Published online Jun 15, 2022. doi: 10.4251/wjgo.v14.i6.1115
Peer-review started: February 23, 2021
First decision: May 3, 2021
Revised: May 23, 2021
Accepted: May 7, 2022
Article in press: May 7, 2022
Published online: June 15, 2022
Processing time: 471 Days and 23.2 Hours
Activating mutations in the oncogenes KRAS, BRAF and PI3K define molecular colorectal cancer (CRC) subtypes because they play key roles in promoting CRC development and in determining the efficacy of chemotherapeutic agents such as 5-fluorouracil and anti-epidermal growth factor receptor monoclonal antibodies. Survival of patients with cancers displaying these molecular profiles is low. Given the limited efficacy of therapeutic strategies for CRC presenting mutational activations in mitogen-activated protein kinase and/or PI3K pathways, develop
Core Tip: Given the limited efficacy of therapeutic strategies for colorectal cancer presenting mutational activations in mitogen-activated protein kinase and/or PI3K pathways, developing combination therapies with natural flavonoids with demonstrated effects on these pathways may constitute a valuable path forward. Published results show quercetin and curcumin as possibly the best candidates to be further explored in the context of adjuvant colorectal cancer therapy either as part of dietary prescriptions or as purified compounds in combination treatment. Clinical trial data is still largely missing and is urgently needed to verify relevant effects and for the development of more personalized treatment approaches.
- Citation: Pereira-Wilson C. Can dietary flavonoids be useful in the personalized treatment of colorectal cancer? World J Gastrointest Oncol 2022; 14(6): 1115-1123
- URL: https://www.wjgnet.com/1948-5204/full/v14/i6/1115.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i6.1115
Colorectal cancer (CRC) is the fourth most common cause of cancer related death worldwide and accounts for 9% and 10% of all new cancer diagnoses per year in women and men, respectively[1].
Wide variation in geographical distribution of CRC exists with incidence rates in Australia and New Zealand being 10-fold higher than in Western Africa[1]. The highest mortality rates are, however, registered in Central and Eastern Europe[1]. Both CRC incidence and mortality are associated with higher income countries with westernized lifestyles, and CRC rates are expected to increase by about 60% over the next 15 years[1].
Lifestyle and dietary choices play an important role in CRC development. Smoking and excessive alcohol consumption also increase CRC risk[1]. There is strong evidence that being physically inactive, overweight or obese and consuming red or processed meat increases the risk of developing CRC. There is also moderate evidence that low consumption of non-starchy vegetables and fruits might increase the risk of CRC. On the other hand, consuming foods containing dietary fiber decreases CRC risk[1].
The relevance of lifestyle and dietary choices to CRC risk are reflected in the fact that only 5%-10% of CRC cases have a known hereditary cause. These hereditary types of CRC are classified as Familial Adenomatous Polyposis and Hereditary Non-Polyposis Colorectal Cancer (or Lynch syndrome) and are attributed to germline mutation in the APC gene or in DNA repair genes, respectively. A further 20% of cases have a family history of CRC (but no known germline mutations), and the remaining 70% of CRC cases are sporadic (caused by gene defects that are not germline mutations)[2,3]. In addition to dietary and lifestyle factors, patients with irritable bowel disease syndrome are at significantly higher risk of developing CRC[3].
CRC progression takes place over many years through a series of stages that go from lesions in single cells of the epithelium to benign tumors to malignant invasive carcinomas. The evolution from a precursor lesion to CRC is estimated to take 10-15 years.
Accumulation of mutations due to loss of genomic stability is an important driver of CRC development. Chromosomal instability or a mutator phenotype involving DNA repair defects or aberrant DNA methylation have been identified as major mechanisms in CRC development[2,3].
Chromosomal instability may cause the loss of a wild-type copy or inactivating mutations in tumor suppressor genes such as APC or TP53[2,3]. APC mutations are the most common early mutations in CRC, present in up to 85% of cases. APC germline mutations (cause of Familial Adenomatous Polyposis) or APC somatic mutations or deletions are found in most cases of CRC. TP53 mutations are the second most common in CRC, present in up to 55% of cases, and inactivate the p53 pathway, which compromises cell-cycle arrest and cell death pathways and has strong implications on decreased responsiveness to therapy[2,3]. Other relevant and frequently mutated genes in CRC are the oncogenes KRAS and BRAF, which activate proliferative and antiapoptotic pathways[2,3].
Factors that prevent DNA lesions, induce DNA repair at the cancer initiation stages or modulate the rate of tumor growth and invasiveness have potential in cancer preventive strategies. Many food constituents have been described that exert cancer preventing effects due to their role as antioxidants or to their effects on the activity of genotoxic metabolizing enzymes. Multiple reports have been published on the anticancer potential of plant foods and individual phytochemicals. Molecular targets for dietary constituents that may be responsible for their cancer chemopreventive effects have been identified. This has been the subject of intense research and major results compiled in several recent reviews[4-7]. Green tea, cruciferous vegetables, red grapes, turmeric, garlic, soybeans, apples and citrus fruits are examples of plant foods with established anticancer properties[4].
In spite of the recognized health benefits of the intake of dietary fruits and vegetables, the World Cancer Research Fund/American Institute of Cancer Research report[1] does not classify the evidence gathered from human studies as being convincingly strong with regard to reduced CRC risk. It is possible that the explanation for this is that effects vary according to CRC molecular type and that this precludes generalized conclusions.
In a large pooled analysis by Hidaka et al[8] of associations by CRC molecular type, the authors found that higher fruit intake was associated with lower risk of BRAF mutated CRC and the traditional adenoma-CRC pathway[8]. However, most other epidemiologic studies investigating the association of different molecular CRC subtypes and intake of fruits, vegetables and fiber ingestion have been inconclusive.
With regard to the individual food chemical constituents responsible for the beneficial effects, much attention has been given to polyphenols, and in particular to flavonoids, due in part to their strong antioxidant activity, repeatedly demonstrated in in vitro and animal studies.
Fruits and vegetables are rich sources of flavonoids and other polyphenols (Table 1). These compounds, flavonoids in particular, are potent antioxidants, have anti-inflammatory properties and have been extensively studied with regard to their potential as anticancer dietary constituents[4,5,7].
| Class of polyphenol | Representative compounds | Food sources |
| Flavonol1 | (+)-Catechin; (-)-Epicatechin Epigallocatechin gallate | Cocoa, green tea |
| Flavone1 | Luteolin, Apigenin, Chrysin | Parsley, red peppers |
| Flavonol1 | Quercetin, Rutin, Kaempferol | Onions, broccoli, apples |
| Flavanone1 | Naringin, Naringenin, Hesperidin | Citrus fruits |
| Isoflavones1 | Genistein, Daidzein | Soybean |
| Anthocyanidin1 | Cyanidin, Delphinidin, Pelargonidin | Blueberries, raspberries |
| Curcuminoid | Curcumin | Turmeric |
| Stilbene | Resveratrol | Red grapes |
The effects of human dietary intake of flavonoids on decreased risk of CRC have been the subject of much fewer reports. Procyanidin (oligomeric forms of catechin and epicatechin) and isoflavone ingestion was found by He and Sun[9] in population studies to decreased CRC risk, while total flavonoid intake was not found to associate with decreases in CRC risk[9].
In another report, Chang et al[6] found that high intake of flavonols, flavones and anthocyanidins may decrease CRC risk. However, Djuric et al[10] corroborated that total flavonoid intake did not associate with CRC risk nor did total flavanones or flavan-3-ols. High variability between the studies in this meta-analysis precluded any further conclusions[6].
Previously, Djuric et al[10] found that quercetin (Q) decreased risk of proximal CRC but lost its effect if tea consumption was high. Importantly, the authors found that increased intake of Q was associated with increased risk of distal colon cancer when total fruit intake was low[10]. On the other hand, APC mutations were found to be associated with alcohol and red and processed meat consumption[11].
Endoscopic or surgical lesion removal is the best curative strategy for CRC[3]. In order to increase treatment efficacy, adjuvant fluoropyrimidine based [5-fluorouracil (5-FU) or capecitabine] chemotherapy combined with oxaliplatin and/or irinotecan is also used[3].
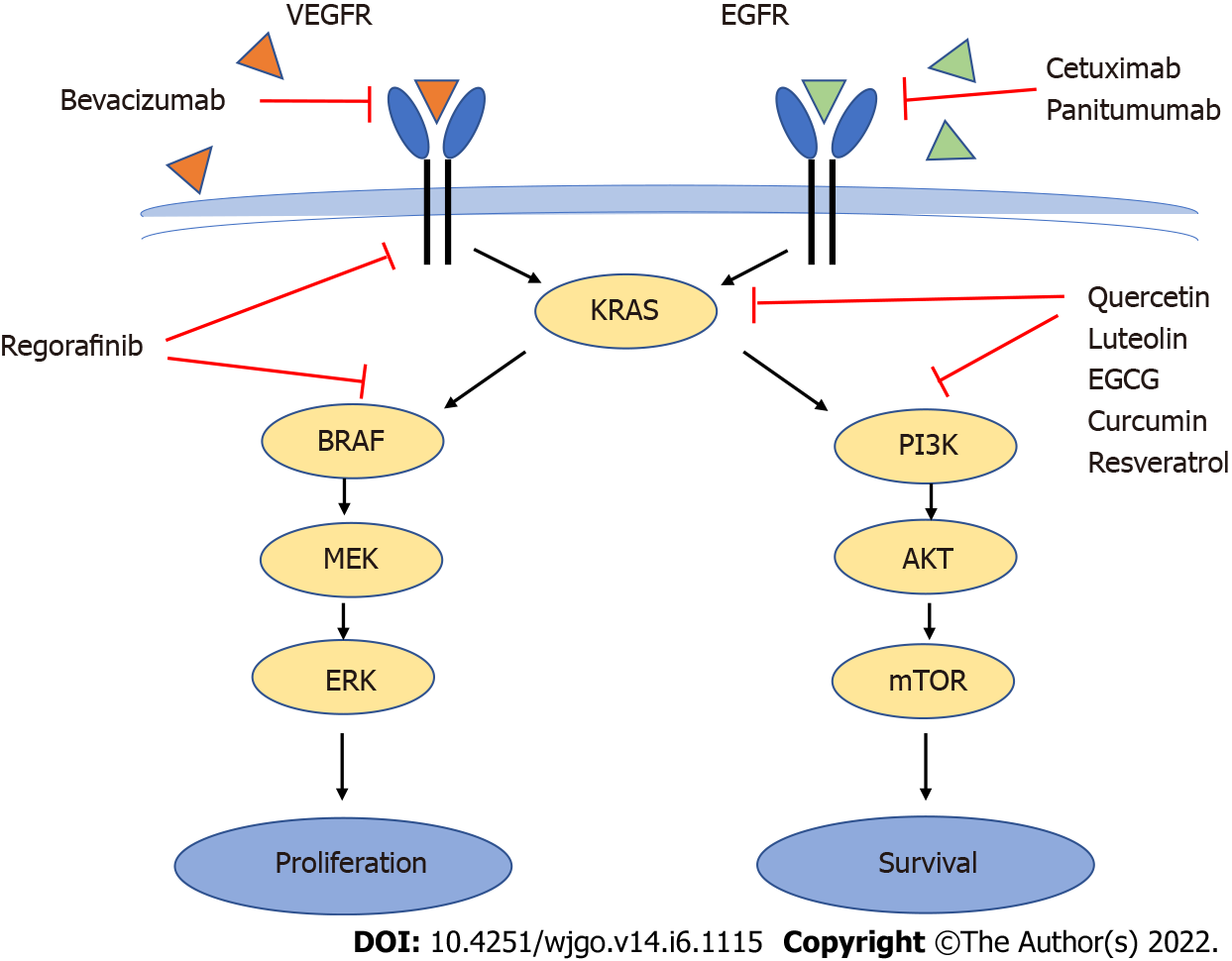
Another recent group of drugs known as “biologics” are increasingly used in the treatment of CRC. These are monoclonal antibodies such as bevacizumab, an anti- vascular endothelial growth factor (VEGF) antibody that targets angiogenesis, or cetuximab and panitumumab, anti-epidermal growth factor receptor (EGFR) antibodies that target proliferative signals mediated by EGFR[3,12] (Figure 1).
FOLFOX (leucovorin, 5-FU and oxaliplatin) or FOLFIRI (leucovorin, 5-FU and irinotecan), frequently combined with EGFR or VEGF receptor inhibitors are the most commonly utilized chemotherapeutic regimes in CRC treatment[12].
Under normal conditions, upon ligand binding to EGFR or the VEGF receptor, both the mitogen-activated protein kinase (MAPK) (RAS/RAF/MEK/ERK) and the PI3K/AKT/mTOR phosphorylation cascades will be activated, leading to cell proliferation and survival[3,13]. Although EGFR is overexpressed in 60%-80% of CRC tumors (which makes it a good anticancer target) favoring the proliferative and antiapoptotic activity of the MAPK and PI3K pathways[14], efficacy of anti-EGFR drugs (and to a smaller extent also anti-VEGF receptor) decreases dramatically in the presence of KRAS and particularly BRAF mutations[3,12,13] due to their overriding downstream effects on the signaling pathways (Figure 1).
Mutations in the oncogene KRAS occur in 37% of CRCs and constitutively activate proliferative and antiapoptotic pathways such as the MAPK and the PI3K pathway. Mutations in the oncogene BRAF occur in 13% of CRCs activate the MAPK pathway downstream of KRAS but not the PI3K pathway[2,15]. KRAS mutations do not co-occur with BRAF mutations in CRC[13,16]. Activating mutations of PI3K are present in 15%-20% of CRC and loss of PTEN, an inhibitor of the PI3K pathway, also contribute to increased activity of the PI3K/AKT pathway[3,13].
Therefore, mutations in the key players KRAS, BRAF and PI3K promote CRC development, define molecular subtypes and are relevant for the efficacy of chemotherapeutic agents such as anti-EGFR monoclonal antibodies[2,3,12,14,15].
In addition, targeted anti-EGFR therapy with cetuximab or panitumumab often leads to secondary resistance through selection of resistant cells even in patients wildtype for EGFR, KRAS and BRAF[13,14]. More recently, regorafinib has shown to be of use as third-line treatment for KRAS and BRAF mutated tumors[15,17] (Figure 1).
Genetic profiling to determine the presence of EGFR, KRAS or BRAF mutation is, therefore, important to predict treatment outcome, although other unknown factors play important roles in a patient’s response to therapy[14]. Targeted chemotherapy tailored to improve patient response with minimal side effects remains a goal, but much work remains in order to achieve it since the 5-year survival rate of CRC patients is still less than 15% for those diagnosed in advanced stages of the disease with metastatic CRC[14].
Therapeutic strategies to treat tumors with mutations in these key genes remain limited, and improving treatment efficacy for these patients constitutes a pressing clinical need. Despite this, dietary and other plant derived phytochemicals remain an untapped resource despite the large body of evidence of their acting on the relevant molecular targets.
Several compounds have been shown to target the MAPK and/or the PI3K pathways. Epigallocatechin-3-gallate is a green tea polyphenol also present in chocolate. Epigallocatechin-3-gallate has been shown to inhibit EGFR signaling and ERK1 (MAPK pathway) and PI3K/AKT activation, with effects on cell proliferation and survival[14,18].
Curcumin, another natural polyphenol present in turmeric, has also been shown to decrease EGFR gene expression thereby decreasing ERK-mediated signaling and gene expression[14]. In addition, curcumin suppressed the PI3K/Akt pathway in vitro with induction of apoptosis[19,20] and decreased NF-kB activation by traditional chemotherapeutic drugs thereby contributing to overcoming treatment resistance[21]. Inhibitory effects on PI3K/AKT and NF-kB seem to be involved in sensitization to treatments with 5-FU and capecitabine[22,23].
In other studies, curcumin was shown to produce a similar effect to that of the MEK inhibitor U0126 and to synergistically enhance the efficacy of the multi-kinase inhibitor regorafinib in HCT116 cells[16,24]. New delivery formulations that address curcumin’s low bioavailability look promising and will increase the compound’s clinical use[21].
Q, is a flavonoid particularly abundant in onions, apples and broccoli. The health benefits of Q have been widely reported and include antioxidant, anti-inflammatory and cancer chemopreventive activity. Recent reviews of Q cancer chemopreventive mechanisms have been published by Kashyap et al[25] and Rather and Bhagat[26]. These include scavenging of reactive oxygen species and induction of antioxidant defenses, modulation of signaling pathways resulting in decreased cell proliferation and increased apoptotic cell death, cytochrome P450 enzyme activity modulation, induction of Nrf2-mediated phase II enzymes and inhibitory effects on inflammatory markers such as iNOS, COX2, IL-6 and TNF-alpha among others. Q has also been shown to decrease signaling through the MAPK and PI3K pathways as well as improve the response to chemotherapeutic drugs[25-29].
In studies with human CRC-derived cell lines harboring either KRAS or BRAF mutations, it is possible to test the impact of individual compounds (or complex plant extracts) on the activity of pathways caused by that particular mutation thereby identifying the subset of patients that would benefit the most from strategies involving that particular flavonoid.
Q and luteolin (L) are two of the best studied flavonoids and two of the most abundant in plant foods. They have demonstrated anti-CRC activity in numerous in vitro and in vivo studies[28].
In a study using the human CRC cells lines HCT15, (harboring a KRAS mutation and wild-type
Crosstalk between MAPK and PI3K/AKT suggests that levels of PI3K pathway activity may also be affected in KRAS-mutated cells and contribute to the overall effects on cell proliferation and apoptotic cell death. However, PI3K activity may also be regulated independently of its upstream regulator KRAS.
Interesting in the Xavier et al[27] study was the fact that the specific MEK inhibitor PD-98059 (MEK a downstream link of both KRAS and BRAF in the MAPK pathway) significantly reduced phosphor-ERK levels but did not significantly decrease cell proliferation or induce apoptotic cell death[27]. Also wortmanin, a reference PI3K inhibitor, decreased phosphor-AKT levels, an indication of pathway inhibition, without producing significant effects on proliferation or apoptotic cell death. Of relevance here, in the comparison between the effects of the reference compounds PD-98059 and wortmanin and Q or L is the fact that the flavonoids had significant effects on cell proliferation and cell death, a clear indication that the multitarget nature of the flavonoids’ actions was beneficial and not detrimental to the overall effect[27].
In another study by Yang et al[30], Q induced apoptosis in KRAS-mutated cells in a c-Jun N-terminal kinase activation-dependent way. Both the G13D and G12V mutations of KRAS rendered CRC cells more sensitive to Q than KRAS wild-type cells[30].
Studies where flavonoids are tested in combination with chemotherapeutical drugs also demonstrate possible enhancement of therapeutic effects and consequent benefits of co-administration regimens. The majority of studies involving the role of phytochemicals in combination with chemotherapeutic drugs, use 5-FU and address the involvement of p53 in the response, due to the key role of p53 in apoptosis induction and treatment sensitivity[29,31-39]. Tumor progression and low treatment efficacy is strongly dependent on successful evasion of cell death due to mutations in TP53[31], present in 35%-55% of CRC cases.
In addition to p53 status, CRC displaying DNA mismatch repair defects and microsatellite instability show lower sensitivity to 5-FU and poorer treatment outcome. In this scenario of increased treatment resistance, Q was tested, and the dependence on p53 status for the cell’s response to 5-FU was determined[29]. Q significantly increased apoptotic cell death in response to 5-FU in p53 wild-type CO115 and HCT116 comparatively to effects on HCT15 p53-mutated cells. P53 silencing in CO115 completely abrogated the apoptotic effects of Q + 5-FU. This p53 dependence was further corroborated in the isogenic HCT116 p53-/- cells, where both the effects of the drug alone and of the combination Q + 5-FU were lost. This constituted a clear indication that wild-type p53 was required for Q enhancement of 5-FU apoptotic effects and suggested that tumors displaying wild-type p53 are more likely to benefit from combination treatment of 5-FU with Q than tumors harboring inactivating mutations of p53[29].
Resveratrol, on the other hand, increased 5-FU-induced apoptosis in a p53-independent manner in both HCT116 wild-type p53 and p53-deficient cells. Resveratrol also decreased MAPK and PI3K/AKT signaling and upregulated miR-34a expression, in a new mechanism of inhibition of HCT116 proliferation induced by the combination of resveratrol + 5-FU[40]. Upregulated miR-34a expression was also involved in chemosensitization of HCT116 and HT-29 to oxaliplatin by resveratrol[41].
Combination exposures to FOLFOX and curcumin showed reduction of tumor explant cell survival due to enhanced antiproliferative effects[42,43]. Information on the anti-CRC effects of curcumin including clinical trials involving curcumin are the subject of a recent publication[21]. Molecular targets for curcumin include MAPK and PI3K pathways and decreases in chemotherapy induction of NF-kB and antiapoptotic gene expression.
Yue et al[44] showed that turmeric extract in combination with bevacizumab produced comparable effects to bevacizumab plus FOLFOX with the advantage that it increased survival of tumor-bearing mice. In another study[45], the combination of curcumin with the multi-kinase inhibitor regorafinib produced an increase in cell death in KRAS-mutated CRC cells and not in wild-type KRAS CRC cells.
With regard to clinical trials[21], most involve curcumin and address the pharmacokinetic profile and toxicity of curcumin formulations. Some trials test curcumin in combination with drugs such as 5-FU or irinotecan and will provide useful information, even if enrollment is low and patients are most likely not stratified by tumor molecular subtype.
Much remains to be done with regard to the effects of phytochemicals on patients, whether isolated or in combination with chemotherapeutical drugs.
Although a diet rich in fruits and vegetables is generally beneficial with regard to CRC, variations in response among individuals to similar dietary choices strongly suggest that there may be interactions of food constituents with particular molecular targets that will benefit patients differently according to their tumor’s molecular signature.
The capacity to inhibit proliferation and induce apoptosis through effects on molecular targets of the MAPK and/or the PI3K/AKT pathways makes flavonoids potentially strong allies if used as adjuvants in combination treatment improving therapeutic efficacy and patient survival. However, in order to take full advantage of the anticancer potential of these natural compounds, a detailed and systematic characterization of the compound’s mechanisms of action is required. Although much has been published on the anticancer effects of dietary phytochemicals, such an exhaustive characterization of potential beneficial and adverse effects in the setting of cancer treatment in humans is still lacking. Results from in vitro studies cannot be extrapolated to effects in humans. Also, care should be taken that some flavonoids’ and other phytochemicals’ potential to prevent DNA damage or induce repair of the lesions produced by the therapeutical agent do not hinder treatment efficacy.
Clinical trials are needed to verify relevant effects and those remain insufficient in number and in patient enrollment. Also, patients are generally not selected on the basis of their tumor molecular profiles, which would be a requirement for a more personalized treatment approach.
Moreover, efforts should be concerted towards establishing nutritional guidelines that prescribe the ingestion of the right plant food sources or of individual flavonoids as part of nutraceutical formulations in the several phases of CRC progression and treatment. Much work remains to be done, not least the introduction of standardization and regulatory systems as well as extensive testing through clinical trials of possible toxicity of the new formulations.
Reduced bioavailability often mean that polyphenols have limited efficacy in in vivo studies. However, these issues can today be addressed by nanoencapsulation, which may increase bioavailability and increase circulating concentrations or enable delivery into the colon and cause direct exposure of colonocytes to the active compounds limiting possible systemic side effects.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Portugal
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zheng YW, Japan S-Editor: Gao CC L-Editor: Filipodia P-Editor: Gao CC
| 1. | World Cancer Research Fund/American Institute for Cancer Research. Continuous update project Expert Report 2018. Diet, nutrition, physical activity and colorectal cancer. [cited 25 Feb 2021]. In: World Cancer Research Fund/American Institute for Cancer Research [Internet]. Available from: https://www.wcrf.org/diet-and-cancer/. |
| 2. | Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1392] [Article Influence: 87.0] [Reference Citation Analysis (2)] |
| 3. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3024] [Article Influence: 504.0] [Reference Citation Analysis (3)] |
| 4. | Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1174] [Cited by in RCA: 1097] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 5. | Alam MN, Almoyad M, Huq F. Polyphenols in Colorectal Cancer: Current State of Knowledge including Clinical Trials and Molecular Mechanism of Action. Biomed Res Int. 2018;2018:4154185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Chang H, Lei L, Zhou Y, Ye F, Zhao G. Dietary Flavonoids and the Risk of Colorectal Cancer: An Updated Meta-Analysis of Epidemiological Studies. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 7. | Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J. Flavonoids as Anticancer Agents. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 633] [Article Influence: 126.6] [Reference Citation Analysis (0)] |
| 8. | Hidaka A, Harrison TA, Cao Y, Sakoda LC, Barfield R, Giannakis M, Song M, Phipps AI, Figueiredo JC, Zaidi SH, Toland AE, Amitay EL, Berndt SI, Borozan I, Chan AT, Gallinger S, Gunter MJ, Guinter MA, Harlid S, Hampel H, Jenkins MA, Lin Y, Moreno V, Newcomb PA, Nishihara R, Ogino S, Obón-Santacana M, Parfrey PS, Potter JD, Slattery ML, Steinfelder RS, Um CY, Wang X, Woods MO, Van Guelpen B, Thibodeau SN, Hoffmeister M, Sun W, Hsu L, Buchanan DD, Campbell PT, Peters U. Intake of Dietary Fruit, Vegetables, and Fiber and Risk of Colorectal Cancer According to Molecular Subtypes: A Pooled Analysis of 9 Studies. Cancer Res. 2020;80:4578-4590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | He X, Sun LM. Dietary intake of flavonoid subclasses and risk of colorectal cancer: evidence from population studies. Oncotarget. 2016;7:26617-26627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Djuric Z, Severson RK, Kato I. Association of dietary quercetin with reduced risk of proximal colon cancer. Nutr Cancer. 2012;64:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Gay LJ, Mitrou PN, Keen J, Bowman R, Naguib A, Cooke J, Kuhnle GG, Burns PA, Luben R, Lentjes M, Khaw KT, Ball RY, Ibrahim AE, Arends MJ. Dietary, lifestyle and clinicopathological factors associated with APC mutations and promoter methylation in colorectal cancers from the EPIC-Norfolk study. J Pathol. 2012;228:405-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | DeStefanis RA, Kratz JD, Emmerich PB, Deming DA. Targeted Therapy in Metastatic Colorectal Cancer: Current Standards and Novel Agents in Review. Curr Colorectal Cancer Rep. 2019;15:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Morkel M, Riemer P, Bläker H, Sers C. Similar but different: distinct roles for KRAS and BRAF oncogenes in colorectal cancer development and therapy resistance. Oncotarget. 2015;6:20785-20800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 14. | Pabla B, Bissonnette M, Konda VJ. Colon cancer and the epidermal growth factor receptor: Current treatment paradigms, the importance of diet, and the role of chemoprevention. World J Clin Oncol. 2015;6:133-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (3)] |
| 15. | Graham DM, Coyle VM, Kennedy RD, Wilson RH. Molecular Subtypes and Personalized Therapy in Metastatic Colorectal Cancer. Curr Colorectal Cancer Rep. 2016;12:141-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Li ZN, Zhao L, Yu LF, Wei MJ. BRAF and KRAS mutations in metastatic colorectal cancer: future perspectives for personalized therapy. Gastroenterol Rep (Oxf). 2020;8:192-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 17. | Yan Y, Grothey A. Molecular profiling in the treatment of colorectal cancer: focus on regorafenib. Onco Targets Ther. 2015;8:2949-2957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Sharifi-Rad M, Pezzani R, Redaelli M, Zorzan M, Imran M, Ahmed Khalil A, Salehi B, Sharopov F, Cho WC, Sharifi-Rad J. Preclinical Pharmacological Activities of Epigallocatechin-3-gallate in Signaling Pathways: An Update on Cancer. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 19. | Jiang QG, Li TY, Liu DN, Zhang HT. PI3K/Akt pathway involving into apoptosis and invasion in human colon cancer cells LoVo. Mol Biol Rep. 2014;41:3359-3367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Rana C, Piplani H, Vaish V, Nehru B, Sanyal SN. Downregulation of PI3-K/Akt/PTEN pathway and activation of mitochondrial intrinsic apoptosis by Diclofenac and Curcumin in colon cancer. Mol Cell Biochem. 2015;402:225-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Ruiz de Porras V, Layos L, Martínez-Balibrea E. Curcumin: A therapeutic strategy for colorectal cancer? Semin Cancer Biol. 2021;73:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Shakibaei M, Mobasheri A, Lueders C, Busch F, Shayan P, Goel A. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-κB and Src protein kinase signaling pathways. PLoS One. 2013;8:e57218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 23. | Shakibaei M, Kraehe P, Popper B, Shayan P, Goel A, Buhrmann C. Curcumin potentiates antitumor activity of 5-fluorouracil in a 3D alginate tumor microenvironment of colorectal cancer. BMC Cancer. 2015;15:250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 24. | Wu CS, Wu SY, Chen HC, Chu CA, Tang HH, Liu HS, Hong YR, Huang CF, Huang GC, Su CL. Curcumin functions as a MEK inhibitor to induce a synthetic lethal effect on KRAS mutant colorectal cancer cells receiving targeted drug regorafenib. J Nutr Biochem. 2019;74:108227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Kashyap D, Mittal S, Sak K, Singhal P, Tuli HS. Molecular mechanisms of action of quercetin in cancer: recent advances. Tumour Biol. 2016;37:12927-12939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Rather RA, Bhagat M. Quercetin as an innovative therapeutic tool for cancer chemoprevention: Molecular mechanisms and implications in human health. Cancer Med. 2020;9:9181-9192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 27. | Xavier CP, Lima CF, Preto A, Seruca R, Fernandes-Ferreira M, Pereira-Wilson C. Luteolin, quercetin and ursolic acid are potent inhibitors of proliferation and inducers of apoptosis in both KRAS and BRAF mutated human colorectal cancer cells. Cancer Lett. 2009;281:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Xavier CPR, Pereira-Wilson C. Medicinal plants of the genuses Salvia and Hypericum are sources of anticolon cancer compounds: Effects on PI3K/Akt and MAP kinases pathways. Pharma Nutrit. 2016;4:112-122. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Xavier CPR, Lima CF, Rohde M, Pereira-Wilson C. Quercetin enhances 5-fluorouracil induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother Pharmacol. 2011;68:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 30. | Yang Y, Wang T, Chen D, Ma Q, Zheng Y, Liao S, Wang Y, Zhang J. Quercetin preferentially induces apoptosis in KRAS-mutant colorectal cancer cells via JNK signaling pathways. Cell Biol Int. 2019;43:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 31. | Boyer J, McLean EG, Aroori S, Wilson P, McCulla A, Carey PD, Longley DB, Johnston PG. Characterization of p53 wild-type and null isogenic colorectal cancer cell lines resistant to 5-fluorouracil, oxaliplatin, and irinotecan. Clin Cancer Res. 2004;10:2158-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 180] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 32. | Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3255] [Cited by in RCA: 3629] [Article Influence: 165.0] [Reference Citation Analysis (0)] |
| 33. | Blondy S, David V, Verdier M, Mathonnet M, Perraud A, Christou N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 2020;111:3142-3154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 315] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 34. | Redondo-Blanco S, Fernández J, Gutiérrez-Del-Río I, Villar CJ, Lombó F. New Insights toward Colorectal Cancer Chemotherapy Using Natural Bioactive Compounds. Front Pharmacol. 2017;8:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 35. | Liu R, Ji P, Liu B, Qiao H, Wang X, Zhou L, Deng T, Ba Y. Apigenin enhances the cisplatin cytotoxic effect through p53-modulated apoptosis. Oncol Lett. 2017;13:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Aranda-Olmedo I, Rubio LA. Dietary legumes, intestinal microbiota, inflammation and colorectal cancer. J Funct Foods. 2020;64:103707. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Teixeira-Guedes CI, Oppolzer D, Barros, AI, Pereira-Wilson C. Phenolic rich extracts from cowpea sprouts decrease cell proliferation and enhance 5-fluorouracil effect in human colorectal cancer cell lines. J Funct Foods. 2019;60:103452. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Jesus MS, Carvalho AC, Teixeira JA, Domingues L, Pereira-Wilson C. Ohmic Heating Extract of Vine Pruning Residue Has Anti-Colorectal Cancer Activity and Increases Sensitivity to the Chemotherapeutic Drug 5-FU. Foods. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Liang L, Wu J, Luo J, Wang L, Chen ZX, Han CL, Gan TQ, Huang JA, Cai ZW. Oxymatrine reverses 5-fluorouracil resistance by inhibition of colon cancer cell epithelial-mesenchymal transition and NF-κB signaling in vitro. Oncol Lett. 2020;19:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Kumazaki M, Noguchi S, Yasui Y, Iwasaki J, Shinohara H, Yamada N, Akao Y. Anti-cancer effects of naturally occurring compounds through modulation of signal transduction and miRNA expression in human colon cancer cells. J Nutr Biochem. 2013;24:1849-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 41. | Yang S, Li W, Sun H, Wu B, Ji F, Sun T, Chang H, Shen P, Wang Y, Zhou D. Resveratrol elicits anti-colorectal cancer effect by activating miR-34c-KITLG in vitro and in vivo. BMC Cancer. 2015;15:969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 42. | Patel BB, Sengupta R, Qazi S, Vachhani H, Yu Y, Rishi AK, Majumdar AP. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 155] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 43. | James MI, Iwuji C, Irving G, Karmokar A, Higgins JA, Griffin-Teal N, Thomas A, Greaves P, Cai H, Patel SR, Morgan B, Dennison A, Metcalfe M, Garcea G, Lloyd DM, Berry DP, Steward WP, Howells LM, Brown K. Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy. Cancer Lett. 2015;364:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 44. | Yue GG, Kwok HF, Lee JK, Jiang L, Wong EC, Gao S, Wong HL, Li L, Chan KM, Leung PC, Fung KP, Zuo Z, Lau CB. Combined therapy using bevacizumab and turmeric ethanolic extract (with absorbable curcumin) exhibited beneficial efficacy in colon cancer mice. Pharmacol Res. 2016;111:43-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 45. | Lamba S, Russo M, Sun C, Lazzari L, Cancelliere C, Grernrum W, Lieftink C, Bernards R, Di Nicolantonio F, Bardelli A. RAF suppression synergizes with MEK inhibition in KRAS mutant cancer cells. Cell Rep. 2014;8:1475-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |