs in fewer than half of patients in the TKI era[28,29]. The only therapeutic options in France in this situation are REG or CBZ, but no head-to-head phase III trial is available for reference. We do not have ramucirumab (which is recommended for HCC patients with AFP > 400 ng/mL) or an immune checkpoint inhibitor against PD-1, although pembrolizumab is approved by the Food Drug Administration in this setting. This French multicenter series is one of the first indirect real-life comparison studies between REG and CBZ as second-line systemic treatment for HCC. Despite different mechanisms of action, this study found no difference in efficacy before and after matching, which contrasts the indirect comparison studies from CELESTIAL and RESORCE populations on PFS[30]. REG[31] is a multiple protein kinase inhibitor that targets angiogenesis (the VEGFR 1-3 and the angiopoietin 1 receptor TIE2) more intensively than sorafenib, tumor cells (especially the oncogenic kinases KIT and RET and the intracellular kinases Raf), and fibroblast growth factor receptors in contrast to sorafenib. CBZ[32] also targets key angiogenesis receptors, including VEGFR-2, AXL, and MET, which exhibits expression increased after sorafenib as an escape mechanism driven by hypoxia inducible factor (HIF)-1. The mortality rate was higher in the REG group most likely because the initiation of CBZ treatment was more recent (molecule available in France from July 2019 vs November 2017 for REG). Therefore, an OS assessment would be biased, especially because patients on REG as second line received CBZ as 3rd line. Notably, the PFS of patients treated with CBZ as second- or third-line treatment was similar, which suggests comparable efficacy in these two situations, as observed in the CELESTIAL study.
Most patients were out of control at the end of the follow-up period (nearly two-thirds). The therapeutic landscape in advanced HCC has profoundly changed since 2020, with the success of combination therapies in first-line (anti-PDL1 antibody + bevacizumab)[10] and second-line (anti-CTLA-4 + anti-PD1 antibodies)[9] treatments, which exhibit increased response rates and prolonged survival, despite the lack of available biomarkers. Beyond a simple association, a synergistic action exists between these molecules. Interfering with VEGF-VEGFR signaling improves the anti-tumor immune response by enhancing T-cell recruitment and functionality and reducing immunosuppressive cells, such as MDSCs and regulatory T cells[11]. The tumor microenvironment composed of endothelial cells, pericytes, fibroblasts and various immune cells adopting a pro-tumor phenotype plays a key role in the inhibition of the lymphocyte effective response. Some of the various mechanisms include the expression of PD-L1 co-inhibitory molecules, the presence of PD-1 and CTLA-4 inhibitory immune checkpoint molecules, and others, such as T-cell immunoglobulin and mucin-domain containing-3 and lymphocyte activation gene 3, on T cells and a decrease in functional dendritic cells and the presence of immunosuppressive populations. Therefore, it is likely that other alternatives will be available in the near future for second-line treatment. Based on this interaction between neoangiogenesis and anti-tumor immunity, the combination of TKIs, such as cabometyx or REG, with immunotherapies targeting PD-1 and CTLA-4 is relevant. Trials evaluating various combinations are underway.
Overall, we found the most common side effects observed with TKIs in phase III trials[5,6] and real-life studies[21,22], namely fatigue, diarrhea, hand-foot skin reaction, loss of appetite, weight loss and hypertension. The general signs were associated with most patients in both groups. Differences were observed in the severity of hand-foot syndrome with REG and in the frequency of diarrhea with CBZ, but without significance. The phase III trials[5,6] and real-life studies[21] found dose reductions and interruptions in most patients (RESORCE: 68%, CELESTIAL: 62%). The frequency and magnitude of side effects associated with TKIs may be a limitation to the long-term use of this therapy, especially when used at full doses[33], despite improved clinician experience[34].
The present study also highlights the relevance of inflammation-related serum factors in the setting of advanced HCC, such as CRP and the NLR, which was shown in other studies[35,36]. These factors were independent prognostic variables that correlated with disease progression over time in our study. We focused on inflammatory markers to assess their role in predicting clinical outcome in this multicenter HCC cohort treated with TKI as second-line therapy because no markers, other than AFP, are currently used. Systemic inflammation is associated with tumor progression[16], the promotion of genomic instability[37], angiogenesis, and cell proliferation[38]. This systemic inflammation, as measured by a high CRP serum level or increased NLR, was reported as a worse prognostic marker in various types of cancer[15]. Tumor necrosis and inflammation are closely linked[39] and enable a hypoxic environment prone to mutations, which is driven by the release of reactive oxygen species[40]. Some of the mediators of this cancer-related inflammatory process involve transcription factors, such as nuclear factor-kappaB, the pro-angiogenic factor HIF-1 alpha, and their effects on interleukin (IL)-6 production, a multifunctional pro-inflammatory cytokine, and the IL-6/Janus kinase/signal transducer and activator of transcription 3 pathway, which promotes cell proliferation, survival and migration[40]. High levels of IL-6 are associated with tumor growth, and it contributes to angiogenesis[41] and the inhibition of apoptosis[42]. The over-expression of IL-6 also affects the immune response via the functional impairment of lymphocytes and the recruitment of immunosuppressive cells[43]. IL-6 is found in the epithelium and tumor stroma of various solid tumors[42]. IL-6 plays an important role in the hepatic overproduction of CRP, and some studies found a positive correlation between increased blood levels of IL-6 and CRP[44,45]. High CRP serum levels are also associated with hypoalbuminemia in cancer[46]. High neutrophil counts and low lymphocyte counts, which mirror this inflammatory process, are also prognostic markers in various cancers at different stages[47]. The prognostic value of NLR is now strongly suggested in the setting of immunotherapy[48], especially during the course of treatment[49]. Therefore, inflammatory scores, such as the Glasgow Prognostic Score or the NLR, demonstrated their prognostic value regardless of cancer type and stage[14], including in controlled studies[15,50]. In summary, these scores reflect this cancer-related inflammatory response.
We investigated baseline variables associated with 2-mo tumor progression risk, considering the short action time of TKI treatment. Our study suggests that higher inflammatory markers and increased AST, which may reflect deterioration of liver function and/or liver tumor growth, are associated with a higher risk of early progression under TKI, i.e., nonresponse. Therefore, careful tumor assessment using imaging and a safety evaluation are required in these patients due to the high adverse event rate related to TKI. Because these parameters are easily available on a routine blood test, we developed a progression risk score based on these variables.
Limitations of the present study include the limited sample size, the retrospective design of the study and the lack of a control group, which prevent definitive conclusions of our model. However, our results are consistent with prior publications, and other studies after sorafenib included comparable population sizes[21,26]. Given the limited response rate to first-line TKIs and the time to control is frequently less than six months[1], few patients will complete a second-line regimen[29]. A previous study of first-line sorafenib therapy[34] had 188 patients in one center compared to 86 patients in three centers for the present study. The retrospective character necessarily leads to various biases, but we considered only patients with all data, and because the data were biological or radiological data, the risk of error was limited, especially because these data were collected in a recent period. Obviously, this model must be evaluated in an independent cohort, but it is based on robust variables.
This indirect comparison from a real-life multicenter cohort found no difference in PFS with the use of REG or CBZ as second-line therapy for advanced HCC. Most patients did not achieve controlled disease at the end of follow-up, particularly patients with vascular invasion. Our results also show that TKIs are not indicated for CP-B patients. Inflammation-related factors (CRP and NLR ratio) and AST increased over time were associated with a higher risk of TKI failure. We propose an online score to assess progression risk based on these variables after two months of treatment.
Switching to a second line of systemic therapy will theoretically concern most patients with advanced hepatocellular carcinoma (HCC), especially after sorafenib. The strict selection criteria in phase III trials result in a lack of data for many patients from current practice. Inflammation acts as a powerful tumor promoter.
Two multi-targeted tyrosine kinase inhibitors (TKIs) [Regorafenib (REG), Cabozantinib (CBZ)] are currently the only available therapeutic options in France in this situation based on phase III trials after sorafenib. There are also no direct comparative studies between the "approved" second-line molecules or any predictive biomarker correlated with treatment activity.
To assess both efficacy and safety of REG and CBZ as second-line systemic treatment after sorafenib in a "real-life" study. To investigate the relevance of serum inflammation-related markers as predictive factors for tumor progression over time in this setting. The current lack of treatment-guiding biomarkers and the safety profile of TKIs are limiting factors for this sequencing.
This is an indirect propensity score-matched comparative study based on recent retrospective data recorded in three French centers. We focused on progression-free survival and disease control rates of patients treated with REG or CBZ, and on factors associated with tumor progression over time.
Both efficacy and safety of REG and CBZ are comparable in this real-life study, and CBZ is still a third-line therapeutic option. Elevated levels of pretherapeutic inflammation-related markers [C-reactive protein (CRP) serum level, neutrophil-to-lymphocyte ratio (NLR)] are associated with poorer survival by using TKIs as second-line treatment for HCC.
In light of the limited tumor control rate with TKIs and the positive results of first- (anti-programmed death ligand-1 + anti-vascular endothelial growth factor) and second-line (anti-human cytotoxic T-lymphocyte antigen-4 + anti-programmed death receptor-1) combination therapies, the therapeutic "landscape" of advanced HCC will be changed in the second-line setting. We propose a 2-mo online progression risk calculation based on CRP serum level, NLR, and aspartate aminotransferase level to estimate the disease course under ITKs treatment.
The tumor microenvironment plays a key role in the suppression of an effective lymphocyte response. TKIs exhibit anti-angiogenic and immunomodulatory properties. Combinations of TKIs and immune checkpoint inhibitors are currently being evaluated as second-line systemic therapy for HCC.
We thank Rahamia Ahamada for her assistance and Doctor Manuela Campanile for her support.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bredt LC, Brazil; Serban D, Romania S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [PubMed] [DOI] [Full Text] |
| 2. | McNamara MG, Le LW, Horgan AM, Aspinall A, Burak KW, Dhani N, Chen E, Sinaei M, Lo G, Kim TK, Rogalla P, Bathe OF, Knox JJ. A phase II trial of second-line axitinib following prior antiangiogenic therapy in advanced hepatocellular carcinoma. Cancer. 2015;121:1620-1627. [PubMed] [DOI] [Full Text] |
| 3. | Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, Kang YK, Assenat E, Lim HY, Boige V, Mathurin P, Fartoux L, Lin DY, Bruix J, Poon RT, Sherman M, Blanc JF, Finn RS, Tak WY, Chao Y, Ezzeddine R, Liu D, Walters I, Park JW. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31:3509-3516. [PubMed] [DOI] [Full Text] |
| 4. | Rimassa L, Assenat E, Peck-Radosavljevic M, Pracht M, Zagonel V, Mathurin P, Rota Caremoli E, Porta C, Daniele B, Bolondi L, Mazzaferro V, Harris W, Damjanov N, Pastorelli D, Reig M, Knox J, Negri F, Trojan J, López López C, Personeni N, Decaens T, Dupuy M, Sieghart W, Abbadessa G, Schwartz B, Lamar M, Goldberg T, Shuster D, Santoro A, Bruix J. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018;19:682-693. [PubMed] [DOI] [Full Text] |
| 5. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [PubMed] [DOI] [Full Text] |
| 6. | Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54-63. [PubMed] [DOI] [Full Text] |
| 7. | Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282-296. [PubMed] [DOI] [Full Text] |
| 8. | Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL; KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193-202. [PubMed] [DOI] [Full Text] |
| 9. | Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. [PubMed] [DOI] [Full Text] |
| 10. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [PubMed] [DOI] [Full Text] |
| 11. | Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front Immunol. 2018;9:978. [PubMed] [DOI] [Full Text] |
| 12. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [PubMed] [DOI] [Full Text] |
| 13. | Rimassa L, Wörns MA. Navigating the new landscape of second-line treatment in advanced hepatocellular carcinoma. Liver Int. 2020;40:1800-1811. [PubMed] [DOI] [Full Text] |
| 14. | Dolan RD, Lim J, McSorley ST, Horgan PG, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: Systematic review and meta-analysis. Sci Rep. 2017;7:16717. [PubMed] [DOI] [Full Text] |
| 15. | Dolan RD, McSorley ST, Horgan PG, Laird B, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: Systematic review and meta-analysis. Crit Rev Oncol Hematol. 2017;116:134-146. [PubMed] [DOI] [Full Text] |
| 16. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [PubMed] [DOI] [Full Text] |
| 17. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [PubMed] [DOI] [Full Text] |
| 18. | Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [PubMed] [DOI] [Full Text] |
| 19. | De Wit M, Boers-Doets CB, Saettini A, Vermeersch K, de Juan CR, Ouwerkerk J, Raynard SS, Bazin A, Cremolini C. Prevention and management of adverse events related to regorafenib. Support Care Cancer. 2014;22:837-846. [PubMed] [DOI] [Full Text] |
| 20. | Yoo C, Byeon S, Bang Y, Cheon J, Kim JW, Kim JH, Chon HJ, Kang B, Kang MJ, Kim I, Hwang JE, Kang JH, Lee MA, Hong JY, Lim HY, Ryoo BY. Regorafenib in previously treated advanced hepatocellular carcinoma: Impact of prior immunotherapy and adverse events. Liver Int. 2020;40:2263-2271. [PubMed] [DOI] [Full Text] |
| 21. | Tovoli F, Dadduzio V, De Lorenzo S, Rimassa L, Masi G, Iavarone M, Marra F, Garajova I, Brizzi MP, Daniele B, Trevisani F, Messina C, Di Clemente F, Pini S, Cabibbo G, Granito A, Rizzato MD, Zagonel V, Brandi G, Pressiani T, Federico P, Vivaldi C, Bergna I, Campani C, Piscaglia F. Real-Life Clinical Data of Cabozantinib for Unresectable Hepatocellular Carcinoma. Liver Cancer. 2021;10:370-379. [PubMed] [DOI] [Full Text] |
| 22. | Merle P, Finn HY, Ikeda RS, Kudo M, Frenette C, Masi G, Kim YJ, Gerolami R, Kurosaki M, Numata K, Klümpen HJ, Zebger-Gong H, Fiala-Buskies S, Ozgurdal K, Qin S. 1010P Real-world dosing of regorafenib (REG) in patients (pts) with unresectable hepatocellular carcinoma (uHCC): Interim analysis (IA) of the observational REFINE study. Ann Oncol. 2020;31:S699-S700. [DOI] [Full Text] |
| 23. | Kelley RK, Ryoo BY, Merle P, Park JW, Bolondi L, Chan SL, Lim HY, Baron AD, Parnis F, Knox J, Cattan S, Yau T, Lougheed JC, Milwee S, El-Khoueiry AB, Cheng AL, Meyer T, Abou-Alfa GK. Second-line cabozantinib after sorafenib treatment for advanced hepatocellular carcinoma: a subgroup analysis of the phase 3 CELESTIAL trial. ESMO Open. 2020;5. [PubMed] [DOI] [Full Text] |
| 24. | Casadei-Gardini A, Rimassa L, Rimini M, Yoo C, Ryoo BY, Lonardi S, Masi G, Kim HD, Vivaldi C, Ryu MH, Rizzato MD, Salani F, Bang Y, Pellino A, Catanese S, Burgio V, Cascinu S, Cucchetti A. Regorafenib versus cabozantinb as second-line treatment after sorafenib for unresectable hepatocellular carcinoma: matching-adjusted indirect comparison analysis. J Cancer Res Clin Oncol. 2021;147:3665-3671. [PubMed] [DOI] [Full Text] |
| 25. | Marrero JA, Kudo M, Venook AP, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JH, de Guevara LL, Papandreou C, Takayama T, Sanyal AJ, Yoon SK, Nakajima K, Lehr R, Heldner S, Lencioni R. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J Hepatol. 2016;65:1140-1147. [PubMed] [DOI] [Full Text] |
| 26. | Kim HD, Bang Y, Lee MA, Kim JW, Kim JH, Chon HJ, Kang B, Kang MJ, Kim I, Cheon J, Hwang JE, Kang JH, Byeon S, Hong JY, Ryoo BY, Lim HY, Yoo C. Regorafenib in patients with advanced Child-Pugh B hepatocellular carcinoma: A multicentre retrospective study. Liver Int. 2020;40:2544-2552. [PubMed] [DOI] [Full Text] |
| 27. | Scheiner B, Kirstein MM, Hucke F, Finkelmeier F, Schulze K, von Felden J, Koch S, Schwabl P, Hinrichs JB, Waneck F, Waidmann O, Reiberger T, Müller C, Sieghart W, Trauner M, Weinmann A, Wege H, Trojan J, Peck-Radosavljevic M, Vogel A, Pinter M. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: efficacy and safety data from an international multicentre real-world cohort. Aliment Pharmacol Ther. 2019;49:1323-1333. [PubMed] [DOI] [Full Text] |
| 28. | Uchikawa S, Kawaoka T, Aikata H, Kodama K, Nishida Y, Inagaki Y, Hatooka M, Morio K, Nakahara T, Murakami E, Hiramatsu A, Tsuge M, Imamura M, Kawakami Y, Chayama K. Clinical outcomes of sorafenib treatment failure for advanced hepatocellular carcinoma and candidates for regorafenib treatment in real-world practice. Hepatol Res. 2018;48:814-820. [PubMed] [DOI] [Full Text] |
| 29. | Fung AS, Tam VC, Meyers DE, Sim HW, Knox JJ, Zaborska V, Davies J, Ko YJ, Batuyong E, Samawi H, Cheung WY, Lee-Ying R. Second-line treatment of hepatocellular carcinoma after sorafenib: Characterizing treatments used over the past 10 years and real-world eligibility for cabozantinib, regorafenib, and ramucirumab. Cancer Med. 2020;9:4640-4647. [PubMed] [DOI] [Full Text] |
| 30. | Kelley RK, Mollon P, Blanc JF, Daniele B, Yau T, Cheng AL, Valcheva V, Marteau F, Guerra I, Abou-Alfa GK. Comparative Efficacy of Cabozantinib and Regorafenib for Advanced Hepatocellular Carcinoma. Adv Ther. 2020;37:2678-2695. [PubMed] [DOI] [Full Text] |
| 31. | Granito A, Forgione A, Marinelli S, Renzulli M, Ielasi L, Sansone V, Benevento F, Piscaglia F, Tovoli F. Experience with regorafenib in the treatment of hepatocellular carcinoma. Therap Adv Gastroenterol. 2021;14:17562848211016959. [PubMed] [DOI] [Full Text] |
| 32. | Personeni N, Pressiani T, Rimassa L. Cabozantinib in patients with hepatocellular carcinoma failing previous treatment with sorafenib. Future Oncol. 2019;15:2449-2462. [PubMed] [DOI] [Full Text] |
| 33. | Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, Lee HC, Song T, Roayaie S, Bolondi L, Lee KS, Makuuchi M, Souza F, Berre MA, Meinhardt G, Llovet JM; STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344-1354. [PubMed] [DOI] [Full Text] |
| 34. | Raoul JL, Adhoute X, Penaranda G, Perrier H, Castellani P, Oules V, Bourlière M. Sorafenib: Experience and Better Manage-ment of Side Effects Improve Overall Survival in Hepatocellular Carcinoma Patients: A Real-Life Retrospective Analysis. Liver Cancer. 2019;8:457-467. [PubMed] [DOI] [Full Text] |
| 35. | Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J Hepatol. 2017;67:999-1008. [PubMed] [DOI] [Full Text] |
| 36. | Zheng Z, Zhou L, Gao S, Yang Z, Yao J, Zheng S. Prognostic role of C-reactive protein in hepatocellular carcinoma: a systematic review and meta-analysis. Int J Med Sci. 2013;10:653-664. [PubMed] [DOI] [Full Text] |
| 37. | Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184-190. [PubMed] |
| 38. | Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997;11:457-465. [PubMed] |
| 39. | Richards CH, Roxburgh CS, Anderson JH, McKee RF, Foulis AK, Horgan PG, McMillan DC. Prognostic value of tumour necrosis and host inflammatory responses in colorectal cancer. Br J Surg. 2012;99:287-294. [PubMed] [DOI] [Full Text] |
| 40. | Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073-1081. [PubMed] [DOI] [Full Text] |
| 41. | Benoy I, Salgado R, Colpaert C, Weytjens R, Vermeulen PB, Dirix LY. Serum interleukin 6, plasma VEGF, serum VEGF, and VEGF platelet load in breast cancer patients. Clin Breast Cancer. 2002;2:311-315. [PubMed] [DOI] [Full Text] |
| 42. | Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer. 2007;110:1911-1928. [PubMed] [DOI] [Full Text] |
| 43. | Guthrie GJ, Roxburgh CS, Horgan PG, McMillan DC. Does interleukin-6 link explain the link between tumour necrosis, local and systemic inflammatory responses and outcome in patients with colorectal cancer? Cancer Treat Rev. 2013;39:89-96. [PubMed] [DOI] [Full Text] |
| 44. | Ramsey S, Lamb GW, Aitchison M, McMillan DC. The longitudinal relationship between circulating concentrations of C-reactive protein, interleukin-6 and interleukin-10 in patients undergoing resection for renal cancer. Br J Cancer. 2006;95:1076-1080. [PubMed] [DOI] [Full Text] |
| 45. | McKeown DJ, Brown DJ, Kelly A, Wallace AM, McMillan DC. The relationship between circulating concentrations of C-reactive protein, inflammatory cytokines and cytokine receptors in patients with non-small-cell lung cancer. Br J Cancer. 2004;91:1993-1995. [PubMed] [DOI] [Full Text] |
| 46. | McMillan DC, Elahi MM, Sattar N, Angerson WJ, Johnstone J, McArdle CS. Measurement of the systemic inflammatory response predicts cancer-specific and non-cancer survival in patients with cancer. Nutr Cancer. 2001;41:64-69. [PubMed] [DOI] [Full Text] |
| 47. | Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218-230. [PubMed] [DOI] [Full Text] |
| 48. | Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, Kosteva JA, Ciunci CA, Gabriel PE, Thompson JC, Stonehouse-Lee S, Sherry VE, Gilbert E, Eaby-Sandy B, Mutale F, DiLullo G, Cohen RB, Vachani A, Langer CJ. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1-7. [PubMed] [DOI] [Full Text] |
| 49. | Kiriu T, Yamamoto M, Nagano T, Hazama D, Sekiya R, Katsurada M, Tamura D, Tachihara M, Kobayashi K, Nishimura Y. The time-series behavior of neutrophil-to-lymphocyte ratio is useful as a predictive marker in non-small cell lung cancer. PLoS One. 2018;13:e0193018. [PubMed] [DOI] [Full Text] |
| 50. | Dolan RD, Laird BJA, Horgan PG, McMillan DC. The prognostic value of the systemic inflammatory response in randomised clinical trials in cancer: A systematic review. Crit Rev Oncol Hematol. 2018;132:130-137. [PubMed] [DOI] [Full Text] |