Published online Mar 15, 2022. doi: 10.4251/wjgo.v14.i3.678
Peer-review started: July 28, 2021
First decision: September 5, 2021
Revised: September 10, 2021
Accepted: January 22, 2022
Article in press: January 22, 2022
Published online: March 15, 2022
Processing time: 225 Days and 4 Hours
Colorectal cancer (CRC) is a commonly diagnosed cancer of the digestive system worldwide. Although chemotherapeutic agents and targeted therapeutic drugs are currently available for CRC treatment, drug resistance is a problem that cannot be ignored and needs to be solved.
To explore the relationship between circular RNA (circRNA) and CRC drug resistance. circRNA plays a key role in the occurrence and development of cancers, but its function in the process of drug resistance has not been widely revealed.
To explore the role of circRNA in 5-fluorouracil (5-Fu) resistance, we performed the circRNA expression profile in two CRC cell lines and their homologous 5-Fu resistant cells by high-throughput sequencing.
We validated the differentially expressed circRNAs in other two paired CRC cells, confirmed that circ_0002813 and circ_0000236 could have a potential competitive endogenous RNA mechanism and be involved in the formation of 5-Fu resistance. And we combined the sequencing results of mRNA to construct the regulatory network of circRNA-miRNA-mRNA.
Our study revealed that circ_0002813 and circ_0000236 may as the biomarkers to predict the occurrence of 5-Fu resistance in CRC.
Core Tip: Therapy resistance has been a culprit for colorectal cancer (CRC) treatment. 5-fluorouracil is a first-line chemotherapeutic agent for CRC, and it is very important to reveal the potential biomarkers and mechanisms of resistance. Circular RNA (circRNA) plays a key role in the occurrence and development of cancers, but its function in the process of drug resistance has not been widely revealed. In this study, through the construction of drug-resistant cell lines and high-throughput sequencing technology, we revealed the changes in the expression of circRNAs during the process of drug resistance, and searched for circRNAs that could predict the occurrence of drug resistance as potential biomarkers.
- Citation: Cheng PQ, Liu YJ, Zhang SA, Lu L, Zhou WJ, Hu D, Xu HC, Ji G. RNA-Seq profiling of circular RNAs in human colorectal cancer 5-fluorouracil resistance and potential biomarkers. World J Gastrointest Oncol 2022; 14(3): 678-689
- URL: https://www.wjgnet.com/1948-5204/full/v14/i3/678.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i3.678
Colorectal cancer (CRC) is the third most common cancer worldwide and ranks as the second leading cause of cancer-related death[1,2]. In addition, the incidence of CRC has increased in recent years, and the number of patients has increased significantly[3]. Regarding the treatment of CRC, 5-fluorouracil (5-Fu), a fluorinated analogue of uracil, is the basic component and first-line chemotherapeutic agent for CRC[4,5]. Clinically, combination chemotherapy regimens based on 5-Fu, such as FOLFOX (5-Fu, leucovorin, and oxaliplatin) or FOLFIRI (5-Fu, leucovorin and irinotecan), have been shown to increase the survival rate and improve the response rate of patients with CRC; however, the emergence of resistance to 5-Fu was a major bottleneck in treatment[6-8]. Therefore, continuous and in-depth exploration of the 5-Fu resistance mechanism is an essential step to improve the survival benefit of 5-Fu-based therapy for CRC.
The development of 5-Fu resistance involves genetic and epigenetic alterations. In recent years, continuous studies have revealed changes in genes involved in the process of 5-Fu resistance and clarified the regulatory mechanisms of some genes involved in the development of resistance, such as EZH2, FOXM1, and YAP[9-12]. In terms of the role of noncoding RNAs in drug resistance, specific microRNA and lncRNA profiles were identified by RNA sequencing, and mechanisms have also been gradually revealed[13,14].
Circular RNAs (circRNAs), members of the noncoding RNA family, are characterized by covalently closed continuous loop structures without 3’ end poly(A) tails and 5’ end caps[15]. CircRNAs are widely expressed in multiple species and different cell types, and more than 20000 circRNAs have been detected in eukaryotes[16,17]. With the application of high-throughput sequencing, an increasing number of circRNAs have been identified, and at the same time, the functions of circRNAs in diseases have been elucidated, especially in cancers[18,19]. CircRNAs regulate gene expression mainly at the transcriptional and posttranscriptional levels by acting as miRNA sponges or binding to other molecules as their main mechanism of action[20]. Studies have shown important roles for circRNAs in the occurrence and malignant progression of almost all types of cancers[21-23]. However, previous studies were limited to the regulation of circRNAs in the malignant progression of cancers, and the mechanism of circRNAs in chemotherapy resistance has not been clearly studied. Therefore, little is known about the role of circRNA-related competitive endogenous RNA (ceRNA) in 5-Fu resistance in CRC.
In this study, we constructed multiple CRC 5-Fu-resistant cell lines and explored the expression profiles of circRNAs and mRNAs in 5-Fu-resistant cell lines and their parental cell lines using RNA sequencing. After verifying some candidate circRNAs in two additional pairs of cell lines, circRNA-miRNA-mRNA regulatory networks were constructed using Cytoscape software. Our study identified potential circRNAs involved in 5-Fu resistance in CRC and suggested that circRNAs play an important role in the generation of 5-Fu resistance.
Human CRC cell lines (HCT116, Lovo, HT29 and SW480) were purchased from the Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). HCT116 cells were cultured in DMEM (Gibco, Carlsbad, CA, United States), Lovo cells were cultured in F12 medium (Gibco, Beijing, China), HT29 cells were cultured in McCoy’s 5A medium (Gibco, Carlsbad, CA, United States), and SW480 cells were cultured in L-15 medium (Gibco, Bleiswijk, Netherlands). All culture media contained 10% foetal bovine serum and 1% penicillin, and these cell lines were maintained in a humidified atmosphere of 5% CO2 at 37 °C. The WST-1 cell proliferation and cytotoxicity detection kit was purchased from Beyotime, China.
The HCT116, Lovo, HT29 and SW480 cells were exposed to an initial 5-Fu concentration of 0.1 μg/mL in medium supplemented with 10% FBS. The surviving population of cells was grown to 80% confluence. 5-Fu-resistant cells were established after sequential treatments with increasing concentrations of 5-Fu (0.2, 0.5, 1, 1.5 and 2 μg/mL). Cells were able to survive at least 3 d of stimulation with 2 μg/mL 5-Fu. Resistance to 5-Fu was confirmed by the WST-1 assay.
Total RNA was isolated from the cell lines using TRIzol reagent (Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. Then, we assessed RNA integrity and DNA contamination using electrophoresis on a denaturing agarose gel. High-throughput RNA sequencing was performed by Cloud-Seq Biotech (Shanghai, China). Briefly, rRNAs were removed from total RNA with the NEBNext rRNA Depletion Kit (New England Biolabs, Inc., Massachusetts, United States) according to the manufacturer's instructions. RNA libraries were constructed using the NEBNext® Ultra™ II Directional RNA Library Prep Kit (New England Biolabs, Inc., Massachusetts, United States) according to the manufacturer’s instructions. Libraries were controlled for quality and quantified using the BioAnalyzer 2100 system (Agilent Technologies, Inc., United States). Library sequencing was performed on an Illumina NovaSeq 6000 instrument to obtain 150 bp paired end reads. The quality of paired end reads was controlled by Q30. After 3’ adaptor trimming and low-quality read removal, cutadapt software (v1.9.3) was used. High-quality trimmed reads were used to analyse circRNAs and mRNAs. CircRNAs: The high-quality reads were aligned to the reference genome/transcriptome with STAR software (v2.5.1b), and circRNAs were detected and identified with DCC software (v0.4.4). EdgeR software (v3.16.5) was used to normalize the data and analyse differentially expressed circRNAs. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were performed on the differentially expressed circRNA-associated genes. For mRNAs, the high-quality reads were aligned to the human reference genome (UCSC hg19) with hisat2 software (v2.0.4). Then, guided by the Ensembl Gene transfer format (GTF) gene annotation file, cuffdiff software (v2.2.1, part of cufflinks) was used to obtain the fragments per kilobase per million mapped reads (FPKM) as the expression profiles of lncRNAs and mRNAs, and fold changes and P values were calculated based on FPKM. Differentially expressed lncRNAs and mRNAs were identified. The target genes of lncRNAs were predicted based on the locations to nearby genes. GO and pathway enrichment analyses were performed on these target genes and the differentially expressed mRNAs.
CircRNA-miRNA interactions were predicted using popular target prediction software programs, including circRNA Interactome and RegRNA. Specific predictions of the target genes of miRNAs were based on the miRanda, miRDB, miRWalk, RNA22 and TargetScan databases. All circRNA-miRNA-mRNA networks were constructed using Cytoscape software.
Total RNA was extracted using TRIzol reagent (Life Technologies, Carlsbad, CA, United States) and then reverse-transcribed into cDNAs using the SuperScript First-Strand Synthesis System (Invitrogen, Carlsbad, CA, United States). The cDNA templates were used for quantitative real-time polymerase chain reaction (qPCR) with SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, United States) and gene-specific primers, and the results were normalized to β-actin as a control. PCR primers are listed in Supplementary Table 1.
Statistical analyses were performed using GraphPad Prism 7.0 (GraphPad Software Inc., CA, United States). Student’s t test and one-way ANOVA were used to compare differences between groups, as appropriate. Data are presented as the means ± SD, and P < 0.05 was considered statistically significant.
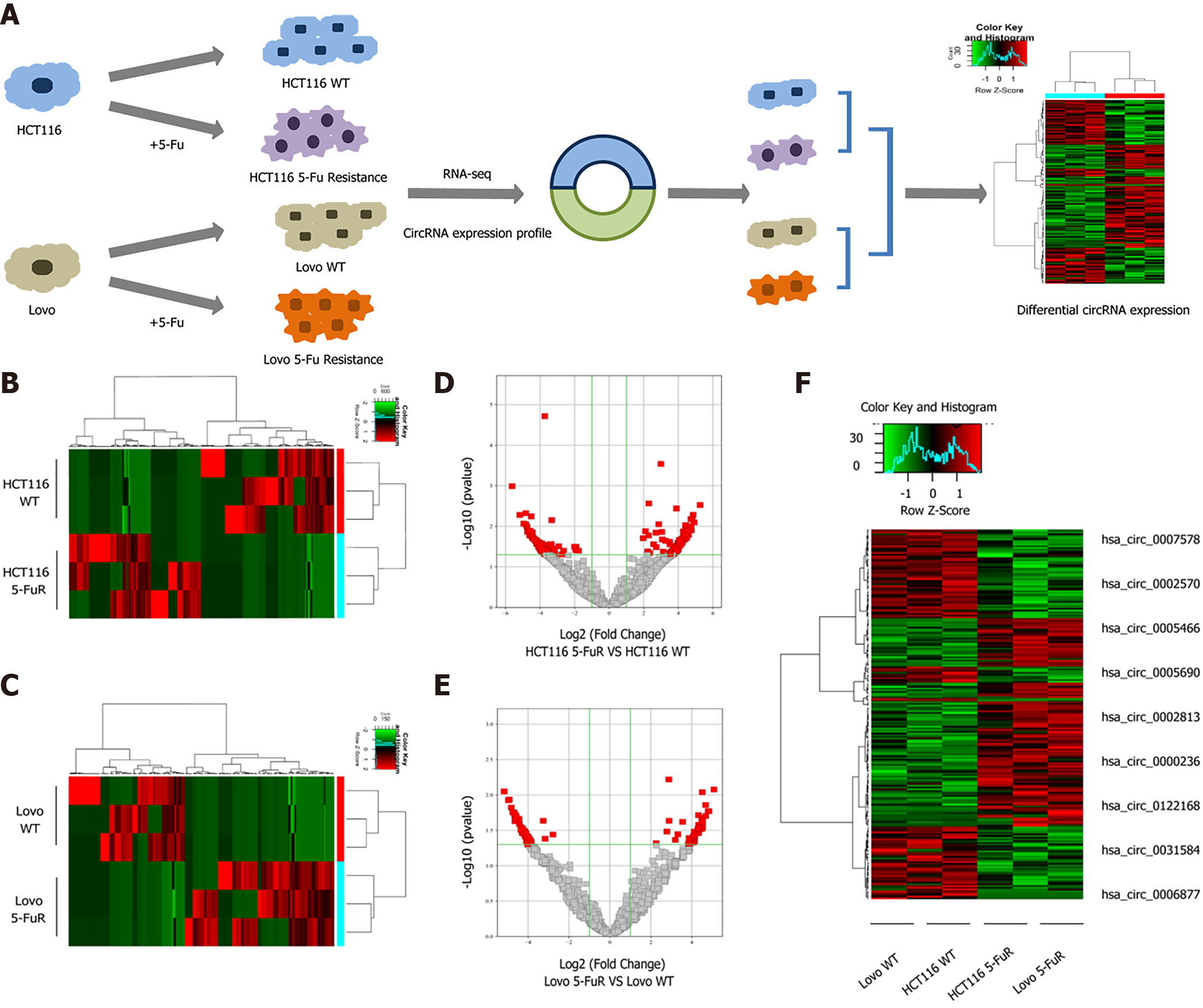
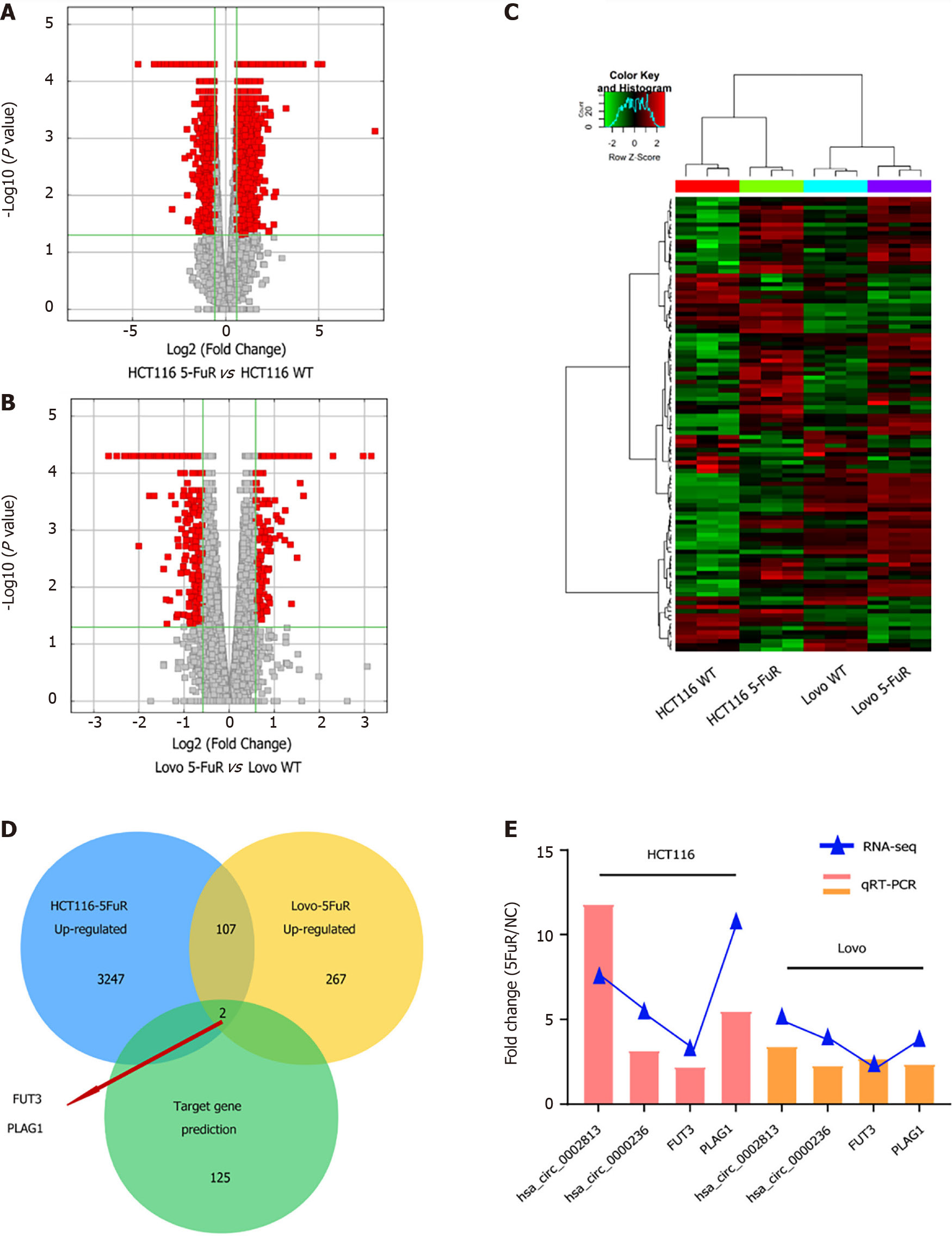
We first needed to determine the differentially expressed circRNAs in 5-Fu-resistant cells to explore the roles of circRNAs in the process of 5-Fu resistance in CRC. Secondary sequencing was used to profile circRNA and mRNA expression in two paired CRC 5-Fu resistant cell lines and parental cell lines, with three technical replicates in each group (Figure 1A). A total of 17939 circRNAs were detected in CRC cells, and the list of total circRNA expression profiles. Hierarchical clustering analysis showed significant differences in circRNA expression in the two pairs of cells individually (Figure 1B and C). We also constructed volcano plots (Figure 1D and E) to depict the significantly differentially expressed circRNAs (fold change > 2, and P < 0.05); the scatter plot shows the variation in circRNA expression levels (Supplementary Figure 1). After the intersection and merge of the differentially expressed circRNAs in two pairs of CRC cells, only 9 circRNAs showed significant differences in expression, among which 4 circRNAs were downregulated in 5-Fu-resistant cells and 5 circRNAs were upregulated in 5-Fu-resistant cells (Figure 1F). Sequencing results and analysis suggested that the expression levels of some circRNAs changed during the process of 5-Fu resistance.
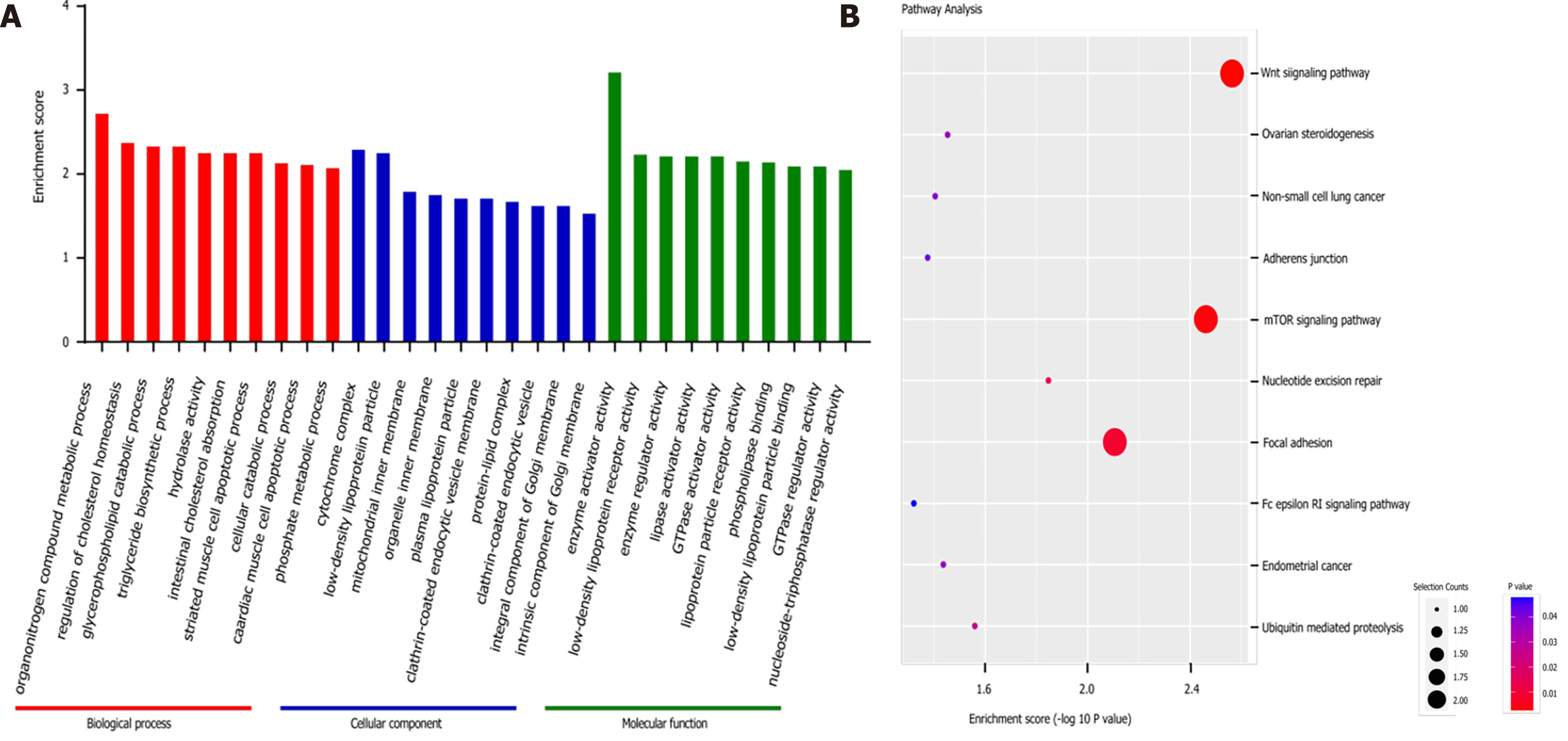
The GO analysis of differentially expressed circRNAs included three categories: Biological process (BP), cell component (CC), and molecular function (MF). We listed the top ten terms in the BP, CC, and MF categories (Figure 2A). In the KEGG analysis, we also listed the 10 enriched pathways among the upregulated circRNAs, among which “Wnt signalling pathway”, “mTOR signalling pathway” and “focal adhesion” were the three most noteworthy pathways identified after combining the selection counts, enrichment scores and P values (Figure 2B).
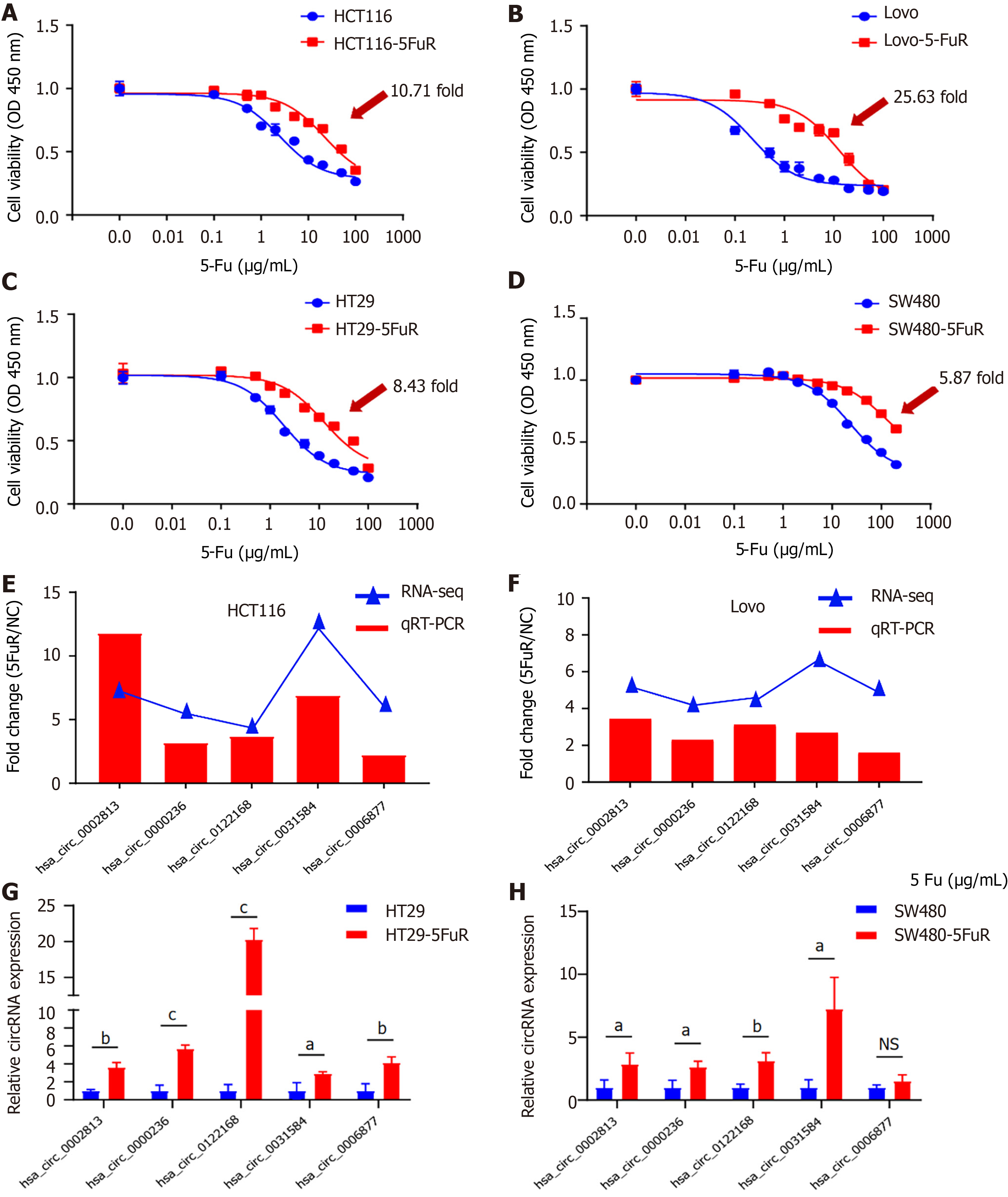
We focused on and verified the differentially expressed circRNAs obtained from the sequencing results using qRT–PCR in 4 pairs of CRC cell lines: HCT116, Lovo, HT29, and SW480. Before validation, we confirmed the drug resistance of the four types of 5-Fu-resistant cells by performing a WST-1 assay. As shown in Figure 3A-D, after intervention with different concentrations of 5-Fu, the IC50 values of the four 5-Fu-resistant cells increased by 5- to 15-fold compared with their parental cells. Based on the results shown in Figure 1F, we selected the 5 circRNAs that were significantly upregulated in 5-Fu-resistant cells and verified them in these 4 pairs of cell lines. For the HCT116 and Lovo cell lines, the qRT–PCR results were consistent with the results of RNA sequencing, and all 5 circRNAs (hsa_circ_0002813, hsa_circ_0000236, hsa_circ_0122168, hsa_circ_0031584, and hsa_circ_0006877) were expressed at high levels in 5-Fu-resistant cells (Figure 3E and F). The results from the HT29 and SW480 cell lines also confirmed that the expression of 4 of 5 circRNAs was significantly increased in 5-Fu-resistant cells, except for hsa_circ_0006877 in SW480 cells, which was not significantly different (Figure 3G and H).
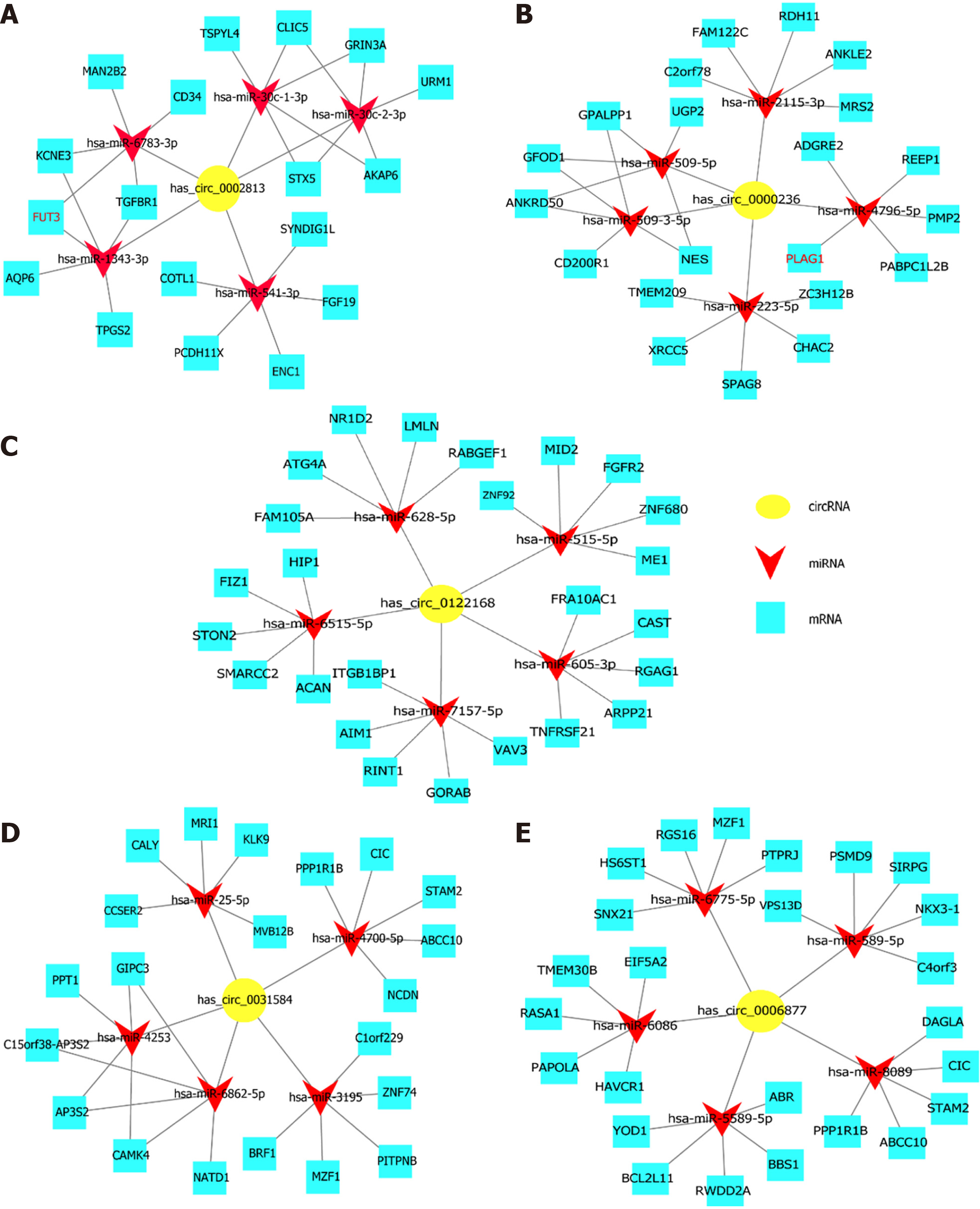
Based on the previous verification results, the circRNA-miRNA-mRNA regulatory networks were constructed by prediction and bioinformatics analysis using Cytoscape software for these 5 circRNAs with significantly altered expression levels. As shown in Figure 4, in the prediction network, we chose the top 5 miRNAs that potentially bind to the circRNAs and the 5 most likely target genes of each miRNA. From the prediction network, we clearly see the potential regulatory targets of the 5 circRNAs. This result provided a clear direction for us to further study the specific mechanism of circRNAs in 5-Fu resistance in CRC.
While circRNA sequencing was performed on the two paired CRC 5-Fu-resistant cell lines, mRNA expression levels were also measured (Figure 5A and B and Supplementary Figure 2). Clustering analysis was performed on the differentially expressed mRNAs. In 5-Fu-resistant HCT116 cells, 3247 genes were significantly upregulated, and 267 genes were significantly upregulated in 5-Fu-resistant Lovo cells. An analysis combining the results from the two pairs of cell lines showed that 107 genes were upregulated in the 5-Fu-resistant variants (Figure 5C). The interactions of the 107 genes that were upregulated in both 5-Fu-resistant cell lines with the potential target genes predicted based on the circRNA-miRNA-mRNA regulatory network were analysed. The results focused on two genes, FUT3 and PLAG1, that were expressed at high levels in 5-Fu-resistant cells and predicted to be targets of differentially expressed circRNAs (Figure 5D). Then, we verified the mRNA expression levels of the two genes using a qRT–PCR assay, and the results were consistent with the RNA sequencing results (Figure 5E). The expression levels of both FUT3 and PLAG1 were significantly increased in 5-Fu-resistant cells, consistent with the expression of circRNAs (hsa_circ_0002813 and hsa_circ_0000236) that regulate them in the predicted network. Thus, the regulatory axes composed of circ_0002813-miR-1343-3p-FUT3 and circ_0000236-miR4769-5p-PLAG1 may play an important role in 5-Fu resistance in CRC.
With the advent of targeted therapy and immunotherapy, the treatment of CRC has achieved great advances. However, 5-Fu chemotherapy is still the main clinical treatment for CRC[5,24]. The main problem of chemotherapy for CRC is 5-Fu resistance, and 50% of patients with advanced CRC show 5-Fu resistance[10]. Therefore, continuous and thorough investigations of the potential mechanism of 5-Fu resistance are urgently needed. As members of the noncoding RNA family, circRNAs have become the focus of tumour research in recent years. Notably, circRNAs are involved in the malignant processes of a variety of human tumours, such as lung cancer, CRC and breast cancer[21,25,26]. Moreover, the role of circRNAs in chemotherapy resistance in cancers has also been reported. For instance, the circRNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer by suppressing miR-198[27]. Another study showed that circRNA-SORE mediates sorafenib resistance in hepatocellular carcinoma by stabilizing YBX1[28].
In this study, we performed high-throughput sequencing to investigate the relationship between circRNAs and 5-Fu resistance, the most common chemotherapeutic drug used to treat CRC. A total of 17939 circRNAs were detected in two 5-Fu-resistant lines and their parental cell lines. We conducted an in-depth analysis of the sequencing results and combined the results of the two paired cell lines and identified 9 circRNAs with significant differences in expression. This study is the first to reveal circRNAs with a potential role in 5-Fu resistance in CRC at the cellular level. Moreover, we postulate that these 9 differentially expressed circRNAs may play a regulatory role in the process of 5-Fu resistance. We focused on 5 circRNAs that were significantly upregulated in 5-Fu-resistant cells and verified them in two paired cell lines selected for sequencing and two other paired cell lines using qRT–PCR assays to further confirm the accuracy of our findings. Four of the 5 circRNAs (hsa_circ_0002813, hsa_circ_0000236, hsa_circ_0122168, and hsa_circ_0031584) were expressed at high levels in the 4 paired cell lines with 5-Fu resistance. Moreover, the remaining circRNA, hsa_circ_0006877, showed significantly higher expression in 5-Fu-resistant variants of all cell lines except SW480 cells. Based on these results, hsa_circ_0002813, hsa_circ_0000236, hsa_circ_0122168, hsa_circ_0031584 and hsa_circ_0006877 may play a role in 5-Fu resistance in CRC.
Studies examining the function of circRNAs have indicated that circRNAs function as miRNA sponges and then regulate the expression of their target genes[20]. This function is the key mechanism by which circRNAs participate in various BP[29,30]. Therefore, for these 5 verified circRNAs, we predicted the miRNAs that they might sponge and their downstream target genes by analysing different databases to explore the potential regulatory mechanism of circRNAs in drug resistance. According to the 5 circRNAs upregulated in the 5-Fu-resistant cells, we first predicted circRNA-miRNA-mRNA interactions through target prediction software and selected the top 5 interactions to construct the networks. The networks established in our study provided a scientific basis for the subsequent study of the mechanism underlying circRNA function in CRC drug resistance.
In our study, we performed high-throughput sequencing of two paired cell lines, and in addition to including circRNAs, we also measured mRNA expression levels. In the process of exerting their biological functions, circRNAs mostly regulate downstream mRNAs by sponging miRNAs. Therefore, we analysed the mRNA expression profiles using the sequencing results. The differentially expressed mRNAs involved in drug resistance were comprehensively analysed with the mRNAs in the previously constructed circRNA-miRNA-mRNA regulatory network. We found that two mRNAs (FUT3 and TNS4) that we predicted to be regulated by potential circRNAs showed significantly increased expression in the mRNA sequencing results from the drug-resistant cells. FUT3 is an α-1,3/4 fucosyltransferase that is absorbed by red blood cells and leads to a Lewis phenotype. The biological functions of FUT3 in tumorigenesis and metastasis have been documented in a variety of tumours[31-33]. TNS4, a member of the tensin protein family, is involved in key cellular processes, including cell adhesion, migration, and proliferation[34,35]. Accumulating evidence has suggested that TNS4 may be involved in the pathogenesis of cancers by interacting with miRNAs. For instance, miR-1224-5p inhibits TNS4, subsequently affecting the progression of oesophageal squamous cell carcinoma[36]. According to a recent report, TNS4 was identified as a key effector of cetuximab and a regulator of the oncogenic activity of KRAS mutant CRC[37]. We consistently found that FUT3 and TNS4 were expressed at higher levels in 5-Fu-resistant CRC cells and were target genes in the regulatory network of two significantly differentially expressed circRNAs that we identified. We strongly speculate that the hsa_circ_0002813- miR-541-3p- FUT3 and hsa_circ_0000236- miR-4796-5p-TNS4 axes may be involved in the regulatory mechanisms of drug resistance and may be potential therapeutic targets.
In conclusion, we comprehensively analysed the circRNA and mRNA expression profiles in paired 5-Fu-resistant cells. We identified circRNAs and mRNAs that are commonly altered during the development of 5-Fu resistance and suggested that hsa_circ_0002813, hsa_circ_0000236, hsa_circ_0122168, hsa_circ_0031584 and hsa_circ_0006877 may play important regulatory roles in the process of 5-Fu resistance in CRC. These circRNAs may represent potential predictive biomarkers and possible therapeutic targets of 5-Fu resistance. Based on our circRNA-related ceRNA networks, two potential regulatory mechanisms have been identified. Our findings may provide new perspectives for understanding the occurrence of 5-Fu resistance in CRC, provide new biomarkers for predicting 5-Fu resistance, and reveal candidate targets for reversing drug resistance.
Therapy resistance has been a culprit for colorectal cancer (CRC) treatment. 5-fluorouracil (5-Fu) is a first-line chemotherapeutic agent for CRC, and it is very important to reveal the potential biomarkers and mechanisms of resistance.
Circular RNA (circRNA) plays a key role in the development and progression of cancer, but its role in the process of drug resistance has not been widely revealed. Therefore, we attempted to explore the relationship between circRNA and CRC drug resistance
Search for circRNAs that can predict the occurrence of CRC 5-Fu resistance, and explore its possibility as potential biomarkers.
In this study, through the construction of drug-resistant cell lines and high-throughput sequencing technology, we revealed the changes in the expression of circRNAs during the process of drug resistance, and the potential circRNAs were verified by quantitative real-time polymerase chain reaction.
We identified circRNAs and mRNAs that are commonly altered in the development of 5-Fu drug resistance and suggested that hsa_circ_0002813, hsa_circ_0000236, hsa_circ_0122168, hsa_circ_0031584 and hsa_circ_0006877 may play an important regulatory role in the process of 5-Fu resistance in CRC. These circRNAs may act as potential predictive biomarkers and possible therapeutic targets of 5-Fu resistan.
Our findings may offer new perspectives for understanding the occurrence of 5-Fu resistance in CRC, provide new biomarkers for predicting 5-Fu resistance, and reveal candidate targets for reversing drug resistance.
Potential circRNA expression differences in drug resistance were sought from the perspective of high-throughput sequencing of paired drug-resistant and parental cell lines.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elkady N, Kołat D, Socea B S-Editor: Fan JR L-Editor: A P-Editor: Guo X
| 1. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3265] [Article Influence: 653.0] [Reference Citation Analysis (2)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55782] [Article Influence: 7968.9] [Reference Citation Analysis (132)] |
| 3. | Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, Jemal A. Colorectal Cancer Incidence Patterns in the United States, 1974-2013. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 833] [Article Influence: 104.1] [Reference Citation Analysis (0)] |
| 4. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 3010] [Article Influence: 501.7] [Reference Citation Analysis (3)] |
| 5. | Vodenkova S, Buchler T, Cervena K, Veskrnova V, Vodicka P, Vymetalkova V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol Ther. 2020;206:107447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 598] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 6. | Stintzing S, Modest DP, Rossius L, Lerch MM, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Held S, Giessen-Jung C, Moehler M, Jagenburg A, Kirchner T, Jung A, Heinemann V; FIRE-3 investigators. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016;17:1426-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 315] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 7. | Van der Jeught K, Xu HC, Li YJ, Lu XB, Ji G. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol. 2018;24:3834-3848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 448] [Cited by in RCA: 413] [Article Influence: 59.0] [Reference Citation Analysis (5)] |
| 8. | Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat Rev Drug Discov. 2017;16:487-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 650] [Article Influence: 81.3] [Reference Citation Analysis (0)] |
| 9. | Wang C, Li X, Zhang J, Ge Z, Chen H, Hu J. EZH2 contributes to 5-FU resistance in gastric cancer by epigenetically suppressing FBXO32 expression. Onco Targets Ther. 2018;11:7853-7864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Varghese V, Magnani L, Harada-Shoji N, Mauri F, Szydlo RM, Yao S, Lam EW, Kenny LM. FOXM1 modulates 5-FU resistance in colorectal cancer through regulating TYMS expression. Sci Rep. 2019;9:1505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 11. | Intuyod K, Saavedra-García P, Zona S, Lai CF, Jiramongkol Y, Vaeteewoottacharn K, Pairojkul C, Yao S, Yong JS, Trakansuebkul S, Waraasawapati S, Luvira V, Wongkham S, Pinlaor S, Lam EW. FOXM1 modulates 5-fluorouracil sensitivity in cholangiocarcinoma through thymidylate synthase (TYMS): implications of FOXM1-TYMS axis uncoupling in 5-FU resistance. Cell Death Dis. 2018;9:1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Huang C, Chen Z, Yang C, Chen L, Lai C, Zhang Y, Yuan W, Jeong JH. Combinational inhibition of EGFR and YAP reverses 5-Fu resistance in colorectal cancer. J Cancer. 2020;11:5432-5439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Feng C, Zhang L, Sun Y, Li X, Zhan L, Lou Y, Wang Y, Liu L, Zhang Y. GDPD5, a target of miR-195-5p, is associated with metastasis and chemoresistance in colorectal cancer. Biomed Pharmacother. 2018;101:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Wang X, Lan Z, He J, Lai Q, Yao X, Li Q, Liu Y, Lai H, Gu C, Yan Q, Fang Y, Zhang Y, Li A, Liu S. LncRNA SNHG6 promotes chemoresistance through ULK1-induced autophagy by sponging miR-26a-5p in colorectal cancer cells. Cancer Cell Int. 2019;19:234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 15. | Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6253] [Cited by in RCA: 6020] [Article Influence: 501.7] [Reference Citation Analysis (0)] |
| 16. | Arnberg AC, Van Ommen GJ, Grivell LA, Van Bruggen EF, Borst P. Some yeast mitochondrial RNAs are circular. Cell. 1980;19:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 204] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Fan X, Zhang X, Wu X, Guo H, Hu Y, Tang F, Huang Y. Single-cell RNA-seq transcriptome analysis of linear and circular RNAs in mouse preimplantation embryos. Genome Biol. 2015;16:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 300] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 18. | Lukiw WJ. Circular RNA (circRNA) in Alzheimer's disease (AD). Front Genet. 2013;4:307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 361] [Cited by in RCA: 501] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 19. | Bachmayr-Heyda A, Reiner AT, Auer K, Sukhbaatar N, Aust S, Bachleitner-Hofmann T, Mesteri I, Grunt TW, Zeillinger R, Pils D. Correlation of circular RNA abundance with proliferation--exemplified with colorectal and ovarian cancer, idiopathic lung fibrosis, and normal human tissues. Sci Rep. 2015;5:8057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 518] [Cited by in RCA: 609] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 20. | Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4631] [Cited by in RCA: 6044] [Article Influence: 503.7] [Reference Citation Analysis (0)] |
| 21. | Vo JN, Cieslik M, Zhang Y, Shukla S, Xiao L, Wu YM, Dhanasekaran SM, Engelke CG, Cao X, Robinson DR, Nesvizhskii AI, Chinnaiyan AM. The Landscape of Circular RNA in Cancer. Cell. 2019;176:869-881.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1086] [Cited by in RCA: 1181] [Article Influence: 196.8] [Reference Citation Analysis (0)] |
| 22. | Li X, Ding J, Wang X, Cheng Z, Zhu Q. NUDT21 regulates circRNA cyclization and ceRNA crosstalk in hepatocellular carcinoma. Oncogene. 2020;39:891-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 23. | Xu H, Wang C, Song H, Xu Y, Ji G. RNA-Seq profiling of circular RNAs in human colorectal Cancer liver metastasis and the potential biomarkers. Mol Cancer. 2019;18:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 24. | Katona BW, Weiss JM. Chemoprevention of Colorectal Cancer. Gastroenterology. 2020;158:368-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 25. | Sang Y, Chen B, Song X, Li Y, Liang Y, Han D, Zhang N, Zhang H, Liu Y, Chen T, Li C, Wang L, Zhao W, Yang Q. circRNA_0025202 Regulates Tamoxifen Sensitivity and Tumor Progression via Regulating the miR-182-5p/FOXO3a Axis in Breast Cancer. Mol Ther. 2019;27:1638-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 320] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 26. | Yang X, Tian W, Wang S, Ji X, Zhou B. CircRNAs as promising biomarker in diagnostic and prognostic of lung cancer: An updated meta-analysis. Genomics. 2021;113:387-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Huang X, Li Z, Zhang Q, Wang W, Li B, Wang L, Xu Z, Zeng A, Zhang X, He Z, Li Q, Sun G, Wang S, Zhang L, Xu H. Circular RNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer via miR-198 suppression. Mol Cancer. 2019;18:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 312] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 28. | Xu J, Ji L, Liang Y, Wan Z, Zheng W, Song X, Gorshkov K, Sun Q, Lin H, Zheng X, Chen J, Jin RA, Liang X, Cai X. CircRNA-SORE mediates sorafenib resistance in hepatocellular carcinoma by stabilizing YBX1. Signal Transduct Target Ther. 2020;5:298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 307] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 29. | Chen X, Chen RX, Wei WS, Li YH, Feng ZH, Tan L, Chen JW, Yuan GJ, Chen SL, Guo SJ, Xiao KH, Liu ZW, Luo JH, Zhou FJ, Xie D. PRMT5 Circular RNA Promotes Metastasis of Urothelial Carcinoma of the Bladder through Sponging miR-30c to Induce Epithelial-Mesenchymal Transition. Clin Cancer Res. 2018;24:6319-6330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 264] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 30. | Wu Y, Xie Z, Chen J, Ni W, Ma Y, Huang K, Wang G, Wang J, Ma J, Shen S, Fan S. Circular RNA circTADA2A promotes osteosarcoma progression and metastasis by sponging miR-203a-3p and regulating CREB3 expression. Mol Cancer. 2019;18:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 31. | Zhan L, Chen L, Chen Z. Knockdown of FUT3 disrupts the proliferation, migration, tumorigenesis and TGF-β induced EMT in pancreatic cancer cells. Oncol Lett. 2018;16:924-930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | He C, Li A, Lai Q, Ding J, Yan Q, Liu S, Li Q. The DDX39B/FUT3/TGFβR-I axis promotes tumor metastasis and EMT in colorectal cancer. Cell Death Dis. 2021;12:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 33. | Gao HF, Wang QY, Zhang K, Chen LY, Cheng CS, Chen H, Meng ZQ, Zhou SM, Chen Z. Overexpressed N-fucosylation on the cell surface driven by FUT3, 5, and 6 promotes cell motilities in metastatic pancreatic cancer cell lines. Biochem Biophys Res Commun. 2019;511:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Kwon SH, Nedvetsky PI, Mostov KE. Transcriptional profiling identifies TNS4 function in epithelial tubulogenesis. Curr Biol. 2011;21:161-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Seo EY, Jin SP, Kim YK, Lee H, Han S, Lee DH, Chung JH. Integrin-β4-TNS4-Focal Adhesion Kinase Signaling Mediates Keratinocyte Proliferation in Human Skin. J Invest Dermatol. 2017;137:763-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Shi ZZ, Wang WJ, Chen YX, Fan ZW, Xie XF, Yang LY, Chang C, Cai Y, Hao JJ, Wang MR, Bai J. The miR-1224-5p/TNS4/EGFR axis inhibits tumour progression in oesophageal squamous cell carcinoma. Cell Death Dis. 2020;11:597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Kim S, Kim N, Kang K, Kim W, Won J, Cho J. Whole Transcriptome Analysis Identifies TNS4 as a Key Effector of Cetuximab and a Regulator of the Oncogenic Activity of KRAS Mutant Colorectal Cancer Cell Lines. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |