Published online Jul 15, 2022. doi: 10.4251/wjgo.v14.i7.1295
Peer-review started: January 11, 2022
First decision: March 13, 2022
Revised: March 18, 2022
Accepted: May 28, 2022
Article in press: May 28, 2022
Published online: July 15, 2022
Processing time: 182 Days and 23.6 Hours
Most gastric cancer (GC) patients are diagnosed at middle or late stage because the symptoms in early stage are obscure, which causes higher mortality rates of GC. Helicobacter pylori (H. pylori) was identified as a class I carcinogen and leads to aberrant DNA methylation/hydroxymethylation. 5-hydroxymethylcytosine (5-hmC) plays complex roles in gene regulation of tumorigenesis and can be considered as an activating epigenetic mark of hydroxymethylation.
To explore the association between 5-hmC levels and the progression and prognosis of GC patients with or without H. pylori infection.
A retrospective cohort study was conducted to estimate the predicted value of 5-hmC level in the progression and prognosis of GC patients with different H. pylori infection status. A total of 144 GC patients were recruited.
The levels of 5-hmC were significantly decreased in tumor tissues (0.076 ± 0.048) compared with the matched control tissues (0.110 ± 0.057, P = 0.001). A high level of 5-hmC was an independent significant favorable predictor of overall survival in GC patients (hazard ratio = 0.61, 95% confidence interval: 0.38-0.98, P = 0.040), the H. pylori-negative GC subgroup (hazard ratio = 0.30, 95% confidence interval: 0.13-0.68, P = 0.004) and the GC patients with TNM stage Ⅰ or Ⅱ (hazard ratio = 0.32, 95% confidence interval: 0.13-0.77, P = 0.011).
Increased 5-hmC is a favorable prognostic factor in GC, especially for H. pylori-negative subgroups.
Core Tip: Helicobacter pylori (H. pylori) was identified as a class I carcinogen and leads to aberrant DNA methylation/hydroxymethylation. 5-hydroxymethylcytosine plays complex roles in the gene regulation of tumorigenesis and is considered an activating epigenetic mark of hydroxymethylation. We conducted a retrospective cohort study to estimate the predictive value of 5-hydroxymethylcytosine levels in the progression and prognosis of gastric cancer patients with different H. pylori infection statuses. The results indicated that increasing 5-hydroxymethylcytosine is a favorable prognostic factor in gastric cancer patients who were not infected with H. pylori, but no associations were observed in H. pylori-positive gastric cancer patients.
- Citation: Fu YL, Wu YH, Cao DH, Jia ZF, Shen A, Jiang J, Cao XY. Increased 5-hydroxymethylcytosine is a favorable prognostic factor of Helicobacter pylori-negative gastric cancer patients. World J Gastrointest Oncol 2022; 14(7): 1295-1306
- URL: https://www.wjgnet.com/1948-5204/full/v14/i7/1295.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i7.1295
Gastric cancer (GC) is a serious disease with over 1 million estimated new cases annually around the world, and it is the fifth most diagnosed malignancy worldwide[1]. Due to the symptoms in early stage being obscure, most GC patients are diagnosed at middle or late stage, which causes higher mortality rates, and accounted for 769000 deaths globally in 2020[1].
Recent comprehensive analyses showed that many GC-related pathways are more frequently altered by aberrant DNA methylation than by mutations[2], and the degree of accumulation of aberrant DNA methylation is highly correlated with GC risk[3,4].
Helicobacter pylori (H. pylori) was identified as a class I carcinogen leading to gastric adenocarcinoma by the World Health Organization[5]. H. pylori-induced chronic inflammation plays a direct role in the induction of aberrant DNA methylation. The methylation level in an H. pylori-positive group was 2.5-34.1 times higher than a negative group. H. pylori eradication leads to a decrease in DNA methylation levels[6,7].
A promising method to reverse the progression of GC is effective demethylation treatment. The passive demethylation agents (5-azacytidine/decitabine), which relies on DNA methyltransferase, are not effective in the treatment of solid tumors and have serious side effects. A newly proposed classical active demethylation process involves oxidizing 5-methylcytosine to 5-hydroxymethylcytosine (5-hmC) and further downstream products by the ten-eleven translocation (TET) family. The median product, 5-hmC, is considered an activating epigenetic marker, and it plays complex roles in gene regulation of tumorigenesis[8-10]. Significant reductions in 5-hmC levels have been found in hematological malignancies, such as breast cancer, colon cancer, prostate cancer and melanoma. A few small size studies analyzed the association between 5-hmC levels and GC, but the evidence is still lacking[11,12], especially for the H. pylori-induced GC.
In the current study, we explored the level of 5-hmC and H. pylori infection in a relatively large scale GC patient cohort to assess the association between 5-hmC level and the malignant progression of the tumor and the overall survival of GC patients with different H. pylori infection status.
This study was approved by the Institutional Review Board of the First Hospital of Jilin University. All participants provided written informed consent prior to joining the study.
A total of 158 patients with histologically diagnosed GC who underwent radical gastrectomy at the Department of Gastric and Colorectal Surgery in the First Hospital of Jilin University (Changchun, China) during 2007 to 2017 were recruited in this cohort study. For each patient, 5 mL of peripheral blood before surgery and 0.5 cm3 of tumor tissue were collected. Among the patients, 38 specimens of 0.5 cm3 adjacent tissue were collected during the operation. All patients did not undergo chemotherapy or radiotherapy before surgery. Demographic information (sex, age) and principal clinical pathological information (histological grade, TNM stage, tumor size, neural invasion, vascular invasion, etc.) were collected. The tumor histological grade was evaluated by the World Health Organization criteria. TNM stages were classified according to the 8th edition of the TNM staging system of the Union for International Cancer Control/American Joint Committee on Cancer (2017). Patients with the following conditions were excluded from this study: (1) Patients with distant metastasis or a positive surgical margin; (2) Patients who died due to complications of the surgical procedure during the perioperative period; and (3) Patients who were lost at the first time of interview.
Follow-up for all patients was implemented at 3 mo, 6 mo, 12 mo and annually afterwards until death or the end of the follow-up. Information on general status and postoperative chemotherapy were collected during each follow-up. If the patients had died, the date of death and potential cause were recorded. The duration from the date of surgery to the date of death or the last successful interview date was defined as the survival time. If the patient was lost to follow-up, survival time was defined as the duration from the date of surgery to the date of the last successful interview.
The genomic DNA from primary tumors and paired noncancerous mucosa tissues were extracted using a QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The 5-hmC content of genomic DNA was determined with a Quest 5-hmC DNA enzyme-linked immunosorbent assay (ELISA) Kit (Zymo Research, Irvine, CA, United States) according to the manufacturer’s instructions. Assays were performed using 4 μg/mL anti-5-hmC polyclonal antibodies, loading 200 ng of DNA per well. Absorbance at 405 nm was evaluated using a SynergyH1 microplate reader and Gen5 software (BioTek, Winooski, VT, United States). The amount of 5-hmC was calculated as a percentage based on a standard curve generated using kit controls and the median value was used as the cutoff of 5-hmC level category. Values above the median value were considered to be the 5-hmC high group (≥ 0.106%), and those below the median value were considered the 5-hmC low group (< 0.106%).
A commercial ELISA kit for H. pylori immunoglobulin G (Biohit, Helsinki, Finland) was used to detect the serum H. pylori immunoglobulin G antibodies. The antibody titers were quantified by optical density readings according to the manufacturer’s protocol, and titers higher than the threshold value of 30 EIU were considered as positive for H. pylori infection.
Continuous variables that followed a normal distribution were shown as the mean ± standard deviation. Independent samples were compared by two-sample t-test, and matched-paired samples were compared by paired t-test. Categorical variables were presented as frequencies with percentages and were compared with the χ2 test or Fisher’s exact test when appropriate.
Survival curves within each stratification of variables were plotted by the Kaplan-Meier method and compared by log-rank test. The forward stepwise multivariate Cox proportional hazard model was used to evaluate the prognostic role of clinical characteristics and 5-hmC level. Hazard ratios (HRs) with their 95% confidence intervals (Cis) were calculated. All analyses were conducted with the SPSS program (version 21.0; IBM Corp., Armonk, NY, United States) or GraphPad Prism 5.0 (La Jolla, CA, United States). A two-tailed P value < 0.05 indicated statistical significance.
In the present study, 144 GC patients were involved for the final prognostic analysis and were followed up until August 2021. The median survival time was 73.59 mo. During the follow-up period, 75 (52.1%) patients died, 68 (47.2%) patients remained alive, and 1 (0.7%) patient was lost to follow-up (Figure 1).
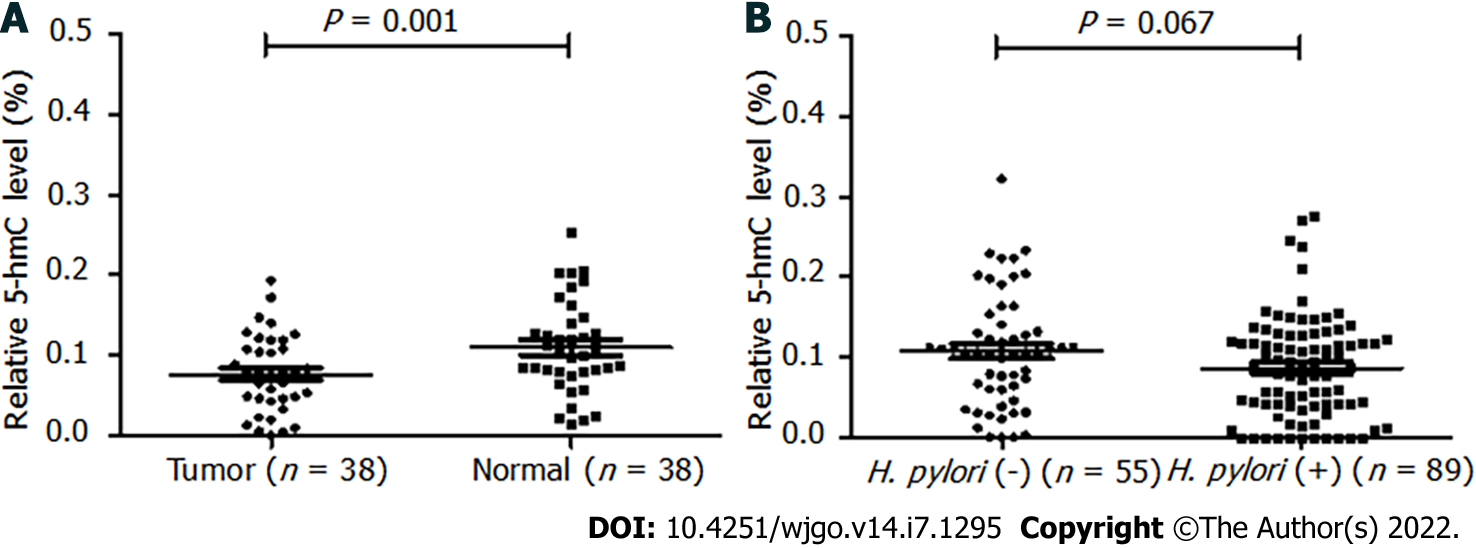
Among the 38 paired tissues, the 5-hmC levels were significantly reduced in tumor tissues (0.076 ± 0.048) compared with the matched control tissues (0.110 ± 0.057, P = 0.001) (Figure 2A).
Among the 144 subjects, there were 99 (68.7%) males, and the median age was 62.82 (range 39–90) years old. The mean 5-hmC level of the 144 GC patients was 0.104 ± 0.062. We investigated possible correlations between 5-hmC levels and general demographic characteristics/routine clinicopathological parameters in the GC patients. The TNM stages (P = 0.012), neural invasion (P = 0.008), age (P = 0.008) and H. pylori infection (P = 0.049) were associated with 5-hmC level. Details were shown in Table 1.
| Characteristics | Total, n (%) | 5-hmC low, n (%) | 5-hmC high, n (%) | P value |
| Sex | ||||
| Male | 45 (31.3) | 17 (24.3) | 28 (37.8) | 0.079 |
| Female | 99 (68.7) | 53 (75.7) | 46 (62.2) | |
| Age in yr | ||||
| < 60 | 56 (38.9) | 35 (50.0) | 21 (28.4) | 0.008 |
| ≥ 60 | 88 (61.1) | 35 (50.0) | 53 (71.6) | |
| Histological grade | ||||
| Low | 106 (73.6) | 47 (67.1) | 59 (79.7) | 0.087 |
| High | 38 (26.4) | 23 (32.9) | 15 (20.3) | |
| WHO Classification | ||||
| Tubular adenocarcinoma | 115 (79.9) | 55 (78.6) | 60 (81.1) | 0.707 |
| Others | 29 (20.1) | 15 (21.4) | 14 (18.9) | |
| Tumor size in cm | ||||
| < 5 | 62 (43.1) | 32 (45.7) | 30 (40.5) | 0.531 |
| ≥ 5 | 82 (56.9) | 38 (54.3) | 44 (59.5) | |
| Vascular invasion | ||||
| Negative | 39 (27.1) | 23 (32.9) | 16 (21.6) | 0.129 |
| Positive | 105 (72.9) | 47 (67.1) | 58 (78.4) | |
| Neural invasion | ||||
| Negative | 64 (44.4) | 39 (55.7) | 25 (33.8) | 0.008 |
| Positive | 80 (55.6) | 31 (44.3) | 49 (66.2) | |
| Depth of invasion | ||||
| T1/T2 | 54 (37.5) | 30 (42.9) | 24 (32.4) | 0.197 |
| T3/T4 | 90 (62.5) | 40 (57.1) | 50 (67.6) | |
| Lymph metastasis | ||||
| N0 | 41 (28.5) | 24 (34.3) | 17 (23.0) | 0.133 |
| N1/N2/N3 | 103 (71.5) | 46 (65.7) | 57 (77.0) | |
| TNM stage | ||||
| Ⅰ + Ⅱ | 73 (50.7) | 43 (61.4) | 30 (40.5) | 0.012 |
| Ⅲ | 71 (49.3) | 27 (38.6) | 44 (59.5) | |
| Chemotherapy | ||||
| No | 75 (52.1) | 33 (47.1) | 42 (56.8) | 0.248 |
| Yes | 69 (47.9) | 37 (52.9) | 32 (43.2) | |
| H. pylori | ||||
| Negative | 55 (38.2) | 21 (30.0) | 34 (45.9) | 0.049 |
| Positive | 89 (61.8) | 49 (70.0) | 40 (54.1) |
For the 144 GC patients, the results of H. pylori infection examination showed that 89 (61.8%) subjects were positive and 55 (38.2%) subjects were negative. We compared the 5-hmC level between H. pylori-positive infection and H. pylori-negative infection groups. It showed that the 5-hmC level was reduced in the H. pylori-positive group, but the P value was at the boundary of significance (P = 0.067, Figure 2B).
We further investigated the association between 5-hmC level and characteristics stratified by H. pylori infection status. We found that 5-hmC levels were higher in patients aged more than 60 years (P = 0.009), with neural invasion positive (P = 0.002), with low histological grade (P = 0.042) or with later TNM stage (P = 0.012) in the H. pylori-positive subset, but no significant associations were observed in the H. pylori-negative subset except sex (Table 2).
| Characteristics | H. pylori (-), n = 55 | H. pylori (+), n = 89 | ||
| 5-hmC high, n (%) | P value | 5-hmC high, n (%) | P value | |
| Sex | ||||
| Male | 18 (50.0) | 0.013 | 28 (44.4) | 0.883 |
| Female | 16 (84.2) | 12 (46.2) | ||
| Age in yr | ||||
| < 60 | 10 (55.6) | 0.505 | 11 (28.9) | 0.009 |
| ≥ 60 | 24 (64.9) | 29 (56.9) | ||
| Histological grade | ||||
| High | 10 (55.6) | 0.505 | 5 (25.0) | 0.042 |
| Low | 24 (64.9) | 35 (50.7) | ||
| WHO classification | ||||
| Tubular adenocarcinoma | 31 (60.8) | 11 | 29 (45.3) | 0.911 |
| Others | 3 (75.0) | 11 (44.0) | ||
| Tumor size in cm | ||||
| < 5 | 13 (61.9) | 0.992 | 17 (41.5) | 0.542 |
| ≥ 5 | 21 (61.8) | 23 (47.9) | ||
| Vascular invasion | ||||
| Negative | 10 (52.6) | 0.308 | 6 (30.0) | 0.127 |
| Positive | 24 (66.7) | 34 (49.3) | ||
| Neural invasion | ||||
| Negative | 16 (57.1) | 0.467 | 9 (25.0) | 0.002 |
| Positive | 18 (66.7) | 31 (58.5) | ||
| Depth of invasion | ||||
| T1/T2 | 12 (50.0) | 0.112 | 12 (40.0) | 0.504 |
| T3/T4 | 22 (71.0) | 28 (47.5) | ||
| Lymph metastasis | ||||
| N0 | 9 (50.0) | 0.208 | 8 (34.8) | 0.255 |
| N1/N2/N3 | 25 (67.6) | 32 (48.5) | ||
| TNM stage | ||||
| Ⅰ + Ⅱ | 17 (54.8) | 0.226 | 13 (31.0) | 0.012 |
| Ⅲ | 17 (70.8) | 27 (57.4) | ||
| Chemotherapy | ||||
| No | 19 (61.3) | 0.927 | 23 (52.3) | 0.169 |
| Yes | 15 (62.5) | 17 (37.8) | ||
Overall survival analyses were performed in total patients and patient stratification by H. pylori infection status or TNM stage. The results of the Kaplan-Meier analysis showed that the 5-hmC level was not associated with overall survival in total patients or H. pylori-negative or positive groups (log rank P values were 0.406, 0.094 and 0.763, respectively, Figure 3A-C). Furthermore, the 5hmC high level was associated with longer overall survival time compared with the 5hmC low group in the TNM stage Ⅰ and Ⅱ subgroup, and log rank test showed the survival curves were significantly different (log rank P = 0.037, Figure 3D), but the association was not significant in the TNM stage Ⅲ subgroup (log rank P = 0.547, Figure 3E).
In the full patient set, 5-hmC high level was a significant favorable predictor of overall survival in multivariate Cox regression analysis (HR = 0.61, 95%CI: 0.38-0.98, P = 0.040) after adjustment for tumor size, histological grade and TNM stage (Table 3).
| Characteristics | HR (95%CI) | P value | |
| Tumor size in cm | < 5 | 1 | 0.027 |
| ≥ 5 | 1.78 (1.07-2.95) | ||
| Histological grade | High | 1 | 0.011 |
| Low | 2.25 (1.21-4.18) | ||
| TNM stage | Ⅰ + Ⅱ | 1 | < 0.001 |
| Ⅲ | 2.84 (1.72-4.70) | ||
| 5-hmC | Low | 1 | 0.040 |
| High | 0.61 (0.38-0.98) |
Multivariate Cox regression analysis for overall survival was also performed in GC patients stratified by H. pylori infection status or TNM stage. In the H. pylori-negative GC subgroup, increased 5-hmC level was a favorable prognostic factor in the multivariate Cox regression analysis (HR = 0.30, 95%CI: 0.13-0.68, P = 0.004) (Table 4), which indicated that higher 5-hmC level was an independent significant protective factor of overall survival time in patients without H. pylori infection. However, within the H. pylori-positive group, we did not observe any significant association between 5-hmC level and GC patient prognosis.
| Characteristics | HR (95%CI) | P value | |
| Neural invasion | Negative | 1 | < 0.001 |
| Positive | 5.45 (2.28-13.07) | ||
| Tumor size in cm | < 5 | 1 | 0.031 |
| ≥ 5 | 2.63 (1.09-6.32) | ||
| 5-hmC | Low | 1 | 0.004 |
| High | 0.30 (0.13-0.68) |
Among patients with TNM stage I or II, increased 5-hmC level was associated with favorable prognosis after adjustment for sex in the multivariate Cox regression analysis (HR = 0.32, 95%CI: 0.13-0.77, P = 0.011). However, no significant association was observed between 5-hmC level and the prognosis in patients with TNM stage Ⅲ (Table 5).
| Characteristics | HR (95%CI) | P value | |
| Sex | Female | 1 | 0.047 |
| Male | 0.43 (0.19-0.99) | ||
| 5-hmC | Low | 1 | 0.011 |
| High | 0.32 (0.13-0.77) |
Long-time H. pylori infection leads to chronic inflammation and further aberrant DNA methylation, which plays an important role in tumorigenesis of GC. The global prevalence of H. pylori reported by a meta-analysis across individual countries varied from 18.9% to 87.7%, and the prevalence in China was 55.8% (95%CI: 51.8%-59.9%)[13]. Among our 144 GC patients, 89 (61.8%) patients were defined as H. pylori-positive by ELISA. The infection rate was slightly higher than the prevalence in the general Chinese population but was similar to the previously reported prevalence in GC patients[14,15], indicating that our study cohort was representative.
DNA methylation/hydroxymethylation is one of the most widely studied epigenetic modifications and has been shown to play significant roles in tumorigenesis and prognosis[16]. Previous studies have shown that aberrant DNA methylation is a common event and a strong candidate mechanism for early nongenetic alterations in GC[17]. Nevertheless, the reports of DNA hydroxymethylation and GC are limited to several small studies.
We estimated the 5-hmC level with an absolutely quantitative method ELISA, which is more objective than the semi-quantitative evaluation system of immunohistochemistry. The results showed that the 5-hmC level was downregulated in GC tissues compared with matched control tissues, which revealed that it was associated with the occurrence of GC and is consistent with previous reports[18]. Although some evidence has emerged about the potential progression and prognostic implications of 5-hmC level in GC[12], very few studies have evaluated the association stratified by H. pylori infection status. The present study was performed on a well-characterized cohort to simultaneously evaluate the level of 5-hmC in GC patients and subsets stratified by H. pylori infection to assess the association between 5-hmC levels and the susceptibility or prognosis of GC in order to provide more evidence for the effect of H. pylori-infection DNA hydroxymethylation on GC.
The 5-hmC level was slightly decreased in the H. pylori-positive subset compared to the H. pylori-negative group in our study. It is hypothesized that H. pylori infection affects TET1 expression in normal gastric epithelial cells and reduces the genome hydroxymethylation level[19]. Interestingly, higher global 5-hmC levels were associated with GC progression in the H. pylori-positive subset. A similar phenomenon was reported that the 5-hmC level in ERG- prostate cancer patients was lower than ERG+ patients, but a higher 5hmC level was associated with tumor progression in ERG- prostate cancer patients[20]. This could be explained by cells responding to hypoxia inducing a transcriptional program regulated by the TET family. Hypoxia together with reactive oxygen species increase global 5-hmC levels by transcriptional activation of TET1[21,22]. H. pylori infection induced the expression of hypoxia-inducible factor[23], which is required for hypoxic induction of TET1 and global increase of 5-hmC. The proliferation rate of H. pylori under aerobic conditions was 3-fold higher than under microaerophilic conditions, and the bacterial growth was more dependent on carbon dioxide than on oxygen[24].
This interesting phenomenon and potential mechanism suggested to us that the 5-hmC level changed due to H. pylori infection and was not simply one direction but complicated. Therefore, it was essential to assess the association between 5-hmC and the prognosis of GC patients in negative or positive H. pylori infection. Our results first showed that reduced 5-hmC was associated with poor prognosis in all GC patients, which was consistent with previous studies[11,12]. Furthermore, in H. pylori-negative GC patients, the 5-hmC level was a significant predictor of prognosis, independent of routine clinicopathological factors. But in contrast, 5-hmC had no prediction value of prognosis in H. pylori-positive GC patients. These results highlight the importance of H. pylori stratification in GC biomarker studies. Similarly to our results, the study conducted in prostate cancer patients also showed that the prognostic predictor value of 5-hmC was discrepant in ERG- and ERG+ prostate cancer patients[20]. Together with our results, it supports potential prognostic implications of 5-hmC as cancer subtype-specific.
In this study, the association between 5-hmC level and the prognosis of GC patients was not significant in the Kaplan-Meier analysis, which could not be adjusted for potential confounders. However, it showed significant association in the multivariate Cox regression after the confounders such as TNM stage were adjusted. This indicated that the clinical characteristics such as TNM stage (which is strongly associated with the prognosis of GC patients) confused the relationship between 5-hmC level and the prognosis. This conclusion was further supported by the Cox regression results of TNM stage stratified analysis.
Several limitations should be mentioned of the present study. First, our study was based at a single center. The prognostic value of 5-hmC in H. pylori-negative but not positive GC patients’ needs to be validated in larger and multicenter GC patient cohorts. Another limitation of our study is the lack of data of 5-methylcytosine and enzymes related to 5-hmC regulation for our sample set. Thus, we have not investigated the correlation between them, which should be investigated in future studies.
5-hmC level was a significant predictor of the prognosis of GC patients without H. pylori infection, independent of routine clinicopathological factors.
Most gastric cancer (GC) patients are diagnosed at middle or late stage because the symptoms in early stage are obscure, which causes higher mortality rates of GC. Analyses show that aberrant DNA methylation is highly correlated with GC risk. Helicobacter pylori (H. pylori) was identified as a class I carcinogen leading to gastric adenocarcinoma, and H. pylori-induced chronic inflammation plays a direct role in the induction of aberrant DNA methylation. The median demethylation product 5-hydroxymethylcytosine (5-hmC) is considered as an activating epigenetic marker, and it plays complex roles in gene regulation of tumorigenesis.
A few small studies analyzed the association between 5-hmC levels and GC, but the evidence is lacking, especially for H. pylori-induced GC.
Exploring the association between 5-hmC level and the progression and prognosis of GC patients with or without H. pylori infection.
This was a retrospective cohort study to estimate the predicted value of 5-hmC level in the progression and prognosis of GC patients with different H. pylori infection status.
A high level of 5-hmC was an independent significant favorable predictor of overall survival in the entire GC patient cohort (hazard ratio = 0.61, 95% confidence interval: 0.38-0.98, P = 0.040), the H. pylori-negative GC subgroup (hazard ratio = 0.30, 95% confidence interval: 0.13-0.68, P = 0.004) and GC patients with early TNM stage (hazard ratio = 0.32, 95% confidence interval: 0.13-0.77, P = 0.011).
5-hmC level was a significant predictor of the prognosis of GC patients without H. pylori infection.
A large-scale GC patient cohort to assess the association between the level of 5-hmC and the prognosis of GC patients, especially for different H. pylori infection status, should be conducted.
We are grateful to all sample donors and the research assistants who helped with sample collection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jeong SH, South Korea; Kirkik D, Turkey A-Editor: Zhu JQ, China S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64636] [Article Influence: 16159.0] [Reference Citation Analysis (176)] |
| 2. | Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi ST, Siu HC, Deng S, Chu KM, Law S, Chan KH, Chan AS, Tsui WY, Ho SL, Chan AK, Man JL, Foglizzo V, Ng MK, Ching YP, Cheng GH, Xie T, Fernandez J, Li VS, Clevers H, Rejto PA, Mao M, Leung SY. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 835] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 3. | Nakajima T, Maekita T, Oda I, Gotoda T, Yamamoto S, Umemura S, Ichinose M, Sugimura T, Ushijima T, Saito D. Higher methylation levels in gastric mucosae significantly correlate with higher risk of gastric cancers. Cancer Epidemiol Biomarkers Prev. 2006;15:2317-2321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Maekita T, Nakazawa K, Mihara M, Nakajima T, Yanaoka K, Iguchi M, Arii K, Kaneda A, Tsukamoto T, Tatematsu M, Tamura G, Saito D, Sugimura T, Ichinose M, Ushijima T. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12:989-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 480] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 5. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2805] [Cited by in RCA: 2739] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 6. | Shimazu T, Asada K, Charvat H, Kusano C, Otake Y, Kakugawa Y, Watanabe H, Gotoda T, Ushijima T, Tsugane S. Association of gastric cancer risk factors with DNA methylation levels in gastric mucosa of healthy Japanese: a cross-sectional study. Carcinogenesis. 2015;36:1291-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Nakajima T, Enomoto S, Yamashita S, Ando T, Nakanishi Y, Nakazawa K, Oda I, Gotoda T, Ushijima T. Persistence of a component of DNA methylation in gastric mucosae after Helicobacter pylori eradication. J Gastroenterol. 2010;45:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Song CX, Yi C, He C. Mapping recently identified nucleotide variants in the genome and transcriptome. Nat Biotechnol. 2012;30:1107-1116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 9. | Mellén M, Ayata P, Dewell S, Kriaucionis S, Heintz N. MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell. 2012;151:1417-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 732] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 10. | Thomson JP, Lempiäinen H, Hackett JA, Nestor CE, Müller A, Bolognani F, Oakeley EJ, Schübeler D, Terranova R, Reinhardt D, Moggs JG, Meehan RR. Non-genotoxic carcinogen exposure induces defined changes in the 5-hydroxymethylome. Genome Biol. 2012;13:R93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Park JL, Kim HJ, Seo EH, Kwon OH, Lim B, Kim M, Kim SY, Song KS, Kang GH, Choi BY, Kim YS. Decrease of 5hmC in gastric cancers is associated with TET1 silencing due to with DNA methylation and bivalent histone marks at TET1 CpG island 3'-shore. Oncotarget. 2015;6:37647-37662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Yang Q, Wu K, Ji M, Jin W, He N, Shi B, Hou P. Decreased 5-hydroxymethylcytosine (5-hmC) is an independent poor prognostic factor in gastric cancer patients. J Biomed Nanotechnol. 2013;9:1607-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2049] [Article Influence: 256.1] [Reference Citation Analysis (0)] |
| 14. | Alipour M. Molecular Mechanism of Helicobacter pylori-Induced Gastric Cancer. J Gastrointest Cancer. 2021;52:23-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 153] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 15. | Elzouki AN, Buhjab SI, Alkialani A, Habel S, Sasco AJ. Gastric cancer and Helicobacter pylori infection in the eastern Libya: a descriptive epidemiological study. Arab J Gastroenterol. 2012;13:85-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Flavahan WA, Gaskell E, Bernstein BE. Epigenetic plasticity and the hallmarks of cancer. Science. 2017;357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 923] [Article Influence: 131.9] [Reference Citation Analysis (0)] |
| 17. | Do C, Shearer A, Suzuki M, Terry MB, Gelernter J, Greally JM, Tycko B. Genetic-epigenetic interactions in cis: a major focus in the post-GWAS era. Genome Biol. 2017;18:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 18. | Liu H, Xu T, Cheng Y, Jin MH, Chang MY, Shu Q, Allen EG, Jin P, Wang X. Altered 5-Hydroxymethylcytosine Landscape in Primary Gastric Adenocarcinoma. DNA Cell Biol. 2019;38:1460-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Zhao R, Liu Z, Xu W, Song L, Ren H, Ou Y, Liu Y, Wang S. Helicobacter pylori infection leads to KLF4 inactivation in gastric cancer through a TET1-mediated DNA methylation mechanism. Cancer Med. 2020;9:2551-2563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Strand SH, Hoyer S, Lynnerup AS, Haldrup C, Storebjerg TM, Borre M, Orntoft TF, Sorensen KD. High levels of 5-hydroxymethylcytosine (5hmC) is an adverse predictor of biochemical recurrence after prostatectomy in ERG-negative prostate cancer. Clin Epigenetics. 2015;7:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Mariani CJ, Vasanthakumar A, Madzo J, Yesilkanal A, Bhagat T, Yu Y, Bhattacharyya S, Wenger RH, Cohn SL, Nanduri J, Verma A, Prabhakar NR, Godley LA. TET1-mediated hydroxymethylation facilitates hypoxic gene induction in neuroblastoma. Cell Rep. 2014;7:1343-1352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Tsai YP, Chen HF, Chen SY, Cheng WC, Wang HW, Shen ZJ, Song C, Teng SC, He C, Wu KJ. TET1 regulates hypoxia-induced epithelial-mesenchymal transition by acting as a co-activator. Genome Biol. 2014;15:513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 23. | Valenzuela-Valderrama M, Cerda-Opazo P, Backert S, González MF, Carrasco-Véliz N, Jorquera-Cordero C, Wehinger S, Canales J, Bravo D, Quest AFG. The Helicobacter pylori Urease Virulence Factor Is Required for the Induction of Hypoxia-Induced Factor-1α in Gastric Cells. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Abass A, Okano T, Boonyaleka K, Kinoshita-Daitoku R, Yamaoka S, Ashida H, Suzuki T. Effect of low oxygen concentration on activation of inflammation by Helicobacter pylori. Biochem Biophys Res Commun. 2021;560:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |