Published online Mar 15, 2022. doi: 10.4251/wjgo.v14.i3.716
Peer-review started: September 29, 2021
First decision: November 7, 2021
Revised: November 16, 2021
Accepted: January 27, 2022
Article in press: January 27, 2022
Published online: March 15, 2022
Processing time: 161 Days and 17.2 Hours
The impact of pancreatic tumor location on patient survival has been studied in large national data-based analyses which yielded controversial results.
To explore if pancreatic head cancer (PHC) and pancreatic body/tail cancer (PBTC) have different overall survival (OS), molecular signature and response to chemotherapy.
We retrospectively queried patient records from July 2016 to June 2020 in our institution. Patient demographics, cancer stage on diagnosis, tumor location, somatic mutations, treatment, and survival are recorded and analyzed. A test is considered statistically significant if the P value was < 0.05.
We reviewed 101 patients with complete records, among which 67 (66.34%) were PHC and 34 (33.66%) were PBTC. More PHC were diagnosed at younger age [61.49 vs 68.97, P = 0.010], earlier stages (P = 0.006) and underwent surgical resection (P = 0.025). There were no significant differences among all mutations and pathways studied except for TP53 mutations (37.0% in PHC vs 70.0% in PBTC, P = 0.03). OS was not statistically different between PHC and PBTC (P = 0.636) in the overall population and in subgroups according to surgical resection status or stages. In terms of response to chemotherapy, chemotherapy regimens (FOLFIRINOX-based vs gemcitabine-based) didn’t impact disease free interval in those who had surgical resection in either PHC (P = 0.546) or PBTC (P = 0.654), or the duration of response to first line palliative treatment in those with advanced disease in PHC (P = 0.915) or PBTC (P = 0.524).
Even though PHC and PBTC have similar poor OS and response to chemotherapy, the different presentations and molecular profiles indicate they are different diseases. Utilization of molecular profiling to develop targeted therapy for individualization of treatment is needed.
Core Tip: The study is a retrospective study of the impact of pancreatic cancer location on survival, molecular profiling and response to chemotherapy among patients who were diagnosed with pancreatic cancer in our institution. Even though there was no significant difference in survival or response to chemotherapy between pancreatic head and pancreatic body/tail cancer, we did observe a trend of long-term survival in stage I/II pancreatic tail patients who underwent surgical resection. TP53 mutations were significantly more in pancreatic body/tail cancer than that in pancreatic head cancer and we propose that gemcitabine-based chemotherapy should be considered in those patients.
- Citation: Sun K, Mylavarapu C, Crenshaw A, Zhang Y, Hsu E, Xu J, Niravath M, Jones SL, Ordonez A, Abdelrahim M. Pancreatic head vs pancreatic body/tail cancer: Are they different? World J Gastrointest Oncol 2022; 14(3): 716-723
- URL: https://www.wjgnet.com/1948-5204/full/v14/i3/716.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i3.716
Pancreatic cancer is the fourth-leading cause of cancer deaths in the United States. Even though the survival rates have improved slightly over the past four decades, the outcome of pancreatic cancer is still dismal. Anatomically pancreatic cancer can be divided into pancreatic head cancer (PHC) and pancreatic body/tail cancer (PBTC). The lower part of head and uncinate process of pancreas has different embryological origins from the rest of the pancreas[1]. This embryological difference leads to significant differences in cell composition, blood supply, lymphatic and venous drainage and innervations between the head and body/tail of pancreas.
Pancreatic tumor location impacts patient presentation and survival, which has been shown in large data-based analyses, even though with conflicting results. 49%-77.5% pancreatic cancers are PHC[2-4], which tend to present at earlier stages than PBTC. Historically, survival of PBTC cancer is believed to be worse than PHC; and PBTC is considered as an independent poor prognostic risk factor. However, PBTC was found to have much better survival over PHC (20% vs 9%) when the tumor is localized[2].
Genetic analyses of pancreatic cancer have suggested that PHC and PBTC are different tumors. Advanced technology including whole genome sequencing and RNA sequencing further classified pancreatic cancer into four subtypes: Classical, squamous, ADEX and immunogenic[5]. The squamous subtype is characterized by genes highly expressed in the C2-squamous-like class of tumors (e.g., lung and head and neck cancer)[6]. PBTC is found to have more squamous subtypes[7,8].
Pancreatic squamous subtype shares similar molecular abnormalities with lung squamous subtype, which include loss of TP53, RB1, CDKN2A and PIK3CA, NOTCH1, NFE2L2, KDM6A and EP300 mutations. Squamous cell lung cancer was found to be more sensitive to platinum and gemcitabine combination therapy[9]. When the combination therapy was tested in advanced pancreatic cancer, there was an improvement in overall survival, disease free survival and response rate, even though not statistically significant[10]. Given that PBTC had more squamous subtype, it is possible that PBTC is more responsive to gemcitabine-based treatment.
We hypothesize that PHC and PBTC are distinct diseases based on their embryological origins and current genetic profiling evidence. To further explore the impact of tumor location on molecular profiling, survival, and response to chemotherapy, we retrospectively reviewed patients with pancreatic cancer in our institution.
The study was approved by the institutional review boards. Informed consent was waived given the retrospective nature of the study. The patient data was queried from Epic electronic medical record system of Houston Methodist Hospital. Patients who had a diagnosis of pancreatic cancer from July 2016 through June 2020 were included. Patient demographics, tumor location, pathology, staging, molecular profiles, treatment history and survival were collected retrospectively. Molecular profiles were performed through a multiplatform approach including next gene sequencing (NGS) and RNA sequencing by commercially available testing from Caris Life Sciences (Phoenix, AZ), FoundationOne (Cambridge, MA), Guardant360 (Redwood City, CA), Tempus (Chicago, IL) and NeoGenomics (Fort Meyers, FL), and in house 50 gene or 70 gene panel that was developed and validated in our institution. Panels of gene mutations are available on each company’s website. Duration of response is defined as the duration of having complete response, partial response or stable disease. Overall survival is defined as the time from pancreatic cancer diagnosis to the date of death or date of last follow-up. Disease free interval is defined as the time from definitive treatment to the date of disease recurrence. Patients who had response and survival data were included in the survival and response analysis. Patients who had molecular profiling data were included in the tumor location analysis. Those who didn’t have either records were excluded from the study.
Mean and standard deviation were calculated for the continuous variables and frequency and percentage [n (%)] were calculated for those categorical variables. T-test was used to compare the mean of a continuous variables between the 2 groups of pancreatic cancer and the Fisher’s exact test was used to find the association between a patient’s characteristic and the pancreatic cancer’s groups. Kaplan Meier curves and Log-rank test were used to compare the survival time between the two location groups of pancreatic cancer. Stata/MP 16.1 for Windows was used to analyze the data. A test was considered statistically significant if the P value was < 0.05.
From July, 2016 to June, 2020, a total of 500 records was retrieved and 101 patients with complete medical records were included in the analysis. 67 patients had PHC and 34 patients had PBTC. Compared to patients with PHC, patients with PBTC are older at diagnosis (68.97 vs 61.49, P = 0.01), diagnosed at more advanced stage (P = 0.006) and are less likely to undergo surgical resection (P = 0.025) (Table 1). There is no significant difference in gender, ethnicity between patients with PHC and patients with PBTC (P = 0.10 and 0.53 respectively).
| Total | Head | Body/tail | P value | |
| n = 101, % | n = 67, % | n = 34, % | ||
| Age at diagnosis | 63.90 (13.03) | 61.49 (13.96) | 68.97 (9.12) | 0.010 |
| Gender | 0.095 | |||
| Female | 51 (50.50) | 38 (56.72) | 13 (38.24) | |
| Male | 50 (49.50) | 29 (43.28) | 21 (61.76) | |
| Ethnicity | 0.53 | |||
| Caucasian | 76 (75.25) | 48 (71.64) | 28 (82.35) | |
| Black | 11 (10.89) | 8 (11.94) | 3 (8.82) | |
| Other | 14 (13.86) | 11 (16.42) | 3 (8.82) | |
| Pathologic initial stage | 0.006 | |||
| I | 5 (4.95) | 3 (4.48) | 2 (5.88) | |
| II | 19 (18.81) | 18 (26.87) | 1 (2.94) | |
| III | 17 (16.83) | 13 (19.40) | 4 (11.76) | |
| IV | 51 (50.50) | 28 (41.79) | 23 (67.65) | |
| Missing | 9 (8.91) | 5 (7.46) | 4 (11.76) | |
| Surgical resection | 0.025 | |||
| Yes | 34 (33.66) | 28 (41.79) | 6 (17.65) | |
| No | 67 (66.34) | 39 (58.21) | 28 (82.35) |
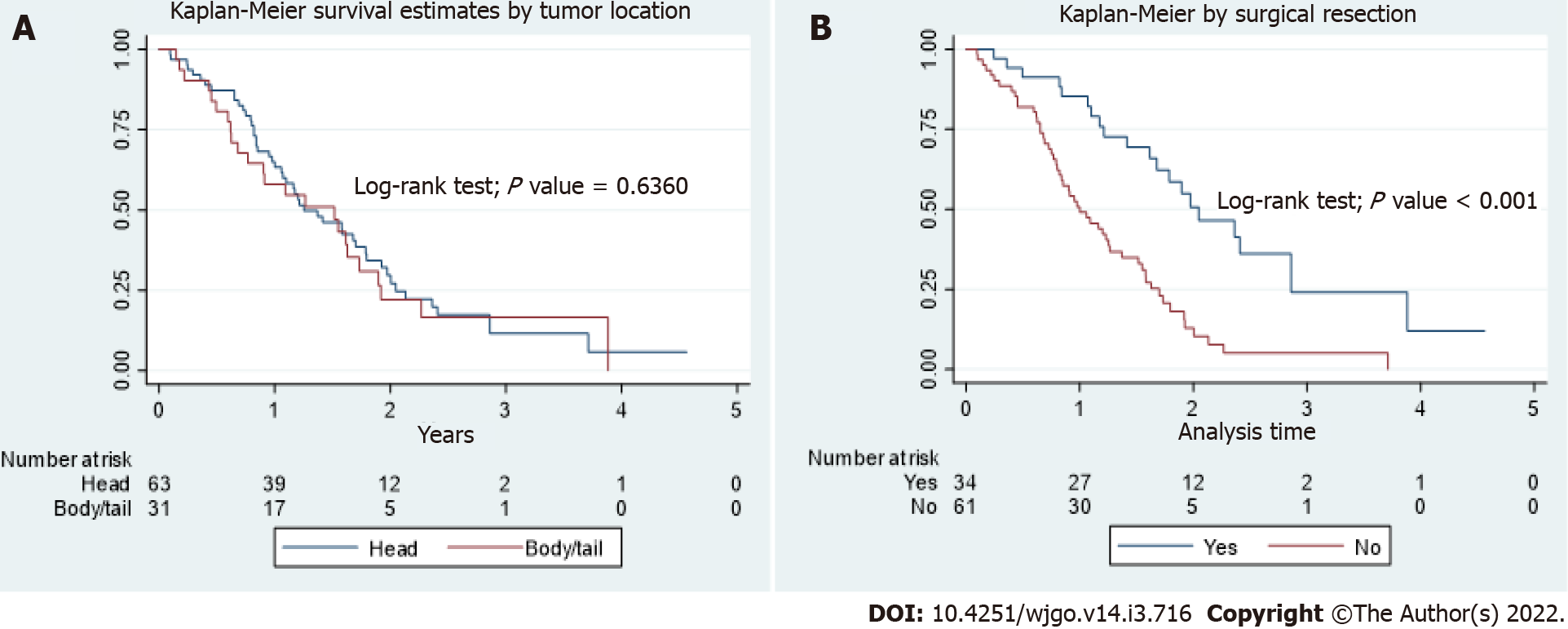
In the total population, the OS between PHC and PBTC was not statistically different (P = 0.64, Figure 1A). Patients who underwent surgical resection had better OS than patients who did not (P < 0.001, Figure 1B), with a median OS of 2.05 years (interquartile range, 1.21 to 2.86) and 1.00 year (interquartile range, 0.77 to 1.70) respectively. There were no differences in survival between PHC and PBTC in those who underwent surgical resection, those who didn’t undergo surgical resection, or those who had Stage IV disease on presentation. In the subgroup of patients who had stage I and II disease, there were 3 patients with PBTC and those were long-term survivors. However, due to small number of patients, no definite conclusion can be made.
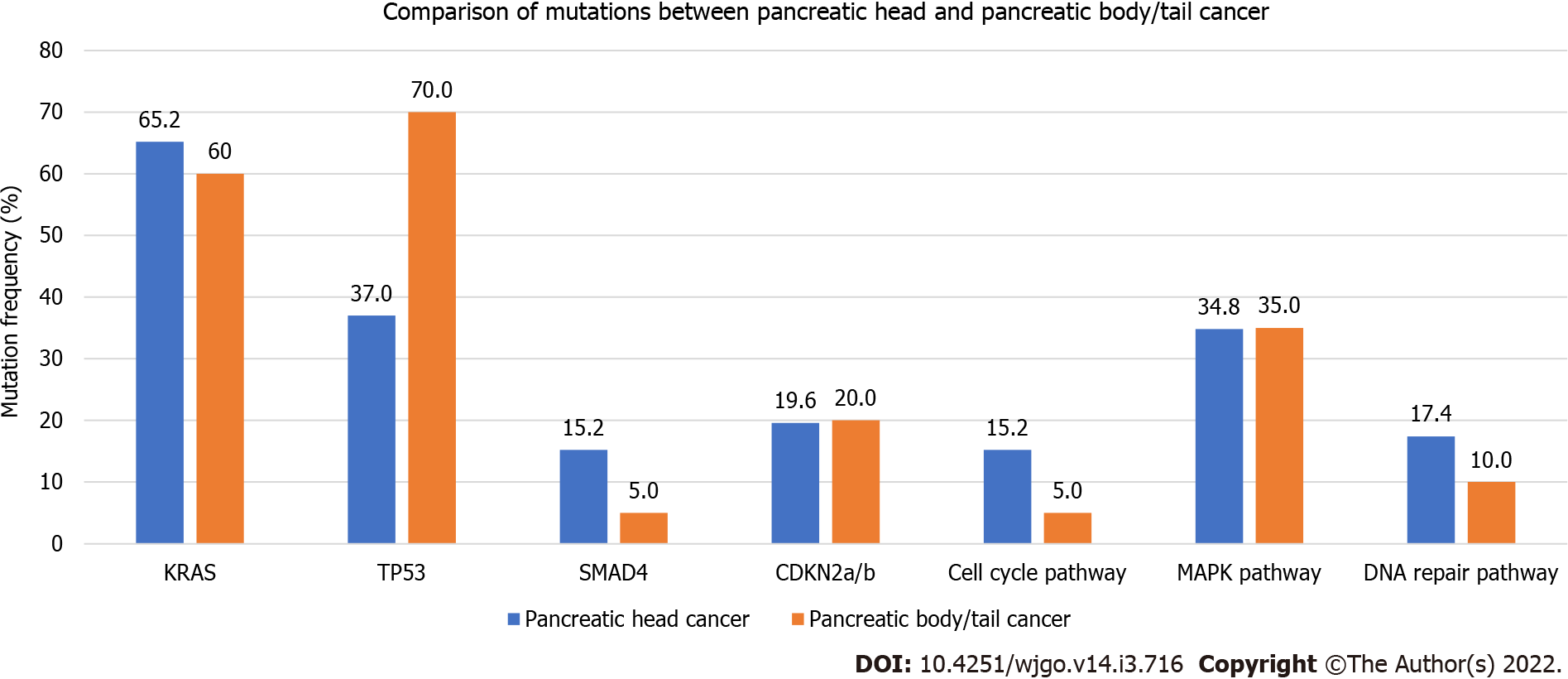
A total of 66 patients (46 PHC and 20 PBTC) who had complete medical records and molecular profiling were reviewed. 20/66 (30.3%) had molecular testing performed on biopsy specimen, 24/66 (36.4%) on peripheral blood, 14/66 (21.2%) on surgical resection specimen and the remaining on samples with unknown sources. Rates of pathogenic mutations were recorded and compared between PHC and PBTC (Figure 2). PHC and PBTC have similar tumor mutation numbers (P = 0.79). The most common mutations were KRAS mutations (63.6% in total, 65.2% in PHC vs 60.6% in PBTC, P = 0.78), TP53 mutations (47.0% in total, 37.0% in PHC vs 70.0% in PBTC, P = 0.03), SMAD mutations (12.1% in total, 15.2% in PHC vs 5.0% in PBTC, P = 0.42) and CDKN2a/b mutations (19.7% in total, 19.6% in PHC vs 20.2% in PBTC, P = 1.00). Only TP53 mutations were significantly different. 12.1%% of the mutations were involved in cell cycle pathway (15.2% in PHC vs 5%% in PBTC, P = 0.47), 34.9% in MAPK pathway (34.8% in PHC vs 35.0% in PBTC, P = 0.99) and 15.1% in DNA repair pathway (17.4% in PHC vs 10.0% in PBTC, P = 0.71). There were no differences in the mutations involved in these pathways.
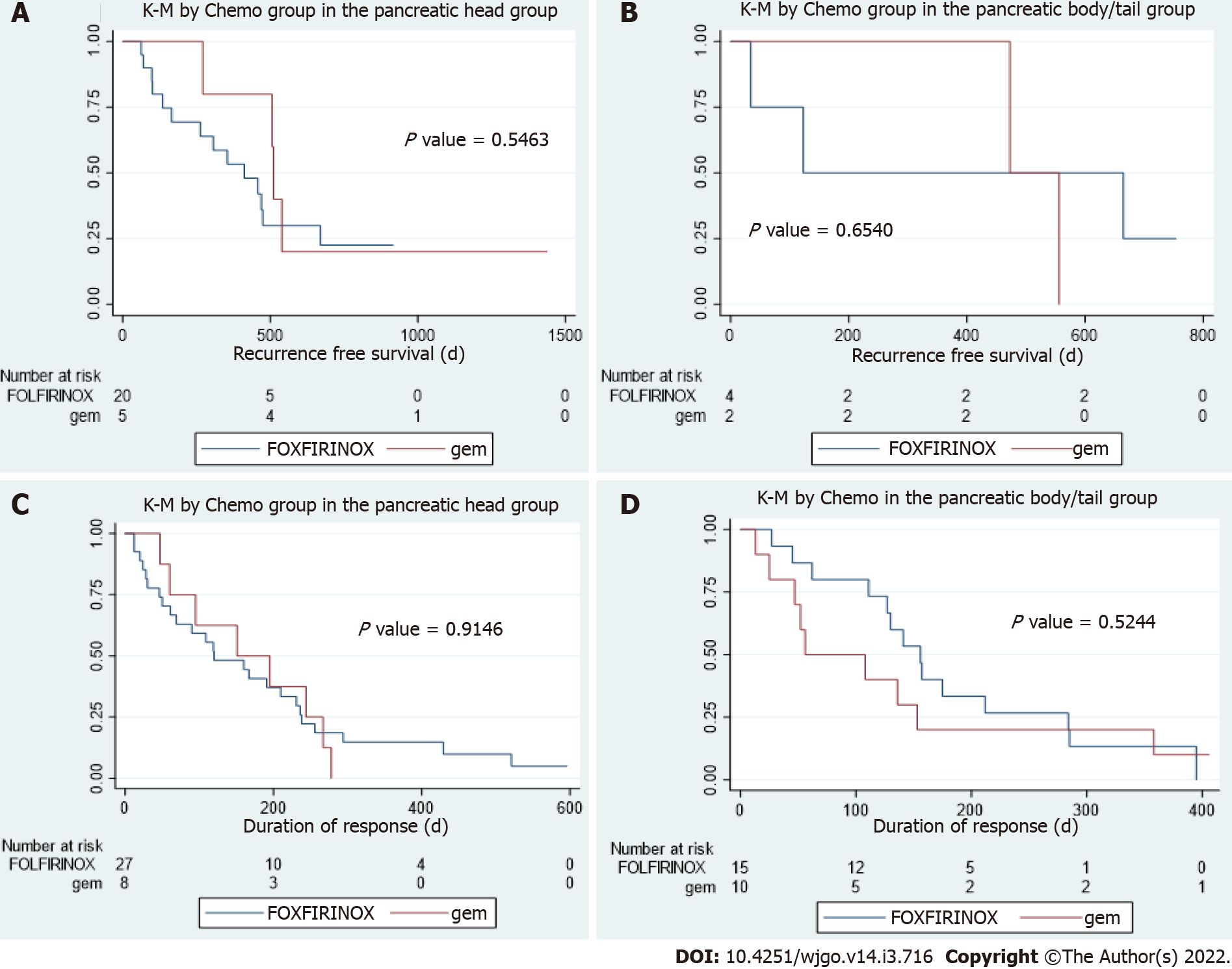
In patients who underwent resection, there were no statistical differences in disease free interval between patients with PHC or PBTC who received FOLFIRINOX based chemotherapy and those who received gemcitabine-based chemotherapy (P values are 0.55 and 0.65 respectively, Figure 3A and B). In patients with metastatic disease, there were no statistical differences in duration of response to first line palliative chemo between patients with PHC or PBTC who received FOLFIRINOX-based chemotherapy and those who received gemcitabine-based chemotherapy (P values are 0.91 and 0.52 respectively, Figure 3C and D).
In this retrospective study, patients with PHC and PBTC were retrieved and the relationships of tumor locations with molecular profiling, overall survival, or response to chemotherapy were explored. Our study showed that patients with PBTC tend to present at later stages and are less likely to undergo resection, consistent with previous studies[11-13]. PBTC are older at diagnosis in our study, which could have explained the lower resection rate.
The impact of tumor location on survival has been a controversy. Several population-based studies[2,3,11,14,15] reported contradictory survival of PBTC and PHC in the overall population; however, better survival in PBTC is seen in early stage patients, especially stage I and II. Winer et al[14] examined the relationship of survival and tumor location in patients who had tumor resection and found that even though PHC were more of early stage at presentation and more likely to be resected, they tended to have higher grade, more positive lymph nodes and worse overall survival. The survival advantage of PBTC from this study was further supported by a single center study[16] which examined survival in matched stage II PBTC and PHC. Only one single center study[12] reported worse outcome for PBTC patients who had resection and among those patients with Stage I disease, the survival seemed to be better in PBTC however not statistically significant. Even though our study didn’t show a survival difference in PHC and PBTC, long-term survival was seen in three patients with Stage I/II PBTC who underwent resection. The findings from previous studies and our study suggest that resected early stage PBTC have better survival than those with PHC.
The four most common mutations in our study were KRAS, TP53, SMAD and CDKN2a/b mutations, which is consistent with previous reports[13,17]. Among these most common mutations, only TP53 mutations were found to be significantly higher in PBTC in our study. Even though the frequency of SMAD mutations was higher in PHC (15.5%) compared to PBTC (5%), this result was not statistically significant because the total number of SMAD mutations observed was only 12.12% in our study. The lower frequencies of those mutations detected in our study could be explained by NGS being performed on insufficient tissues, as majority of samples were biopsy specimens or peripheral blood (66.7%). TP53 mutation is enriched in pancreatic squamous cell type[5] that is similar to lung squamous subtype, and might be similarly more sensitive to gemcitabine therapy. TP53 was also found to predict sensitivity to gemcitabine-based adjuvant therapy in a survival and mutational analysis from CONKOO-001 study[17]. Based on these, our finding of more TP53 mutations in PBTC suggest gemcitabine-based adjuvant therapy to be considered in PBTC, especially in those who can’t tolerate FOLFIRINOX therapy.
To our knowledge, our study is the first study that looked at the impact of tumor location on response to different chemotherapy regimens. Neither PHC or PBTC responded differently to FOLFIRINOX based or gemcitabine-based chemotherapies, suggesting a universal poor prognosis of pancreatic cancer regardless of tumor location when compared based on chemotherapy response. However, differential response to targeted therapy or immunotherapy is yet to be explored given the different distribution of pancreatic cancer subtype like immunogenic.
Our study was a retrospective study in a single institution and a relatively small number of patients was retrieved. Due to the retrospective nature, the NGS platforms utilized, and the depths of sequencing were not uniform. This added another layer of bias in data interpretation. However, this reflects the real-world experience in many community and academic cancer centers that often rely heavily on commercial NGS platforms. Even though there is a trend to suggest better survival in early stage PBTC patients after resection, there were few patients and events in this subgroup and no definitive conclusion can be made.
There is no difference in OS between PHC and PBTC but the long-term survival observed in early stage PBTC after resection suggests better survival in this subgroup of patients. PBTC has significantly more mutations involved in TP53 mutations and its predictive role in gemcitabine sensitivity should be explored in future studies.
Pancreatic head and pancreatic body/tail have different embryological origins. Tumors arising at different locations of pancreas might carry different mutations and respond differently to chemotherapy.
To better define pancreatic cancer and search for precision oncological targets that yield better outcomes.
To study the relationships of pancreatic cancer location with molecular profiling, response to chemotherapy and survival.
This is a single institution retrospective study that retrieved patients who carry a diagnosis of pancreatic cancer from July 2016 to June 2020. Patient demographics and molecular profiling information were reviewed and the relationship between tumor location and molecular profiling, response to chemotherapy and survival were analyzed.
Pancreatic head cancer and pancreatic body/tail cancer (PBTC) have different presentations but similar overall survival and response to chemotherapy. PBTC have significantly more TP53 mutations.
Given that TP53 mutations predict gemcitabine sensitivity, gemcitabine containing chemotherapy should be considered for PBTC as first line.
A larger and prospective study should be performed to explore the role of gemcitabine in PBTC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Luyer MDP, Reshkin SJ, Xu K S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Bastidas-Ponce A, Scheibner K, Lickert H, Bakhti M. Cellular and molecular mechanisms coordinating pancreas development. Development. 2017;144:2873-2888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 2. | Lau MK, Davila JA, Shaib YH. Incidence and survival of pancreatic head and body and tail cancers: a population-based study in the United States. Pancreas. 2010;39:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Matsuno S, Egawa S, Fukuyama S, Motoi F, Sunamura M, Isaji S, Imaizumi T, Okada S, Kato H, Suda K, Nakao A, Hiraoka T, Hosotani R, Takeda K. Pancreatic Cancer Registry in Japan: 20 years of experience. Pancreas. 2004;28:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 248] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 4. | Saad AM, Turk T, Al-Husseini MJ, Abdel-Rahman O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer. 2018;18:688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 188] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 5. | Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grützmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM; Australian Pancreatic Cancer Genome Initiative, Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Waddell N, Biankin AV, Grimmond SM. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2848] [Cited by in RCA: 2548] [Article Influence: 283.1] [Reference Citation Analysis (0)] |
| 6. | Hoadley KA, Yau C, Wolf DM, Cherniack AD, Tamborero D, Ng S, Leiserson MDM, Niu B, McLellan MD, Uzunangelov V, Zhang J, Kandoth C, Akbani R, Shen H, Omberg L, Chu A, Margolin AA, Van't Veer LJ, Lopez-Bigas N, Laird PW, Raphael BJ, Ding L, Robertson AG, Byers LA, Mills GB, Weinstein JN, Van Waes C, Chen Z, Collisson EA; Cancer Genome Atlas Research Network, Benz CC, Perou CM, Stuart JM. Multiplatform analysis of 12 cancer types reveals molecular classification within and across tissues of origin. Cell. 2014;158:929-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1229] [Cited by in RCA: 1055] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 7. | Birnbaum DJ, Bertucci F, Finetti P, Birnbaum D, Mamessier E. Head and Body/Tail Pancreatic Carcinomas Are Not the Same Tumors. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Dreyer SB, Jamieson NB, Upstill-Goddard R, Bailey PJ, McKay CJ; Australian Pancreatic Cancer Genome Initiative, Biankin AV, Chang DK. Defining the molecular pathology of pancreatic body and tail adenocarcinoma. Br J Surg. 2018;105:e183-e191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP, Gandara D. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543-3551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2484] [Cited by in RCA: 2488] [Article Influence: 146.4] [Reference Citation Analysis (0)] |
| 10. | Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schönekäs H, Rost A, Neuhaus H, Haag C, Clemens M, Heinrich B, Vehling-Kaiser U, Fuchs M, Fleckenstein D, Gesierich W, Uthgenannt D, Einsele H, Holstege A, Hinke A, Schalhorn A, Wilkowski R. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946-3952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 495] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 11. | van Erning FN, Mackay TM, van der Geest LGM, Groot Koerkamp B, van Laarhoven HWM, Bonsing BA, Wilmink JW, van Santvoort HC, de Vos-Geelen J, van Eijck CHJ, Busch OR, Lemmens VE, Besselink MG; Dutch Pancreatic Cancer Group. Association of the location of pancreatic ductal adenocarcinoma (head, body, tail) with tumor stage, treatment, and survival: a population-based analysis. Acta Oncol. 2018;57:1655-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 12. | Sheng W, Dong M, Wang G, Shi X, Gao W, Wang K, Song H, Shi G, Tan X. The diversity between curatively resected pancreatic head and body-tail cancers based on the 8th edition of AJCC staging system: a multicenter cohort study. BMC Cancer. 2019;19:981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Zhang X, Feng S, Wang Q, Huang H, Chen R, Xie Q, Zhang W, Wang A, Zhang S, Wang L, Yao M, Ling Q. Comparative genomic analysis of head and body/tail of pancreatic ductal adenocarcinoma at early and late stages. J Cell Mol Med. 2021;25:1750-1758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Winer LK, Dhar VK, Wima K, Morris MC, Lee TC, Shah SA, Ahmad SA, Patel SH. The Impact of Tumor Location on Resection and Survival for Pancreatic Ductal Adenocarcinoma. J Surg Res. 2019;239:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Zheng Z, Wang M, Tan C, Chen Y, Ping J, Wang R, Liu X. Disparities in survival by stage after surgery between pancreatic head and body/tail in patients with nonmetastatic pancreatic cancer. PLoS One. 2019;14:e0226726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Ling Q, Xu X, Ye P, Xie H, Gao F, Hu Q, Liu Z, Wei X, Röder C, Trauzold A, Kalthoff H, Zheng S. The prognostic relevance of primary tumor location in patients undergoing resection for pancreatic ductal adenocarcinoma. Oncotarget. 2017;8:15159-15167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Sinn M, Sinn BV, Treue D, Keilholz U, Damm F, Schmuck R, Lohneis P, Klauschen F, Striefler JK, Bahra M, Bläker H, Bischoff S, Pelzer U, Oettle H, Riess H, Budczies J, Denkert C. TP53 Mutations Predict Sensitivity to Adjuvant Gemcitabine in Patients with Pancreatic Ductal Adenocarcinoma: Next-Generation Sequencing Results from the CONKO-001 Trial. Clin Cancer Res. 2020;26:3732-3739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |