Published online Mar 15, 2022. doi: 10.4251/wjgo.v14.i3.734
Peer-review started: May 17, 2021
First decision: July 4, 2021
Revised: July 16, 2021
Accepted: February 22, 2022
Article in press: February 22, 2022
Published online: March 15, 2022
Processing time: 296 Days and 23.8 Hours
Recently, there have been several findings that showed intestinal colonisation of Blastocystis hominis (Blastocystis) as a risk factor to the worsening of colorectal cancer (CRC). However, studies have shown controversial results in the pathogenicity of Blastocystis.
To review systematically the evidence available on the association between CRC and Blastocystis and the prevalence of Blastocystis in CRC patients and to investigate cytopathic and immunological effects of Blastocystis in in vitro and in vivo studies.
PRISMA guidelines were utilised in conducting this systematic review. Original articles published before February 2, 2020 were included. PubMed, Science Direct, Scopus and Google scholar databases were searched. Manual searching was carried out to find articles missed during the online search.
Out of 12 studies selected for this systematic review, seven studies confirmed the prevalence of Blastocystis and found it to be between 2%-28% in CRC patients, whereby subtype 1 and subtype 3 were predominantly seen. A total of four studies employing in vitro human colorectal carcinoma cell line study models showed significant cytopathic and immunological effects of Blastocystis. In addition, one in vivo experimental animal model study showed that there was a significant effect of infection with Blastocystis on exacerbation of colorectal carcinogenesis.
Blastocystis is a commonly identified microorganism in CRC patients. These studies have provided supportive data that Blastocystis could exacerbate existing CRC via alteration in host immune response and increased oxidative damage. Future studies of CRC and Blastocystis should attempt to determine the various stages of CRC that are most likely to be associated with Blastocystis and its relationship with other intestinal bacteria.
Core Tip: Certain gut microorganisms are known to be important factors associated with initiation and development of colorectal cancer (CRC). However, data on the roles of parasites are vague and restricted. Blastocystis hominis (Blastocystis) is one of the most commonly recovered microorganisms in faecal specimens, and its widespread presence is found in CRC patients. This systematic review aims to quantify the studies published so far that revealed the association of Blastocystis and CRC. We sought to identify the prevalence of Blastocystis and its subtypes among CRC patients, in vitro studies using Blastocystis antigen and in vivo studies using animal models.
- Citation: Kumarasamy V, Atroosh WM, Anbazhagan D, Abdalla MMI, Azzani M. Association of Blastocystis hominis with colorectal cancer: A systematic review of in vitro and in vivo evidences. World J Gastrointest Oncol 2022; 14(3): 734-745
- URL: https://www.wjgnet.com/1948-5204/full/v14/i3/734.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i3.734
Blastocystis hominis (Blastocystis) is one of the most commonly recovered microorganisms in faecal specimens, and its widespread presence is found in colorectal cancer (CRC) patients[1]. Its distribution is known to be prevalent in both rural and urban areas[2]. This microorganism has been in discussion since early 1900s[3,4]; however, the taxonomic position of Blastocystis remains unanswered. Blastocystis treatment is often difficult due to its drug resistance and the failure of the host defenses to counter the infection[5]. Previous studies suggest that Blastocystis is a common and diverse element of microbiota in human host, as it has been highly prevalent in healthy individuals[6-8]. Blastocystis is commonly found in both patients with gastrointestinal symptoms and in healthy people widely across the world. More recently, researchers consider Blastocystis as an emerging zoonotic disease, and its pathogenic potential in human is somewhat controversial[9]. Although accumulating data suggest that Blastocystis is a pathogen, the pathogenic role in humans is still a matter of debate.
It is suggested that around 20% of cancer reported worldwide could have been due to infectious agents[10,11]. Viruses such as hepatitis B virus, human papilloma virus and Epstein-Barr virus have been associated with carcinogenesis. Various other bacteria also have been described previously to exacerbate cancer[12]. There are numerous epidemiological evidences that strongly support the fact that parasites can be a factor of various malignant tumours[13], but it is challenging to validate this relationship. Previously, a review article highlighted the correlation of various protozoan parasites including Blastocystis with carcinogenesis[13]. In addition, there was a case report in India that demonstrated a possible association of subtype 3 Blastocystis in the worsening of CRC[14].
There are a few other systematic reviews on the interventional studies done on Blastocystis, but we did not find any systematic reviews on the association between Blastocystis and CRC. Therefore, this systematic review aimed to (1) identify prevalence of Blastocystis in CRC patients; (2) review in vitro colorectal carcinoma cell line study models on the cytopathic and immunological effects of Blastocystis antigens; and (3) review an in vivo experimental animal model study to investigate the effect of infection with Blastocystis on exacerbation of colorectal carcinogenesis.
PRISMA guidelines were utilised in conducting this review[15].
Original articles that reported the prevalence and association of Blastocystis subtypes with CRC patients, in vitro studies using Blastocystis antigen and an in vivo study using an animal model published before February 2, 2020 were included.
Articles that reported the association of Blastocystis with cancer in general without specific findings on its association with CRC, reviews papers, conference proceedings and case reports were excluded from this review.
PubMed, Science Direct, Scopus and Google scholar databases were searched.
The various keywords used were: Blastocystis infection and CRC and their MESH terms and synonyms. Manual searching through reference lists of included journal articles was done to find the missed studies during online search.
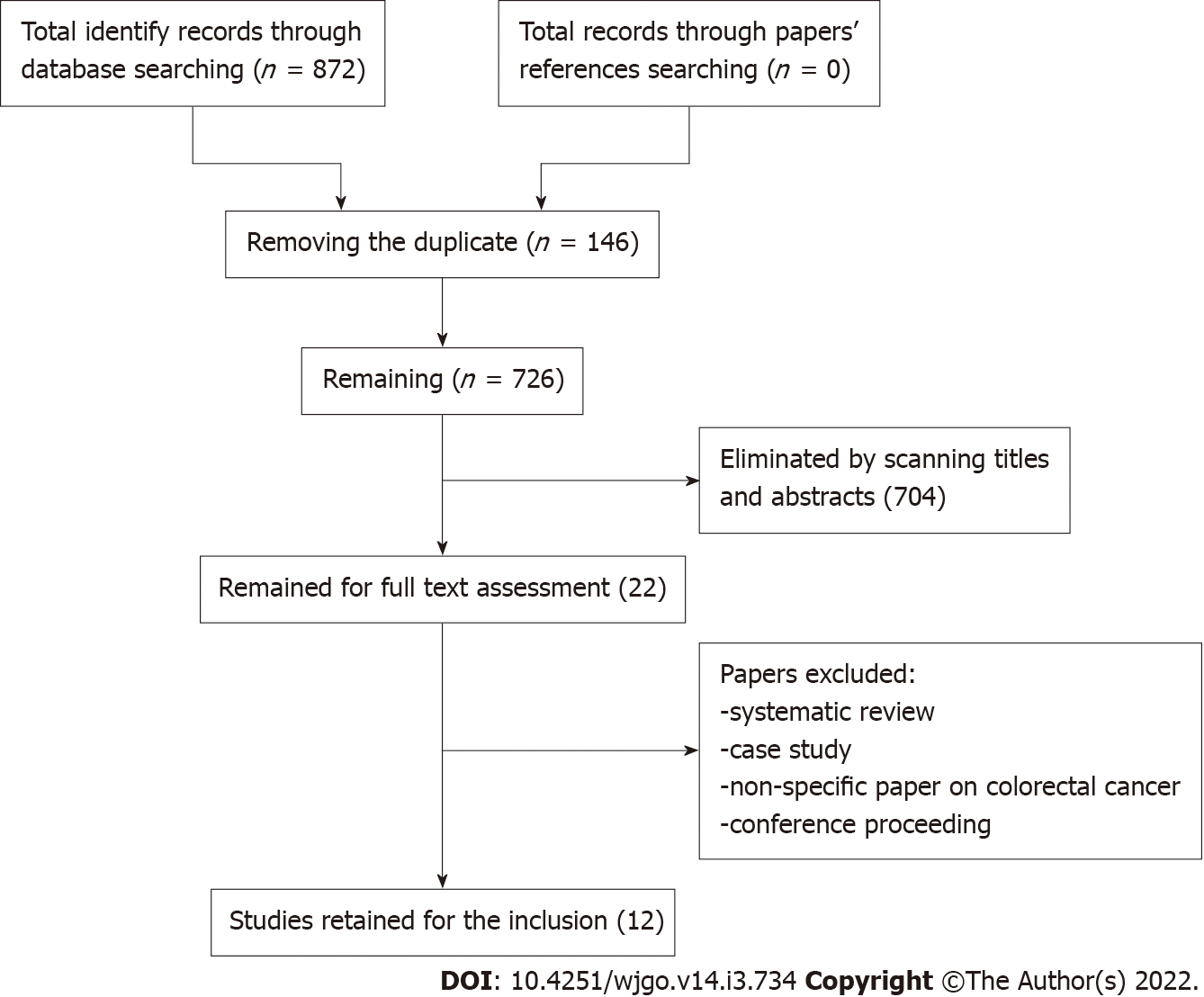
We identified 872 papers in the initial screening (Figure 1).
First, two authors developed a search strategy with different key words and their synonyms. All articles were moved to the Endnote X7 software (Clarivate Analytics, Philadelphia, PA, United States), and 146 duplicate papers were removed. Two authors independently went through the titles and abstracts of the remaining 726 papers. Subsequently, a total of 22 papers were retained for full text review. The eligibility of retained papers was evaluated by two other authors. Authors checked the reference lists of all included articles for any relevant studies that met this systematic review inclusion criteria and had not been found during the database searches.
After full review of the 22 papers, 12 were selected. All authors have completed data extraction and agreed eligibility of included papers (Figure 1).
For in vivo studies, the quality of the studies was assessed using Quasi experimental appraisal tool[16], which contained nine questions. For in vitro studies, checklist of Systematic Review Center for Laboratory Animal Experimentation’s Risk of Bias tool for assessing risk of bias as quality assessment was used[17]. The quality of all included studies was acceptable.
The data extracted included author-year, country, sampling, setting, methods, results and study conclusion (Table 1).
| No. | Ref. | Country | Sampling | Setting | Method | Main results | Conclusion |
| Please note: (1) Main outcomes assessed; (2) If the protocol is published; and (3) If risk of bias is reported | |||||||
| Prevalence studies | |||||||
| 1 | Esteghamati et al[35], 2019 | Tehran, Iran | Study design: cross-sectional. Study duration: July 2016 and November of 2017. Sample size (190): 80 patients (Primary Immunodeficiency), 85 (cancer patients) and 25 (organ transplant recipients) | 3 hospitals in in Tehran, Iran | The aim of this study to determine the prevalence of intestinal parasites in 3 different groups of patients referred to 3 hospitals. Method used for parasite identification: Conventional methods, nested PCR and amplification of the 18S rRNA gene | The prevalence of Blastocystis hominis among CRC patient was 13/39 (28.2%) | The prevalence of Blastocystis hominis was found high in cancer patients, especially CRC patients |
| 2 | Zhang et al[18], 2017 | China | Sample size: 381 faecal specimens were collected from cancer patients including CRC. Study duration: 2016 to 2017 | Tumor Hospitalof Harbin Medical University | The aim of this study to determine the prevalence and genotypes/subtypes-Blastocystis in CP and analysed for the Blastocystis by PCR amplifying and sequencing | Prevalence of Blastocystis was 4 (8.1%) among CRC patient | Blastocystis subtype 1 and 3 have been identified in humans and animals |
| 3 | Mohamed et al[19], 2017 | Saudi Arabia | Total sample size: 218. Two groups of participants: (1) CP (138) of which 74 had CRC and 46 had cancers outside gastrointestinal tract; and (2) NCP (80). Exclusion criteria: (1) Patient started chemotherapy regime; and (2) Receiving any anti-parasitic medication. Study duration: 2013-2015 | King Abdulla Medical city (KAMC), Makkah | Case control study design: Aim, to determine the prevalence of Blastocystis among CRC patients compared to patients who had cancers outside gastrointestinal tract and control group. Obtained Blastocystis isolates were grouped into 2 categories (A and C), then subtyped into 3 various subtypes; subtype-I, subtype-II and subtype-V | Prevalence of Blastocystis among CRC = 22 (29%). Blastocystis infection frequency was significantly different between CP group and NC group. There was a higher probability of Blastocystis sp. among CP. Subtype I was the common subtype among CRC patients (54.5%). Interestingly, an association risk between Blastocystis subtype 1 with a greater risk of association in CRC group | The study revealed a probable association between subtype 1 of Blastocystis and CRC |
| 4 | Toychiev et al[20], 2018 | Uzbekistan | A total sample of 400 participants, two groups of participants: (1) 200 CRC patients; and (2) 200 of Tashkent residents (without any gastrointestinal tract complaints). Exclusion criteria: (1) patient had problems with stool sample collection; and (2) received any treatment 2–3 wk before the study. Study duration: 2015-2017 | Research Institute of Epidemiology, Microbiology and Infectious Diseases and the Research Center of Oncology, Tashkent, Uzbekistan, during the period | Study design: Prospective cohort: Prevalence of some parasites including Blastocystis sp. in CRC patients before and after surgery and chemotherapy compared to control group. Methods: “3 stool samples for parasitological examination were taken at 2-d intervals during CRC diagnosis before and after surgery and chemotherapy” | A significantly higher prevalence of protozoa was found in CRC patients than in control population “the prevalence of Blastocystis in CRC patients is 4 times as high as in the control population. The overall prevalence of Blastocystis sp. was 2.8% and was higher than the other protozoa” | Data revealed a potential role for Blastocystis sp. in CRC pathogenesis |
| 5 | Kumarasamy et al[21], 2014 | Malaysia | Sample size: 425 patients who go through diagnostic colonoscopy. faecal samples and colonic washouts were obtained from 221 control patients and 204 patients with CRC. Study duration: 2010 and 2012 | University of Malaya Medical Centre | To determine the Blastocystis genotype present by comparing the prevalence using colonic washouts and faecal samples PCR and standard stool culture. Both techniques were used to detect Blastocystis from control and patients with CRC. | The prevalence of Blastocystis was 15.29% (65/425). “Colonic washouts and faecal samples showed 12.24% (n = 52) and 5.65% (n = 24) of Blastocystis infection respectively”. A total of 43 individuals were positive for Blastocystis in CRC patients and was significantly higher compared to control group. Subtype 3 was predominant compared to other subtypes. It was significantly higher in CRC group as compared with control group | Blastocystis sp. is common in CRC patients. Subtype 3 is the most common genotype in the infected individuals |
| 6 | Chandramathi et al[1], 2012 | Malaysia | Stool samples were obtained from 46 and 15 breast cancer and CRC patients, respectively | Department of Parasitology, Faculty of Medicine, University of Malaya | Aim: To investigate whether intestinal parasites can be an opportunistic infection in breast cancer and CRC patients who are undergoing chemotherapy treatment. Molecular detection of microsporidia species was done using a PCR technique. The presence of Blastocystis hominis was further confirmed by culturing stool samples | This study found that 7 out of 15 CRC patients were positive Blastocystis in various chemotherapy cycles accounting for 46.7% | Blastocystis hominis and microsporidia could appear as opportunistic infections during chemotherapy treatment of CP. This infection may diminish the efficacy of chemotherapy treatments and consequently advance the progression of cancer |
| 7 | Majeed et al[22], 2019 | Iraq | 116 faecal specimens with Blastocystis and Helicobacter pylori infection, 15 biopsy specimens from CRC patients | Middle Technical University/Baghdad 1st Feb 2018-15th June 2018 | Faecal specimens were screened for Blastocystis and Helicobacter pylori. Direct DNA sequencing was done to evaluate mutations in CRC-associated molecular pathways | Prevalence of Blastocystis infection statistically insignificant in various age groups. Prevalence of Blastocystis infection was more in females [females 29 (46.9 %), males 22(43.1%)]. Prevalence of mixed infection (Blastocystis and Helicobacter pylori) was 27 (23.32%) | Prevalence of Blastocystis infection was more in females. KRAS and TP53 gene mutation was observed in the CRC patients with mixed infection (Blastocystis and H. pylori) |
| In vitro studies | |||||||
| 8 | Chandramathi et al[7], 2010 | Malaysia | In vitro study model. PBMCs were isolated from blood collected from healthy persons. Solubilised antigen of Blastocystis isolate was obtained from a human subject. Human colorectal carcinoma cell line, HCT116, was used | University of Malaya, Kuala Lumpur | Effect solubilised antigen of Blastocystis on the HCT116 proliferation quantified. Gene expressions of certain genes in HCT116 and PBMCs evaluated via real-time reverse transcription PCR. PBMCs were isolated from blood using Histopaque technique. Cell proliferations were measured using MTT assay | Increased number of PBMCs/ HCT116 cells observed with Blastocystis antigen. IFN-γ and TNF-α were downregulated and IL-6, IL-8 and NF-κB, p53 were upregulated in the PBMCs treated with the antigen. IFN-γ was downregulated and IL-6 and NF-κB was upregulated in HCT116 cells | Solubilised antigen of Blastocystis could facilitate increased number of PBMCs/ HCT116 cell and has the ability to downregulate immune cell responses |
| 9 | Chan et al[23], 2012 | Malaysia | In vitro study model. Solubilised antigen of Blastocystis isolate was obtained from symptomatic and asymptomatic human subject. HCT116 was used | University of Malaya, Kuala Lumpur | Effects of solubilised antigen of Blastocystis isolate was obtained from symptomatic and asymptomatic human subject on HCT116. Gene expressions of certain genes in HCT116 and PBMCs evaluated via real-time reverse transcription PCR | Increased number of HCT116 cells observed with symptomatic Blastocystis antigen. Th2 cytokines/CTSB were upregulated in HCT116. NF-κB was observed upregulated in HCT116 exposed to symptomatic Blastocystis antigen | Solubilized antigen of Blastocystis from symptomatic individual was more virulent than that in asymptomatic. Higher inflammatory reaction and increased proliferation of cancer cells was observed |
| 10 | Kumarasamy et al[24], 2013 | Malaysia, | In vitro study model using HCT116 treated with solubilised Blastocystis antigen from 5 Blastocystis subtypes | University Malaya research Lab | In vitro study. HCT116 treated with solubilised antigen from Blastocystis. Following Assays: Proliferation of the cell line, HCT116 on exposure to different Blastocystis subtypes; Gene expression profile of apoptotic genes like p53 and CTSB; Transcription factor gene expression profile | Blastocystis subtypes (5) increased the proliferation of HCT116, especially subtype 3. Blastocystis antigen caused the upregulation of Th2 and Th1 cytokines, and downregulation of IFN-γ and p53 in HCT116 cells. Blastocystis antigen caused a higher stimulation of gene expression of CTSB and TGF-β genes | Infection with Blastocystis caused exacerbation of existing colon cancer cells. The effect may be due to weakening of the cellular immune response and dysregulation of IFN-γ and p53 expression. Infection with Blastocystis subtype 3 has a higher pathogenic potential |
| 11 | Ahmed et al[25], 2019 | Cairo, Egypt | Seven Blastocystis isolates were from stools specimen from patients with early diagnosed CRC (Oncology and Surgery and Colonoscopy unit) of a Hospital in Egypt. The different groups were: Group I (GI), 12 isolates from infected non-CRC; Group II (GII), 6 from infected symptomatic patients and Group III (GIII), 6 from infected non-symptomatic carriers | Department of Parasitology lab, Faculty of Medicine, Ain Shams University, Cairo, Egypt | Aim: To investigate some phenotypic characters like the surface ultrastructure, protein profiles and protease activity of Blastocystis from three different clinical groups. Techniques performed: Scanning electron microscopy to study morphology of the organism; SDS-PAGE to analyse the Blastocystis protein profiles and their protease activities | Observations: All CRC Blastocystis isolates showed a very rough intensely folded surface when compared to less rough and smooth surface of isolates from symptomatic and asymptomatic and non-CRC isolates; SDS-PAGE showed presence of 2 protein bands of 230 and 32 KDa in 42.9% of Blastocystis CRC isolates and these proteins were absent in Non-CRC isolates. When the protease activity of the parasite was tested, no significant difference existed between isolates of the three groups | There was significant difference in the surface structure and the protein profiles between different clinical isolates of Blastocystis. Differences indicate that it may be: (1) secondary to the altered gut environment in the presence of CRC or (2) indicators of a different pathogenic potential of the parasite in inducing malignancy |
| In Vivo studies | |||||||
| 12 | Kumarasamy et al[26], 2017 | Malaysia | Different specimens collected: Blood, urine, faecal samples and gastrointestinal tract sections from 24 male Wistar rats. Age of the rats: 3 wk. Weight of each rat: Average of 65 g/rat | University Malaya research Lab | In vivo experimental study. Aim: To investigate the effect of infection with Blastocystis cyst on exacerbation of carcinogenesis. Twenty-four rats divided into different groups for the study (4 groups, 6 rats each): Control group, AOM group, group inoculated with Blastocystis cyst, the group inoculated with Blastocystis cyst and AOM injection. Body weights recorded once a week. Rat faecal samples screened for presence of Blastocystis post-inoculation. Histopathological assessment of the rat colon for aberrant crypts. Urine and blood samples assessed for oxidative stress | Observations: lower body weight showed by Blastocystis infected rats than rats infected with Blastocystis and injected with AOM (P < 0.05). Stools from AOM-rats with Blastocystis infection were softer and watery compared to the AOM-rats without Blastocystis infection. Blastocystis was present in the stool of all infected rats from Day 3 to 7 post-inoculation. All the rats injected with AOM developed numerous abnormal, hyperplastic colonic crypts. Co-administration of Blastocystis cyst showed a 1.6-fold increase in the number of crypts when compared with control rats treated with AOM only. Two of the co-Blastocystis infected AOM-rats were found to have adenomas. Major dysplasia and presence of hyperplastic aberrant crypts were observed in rats injected with AOM and co-infected with Blastocystis | Blastocystis infection considerably enhanced the AOM-induced carcinogenesis because of the oxidative damage of the intestinal epithelium |
Based on the seven reviewed articles, the prevalence of Blastocystis sp. in CRC patients were found to be between 2.8%-46.7%, whereby subtype 1 and subtype 3 were predominantly isolated.
A study by Esteghamati et al[35] evaluated the prevalence of Blastocystis in 85 cancer patients, including 39 CRC and 46 cancers outside gastrointestinal tract (COGT). In this study, Blastocystis was identified in 11/39 (28.2%) among CRC group. Another study in China by Zhang et al[18] showed the prevalence of Blastocystis in 4 among 49 (8.1%) patients with CRC. In another study conducted in Saudi Arabia, the prevalence of Blastocystis among CRC patients was 29.7%[19]. Subtype I was the predominant (54.5%) among CRC patients, while subtype II was predominant (43.7%) among COGT patients[19]. Higher prevalence of intestinal helminths and protozoa was observed in CRC patients than in the control population in a study conducted in Uzbekistan. The prevalence of Blastocystis in CRC patients was four times higher than that in the control population. The overall prevalence of Blastocystis (2.8%) was significantly higher than the other protozoa[20]. In a study by Kumarasamy et al[21] in Malaysia, among 221 control patients and 204 CRC patients with colorectal malignancies, the overall prevalence of Blastocystis infection was 15.29% (65/425). A total of 43 (21.08%) samples were positive for Blastocystis infection in CRC patients and was significantly higher compared to normal individuals (n = 22, 9.95%, P < 0.01). Subtype 3 was present at higher levels compared to other subtypes detected in both groups and was significantly higher in CRC patients as compared with control patients[21].
Another study was designed to investigate the emergence of Blastocystis and Microsporidia infections in breast and CRC patients undergoing chemotherapy treatment. This study found that 7 out of 15 CRC patients were positive Blastocystis in various chemotherapy cycles, accounting for 46.7%. However, the researchers did not mention whether the isolate was from the same patients in different cycles[1]. In a study carried out in Iraq, stool samples from 116 patients with Blastocystis and H. pylori infections were investigated. Fifteen tissue samples of CRC were taken from 15 suspected patients out of 116 infected cases, and it was shown that the infection with Blastocystis and H. pylori was associated with pathological gene mutation in the CRC patients[22].
Three of the reviewed articles used in vitro study models and observed considerable cytopathic and immunological effects induced by the solubilised antigen of Blastocystis to the human colorectal carcinoma cell line[7,23,24]. These findings speculated that Blastocystis infection may enhance the proliferation, invasiveness and metastatic properties of CRC cells. One study investigated some phenotypic characteristics of Blastocystis isolated from CRC patients[25].
The three in vitro model studies used the human colorectal carcinoma cell line HCT116 and the Blastocystis isolated from a human subject[7,23,24]. One of the studies demonstrated the cytopathic effect of Blastocystis antigen on peripheral blood mononuclear cells. This study findings showed increased cell proliferations in Blastocystis antigen-stimulated HCT116 cell-lines, which suggested that the infection by Blastocystis may facilitate the growth of colon cancer cells[7]. Another in vitro study showed that the five subtypes of Blastocystis significantly increased the proliferation of HCT116, especially subtype 3. Blastocystis antigen caused the upregulation of T helper (Th)2 and Th1 cytokine gene expressions, and downregulation of interferon gamma and p53 gene expressions in HCT116 cells. In addition, Blastocystis antigen caused a significantly higher stimulation of Cathepsin B (CTSB) and Transforming growth factor beta (TGF-β) gene expression, which indicates the pathogenic potential of this protozoan[24]. Another study showed an increase in cell proliferation in HCT116 cells inoculated with the symptomatic Blastocystis antigen. Gene expression studies carried out in this research also showed a significant upregulation of Th2 cytokines, which indicates the parasites’ potential in weakening the cellular immune response[23]. HCT116 cells exposed to symptomatic and asymptomatic Blastocystis antigen caused a significant upregulation of CTSB, which led to the postulation that the Blastocystis antigen may enhance the invasive and metastasis properties of CRC[23].
Another study sought to investigate some phenotypic characteristics such as the surface ultrastructure, protein profiles and protease activity of Blastocystis isolated from three different clinical groups: CRC patients, non-CRC symptomatic and asymptomatic infected persons. This study showed the presence of two protein bands of 230 and 32 KDa in 42.9% of Blastocystis CRC isolates with their complete absence from non-CRC isolates. There was no significant difference in the protease activity of the protein among isolates of the three groups, CRC and Non-CRC Blastocystis isolates[25].
An animal model study compared the effects of Blastocystis infected rats and rats infected with Blastocystis co-administered with Azoxymethane (AOM), a potent carcinogen. This finding showed that the co-administration of Blastocystis cyst resulted in a 1.6-fold increase in the number of colonic crypts when compared with control rats treated with AOM only. Two of the co-Blastocystis infected AOM rats were found to have adenomas. Major dysplasia and the presence of hyperplastic aberrant crypts were also observed in rats injected with AOM and co-infected with Blastocystis[26].
Blastocystis is one of the most common gut microorganisms found in healthy individuals[27]. Besides being associated with a healthy gut microbiota[27,28], Blastocystis infection is also known to be opportunistic in immunocompromised patients[29]. CRC is the third most common cancer diagnosed worldwide and one of the major causes of cancer-associated fatality[30]. The reason for high mortality is due to the asymptomatic progression of the disease that usually results in late diagnosis[31,32]. Certain gut microorganisms are known to be one of the important factors that had been associated with initiation and development of CRC[33]. However, data on the roles of parasites are vague and restricted. Various findings have been reported regarding the association between Blastocystis among CRC patients, whereby positive association was shown in all the studies[1,23,24]. Therefore, the aim of this systematic review was to quantify the studies published so far that revealed the direct association of Blastocystis and CRC. This paper outlines the results of a systematic review to evaluate the prevalence of Blastocystis in CRC patients, in vitro studies using Blastocystis antigen and an in vivo study using animal models.
Out of the data extracted from 12 studies relevant to this topic, all the studies showed positive association between Blastocystis and CRC. Prevalence studies, in vitro investigations and in vivo studies were used to evaluate the pathogenicity of Blastocystis with CRC.
The global prevalence of Blastocystis infection ranged from 1.5%-20% in developed countries, which was much less than that in developing countries, which was 30%-50%[34]. Based on our review, the prevalence of Blastocystis infection in CRC patients ranged between 2.8%-46.7%. It has been widely reported in the world, in developing countries such as Iran, China, Saudi Arabia, Uzbekistan and Malaysia[18-20,24,35]. The first demonstration of Blastocystis infection in Iran was reported in 11 CRC patients in Tehran province[35]. In Malaysia, a total of 43 samples were positive for Blastocystis from 204 CRC patients[21]. This study utilised colonic washout in addition to stool sample to recover the parasites. Subtype 3 Blastocystis was detected predominantly as compared to other subtypes[21]. Some of these findings highlighted the high prevalence of certain subtypes of Blastocystis among these patients[19,21]. A previous study showed that subtype 1 was the most common genotype identified (54.5%) among CRC patients[19]. DNA was extracted from Blastocystis cultures via conventional method and subtyped using multiplex polymerase chain reaction (PCR) with restriction fragment length polymorphism and sequence-tagged site primers-based PCR. In another study, subtype 3 was predominant compared to other subtypes found in both CRC patients and healthy individuals[21]. Subtype 3 is speculated as the most pathogenic subtype in symptomatic individuals[36]. Some researchers have attributed subtype 3 to be more pathogenic compared to other subtypes[37,38]. Blastocystis was initially screened via in vitro culture and conventional PCR using stool samples and colonic washouts. The presence of Blastocystis infection in CRC patients could be contributed by various reasons including health status. For instance, positive cases were more likely in patients with gastrointestinal symptoms compared to healthy individuals[18]. Besides, Blastocystis was also identified in higher frequency in immunosuppressed CRC patients who were undergoing chemotherapy treatment[1,18].
A total of three in vitro studies were carried out using colorectal carcinoma cell line models to study cytopathic and immunological effects of Blastocystis antigen[7,23,24]. Some of the research studies suggested that solubilised antigen of Blastocystis could facilitate the exacerbation of CRC cells, HCT116[7,23]. In another study by Kumarasamy et al[24], Blastocystis subtype 3 stimulated significantly higher CTSB and TGF-β gene expression in HCT116, which indicates the pathogenic potential of this protozoan. Result of in vitro studies that were performed in Malaysia were similar[7,23,24].
Blastocystis is commonly found in both patients with gastrointestinal symptoms and in healthy people widely across the world. More recently, researchers consider Blastocystis as an emerging zoonotic disease, and its pathogenic potential in human is unclear[9]. The pathogenic potential of Blastocystis was widely debated, as they are found in both symptomatic and asymptomatic patients. The significant expression of nuclear factor kappa light chain enhancer of activated B cells was observed in HCT116 exposed to Blastocystis antigen isolated from individuals with gastrointestinal symptoms, but such observations were not found when the colon cells treated with Blastocystis antigen isolated from asymptomatic individuals. This finding shows the potential pathogenicity of symptomatic Blastocystis in CRC patients[23]. Similarly, HCT116 cells exposed to symptomatic and asymptomatic Blastocystis antigen caused a significant upregulation of CTSB. These gene expression findings lead to a postulation that the Blastocystis antigen may enhance the invasive and metastasis properties of CRC[23]. Besides, proliferation of HCT116 when exposed with Blastocystis antigen could be a result of higher levels of interleukin (IL)-6 and IL-8 expression[23].
Another study revealed that solubilised antigen isolated from subtype 2 and 3 isolates introduced to colon cancer cells showed significant IL-8 and IL-6 expression[24]. The production of inflammatory cytokines such as IL-8 together with reactive oxygen species could contribute to the pathogenesis of cancer[38]. In a few studies conducted previously, IL-6 expression was associated with proliferation of colon carcinoma[39,40]. Besides that, subtype 3 Blastocystis also triggered positive expression of CTSB in cancer cells. A previous study showed that CTSB expression is significant in CRC patients[41].
Only one study was conducted to investigate the in vivo effect of Blastocystis in Wistar rats. In parallel with in vitro studies, an in vivo study showed similar findings on the possible role of Blastocystis to exacerbate CRC. The results demonstrated that Blastocystis may cause damage to the intestinal mucosal layer and result in increased crypts formation. Furthermore, an increased oxidative stress was also observed in these rats. There have been numerous animal models of human CRC and animal model of tumour carried out via quantification of aberrant crypt foci[42,43]. This allows the study of gut microbiome and its role in pathogenesis. In humans, there are many potential pathogenic and non-pathogenic gut microbial infections, and various animal models have been used for such studies. Aberrant crypt foci are known as putative precancerous lesions of the colon in both animal models and humans[44,45]. Even though various studies associating Blastocystis and CRC were carried out via in vitro model using colon cancer cells, this study utilised animal model to bridge between in vitro findings in the laboratory and studies in humans. As such, this extensive in vivo study showed that Blastocystis had a major impact on normal intestinal function in Wistar rats resulting in damage to the intestinal mucosal layer and inducing oxidative stress, which caused increase in crypts formation in AOM-treated rat models. The study establishes that Blastocystis is a pathogen, and there is a need to screen cancer patients for harbouring this parasite.
This systematic review has some limitations. According to these investigations, a greater prevalence of Blastocystis was found in CRC patients, but the question whether increased prevalence of Blastocystis could be linked with increased high risk to CRC is unclear. The studies discussed in this review did not highlight the association of Blastocystis according to cancer stages, and it was unclear if Blastocystis itself could result in the initiation of malignancy as Blastocystis acquisition alone is insufficient for cancer development. Even though strong association between Blastocystis and CRC is apparent, some questions remain unanswered. Therefore, we propose future studies should focus on the pathogenicity of Blastocystis in various stages of CRC by concentrating on the molecular pathways involved in tumorigenesis.
In conclusion, according to various recent studies, Blastocystis is one of the most commonly identified microorganisms in CRC patients, whereby subtype 1 and subtype 3 were predominantly isolated. It is apparent in most cases that the prevalence is higher in developing countries compared to developed countries. These studies have provided supportive data that Blastocystis could exacerbate existing CRC via alteration in host immune response and increased oxidative damage. An in vivo study well-established that Blastocystis infections resulted in tissue damage from host inflammatory responses that may predispose the host towards neoplasm exacerbation. Upregulation of gene expression responsible for proinflammatory cytokines and downregulation of apoptotic genes was observed in in vitro studies. Through continued research in Blastocystis and CRC, we may discover new findings as well as develop new effective means of prevention. Future studies of CRC and Blastocystis should attempt to determine the various stages of CRC that are most likely to be associated with Blastocystis and its association with other intestinal bacteria. In addition, future in vivo studies should evaluate exposure to various subtypes of Blastocystis.
Intestinal colonisation of Blastocystis hominis (Blastocystis) as a risk factor to the worsening of colorectal cancer (CRC).
There has been an increase in the prevalence of Blastocystis in CRC patients. Besides, various in vitro and in vivo studies have highlighted Blastocystis as an important risk factor for the worsening of CRC.
To perform a systematic review on all evidence on the association between CRC and Blastocystis.
A systematic review of the literature was performed by searching PubMed, Science Direct, Scopus and Google scholar databases up to February 2020.
Out of 12 studies selected for this systematic review, seven studies have confirmed the prevalence of Blastocystis. A total of four studies employing in vitro human colorectal carcinoma cell line study models showed significant cytopathic and immunological effects of Blastocystis. One in vivo experimental animal model study showed that there was a significant effect of infection with Blastocystis on exacerbation of colorectal carcinogenesis.
Blastocystis is a commonly identified microorganisms in CRC patients. These studies have provided supportive data that Blastocystis could exacerbate existing CRC via alteration in host immune response and increased oxidative damage.
Future studies of CRC and Blastocystis should attempt to determine the various stages of CRC that are most likely to be associated with Blastocystis and its association with other intestinal diseases.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Farouk S S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL
| 1. | Chandramathi S, Suresh K, Anita ZB, Kuppusamy UR. Infections of Blastocystis hominis and microsporidia in cancer patients: are they opportunistic? Trans R Soc Trop Med Hyg. 2012;106:267-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 2. | Aksoy U, Akisü C, Bayram-Delibaş S, Ozkoç S, Sahin S, Usluca S. Demographic status and prevalence of intestinal parasitic infections in schoolchildren in Izmir, Turkey. Turk J Pediatr. 2007;49:278-282. [PubMed] |
| 3. | Brumpt E. Blastocystis hominis n. sp. et formes voisines. Bull Soc Pathol. 1912;5:725-730. |
| 4. | Low GC. Two Chronic Amoebic Dysentery Carriers treated by Emetine, with some Remarks on the Treatment of Lamblia, Blastocystis and E. coli Infections. J Trop Med Hyg. 1916;19:29-34. |
| 5. | Roberts T, Ellis J, Harkness J, Marriott D, Stark D. Treatment failure in patients with chronic Blastocystis infection. J Med Microbiol. 2014;63:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 6. | Borralho PM, Kren BT, Castro RE, da Silva IB, Steer CJ, Rodrigues CM. MicroRNA-143 reduces viability and increases sensitivity to 5-fluorouracil in HCT116 human colorectal cancer cells. FEBS J. 2009;276:6689-6700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 7. | Chandramathi S, Suresh K, Kuppusamy UR. Solubilized antigen of Blastocystis hominis facilitates the growth of human colorectal cancer cells, HCT116. Parasitol Res. 2010;106:941-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 8. | Cirioni O, Giacometti A, Drenaggi D, Ancarani F, Scalise G. Prevalence and clinical relevance of Blastocystis hominis in diverse patient cohorts. Eur J Epidemiol. 1999;15:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 9. | Basak S, Rajurkar MN, Mallick SK. Detection of Blastocystis hominis: a controversial human pathogen. Parasitol Res. 2014;113:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 10. | Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V; WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1935] [Cited by in RCA: 2161] [Article Influence: 135.1] [Reference Citation Analysis (1)] |
| 11. | zur Hausen H. Streptococcus bovis: causal or incidental involvement in cancer of the colon? Int J Cancer. 2006;119:xi-xii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 12. | Lax AJ. Opinion: Bacterial toxins and cancer--a case to answer? Nat Rev Microbiol. 2005;3:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | El-Gayar EK, Mahmoud MM. Do protozoa play a role in carcinogenesis? Parasitol United J. 2014;7:80. [DOI] [Full Text] |
| 14. | Padukone S, Mandal J, Parija SC. Severe Blastocystis subtype 3 infection in a patient with colorectal cancer. Trop Parasitol. 2017;7:122-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (1)] |
| 15. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47111] [Article Influence: 2944.4] [Reference Citation Analysis (0)] |
| 16. | (JBI) TJBI. Critical Appraisal Tools. [cited 1 January 2021]. Available from: http://joannabriggs.org/research/critical-appraisal-tools.html. |
| 17. | Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2511] [Cited by in RCA: 2461] [Article Influence: 223.7] [Reference Citation Analysis (2)] |
| 18. | Zhang W, Ren G, Zhao W, Yang Z, Shen Y, Sun Y, Liu A, Cao J. Genotyping of Enterocytozoon bieneusi and Subtyping of Blastocystis in Cancer Patients: Relationship to Diarrhea and Assessment of Zoonotic Transmission. Front Microbiol. 2017;8:1835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Mohamed AM, Ahmed MA, Ahmed SA, Al-Semany SA, Alghamdi SS, Zaglool DA. Predominance and association risk of Blastocystis hominis subtype I in colorectal cancer: a case control study. Infect Agent Cancer. 2017;12:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Toychiev A, Abdujapparov S, Imamov A, Navruzov B, Davis N, Badalova N, Osipova S. Intestinal helminths and protozoan infections in patients with colorectal cancer: prevalence and possible association with cancer pathogenesis. Parasitol Res. 2018;117:3715-3723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Kumarasamy V, Roslani AC, Rani KU, Kumar Govind S. Advantage of using colonic washouts for Blastocystis detection in colorectal cancer patients. Parasit Vectors. 2014;7:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Majeed GH, Mohammed NS, Muhsen SS. The synergistic effect of Blastocystis hominis and H. pylori in Iraqi colorectal cancer patients. J. Pharm. Sci. & Res. 2019;11:523-526. |
| 23. | Chan KH, Chandramathi S, Suresh K, Chua KH, Kuppusamy UR. Effects of symptomatic and asymptomatic isolates of Blastocystis hominis on colorectal cancer cell line, HCT116. Parasitol Res. 2012;110:2475-2480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Kumarasamy V, Kuppusamy UR, Samudi C, Kumar S. Blastocystis sp. subtype 3 triggers higher proliferation of human colorectal cancer cells, HCT116. Parasitol Res. 2013;112:3551-3555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Ahmed MM, Habib FSM, Saad GA, El Naggar HM. Surface ultrastructure, protein profile and zymography of Blastocystis species isolated from patients with colorectal carcinoma. J Parasit Dis. 2019;43:294-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Kumarasamy V, Kuppusamy UR, Jayalakshmi P, Samudi C, Ragavan ND, Kumar S. Exacerbation of colon carcinogenesis by Blastocystis sp. PLoS One. 2017;12:e0183097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Scanlan PD, Stensvold CR, Rajilić-Stojanović M, Heilig HG, De Vos WM, O'Toole PW, Cotter PD. The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiol Ecol. 2014;90:326-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 201] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 28. | Audebert C, Even G, Cian A; Blastocystis Investigation Group, Loywick A, Merlin S, Viscogliosi E, Chabé M. Colonization with the enteric protozoa Blastocystis is associated with increased diversity of human gut bacterial microbiota. Sci Rep. 2016;6:25255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 202] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 29. | Bednarska M, Jankowska I, Pawelas A, Piwczyńska K, Bajer A, Wolska-Kuśnierz B, Wielopolska M, Welc-Falęciak R. Prevalence of Cryptosporidium, Blastocystis, and other opportunistic infections in patients with primary and acquired immunodeficiency. Parasitol Res. 2018;117:2869-2879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 30. | Favoriti P, Carbone G, Greco M, Pirozzi F, Pirozzi RE, Corcione F. Worldwide burden of colorectal cancer: a review. Updates Surg. 2016;68:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 479] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 31. | O'Connell JB, Maggard MA, Liu JH, Etzioni DA, Ko CY. Are survival rates different for young and older patients with rectal cancer? Dis Colon Rectum. 2004;47:2064-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Siegel RL, Ward EM, Jemal A. Trends in colorectal cancer incidence rates in the United States by tumor location and stage, 1992-2008. Cancer Epidemiol Biomarkers Prev. 2012;21:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 33. | Zhu Q, Gao R, Wu W, Qin H. The role of gut microbiota in the pathogenesis of colorectal cancer. Tumour Biol. 2013;34:1285-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 34. | Su FH, Chu FY, Li CY, Tang HF, Lin YS, Peng YJ, Su YM, Lee SD. Blastocystis hominis infection in long-term care facilities in Taiwan: prevalence and associated clinical factors. Parasitol Res. 2009;105:1007-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Esteghamati A, Khanaliha K, Bokharaei-Salim F, Sayyahfar S, Ghaderipour M. Prevalence of Intestinal Parasitic Infection in Cancer, Organ Transplant and Primary Immunodeficiency Patients in Tehran, Iran. Asian Pac J Cancer Prev. 2019;20:495-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Souppart L, Sanciu G, Cian A, Wawrzyniak I, Delbac F, Capron M, Dei-Cas E, Boorom K, Delhaes L, Viscogliosi E. Molecular epidemiology of human Blastocystis isolates in France. Parasitol Res. 2009;105:413-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Ragavan ND, Govind SK, Chye TT, Mahadeva S. Phenotypic variation in Blastocystis sp. ST3. Parasit Vectors. 2014;7:404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Rajamanikam A, Hooi HS, Kudva M, Samudi C, Kumar S. Resistance towards metronidazole in Blastocystis sp.: A pathogenic consequence. PLoS One. 2019;14:e0212542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Chung YC, Chang YF. Serum interleukin-6 Levels reflect the disease status of colorectal cancer. J Surg Oncol. 2003;83:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 265] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 40. | Galizia G, Orditura M, Romano C, Lieto E, Castellano P, Pelosio L, Imperatore V, Catalano G, Pignatelli C, De Vita F. Prognostic significance of circulating IL-10 and IL-6 serum levels in colon cancer patients undergoing surgery. Clin Immunol. 2002;102:169-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 170] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 41. | Herszényi L, Farinati F, Cardin R, István G, Molnár LD, Hritz I, De Paoli M, Plebani M, Tulassay Z. Tumor marker utility and prognostic relevance of cathepsin B, cathepsin L, urokinase-type plasminogen activator, plasminogen activator inhibitor type-1, CEA and CA 19-9 in colorectal cancer. BMC Cancer. 2008;8:194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 42. | Kim J, Ng J, Arozulllah A, Ewing R, Llor X, Carroll RE, Benya RV. Aberrant crypt focus size predicts distal polyp histopathology. Cancer Epidemiol Biomarkers Prev. 2008;17:1155-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Reddy BS. Studies with the azoxymethane-rat preclinical model for assessing colon tumor development and chemoprevention. Environ Mol Mutagen. 2004;44:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987;37:147-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 740] [Cited by in RCA: 728] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 45. | Roncucci L, Stamp D, Medline A, Cullen JB, Bruce WR. Identification and quantification of aberrant crypt foci and microadenomas in the human colon. Hum Pathol. 1991;22:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 210] [Article Influence: 6.2] [Reference Citation Analysis (0)] |