Published online Jun 15, 2022. doi: 10.4251/wjgo.v14.i6.1199
Peer-review started: December 27, 2021
First decision: March 13, 2022
Revised: March 26, 2022
Accepted: May 27, 2022
Article in press: May 27, 2022
Published online: June 15, 2022
Processing time: 164 Days and 8.7 Hours
The effect of chronic kidney disease (CKD) on the outcomes of colorectal cancer (CRC) patients after primary CRC surgery is controversial.
To analyze whether CKD had specific effect on the outcomes after CRC surgery.
We searched the PubMed, Embase, Cochrane Library databases and CNKI, from inception to March 14, 2022. Newcastle-Ottawa Scale was used for the quality assessment in this meta-analysis, and we used RevMan 5.3 was used for data analysis.
A total of nine studies including 47771 patients were eligible for this meta-analysis. No significant difference was found in terms of overall postoperative complications [odds ratio (OR) = 1.78, 95%CI: 0.64-4.94, P = 0.27]. We analyzed the specific complications and found that the CKD group had higher rates of pulmonary infection (OR = 2.70, 95%CI: 1.82-4.00, P < 0.01), cardiovascular complications (OR = 3.39, 95%CI: 2.34-4.91, P < 0.01) and short-term death (OR = 3.01, 95%CI: 2.20-4.11, P < 0.01). After pooling the hazard ratio (HR), the CKD group had worse overall survival (OS) (HR = 1.51, 95%CI: 1.04-2.20, P = 0.03). We performed subgroup analyses of the dialysis and non-dialysis groups, and no significant difference was found in the non-dialysis group (HR = 1.20, 95%CI: 0.98-1.47, P = 0.08). The dialysis group had worse OS (HR = 3.36, 95%CI: 1.92-5.50, P < 0.01) than the non-dialysis group. The CKD group had worse disease-free survival (DFS) (HR = 1.41, 95%CI: 1.12-1.78, P < 0.01), and in the subgroup analysis of the dialysis and non-dialysis groups, no significant difference was found in the non-dialysis group (HR = 1.27, 95%CI: 0.97-1.66, P = 0.08). The dialysis group had worse OS (HR = 1.95, 95%CI: 1.23-3.10, P < 0.01) than the non-dialysis group.
Preexisting CKD was associated with higher rates of pulmonary infection, higher rates of short-term death, and worse OS and poorer DFS following CRC surgery.
Core Tip: Previous studies have shown that patients with chronic kidney disease might have an increased risk of colorectal cancer, however, the impact of chronic kidney disease on complications and prognosis after colorectal cancer surgery is controversial. Furthermore, the prognosis remained unclear as well. Therefore, this study aimed to analyze whether chronic kidney disease had specific effect on the outcomes after colorectal cancer surgery. In conclusion, preexisting chronic kidney disease was associated with higher rates of pulmonary infection, higher rates of short-term death, poorer overall survival rates, and poorer disease-free survival rates following colorectal cancer surgery.
- Citation: Liu XY, Zhang B, Cheng YX, Tao W, Yuan C, Wei ZQ, Peng D. Does chronic kidney disease affect the complications and prognosis of patients after primary colorectal cancer surgery? World J Gastrointest Oncol 2022; 14(6): 1199-1209
- URL: https://www.wjgnet.com/1948-5204/full/v14/i6/1199.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i6.1199
Colorectal cancer (CRC) is the third most common malignant tumor and ranks the second leading cause of cancer-related deaths around the world. Nearly 1.8 million new CRC patients and 0.7 million cancer-related deaths occur each year[1]. Radical resection is the only curative treatment for early-stage CRC[2,3], and comprehensive therapy (such as immune therapy, radiotherapy, chemotherapy and surgery) is recommended for advanced-stage CRC[4-8].
It is estimated that approximately 500 million adults worldwide are diagnosed with chronic kidney disease (CKD), but the prevalence varies greatly among different countries[9,10]. CKD can increase mortality and morbidity, and it involves an economic burden as well[11,12]. Some key pathophy
As previous studies reported, patients with CKD might increase the risk of CRC[14]; however, the effect of CKD on complications and prognosis after CRC surgery remains controversial. Some studies held the view that CKD had no effect on surgical complications[15,16]. Moreover, other studies believed that CKD increased postoperative complications[17,18]. Furthermore, the prognosis remained unclear as well. Therefore, our study aimed to analyze whether CKD had an effect on the complications and prognosis of CRC patients who underwent primary CRC surgery.
This meta-analysis conformed to the Preferred Reporting method for Systematic Reviews and Meta-Analyses (PRISMA) statement[19]. The register number of this meta-analysis was CRD42021266160.
We searched the PubMed, Embase, Cochrane Library databases and CNKI from inception to March 14, 2022. There were two key items in this search strategy: chronic kidney disease and colorectal cancer. To expand the search scope, in terms of chronic kidney disease, we used "kidney" OR "dialysis" OR "hemodialysis" OR "estimated glomerular filtration rate". Colorectal cancer was searched as follows: "rectal cancer" OR "colorectal cancer" OR "colon cancer" OR "rectal neoplasm" OR "colorectal neoplasm" OR "colon neoplasm" OR "rectal tumor" OR "colorectal tumor" OR "colon tumor". Then, two key items were combined with "AND". We restricted the search language to Chinese and English, and we limited the searching scope to titles and abstracts. In addition, we use Reference Citation Analysis (https://www.referencecitationanalysis.com) to retrieve relevant literature.
The inclusion criteria were as follows: (1) Studies that included patients undergoing colorectal surgery; (2) Studies that compared the CKD group and the non-CKD group of CRC patients; and (3) Study outcomes included the complications or prognosis. The exclusion criteria were as follows: (1) Studies with insufficient data on complications or prognosis; and (2) Case reports, comments, letter to editor, conferences and reviews. The procedures of inclusion and exclusion criteria were carried out by two reviewers separately, and if there was a disagreement, it was settled by discussion with another reviewer.
Two reviewers searched the databases separately. The titles and abstracts were screened after removing duplicates, after which full texts were evaluated for eligibility. Disagreement was settled by another reviewer.
Two reviewers extracted and cross-checked the data. The data which were extracted were: (1) Publication year, first author, country, sample size, study design, Newcastle-Ottawa Scale (NOS) score and the definition of the CKD group and the non-CKD group; (2) The baseline information included age, sex, comorbidities, American Society of Anesthesiologists (ASA) score and tumor stage; (3) The surgery-related information included surgery type and surgery method; (4) Postoperative complications included anastomotic leakage (divided into three groups: grade A, grad B and grade C. Grade A needed no active intervention, grade B needed active intervention and grade C needed reoperation)[20], pulmonary complications, intestinal obstruction, surgical site infection, postoperative bleeding and short-term death ; and (5) Overall survival (OS) and disease-free survival (DFS).
The primary outcome referred to the short-term outcome, which was equal to postoperative complications (including anastomotic leakage, intestinal obstruction, surgical site infection, postoperative bleeding, pulmonary complications and short-term death during the hospital stay). The second outcome was the long-term prognosis of OS and DFS.
The assessment of the included studies was according to the Newcastle-Ottawa Scale (NOS)[21]. Nine points represented high-quality studies, seven to eight points represented medium-quality studies and scores less than seven points represented low-quality studies.
In the current meta-analysis, the pooled prognosis (OS and DFS) used hazard ratios (HRs) and 95%CIs, and the HRs were extracted from COX analyses. If no available data reported the HRs from COX analyses, then we extracted the HRs from Kaplan-Meier survival curves[22]. The mean ± SD was used for continuous variables, and proportions were used for categorical variables. Mean differences (MDs)/ odds ratios (ORs) plus 95%CIs were calculated for continuous and dichotomous variables. Statistical heterogeneity was analyzed using the I2 and the chi-square test[23,24]. When I2 > 50%, we used the random effects model, in this model, P < 0.1 was considered to be statistically significant. We used the fixed effects model, in this model, when I2 ≤ 50%, and P < 0.05 was considered to be statistically significant. All the statistical analysis was performed using RevMan 5.3 (The Cochrane Collaboration, London, United Kingdom).
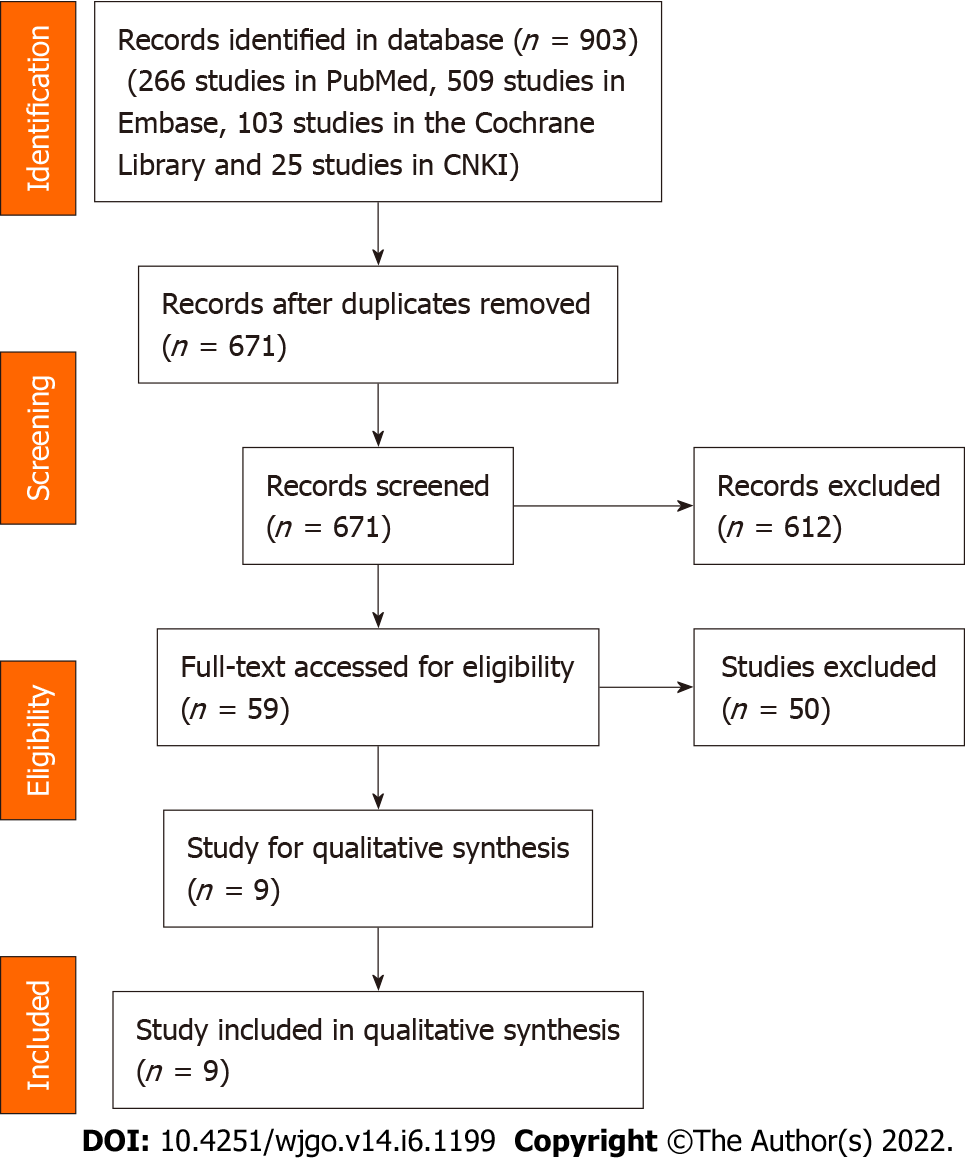
We identified 903 studies (266 studies were obtained from PubMed, 509 studies were obtained from Embase, 103 studies were obtained from the Cochrane Library and 25 studies were obtained from CNKI). A total of 903 studies were included after removed duplicate, 671 studies were left for initial evaluation. After titles and abstracts were screened, 9 studies were left for full-text screening. Finally, nine studies[15-18,25-29] were included (Figure 1).
A total of nine studies[15-18,25-29] including 47771 patients, were enrolled in the current meta-analysis. As for publishing countries, five of nine studies were conducted in Japan, and the other studies were in the United States, the United Kingdom, Canada and China. The publication years were from 2012 to 2022, eight studies were retrospective studies, only one study was a prospective study. The study dates were from 2001 to 2020. The NOS score and definitions of the CKD group and the non-CKD group are shown in Table 1. Six studies reported that the modification of diet in renal disease equation was used to estimate the estimated glomerular filtration rates (eGFR)[16,18,25,27-29]; however, the method used in the other studies was unclear[15,17,26] (see Table 1).
| Ref. | Country | Study design | Study date | Sample size | Definition of CKD and non-CKD | NOS | |||
| CKD group | Non-CKD group | Total | CKD group | Non-CKD group | |||||
| Currie et al[15], 2014 | United Kingdom | Prospective | 2006-2011 | 126 | 582 | 708 | 15 < eGFR < 60 | eGFR ≥ 60 | 7 |
| Nozawa et al[17], 2012 | Japan | Retrospective | 2001-2010 | 245 | 882 | 1127 | eGFR < 60 | eGFR ≥ 60 | 8 |
| Higashino et al[16], 2020 | Japan | Retrospective | 2008-2015 | 14 | 567 | 581 | Dialysis | Non-dialysis | 7 |
| Hu et al[18], 2015 | United States | Retrospective | 2009-2013 | 265 | 42138 | 42401 | Dialysis | Non-dialysis | 8 |
| Chen et al[26], 2016 | China | Retrospective | 2012-2015 | 5 | 10 | 15 | eGFR < 60 | eGFR > 60 | 6 |
| Obara et al[25], 2021 | Japan | Retrospective | 2007-2016 | 24 | 24 | 48 | Dialysis | Non-dialysis | 8 |
| Obara et al[27], 2022 | Japan | Retrospective | 2011-2015 | 59 | 204 | 263 | eGFR < 55 | eGFR ≥ 55 | 7 |
| Shiraishi et al[28], 2022 | Japan | Retrospective | 2016-2020 | 78 | 1294 | 1372 | eGFR < 60 | eGFR ≥ 60 | 8 |
| Dudani et al[29], 2021 | Canada | Retrospective | 2005-2013 | 136 | 1118 | 1254 | eGFR < 60 | eGFR > 60 | 8 |
Sex, age, ASA score, T staging and N staging were included for baseline information analysis. The CKD group had an older age (OR = 8.67, 95%CI: 5.73-11.61, P < 0.01) and a higher proportion of ASA 3-4 grade (OR = 10.27, 95%CI: 2.98-35.35, P < 0.01) after pooling the baseline information. As for other baseline information, no significant difference was found between the two groups (P > 0.05) (Table 2).
| Characteristics | Studies | Participants (CKD/non-CKD) | Mean difference/odds ratio (95%CI) | Heterogeneity |
| Baseline information | ||||
| Male | 9 | 952/46819 | 0.92 (0.63, 1.35); P = 0.67 | I2 = 82%; P < 0.01 |
| Age, yr | 3 | 190/796 | 8.67 (5.73, 11.61); P < 0.01 | I2 = 60%; P = 0.08 |
| ASA1-ASA2 | 3 | 218/2443 | 0.10 (0.03, 0.34); P < 0.01 | I2 = 88%; P < 0.01 |
| ASA3-ASA4 | 3 | 218/2443 | 10.27 (2.98, 35.35); P < 0.01 | I2 = 88%; P < 0.01 |
| T0-T2 | 4 | 508/2962 | 0.71 (0.46, 1.08); P = 0.11 | I2 = 55%; P = 0.08 |
| T3-T4 | 4 | 508/2962 | 1.41 (0.92, 2.16); P = 0.11 | I2 = 55%; P = 0.08 |
| N0 | 4 | 508/2962 | 1.09 (0.89, 1.33); P = 0.42 | I2 = 0%; P = 0.40 |
| N1-N3 | 4 | 508/2962 | 0.92 (0.75, 1.33); P = 0.42 | I2 = 0%; P = 0.40 |
| Surgery-related information | ||||
| Colon cancer | 4 | 409/2055 | 2.10 (0.91, 4.85); P = 0.08 | I2 = 80%; P < 0.01 |
| Rectal cancer | 4 | 409/2055 | 0.48 (0.21, 1.10); P = 0.08 | I2 = 80%; P < 0.01 |
| Laparoscopy surgery | 6 | 566/44809 | 0.88 (0.54, 1.42); P = 0.59 | I2 = 72%; P < 0.01 |
| Open surgery | 6 | 566/44809 | 1.14 (0.70, 1.86); P = 0.59 | I2 = 72%; P < 0.01 |
| Emergency operation | 2 | 324/42342 | 1.31 (0.84, 2.05); P = 0.23 | I2 = 0%; P = 0.49 |
| Operative time | 3 | 309/1096 | -3.58 (-18.95, 11.79); P = 0.65 | I2 = 60%; P = 0.08 |
| Intraoperative blood loss | 3 | 309/1096 | 1.40 (-30.34, 33.15); P = 0.93 | I2 = 0%; P = 0.96 |
| Postoperative complications | ||||
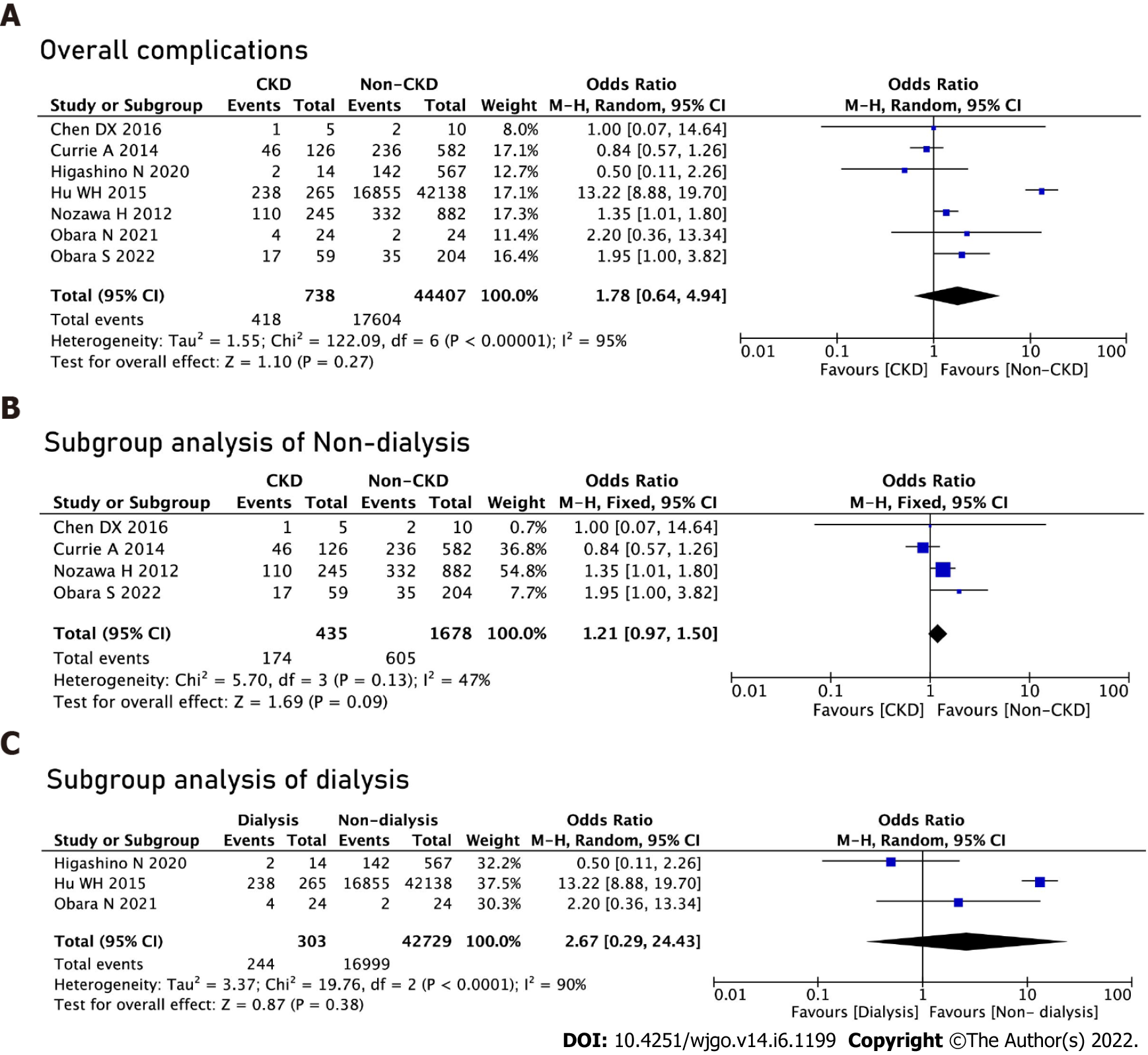
| Overall complication | 7 | 738/44407 | 1.78 (0.64, 4.94); P = 0.27 | I2 = 95%; P < 0.01 |
| Specific complications | ||||
| Cardiovascular | 4 | 641/43612 | 3.39 (2.34, 4.91); P < 0.01 | I2 = 36%; P = 0.20 |
| Anastomotic leakage | 4 | 508/2962 | 1.44 (0.89, 2.32); P = 0.14 | I2 = 35%; P = 0.21 |
| Pulmonary infection | 5 | 607/44891 | 2.70 (1.82, 4.00); P < 0.01 | I2 = 0%; P = 0.84 |
| Intestinal obstruction | 5 | 420/2971 | 0.89 (0.58, 1.37); P = 0.60 | I2 = 6%; P = 0.37 |
| Surgical site infection | 6 | 685/45109 | 1.29 (1.00, 1.65); P = 0.05 | I2 = 0%; P = 0.86 |
| Other site infection | 3 | 515/43030 | 1.07 (0.64, 1.80); P = 0.79 | I2 = 0%; P = 0.60 |
| Postoperative bleeding | 3 | 347/2200 | 0.63 (0.17, 2.36); P = 0.49 | I2 = 0%; P = 0.50 |
| Short-term death | 4 | 650/44169 | 3.01 (2.20, 4.11); P < 0.01 | I2 = 0%; P = 0.50 |
We compared the surgery-related information between the two groups, and it was found no significant difference in terms of laparoscopic surgery, open surgery, emergency operation, intraoperative blood loss or operation time (P > 0.05). But, the CKD group had higher proportion of patients who underwent colon cancer surgery (OR = 2.10, 95%CI: 0.91-4.85, P = 0.08) (Table 2).
There was no significant difference in terms of overall postoperative complications (OR = 1.78, 95%CI: 0.64-4.94, P = 0.27). We performed subgroup analyses of the dialysis and non-dialysis groups and no significant difference was found between the non-dialysis group (OR = 1.21, 95%CI: 0.97-1.50, P = 0.09) and the dialysis group (OR = 2.67, 95%CI: 0.29-24.23, P = 0.38) (Figure 2).
We conducted the analysis of specific complications, and we found that the CKD group had higher rates of pulmonary infections (OR = 2.70, 95%CI: 1.82-4.00, P < 0.01), cardiovascular complications (OR = 3.39, 95%CI: 2.34-4.91, P < 0.01) and short-term death (OR = 3.01, 95%CI: 2.20-4.11, P < 0.01) (Table 2).
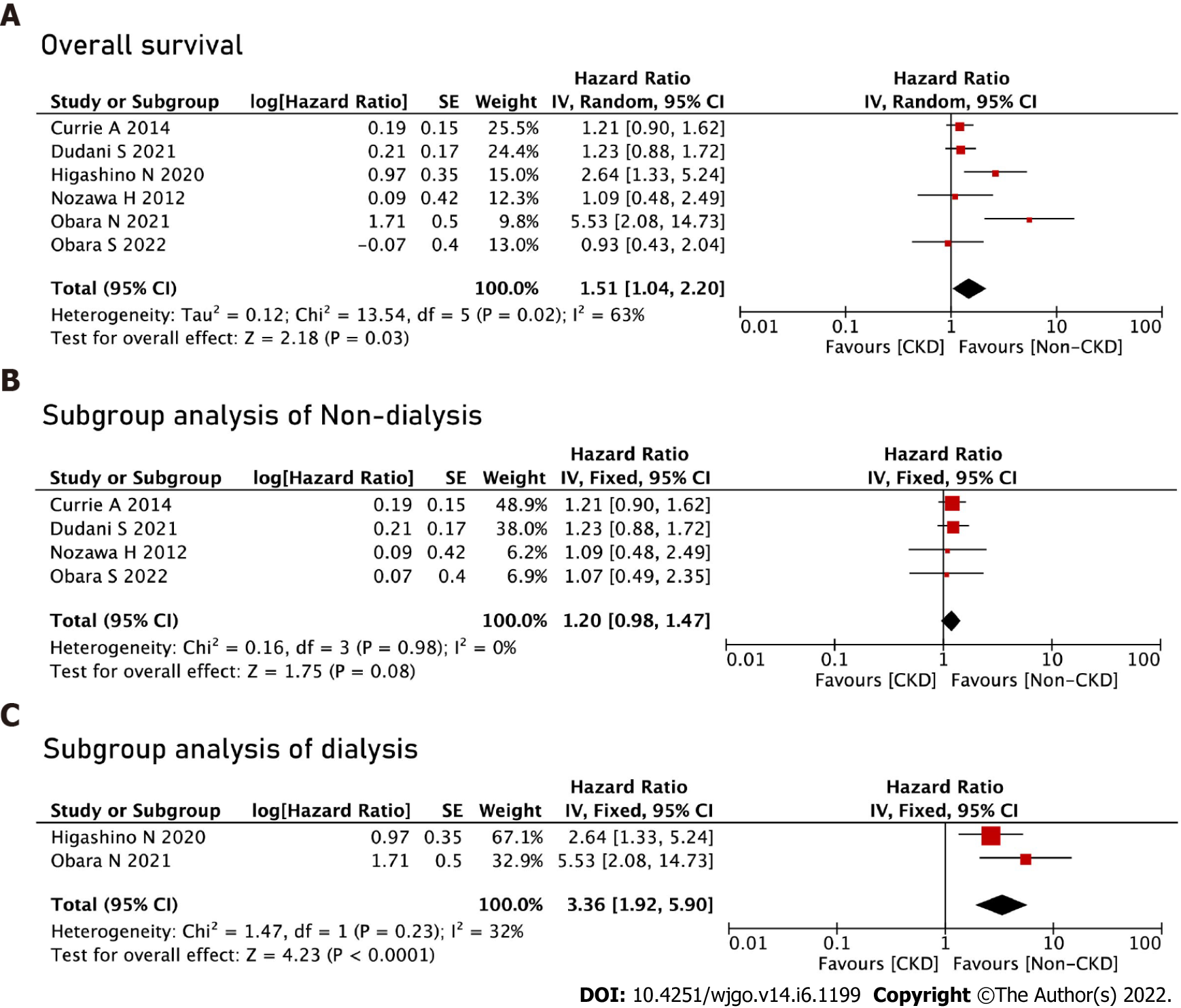
The CKD group had worse OS (HR = 1.51, 95%CI: 1.04-2.20, P = 0.03) after pooling up the HRs. We performed subgroup analyses of the dialysis and non-dialysis groups and no significant difference was found in the non-dialysis group (HR = 1.20, 95%CI: 0.98-1.47, P = 0.08). The dialysis group had worse OS (HR = 3.36, 95%CI: 1.92-5.90, P < 0.01) than the non-dialysis group (Figure 3).
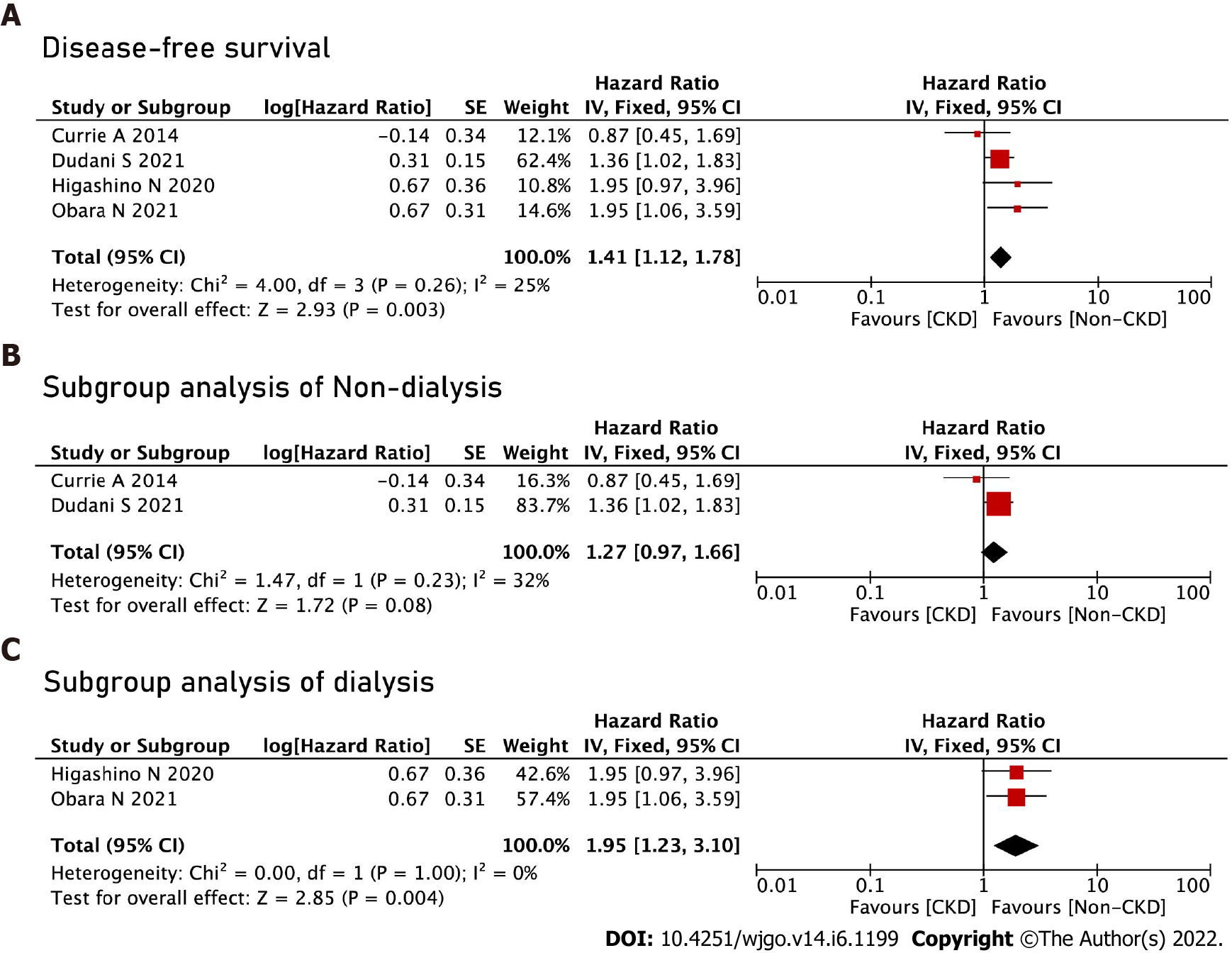
The CKD group had worse DFS (HR = 1.41, 95%CI: 1.12-1.78, P < 0.01). We performed subgroup analyses of the dialysis and non-dialysis groups, and no significant difference was found in the non-dialysis group (HR = 1.27, 95%CI: 0.97-1.66, P = 0.08). The dialysis group had worse OS (HR = 1.95, 95%CI: 1.23-3.10, P < 0.01) than the non-dialysis group (Figure 4).
We performed repeated meta-analysis by omitting each study at a time, in our meta-analysis, the exclusion of any one of the included studies did not alter the results.
A total of nine studies were included in this meta-analysis. There was no significant difference in terms of overall postoperative complications after pooling all of the data. As for prognosis, the CKD group had worse OS and DFS. We performed subgroup analysis of the dialysis and non-dialysis groups. Dialysis was associated with a worse OS and DFS as well.
The classification of renal function is mainly based on the eGFR[30] and different renal function classifications might have an impact on surgery outcomes. As previous studies have reported, CKD could increase postoperative complications after gastric cancer surgery and hepatocellular carcinoma surgery, and the complications included anastomotic leakage and short-term postoperative death[31-33]. In addition, patients with concurrent CKD and cancer have a poor prognosis[32]. In terms of CRC, although an impact of renal function on postoperative complications and prognosis has been reported, it remains controversial.
In this study, there was no significant difference in terms of overall postoperative complications, and we chose the random effect model to conduct the analysis because of the high heterogeneity among studies. We conducted the analysis of specific complications, and we found that the CKD group had higher rates of pulmonary infection, cardiovascular complications and short-term death. The possible reason was that patients undergoing colorectal surgery might experience fluid overload due to nutritional support during non-oral periods. Therefore, these events might increase the risk of lung-related complications[18]. Cardiovascular complications might be associated with endothelial dysfunction in CKD patients[16,34]. Furthermore, patients with CKD are in a relatively immunosuppressive state due to nutritional deficiencies, the lymphocyte suppression and the loss of serum immune system components, which might result in an increase in infectious diseases and short-term deaths after surgery[35].
The CKD group had worse OS rates DFS rates. The probable reason was that CKD was associated with endothelial dysfunction, malnutrition, volume overload or changes in calcium and phosphorus metabolism, and the dysfunction would cause higher rates of cardiovascular events[13,36]. In addition, an association between postoperative complications and cancer-related poor prognosis has been reported in esophageal, gastric and colorectal cancer; therefore, higher rates of postoperative complications might result in a poor prognosis[37,38]. Moreover, postoperative complications and perioperative blood loss can suppress immune function, which might be a factor for promoting cancer recurrence[39,40]. We performed subgroup analyses of the dialysis and non-dialysis groups. Dialysis was associated with worse OS and DFS. Therefore, the distinction in OS and DFS between the two groups might be mainly determined by dialysis.
Some parameters that were insufficient for meta-analysis included cancer-related death, blood transfusion, reoperation rate, adjuvant chemotherapy and the R0 resection rate. However, attention should be given to these parameters. In addition to OS and DFS, cancer-specific survival (CSS) could reflect tumor-related deaths. Obara et al[25] reported that CSS was a negative parameter between the two groups, and the major long-term cause of death in CKD patients might be cardiovascular death. Blood transfusion, a factor that influences the prognosis, might have been more common in the CKD group. Therefore, for surgeons, careful control during the operation and perioperative management of CKD are necessary to reduce blood transfusion.
Some limitations were existing. First, only nine studies were included, and the sample size was relatively small. Second, the cutoff for CKD differed among the included study. Five studies reported that the cutoff eGFR was 60 mL/min/1.73 m2, one study reported that the cutoff value was 55 mL/min/1.73 m2 and the remaining three studies used hemodialysis as the cutoff. Obvious heterogeneity was seen after pooling the data in terms of complications, OS and DFS, therefore, we performed a subgroup analysis, and heterogeneity was reduced. Third, we extracted all of the data that could be analyzed in this study, however, some information was lacking, such as postoperative hospital stay, cancer-related death, blood transfusion, reoperation rate, adjuvant chemotherapy, R0 resection rate, perioperative chemo-radiotherapy and completion of the schedule of chemo-radiotherapy. Fourth, some of the survival data were extracted from the Kaplan-Meier survival curves, which might result in inaccuracies. Finally, among the specific postoperative complications, it was found that pulmonary infection, cardiovascular complications and short-term death were significantly different between the groups; however, this conclusion was based on only four studies, including two CKD studies and two dialysis studies, which would result in heterogeneity. Therefore, multicenter, high-quality and well-controlled prospective studies including comprehensive baseline information comparing the complications, OS, DFS and CSS should be conducted.
In conclusion, preexisting CKD was associated with higher rates of pulmonary infection, higher rates of short-term death, and worse OS and DFS following CRC surgery.
Colorectal cancer (CRC) is the third most common malignant tumor and the second leading cause of cancer deaths worldwide. Several key pathophysiological causes of chronic kidney disease (CKD) may lead to increased postoperative morbidity, including excessive arterial calcification, endothelial dysfunction and increased levels of inflammatory factors. Previous studies have shown that patients with CKD might have an increased risk of CRC; however, the impact of CKD on complications and prognosis after CRC surgery is controversial.
The aim of this study was to conduct meta-analysis of current studies and to analyze whether CKD had specific effect on the outcomes after CRC surgery.
The aim of this study is to provide some recommendations for clinical work by investigating the impact of CKD on postoperative complications and prognosis in colorectal cancer.
We searched the PubMed, Embase, Cochrane Library databases and CNKI, from inception to March 14, 2022. Newcastle-Ottawa Scale was used for the quality assessment in this meta-analysis, and we used RevMan 5.3 was used for data analysis.
A total of nine studies including 47771 patients were included in this meta-analysis. No significant difference was found in terms of overall postoperative complications. We analyzed the specific complications and found that the CKD group had higher rates of pulmonary infection, cardiovascular complications and short-term death. After pooling the hazard ratios, the CKD group had worse overall survival (OS).
Preexisting CKD was associated with higher rates of pulmonary infection, higher rates of short-term death, and worse OS and poorer disease-free survival (DFS) following CRC surgery.
Based on the results and limitations of this research, multicenter, high-quality and well-controlled prospective studies including comprehensive baseline information comparing the complications, OS, DFS and CSS should be performed in the future.
We thank the Department of Gastrointestinal Surgery, of The First Affiliated Hospital of Chongqing Medical University for their contributions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ekine-Afolabi B, United Kingdom; Matsumoto T, Japan; Rathnaswami A, India A-Editor: Liu X, China S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55827] [Article Influence: 7975.3] [Reference Citation Analysis (132)] |
| 2. | Ergun Y, Bal O, Dogan M, Ucar G, Dirikoc M, Acikgoz Y, Bacaksiz F, Uncu D. Does primary tumor resection contribute to overall survival in unresectable synchronous metastatic colorectal cancer? J Res Med Sci. 2020;25:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Bundred NJ. Erratum to "Impact of Oncotype DX Breast Recurrence Score testing on adjuvant chemotherapy use in early breast cancer: Real world experience in Greater Manchester, UK" [Eur J Surg Oncol 43 (5) (2017) 931-937]. Eur J Surg Oncol. 2018;44:194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Peng D, Cheng YX, Cheng Y. Improved Overall Survival of Colorectal Cancer under Multidisciplinary Team: A Meta-Analysis. Biomed Res Int. 2021;2021:5541613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Feliu J, Espinosa E, Basterretxea L, Paredero I, Llabrés E, Jiménez-Munárriz B, Antonio-Rebollo M, Losada B, Pinto A, Custodio AB, Del Mar Muñoz M, Gómez-Mediavilla J, Torregrosa MD, Soler G, Cruz P, Higuera O, Molina-Garrido MJ. Prediction of Chemotoxicity, Unplanned Hospitalizations and Early Death in Older Patients with Colorectal Cancer Treated with Chemotherapy. Cancers (Basel). 2021;14:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Lee J, Koom WS, Byun HK, Yang G, Kim MS, Park EJ, Ahn JB, Beom SH, Kim HS, Shin SJ, Kim K, Chang JS. Metastasis-Directed Radiotherapy for Oligoprogressive or Oligopersistent Metastatic Colorectal Cancer. Clin Colorectal Cancer. 2021;S1533-0028(21)00120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Wang D, Zhang H, Xiang T, Wang G. Clinical Application of Adaptive Immune Therapy in MSS Colorectal Cancer Patients. Front Immunol. 2021;12:762341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Naumann RW, Coleman RL, Brown J, Moore KN. Corrigendum to "Phase III trials in ovarian cancer: The evolving landscape of front line therapy" [Gynecol. Oncol. 153 (2019) 436-444]. Gynecol Oncol. 2020;156:512-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Mills KT, Xu Y, Zhang W, Bundy JD, Chen CS, Kelly TN, Chen J, He J. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88:950-957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 588] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 10. | Brück K, Stel VS, Gambaro G, Hallan S, Völzke H, Ärnlöv J, Kastarinen M, Guessous I, Vinhas J, Stengel B, Brenner H, Chudek J, Romundstad S, Tomson C, Gonzalez AO, Bello AK, Ferrieres J, Palmieri L, Browne G, Capuano V, Van Biesen W, Zoccali C, Gansevoort R, Navis G, Rothenbacher D, Ferraro PM, Nitsch D, Wanner C, Jager KJ; European CKD Burden Consortium. CKD Prevalence Varies across the European General Population. J Am Soc Nephrol. 2016;27:2135-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 375] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 11. | Cirakoglu A, Yuce A, Benli E, Kasko Arici Y, Dugeroglu H, Ogreden E. Is erectile dysfunction an early clinical symptom of chronic kidney disease? Aging Male. 2021;24:24-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Chen TK, Knicely DH, Grams ME. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA. 2019;322:1294-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 1015] [Article Influence: 169.2] [Reference Citation Analysis (0)] |
| 13. | Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1265] [Cited by in RCA: 1538] [Article Influence: 128.2] [Reference Citation Analysis (0)] |
| 14. | Wu MY, Chang TC, Chao TY, Huang MT, Lin HW. Risk of colorectal cancer in chronic kidney disease: a matched cohort study based on administrative data. Ann Surg Oncol. 2013;20:3885-3891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Currie A, Malietzis G, Askari A, Nachiappan S, Swift P, Jenkins JT, Faiz OD, Kennedy RH. Impact of chronic kidney disease on postoperative outcome following colorectal cancer surgery. Colorectal Dis. 2014;16:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Higashino N, Matsuda T, Hasegawa H, Yamashita K, Sakamoto H, Fujikawa M, Yamamoto M, Kanaji S, Oshikiri T, Nakamura T, Suzuki S, Kakeji Y. Outcomes of Laparoscopic Surgery in Colorectal Cancer Patients With Dialysis. Anticancer Res. 2020;40:2165-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Nozawa H, Kitayama J, Sunami E, Watanabe T. Impact of chronic kidney disease on outcomes of surgical resection for primary colorectal cancer: a retrospective cohort review. Dis Colon Rectum. 2012;55:948-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Hu WH, Cajas-Monson LC, Eisenstein S, Parry L, Ramamoorthy S. Association of dialysis with adverse postoperative outcomes in colorectal cancer-an analysis of ACS-NSQIP. Int J Colorectal Dis. 2015;30:1557-1562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47183] [Article Influence: 2948.9] [Reference Citation Analysis (0)] |
| 20. | Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Büchler MW. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 1032] [Article Influence: 68.8] [Reference Citation Analysis (4)] |
| 21. | Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8858] [Cited by in RCA: 12663] [Article Influence: 844.2] [Reference Citation Analysis (0)] |
| 22. | Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4738] [Cited by in RCA: 4954] [Article Influence: 275.2] [Reference Citation Analysis (0)] |
| 23. | Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. 2008;14:951-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 461] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 24. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46535] [Article Influence: 2115.2] [Reference Citation Analysis (3)] |
| 25. | Obara N, Hirano Y, Ishii T, Kondo H, Hara K, Wang L, Asari M, Ito M, Yamaguchi S. Laparoscopic Surgery for Colorectal Cancer in Patients on Hemodialysis: A Propensity Score-matched Analysis. Anticancer Res. 2021;41:993-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Chen DX, Zhan XA, Guo QS, Zhou XF, Xu XJ. [Clinical analysis of laparoscopic surgery in patients with maintenance hemodialysis and colon cancer]. Zhonghua Yi Xue Za Zhi. 2016;96:1441-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Obara S, Koyama F, Kuge H, Nakamoto T, Ikeda N, Iwasa Y, Takei T, Sadamitsu T, Fujimoto K, Harada S, Sho M. Effect of preoperative asymptomatic renal dysfunction on the clinical course after colectomy for colon cancer. Surg Today. 2022;52:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Shiraishi T, Tominaga T, Nonaka T, Hashimoto S, Hamada K, Araki M, Sumida Y, Takeshita H, Fukuoka H, Wada H, To K, Yamashita M, Tanaka K, Sawai T, Nagayasu T. Effect of hemodialysis on short-term outcomes after colon cancer surgery. PLoS One. 2022;17:e0262531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Dudani S, Marginean H, Gotfrit J, Tang PA, Monzon JG, Dennis K, Kennecke HF, Powell ED, Babak S, Cheung WY, Vickers MM. The Impact of Chronic Kidney Disease in Patients With Locally Advanced Rectal Cancer Treated With Neoadjuvant Chemoradiation. Dis Colon Rectum. 2021;64:1471-1478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67:2089-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2145] [Cited by in RCA: 2521] [Article Influence: 126.1] [Reference Citation Analysis (0)] |
| 31. | Sakurai K, Kubo N, Tamamori Y, Aomatsu N, Nishii T, Tachimori A, Maeda K. Impact of chronic kidney disease on the short- and long-term outcomes of laparoscopic gastrectomy for gastric cancer patients. PLoS One. 2021;16:e0250997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Yoshikawa T, Nomi T, Hokuto D, Kamitani N, Matsuo Y, Sho M. Outcomes in Patients with Chronic Kidney Disease After Liver Resection for Hepatocellular Carcinoma. World J Surg. 2021;45:598-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Cheng YX, Tao W, Liu XY, Zhang H, Yuan C, Zhang B, Zhang W, Peng D. Does Chronic Kidney Disease Affect the Surgical Outcome and Prognosis of Patients with Gastric Cancer? Nutr Cancer. 2021;1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Stafford-Smith M. Heart and kidneys: sharing more than just blood. Curr Opin Anaesthesiol. 2007;20:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Vollmer CM Jr, Lewis RS, Hall BL, Allendorf JD, Beane JD, Behrman SW, Callery MP, Christein JD, Drebin JA, Epelboym I, He J, Pitt HA, Winslow E, Wolfgang C, Strasberg SM. Establishing a quantitative benchmark for morbidity in pancreatoduodenectomy using ACS-NSQIP, the Accordion Severity Grading System, and the Postoperative Morbidity Index. Ann Surg. 2015;261:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | Drolet S, Maclean AR, Myers RP, Shaheen AA, Dixon E, Donald Buie W. Morbidity and mortality following colorectal surgery in patients with end-stage renal failure: a population-based study. Dis Colon Rectum. 2010;53:1508-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Maizel J, Deransy R, Dehedin B, Secq E, Zogheib E, Lewandowski E, Tribouilloy C, Massy ZA, Choukroun G, Slama M. Impact of non-dialysis chronic kidney disease on survival in patients with septic shock. BMC Nephrol. 2013;14:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Lagarde SM, de Boer JD, ten Kate FJ, Busch OR, Obertop H, van Lanschot JJ. Postoperative complications after esophagectomy for adenocarcinoma of the esophagus are related to timing of death due to recurrence. Ann Surg. 2008;247:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 134] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 39. | Aoyama T, Oba K, Honda M, Sadahiro S, Hamada C, Mayanagi S, Kanda M, Maeda H, Kashiwabara K, Sakamoto J, Saji S, Yoshikawa T. Impact of postoperative complications on the colorectal cancer survival and recurrence: analyses of pooled individual patients' data from three large phase III randomized trials. Cancer Med. 2017;6:1573-1580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 40. | Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1575-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |