Published online Aug 15, 2022. doi: 10.4251/wjgo.v14.i8.1406
Peer-review started: January 26, 2022
First decision: April 17, 2022
Revised: May 8, 2022
Accepted: July 16, 2022
Article in press: July 16, 2022
Published online: August 15, 2022
Processing time: 195 Days and 16.6 Hours
While the incidence of gastric cancer (GC) in general has decreased worldwide in recent decades, the incidence of diffuse cancer historically comprising poorly cohesive cells-GC (PCC-GC) and including signet ring cell cancer is rising. Literature concerning PCC-GC is scarce and unclear, mostly due to a large variety of historically used definitions and classifications. Compared to other histological subtypes of GC, PCC-GC is nevertheless characterized by a distinct set of epidemiological, histological and clinical features which require a specific diagnostic and therapeutic approach. The aim of this review was to provide an update on the definition, classification and therapeutic strategies of PCC-GC. We focus on the updated histological definition of PCC-GC, along with its implications on future treatment strategies and study design. Also, specific considerations in the diagnostic management are discussed. Finally, the impact of some recent developments in the therapeutic management of GC in general such as the recently validated taxane-based regimens (5-Fluorouracil, leucovorin, oxaliplatin and docetaxel), the use of hyperthermic intraperitoneal chemotherapy as well as pressurized intraperitoneal aerosol chemotherapy and targeted therapy have been reviewed in depth for their relative importance for PCC-GC in particular.
Core Tip: Although the worldwide incidence of gastric cancer (GC) has decreased in recent decades, the incidence of diffuse cancer historically comprising poorly cohesive cells-GC (PCC-GC) and including signet ring cell cancer is rising. While the existing literature concerning PCC-GC is scarce, this narrative review aims to provide an update on the classification and management of PCC-GC in light of several recent developments: (1) The updated definition according to World Health Organization classification and Verona consensus; (2) An update in curative approaches following the recent validation of 5-Fluorouracil, leucovorin, oxaliplatin and docetaxel regimen and development of hyperthermic intraperitoneal chemotherapy; and (3) Role of chemotherapy and targeted therapies in the treatment of PCC-GC.
- Citation: Drubay V, Nuytens F, Renaud F, Adenis A, Eveno C, Piessen G. Poorly cohesive cells gastric carcinoma including signet-ring cell cancer: Updated review of definition, classification and therapeutic management. World J Gastrointest Oncol 2022; 14(8): 1406-1428
- URL: https://www.wjgnet.com/1948-5204/full/v14/i8/1406.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i8.1406
Worldwide, gastric cancer (GC) is ranked as the 5th most frequently diagnosed cancer. Because of its poor prognosis, it is responsible for the 3rd highest cancer-related death rate[1]. Despite a global decline in the overall incidence of GC, the relative incidence of diffuse-type GC historically comprising poorly cohesive cells-GC (PCC-GC) and including signet ring cell (SRC) cancer has shown a steady increase in the past few decades, especially in the United States and Europe[2-4]. Based on data from the Surveillance, Epidemiology and End Results (SEER) database, collected between 1973 and 2000, an increase of 400% of the diffuse type GC has been noted[4]. In contrast to other histological types of GC, SRC-GC is known to be associated with a younger age at the time of diagnosis along with a more female sex distribution[5-8]. Since the publication of the first edition of the World Health Organization (WHO) classification of GC in 1977, the definition of SRC-GC has changed several times until the 5th edition in 2019[9-13]. Before 2010, SRC-GC was classified as a separate specific subtype of GC[9,10,13]. In the edition of 2010, the SRC-GC category was redefined entirely as a subtype of PCC-GC[10]. Previously, alternative classification systems such as the Lauren and the Ming classification, categorized SRC-GC as ‘diffuse/mixed’ and ‘infiltrative’ type carcinoma, respectively[14,15]. As such, these multiple definitions and classifications render correct assessment and comparison of this histological subtype in the current literature challenging to make. In this context, an updated review on PCC-GC was needed to address the following topics: (1) Recent definition according to WHO classification[12] and Verona consensus[16]; (2) Update in curative approaches following validation of the new perioperative chemotherapy (CT) regimen 5-Fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT)[17,18] and the increasing role of hyperthermic intraperitoneal chemotherapy (HIPEC) in the prevention of, or as a curative treatment for, peritoneal metastases; and (3) Recent developments in future-based therapeutic strategies including CT, pressurized intraperitoneal aerosol chemotherapy (PIPAC) and targeted therapies including immunotherapy.
A literature search in the MEDLINE/PubMed and Reference Citation Analysis (https://www.referencecitationanalysis.com/) database was conducted with the use of the following search terms: ‘Signet ring cell carcinoma’ (n = 3345), ‘PCC’ (n = 136), ‘Lauren and diffuse type’ (n = 257), ‘linitis plastica’ (n = 423) and ‘Bormann type IV’ (n = 178) up to 2021. Only studies in the English language published after January 1980 were eligible for inclusion. Studies were screened based on the abstract. Additional studies were retrieved by screening the references of each article. Case reports and studies including patients < 18-years-old were excluded as well as studies reporting on non-gastric PCC-GC. Studies reporting on < 30 cases were also excluded. Abstracts and meeting reports were only included if the information was found to be relevant enough in the context of the subject. Studies were only included after the agreement of both VD and GP.
Overview and update on histological and molecular classifications of SRC- and PCC-GC.
The most commonly used classifications in GC are the WHO and the Laurén classifications[10,11,14].
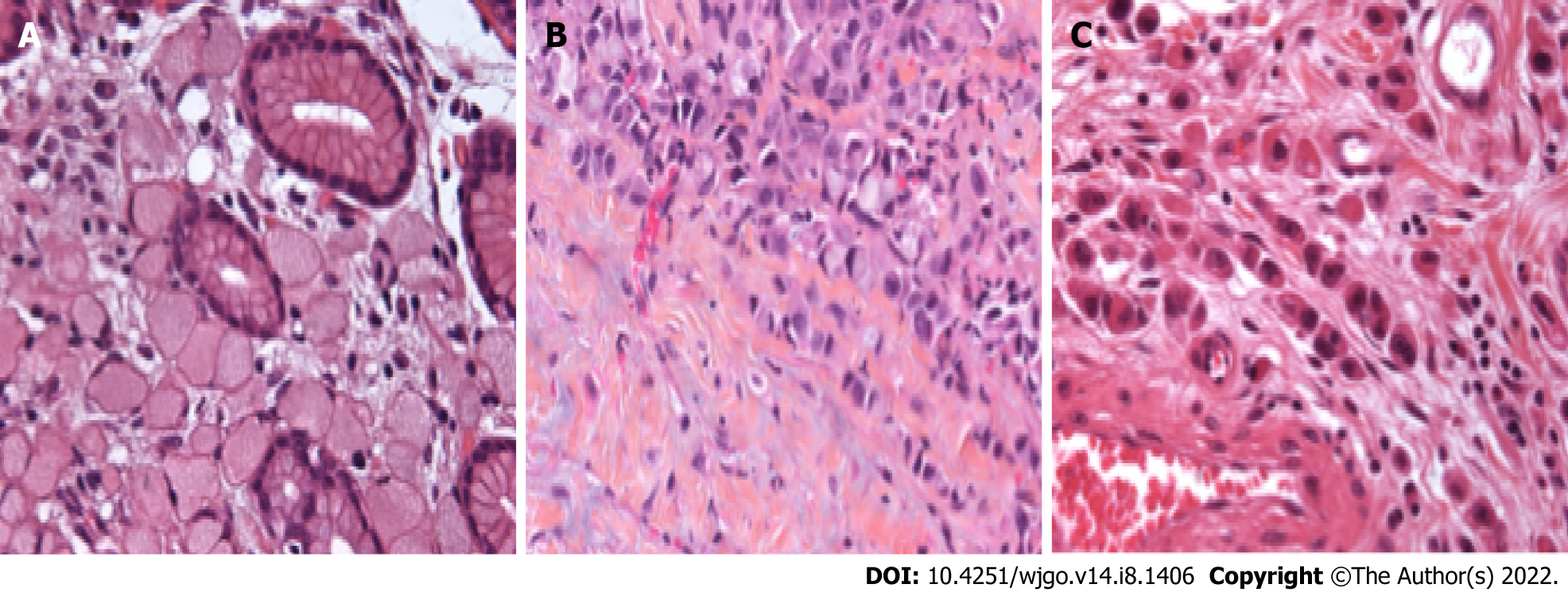
The WHO definition of SRC-GC and-more recently-PCC-GC has evolved in function of the different published editions of the WHO classification. In the very first edition, published in 1977, SRC-GC was considered as a separate subtype of GC and was defined as ‘a tumor which contained more than 50% of isolated or small groups of malignant cells containing intracytoplasmic mucin’. As such, four morphological SRC types were defined[9]. By the time the 3rd edition of the WHO classification was published in 2000, this was extended to 5 morphological SRC types[11]. In the 4th edition in 2010, the SRC-GC category was completely redefined as a subtype of PCC-GC[10]. PCC-GC is composed of neoplastic cells that are isolated or arranged in small aggregates without well-formed glands. The definition of the extent of SRC to qualify as SRC-GC evolved to “predominantly” or “exclusively” in the 4th and 5th editions of the WHO[10,12]. SRCs are characterized by a central optically clear, globoid droplet of cytoplasmic mucin with an eccentrically placed nucleus[10]. Other cellular subtypes not fulfilling the requirements of this definition should be defined as PCC not otherwise specified (PCC-NOS). PCC-NOS include tumors composed of neoplastic cells resembling histiocytes or lymphocytes; others have deeply eosinophilic cytoplasm; some PCC are pleomorphic with bizarre nuclei. A mixture of the different cell types can be seen, including a mixture of PCC-NOS and SRC. Historically, mucinous adenocarcinoma has frequently been misclassified as SRCC due to the frequent observation of SRC in this subtype[19,20]. Overall, this added a lot of confusion in analyzing data from the literature.
Invited by the European chapter of the International Gastric Cancer Association (IGCA), a multidisciplinary expert panel convened in 2017 with the intent to clarify the pathological definition of PCC-GC[16]. In a consented conclusion, it was proposed that only PCC-GC with more than 90% of cells representing an SRC morphology should be classified as SRC-type. The two other categories were PCC with SRC component (< 90% but > 10% of SRC) and PCC-NOS: < 10% of SRC[16]. An overview of the proposed definition and classification is shown in Table 1 and Figure 1. On another level, this newly defined classification also incorporates the theory that the extent of SRC in the tumor may be an expression of the differentiation grade of PCC[16]. The importance of this consensus definition cannot be underestimated since it will enable future studies to standardize results and facilitate comparison between studies in order to avoid the major heterogeneity that has characterized studies concerning SRC-GC for the past few decades.
The Laurén classification, which is the oldest and most general classification, categorizes tumors into two major categories: Intestinal-type tumors, characterized by cohesive neoplastic cells organized in well-differentiated glandular structures and diffuse tumors, diffusely infiltrating the gastric wall, with little to no gland formation. The latter type consists of PCCs, with or without SRC morphology and thus corresponds most with the PCC category of the WHO classification[14]. Comparative studies are shown in Table 2. Tumors exhibiting features of both the intestinal and diffuse types (> 25% of either component) are designated as mixed-type adenocarcinoma and account for approximately 10% of all gastric adenocarcinomas[21,22]. Some tumors may be unclassified. Although widely implemented, the Laurén classification does not allow for any clinical or pathological evaluation according to the proportion of the SRC component, which is an additional justification for the implementation of the recently proposed renewed definition of PCC by the WHO[12] and the European chapter of IGCA[16].
| Ref. | Reclassification of SRC and PCC-GC according to Laurén classification | |||
| n | % Intestinal | % Diffuse | % Mixed | |
| Pyo et al[7], 2016 | 3170 | 0.6 | 96.3 | 3.1 |
| Pyo et al[173], 2017 | 5309 | 0.0 | 96.1 | 3.9 |
| Wanebo et al[174],1993 | 187 | 2 | 87 | 11 |
| Hass et al[175], 2011 | 160 | 7.6 | 66.2 | 26.2 |
| Lee et al[176], 2012 | 320 | 0.0 | 90.6 | 9.4 |
| Heger et al[58], 2014 | 235 | 0.0 | 75.3 | 20.0 |
| Chon et al[53], 2017 | 1646 | 1.2 | 96.4 | 2.4 |
The original Japanese classification system categorized GC into differentiated and undifferentiated tumors, with undifferentiated type corresponding to diffuse type[23]. A more recent version of the classification proposed by the Japanese Gastric Cancer Association (JCGA) is however mainly based on the WHO classification and distinguishes between papillary, tubular, poorly differentiated and mucinous adenocarcinoma as well as SRC tumors[24]. Finally, the Ming classification describes an expanding and infiltrative type, the latter being strongly correlated to diffuse type[25,26].
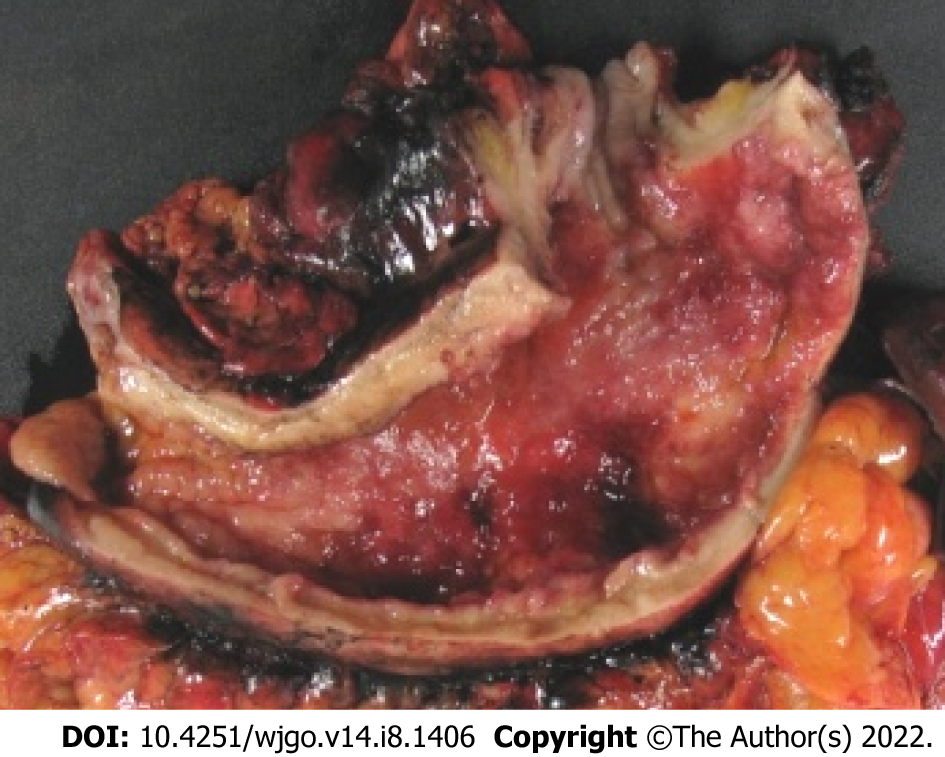
Linitis plastica (LP) is macroscopically described as an increased thickening and rigidity of the gastric wall with an aspect of linen. From a histological point of view, it corresponds to involvement of the entire stomach wall by carcinoma cells, mostly SRC, with a very abundant sclerous stroma. LP is an uncommon variant of gastric adenocarcinoma occurring in 7%–17.4% of cases[27-31]. LP is rarely individualized in studies for two main reasons; (1) Some authors confuse the histological and macroscopical definition[32-34] assimilating SRC-GC with LP, thus adding to the confusion; and (2) LP is also referred to as Borrmann type IV or scirrhous gastric carcinoma in the Eastern literature. An illustration of gastric LP is presented in Figure 2. In one study at our center, among 159 patients with SRC-GC and non-SRC_GC, LP occurred in 35.6% in the SRC group vs 6% in the non-SRCC group (P < 0.001)[35]. Most LP in the non-SRC-group had a minor component of SRC. In other words, LP and SRCC are not synonyms[36] but are closely associated. However, we believe that the current definition of SCR-GC should be used systematically. The term ‘linitis plastica’ can be additionally used when applicable.
From a molecular point of view, GC has been classified into four genomic subtypes in a landmark project by The Cancer Genome Atlas[37]. These four subtypes comprise: (1) The Epstein-Barr virus (EBV) subtype (9%), characterized by extreme DNA hypermethylation, recurrent PIK3CA mutations and amplification of JAK2, programmed death-ligand 1 (PD-L1) and PD-L2; (2) The microsatellite instability (MSI) subtype (21%), containing mutations in genes encoding for targetable oncogenic signaling proteins and associated with a more favorable oncological outcome; (3) A genomically stable (GS) subtype (20%), in which most but not all PCC-GC are categorized; and (4) The chromosomal instability (CIN) subtype (50%), associated with aneuploidy and amplification of genes involved in receptor tyrosine kinase/RAS/MAPK signaling[38]. More recently, another molecular analysis for GC identified four subgroups of tumors associated with distinct clinical outcomes: (1) A mesenchymal-type, including diffuse-subtype tumors and most PCC-GC tumors; (2) An MSI subtype, characterized by numerous mutations and a better prognosis; (3) A tumor protein 53 (TP53)-active subtype, associated with higher rates of EBV infection; and (4) A TP53-inactive subtype, similar to the CIN subgroup[39]. The importance of these molecular classifications cannot be underestimated as they provide a roadmap for patient stratification. In addition to the prognostic impact, it has been proven that these genomic subtypes are associated with distinct features regarding tumor response. As such, this subtype classification is primordial in the implementation of current and future clinical trials that evaluate the role of targeted therapies, among others[40,41]. However, we have to bear in mind that GC consists of heterogeneous tumors and that several histological and molecular components can be present in the same tumor and may be modified by the treatment applied[42]. In addition, there is no strict correlation between the histological types and molecular subtypes. PCC-GC are mostly GS but can also be MSI or EBV type with potential therapeutic implications since both molecular subtypes are associated with response to immune checkpoint inhibitors[43].
All stages studies: Although most studies agree about the poor prognosis of diffuse GC according to the Laurén classification, more discrepancies exist about the specific prognosis of PCC-GC[22,35,44,45]. An overview of studies reporting on the prognosis of all stages of SRC- and PCC-GC, is shown in Table 3. The reported prognosis of PCC-GC in Western studies is in general worse compared to that of most Eastern studies with, however, significant differences in terms of tumor stages; the majority of studies in early gastric cancer (EGC) (i.e. GC pT1a or pT1b regardless of lymph node status)[46] originate from Eastern series.
| Ref. | n | n SRC-CG (%) | % LNM | 5-yr survival rate %, SR-CGC vs other | Univariate | Multivariate | Compared to | |||
| Eastern studies | ||||||||||
| Maehara et al[49], 1992 | 1500 | 51 (3.4) | 33.3 | 74.5 vs 52.4 | P < 0.01 | - | Non SRC-GC | |||
| Kim et al[177], 1994 | 3702 | 450 (12.2) | 50.6 | 59.7 vs 57.7/48.6/43.1 | NS | - | WD/MD/PD | |||
| Otsuji et al[178], 1998 | 1498 | 154 (10.3) | 27.9 | 68.2 vs 43.9 (10-yr survival rate) | P < 0.05 | - | Non SRC-GC | |||
| Yokota et al[179], 1998 | 923 | 93 (10.1) | 43 | Worse | NS | - | Non SRC-GC | |||
| Kim et al[180], 2004 | 2358 | 204 (8.7) | 26.5 | 60.2 vs 48.9 | P < 0.01 | NS | Non SRC-GC | |||
| Park et al[181], 2008 | 2275 | 251 (11.0) | 46.2 | 66.2 vs 66.7/54.5/51.0 | WD: NS; PD/MC: P < 0.001 | P = 0.002a | WD/PD/MC | |||
| Zhang et al[45], 2010 | 1439 | 218 (15.1) | 76.1 | 44.9 vs 36 | P = 0.013 | NS | Non SRC-GC | |||
| Chiu et al[182], 2011 | 2439 | 505 (20.7) | 53.7 | 57.6 vs 56 | NS | - | Non SRC-GC | |||
| Jiang et al[55], 2011 | 1439 | 211 (14.7) | 52,0 | 49.8 vs 41.4 | P = 0.001 | - | Non SRC-GC | |||
| Lee et al[176], 2012 | 1002 | 320 (31.9) | 37.2 | 84.8 vs 71.9/57.8 | P < 0.001 | NS | PD/MC | |||
| Kwon et al[50], 2014 | 769 | 108 (14.0) | 43.5 | 55.4 vs 64.5/46.2 (10-yr survival rate) | P < 0.001 | NS | WD-MD/PD-MC | |||
| Liu et al[162], 2015 | 1464 | 138 (9.4) | 30.4 | 36.2 vs 49.5 | P < 0.001 | P < 0.001 | Non SRC-GC | |||
| Chon et al[53], 2017 | 7667 | 1646 (21.5) | 25.8 | 80.0 vs 70.0 (10-y survival rate) | P < 0.001 | NS | WMD/PD | |||
| Lu et al[183], 2016 | 2199 | 354 (16.1) | - | 15.9 mo vs 22.1 mo | P = 0.002 | < 0.001 | Non SRC-GC | |||
| Western studies | ||||||||||
| Theuer et al[184], 1999 | 3020 | 453 (15.0) | NR | Similar | NS | NS | Non SRC-GC | |||
| Piessen et al[35], 2009 | 180 | 59 (32.8) | 83.1 | 28 vs 46 | P = 0.004 | P = 0.004 | Non SRC-GC | |||
| Taghavi et al[44], 2012 | 10246 | 2666 (26) | 59.7 | Similar (Disease-specific survival) | NS | P = 0.15 | Non SRC-GC | |||
| Bamboat et al[51], 2014 | 569 | 210 (36.9) | 61.0 | 49 vs 24/43 (5-y cumulative-mortality) | P < 0.0001 | - | WMD/PD | |||
| Postlewait et al[6], 2015 | 768 | 312 (40.6) | 66.3 | 33.7 mo vs 46.6 mo (OS) | P = 0.011 | NS | Non SRC-GC | |||
| Voron et al[8], 2016 | 1799 | 899 (50) | 73.2 | 26 mo vs 51 mo (median survival) | P < 0.001 | P < 0.041 | Non SRC-GC | |||
Among PCC tumors, the prognostic impact of the relative percentages of an SRC component within the tumors remains controversial[40]. Two studies evaluated the prognostic role of the Verona consensus with marked differences between the distribution of the three categories questioning the reproducibility of the classification (Table 1)[40,47,48]. Bencivenga et al[47] showed that the percentage of SRC was associated with tumor stage and survival in PCC-GC: The percentage of SRC was inversely related to tumor aggressiveness, pT stage (P < 0.001) and the number of positive nodes coded as a continuous variable (P = 0.009). Long-term survival was significantly higher in SRC-type (> 90% SRC) compared with PCC with SRC component (< 90% but > 10% of SRC) and PCC-NOS (< 10% of SRC) tumors[47]. In the other study, on pathological revision no patients with SRC-type (> 90% SRC) were identified[48]. The 5-year overall survival (OS) was significantly higher in PCC with an SRC component (< 90% but > 10% of SRC) compared with PCC-NOS (< 10% of SRC) (63.3% vs 12.7%)[48].
EGC: An overview of studies reporting on the prognostic outcomes of SRC- or PCC-EGC is shown in Table 4. Most studies demonstrated that the prognosis of SRC- or PCC-EGC is similar to or even better than that of other EGC[49-52]. The largest of these studies, including data on 3272 patients, concluded that the prognosis of SRC-EGC was better than that of well-and moderately-differentiated EGC [hazard ratio (HR) for OS = 0.66, 95%CI: 0.44-0.98][53]. In one of the few Western studies, Gronnier et al[54] showed that SRC-EGC was associated with a 5 year-OS benefit (85% vs 76%, P = 0.035) compared to non-SRCEGC, although SRC-EGC was more frequently associated with submucosal invasion[54]. However, the survival benefit in this study was no longer objectivated after multivariable analysis, possibly because of the lower rate of non-cancer-related deaths in the younger SRC group. More studies in Western populations are required to validate further the superior prognostic results of PCC- or SRC-EGC as reported by the Eastern series and should include an analysis according to the new WHO classification and Verona consensus[12,16].
| Ref. | n | n SRC-GC (%) | % LNM | 5-yr survival rate %, SRC-GC vs other | Univariate | Multivariate | Compared to |
| Eastern studies | |||||||
| Maehara et al[49], 1992 | 384 | 28 (7.3) | 10.7 | 100 vs 94.8 | NS | - | Non SRC-GC |
| Kim et al[177], 1994 | 785 | 185 (23.6) | 7.6 | 92.9 vs 83.9/87.3/93.6 | NS | - | WD/MD/PD |
| Otsuji et al[178], 1998 | 568 | 94 (16.5) | 5.3 | 93 vs 76.3 | P < 0.05 | - | Non SRC-GC |
| Yokota et al[179], 1998 | 253 | 41 (16.2) | - | Similar | NS | - | Non SRC-GC |
| Hyung et al[80], 2002 | 933 | 263 (28.2) | 5.7 | 94.2 vs 91.6 | P = 0.01 | - | Non SRC-GC |
| Kim et al[180], 2004 | 561 | 94 (16.8) | 2.1 | 96.3 vs 90.8 | NS | NS | Non SRC-GC |
| Kunisaki et al[185], 2004 | 513 | 120 (23.4) | 9.2 | Better | P = 0.033 | P = 0.036 | Non SRC-GC |
| Ha et al[186], 2008 | 1520 | 388 (25.5) | 9.5 | 99.7 vs 99.1/97.2 | NS/P = 0.019 | - | WMD-PA/PD-MC |
| Zhang et al[45], 2010 | 138 | 49 (35.5) | - | Similar | NS | - | Non SRC-GC |
| Chiu et al[182], 2011 | 579 | 149 (25.7) | 10.7 | 96.1 vs 89.6 | P = 0.01 | - | Non SRC-GC |
| Jiang et al[55], 2011 | 269 | 54 (20.1) | 16.7 | 94.3 vs 90.6 | P = 0.007 | P = 0.011 | Non SRC-GC |
| Kwon et al[50], 2014 | 326 | 51 (15.6) | 9.8 | 84.0 vs 76.0/65.7 (10-yr survival rate) | NS | - | WD-MD/PD-MC |
| Kim et al[52], 2014 | 2085 | 345 (16.5) | 9.0% | Similar (disease-related survival) | NS | - | WD/MD/PD |
| Wang et al[187], 2015 | 334 | 115 (34.4) | 8.5 | 93.9 vs 85.8 | P = 0.027 | 0.001 | UD |
| Chon et al[53], 2017 | 3272 | 1091 (33.3) | - | 95 vs 85 (10-yr survival rate) | P < 0.001 | P = 0.041 (WMD) | WMD-PD |
| Imamura et al[188], 2016 | 746 | 152 (20.4) | 2.0 | 97.4 vs 89.9 | P = 0.012 | P = 0.038 | Non SRC-GC |
| Western studies | |||||||
| Gronnier et al[54], 2013 | 421 | 104 (24.7) | 24,0 | 85 vs 76 | P = 0.035 | NS | Non SRC-GC |
| Bamboat et al[51], 2014 | 437 | 174 (39.8) | - | 0 vs 8/24 (5-disease-specific mortality) | P = 0.001 | - | WMD/PD |
Table 5 presents an overview of studies reporting on the prognostic characteristics of SRC- or PCC-advanced GC (AGC). At an advanced stage, SRC-AGC is associated with deeper tumor invasion, a higher rate of lymph node involvement, an increased potential for diffuse infiltration of the gastric wall (LP), a greater risk of metastatic peritoneal disease, lower rates of R0 resection and higher rates of early disease recurrence[44,55-57]. Whether the dismal prognosis of PCC-GC is related to a more advanced stage of the disease at the time of diagnosis or to inherently more aggressive tumor biology is much debated[35,44]. Results from a large population-based study in the United States demonstrated that after adjustment for stage, SRC histology was not independently associated with a worse prognosis[44]. These findings seem to be confirmed by several other studies that reported a worse prognosis in univariable analysis, but not in multivariable analysis after adjustment for tumor stage[6,56-58]. Critics, however, state that a posteriori adjustment by multivariable analysis results in an oversimplification of the issue. In the absence of any possibility for prospective randomization, some authors noted that a matched case-control analysis should be the methodological tool of choice to clarify this debate[59]. Piessen et al[35] confirmed that SRC histology entailed a worse stage-independent prognosis in patients with GC than other histological subtypes[35].
| Studies | n | n SRC (%) | % LNM | 5-yr survival rate % (PCC-GC vs other) | Univariate | Multivariate | Compared to |
| Eastern studies | |||||||
| Maehara et al[49], 1992 | 1116 | 23 (2.1) | 60.8 | 42.5 vs 37.6 | NS | - | Non SRC-GC |
| Kim et al[177], 1994 | 2917 | 265 (9.1) | 80.8 | 33 vs 45.4/38.8/35.3 | P < 0.05 | - | WD/MD/PD |
| Otsuji et al[178], 1998 | 930 | 60 (6.4) | 63.3 | 44.4 vs 27.5 (10-yr survival rate) | NS | - | Non SRC-GC |
| Yokota et al[179], 1998 | 430 | 52 (12.1) | - | Worse | NS | - | Non SRC-GC |
| Kunisaki et al[185], 2004 | 600 | 54 (9.0) | 57.4 | Similar | NS | - | Non SRC-GC |
| Kim et al[180], 2004 | 1797 | 110 (6.1) | 47.3 | 35.1 vs 39.5 | NS | - | Non SRC-GC |
| Li et al[56], 2007 | 4759 | 662 (13.9) | 75.7 | 42.4 vs 50.1 | 0.009 | NS | Non SRC-GC |
| Chiu et al[182], 2011 | 1860 | 356 (19.1) | 71.6 | 41.5 vs 46.3 | P = 0.018 | - | Non SRC-GC |
| Jiang et al[55], 2011 | 2046 | 157 (7.7) | 64.3 | 31.5 vs 35.7 | NS | NS | Non SRC-GC |
| Zu et al[57], 2014 | 741 | 44 (5.9) | 56.8 | 43.4 vs 87.1/57.1/50.6/62.7 | P = 0.012 | 0.028 | WD/MD/PD/MC |
| Kwon et al[50], 2014 | 443 | 57 (12.9) | 73.7 | 26.0 vs 50.5/38.4 (10-yr survival rate) | P = 0.044 | NS | WD-MD/PD-MC |
| Chon et al[53], 2017 | 1777 | 555 (31.2) | - | 53 vs 58/52 (10-yr survival rate) | P < 0.001 | P < 0.001 | WMD/PD |
| Western studies | |||||||
| Heger et al[58], 2014 | 723 | 235 (32.5) | 63.0 | 26.3 vs 46.6 mo (median survival) | P < 0.001 | P = 0.02 (backward analysis) | Non SRC-GC |
The underlying factors that may cause the discrepancy between the prognostic characteristics of early and advanced PCC-GC remain uncertain. This topic is even more complicated by the geographical differences and potential variability in the molecular tumor characteristics between Western and Eastern populations[60]. Within the group of GCs, early and advanced PCC-GC may represent two distinct entities, each with its own prognostic features[61].
A thorough anamnestic evaluation with emphasis on family history should be performed to detect clinical criteria for hereditary diffuse GC[62]. Because the tumoral spread in PCC-GC mainly occurs within the deeper tissue layers, mostly in the absence of any mucosal alterations, conventional endoscopy and superficial biopsies may miss the diagnosis. Repeated endoscopies should consequently be performed with deep biopsies guided by endoscopic ultrasonography. A CT scan can give useful additional information by identifying areas of the stomach characterized by an increased wall thickness in the case of LP.
In light of the WHO criteria from 2000 for SRC-GC (i.e. more than 50% SRC), the overall reliability of pretherapeutic biopsies to predict specimen histology has been evaluated. Among 254 patients, the presence of SRC in routine pre-therapeutic endoscopic biopsies could accurately predict SRC histology and its associated poor prognosis (Sensitivity: 88.1%, Specificity: 95.4%, Positive predictive value: 92.7%, Negative predictive value: 92.4%)[5]. Future studies evaluating the concordance between pretherapeutic biopsies and specimens in PCC-GC will have to be performed using the new WHO definition and the Verona consensus[12,16].
Positron emission tomography (PET) imaging using fluoro-2-deoxy-D-glucose (FDG) may be helpful to eliminate distant metastases in the case of advanced disease[63,64]. However, PCC-GC has proven to be associated with a lower PET sensitivity and a lower standard uptake value (SUV) than no PCC-GC, with a potential risk of false-negative results[65-67]. In addition, two studies suggested that a higher SUVmax was a predictive factor of poor prognosis in SRC histology[68,69].
Staging laparoscopy is currently recommended by the European Society for Medical Oncology (ESMO) for tumors ≥ stage Ib[70] and by the National Comprehensive Cancer Network (NCCN) for tumors ≥ T1b[71]. Several studies reported high rates of peritoneal carcinomatosis (5%-21%) discovered during surgical exploration after a standard workup, including CT scan in advanced PCC-GC or diffuse tumors[35,72-74]. In the PlASTIC-study, comparing staging laparoscopy and FDG-PET/CT in preoperative workup of locally AGC, treatment intent changed from curative to palliative in 73 patients (19%) after staging laparoscopy (detecting peritoneal or locally non-resectable disease) vs in 12 patients (3%) after FDG-PET/CT (detecting distant metastases)[74]. This risk was 1.5 to 3 times higher than in other tumors[35,74]. Staging laparoscopy has been consequently proposed as an essential tool for pretherapeutic evaluation of PCC-GC[75]. In addition to a complete and systematic exploration of the abdominal cavity, staging laparoscopy provides the possibility to perform a peritoneal lavage with cytology. A positive cytology classifies the disease as stage IV, necessitating a change in therapeutic strategy[30,76,77]. Alternative procedures such as laparo-endoscopic single-site surgery are currently being evaluated to optimize the detection of peritoneal disease. Even with standard staging laparoscopy, lesions on the mesenteric side of the small bowel are still frequently missed[78,79]. A small periumbilical incision to explore the small bowel by means of palpation may be helpful in advanced PCC-GC.
Endoscopic resection: An increasing amount of evidence has been gathered that endoscopic treatment using an endoscopic submucosal dissection could represent a valid option for non-ulcerated undifferentiated lesions, ≤ 2 cm in diameter, limited to the mucosa and without LVI[50,80-82]. Lesions in this category are currently excluded from the absolute indication by the JGCA recommendations due to the lack of sufficient evidence for long-term outcome. Still, they may in the future be included pending the results of the JCOG1009/1010 study[83]. For Western countries, the European Organisation for Research and Treatment of Cancer has defined the indications for endoscopic resection for EGC during the St. Gallen international consensus meeting. For diffuse EGC, gastrectomy is considered mandatory[84]. In the NCCN and ESMO guidelines, undifferentiated tumors (including PC-GC) are contra-indicated for endoscopic treatment[71].
Surgery: Multiple studies have demonstrated a higher risk of positive resection margins due to the specific infiltrative characteristics of PCC-GC and a higher risk of lymph node involvement[6,8,35]. Consequently, some surgical specificities should be proposed.
According to the JCGA, a proximal margin of 5 cm is recommended in cases of AGC with an infiltrative growth pattern (i.e. PCC-GC). A frozen section is advisable in case of doubt. For EGC, a gross resection margin of 2 cm should be respected[83]. A margin of 4 cm is recommended by the NCCN regardless of histological type[71]. According to the ESMO guidelines, a subtotal gastrectomy is indicated if a macroscopic proximal margin of 5 cm can be achieved. For diffuse GC and consequently PCC-GC, a margin of 8 cm should be respected. If not, a total gastrectomy is advised[70]. In the case of an antropyloric location of PCC-GC, a frozen section of the distal margin should be proposed since there is a significant risk of duodenal invasion due to submucosal and subserosal spreading of the tumor[40].
Neither JCGA nor ESMO, nor NCCN guidelines advocate a modification of the D2 Lymphadenectomy without systematic splenectomy for AGC in PCC-GC[70,71,83]. Only the guidelines of the Italian Research Group for Gastric Cancer recommend a D2+ lymphadenectomy (D2 + stations 8p, 12p/b, 13, station 14 v along the mesenteric vein and para-aortic lymph node station 16a2/16b1) for tumors classified as diffuse-type according to the Laurén classification and located in the distal two-thirds of the stomach[85]. Whether or not the extent of lymphadenectomy should be adapted to the higher potential of lymph node metastasis in PCC-GC is questionable and has so far not been investigated by any randomised controlled trial (RCT).
In Western countries, before the FLOT era: The added value of perioperative CT for GC has been demonstrated in two randomized trials[17,86,87]. Perioperative CT allows for an increased R0-resection rate, tumor- and lymph node downstaging and significant improvement in OS. In a post hoc analysis of the MAGIC trial, no statistically significant difference in pathological response rate could be identified between the different histological types according to the Lauren classification. Of note, only 18 % of included patients presented with diffuse-type GC and SRC-presence was not specifically evaluated[88]. Other studies, mainly retrospective, have suggested that Laurén diffuse-type GC and SRC-GC specifically were less chemosensitive than other histological subtypes[8,89-92]. In a large multicentric retrospective cohort study among 1050 patients with SRC-GC defined as tumors with > 50% SRC, Messager et al[92] found that perioperative CT (ECF or 5FU/Cisplatin) did not result in tumor- or lymph node downstaging, nor did it entail any benefit in terms of R0 resection[92]. Perioperative administration of CT was even identified as an independent factor of poor prognosis in the SRC-GC group (HR = 1.4, 95%CI: 1.1-1.9). Several hypotheses could account for these findings: (1) Innate chemoresistance of SRC-GC; (2) Disease progression during neoadjuvant CT; or (3) Toxicity resulting in relative immunodepression with subsequent facilitation of disease progression[93]. The results found by Messager et al[92] highlighted the urgent need for a randomized controlled trial dedicated to identifying optimal therapeutic strategies in the management of SRC-GC. In this context, the phase II/III PRODIGE 19 randomized controlled trial was designed to evaluate whether upfront surgery with adjuvant CT (6 cycles of ECF regimen) would provide a survival benefit compared to perioperative CT (perioperative ECF regimen) in patients with stage Ib-III SRC-GC[94]. The phase II study met its primary endpoint of > 26 mo of 2-year OS in the upfront surgery + adjuvant CT arm. However, 2-year OS rates were 60% in the perioperative arm vs 53.5% in the upfront surgery arm, with a median survival of 39 mo vs 28 mo respectively (exploratory HR = 0.71, 95%CI: 0.40-2.64). Subsequently, phase III was not launched[18].
Another retrospective study, including 235 patients with SRC-GC, defined as tumors with any percentage of SRC, suggested that SRC-GC had a lower clinical (21.1% vs 33.7%, P = 0.001) and histopathological (16.3% vs 28.9%, P < 0.001) response rate to neoadjuvant CT than non-SRC-GC[58]. However, within the cohort of SRC-GC patients that displayed a clinical or histopathological response, the outcome was favorable which led to the conclusion that perioperative CT should not be abandoned for SRC-GC. In the same study, the addition of a taxane-based CT regimen did not have any positive influence on prognosis in SRC-GC patients.
In Western countries in the FLOT era: Taxane-based CT regimens and more specifically the FLOT regimen, have in recent years proven their added value in the peri-operative treatment of GC[17,95,96]. Results concerning the benefit of the FLOT regimen in the treatment of PCC-GC remain, however, controversial: Homan et al[97] found that the pathological complete response rate to FLOT-therapy in intestinal-type GC was higher as compared to diffuse/mixed type GC (30.8% vs 0%, P < 0.05)[97]. Likewise, in the phase II NeoFLOT study, it was demonstrated that when considering near-complete responders (< 10% residual tumor), 85% had an intestinal-type GC in contrast to only 10% and 5% of these patients that exhibited a diffuse and mixed type tumor, respectively[98]. However, the results from the FLOT4 trial demonstrated a beneficiary treatment effect of the FLOT regimen vs ECF regardless of histological type and presence of an SRC component[17]. The definition of SRC in the FLOT trial, was the presence of any SRC in the pathological report, which does not correlate with the recent definition of PCC-GC[12]. The beneficial effect on OS was more pronounced in the SRC-GC than in diffuse GC. These findings are difficult to analyze in the absence of pathological reassessment of the pathological specimen. However, this was an additional argument not to launch the phase III of PRODIGE 19 trial.
In Eastern countries: In Eastern countries where primary surgery followed by adjuvant CT is the standard treatment, three trials evaluating preoperative CT dedicated to LP have been identified[99-102]. The first study with S1 (JCOG02) did not reach its expected survival rate and consequently, no phase III study was performed; the second study with S1+ cisplatin showed interesting tumor response (JOG0210) but did not show any superiority of the neoadjuvant arm in the long term in the phase III (JCOG0501).
Impact of PCC-GC on adjuvant CT: In Eastern countries, adjuvant CT is the preferred therapeutic strategy in GC based on two major trials: The ACTS-GC (Adjuvant CT Trial of TS-1 for GC) trial and the CLASSIC study with CAPOX[103,104]. There was no subgroup analysis based on diffuse or SRC-GC type in both trials. However, in the ACTS-GC trial, the S-1 setting had a significant favorable HR for death in the undifferentiated group (that includes PCC-GC) compared to surgery alone, contrary to the differentiated group, where the effect was not significant[103]. After 5 years, the results were maintained in both subgroups[105]. A retrospective study suggested no tumor response of SRC-GC to either oxaliplatin or docetaxel adjuvant-based CT. In contrast, the mixed SRC-GC group responded to both regimens with even more improved survival with the docetaxel-based regimen[90]. Although the exact definition of SRC-GC and mixed SRC-GC was not mentioned in this study, it supports the fact that PCC-GC could behave differently according to the percentage of SRC and underlines the potential benefit of taxane-based CT in PCC-GC.
Impact of PCC-GC on adjuvant radiotherapy: Several RCT’s evaluated the potential benefit of adjuvant CRT in GC (Intergroup 0116, ARTIST, ARTIST2, CRITICS)[106-110]. They failed to show a favorable outcome in PCC or diffuse GC subgroups. An analysis of the SEER database using a propensity score however showed favorable outcome of adjuvant RT in patients with diffuse-type GC (median survival time: 30 mo with adjuvant RT vs 18 mo without adjuvant RT, P < 0.001, HR: 0.75, P < 0.001). A major bias was the absence of data regarding the use of CT[111].
Impact of PCC-GC on neo adjuvant chemoradiotherapy: Phase III trials evaluating RT or preoperative CRT in GC, excluding the gastroesophageal junction (GEJ), are scarce and small[112-114]. Several phase II trials showed encouraging results in tumor response and survival but this type of strategy has so far been limited by the related toxicity[115-119]. At least two trials are ongoing: TOPGEAR[120] and CRITICS-II[121] with a planned subgroup analysis according to histological type in the CRITICS-II study.
A study analyzing 107 localized GA (n = 45 non-SRC-GC and n = 62 SRC-GC) treated with preoperative CRT showed that the presence of SRC was associated with a lower rate of pCR (11% vs 36%, P = 0.004) which remained significant even with a low percentage of SRC (1%–10%; P = 0.014). The higher the fraction of SRC, the lower the probability of pCR (P = 0.03). Poorly differentiated and SRCC led to shorter OS (P = 0.046 and P = 0.038, respectively)[89].
Preventive setting: The high failure rate of surgical curative therapy for GC and PCC-GC in particular, is mainly due to a high rate of peritoneal recurrence. In this context, a strategy of preventive intraperitoneal chemotherapy (IPC) during the surgical intervention has been hypothesized. Two meta-analyses (including mostly Asian studies) showed a clear benefit of preventive IPC in terms of survival[122,123]. However, no subgroup analysis for PCC-GC was performed. The phase III GASTRICHIP trial (NCT01882933) is currently evaluating the role of oxaliplatin-based HIPEC in addition to curative gastrectomy in patients with GC or Siewert II/III cardia adenocarcinoma with either serosal infiltration, LN positivity, positive peritoneal cytology or perforated tumor. Stratification according to the presence of SRC on pretherapeutic biopsies, has been anticipated[124]. The ongoing PREVENT trial (FLOT-9) (NCT04447352) is a multicenter, randomized, controlled, open-label study including a total of 200 patients with localized and locally advanced non-metastatic diffuse or mixed type (Laurens’s classification) adenocarcinoma of the stomach and Type II/III esogastric junction tumors. Patients undergo perioperative FLOT and are randomized between curative gastrectomy alone and curative gastrectomy + intra operative cisplatin-based HIPEC[125]. In Japan, the PHOENIX-GC2 Trial will evaluate the impact of IPC as adjuvant or perioperative CT for patients with type 4 scirrhous GC in addition to S1 CT[126].
Curative setting: In a curative setting, cytoreductive surgery (CRS) plus HIPEC has been strongly recommended for AGC by a panel of international experts[127,128]. However, controversy concerning this topic remains, with further high-quality evidence being expected to confirm the value of this treatment strategy, which could be of particular interest for PCC-GC.
At present, no published RCT has compared CRS + HIPEC vs CT alone. Two ongoing randomized phase III trials evaluate the role of surgery in limited- metastatic adenocarcinoma of the stomach or esophagogastric junction in patients responding to CT and will include patients with peritoneal carcinomatosis[129,130]. In the RENAISSANCE trial no stratification based on histological type has been anticipated and HIPEC is not described in the protocol (NCT02578368)[129].In the SURGIGAST trial, stratification based on histological type (PCC-GC on biopsy) has been anticipated (NCT03042169)[130].
In the multicenter, open-label, phase III PERISCOPE II trial, patients with peritoneal metastasis are currently randomized between CT alone vs CRS + HIPEC with CT. Study completion is expected by October 2022[131]. Stratification based on the main histological subtype (diffuse vs intestinal) has been anticipated.
Based upon the available evidence, it is presumed that for GC in general, only patients with a peritoneal cancer index (PCI) < 12, who display a clinical response after neoadjuvant CT and in whom no diffuse bowel involvement is found, may benefit from the added value of CRS + HIPEC[132,133]. For PCC-GC, little to no specific selection criteria have been proposed so far. In a retrospective study on 89 patients, Chia et al[134] demonstrated that after treatment with CRS + HIPEC, non-PCC-GC patients had a better OS (21.8 mo vs 13.2 mo, P = 0.0214) compared to PCC-GC patients. The authors suggested that if complete CRS was achievable in patients with a PCI < 7, the presence of an SRC component should not be considered as a contra-indication for CRS + HIPEC[134].
In 2018, Bonnot et al[135] published the results from the large multicenter retrospective CYTO-CHIP study, which evaluated the survival results of CRS compared to CRS + HIPEC in patients with AGC with peritoneal involvement[135]. Only patients with a complete CRS (CC-0 or CC-1) were included in the study. After propensity scored weighting, this study showed that CRS + HIPEC was associated with an increased OS and the potential of disease eradication compared to CRS alone. Subgroup analysis confirmed the superiority of CRS + HIPEC in patients with PCC-GC defined according to WHO classification[11]. An ancillary study recently published showed that PCC-GC was associated with poorer OS (HR: 0.43, P = 0.003), as were pN3, PCI, and resection with a completeness of cytoreduction score of 1, whereas HIPEC was associated with improved OS (HR: 0.52; P < 0.001). The benefit of CRS-HIPEC over CRS alone was consistent, irrespective of histology, with a median OS of 16.7 mo vs 11.3 mo (HR: 0.60, P = 0.018) in the PCC-GC group, and 34.5 mo vs 14.3 mo (HR: 0.43, P = 0.003) in the non-PCC-GC group. Non PCC-GC and HIPEC were independently associated with improved recurrence-free survival and fewer peritoneal recurrences. In patients who underwent HIPEC, PCI values < 7 and < 13 were predictive of OS in PCC-GC and non PCC-GC populations, respectively[136]. Consequently, those patients should be well-selected to avoid the excess morbidity rate associated with an unnecessary exploratory laparotomy[137].
CT: Several studies demonstrated that SRC-GC had different infiltrative and metastatic mechanisms than non-SRC-CG. It lacked free ribosomes but were rich in lysosomes and mucus impeding anticancer drugs from getting to the cell[20,138]. In a metastatic setting, there are few data concerning the chemosensitivity of PCC-GC. Rougier et al[139] reported among 87 patients with metastatic or recurrent tumor (n = 57) or with locally AGC (n = 30) a significantly poorer response rate of CT using infusional 5-FU and cisplatinum for linitis plastic or SRC histology (P = 0.003 and P = 0.16, respectively)[139].
A retrospective analysis of the FLAGS trial suggested that survival was improved among patients with advanced diffuse GC treated with S-1 and cisplatin compared to 5-FU and cisplatin[140]. A dedicated phase III trial compared both regimens in patients with metastatic diffuse gastric and GEJ adenocarcinoma previously untreated[141]. However, both regimens were similar in efficacy and safety and the primary endpoint was not met. A study of the AGEO evaluated the place of docetaxel added to 5-FU, leucovorin and oxaliplatin (TEFOX) as first-line treatment in 65 patients with metastatic or locally advanced non-resectable gastric or GEJ SRC-GC including 17 LP. This regimen gave an interesting response rate of 66% with an OS of 14.3 mo. Interestingly, 26 patients (40%) initially unresectable had secondary resection (n = 24) or radiotherapy (n = 2) with curative intent[142].
PIPAC: PIPAC is a recently developed promising technique that allows for homogeneous loco-regional application of intraperitoneal CT at lower doses than achievable in conventional HIPEC[143]. This technique could offer a valuable alternative for patients with unresectable peritoneal disease from GC and with PCI-scores that are considered as too high for CRS + HIPEC (PCI > 7 or 12 depending on histological type). Several retrospective studies have evaluated the feasibility of this technique on patients with unresectable peritoneal metastasis from GC. The majority of patients included in these studies were affected by an SRC histology and the results show that PIPAC treatment (with low-dose cisplatin + doxorubicin) is associated with improved survival, without compromising the quality of life[143-145]. Further results from the randomized controlled multicenter phase II PIPAC EstoK 01 trial evaluated the interest of PIPAC in addition to intravenous CT and are awaited[143].
Targeted drugs in gastric SRCC: Due to some specific oncogenic pathways in GC, the efficacy of several targeted agents has been tested in recent trials, in which SRC histology has only rarely been the subject of subanalysis. On the other hand, diffuse type GC has been evaluated frequently within these trials.
Human epidermal growth factor receptor 2 targeting agents: The incidence of human epidermal growth factor receptor 2 (HER2) amplification in GC ranges from 12% to 22.1%. It is more often noted in intestinal GC than diffuse-type GC and characterized by a more frequent location in the proximal stomach and gastroesophageal junction[146-150]. Although still controversial, HER2 positive status is, in general, associated with a poor outcome and more aggressive disease[147,149,150]. Some authors found that the unfavorable prognostic value of HER2 positivity was present in intestinal-type GC, but not in diffuse-type GC[151,152]. In PCC-GC, the diagnosis of HER2 status can be somewhat troublesome due to the presence of a marginalized cytoplasm and nucleus, entailing a frequent misinterpretation of intense, non-specific staining[153-155]. The phase III ToGa trial demonstrated the added value of the humanized monoclonal antibody against HER2 (Trastuzumab) in combination with CT (capecitabine or 5-FU and cisplatin) compared to CT alone in HER2-positive AGC[156]. Of note, a sub-group analysis among patients with a diffuse-type tumor showed no benefit of trastuzumab, although the number of patients in this sub-analysis was quite low. A Korean study found resistance to trastuzumab of more than 50% among 13 patients with SRC-GC who were HER2 positive, with a low HER2 amplification index being identified as an independent molecular predictor for trastuzumab resistance in a multivariate analysis[157]. Despite these findings, it remains recommended to routinely test all patients with GC for HER2 amplification, regardless of the histological type[146,156,158]. Future studies are required to investigate more profoundly a potential benefit of trastuzumab in PCC-GC.
Anti-angiogenic agents: The randomized phase III AVAGAST trial evaluated the effect of bevacizumab [a humanized anti-vascular endothelial growth factor (VEGF) monoclonal antibody] in combination with CT (fluoropyrimidine-cisplatin) as first-line therapy in AGC. Although AVAGAST did not reach its primary objective (OS of 10.1 mo in the placebo arm vs 12.1 mo in the bevacizumab arm, P = 0.1002), the addition of bevacizumab to CT was found to be associated with a significant increase in progression-free survival (PFS) and overall response rate[159]. An additional analysis according to disease subtype, suggested a benefit of bevacizumab in a subset of non-Asian patients with the diffuse histologic type (HR = 0.68; 95%CI: 0.48-0.97)[159]. The phase III REGARDS trial compared ramucirumab (an anti-VEGF-R2 antibody) vs best supportive care after first-line platinum-containing or fluoropyrimidine-containing CT in AGC or gastro-esophageal junction adenocarcinoma. Ramucirumab provided a significant benefit in terms of OS (5.2 mo vs 3.8 mo, HR = 0.78, 95%CI: 0.603-0.998)[160]. In subgroup analysis, a significant benefit was found for diffuse-type GC (HR = 0.56; 95%CI: 0.36-0.85), but not for the intestinal-type (HR = 1.009, 95%CI: 0.583-1.745), suggesting a higher sensitivity to anti-angiogenics. Conversely, the RAINBOW trial showed that for ramucirumab in combination with paclitaxel in a second-line treatment, the OS benefit concerned only the intestinal histological subtype [HR: 0.705 (0.534–0.932)][161]. Supplemental data are needed to establish the role of anti-angiogenic targeted therapies in patients with diffuse-type GC. Currently, no data concerning the role of anti-angiogenic therapies in the therapy of PCC-GC are available.
Anti-epidermal growth factor receptor: Epidermal growth factor receptor (EGFR) expression has been identified as an independent predictor of poor prognosis in patients with PCC-GC compared to non-PCC-GC patients[162]. Data from the EXPAND and REAL3 trials have suggested no additional benefit of anti-EGFR treatment in combination with CT for AGC[163,164]. In a subgroup analysis of the EXPAND trial in function of the histological subtype, it was even found that anti-EGFR could be harmful in diffuse-type tumors (HR for OS: 1.44, 95%CI: 1.01-2.03)[163].
Since phospho-mammalian target of rapamycin (mTOR) is expressed in 60% of intestinal and 64% of diffuse-type GC, mTOR inhibitors were considered an interesting therapeutic option from a biological point of view[165]. However, results from the phase III GRANITE-1 trial showed no benefit of everolimus (an oral mTOR-inhibitor) on OS compared to best supportive care for previously treated AGC[166]. In a subgroup analysis, no benefit in diffuse-type GC was found either.
In advanced gastric/gastro-esophageal junction and esophageal adenocarcinoma patients expressing CLDN18.2, adding zolbetuximab to first-line EOX provided longer PFS and OS vs EOX alone in a phase 2 trial[167]. Interestingly, the vast majority of these populations had diffuse- or mixed type GC. Zolbetuximab is being evaluated in phase III studies based on clinical benefits observed in the overall population and in patients with moderate-to-strong CLDN18.2 expression in > 70% of tumor cells.
Among new treatment strategies for GC, immunotherapy, and more specifically, PD-L1 inhibitors have proven to be the most promising. PD-L1 is expressed in 30% to 63% of GC[168,169]. The results of the CheckMate 649 study demonstrated the superiority of nivolumab in combination with CT compared to CT alone. In a study population of patients with HER2 negative, previously untreated, unresectable advanced or metastatic GC or gastro-esophageal junction cancer, nivolumab in combination with CT (XELOX or FOLFOX) resulted in significantly improved OS and PFS vs CT in patients whose tumors expressed a PD-L1 combined positive score (CPS) ≥ 5 (HR for OS = 0.71, 98.4%CI: 0.59–0.86 and HR for PFS = 0.68, 98%CI: 0.56–0.81). This survival benefit was also observed in patients with a PD-L1 CPS ≥ 1 and in the all-randomized population[170]. The rate of patients with SRC-GC or diffuse tumors was close between patients with a CPS ≥ 5 and the overall population[170]. However, other studies found that in SRC histology, PD-L1 CPS > 1 was significantly less observed[171]. The question remains how the recent findings of the CheckMate 649 trial could be applied to PCC-GC. A group of specifically selected PCC-GC patients with S-I may benefit from immunotherapy. However, Hirotsu et al[172] reported that PCC-GC exhibits high MSI at low frequencies[172].
In contrast to GC in general, the relative incidence of PCC-GC has risen over the past few decades. PCC-GC represents a distinct pathological entity within the GC spectrum, characterized by specific epidemiological and clinical features, including younger age at presentation and a significantly worse prognosis, primarily due to peritoneal dissemination early in the disease. In light of these distinct features, the recently redefined pathological definition of PCC-GC by the WHO and the European chapter of IGCA will facilitate methodological standardization in future studies which in turn will help to identify which therapeutic strategies for GC in general apply to PCC-GC. We believe that the updated definition will help standardize future research concerning the prognostic results of SRC-ECG in Western populations and evaluate the correlation between pre-therapeutic biopsies and the final pathology result. Con
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kotelevets SM, Russia; Marano L, Italy S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol. 2020;18:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 982] [Article Influence: 196.4] [Reference Citation Analysis (1)] |
| 2. | Amorosi A, Bianchi S, Buiatti E, Cipriani F, Palli D, Zampi G. Gastric cancer in a high-risk area in Italy. Histopathologic patterns according to Lauren's classification. Cancer. 1988;62:2191-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Marrelli D, Pedrazzani C, Morgagni P, de Manzoni G, Pacelli F, Coniglio A, Marchet A, Saragoni L, Giacopuzzi S, Roviello F; Italian Research Group for Gastric Cancer. Changing clinical and pathological features of gastric cancer over time. Br J Surg. 2011;98:1273-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973-2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Piessen G, Amielh D, Messager M, Vinatier E, Leteurtre E, Triboulet JP, Mariette C. Is pretreatment endoscopic biopsy a good predictor of signet ring cell histology in gastric carcinoma? World J Surg. 2012;36:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Postlewait LM, Squires MH 3rd, Kooby DA, Poultsides GA, Weber SM, Bloomston M, Fields RC, Pawlik TM, Votanopoulos KI, Schmidt CR, Ejaz A, Acher AW, Worhunsky DJ, Saunders N, Swords D, Jin LX, Cho CS, Winslow ER, Cardona K, Staley CA, Maithel SK. The Prognostic Value of Signet-Ring Cell Histology in Resected Gastric Adenocarcinoma. Ann Surg Oncol. 2015;22 Suppl 3:S832-S839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Pyo JH, Ahn S, Lee H, Min BH, Lee JH, Shim SG, Choi MG, Sohn TS, Bae JM, Kim KM, Yeon S, Jung SH, Kim JJ, Kim S. Clinicopathological Features and Prognosis of Mixed-Type T1a Gastric Cancer Based on Lauren's Classification. Ann Surg Oncol. 2016;23:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Voron T, Messager M, Duhamel A, Lefevre J, Mabrut JY, Goere D, Meunier B, Brigand C, Hamy A, Glehen O, Mariette C, Paye F. Is signet-ring cell carcinoma a specific entity among gastric cancers? Gastric Cancer. 2016;19:1027-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Oota K, Sobin H. Histological typing of gastric and oesophageal tumors, in international classification of tumors. WHO Editor WHO : Geneva, 1977. |
| 10. | Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. Fourth Edition, 2010. |
| 11. | Hamilton SR, Aaltonen L. Pathology and Genetics of Tumours of the Digestive System. World Health Organization Classification of Tumours. IARC, Lyon: France, 2000. |
| 12. | WHO Classification of Tumours Editorial Board (eds). Digestive system tumours. 5th ed. Lyon: IARC Press, 2019. |
| 13. | Watanabe H, Jass JR, Sobin LH (eds). Histological typing of oesophageal and gastric tumours. 2nd ed. WHO: International histological classification of tumours. Springer-Verlag, Berlin Heidelberg, 1990. |
| 14. | Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a hiato-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4323] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 15. | Ming SC. Gastric carcinoma. A pathobiological classification. Cancer. 1977;39:2475-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 16. | Mariette C, Carneiro F, Grabsch HI, van der Post RS, Allum W, de Manzoni G; European Chapter of International Gastric Cancer Association. Consensus on the pathological definition and classification of poorly cohesive gastric carcinoma. Gastric Cancer. 2019;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 17. | Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, Kopp HG, Mayer F, Haag GM, Luley K, Lindig U, Schmiegel W, Pohl M, Stoehlmacher J, Folprecht G, Probst S, Prasnikar N, Fischbach W, Mahlberg R, Trojan J, Koenigsmann M, Martens UM, Thuss-Patience P, Egger M, Block A, Heinemann V, Illerhaus G, Moehler M, Schenk M, Kullmann F, Behringer DM, Heike M, Pink D, Teschendorf C, Löhr C, Bernhard H, Schuch G, Rethwisch V, von Weikersthal LF, Hartmann JT, Kneba M, Daum S, Schulmann K, Weniger J, Belle S, Gaiser T, Oduncu FS, Güntner M, Hozaeel W, Reichart A, Jäger E, Kraus T, Mönig S, Bechstein WO, Schuler M, Schmalenberg H, Hofheinz RD; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948-1957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 974] [Cited by in RCA: 1646] [Article Influence: 274.3] [Reference Citation Analysis (0)] |
| 18. | Eveno C, Adenis A, Bouche O, Le Malicot K, Hautefeuille V, Faroux R, Bidault AT, Egreteau J, Meunier B, Mabro M, Carrere N, Barriere N, Ben Abdelghani M, Mauvais F, Di Fiore F, Malka D, Manfredi S, Piessen G. Adjuvant chemotherapy vs perioperative chemotherapy (CTx) for resectable gastric signet ring cell (SRC) gastric cancer: A multicenter, randomized phase II study (PRODIGE 19). J Clin Oncol. 2019;37:4019-4019. |
| 19. | Yao JC, Tseng JF, Worah S, Hess KR, Mansfield PF, Crane CH, Schnirer II, Reddy S, Chiang SS, Najam A, Yu C, Giacco GG, Xie K, Wu TT, Feig BW, Pisters PW, Ajani JA. Clinicopathologic behavior of gastric adenocarcinoma in Hispanic patients: analysis of a single institution's experience over 15 years. J Clin Oncol. 2005;23:3094-3103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Yang XF, Yang L, Mao XY, Wu DY, Zhang SM, Xin Y. Pathobiological behavior and molecular mechanism of signet ring cell carcinoma and mucinous adenocarcinoma of the stomach: a comparative study. World J Gastroenterol. 2004;10:750-754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Bringeland EA, Wasmuth HH, Mjønes P, Myklebust TÅ, Grønbech JE. A population-based study on incidence rates, Lauren distribution, stage distribution, treatment, and long-term outcomes for gastric adenocarcinoma in Central Norway 2001-2011. Acta Oncol. 2017;56:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Baiocchi GL, Tiberio GA, Minicozzi AM, Morgagni P, Marrelli D, Bruno L, Rosa F, Marchet A, Coniglio A, Saragoni L, Veltri M, Pacelli F, Roviello F, Nitti D, Giulini SM, De Manzoni G. A multicentric Western analysis of prognostic factors in advanced, node-negative gastric cancer patients. Ann Surg. 2010;252:70-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gan. 1968;59:251-258. [PubMed] |
| 24. | Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C, Yamamoto Y, Ohashi Y. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 344] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 25. | Davessar K, Pezzullo JC, Kessimian N, Hale JH, Jauregui HO. Gastric adenocarcinoma: prognostic significance of several pathologic parameters and histologic classifications. Hum Pathol. 1990;21:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Luebke T, Baldus SE, Grass G, Bollschweiler E, Thiele J, Dienes HP, Hoelscher AH, Moenig SP. Histological grading in gastric cancer by Ming classification: correlation with histopathological subtypes, metastasis, and prognosis. World J Surg. 2005;29:1422-7; discussion 1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Kitamura K, Beppu R, Anai H, Ikejiri K, Yakabe S, Sugimachi K, Saku M. Clinicopathologic study of patients with Borrmann type IV gastric carcinoma. J Surg Oncol. 1995;58:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Kodera Y, Ito S, Mochizuki Y, Yamamura Y, Misawa K, Ohashi N, Nakayama G, Koike M, Fujiwara M, Nakao A. The number of metastatic lymph nodes is a significant risk factor for bone metastasis and poor outcome after surgery for linitis plastica-type gastric carcinoma. World J Surg. 2008;32:2015-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Maehara Y, Moriguchi S, Orita H, Kakeji Y, Haraguchi M, Korenaga D, Sugimachi K. Lower survival rate for patients with carcinoma of the stomach of Borrmann type IV after gastric resection. Surg Gynecol Obstet. 1992;175:13-16. [PubMed] |
| 30. | Schauer M, Peiper M, Theisen J, Knoefel W. Prognostic factors in patients with diffuse type gastric cancer (linitis plastica) after operative treatment. Eur J Med Res. 2011;16:29-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Kim EY, Yoo HM, Song KY, Park CH. Limited significance of curative surgery in Borrmann type IV gastric cancer. Med Oncol. 2016;33:69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Feng J, Al-Abbadi M, Kodali U, Dhar R. Cytologic diagnosis of gastric linitis plastica by endoscopic ultrasound guided fine-needle aspiration. Diagn Cytopathol. 2006;34:177-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Wachtel MS, Zhang Y, Chiriva-Internati M, Frezza EE. Different regression equations relate age to the incidence of Lauren types 1 and 2 stomach cancer in the SEER database: these equations are unaffected by sex or race. BMC Cancer. 2006;6:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1468] [Article Influence: 163.1] [Reference Citation Analysis (0)] |
| 35. | Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C. Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation. Ann Surg. 2009;250:878-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 36. | Endo K, Sakurai M, Kusumoto E, Uehara H, Yamaguchi S, Tsutsumi N, Ikejiri K. Biological significance of localized Type IV scirrhous gastric cancer. Oncol Lett. 2012;3:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 37. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4850] [Article Influence: 440.9] [Reference Citation Analysis (2)] |
| 38. | dos Santos NR, Seruca R, Constância M, Seixas M, Sobrinho-Simões M. Microsatellite instability at multiple loci in gastric carcinoma: clinicopathologic implications and prognosis. Gastroenterology. 1996;110:38-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 159] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 39. | Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, Ye XS, Do IG, Liu S, Gong L, Fu J, Jin JG, Choi MG, Sohn TS, Lee JH, Bae JM, Kim ST, Park SH, Sohn I, Jung SH, Tan P, Chen R, Hardwick J, Kang WK, Ayers M, Hongyue D, Reinhard C, Loboda A, Kim S, Aggarwal A. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med. 2015;21:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1071] [Cited by in RCA: 1579] [Article Influence: 157.9] [Reference Citation Analysis (0)] |
| 40. | Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 256] [Reference Citation Analysis (0)] |
| 41. | Lei Z, Tan IB, Das K, Deng N, Zouridis H, Pattison S, Chua C, Feng Z, Guan YK, Ooi CH, Ivanova T, Zhang S, Lee M, Wu J, Ngo A, Manesh S, Tan E, Teh BT, So JB, Goh LK, Boussioutas A, Lim TK, Flotow H, Tan P, Rozen SG. Identification of molecular subtypes of gastric cancer with different responses to PI3-kinase inhibitors and 5-fluorouracil. Gastroenterology. 2013;145:554-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 340] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 42. | Pectasides E, Stachler MD, Derks S, Liu Y, Maron S, Islam M, Alpert L, Kwak H, Kindler H, Polite B, Sharma MR, Allen K, O'Day E, Lomnicki S, Maranto M, Kanteti R, Fitzpatrick C, Weber C, Setia N, Xiao SY, Hart J, Nagy RJ, Kim KM, Choi MG, Min BH, Nason KS, O'Keefe L, Watanabe M, Baba H, Lanman R, Agoston AT, Oh DJ, Dunford A, Thorner AR, Ducar MD, Wollison BM, Coleman HA, Ji Y, Posner MC, Roggin K, Turaga K, Chang P, Hogarth K, Siddiqui U, Gelrud A, Ha G, Freeman SS, Rhoades J, Reed S, Gydush G, Rotem D, Davison J, Imamura Y, Adalsteinsson V, Lee J, Bass AJ, Catenacci DV. Genomic Heterogeneity as a Barrier to Precision Medicine in Gastroesophageal Adenocarcinoma. Cancer Discov. 2018;8:37-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 242] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 43. | Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, Liu XQ, Sher X, Jung H, Lee M, Lee S, Park SH, Park JO, Park YS, Lim HY, Lee H, Choi M, Talasaz A, Kang PS, Cheng J, Loboda A, Lee J, Kang WK. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 1165] [Article Influence: 166.4] [Reference Citation Analysis (0)] |
| 44. | Taghavi S, Jayarajan SN, Davey A, Willis AI. Prognostic significance of signet ring gastric cancer. J Clin Oncol. 2012;30:3493-3498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 197] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 45. | Zhang M, Zhu G, Zhang H, Gao H, Xue Y. Clinicopathologic features of gastric carcinoma with signet ring cell histology. J Gastrointest Surg. 2010;14:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Murakami T. Early cancer of the stomach. World J Surg. 1979;3:685-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Bencivenga M, Treppiedi E, Verlato G, Mengardo V, Giacopuzzi S, de Manzoni G. The amount of cells with Signet Ring Cell morphology has a prognostic impact in poorly cohesive gastric carcinoma. Eur J Cancer. 2018;92 Suppl 2:S6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Roviello F, Marano L, Ambrosio MR, Resca L, D'Ignazio A, Petrelli F, Petrioli R, Costantini M, Polom K, Macchiarelli R, Biviano I, Marrelli D. Signet ring cell percentage in poorly cohesive gastric cancer patients: A potential novel predictor of survival. Eur J Surg Oncol. 2022;48:561-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 49. | Maehara Y, Sakaguchi Y, Moriguchi S, Orita H, Korenaga D, Kohnoe S, Sugimachi K. Signet ring cell carcinoma of the stomach. Cancer. 1992;69:1645-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 50. | Kwon KJ, Shim KN, Song EM, Choi JY, Kim SE, Jung HK, Jung SA. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer. 2014;17:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 51. | Bamboat ZM, Tang LH, Vinuela E, Kuk D, Gonen M, Shah MA, Brennan MF, Coit DG, Strong VE. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Ann Surg Oncol. 2014;21:1678-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 52. | Kim BS, Oh ST, Yook JH, Kim BS. Signet ring cell type and other histologic types: differing clinical course and prognosis in T1 gastric cancer. Surgery. 2014;155:1030-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Chon HJ, Hyung WJ, Kim C, Park S, Kim JH, Park CH, Ahn JB, Kim H, Chung HC, Rha SY, Noh SH, Jeung HC. Differential Prognostic Implications of Gastric Signet Ring Cell Carcinoma: Stage Adjusted Analysis From a Single High-volume Center in Asia. Ann Surg. 2017;265:946-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 54. | Gronnier C, Messager M, Robb WB, Thiebot T, Louis D, Luc G, Piessen G, Mariette C; FREGAT working group-FRENCH. Is the negative prognostic impact of signet ring cell histology maintained in early gastric adenocarcinoma? Surgery. 2013;154:1093-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Jiang CG, Wang ZN, Sun Z, Liu FN, Yu M, Xu HM. Clinicopathologic characteristics and prognosis of signet ring cell carcinoma of the stomach: results from a Chinese mono-institutional study. J Surg Oncol. 2011;103:700-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 56. | Li C, Kim S, Lai JF, Hyung WJ, Choi WH, Choi SH, Noh SH. Advanced gastric carcinoma with signet ring cell histology. Oncology. 2007;72:64-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 57. | Zu H, Wang H, Li C, Xue Y. Clinicopathologic characteristics and prognostic value of various histological types in advanced gastric cancer. Int J Clin Exp Pathol. 2014;7:5692-5700. [PubMed] |
| 58. | Heger U, Blank S, Wiecha C, Langer R, Weichert W, Lordick F, Bruckner T, Dobritz M, Burian M, Springfeld C, Grenacher L, Siewert JR, Büchler M, Ott K. Is preoperative chemotherapy followed by surgery the appropriate treatment for signet ring cell containing adenocarcinomas of the esophagogastric junction and stomach? Ann Surg Oncol. 2014;21:1739-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 59. | Piessen G, Messager M, Robb WB, Bonnetain F, Mariette C. Gastric signet ring cell carcinoma: how to investigate its impact on survival. J Clin Oncol. 2013;31:2059-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Lin SJ, Gagnon-Bartsch JA, Tan IB, Earle S, Ruff L, Pettinger K, Ylstra B, van Grieken N, Rha SY, Chung HC, Lee JS, Cheong JH, Noh SH, Aoyama T, Miyagi Y, Tsuburaya A, Yoshikawa T, Ajani JA, Boussioutas A, Yeoh KG, Yong WP, So J, Lee J, Kang WK, Kim S, Kameda Y, Arai T, Zur Hausen A, Speed TP, Grabsch HI, Tan P. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut. 2015;64:1721-1731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 187] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 61. | Pernot S, Voron T, Perkins G, Lagorce-Pages C, Berger A, Taieb J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J Gastroenterol. 2015;21:11428-11438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 178] [Cited by in RCA: 231] [Article Influence: 23.1] [Reference Citation Analysis (3)] |
| 62. | van der Post RS, Vogelaar IP, Carneiro F, Guilford P, Huntsman D, Hoogerbrugge N, Caldas C, Schreiber KE, Hardwick RH, Ausems MG, Bardram L, Benusiglio PR, Bisseling TM, Blair V, Bleiker E, Boussioutas A, Cats A, Coit D, DeGregorio L, Figueiredo J, Ford JM, Heijkoop E, Hermens R, Humar B, Kaurah P, Keller G, Lai J, Ligtenberg MJ, O'Donovan M, Oliveira C, Pinheiro H, Ragunath K, Rasenberg E, Richardson S, Roviello F, Schackert H, Seruca R, Taylor A, Ter Huurne A, Tischkowitz M, Joe ST, van Dijck B, van Grieken NC, van Hillegersberg R, van Sandick JW, Vehof R, van Krieken JH, Fitzgerald RC. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet. 2015;52:361-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 395] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 63. | Sim SH, Kim YJ, Oh DY, Lee SH, Kim DW, Kang WJ, Im SA, Kim TY, Kim WH, Heo DS, Bang YJ. The role of PET/CT in detection of gastric cancer recurrence. BMC Cancer. 2009;9:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 64. | Chen J, Cheong JH, Yun MJ, Kim J, Lim JS, Hyung WJ, Noh SH. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer. 2005;103:2383-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 146] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 65. | Dassen AE, Lips DJ, Hoekstra CJ, Pruijt JF, Bosscha K. FDG-PET has no definite role in preoperative imaging in gastric cancer. Eur J Surg Oncol. 2009;35:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 66. | Stahl A, Ott K, Weber WA, Becker K, Link T, Siewert JR, Schwaiger M, Fink U. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging. 2003;30:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 225] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 67. | Ott K, Herrmann K, Lordick F, Wieder H, Weber WA, Becker K, Buck AK, Dobritz M, Fink U, Ulm K, Schuster T, Schwaiger M, Siewert JR, Krause BJ. Early metabolic response evaluation by fluorine-18 fluorodeoxyglucose positron emission tomography allows in vivo testing of chemosensitivity in gastric cancer: long-term results of a prospective study. Clin Cancer Res. 2008;14:2012-2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 68. | Chon HJ, Kim C, Cho A, Kim YM, Jang SJ, Kim BO, Park CH, Hyung WJ, Ahn JB, Noh SH, Yun M, Rha SY. The clinical implications of FDG-PET/CT differ according to histology in advanced gastric cancer. Gastric Cancer. 2019;22:113-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 69. | Pak KH, Yun M, Cheong JH, Hyung WJ, Choi SH, Noh SH. Clinical implication of FDG-PET in advanced gastric cancer with signet ring cell histology. J Surg Oncol. 2011;104:566-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38-v49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1118] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 71. | NCCN. Clinical Practice Guidelines in Oncology (NCCN Guidelines®)Gastric CancerVersion 2.2022. [cited 11 January 2022]. Available from: https://www.nccn.org/. |
| 72. | Ikeguchi M, Oka A, Tsujitani S, Maeta M, Kaibara N. Relationship between area of serosal invasion and intraperitoneal free cancer cells in patients with gastric cancer. Anticancer Res. 1994;14:2131-2134. [PubMed] |
| 73. | Kuramoto M, Shimada S, Ikeshima S, Matsuo A, Yagi Y, Matsuda M, Yonemura Y, Baba H. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg. 2009;250:242-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 74. | Gertsen EC, Brenkman HJF, van Hillegersberg R, van Sandick JW, van Berge Henegouwen MI, Gisbertz SS, Luyer MDP, Nieuwenhuijzen GAP, van Lanschot JJB, Lagarde SM, Wijnhoven BPL, de Steur WO, Hartgrink HH, Stoot JHMB, Hulsewe KWE, Spillenaar Bilgen EJ, van Det MJ, Kouwenhoven EA, van der Peet DL, Daams F, van Grieken NCT, Heisterkamp J, van Etten B, van den Berg JW, Pierie JP, Eker HH, Thijssen AY, Belt EJT, van Duijvendijk P, Wassenaar E, van Laarhoven HWM, Wevers KP, Hol L, Wessels FJ, Haj Mohammad N, van der Meulen MP, Frederix GWJ, Vegt E, Siersema PD, Ruurda JP; PLASTIC Study Group. 18F-Fludeoxyglucose-Positron Emission Tomography/Computed Tomography and Laparoscopy for Staging of Locally Advanced Gastric Cancer: A Multicenter Prospective Dutch Cohort Study (PLASTIC). JAMA Surg. 2021;156:e215340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 75. | Lowy AM, Mansfield PF, Leach SD, Ajani J. Laparoscopic staging for gastric cancer. Surgery. 1996;119:611-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 76. | Ikeguchi M, Yamamoto O, Kaibara N. Management protocol for scirrhous gastric cancer. In Vivo. 2004;18:577-580. [PubMed] |
| 77. | Kodera Y, Yamamura Y, Ito S, Kanemitsu Y, Shimizu Y, Hirai T, Yasui K, Kato T. Is Borrmann type IV gastric carcinoma a surgical disease? J Surg Oncol. 2001;78:175-81; discussion 181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 78. | Najah H, Lo Dico R, Grienay M, Dohan A, Dray X, Pocard M. Single-incision flexible endoscopy (SIFE) for detection and staging of peritoneal carcinomatosis. Surg Endosc. 2016;30:3808-3815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Najah H, Lo Dico R, Eveno C, Pocard M. Laparo-endoscopic single site surgery for peritoneal carcinomatosis detection and staging (with video). J Visc Surg. 2017;154:133-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 80. | Hyung WJ, Noh SH, Lee JH, Huh JJ, Lah KH, Choi SH, Min JS. Early gastric carcinoma with signet ring cell histology. Cancer. 2002;94:78-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 139] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 81. | Yamamoto Y, Fujisaki J, Hirasawa T, Ishiyama A, Yoshimoto K, Ueki N, Chino A, Tsuchida T, Hoshino E, Hiki N, Fukunaga T, Sano T, Yamaguchi T, Takahashi H, Miyata S, Yamamoto N, Kato Y, Igarashi M. Therapeutic outcomes of endoscopic submucosal dissection of undifferentiated-type intramucosal gastric cancer without ulceration and preoperatively diagnosed as 20 millimetres or less in diameter. Dig Endosc. 2010;22:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Wang Z, Zhang X, Hu J, Zeng W, Liang J, Zhou H, Zhou Z. Predictive factors for lymph node metastasis in early gastric cancer with signet ring cell histology and their impact on the surgical strategy: analysis of single institutional experience. J Surg Res. 2014;191:130-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1336] [Article Influence: 334.0] [Reference Citation Analysis (2)] |
| 84. | Lutz MP, Zalcberg JR, Ducreux M, Ajani JA, Allum W, Aust D, Bang YJ, Cascinu S, Hölscher A, Jankowski J, Jansen EP, Kisslich R, Lordick F, Mariette C, Moehler M, Oyama T, Roth A, Rueschoff J, Ruhstaller T, Seruca R, Stahl M, Sterzing F, van Cutsem E, van der Gaast A, van Lanschot J, Ychou M, Otto F; First St Gallen EORTC Gastrointestinal Cancer Conference 2012 Expert Panel. Highlights of the EORTC St. Gallen International Expert Consensus on the primary therapy of gastric, gastroesophageal and oesophageal cancer - differential treatment strategies for subtypes of early gastroesophageal cancer. Eur J Cancer. 2012;48:2941-2953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 85. | De Manzoni G, Marrelli D, Baiocchi GL, Morgagni P, Saragoni L, Degiuli M, Donini A, Fumagalli U, Mazzei MA, Pacelli F, Tomezzoli A, Berselli M, Catalano F, Di Leo A, Framarini M, Giacopuzzi S, Graziosi L, Marchet A, Marini M, Milandri C, Mura G, Orsenigo E, Quagliuolo V, Rausei S, Ricci R, Rosa F, Roviello G, Sansonetti A, Sgroi G, Tiberio GA, Verlato G, Vindigni C, Rosati R, Roviello F. The Italian Research Group for Gastric Cancer (GIRCG) guidelines for gastric cancer staging and treatment: 2015. Gastric Cancer. 2017;20:20-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 86. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4609] [Article Influence: 242.6] [Reference Citation Analysis (0)] |
| 87. | Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G, Ducourtieux M, Bedenne L, Fabre JM, Saint-Aubert B, Genève J, Lasser P, Rougier P. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011;29:1715-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1504] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 88. | Smyth EC, Fassan M, Cunningham D, Allum WH, Okines AF, Lampis A, Hahne JC, Rugge M, Peckitt C, Nankivell M, Langley R, Ghidini M, Braconi C, Wotherspoon A, Grabsch HI, Valeri N. Effect of Pathologic Tumor Response and Nodal Status on Survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J Clin Oncol. 2016;34:2721-2727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 89. | Charalampakis N, Nogueras González GM, Elimova E, Wadhwa R, Shiozaki H, Shimodaira Y, Blum MA, Rogers JE, Harada K, Matamoros A Jr, Sagebiel T, Das P, Minsky BD, Lee JH, Weston B, Bhutani MS, Estrella JS, Badgwell BD, Ajani JA. The Proportion of Signet Ring Cell Component in Patients with Localized Gastric Adenocarcinoma Correlates with the Degree of Response to Pre-Operative Chemoradiation. Oncology. 2016;90:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 90. | Chen L, Shi Y, Yuan J, Wu Q, Han Y, Qin R, Jia B, Wei B, Wei L, Dai G, Jiao S. Evaluation of docetaxel- and oxaliplatin-based adjuvant chemotherapy in postgastrectomy gastric cancer patients reveals obvious survival benefits in docetaxel-treated mixed signet ring cell carcinoma patients. Med Oncol. 2014;31:159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 91. | Lemoine N, Adenis A, Bouche O, Duhamel A, Heurgue A, Leteurtre E, Amela E, Salleron J, Hebbar M. Signet Ring Cells and Efficacy of First-line Chemotherapy in Advanced Gastric or Oesogastric Junction Adenocarcinoma. Anticancer Res. 2016;36:5543-5549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Messager M, Lefevre JH, Pichot-Delahaye V, Souadka A, Piessen G, Mariette C; FREGAT working group - FRENCH. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg. 2011;254:684-93; discussion 693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 93. | Robb WB, Messager M, Gronnier C, Tessier W, Hec F, Piessen G, Mariette C; FREGAT (French EsoGastric Tumor) working group - FRENCH (Fédération de Recherche en Chirurgie). High-Grade Toxicity to Neoadjuvant Treatment for Upper Gastrointestinal Carcinomas: What is the Impact on Perioperative and Oncologic Outcomes? Ann Surg Oncol. 2015;22:3632-3639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 94. | Piessen G, Messager M, Le Malicot K, Robb WB, Di Fiore F, Guilbert M, Moreau M, Christophe V, Adenis A, Mariette C. Phase II/III multicentre randomised controlled trial evaluating a strategy of primary surgery and adjuvant chemotherapy versus peri-operative chemotherapy for resectable gastric signet ring cell adenocarcinomas - PRODIGE 19 - FFCD1103 - ADCI002. BMC Cancer. 2013;13:281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 95. | Al-Batran SE, Hofheinz RD, Pauligk C, Kopp HG, Haag GM, Luley KB, Meiler J, Homann N, Lorenzen S, Schmalenberg H, Probst S, Koenigsmann M, Egger M, Prasnikar N, Caca K, Trojan J, Martens UM, Block A, Fischbach W, Mahlberg R, Clemens M, Illerhaus G, Zirlik K, Behringer DM, Schmiegel W, Pohl M, Heike M, Ronellenfitsch U, Schuler M, Bechstein WO, Königsrainer A, Gaiser T, Schirmacher P, Hozaeel W, Reichart A, Goetze TO, Sievert M, Jäger E, Mönig S, Tannapfel A. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17:1697-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 510] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 96. | Al-Batran SE. Docetaxel, oxaliplatin, and fluorouracil/Leucovorin (FLOT) for resectable esophagogastric cancer: updated results from multicenter, randomized phase 3 FLOT4-AIO trial (German Gastric Group at AIO). ESMO congress Abstract, 2017: LBA27_PR. |
| 97. | Homann N, Pauligk C, Luley K, Werner Kraus T, Bruch HP, Atmaca A, Noack F, Altmannsberger HM, Jäger E, Al-Batran SE. Pathological complete remission in patients with oesophagogastric cancer receiving preoperative 5-fluorouracil, oxaliplatin and docetaxel. Int J Cancer. 2012;130:1706-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 98. | Schulz C, Kullmann F, Kunzmann V, Fuchs M, Geissler M, Vehling-Kaiser U, Stauder H, Wein A, Al-Batran SE, Kubin T, Schäfer C, Stintzing S, Giessen C, Modest DP, Ridwelski K, Heinemann V. NeoFLOT: Multicenter phase II study of perioperative chemotherapy in resectable adenocarcinoma of the gastroesophageal junction or gastric adenocarcinoma-Very good response predominantly in patients with intestinal type tumors. Int J Cancer. 2015;137:678-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 99. | Iwasaki Y, Sasako M, Yamamoto S, Nakamura K, Sano T, Katai H, Tsujinaka T, Nashimoto A, Fukushima N, Tsuburaya A; Gastric Cancer Surgical Study Group of Japan Clinical Oncology Group. Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol. 2013;107:741-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 100. | Kinoshita T, Sasako M, Sano T, Katai H, Furukawa H, Tsuburaya A, Miyashiro I, Kaji M, Ninomiya M. Phase II trial of S-1 for neoadjuvant chemotherapy against scirrhous gastric cancer (JCOG 0002). Gastric Cancer. 2009;12:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 101. | Terashima M, Iwasaki Y, Mizusawa J, Katayama H, Nakamura K, Katai H, Yoshikawa T, Ito Y, Kaji M, Kimura Y, Hirao M, Yamada M, Kurita A, Takagi M, Boku N, Sano T, Sasako M; Stomach Cancer Study Group, Japan Clinical Oncology Group. Randomized phase III trial of gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer, the short-term safety and surgical results: Japan Clinical Oncology Group Study (JCOG0501). Gastric Cancer. 2019;22:1044-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 102. | Iwasaki Y, Terashima M, Mizusawa J, Katayama H, Nakamura K, Katai H, Yoshikawa T, Ito S, Kaji M, Kimura Y, Hirao M, Yamada M, Kurita A, Takagi M, Lee SW, Takagane A, Yabusaki H, Hihara J, Boku N, Sano T, Sasako M. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial. Gastric Cancer. 2021;24:492-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (1)] |
| 103. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K; ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1943] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 104. | Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, Lee JI, Shin DB, Ji J, Chen JS, Lim Y, Ha S, Bang YJ; CLASSIC trial investigators. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1389-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 783] [Cited by in RCA: 776] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 105. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1089] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 106. | Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2465] [Cited by in RCA: 2437] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 107. | Cats A, Jansen EPM, van Grieken NCT, Sikorska K, Lind P, Nordsmark M, Meershoek-Klein Kranenbarg E, Boot H, Trip AK, Swellengrebel HAM, van Laarhoven HWM, Putter H, van Sandick JW, van Berge Henegouwen MI, Hartgrink HH, van Tinteren H, van de Velde CJH, Verheij M; CRITICS investigators. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): an international, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:616-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 379] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 108. | Park SH, Lim DH, Sohn TS, Lee J, Zang DY, Kim ST, Kang JH, Oh SY, Hwang IG, Ji JH, Shin DB, Yu JI, Kim KM, An JY, Choi MG, Lee JH, Kim S, Hong JY, Park JO, Park YS, Lim HY, Bae JM, Kang WK; ARTIST 2 investigators. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial☆. Ann Oncol. 2021;32:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 109. | Park SH, Sohn TS, Lee J, Lim DH, Hong ME, Kim KM, Sohn I, Jung SH, Choi MG, Lee JH, Bae JM, Kim S, Kim ST, Park JO, Park YS, Lim HY, Kang WK. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J Clin Oncol. 2015;33:3130-3136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 300] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 110. | Dikken JL, van Sandick JW, Maurits Swellengrebel HA, Lind PA, Putter H, Jansen EP, Boot H, van Grieken NC, van de Velde CJ, Verheij M, Cats A. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS). BMC Cancer. 2011;11:329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 111. | Stessin AM, Sison C, Schwartz A, Ng J, Chao CK, Li B. Does adjuvant radiotherapy benefit patients with diffuse-type gastric cancer? Cancer. 2014;120:3562-3568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 112. | Shchepotin IB, Evans SR, Chorny V, Osinsky S, Buras RR, Maligonov P, Shabahang M, Nauta RJ. Intensive preoperative radiotherapy with local hyperthermia for the treatment of gastric carcinoma. Surg Oncol. 1994;3:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 113. | Skoropad V, Berdov B, Zagrebin V. Concentrated preoperative radiotherapy for resectable gastric cancer: 20-years follow-up of a randomized trial. J Surg Oncol. 2002;80:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 114. | Skoropad VY, Berdov BA, Mardynski YS, Titova LN. A prospective, randomized trial of pre-operative and intraoperative radiotherapy versus surgery alone in resectable gastric cancer. Eur J Surg Oncol. 2000;26:773-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 115. | Ajani JA, Mansfield PF, Janjan N, Morris J, Pisters PW, Lynch PM, Feig B, Myerson R, Nivers R, Cohen DS, Gunderson LL. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol. 2004;22:2774-2780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 266] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 116. | Ajani JA, Mansfield PF, Crane CH, Wu TT, Lunagomez S, Lynch PM, Janjan N, Feig B, Faust J, Yao JC, Nivers R, Morris J, Pisters PW. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol. 2005;23:1237-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 234] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 117. | Ajani JA, Winter K, Okawara GS, Donohue JH, Pisters PW, Crane CH, Greskovich JF, Anne PR, Bradley JD, Willett C, Rich TA. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24:3953-3958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 282] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 118. | Allal AS, Zwahlen D, Bründler MA, de Peyer R, Morel P, Huber O, Roth AD. Neoadjuvant radiochemotherapy for locally advanced gastric cancer: long-term results of a phase I trial. Int J Radiat Oncol Biol Phys. 2005;63:1286-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 119. | Wydmański J, Suwinski R, Poltorak S, Maka B, Miszczyk L, Wolny E, Bielaczyc G, Zajusz A. The tolerance and efficacy of preoperative chemoradiotherapy followed by gastrectomy in operable gastric cancer, a phase II study. Radiother Oncol. 2007;82:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 120. | Leong T, Smithers BM, Michael M, Gebski V, Boussioutas A, Miller D, Simes J, Zalcberg J, Haustermans K, Lordick F, Schuhmacher C, Swallow C, Darling G, Wong R. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer. 2015;15:532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 121. | Slagter AE, Jansen EPM, van Laarhoven HWM, van Sandick JW, van Grieken NCT, Sikorska K, Cats A, Muller-Timmermans P, Hulshof MCCM, Boot H, Los M, Beerepoot LV, Peters FPJ, Hospers GAP, van Etten B, Hartgrink HH, van Berge Henegouwen MI, Nieuwenhuijzen GAP, van Hillegersberg R, van der Peet DL, Grabsch HI, Verheij M. CRITICS-II: a multicentre randomised phase II trial of neo-adjuvant chemotherapy followed by surgery versus neo-adjuvant chemotherapy and subsequent chemoradiotherapy followed by surgery versus neo-adjuvant chemoradiotherapy followed by surgery in resectable gastric cancer. BMC Cancer. 2018;18:877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 122. | Desiderio J, Chao J, Melstrom L, Warner S, Tozzi F, Fong Y, Parisi A, Woo Y. The 30-year experience-A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur J Cancer. 2017;79:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 123. | Coccolini F, Cotte E, Glehen O, Lotti M, Poiasina E, Catena F, Yonemura Y, Ansaloni L. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol. 2014;40:12-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 124. | Glehen O, Passot G, Villeneuve L, Vaudoyer D, Bin-Dorel S, Boschetti G, Piaton E, Garofalo A. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: a randomized and multicenter phase III study. BMC Cancer. 2014;14:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 125. | Götze TO, Piso P, Lorenzen S, Bankstahl US, Pauligk C, Elshafei M, Amato G, Reim D, Bechstein WO, Königsrainer A, Mönig SP, Rau B, Schwarzbach M, Al-Batran SE. Preventive HIPEC in combination with perioperative FLOT versus FLOT alone for resectable diffuse type gastric and gastroesophageal junction type II/III adenocarcinoma - the phase III "PREVENT"- (FLOT9) trial of the AIO /CAOGI /ACO. BMC Cancer. 2021;21:1158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 126. | Ishigami H, Tsuji Y, Shinohara H, Kodera Y, Kanda M, Yabusaki H, Ito S, Imano M, Yamashita H, Hidemura A, Yamaguchi H, Fukagawa T, Oba K, Kitayama J, Seto Y. Intraperitoneal Chemotherapy as Adjuvant or Perioperative Chemotherapy for Patients with Type 4 Scirrhous Gastric Cancer: PHOENIX-GC2 Trial. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 127. | Sugarbaker PH, Yu W, Yonemura Y. Gastrectomy, peritonectomy, and perioperative intraperitoneal chemotherapy: the evolution of treatment strategies for advanced gastric cancer. Semin Surg Oncol. 2003;21:233-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 128. | Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: Progress toward a new standard of care. Cancer Treat Rev. 2016;48:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 129. | Al-Batran SE, Goetze TO, Mueller DW, Vogel A, Winkler M, Lorenzen S, Novotny A, Pauligk C, Homann N, Jungbluth T, Reissfelder C, Caca K, Retter S, Horndasch E, Gumpp J, Bolling C, Fuchs KH, Blau W, Padberg W, Pohl M, Wunsch A, Michl P, Mannes F, Schwarzbach M, Schmalenberg H, Hohaus M, Scholz C, Benckert C, Knorrenschild JR, Kanngießer V, Zander T, Alakus H, Hofheinz RD, Roedel C, Shah MA, Sasako M, Lorenz D, Izbicki J, Bechstein WO, Lang H, Moenig SP. The RENAISSANCE (AIO-FLOT5) trial: effect of chemotherapy alone vs. chemotherapy followed by surgical resection on survival and quality of life in patients with limited-metastatic adenocarcinoma of the stomach or esophagogastric junction - a phase III trial of the German AIO/CAO-V/CAOGI. BMC Cancer. 2017;17:893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 130. | NIH. Surgical Resection Plus Chemotherapy Versus Chemotherapy Alone in Oligometastatic Stage IV Gastric Cancer (SURGIGAST). Report No.: NCT03042169. [cited 11 January 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT03042169. |
| 131. | Koemans WJ, van der Kaaij RT, Boot H, Buffart T, Veenhof AAFA, Hartemink KJ, Grootscholten C, Snaebjornsson P, Retel VP, van Tinteren H, Vanhoutvin S, van der Noort V, Houwink A, Hahn C, Huitema ADR, Lahaye M, Los M, van den Barselaar P, Imhof O, Aalbers A, van Dam GM, van Etten B, Wijnhoven BPL, Luyer MDP, Boerma D, van Sandick JW. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy versus palliative systemic chemotherapy in stomach cancer patients with peritoneal dissemination, the study protocol of a multicentre randomised controlled trial (PERISCOPE II). BMC Cancer. 2019;19:420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 132. | Glehen O, Gilly FN, Arvieux C, Cotte E, Boutitie F, Mansvelt B, Bereder JM, Lorimier G, Quenet F, Elias D; Association Française de Chirurgie. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17:2370-2377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 133. | Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, Zhou YF, Xiong B, Yonemura Y, Li Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 493] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 134. | Chia CS, You B, Decullier E, Vaudoyer D, Lorimier G, Abboud K, Bereder JM, Arvieux C, Boschetti G, Glehen O; BIG RENAPE Group. Patients with Peritoneal Carcinomatosis from Gastric Cancer Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Is Cure a Possibility? Ann Surg Oncol. 2016;23:1971-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 135. | Bonnot PE, Piessen G, Pocard M, Meunier B, Bereder JM, Abboud K, Marchal F, Quenet F, Goere D, Msika S, Arvieux C, Pirro N, Wernert R, Rat P, Pezet D, Lefevre J, Courvoisier T, Kianmanesh R, Meeus P, Glehen O. CYTO-CHIP: Cytoreductive surgery vs cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis: A propensity-score analysis from BIG RENAPE and FREGAT working groups. JCO. 2018;36:8-8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 136. | Bonnot PE, Lintis A, Mercier F, Benzerdjeb N, Passot G, Pocard M, Meunier B, Bereder JM, Abboud K, Marchal F, Quenet F, Goere D, Msika S, Arvieux C, Pirro N, Wernert R, Rat P, Gagnière J, Lefevre JH, Courvoisier T, Kianmanesh R, Vaudoyer D, Rivoire M, Meeus P, Villeneuve L, Piessen G, Glehen O; FREGAT and BIG-RENAPE Networks. Prognosis of poorly cohesive gastric cancer after complete cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy (CYTO-CHIP study). Br J Surg. 2021;108:1225-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 137. | Königsrainer I, Horvath P, Struller F, Königsrainer A, Beckert S. Initial clinical experience with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in signet-ring cell gastric cancer with peritoneal metastases. J Gastric Cancer. 2014;14:117-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 138. | Huh CW, Jung DH, Kim JH, Lee YC, Kim H, Yoon SO, Youn YH, Park H, Lee SI, Choi SH, Cheong JH, Noh SH. Signet ring cell mixed histology may show more aggressive behavior than other histologies in early gastric cancer. J Surg Oncol. 2013;107:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 139. | Rougier P, Ducreux M, Mahjoubi M, Pignon JP, Bellefqih S, Oliveira J, Bognel C, Lasser P, Ychou M, Elias D. Efficacy of combined 5-fluorouracil and cisplatinum in advanced gastric carcinomas. A phase II trial with prognostic factor analysis. Eur J Cancer. 1994;30A:1263-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 140. | Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A, Lang I, Falcon S. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 431] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 141. | Ajani JA, Abramov M, Bondarenko I, Shparyk Y, Gorbunova V, Hontsa A, Otchenash N, Alsina M, Lazarev S, Feliu J, Elme A, Esko V, Abdalla K, Verma U, Benedetti F, Aoyama T, Mizuguchi H, Makris L, Rosati G; DIGEST Study Group. A phase III trial comparing oral S-1/cisplatin and intravenous 5-fluorouracil/cisplatin in patients with untreated diffuse gastric cancer. Ann Oncol. 2017;28:2142-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 142. | Pernot S, Mitry E, Samalin E, Dahan L, Dalban C, Ychou M, Seitz JF, Turki H, Mazard T, Zaanan A, Lepère C, Vaillant JN, Landi B, Rougier P, Taieb J. Biweekly docetaxel, fluorouracil, leucovorin, oxaliplatin (TEF) as first-line treatment for advanced gastric cancer and adenocarcinoma of the gastroesophageal junction: safety and efficacy in a multicenter cohort. Gastric Cancer. 2014;17:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 143. | Eveno C, Jouvin I, Pocard M. PIPAC EstoK 01: Pressurized IntraPeritoneal Aerosol Chemotherapy with cisplatin and doxorubicin (PIPAC C/D) in gastric peritoneal metastasis: a randomized and multicenter phase II study. Pleura Peritoneum. 2018;3:20180116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 144. | Nadiradze G, Giger-Pabst U, Zieren J, Strumberg D, Solass W, Reymond MA. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with Low-Dose Cisplatin and Doxorubicin in Gastric Peritoneal Metastasis. J Gastrointest Surg. 2016;20:367-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 145. | Alyami M, Bonnot PE, Mercier F, Laplace N, Villeneuve L, Passot G, Bakrin N, Kepenekian V, Glehen O. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for unresectable peritoneal metastasis from gastric cancer. Eur J Surg Oncol. 2021;47:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 146. | Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, Stoss O, Hill J, Ng V, Lehle M, Thomas M, Kiermaier A, Rüschoff J. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 452] [Cited by in RCA: 428] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 147. | Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, Elenius K, Isola J. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 525] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 148. | Kim KC, Koh YW, Chang HM, Kim TH, Yook JH, Kim BS, Jang SJ, Park YS. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol. 2011;18:2833-2840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 149. | Chua TC, Merrett ND. Clinicopathologic factors associated with HER2-positive gastric cancer and its impact on survival outcomes--a systematic review. Int J Cancer. 2012;130:2845-2856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 150. | Park DI, Yun JW, Park JH, Oh SJ, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Yoo CH, Son BH, Cho EY, Chae SW, Kim EJ, Sohn JH, Ryu SH, Sepulveda AR. HER-2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci. 2006;51:1371-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 151. | He C, Bian XY, Ni XZ, Shen DP, Shen YY, Liu H, Shen ZY, Liu Q. Correlation of human epidermal growth factor receptor 2 expression with clinicopathological characteristics and prognosis in gastric cancer. World J Gastroenterol. 2013;19:2171-2178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 152. | Qiu M, Zhou Y, Zhang X, Wang Z, Wang F, Shao J, Lu J, Jin Y, Wei X, Zhang D, Li Y, Yang D, Xu R. Lauren classification combined with HER2 status is a better prognostic factor in Chinese gastric cancer patients. BMC Cancer. 2014;14:823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 153. | Abrahão-Machado LF, Jácome AA, Wohnrath DR, dos Santos JS, Carneseca EC, Fregnani JH, Scapulatempo-Neto C. HER2 in gastric cancer: comparative analysis of three different antibodies using whole-tissue sections and tissue microarrays. World J Gastroenterol. 2013;19:6438-6446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 154. | Warneke VS, Behrens HM, Böger C, Becker T, Lordick F, Ebert MP, Röcken C. Her2/neu testing in gastric cancer: evaluating the risk of sampling errors. Ann Oncol. 2013;24:725-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 155. | Woo CG, Ho WJ, Park YS, Park SR, Ryu MH, Jung HY, Kang YK. A potential pitfall in evaluating HER2 immunohistochemistry for gastric signet ring cell carcinomas. Pathology. 2017;49:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 156. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5324] [Article Influence: 354.9] [Reference Citation Analysis (3)] |
| 157. | Kim C, Lee CK, Chon HJ, Kim JH, Park HS, Heo SJ, Kim HJ, Kim TS, Kwon WS, Chung HC, Rha SY. PTEN loss and level of HER2 amplification is associated with trastuzumab resistance and prognosis in HER2-positive gastric cancer. Oncotarget. 2017;8:113494-113501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 158. | Rüschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 432] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 159. | Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B, Starnawski M, Kang YK. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968-3976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 887] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 160. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J; REGARD Trial Investigators. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1541] [Cited by in RCA: 1574] [Article Influence: 143.1] [Reference Citation Analysis (0)] |
| 161. | Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A; RAINBOW Study Group. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1613] [Cited by in RCA: 1768] [Article Influence: 160.7] [Reference Citation Analysis (0)] |
| 162. | Liu X, Cai H, Sheng W, Yu L, Long Z, Shi Y, Wang Y. Clinicopathological Characteristics and Survival Outcomes of Primary Signet Ring Cell Carcinoma in the Stomach: Retrospective Analysis of Single Center Database. PLoS One. 2015;10:e0144420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 163. | Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, Kurteva G, Volovat C, Moiseyenko VM, Gorbunova V, Park JO, Sawaki A, Celik I, Götte H, Melezínková H, Moehler M; Arbeitsgemeinschaft Internistische Onkologie and EXPAND Investigators. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 680] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 164. | Waddell T, Chau I, Cunningham D, Gonzalez D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G, Wadsley J, Ferry D, Mansoor W, Crosby T, Coxon F, Smith D, Waters J, Iveson T, Falk S, Slater S, Peckitt C, Barbachano Y. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 561] [Cited by in RCA: 581] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 165. | Lang SA, Gaumann A, Koehl GE, Seidel U, Bataille F, Klein D, Ellis LM, Bolder U, Hofstaedter F, Schlitt HJ, Geissler EK, Stoeltzing O. Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model. Int J Cancer. 2007;120:1803-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 166. | Ohtsu A, Ajani JA, Bai YX, Bang YJ, Chung HC, Pan HM, Sahmoud T, Shen L, Yeh KH, Chin K, Muro K, Kim YH, Ferry D, Tebbutt NC, Al-Batran SE, Smith H, Costantini C, Rizvi S, Lebwohl D, Van Cutsem E. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol. 2013;31:3935-3943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 373] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 167. | Sahin U, Türeci Ö, Manikhas G, Lordick F, Rusyn A, Vynnychenko I, Dudov A, Bazin I, Bondarenko I, Melichar B, Dhaene K, Wiechen K, Huber C, Maurus D, Arozullah A, Park JW, Schuler M, Al-Batran SE. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021;32:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 263] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 168. | Böger C, Behrens HM, Mathiak M, Krüger S, Kalthoff H, Röcken C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget. 2016;7:24269-24283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 228] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 169. | Kim JW, Nam KH, Ahn SH, Park DJ, Kim HH, Kim SH, Chang H, Lee JO, Kim YJ, Lee HS, Kim JH, Bang SM, Lee JS, Lee KW. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer. 2016;19:42-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 202] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 170. | Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1962] [Cited by in RCA: 1898] [Article Influence: 474.5] [Reference Citation Analysis (1)] |
| 171. | Liu X, Choi MG, Kim K, Kim KM, Kim ST, Park SH, Cristescu R, Peter S, Lee J. High PD-L1 expression in gastric cancer (GC) patients and correlation with molecular features. Pathol Res Pract. 2020;216:152881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 172. | Hirotsu Y, Mochizuki H, Amemiya K, Ohyama H, Yoshimura D, Amano H, Miura Y, Ashizawa H, Nakagomi K, Takaoka S, Hosoda K, Suzuki Y, Oyama T, Hada M, Kojima Y, Omata M. Deficiency of mismatch repair genes is less frequently observed in signet ring cell compared with non-signet ring cell gastric cancer. Med Oncol. 2019;36:23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 173. | Pyo JH, Lee H, Min BH, Lee JH, Choi MG, Sohn TS, Bae JM, Kim KM, Yeon S, Jung SH, Kim JJ, Kim S. Early gastric cancer with a mixed-type Lauren classification is more aggressive and exhibits greater lymph node metastasis. J Gastroenterol. 2017;52:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 174. | Wanebo HJ, Kennedy BJ, Chmiel J, Steele G Jr, Winchester D, Osteen R. Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg. 1993;218:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 452] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 175. | Hass HG, Smith U, Jäger C, Schäffer M, Wellhäuber U, Hehr T, Markmann HU, Nehls O, Denzlinger C. Signet ring cell carcinoma of the stomach is significantly associated with poor prognosis and diffuse gastric cancer (Lauren's): single-center experience of 160 cases. Onkologie. 2011;34:682-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 176. | Lee HH, Song KY, Park CH, Jeon HM. Undifferentiated-type gastric adenocarcinoma: prognostic impact of three histological types. World J Surg Oncol. 2012;10:254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 177. | Kim JP, Kim SC, Yang HK. Prognostic significance of signet ring cell carcinoma of the stomach. Surg Oncol. 1994;3:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 96] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 178. | Otsuji E, Yamaguchi T, Sawai K, Takahashi T. Characterization of signet ring cell carcinoma of the stomach. J Surg Oncol. 1998;67:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 179. | Yokota T, Kunii Y, Teshima S, Yamada Y, Saito T, Kikuchi S, Yamauchi H. Signet ring cell carcinoma of the stomach: a clinicopathological comparison with the other histological types. Tohoku J Exp Med. 1998;186:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 180. | Kim DY, Park YK, Joo JK, Ryu SY, Kim YJ, Kim SK, Lee JH. Clinicopathological characteristics of signet ring cell carcinoma of the stomach. ANZ J Surg. 2004;74:1060-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 181. | Park JM, Jang YJ, Kim JH, Park SS, Park SH, Kim SJ, Mok YJ, Kim CS. Gastric cancer histology: clinicopathologic characteristics and prognostic value. J Surg Oncol. 2008;98:520-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 182. | Chiu CT, Kuo CJ, Yeh TS, Hsu JT, Liu KH, Yeh CN, Hwang TL, Jan YY, Lin CJ. Early signet ring cell gastric cancer. Dig Dis Sci. 2011;56:1749-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 183. | Lu M, Yang Z, Feng Q, Yu M, Zhang Y, Mao C, Shen L, Tang J. The characteristics and prognostic value of signet ring cell histology in gastric cancer: A retrospective cohort study of 2199 consecutive patients. Medicine (Baltimore). 2016;95:e4052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 184. | Theuer CP, Nastanski F, Brewster WR, Butler JA, Anton-Culver H. Signet ring cell histology is associated with unique clinical features but does not affect gastric cancer survival. Am Surg. 1999;65:915-921. [PubMed] |
| 185. | Kunisaki C, Shimada H, Nomura M, Matsuda G, Otsuka Y, Akiyama H. Therapeutic strategy for signet ring cell carcinoma of the stomach. Br J Surg. 2004;91:1319-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 186. | Ha TK, An JY, Youn HK, Noh JH, Sohn TS, Kim S. Indication for endoscopic mucosal resection in early signet ring cell gastric cancer. Ann Surg Oncol. 2008;15:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 187. | Wang Z, Zhang X, Hu J, Zeng W, Zhou Z. Clinicopathological features and outcomes in patients undergoing radical resection for early gastric cancer with signet ring cell histology. J Visc Surg. 2015;152:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 188. | Imamura T, Komatsu S, Ichikawa D, Kawaguchi T, Kosuga T, Okamoto K, Konishi H, Shiozaki A, Fujiwara H, Otsuji E. Early signet ring cell carcinoma of the stomach is related to favorable prognosis and low incidence of lymph node metastasis. J Surg Oncol. 2016;114:607-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |