Published online Jun 15, 2022. doi: 10.4251/wjgo.v14.i6.1103
Peer-review started: January 15, 2022
First decision: March 13, 2022
Revised: March 19, 2022
Accepted: May 8, 2022
Article in press: May 8, 2022
Published online: June 15, 2022
Processing time: 145 Days and 3.4 Hours
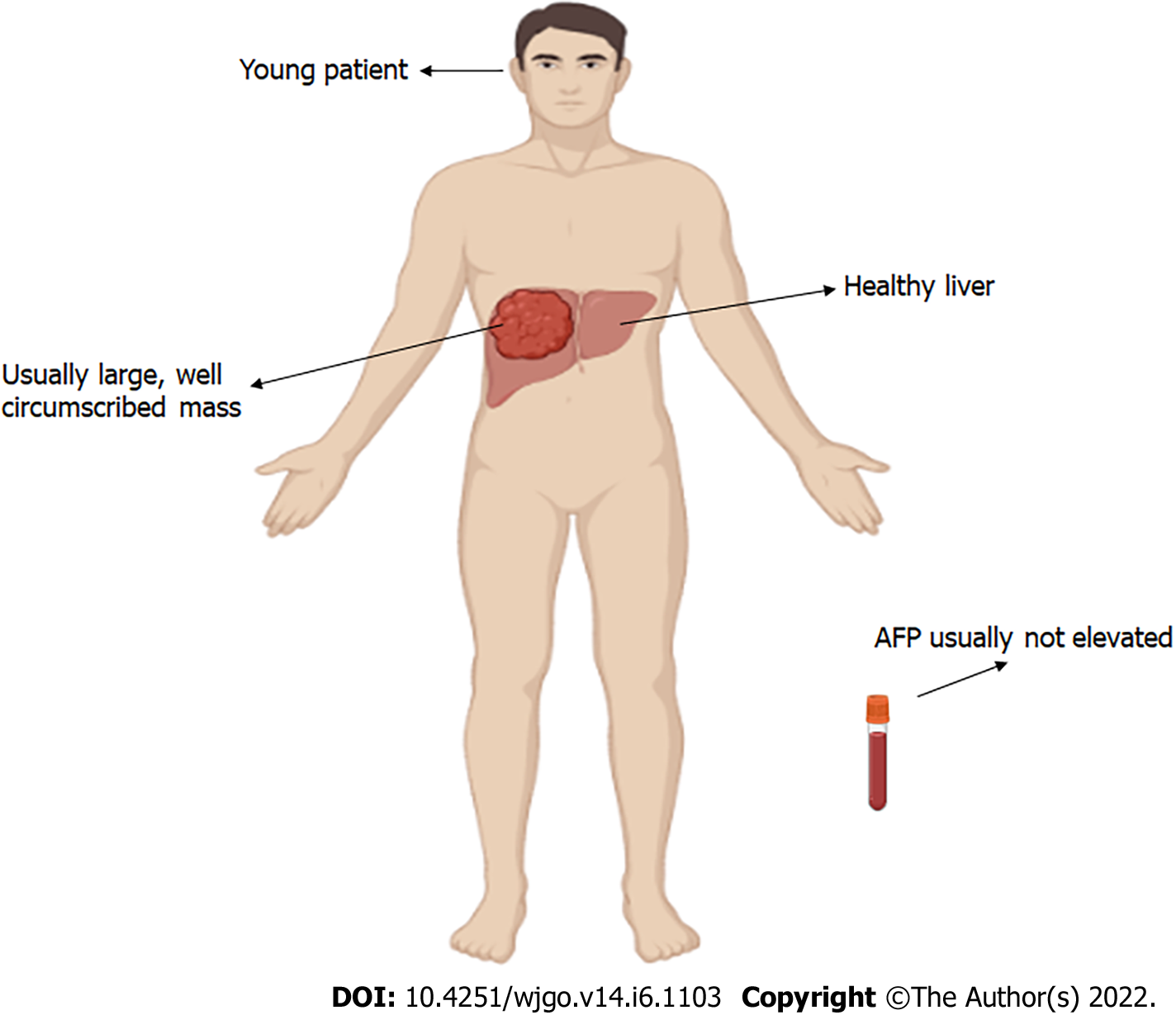
Fibrolamellar carcinoma (FLC) is a rare variant of hepatocellular carcinoma (HCC), comprising 1%–9% of all HCCs. FLC is a poorly understood malignancy, which seems to be more prevalent in young patients with no underlying liver diseases. The term “fibrolamellar” is derived from thick fibrous collagen bands surrounding the tumor cells. Unlike HCC, cirrhosis and viral hepatitis infection are not predisposing to FLC, and it is not associated with elevations in serum alpha-fetoprotein. FLC patients often present with vague abdominal pain, nausea, malaise, and weight loss. Most cases present are at an advanced stage at the time of initial diagnosis. However, curative treatment options can still be offered to up to 70% of patients. Surgery (resection/liver transplantation) is the mainstay of treatment and the only potentially curative option. FLCs have been less chemo-responsive than the conventional HCC, however, in advanced cases, multi
Core Tip: Fibrolamellar carcinoma (FLC) is a rare liver cancer that displays unique features in behavior and clinical findings from conventional hepatocellular carcinoma (HCC). No certain underlying trigger is detected in FLC. Alpha-fetoprotein levels are normal, unlike in traditional HCC. Surgery (resection/liver transplantation) is the current mainstay of treatment and remains the only curative option. FLCs have been less chemo-responsive than the conventional HCC. Controlled trials evaluating checkpoint inhibitors in FLC are lacking. In this review, we collect and summarize current evidence and clinical experience of conversion therapy, highlight remaining problems and challenges for further research.
- Citation: Abdelhamed W, El-Kassas M. Fibrolamellar hepatocellular carcinoma: A rare but unpleasant event. World J Gastrointest Oncol 2022; 14(6): 1103-1114
- URL: https://www.wjgnet.com/1948-5204/full/v14/i6/1103.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i6.1103
Fibrolamellar carcinoma (FLC) is an uncommon liver cancer with behaviour and clinical findings that vary significantly from ordinary hepatocellular carcinoma (HCC)[1]. It comprises 1%–9% of all HCCs, according to the Surveillance, Epidemiology, and End Results SEER database[2]. Edmonson first described FLC in 1956 as an adult type of liver cancer in a 14-year-old female without a background of liver affection[3]. FLC receives its name from the histologically distinct intra tumoral lamellar collagen bands observed between large polygonal cells with abundant eosinophilic cytoplasm, large vesiculated nuclei, and large nucleoli[4]. The majority of FLC patients are in their second or third decade[5,6]. It often affects patients between (10–35 years of age) with no primary liver disease[6,7]. No certain underlying trigger is detected in FLC. Less than 10% of patients with FLC have cirrhotic liver morphology[8]. Unlike HCC, cirrhosis and viral hepatitis infection are not predisposing to FLC, and it is typically not associated with elevations in serum alpha-fetoprotein[9,10]. More than half of patients with FLC are Caucasians, while more than 80% of HCC patients are Caucasians[6]. Tumour markers are increased in less than 10% of affected patient and have no role in the assessment or diagnosis of FLC[11,12].
Most cases with FLC cases are advanced at the time of diagnosis; however, up to 70% of patients may still be treated with curative therapy. The current cornerstone therapy (resection/liver transplantation) is still the sole possibly curative approach[13]. Chemotherapy was utilized as a complimentary treatment before and after postoperative resection. However, because of the low frequency of FLC, no randomized controlled trial (RCT) has explained the most successful regimens[14]. Still, no neo-adjuvant/adjuvant systemic therapies have been reported to improve survival in patients with resected FLC[14]. Therefore, chemotherapeutic agents like gemcitabine, cisplatin, 5-fluorouracil, interferon, and oxaliplatin have been tried and have demonstrated various degrees of response[15,16].
Due to the rarity of this tumor, exact estimates of its occurrence across nations are difficult to come by. FLCs account for less than 1% of primary liver tumors in the United States and 5.8% of liver tumors in Mexico[17]. Incidence rates, on the other hand, are very consistent over the world[6,18]. FLC affects a younger group, with a median age of 21 years, compared to HCC, affecting people between the ages of 14 and 33. The vast majority of cases (64%) are discovered before the age of 40[6]. A bimodal age distribution has been described, with incidence peaks between the ages of 10 and 30, and a second peak between the ages of 60 and 69 years[19,20]. Most research show that both sexes are equally affected, however a few have shown a slight male predominance (Male: Female = 1.7:1)[6,18].
Another interesting study showed a that female gender was more prevalent in the FLC group than in the traditional HCC group (60% vs 37%)[6]. This was also seen in the SEER study, where the authors discovered that FLC had a larger percentage of females (51.5% vs 26.3%)[6].
Furthermore, the United States, Mexico, Sweden, Saudi Arabia, Thailand, France, Canada, South Africa, Japan, South Korea, India, Taiwan, and the United Kingdom have all reported a comparable incidence of FLC. This could exclude the strong association between race and ethnicity with FLC risk[21-24].
The etiology of FLC remains uncertain. It typically occurs in normal livers without a clear background of liver fibrosis or cirrhosis[25]. Unlike HCC, which are usually found in the presence of cirrhosis or chronic hepatitis[26], FLC has been reported to occur in association with focal nodular hyperplasia (FNH), a benign form of liver tumors[27,28]. Pathologically, both have a central stellate scar which appears on imaging studies, and copper accumulation upon histological examination[28,29]. Hepatitis B viral proteins or DNA have been found in FLC on rare occasions, although this seems to be by coincidence given the enormous global frequency of chronic hepatitis B infection, and there is no evidence to identify hepatitis B as an etiological agent[30-33]. Similarly, FLCs have arisen in women who use oral contraceptives, although the link seems to be coincidental[34].
FLC tumors are big, yellow/tan, hypervascular, well-circumscribed lumps in otherwise normal liver parenchyma with patches of necrosis[35]. A central stellate scar and conspicuous fibrous tissue may be seen in up to 75% of tumors[35]. Histological examination generally reveals well-differentiated big polygonal tumor cells with eosinophilic hyaline cytoplasmic particles. Large polygonal or spindle-shaped tumor cells with highly eosinophilic cytoplasm due to numerous mitochondria and conspicuous nuclei grouped in cords surrounded by lamellated collagen fibres describe FLC microscopically[30,36,37].
Usually, there is no cirrhosis in the surrounding liver parenchyma, although mononuclear cells and lymphocytes imply nonspecific inflammation[37]. Electron microscopy often reveals an increase in mitochondrial number, a pathogenic characteristic unique to FLC[30]. FLC immunohistochemistry has several characteristics with HCC, such as staining positive for hepatocyte paraffin[38]. Unlike HCC, however, FLC stains are negative for alpha-fetoprotein and significantly positive for CK7 and epithelial membrane antigen, both of which are indicators of biliary differentiation (CK19 and Ep CAM)[39,40]. Additionally, FLC exhibits the stem cell markers CD133 and CD44[41]. FLC also stains for epithelial growth factor receptor and transforming growth factor-beta more often and diffusely than classic HCC[41,42]. FLC may be distinguished from normal liver parenchyma and HCC thanks to genetic differences revealed. Honeyman and his colleagues found that a 400-kb deletion on chromosome 19 leads in a functional DNAJB1-PRKACA chimeric transcript in 100% of FLC tumors examined, further identifying FLC as a distinct entity[43,44].
Patients frequently report with a variety of symptoms and signs, ranging from discomfort to a liver tumour discovered during a clinical examination for another indication[11,45]. Symptoms commonly seen with the conventional HCC are not seen in FLC[24,46]. FLC patients often complain of nonspecific abdominal pain, nausea, abdominal fullness, malaise, and weight loss[30]. A palpable abdominal mass or hepatomegaly with or without right upper quadrant pain and jaundice due to biliary obstruction[47-49], male gynecomastia[50], fulminant liver failure[7,51-53], recurrent deep venous thrombosis[54], encephalopathy[55], lower limb thrombophlebitis[56], anemia[57], ascites[58], and hypoglycemia[59]. Hepatic transaminases and alkaline phosphatase levels are usually normal or slightly elevated[30,60,61]. Common characteristics of FLC upon presentation are featured in Figure 1.
Alpha-fetoprotein levels are predominately normal, unlike in traditional HCC[62]. Several case reports have described increased levels of blood transcobalamin I (haptocorrin) and vitamin B12 binding capacity[63,64]. To assess their diagnostic function, however, further research is needed. Although serum neurotensin levels have been reported to be high with FLC, this test was not enough sensitive or specific to be used for diagnosing FLC[25,65,66]. Des-gamma carboxy prothrombin is elevated in FLC and conventional HCC, which is less useful[66].
Ultrasound: FLCs exhibit a wide variety of sonographic characteristics and usually appear as well-defined masses with varying echogenicity[67].
Contrast-enhanced computed tomography: FLCs often appear on computed tomography as large heterogeneous well-defined lesions (80%–100%) with a lobulated contour. Calcification and core stellate scarring, as well as tumour necrosis, are found in 65%–70% of cases[39]. In the arterial phase, more than 80% of patients have increased contrast avidity, which reflects the tumors’ primary blood supply. In the venous phase, half of these masses enhance similarly to the background liver, one-third of these tumors show increased contrast avidity, and approximately two-thirds of these tumours enhance similarly to the background liver in the delayed phase, making differentiation difficult[5]. The hepatic hilum and hepatoduodenal ligament are the most prevalent sites for nodal metastatic lesions, accounting for up to 50% of cases[7,35,68]. On imaging, distant FLC metastasis, particularly to the lungs, peritoneum, and adrenal gland, has been recorded in 20%–30% of patients[6,68].
Magnetic resonance imaging: On Magnetic resonance imaging, FLC is hypointense on T1-weighted images and hyperintense on T2-weighted images with no intralesional fat. Unlike the FNH, the fibrous central scar is hypointense on both T1 and T2-weighted imaging[69]. When using Gadolinium as a contrast agent, the enhancement pattern is similar to that of a CT scan, with heterogeneous contrast enhancement on the arterial phase and isointense or hypointense contrast enhancement on the portal venous and delayed phases[70].
Nuclear medicine imaging may help diagnose FLC in certain cases[71]. On delayed phase pictures, these tumors demonstrate enhanced absorption of 99 mTc-labeled RBCs during the arterial phase and washout. They also seem photopenic when 99 mTc-sulfur colloid scanning is performed[71]. Although the relevance of 18FDG PET/CT in FLC is unknown, it may be beneficial for primary staging and restaging in recurring cases[72].
Histologic appearances are the most objective and have widely accepted differences between FLC and HCC[73,74]. So, histologic confirmation is needed to diagnose FLC with certainty[26]. Core biopsy is recommended over fine-needle aspiration for percutaneous biopsy because malignant hepatocytes may be aspirated without the distinctive fibrotic lamellae, resulting in a diagnosis of HCC rather than FLC[75].
Surgical resection is the ideal treatment option that could carry an advantageous prognosis[14]. Over 70% of patients need a major hepatectomy (i.e., semi hepatectomy or extended hepatectomy), with a median tumor size of 10.5 cm. Around 24% of patients undergo partial or minor hepatectomy[76-78]. When compared to older patients, young patients (under 40 years old) had a higher likelihood of resection[6]. Resected patients have 26%–76% postoperative survivals at five years with a median survival of 32–174 mo[7,79-81]. Patients undergoing resection had an overall survival rate of 58.2% (44%–70%) according to a SEER database analysis[6]. Recurrence occurs in a large number of patients (more than two-thirds)[1]. Disease recurrence after complete surgical resection is high, ranging from 33% to 100%[19,77,81,82]. The median time for recurrences to occur ranges between 10 and 33 mo, which is obviously short[7,35,80,83]. While recurrence of the disease after over five years postoperative is a rare event[81,84]. The significant recurrence rate after surgery may come as a surprise, particularly given that these patients were treated at highly skilled hepatobiliary facilities. These patients, on the other hand, were often in late stages, with large primary tumors and lymph node metastases, both of which have been recognized as poor prognostic indicators[81-83]. Surgical resection may also be beneficial for patients with recurrent illness. Yamashita et al[79] found that 86% of patients had recurrent illness following resection in their investigations. Surgical excision of recurrent FLC was linked to a longer median overall survival of 122 mo, compared to 37 mo without surgery.
FLC recurrence occurred in all patients after first surgery in Maniaci et al[83]'s analysis of ten patients, with a median time to recurrence of 2.2 years, and seven patients were surgically handled, with a median survival of 4.7 years and an OS of 48% at five years. Patients who are not surgical candidates, on the other hand, have few therapy alternatives, with a median overall survival of less than 12 mo[7,84,85].
A curative alternative with transplantation has comparable survival rates to transplanted classical HCC in unresectable FLC[86]. Liver transplantation should be regarded a 3-year survival rate if 75%–80% of the liver is transplanted[80]. Because HCC is more prevalent than FLC, and regional lymph node metastases (a relative contraindication to transplant) is more likely in FLC (42.2%) compared to HCC (22.2%), liver transplantation is considerably more typically demonstrated for HCC than FLC[76].
Because of the limited incidence of FLC, no available RCT has shown the most effective chemotherapeutic option. It's worth noting that no neo-adjuvant/adjuvant systemic therapy have yet been found to increase survival in patients with excised FLC[14]. Furthermore, FLC is not normally sensitive to chemotherapy; nevertheless, platinum-based chemotherapy regimens and combination regimens, including interferon alpha-2b, have been utilized successfully[83,87].
A full or partial response has been observed in five out of eight patients treated with fluorouracil plus recombinant interferon alpha-2b in a Phase II trial[87,88]. Gemcitabine, cisplatin, 5-fluorouracil, interferon, and oxaliplatin are examples of agents that must be taken and have varied degrees of response[16]. Better results have been seen with combined treatment regimens that involve surgery, chemotherapy, and radiation[83]. Furthermore, percutaneous radioembolization has been used to reduce the size of the tumor prior to surgical excision[89]. One of the targeted therapies that have shown efficacy in treating HCC, sorafenib, was evaluated in cases with FLC but has shown limited efficiency[16].
Because FLC is not frequently responsive to systemic chemotherapy, only a few cases of FLC treated with radiation treatment have been reported[83,90]. Radiation treatment was utilized to treat unresectable primary tumors[90], to convert unresectable to resectable tumors[91], and to treat metastases or relapses[83] in these reports. One report found that employing targeted internal radiation treatment with Yttrium-90 resulted in a substantial FLC response, enabled the patient to undergo curative surgical resection[89]. Using 40 Gy in ten parts over 13 d, one case report demonstrated an 85% reduction in tumor volume of FLC metastases[92]. Three patients achieved objective partial responses, six patients had tumour volume stability, and one patient had early progression in a separate retrospective analysis of 10 patients with nonresectable metastatic cancer treated with external beam radiation in addition to chemotherapy[90].
In the IMbrave150 study, the combination of atezolizumab and bevacizumab improved survival and considerably delayed deterioration, lowering the chance of death by 42% compared to sorafenib monotherapy in the treatment of patients with unresectable classical cancer (HCC). Patients with FLC, on the other hand, were not included in this study[93]. Another research found that three cases with metastatic FLC progressed after 2–3 mo of initiating PD-L1 antibodies, one of them was treated with pembrolizumab and the other two with nivolumab[16]. Checkpoint inhibitors have been shown to be effective in the treatment of melanoma, lung cancer, renal cell carcinoma, and head and neck cancers[94], and they seem to be a viable therapeutic strategy in HCC[95,96]. Several tumor features seem to encourage a response to checkpoint inhibitors, including tumor-inherent genomic instability and a high mutational burden, both of which are linked to increased overall survival[94,97]. In a Phase II trial of advanced HCC, checkpoint inhibitors showed acceptable efficacy[39]. However, there are no controlled studies testing checkpoint inhibitors in FLC, and case reports are few and contradictory[14,16]. FLC's molecular characterization has recently identified potential targets such as the mTOR pathway and Aurora A kinase. Despite the positive findings of mTOR inhibition in sporadic cases[98], no encouraging results from controlled studies have been revealed to date[99].
DNAJB1-PRKACA: In conventional HCC and cholangiocellular cancers, DNAJB1-PRKACA rearrangements are absent[100]. In primary hepatocellular neoplastic processes, DNAJB1-PRKACA and PRKACA rearrangement detection using a break-apart fluorescence in situ hybridization probe or a polymerase chain reaction provides both sensitive and specific elucidation[100]. Introducing the DNAJB1-PRKACA fusion gene into wild-type mice resulted in hepatic tumors in mice with characteristics similar to human FLC, according to Engelholm et al[101]. The kinase activity of PRKACA, the catalytic subunit of protein kinase A (PKA), has been shown in this newly characterized predominant fusion protein[43,102,103]. This fusion is not unique to FLC, since it has been shown in other cancers[104]. However, significant levels of DNAJB1-PRKACA protein expression (amplified in over 70% of FLC) compared to a normal liver or HCC[103] make DNAJB1-PRKACA a promising therapeutic target. Because PKA regulates so many oncogenic signaling pathways[105,106], kinase inhibitors that bind at the active region of the PKA catalytic subunit may simultaneously target many oncogenic proteins. There are no known clinical studies utilizing such inhibitors against FLC[107].
mTOR: The first randomized Phase II clinical trial for FLC had three arms: The mTOR inhibitor everolimus, estrogen-deprivation therapy with leuprolide plus letrozole, and everolimus plus estrogen-deprivation therapy. This study was discontinued due to a lack of improvement in progression-free survival among the three study arms[108]. The mammalian target of rapamycin (mTOR) is an intracellular protein kinase expressed in mammalian cells and is important for the development of many cancers[109]. When this route is disrupted, mTOR is activated, resulting in enhanced cell proliferation, angiogenesis, and apoptosis evasion[110].
For low and intermediate-grade neuroendocrine tumors, the mTOR inhibitor everolimus coupled with octreotide is helpful. The majority of patients had a partial response or stable disease, with a small percentage having tumor progression[111].
Despite the fact that FLC patients frequently have advanced illness, around 50%to 84% of them are surgically treatable and have a five-year survival rate of up to 76%. FLC patients tend to have a better prognosis than HCC patients, who have a far poorer prognosis, with a 5-year survival rate of just 6.8%[112]. Those with FLC, on the other hand, do not have a better prognosis and do not react to therapy any differently than patients with HCC in non-cirrhotic livers at the same stage of disease[113-116]. The apparent superior result reported in FLC might be due to the lack of liver cirrhosis, as well as the disease's indolent character and younger age, which allows for intensive surgical treatment[6,7,113,117].
Tumor stage, number and size of tumors, vascular invasion, regional lymph node metastases, extrahepatic disease, non-white race, and female gender have all been linked to poor surgical outcomes[7,78,81,82]. However, it seems that the tumor's early stage at the time of treatment is the most important driver of prognosis. Patients with stage I–III illness had a better prognosis than those with stage IV disease[7,84,118,119], and sometimes, this difference attains statistical significance[81,84,119].
FLC is a rare liver cancer and this relative rarity makes data collection and clinical research protocol designing difficult. Collaboration between international institutes and societies in conducting large scale global research addressing epidemiologic aspects of FLC is needed. No predictive standards have been elucidated for FLC. Unfortunately, non-surgical options for FLC patients remain limited. Experimental animal studies may be needed to better understand FLC’s pathogenesis and molecular genetics. Evidence supporting systemic therapies in FLC is scarce, further research is required on the chemotherapeutic compounds used, including cisplatin, epirubicin, 5-fluorouracil. There is a need to expand our understanding of the molecular underpinnings of FLC and outline the current knowledge gaps to reach a consensus regarding effective treatment modalities.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Egyptian Association for Research and Training in Hepatogastroenterology, No. 001.
Specialty type: Oncology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Giacomelli L, Italy; Kang KJ, South Korea S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Riggle KM, Turnham R, Scott JD, Yeung RS, Riehle KJ. Fibrolamellar Hepatocellular Carcinoma: Mechanistic Distinction From Adult Hepatocellular Carcinoma. Pediatr Blood Cancer. 2016;63:1163-1167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM; IMPROVE-IT Investigators. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med. 2015;372:2387-2397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3427] [Cited by in RCA: 3002] [Article Influence: 300.2] [Reference Citation Analysis (0)] |
| 3. | Edmondson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child. 1956;91:168-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 96] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Ross HM, Daniel HD, Vivekanandan P, Kannangai R, Yeh MM, Wu TT, Makhlouf HR, Torbenson M. Fibrolamellar carcinomas are positive for CD68. Mod Pathol. 2011;24:390-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 5. | Ganeshan D, Szklaruk J, Kaseb A, Kattan A, Elsayes KM. Fibrolamellar hepatocellular carcinoma: multiphasic CT features of the primary tumor on pre-therapy CT and pattern of distant metastases. Abdom Radiol (NY). 2018;43:3340-3348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | El-Serag HB, Davila JA. Is fibrolamellar carcinoma different from hepatocellular carcinoma? Hepatology. 2004;39:798-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 192] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Stipa F, Yoon SS, Liau KH, Fong Y, Jarnagin WR, D'Angelica M, Abou-Alfa G, Blumgart LH, DeMatteo RP. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer. 2006;106:1331-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 152] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Sobel ME. Metastasis suppressor genes. J Natl Cancer Inst. 1990;82:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Farber BA, Lalazar G, Simon EP, Hammond WJ, Requena D, Bhanot UK, La Quaglia MP, Simon SM. Non coding RNA analysis in fibrolamellar hepatocellular carcinoma. Oncotarget. 2018;9:10211-10227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Lim II, Farber BA, LaQuaglia MP. Advances in fibrolamellar hepatocellular carcinoma: a review. Eur J Pediatr Surg. 2014;24:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Wahab MA, El Hanafy E, El Nakeeb A, Ali MA. Clinicopathological features and surgical outcome of patients with fibrolamellar hepatocellular carcinoma (experience with 22 patients over a 15-year period). World J Gastrointest Surg. 2017;9:61-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Abdel-Wahab M, El-Husseiny TS, El Hanafy E, El Shobary M, Hamdy E. Prognostic factors affecting survival and recurrence after hepatic resection for hepatocellular carcinoma in cirrhotic liver. Langenbecks Arch Surg. 2010;395:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Chaudhari VA, Khobragade K, Bhandare M, Shrikhande SV. Management of fibrolamellar hepatocellular carcinoma. Chin Clin Oncol. 2018;7:51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Chakrabarti S, Tella SH, Kommalapati A, Huffman BM, Yadav S, Riaz IB, Goyal G, Mody K, Borad M, Cleary S, Smoot RL, Mahipal A. Clinicopathological features and outcomes of fibrolamellar hepatocellular carcinoma. J Gastrointest Oncol. 2019;10:554-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Gras P, Truant S, Boige V, Ladrat L, Rougier P, Pruvot FR, Hebbar M. Prolonged Complete Response after GEMOX Chemotherapy in a Patient with Advanced Fibrolamellar Hepatocellular Carcinoma. Case Rep Oncol. 2012;5:169-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Bauer U, Mogler C, Braren RF, Algül H, Schmid RM, Ehmer U. Progression after Immunotherapy for Fibrolamellar Carcinoma. Visc Med. 2019;35:39-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Arista-Nasr J, Gutierrez-Villalobos L, Nuncio J, Maldonaldo H, Bornstein-Quevedo L. Fibrolamellar hepatocellular carcinoma in mexican patients. Pathol Oncol Res. 2002;8:133-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Eggert T, McGlynn KA, Duffy A, Manns MP, Greten TF, Altekruse SF. Fibrolamellar hepatocellular carcinoma in the USA, 2000-2010: A detailed report on frequency, treatment and outcome based on the Surveillance, Epidemiology, and End Results database. United European Gastroenterol J. 2013;1:351-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Mavros MN, Mayo SC, Hyder O, Pawlik TM. A systematic review: treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma. J Am Coll Surg. 2012;215:820-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Eggert T, McGlynn KA, Duffy A, Manns MP, Greten TF, Altekruse SF. Epidemiology of fibrolamellar hepatocellular carcinoma in the USA, 2000-10. Gut. 2013;62:1667-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Moore SW, Davidson A, Hadley GP, Kruger M, Poole J, Stones D, Wainwright L, Wessels G. Malignant liver tumors in South African children: a national audit. World J Surg. 2008;32:1389-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Al-Matham K, Alabed I, Zaidi SZ, Qushmaq KA. Cold agglutinin disease in fibrolamellar hepatocellular carcinoma: a rare association with a rare cancer variant. Ann Saudi Med. 2011;31:197-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Thirabanjasak D, Sosothikul D, Mahayosnond A, Thorner PS. Fibrolamellar carcinoma presenting as a pancreatic mass: case report and review of the literature. J Pediatr Hematol Oncol. 2009;31:370-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Yen JB, Chang KW. Fibrolamellar hepatocellular carcinoma- report of a case. Chang Gung Med J. 2009;32:336-339. [PubMed] |
| 25. | Collier NA, Weinbren K, Bloom SR, Lee YC, Hodgson HJ, Blumgart LH. Neurotensin secretion by fibrolamellar carcinoma of the liver. Lancet. 1984;1:538-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | McLarney JK, Rucker PT, Bender GN, Goodman ZD, Kashitani N, Ros PR. Fibrolamellar carcinoma of the liver: radiologic-pathologic correlation. Radiographics. 1999;19:453-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 112] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Imkie M, Myers SA, Li Y, Fan F, Bennett TL, Forster J, Tawfik O. Fibrolamellar hepatocellular carcinoma arising in a background of focal nodular hyperplasia: a report of 2 cases. J Reprod Med. 2005;50:633-637. [PubMed] |
| 28. | Vecchio FM, Fabiano A, Ghirlanda G, Manna R, Massi G. Fibrolamellar carcinoma of the liver: the malignant counterpart of focal nodular hyperplasia with oncocytic change. Am J Clin Pathol. 1984;81:521-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 56] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Vecchio FM. Fibrolamellar carcinoma of the liver: a distinct entity within the hepatocellular tumors. A review. Appl Pathol. 1988;6:139-148. [PubMed] |
| 30. | Craig JR, Peters RL, Edmondson HA, Omata M. Fibrolamellar carcinoma of the liver: a tumor of adolescents and young adults with distinctive clinico-pathologic features. Cancer. 1980;46:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Dadke D, Jaganath P, Krishnamurthy S, Chiplunkar S. The detection of HBV antigens and HBx-transcripts in an Indian fibrolamellar carcinoma patient: a case study. Liver. 2002;22:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Morise Z, Sugioka A, Mizoguchi Y, Fujita J, Hoshimoto S, Kato T, Hasumi A. Fibrolamellar carcinoma of the liver in a Japanese hepatitis B virus carrier. J Gastroenterol Hepatol. 2005;20:1136-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Davison FD, Fagan EA, Portmann B, Williams R. HBV-DNA sequences in tumor and nontumor tissue in a patient with the fibrolamellar variant of hepatocellular carcinoma. Hepatology. 1990;12:676-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Malt RA, Galdabini JJ, Jeppsson BW. Abnormal sex-steroid milieu in young adults with hepatocellular carcinoma. World J Surg. 1983;7:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Ichikawa T, Federle MP, Grazioli L, Marsh W. Fibrolamellar hepatocellular carcinoma: pre- and posttherapy evaluation with CT and MR imaging. Radiology. 2000;217:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Berman MM, Libbey NP, Foster JH. Hepatocellular carcinoma. Polygonal cell type with fibrous stroma--an atypical variant with a favorable prognosis. Cancer. 1980;46:1448-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Liu S, Chan KW, Wang B, Qiao L. Fibrolamellar hepatocellular carcinoma. Am J Gastroenterol. 2009;104:2617-24; quiz 2625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Weir HK, Thompson TD, Soman A, Møller B, Leadbetter S, White MC. Meeting the Healthy People 2020 Objectives to Reduce Cancer Mortality. Prev Chronic Dis. 2015;12:E104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Ichikawa T, Federle MP, Grazioli L, Madariaga J, Nalesnik M, Marsh W. Fibrolamellar hepatocellular carcinoma: imaging and pathologic findings in 31 recent cases. Radiology. 1999;213:352-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 142] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Ward SC, Waxman S. Fibrolamellar carcinoma: a review with focus on genetics and comparison to other malignant primary liver tumors. Semin Liver Dis. 2011;31:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Zenali MJ, Tan D, Li W, Dhingra S, Brown RE. Stemness characteristics of fibrolamellar hepatocellular carcinoma: immunohistochemical analysis with comparisons to conventional hepatocellular carcinoma. Ann Clin Lab Sci. 2010;40:126-134. [PubMed] |
| 42. | Orsatti G, Hytiroglou P, Thung SN, Ishak KG, Paronetto F. Lamellar fibrosis in the fibrolamellar variant of hepatocellular carcinoma: a role for transforming growth factor beta. Liver. 1997;17:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Honeyman JN, Simon EP, Robine N, Chiaroni-Clarke R, Darcy DG, Lim II, Gleason CE, Murphy JM, Rosenberg BR, Teegan L, Takacs CN, Botero S, Belote R, Germer S, Emde AK, Vacic V, Bhanot U, LaQuaglia MP, Simon SM. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science. 2014;343:1010-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 363] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 44. | Darcy DG, Chiaroni-Clarke R, Murphy JM, Honeyman JN, Bhanot U, LaQuaglia MP, Simon SM. The genomic landscape of fibrolamellar hepatocellular carcinoma: whole genome sequencing of ten patients. Oncotarget. 2015;6:755-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 45. | Butte JM, Waugh E, Meneses M, Pruzzo R, Carvallo C, Redondo F, Suárez C, Parada H, Amaral H, de La Fuente H. [Fibrolamellar liver carcinoma: report of two cases and review of the literature]. Rev Med Chil. 2009;137:394-400. [PubMed] |
| 46. | Terzis I, Haritanti A, Economou I. Fibrolamellar hepatocellular carcinoma: a case report with distinct radiological features. J Gastrointest Cancer. 2010;41:2-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Albaugh JS, Keeffe EB, Krippaehne WW. Recurrent obstructive jaundice caused by fibrolamellar hepatocellular carcinoma. Dig Dis Sci. 1984;29:762-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 48. | Eckstein RP, Bambach CP, Stiel D, Roche J, Goodman BN. Fibrolamellar carcinoma as a cause of bile duct obstruction. Pathology. 1988;20:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Soyer P, Roche A, Levesque M. Fibrolamellar hepatocellular carcinoma presenting with obstructive jaundice. A report of two cases. Eur J Radiol. 1991;13:196-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | McCloskey JJ, Germain-Lee EL, Perman JA, Plotnick LP, Janoski AH. Gynecomastia as a presenting sign of fibrolamellar carcinoma of the liver. Pediatrics. 1988;82:379-382. [PubMed] |
| 51. | Athanasakis E, Mouloudi E, Prinianakis G, Kostaki M, Tzardi M, Georgopoulos D. Metastatic liver disease and fulminant hepatic failure: presentation of a case and review of the literature. Eur J Gastroenterol Hepatol. 2003;15:1235-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | Myszor MF, Record CO. Primary and secondary malignant disease of the liver and fulminant hepatic failure. J Clin Gastroenterol. 1990;12:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Soreide O, Czerniak A, Bradpiece H, Bloom S, Blumgart L. Characteristics of fibrolamellar hepatocellular carcinoma. A study of nine cases and a review of the literature. Am J Surg. 1986;151:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 70] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 54. | Marrannes J, Gryspeerdt S, Haspeslagh M, van Holsbeeck B, Baekelandt M, Lefere P. Fibrolamellar hepatocellular carcinoma in a 65-year-old woman: CT features. JBR-BTR. 2005;88:237-240. [PubMed] |
| 55. | Sethi S, Tageja N, Singh J, Arabi H, Dave M, Badheka A, Revankar S. Hyperammonemic encephalopathy: a rare presentation of fibrolamellar hepatocellular carcinoma. Am J Med Sci. 2009;338:522-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Mansouri D, Van Nhieu JT, Couanet D, Terrier-Lacombe MJ, Brugières L, Cherqui D, Suciu V, Vielh P. Fibrolamellar hepatocellular carcinoma: a case report with cytological features in a sixteen-year-old girl. Diagn Cytopathol. 2006;34:568-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 57. | Tanaka J, Baba N, Arii S, Fujita K, Tamura J, Kawakami Y, Tsuji S, Imamura M, Yamabe H, Nakai S. Typical fibrolamellar hepatocellular carcinoma in Japanese patients: report of two cases. Surg Today. 1994;24:459-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Gupta P, Dhar S, Strickland NH. Fibrolamellar carcinoma: an unusual clinico-radiological presentation. Eur J Radiol. 1999;32:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Tangkijvanich P, Thong-Ngam D, Kullavanijaya P, Suwangool P. Fibrolamellar hepatocellular carcinoma in a Thai man who presented with hypoglycemia: case report and review of literature. J Med Assoc Thai. 2000;83:809-816. [PubMed] |
| 60. | Hemming AW, Langer B, Sheiner P, Greig PD, Taylor BR. Aggressive surgical management of fibrolamellar hepatocellular carcinoma. J Gastrointest Surg. 1997;1:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Torbenson M. Fibrolamellar carcinoma: 2012 update. Scientifica (Cairo). 2012;2012:743790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 62. | Ward SC, Huang J, Tickoo SK, Thung SN, Ladanyi M, Klimstra DS. Fibrolamellar carcinoma of the liver exhibits immunohistochemical evidence of both hepatocyte and bile duct differentiation. Mod Pathol. 2010;23:1180-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 63. | Wheeler K, Pritchard J, Luck W, Rossiter M. Transcobalamin I as a "marker" for fibrolamellar hepatoma. Med Pediatr Oncol. 1986;14:227-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Lildballe DL, Nguyen KQ, Poulsen SS, Nielsen HO, Nexo E. Haptocorrin as marker of disease progression in fibrolamellar hepatocellular carcinoma. Eur J Surg Oncol. 2011;37:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Ehrenfried JA, Zhou Z, Thompson JC, Evers BM. Expression of the neurotensin gene in fetal human liver and fibrolamellar carcinoma. Ann Surg. 1994;220:484-9; discussion 489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Bertino G, Ardiri AM, Calvagno GS, Bertino N, Boemi PM. Prognostic and diagnostic value of des-γ-carboxy prothrombin in liver cancer. Drug News Perspect. 2010;23:498-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 67. | Friedman AC, Lichtenstein JE, Goodman Z, Fishman EK, Siegelman SS, Dachman AH. Fibrolamellar hepatocellular carcinoma. Radiology. 1985;157:583-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 60] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Ganeshan D, Szklaruk J, Kundra V, Kaseb A, Rashid A, Elsayes KM. Imaging features of fibrolamellar hepatocellular carcinoma. AJR Am J Roentgenol. 2014;202:544-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 69. | Brandt DJ, Johnson CD, Stephens DH, Weiland LH. Imaging of fibrolamellar hepatocellular carcinoma. AJR Am J Roentgenol. 1988;151:295-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 70. | Kadoya M, Matsui O, Takashima T, Nonomura A. Hepatocellular carcinoma: correlation of MR imaging and histopathologic findings. Radiology. 1992;183:819-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 195] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 71. | Blachar A, Federle MP, Ferris JV, Lacomis JM, Waltz JS, Armfield DR, Chu G, Almusa O, Grazioli L, Balzano E, Li W. Radiologists' performance in the diagnosis of liver tumors with central scars by using specific CT criteria. Radiology. 2002;223:532-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Liu S, Wah Chan K, Tong J, Wang Y, Wang B, Qiao L. PET-CT scan is a valuable modality in the diagnosis of fibrolamellar hepatocellular carcinoma: a case report and a summary of recent literature. QJM. 2011;104:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 73. | Mitchell KA. Hepatocellular carcinoma: histologic considerations: pure, mixed, and motley. J Clin Gastroenterol. 2013;47 Suppl:S20-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 74. | Powers C, Ros PR, Stoupis C, Johnson WK, Segel KH. Primary liver neoplasms: MR imaging with pathologic correlation. Radiographics. 1994;14:459-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 80] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 75. | Suen KC, Magee JF, Halparin LS, Chan NH, Greene CA. Fine needle aspiration cytology of fibrolamellar hepatocellular carcinoma. Acta Cytol. 1985;29:867-872. [PubMed] |
| 76. | Mayo SC, Mavros MN, Nathan H, Cosgrove D, Herman JM, Kamel I, Anders RA, Pawlik TM. Treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma: a national perspective. J Am Coll Surg. 2014;218:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 77. | Kaseb AO, Shama M, Sahin IH, Nooka A, Hassabo HM, Vauthey JN, Aloia T, Abbruzzese JL, Subbiah IM, Janku F, Curley S, Hassan MM. Prognostic indicators and treatment outcome in 94 cases of fibrolamellar hepatocellular carcinoma. Oncology. 2013;85:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 78. | Ang CS, Kelley RK, Choti MA, Cosgrove DP, Chou JF, Klimstra D, Torbenson MS, Ferrell L, Pawlik TM, Fong Y, O'Reilly EM, Ma J, McGuire J, Vallarapu GP, Griffin A, Stipa F, Capanu M, Dematteo RP, Venook AP, Abou-Alfa GK. Clinicopathologic characteristics and survival outcomes of patients with fibrolamellar carcinoma: data from the fibrolamellar carcinoma consortium. Gastrointest Cancer Res. 2013;6:3-9. [PubMed] |
| 79. | Yamashita S, Vauthey JN, Kaseb AO, Aloia TA, Conrad C, Hassan MM, Passot G, Raghav KP, Shama MA, Chun YS. Prognosis of Fibrolamellar Carcinoma Compared to Non-cirrhotic Conventional Hepatocellular Carcinoma. J Gastrointest Surg. 2016;20:1725-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 80. | El-Gazzaz G, Wong W, El-Hadary MK, Gunson BK, Mirza DF, Mayer AD, Buckels JA, McMaster P. Outcome of liver resection and transplantation for fibrolamellar hepatocellular carcinoma. Transpl Int. 2000;13 Suppl 1:S406-S409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 81. | Groeschl RT, Miura JT, Wong RK, Bloomston M, Lidsky ML, Clary BM, Martin RC, Belli G, Buell JF, Gamblin TC. Multi-institutional analysis of recurrence and survival after hepatectomy for fibrolamellar carcinoma. J Surg Oncol. 2014;110:412-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 82. | Darcy DG, Malek MM, Kobos R, Klimstra DS, DeMatteo R, La Quaglia MP. Prognostic factors in fibrolamellar hepatocellular carcinoma in young people. J Pediatr Surg. 2015;50:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 83. | Maniaci V, Davidson BR, Rolles K, Dhillon AP, Hackshaw A, Begent RH, Meyer T. Fibrolamellar hepatocellular carcinoma: prolonged survival with multimodality therapy. Eur J Surg Oncol. 2009;35:617-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 84. | Pinna AD, Iwatsuki S, Lee RG, Todo S, Madariaga JR, Marsh JW, Casavilla A, Dvorchik I, Fung JJ, Starzl TE. Treatment of fibrolamellar hepatoma with subtotal hepatectomy or transplantation. Hepatology. 1997;26:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 112] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 85. | Kassahun WT. Contemporary management of fibrolamellar hepatocellular carcinoma: diagnosis, treatment, outcome, prognostic factors, and recent developments. World J Surg Oncol. 2016;14:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 86. | Atienza LG, Berger J, Mei X, Shah MB, Daily MF, Grigorian A, Gedaly R. Liver transplantation for fibrolamellar hepatocellular carcinoma: A national perspective. J Surg Oncol. 2017;115:319-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 87. | Bower M, Newlands ES, Habib N. Fibrolamellar hepatocellular carcinoma responsive to platinum-based combination chemotherapy. Clin Oncol (R Coll Radiol). 1996;8:331-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Patt YZ, Hassan MM, Lozano RD, Brown TD, Vauthey JN, Curley SA, Ellis LM. Phase II trial of systemic continuous fluorouracil and subcutaneous recombinant interferon Alfa-2b for treatment of hepatocellular carcinoma. J Clin Oncol. 2003;21:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 89. | Mafeld S, French J, Tiniakos D, Haugk B, Manas D, Littler P. Fibrolamellar Hepatocellular Carcinoma: Treatment with Yttrium-90 and Subsequent Surgical Resection. Cardiovasc Intervent Radiol. 2018;41:816-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Epstein BE, Pajak TF, Haulk TL, Herpst JM, Order SE, Abrams RA. Metastatic nonresectable fibrolamellar hepatoma: prognostic features and natural history. Am J Clin Oncol. 1999;22:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 91. | Sitzmann JV. Conversion of unresectable to resectable liver cancer: an approach and follow-up study. World J Surg. 1995;19:790-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 92. | G Peacock J, A Call J, R Olivier K. Radiotherapy for metastatic fibrolamellar hepatocellular carcinoma. Rare Tumors. 2013;5:e28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 93. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4691] [Article Influence: 938.2] [Reference Citation Analysis (2)] |
| 94. | Rotte A, Jin JY, Lemaire V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol. 2018;29:71-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 95. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3278] [Cited by in RCA: 3308] [Article Influence: 413.5] [Reference Citation Analysis (1)] |
| 96. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1895] [Article Influence: 270.7] [Reference Citation Analysis (0)] |
| 97. | Yaghmour G, Pandey M, Ireland C, Patel K, Nunnery S, Powell D, Baum S, Wiedower E, Schwartzberg LS, Martin MG. Role of Genomic Instability in Immunotherapy with Checkpoint Inhibitors. Anticancer Res. 2016;36:4033-4038. [PubMed] |
| 98. | Bill R, Montani M, Blum B, Dufour JF, Escher R, Bühlmann M. Favorable response to mammalian target of rapamycin inhibition in a young patient with unresectable fibrolamellar carcinoma of the liver. Hepatology. 2018;68:384-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 99. | Lalazar G, Simon SM. Fibrolamellar Carcinoma: Recent Advances and Unresolved Questions on the Molecular Mechanisms. Semin Liver Dis. 2018;38:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 100. | Dinh TA, Vitucci EC, Wauthier E, Graham RP, Pitman WA, Oikawa T, Chen M, Silva GO, Greene KG, Torbenson MS, Reid LM, Sethupathy P. Comprehensive analysis of The Cancer Genome Atlas reveals a unique gene and non-coding RNA signature of fibrolamellar carcinoma. Sci Rep. 2017;7:44653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 101. | Engelholm LH, Riaz A, Serra D, Dagnæs-Hansen F, Johansen JV, Santoni-Rugiu E, Hansen SH, Niola F, Frödin M. CRISPR/Cas9 Engineering of Adult Mouse Liver Demonstrates That the Dnajb1-Prkaca Gene Fusion Is Sufficient to Induce Tumors Resembling Fibrolamellar Hepatocellular Carcinoma. Gastroenterology. 2017;153:1662-1673.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 102. | Riggle KM, Riehle KJ, Kenerson HL, Turnham R, Homma MK, Kazami M, Samelson B, Bauer R, McKnight GS, Scott JD, Yeung RS. Enhanced cAMP-stimulated protein kinase A activity in human fibrolamellar hepatocellular carcinoma. Pediatr Res. 2016;80:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 103. | Cornella H, Alsinet C, Sayols S, Zhang Z, Hao K, Cabellos L, Hoshida Y, Villanueva A, Thung S, Ward SC, Rodriguez-Carunchio L, Vila-Casadesús M, Imbeaud S, Lachenmayer A, Quaglia A, Nagorney DM, Minguez B, Carrilho F, Roberts LR, Waxman S, Mazzaferro V, Schwartz M, Esteller M, Heaton ND, Zucman-Rossi J, Llovet JM. Unique genomic profile of fibrolamellar hepatocellular carcinoma. Gastroenterology. 2015;148:806-18.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 104. | Vyas M, Hechtman JF, Zhang Y, Benayed R, Yavas A, Askan G, Shia J, Klimstra DS, Basturk O. DNAJB1-PRKACA fusions occur in oncocytic pancreatic and biliary neoplasms and are not specific for fibrolamellar hepatocellular carcinoma. Mod Pathol. 2020;33:648-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 105. | Adams DG, Sachs NA, Vaillancourt RR. Phosphorylation of the stress-activated protein kinase, MEKK3, at serine 166. Arch Biochem Biophys. 2002;407:103-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 106. | Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME. 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell. 2000;6:41-51. [PubMed] |
| 107. | Cheung J, Ginter C, Cassidy M, Franklin MC, Rudolph MJ, Robine N, Darnell RB, Hendrickson WA. Structural insights into mis-regulation of protein kinase A in human tumors. Proc Natl Acad Sci U S A. 2015;112:1374-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 108. | El Dika I, Mayer RJ, Venook AP, Capanu M, LaQuaglia MP, Kobos R, O'Neill AF, Chou JF, Ly M, Ang C, O'Reilly EM, Gordan JD, Abou-Alfa GK. A Multicenter Randomized Three-Arm Phase II Study of (1) Everolimus, (2) Estrogen Deprivation Therapy (EDT) with Leuprolide + Letrozole, and (3) Everolimus + EDT in Patients with Unresectable Fibrolamellar Carcinoma. Oncologist. 2020;25:925-e1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 109. | Samlowski WE, Vogelzang NJ. Emerging drugs for the treatment of metastatic renal cancer. Expert Opin Emerg Drugs. 2007;12:605-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 110. | Yuan R, Kay A, Berg WJ, Lebwohl D. Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. J Hematol Oncol. 2009;2:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 111. | Yao JC, Phan AT, Chang DZ, Wolff RA, Hess K, Gupta S, Jacobs C, Mares JE, Landgraf AN, Rashid A, Meric-Bernstam F. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26:4311-4318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 462] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 112. | Moreno-Luna LE, Arrieta O, García-Leiva J, Martínez B, Torre A, Uribe M, León-Rodríguez E. Clinical and pathologic factors associated with survival in young adult patients with fibrolamellar hepatocarcinoma. BMC Cancer. 2005;5:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 113. | Kakar S, Burgart LJ, Batts KP, Garcia J, Jain D, Ferrell LD. Clinicopathologic features and survival in fibrolamellar carcinoma: comparison with conventional hepatocellular carcinoma with and without cirrhosis. Mod Pathol. 2005;18:1417-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 114. | Wood WJ, Rawlings M, Evans H, Lim CN. Hepatocellular carcinoma: importance of histologic classification as a prognostic factor. Am J Surg. 1988;155:663-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 115. | Njei B, Konjeti VR, Ditah I. Prognosis of Patients With Fibrolamellar Hepatocellular Carcinoma Versus Conventional Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Gastrointest Cancer Res. 2014;7:49-54. [PubMed] |
| 116. | Katzenstein HM, Krailo MD, Malogolowkin MH, Ortega JA, Qu W, Douglass EC, Feusner JH, Reynolds M, Quinn JJ, Newman K, Finegold MJ, Haas JE, Sensel MG, Castleberry RP, Bowman LC. Fibrolamellar hepatocellular carcinoma in children and adolescents. Cancer. 2003;97:2006-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 117. | Berman MA, Burnham JA, Sheahan DG. Fibrolamellar carcinoma of the liver: an immunohistochemical study of nineteen cases and a review of the literature. Hum Pathol. 1988;19:784-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 87] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 118. | Ringe B, Wittekind C, Weimann A, Tusch G, Pichlmayr R. Results of hepatic resection and transplantation for fibrolamellar carcinoma. Surg Gynecol Obstet. 1992;175:299-305. [PubMed] |
| 119. | Malouf GG, Brugières L, Le Deley MC, Faivre S, Fabre M, Paradis V, Aerts I, Le Tourneau C, Dreyer C, Branchereau S, Belghiti J, Raymond E. Pure and mixed fibrolamellar hepatocellular carcinomas differ in natural history and prognosis after complete surgical resection. Cancer. 2012;118:4981-4990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |