Published online Jul 15, 2022. doi: 10.4251/wjgo.v14.i7.1252
Peer-review started: December 31, 2021
First decision: March 10, 2022
Revised: March 22, 2022
Accepted: May 28, 2022
Article in press: May 28, 2022
Published online: July 15, 2022
Processing time: 193 Days and 15.7 Hours
Pancreatic ductal adenocarcinoma (PDAC) is frequently diagnosed and treated in advanced tumor stages with poor prognosis. More effective screening programs and novel therapeutic means are urgently needed. Recent studies have regarded tight junction protein claudin 18.2 (CLDN18.2) as a candidate target for cancer treatment, and zolbetuximab (formerly known as IMAB362) has been developed against CLDN18.2. However, there are few data reported thus far related to the clinicopathological characteristics of CLDN18.2 expression for PDAC.
To investigate the expression of CLDN18.2 in PDAC patients and subsequently propose a new target for the treatment of PDAC.
The Cancer Genome Atlas, Genotype-Tissue Expression, Gene Expression Omnibus, and European Genome-phenome Archive databases were first employed to analyze the CLDN18 gene expression in normal pancreatic tissue compared to that in pancreatic cancer tissue. Second, we analyzed the expression of CLDN18.2 in 93 primary PDACs, 86 para-cancer tissues, and 13 normal pancreatic tissues by immunohistochemistry. Immunostained tissues were assessed applying the histoscore. subsequently, they fell into two groups according to the expression state of CLDN18.2. Furthermore, the correlations between CLDN18.2 expression and diverse clinicopathological characteristics, including survival, were investigated.
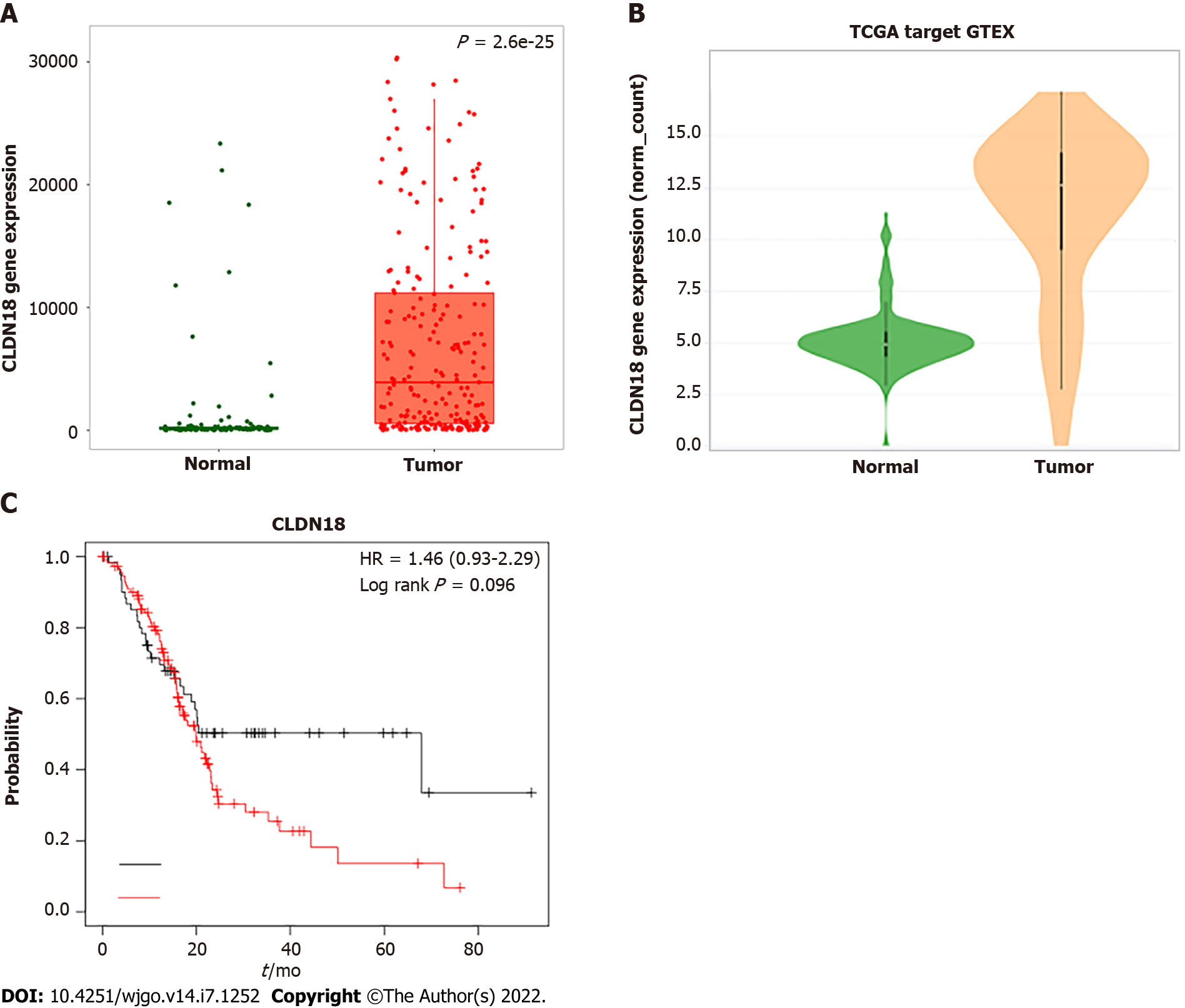
The gene expression of CLDN18 was statistically higher (P < 0.01) in pancreatic tumors than in normal tissues. However, there was no significant correlation between CLDN18 expression and survival in pancreatic cancer patients. CLDN18.2 was expressed in 88 (94.6%) of the reported PDACs. Among these tumors, 50 (56.8%) cases showed strong immunostaining. The para-cancer tissues were positive in 81 (94.2%) cases, among which 32 (39.5%) of cases were characterized for strong staining intensities. Normal pancreatic tissue was identified solely via weak immunostaining. Finally, CLDN18.2 expression significantly correlated with lymph node metastasis, distant metastasis, nerve invasion, stage, and survival of PDAC patients, while there was no correlation between CLDN18.2 expression and localization, tumor size, patient age and sex, nor any other clinicopathological characteristic.
CLDN18.2 expression is frequently increased in PDAC patients. Thus, it may act as a potential therapeutic target for zolbetuximab in PDAC.
Core Tip: Claudin 18.2 (CLDN18.2) shows a high rate of expression in pancreatic ductal adenocarcinoma (PDAC) but displays little expression in normal pancreatic tissue. CLDN18.2 expression significantly correlates with lymph node metastasis, distant metastasis, nerve invasion, stage, and survival of PDAC patients. Thus, CLDN18.2 may act as an ideal therapeutic target, and a considerable number of PDAC patients may be in eligible for a CLDN18.2-targeted therapeutic approach.
- Citation: Wang X, Zhang CS, Dong XY, Hu Y, Duan BJ, Bai J, Wu YY, Fan L, Liao XH, Kang Y, Zhang P, Li MY, Xu J, Mao ZJ, Liu HT, Zhang XL, Tian LF, Li EX. Claudin 18.2 is a potential therapeutic target for zolbetuximab in pancreatic ductal adenocarcinoma. World J Gastrointest Oncol 2022; 14(7): 1252-1264
- URL: https://www.wjgnet.com/1948-5204/full/v14/i7/1252.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i7.1252
Pancreatic cancer is the eleventh most frequently diagnosed cancer and the sixth most common cause of cancer-related deaths in China, being only slightly lower than the rates reported from the United States and United Kingdom[1]. However, the overall incidence and mortality rates of pancreatic cancer are expected to increase further[2]. Pancreatic ductal adenocarcinoma (PDAC) accounts for more than 90% of all pancreatic neoplasms. Yet, there is no effective screening tool for early detection of PDAC, and patients lack specific clinical symptoms at early stages. Thus, most patients are usually diagnosed at the advanced stage with distant metastases and are not suitable for curable surgery, aggravating its poor prognosis[3]. It is therefore urgent to develop nonsurgical therapeutic approaches for effective treatment of PDAC.
For systemic palliative treatment of unresectable PDAC patients, chemotherapy is the first-line approach. The majority of patients are treated with FOLFIRINOX (5-fluorouracil/irinotecan/ oxaliplatin)[4] and gemcitabine-based chemotherapy, including combinations of gemcitabine and nanoparticle albumin-bound paclitaxel (nab-paclitaxel)[5], gemcitabine, and erlotinib[6]. These combination therapies exhibit an improvement in median and 1-year survival rates as compared with gemcitabine alone. However, the chemosensitivity of PDAC is moderate, and as the benefits of adding erlotinib are marginal but the toxicity of the combination is higher, erlotinib has not been widely adopted[6].
Immunotherapy has great success in treating many types of cancers, whereas it has not been very successful against PDAC. Most clinical outcomes of immunotherapy with immune checkpoint inhibitors, chimeric antigen receptor T cells, immunomodulators, and vaccines were not satisfactory[7]. Therefore, immunotherapy is not recommended as a conventional treatment by the guidelines for PDAC. However, we cannot totally deny the immunotherapeutic potential. With a deeper level understanding of the PDAC immunology and mechanisms of immunotherapeutic resistance, immunotherapy may achieve great success in treating PDAC. A clinical trial showed that BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer enhanced the objective response rate, disease control rate, and median duration in PDAC[8]. Another study revealed that combination treatment of a vaccine p53MVA and pembrolizumab (an immune checkpoint inhibitor of programmed death protein 1) had higher cure rate or longer survival time than the control group, but there were still many patients who suffered grade 1-2 adverse events, despite the small sample size[9]. Therefore, combination immunotherapy with or without chemoradiotherapy may be one of the future directions of immunotherapy application for treating PDAC. Novel treatments and early detection tools are still urgently needed for this highly aggressive and lethal disease.
Claudin 18 (CLDN18) is a highly specific tight junction protein, encoded by the CLDN18 gene, regulating paracellular barrier functions. Its two isoforms are known as isoform 1 (CLDN18.1) and isoform 2 (CLDN18.2). Expression of CLDN18.2 has been revealed to be confined to short lived differentiated gastric epithelial cells of the primary gastric carcinoma and normal gastric mucosa, which suggesting its potential as a candidate therapeutic target in cancer treatment[10,11]. CLDN18.2 expre
Zolbetuximab is a highly potent and tumor cell-selective therapeutic antibody that directly targets the tight junction molecule CLDN18.2, a proliferation-promoting transmembrane protein[14]. Zolbetuximab is currently in clinical testing. The phase II clinical trial (FAST: NCT01630083) revealed that zolbetuximab combined with first-line chemotherapy significantly improved the overall survival, progression-free survival and the objective response rate with acceptable safety and tolerability in patients with CLDN18.2-positive advanced/recurrent gastric cancers and gastroesophageal junction cancers[15]. Furthermore, health-related quality of life was sustained for a longer duration in patients who received zolbetuximab plus chemotherapy compared with those who received chemotherapy alone[16]. This prompted us to consider clinical testing of zolbetuximab in PDAC. Since few data are available regarding the clinicopathological characteristics of CLDN18.2 expression for PDAC, this study was designed and carried out as a part of the prefeasibility program for such clinical trials.
Expression of the CLDN18 gene in normal pancreatic tissue and pancreaticcancer was analyzed using TNMplot.com (https://tnmplot.com/analysis/)[17] and Xena (http://xena.ucsc.edu/compare-tissue/)[18], which allow for online analysis of The Cancer Genome Atlas (TCGA), Genotype-Tissue Expression, and Gene Expression Omnibus (GEO) data. In this study, TCGA and GEO offered the pancreatic tumor samples and solid tissue normal samples from individuals with cancer, while the Genotype-Tissue Expression offered normal tissue from individuals who did not have cancer. In addition, we used KM plotter to assess the effect of CLDN18 on survival in pancreatic cancer (https://kmplot.com/analysis/), which is based on the databases of TCGA, GEO, and European Genome-phenome Archive.
The primary tumor samples and para-cancer tissues as well as normal pancreatic tissues were collected between 2018 and 2020 at the Institute of Pathology of the First Affiliated Hospital of Xi’an Jiaotong University (Xi’an, Shaanxi Province, China). We included patients with pathologically confirmed PDAC. Patients with a tumor type different from PDAC were excluded. Each tissue had gone through gross sectioning and histological detection by qualified pathologists. The date of patients’ deaths was collected from the hospital records. Follow-up data of the patients who were still alive were obtained from the telephone follow-up and hospital records. The histopathological diagnosis and grading followed the recommendations of the World Health Organization, and the tumor stage was confirmed in accordance with the 8th edition of American Joint Committee on Cancer staging system. Sampling of tissues and clinical data for scientific purposes was approved by the ethics committee of The First Affiliated Hospital of Xi’an Jiaotong University. Additionally, tissue microarrays spotted with samples of primary PDAC and para-cancer tissues were bought from Shanghai Zhuo hao Pharmaceutical Technology Co., LTD (Shanghai, China) (Cat. No. PAC1602).
Immunohistochemistry (IHC) was performed on slides of 4% buffered formalin-fixed paraffin-embedded samples. Deparaffinized tissue slice were stained with hematoxylin and eosin. Immunohistochemical CLDN18.2 staining used the anti-CLDN18.2 antibody (Rabbit monoclonal EPR19202, Cat No. ab222512; Abcam, Cambridge, United Kingdom) in 1:500 dilution on a BOND-MAX automated staining system with Leica Bond Polymer Refine Detection Kit (Leica Biosystems, Wetzlar, Germany).
Scoring of 93 primary PDACs, 86 para-cancer tissues, and 13 normal pancreatic tissues was assessed by using a semi-quantitative pathology histoscore (H-score), defined as a method combined both percentages of positive-expression cells in the tissue slice and immunostaining intensities (hereinafter referred to as IHC-score). The IHC-score was on account of the membranous staining intensity level of CLDN18.2 from 0 to 1+ (weak), 2+ (intermediate), or 3+ (strong). Only membranous staining was retained for scoring. Nuclear and/or cytoplasmic CLDN18.2 expression was just noted but not scored. Tissue was assessed as IHC-score 0 (no staining was detectable), 1+ (faint membranous staining was partially showed), 2+ (moderate membranous staining was observed), or 3+ (strong membranous staining was present in the tissue section). In brief, the H-score was calculated according to the formula: (0 × percentage of immunonegative cells) + (1 × percentage of weakly stained cells) + (2 × percentage of intermediately stained cells) + (3 × percentage of strongly stained cells). Thus, the H-scoring ranged from 0 (a tissue sample that is completely negative) to a maximum of 300 (a tissue sample in which all the cells show a 3+ staining), which can separate samples with a predominantly high staining intensity from samples with a predominantly low staining intensity more distinctively. All samples of this study were assessed by two pathologists working independently. In case of discrepancies in the assessments, the sections were discussed to reach a final agreement.
For the purpose of finding correlations between CLDN18.2 expression and clinicopathological characteristics of PDAC patients, the tissues were divided into two groups according to the median H-score: negative/low (≤ median) and positive/high (> median).
During the process of reviewing both IHC-score and H-score, we found the obvious intratumoral heterogeneity in PDAC. Due to lack of accredited guideline to evaluate the heterogeneity between PDAC patients, some literature materials were referenced and we classified the heterogeneity according to the IHC-score, if 3+ and 0 were present meanwhile in one tumor tissue and accounted for more than 50% combined, we thought the strong heterogeneous expression was showed[14]. Additionally, we assessed the immunostaining patterns of these heterogeneous tumors. Some tumor cells of PDAC showed diffusely distribution with low or no IHC staining, which we referred to as “scattered”. Another heterogeneity pattern of tumors with a “downward gradient” pattern displayed an obvious decline in intensity of the immunostaining towards the deep of the tissue.
SPSS version 24.0 (IBM Corp., Armonk, NY, United States) was used for statistical analyses. For assessing the correlation between non-ordinal variables, we applied the χ2 test and Fisher’s exact test. To make up for the false discovery rate in the correlations, we used the Simes’ procedure, also known as Benjamini-Hochberg procedure. Multivariate analysis was performed to evaluate if a significant factor correlated to CLDN18.2 expression was an independent factor. The Kaplan-Meier method was used to determine median survival with 95%CIs and Log-rank test was applied to assess the differences between median survivals. Furthermore, Cox’s regression model was performed for the Multivariate survival analysis. P < 0.05 was accepted as demonstration of significant differences.
The TNMplot.comanalysis involved 108 normal tissues and 248 pancreatic tumors. We found that the gene expression of CLDN18 in pancreatic tumors was much higher than that in normal tissues, and the difference was statistically significant (P < 0.01) (Figure 1A). Xena analysis of the gene expression of CLDN18 in 167 normal tissues and 183 pancreatic tumors yielded results that were consistent with those from TNMplot.com (Figure 1B). KM plotter assessment of the effect of CLDN18 expression on survival in 177 pancreatic cancer patients revealed no significant correlation between CLDN18 expression and survival (Figure 1C).
We observed a set of non-neoplastic pancreatic tissue samples (n = 13) for CLDN18.2 expression. All histological cell types and distinct structures of normal pancreatic tissue, such as duct cells, acinar cells, and endocrine cells, were observed. CLDN18.2-specific staining was not detectable in any of the normal pancreatic tissue cells. Representative images are displayed in Supplementary Figure 1.
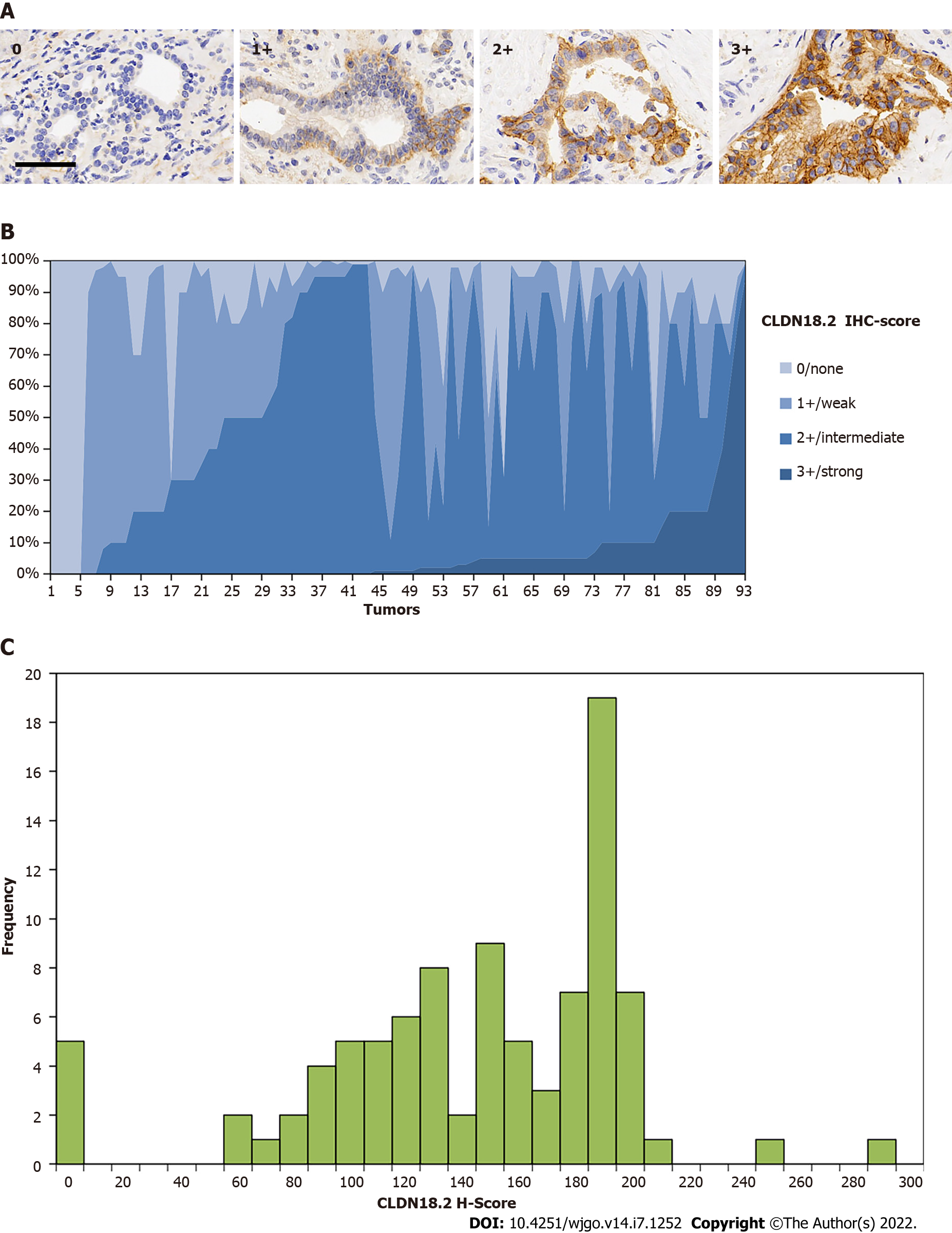
Eighty-six para-cancer tissues of PDAC were analyzed for CLDN18.2 expression. We found that 81 (94.2%) cases showed the positive fraction ≥ 1%, in which 32 (39.5%) cases were characterized as strong staining intensities (Table 1).
| Sample type | Samples, n | CLDN18.2 expression | |
| Positive fraction ≥ 1%, n (%) | Staining intensity = 3+, n (%) | ||
| PDAC | 93 | 88 (94.6) | 50 (56.8) |
| Para-cancerous | 86 | 81 (94.2) | 32 (39.5) |
| Normal | 13 | 0 (0.0) | 0 (0.0) |
In total, 93 cases of primary PDAC were analyzed for CLDN18.2 expression. The average age was 63.48 (51.6%). PDAC samples were poorly differentiated (i.e. grade 3). Twenty-seven (29.0%) cases were classified as pT3/4. Thirty-six (38.7%) cases had already-confirmed lymph node invasion (pN1/2), twenty-three (24.7%) cases were confirmed as nerve invasion, and fourteen (15.1%) cases presented distant metastasis at the time of first diagnosis (Table 2).
| Clinicopathological parameter | Variable | Total valid, n (%) | CLDN18.2 expression | P value | |
| Positive, n (%) | Negative, n (%) | ||||
| Age | < 63 | 49 (52.7) | 23 (46.9) | 26 (53.1) | 0.680 |
| ≥ 63 | 44 (47.3) | 23 (52.3) | 21 (47.7) | ||
| Sex | Female | 44 (47.3) | 24 (54.5) | 20 (45.5) | 0.409 |
| Male | 49 (52.7) | 22 (44.9) | 27 (55.1) | ||
| Localization | Head and neck | 52 (65.0) | 23 (44.2) | 29 (55.8) | 0.350 |
| Body and tail | 28 (35.0) | 16 (57.1) | 12 (42.9) | ||
| CA199 | High1 | 65 (81.3) | 33 (50.8) | 32 (49.2) | 0.570 |
| Normal2 | 15 (18.7) | 6 (40.0) | 9 (60.0) | ||
| T category | T1 | 14 (15.1) | 6 (42.9) | 8 (57.1) | 0.773 |
| T2 | 47 (50.5) | 24 (51.1) | 23 (48.9) | ||
| T3 | 26 (28.0) | 14 (53.8) | 12 (46.2) | ||
| T4 | 6 (6.4) | 2 (33.3) | 4 (66.7) | ||
| T1 + T2 | 61 (65.6) | 30 (49.2) | 31 (50.8) | 1.000 | |
| T3 + T4 | 32 (34.4) | 16 (50.0) | 16 (50.0) | ||
| T1 | 14 (15.1) | 6 (42.9) | 8 (57.2) | 0.773 | |
| T2 + T3 + T4 | 79 (84.9) | 40 (50.6) | 39 (49.4) | ||
| N category | N0 | 57 (61.3) | 23 (40.4) | 34 (59.6) | 0.019a |
| N1 | 27 (29.0) | 15 (55.6) | 12 (44.4) | ||
| N2 | 9 (9.7) | 8 (88.9) | 1 (11.1) | ||
| N0 | 57 (61.3) | 23 (40.4) | 34 (59.6) | 0.034a | |
| N1 + N2 | 36 (38.7) | 23 (63.9) | 13 (36.1) | ||
| M category | M0 | 79 (84.9) | 35 (44.3) | 44 (55.7) | 0.022a |
| M1 | 14 (15.1) | 11 (78.6) | 3 (21.4) | ||
| AJCC stage | I | 38 (40.9) | 16 (42.1) | 22 (57.9) | 0.058 |
| II | 28 (30.1) | 11 (39.3) | 17 (60.7) | ||
| III | 13 (14.0) | 8 (61.5) | 5 (38.5) | ||
| IV | 14 (15.0) | 11 (78.6) | 3 (21.4) | ||
| I | 38 (40.9) | 16 (42.1) | 22 (57.9) | 0.293 | |
| II + III + IV | 55 (59.1) | 30 (54.5) | 25 (45.5) | ||
| I + II | 66 (71) | 27 (40.9) | 39 (59.1) | 0.012a | |
| III + IV | 27 (29) | 19 (70.4) | 8 (29.6) | ||
| I + II + III | 79 (84.9) | 35 (44.3) | 44 (55.7) | 0.022a | |
| IV | 14 (15.1) | 11 (78.6) | 3 (21.4) | ||
| Local infiltration | Yes | 57 (71.3) | 29 (50.9) | 28 (49.1) | 0.625 |
| No | 23 (28.7) | 10 (43.5) | 13 (56.5) | ||
| Vascular invasion | Yes | 12 (15.0) | 7 (58.3) | 5 (41.7) | 0.542 |
| No | 68 (85.0) | 32 (47.1) | 36 (52.9) | ||
| Nerve invasion | Yes | 23 (28.7) | 17 (73.9) | 6 (26.1) | 0.006b |
| No | 57 (71.3) | 22 (38.6) | 35 (61.4) | ||
| Vessel carcinoma embolus | Yes | 27 (33.8) | 13 (48.1) | 14 (51.9) | 1.000 |
| No | 53 (66.2) | 26 (49.1) | 27 (50.9) | ||
| Grading | G1/G2 | 45 (48.4) | 20 (44.4) | 25 (55.6) | 0.409 |
| G3 | 48 (51.6) | 26 (54.2) | 22 (45.8) | ||
CLDN18.2 presented quite high expression rate in PDAC patients, with 88 (94.6%) PDACs showed positive expression (Table 1), in which most patients showed compositive IHC-intensity. Fifty (56.8%) cases were scored up to IHC 3+, eighty-six (97.7%) cases were scored equivalent to but no more than IHC 2+, seventy-seven (87.5%) cases were no higher than IHC 1+ (representative images are displayed in Figure 2A). The supreme expression of CLDN18.2 IHC 3+ was discovered with 94.0% of tumor cells, observable in 1 case. The IHC-score distribution of CLDN18.2 in this study is exhibited in Figure 2B. Figure 2C summed up the distribution and frequency of the H-scores.
Group comparison analysis revealed that CLDN18.2 correlated with lymph node metastasis, distant metastasis, nerve invasion, and stage (Table 2). In our study, the N category was assessed in 93 cases, including N0 (n = 57), N1 (n = 27), and N2 (n= 9). CLDN18.2 positivity showed the following distribution of N categories: N0 in 23 (40.4%) cases; N1 in 15 (55.6%) cases; and N2 in 8 (88.9%) cases. There was a statistically significant difference between them (P = 0.019). When we stratified the lymph node metastasis, we found the difference also existed (P = 0.034). CLDN18.2 expression was predominantly increased in the cases of lymph node invasion (pN1/2). A similar observation was also made for distant metastasis. Compared to patients with M0, the expression of CLDN18.2 significantly increased in PDAC patients with distant metastases (78.6% vs 44.3%, P = 0.022). Moreover, we found that 17 cases with nerve invasion showed positive CLDN18.2 expression, while the patients without nerve invasion showed much lower expression (73.9% vs 38.5%, P = 0.006), the difference between two group was statistically significant.
Furthermore, it is interesting to note that the relative proportion of positive CLDN18.2 expression was not different between the four stage groups (I, II, III and IV). But when we stratified it, the correlation was observed. CLDN18.2 expression was significantly increased in III + IV stages than that in I + II stages (70.4% vs 40.9%, P = 0.012). The cases with stage IV showed significantly higher CLDN18.2 expression than I + II + III stages (78.6% vs 44.3%, P = 0.022).
To evaluate if any of the significant factors correlated to CLDN18.2 expression was an independent factor, we performed multivariate analysis. We found that the significant factors of stage, lymph node metastasis, distant metastasis, and nerve invasion related to the expression of CLDN18.2 as independent factors. Corresponding P values were all less than 0.05.
We demonstrated that the expression of CLDN18.2 had no relevance with T category and grading (Table 2). No other clinicopathological characteristic of PDAC patient, for example, age, sex, tumor site, CA199, local infiltration, vascular invasion, or vessel carcinoma embolus, correlated with CLDN18.2 expression.
In our study, almost all tumors showed compositive IHC-intensity with IHC 3+ and IHC 0 were present meanwhile in one tumor tissue, revealing the expression of CLDN18.2 had a high tendency to heterogeneous expression. In order to elaborate the degree of tumor heterogeneity, it was considered that if both strong and negative expressions were existed simultaneously and accounted for more than 50% combined, the tumor showed strong heterogeneity. Nine (9.7%) tumors met these criteria. We assessed the different immunostaining distribution patterns of these heterogeneous tumors. Six (66.7%) PDACs showed a “scattered” pattern, which had diffusely distribution with low or no IHC staining in tumor cells. Three (33.3%) PDACs displayed a “downward gradient”, with weaker staining intensity towards the depth of the tumor. Representative images are displayed in Supplementary Figure 2.
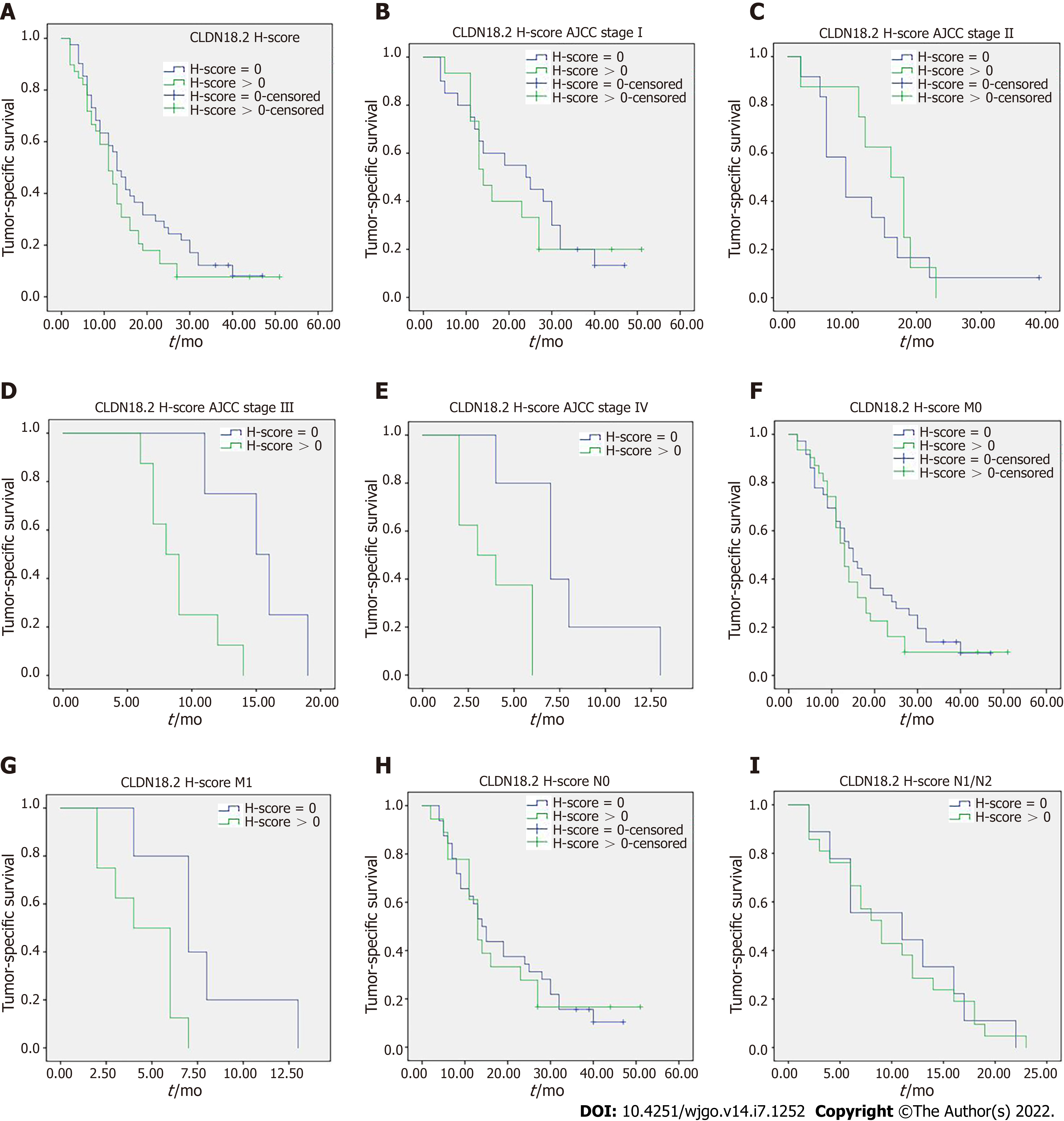
Tumor-specific survival data were available in 80 cases and no correlation was discovered between the cancer specific survival and expression of CLDN18.2 (Figure 3A). Nevertheless, when stratified analysis was applied to verify the influence of diverse CLDN18.2 expression on various tumor stages (American Joint Committee on Cancer) and different N category and M category, the correlation was discovered. The study revealed that the expression of CLDN18.2 correlated with cancer survival of PDAC patients with stage III, stage IV, and distant metastasis meaningfully (Figure 3B-I). This suggests the CLDN18.2-positive patients with late stage and distant metastasis may have a poorer prognosis.
PDAC is still difficult to diagnose and has a poor prognosis. The main aim of this study was to investigate CLDN18.2 expression in a large PDAC patient population using IHC and then find its correlation with diverse clinicopathological characteristics, including survival in order to detect possible distinctive features of CLDN18-positive PDACs and assess whether it is a suitable indication for clinical development of zolbetuximab, the therapeutic antibody directed against CLDN18.2.
Previous studies reported CLDN18 expression in 50%-90% of pancreatic cancer[12,13,19]. These studies involved patients with different cancer types and different stages, and they used different grade staining protocols and various anti-CLDN18 antibodies or sera, which is a drawback because of cross-reactivity to CLDN18.1. Furthermore, different approaches to analyze and score CLDN18 positivity status were implemented. Therefore, it was not suitable to completely rely on these data for a clinical development program. To further add to the validity and reliability of the obtained data, we used H-score to assess the CLDN18.2 expression, which combined both the fraction of stained tumor cells and intensity of cell surface staining. It can separate the sample’s staining intensity more distinctively. Based on this, our study has the following major key findings, which are novel and support indications for clinical testing of zolbetuximab in PDAC patients.
The ideal therapeutic target should show high and specific expression in the tumor and show a very low or no expression in normal tissues. The majority of PDACs in our study showed a high rate of CLDN18.2 positivity, but all normal pancreatic tissue showed CLDN18.2 negativity. Thus, CLDN18.2 may act as an ideal therapeutic target, and a considerable number of PDAC patients would be eligible for a CLDN18.2-targeting therapeutic approach. However, we need to realize that the expression of a target does not necessarily mean that a patient will definitely benefit from the respective targeting drug. The clinical curative effect may depend on the intensity of expression[20], the fraction of positive tumor cells, or may not be associated at all to the target expression state[21]. Well-controlled clinical trials should be designed to investigate the therapeutic agent of our CLDN18.2-targeting approach. It is noteworthy that almost 86 (92.5%) tumors assessed in this study presented at least 2+ cell surface expression of CLDN18.2, and the majority of tumor tissue displayed a relatively high fraction of positive cells (median was 50%). This indicates that even if the clinical benefit requires high expression of CLDN18.2, a considerable number of PDAC patients will still be eligible.
In addition, the correlation analysis revealed that the fractions of positive cells and the intensities of membrane staining of CLDN18.2 were significantly higher in lymph node-positive tumors, distant metastatic tumors, nerve invasion tumors, and stage III/IV PDAC patients. Lymph node positivity and distant metastasis were independent factors for poor prognosis in PDAC[22]. Moreover, CLDN18.2 expression correlated to cancer survival of PDAC patients with stage III, stage IV, and distant metastasis meaningfully, which was not in accordance with the result from the database (Figure 1C and Figure 3). The reason for this inconsistency may be that the database analyzed the relevance between gene expression and cancer survival, whereas our research explored the relationship between protein expression and cancer survival. The survival data from the database was analyzed but not stratified. This result also needs to be verified in more substantial pancreatic cancer patients. Besides, CLDN18.2 expression was not associated with tumor size, differentiation, localization, CA199, local infiltration, vascular invasion, nor vessel carcinoma embolus. These data revealed that CLDN18.2 might play a role as an oncogene in the development and progression of pancreatic cancer, and the expression of this gene could promote the aggressiveness of tumor cells. Therefore, CLDN18.2 has the potential to act as a risk assessment and as a prognostic indicator for PDAC.
While some researchers have reported weak expression of CLDN18 in normal pancreatic tissue[19], others have denied it. Our study confirmed that CLDN18.2 was not expressed in normal pancreatic tissue including all different cell types prevalent in the pancreas. More interestingly, we found that CLDN18.2 expression was increased in para-cancer tissues and higher in PDAC tissues. This gradual upward trend of CLDN18.2 expression has not been reported before, which suggests that CLDN18.2 is silenced in normal pancreatic tissue but strongly activated during the course of malignant occurrence and development. However, there is little research reporting the molecular mechanism of CLDN18.2. Combined with the previous correlation analysis results, we thus hypothesize that CLDN18.2 may be involved in the tumor migration process, but further experiments are needed to test this hypothesis and explore the exact molecular mechanism of CLDN18.2.
Moreover, the differential expression of CLDN18.2 in normal pancreatic tissue and pancreatic neoplasm suggests that CLDN18.2 can be used as a diagnostic marker for PDAC. This has been reported in other studies. Li et al[23] reported the sensitivity of CLDN18 for identifying the gastric and pancreatobiliary tract as primary tumor sites was 79% and the specificity was 93%. The positive and negative predictive values were 76% and 94%, respectively, which indicated that CLDN18 represented a sensitive and specific marker for stomach and pancreatobiliary adenocarcinoma that might be a useful diagnostic tool in routine surgical pathology. However, CLDN18.2 heterogeneity poses a challenge to diagnostic evaluations. In the light of distributions of IHC-score and H-score, this research demonstrated a universal phenomenon of CLDN18.2 expression heterogeneity in PDAC (Supplementary Figure 2), and then we describe heterogeneity types, which likely bring huge challenges to scientific explore and clinical practice. For example, one small tumor specimen with a scattered pattern may lead a serious misjudgment of total expression rate. In addition, the occurrence of the “downward gradient” staining pattern that shows obvious decline in intensity of the immunostaining towards the depth of the tissue may have some impact on biopsy within the deep of PDAC tissue specimen, which mainly allow evaluation of the superficial malignant tumor tissues. Therefore, we should obtain as much tissue as possible when taking a biopsy so that the accuracy of diagnosis can be further improved.
This study suffered from a few limitations that deserve to be underlined. First, our study was limited by the types of samples. We described and illustrated CLDN18.2 expression in PDAC but not in other types of pancreatic tumors, such as adenosquamous carcinoma and pancreatic endocrine neoplasms. Second, we were limited by the numbers of samples. More large-scale studies need to be conducted to further analyze CLDN18.2 expression in PDAC in the future.
In general, this research describes a specified illustration for the expression of CLDN18.2 and its relationship with different clinicopathological elements in PDAC. We conclude CLDN18.2 is a potential therapeutic target for the treatment of PDAC.
Pancreatic ductal adenocarcinoma (PDAC) is frequently diagnosed and treated in advanced tumor stages with a poor prognosis. More effective screening programs and novel therapeutic means are urgently needed. The tight junction protein claudin 18.2 (CLDN18.2) has been proved as a novel candidate drug target for cancer treatment, and zolbetuximab (formerly known as IMAB362) has been developed against CLDN18.2. Due to the few data available for clinicopathological characteristics of CLDN18.2 expression in PDAC, this study was performed to evaluate CLDN18.2 expression and to determine whether it can act as a potential therapeutic target for PDAC patients.
Zolbetuximab is a highly potent and tumor cell-selective therapeutic antibody that directly targets the tight junction molecule CLDN18.2. Zolbetuximab is currently in clinical testing and has shown good therapeutic effect. This prompted us to consider clinical testing of zolbetuximab in PDAC. Since few data are available for clinicopathological characteristics of the expression of CLDN18.2 in PDAC, this study is part of the prefeasibility program for such clinical trials.
The present study designed to investigate the CLDN18.2 expression in PDAC patients, and subsequently analyze its relevance with diverse clinicopathological characteristics of PDAC, and then propose a novel target for the cancer treatment of PDAC.
The databases, including The Cancer Genome Atlas, Genotype-Tissue Expression, Gene Expression Omnibus, and European Genome-phenome Archive, were used to analyze the expression of the CLDN18 gene in normal pancreatic tissue and pancreatic cancer. Immunohistochemistry was used to analyze the expression of CLDN18.2 in 93 primary PDACs, 86 para-cancer tissues, and 13 normal pancreatic tissues. Immunostained tissues were assessed applying the histoscore and subsequently fell into two groups according to detection of any or no CLDN18.2 expression. Furthermore, the correlations between CLDN18.2 expression and diverse clinicopathological characteristics, including survival, were investigated.
Reports found in the searched databases showed that the gene expression of CLDN18 in pancreatic tumors was much higher than that in normal tissues. Moreover, the difference was statistically significant (P < 0.01), and there was no significant correlation between CLDN18 expression and survival in pancreatic cancer patients. CLDN18.2 was expressed in 88 (94.6%) PDACs. Of these tumors, 50 (56.8%) cases showed strong immunostaining. The para-cancer tissues were positive in 81 (94.2%) cases, in which 32 (39.5%) cases were characterized as having strong staining intensities. Normal pancreatic tissue showed only weak immunostaining. CLDN18.2 expression significantly correlated with lymph node metastasis, distant metastasis, nerve invasion, stage, and survival of PDAC patients, while there was no correlation between CLDN18.2 expression and localization, tumor size, patient age and sex, nor any other clinicopathological characteristic.
CLDN18.2 expression is frequently increased in PDAC patients. Thus, it may act as a potential therapeutic target for zolbetuximab in pancreatic ductal adenocarcinoma.
This study is part of the prefeasibility program for some clinical trials that applied zolbetuximab in PDAC patients.
The authors would like to acknowledge all staff members of the department of Cancer Precision Medicine, Med-X Institute for skillful technical assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pathology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Plougmann JI, Denmark; Sato H, Japan A-Editor: Nakaji K, Japan S-Editor: Zhang H L-Editor: A P-Editor: Yu HG
| 1. | Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun (Lond). 2021;41:1037-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 584] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 2. | Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694-9705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1003] [Cited by in RCA: 957] [Article Influence: 106.3] [Reference Citation Analysis (24)] |
| 3. | Yu S, Zhang C, Xie KP. Therapeutic resistance of pancreatic cancer: Roadmap to its reversal. Biochim Biophys Acta Rev Cancer. 2021;1875:188461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 4. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5637] [Article Influence: 402.6] [Reference Citation Analysis (1)] |
| 5. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4887] [Article Influence: 407.3] [Reference Citation Analysis (0)] |
| 6. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W; National Cancer Institute of Canada Clinical Trials Group. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 2776] [Article Influence: 154.2] [Reference Citation Analysis (0)] |
| 7. | Wu J, Cai J. Dilemma and Challenge of Immunotherapy for Pancreatic Cancer. Dig Dis Sci. 2021;66:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Bockorny B, Semenisty V, Macarulla T, Borazanci E, Wolpin BM, Stemmer SM, Golan T, Geva R, Borad MJ, Pedersen KS, Park JO, Ramirez RA, Abad DG, Feliu J, Muñoz A, Ponz-Sarvise M, Peled A, Lustig TM, Bohana-Kashtan O, Shaw SM, Sorani E, Chaney M, Kadosh S, Vainstein Haras A, Von Hoff DD, Hidalgo M. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat Med. 2020;26:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 372] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 9. | Chung V, Kos FJ, Hardwick N, Yuan Y, Chao J, Li D, Waisman J, Li M, Zurcher K, Frankel P, Diamond DJ. Evaluation of safety and efficacy of p53MVA vaccine combined with pembrolizumab in patients with advanced solid cancers. Clin Transl Oncol. 2019;21:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Sahin U, Koslowski M, Dhaene K, Usener D, Brandenburg G, Seitz G, Huber C, Türeci O. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin Cancer Res. 2008;14:7624-7634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 300] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 11. | Türeci O, Koslowski M, Helftenbein G, Castle J, Rohde C, Dhaene K, Seitz G, Sahin U. Claudin-18 gene structure, regulation, and expression is evolutionary conserved in mammals. Gene. 2011;481:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Wöll S, Schlitter AM, Dhaene K, Roller M, Esposito I, Sahin U, Türeci Ö. Claudin 18.2 is a target for IMAB362 antibody in pancreatic neoplasms. Int J Cancer. 2014;134:731-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Türeci Ӧ, Mitnacht-Kraus R, Wöll S, Yamada T, Sahin U. Characterization of zolbetuximab in pancreatic cancer models. Oncoimmunology. 2019;8:e1523096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 14. | Dottermusch M, Krüger S, Behrens HM, Halske C, Röcken C. Expression of the potential therapeutic target claudin-18.2 is frequently decreased in gastric cancer: results from a large Caucasian cohort study. Virchows Arch. 2019;475:563-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 15. | Sahin U, Türeci Ö, Manikhas G, Lordick F, Rusyn A, Vynnychenko I, Dudov A, Bazin I, Bondarenko I, Melichar B, Dhaene K, Wiechen K, Huber C, Maurus D, Arozullah A, Park JW, Schuler M, Al-Batran SE. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol. 2021;32:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 263] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 16. | Lordick F, Al-Batran SE, Ganguli A, Morlock R, Sahin U, Türeci Ö. Patient-reported outcomes from the phase II FAST trial of zolbetuximab plus EOX compared to EOX alone as first-line treatment of patients with metastatic CLDN18.2+ gastroesophageal adenocarcinoma. Gastric Cancer. 2021;24:721-730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Bartha Á, Győrffy B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 630] [Article Influence: 157.5] [Reference Citation Analysis (0)] |
| 18. | Goldman MJ, Craft B, Hastie M, Repečka K, McDade F, Kamath A, Banerjee A, Luo Y, Rogers D, Brooks AN, Zhu J, Haussler D. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38:675-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2228] [Cited by in RCA: 2575] [Article Influence: 515.0] [Reference Citation Analysis (0)] |
| 19. | Tanaka M, Shibahara J, Fukushima N, Shinozaki A, Umeda M, Ishikawa S, Kokudo N, Fukayama M. Claudin-18 is an early-stage marker of pancreatic carcinogenesis. J Histochem Cytochem. 2011;59:942-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, Stoss O, Hill J, Ng V, Lehle M, Thomas M, Kiermaier A, Rüschoff J. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 452] [Cited by in RCA: 428] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 21. | Eich ML, Athar M, Ferguson JE 3rd, Varambally S. EZH2-Targeted Therapies in Cancer: Hype or a Reality. Cancer Res. 2020;80:5449-5458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 22. | Silvestris N, Brunetti O, Bittoni A, Cataldo I, Corsi D, Crippa S, D'Onofrio M, Fiore M, Giommoni E, Milella M, Pezzilli R, Vasile E, Reni M. Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up of Exocrine Pancreatic Ductal Adenocarcinoma: Evidence Evaluation and Recommendations by the Italian Association of Medical Oncology (AIOM). Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Li WT, Jeng YM, Yang CY. Claudin-18 as a Marker for Identifying the Stomach and Pancreatobiliary Tract as the Primary Sites of Metastatic Adenocarcinoma. Am J Surg Pathol. 2020;44:1643-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |