Published online Feb 15, 2022. doi: 10.4251/wjgo.v14.i2.533
Peer-review started: February 22, 2021
First decision: April 19, 2021
Revised: April 27, 2021
Accepted: December 31, 2021
Article in press: December 31, 2021
Published online: February 15, 2022
Processing time: 352 Days and 16.8 Hours
Endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) is emerging as a complementary therapeutic approach for pancreatic solid masses. However, results of published data are difficult to interpret because of a retrospective design and small sample size.
To systematically review data on EUS-RFA for solid lesions and to pool the results of the different experiences in order to provide more consistent evidence in terms of safety and efficacy.
A comprehensive systematic literature search on the main databases was performed to identify articles in which patients with pancreatic solid lesions underwent EUS-RFA. The primary outcomes were procedure-related adverse events (AEs) and mortality. Secondary outcomes were the technical success rate and the effects on primary tumor growth. Statistical analyses were performed using Stata version 14.0.
In total, 14 studies were included, with 120 patients undergoing 153 ablations of 129 solid pancreatic lesions. The STARmed technology was used in seven studies, the Habib system in six studies, and the HybridTherm probe in one study. The pooled technical success rate was 99.0% (I2: 25.82%). The pooled overall AE rate was 8.0% (I2: 11.46%). Excluding mild AEs, the pooled rates of serious AEs was 1.0% (I2: 0%). No mortality related to the procedure was reported.
The present pooled analysis confirms the safety and feasibility of EUS-RFA.
Core Tip: Endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) is emerging as a complementary therapeutic approach for pancreatic solid masses. However, results of published data are difficult to interpret because of a retrospective design and small sample size. Pooling results across 14 published experiences, the technical success rate was 99.0% among over 150 ablations, with an overall adverse event (AE) rate of 8.0%. Excluding mild AEs, the pooled rate of serious AEs was 1.0%, with no mortality. The present pooled analysis confirms the feasibility and safety of EUS-RFA.
- Citation: Spadaccini M, Di Leo M, Iannone A, von den Hoff D, Fugazza A, Galtieri PA, Pellegatta G, Maselli R, Anderloni A, Colombo M, Siersema PD, Carrara S, Repici A. Endoscopic ultrasound-guided ablation of solid pancreatic lesions: A systematic review of early outcomes with pooled analysis. World J Gastrointest Oncol 2022; 14(2): 533-542
- URL: https://www.wjgnet.com/1948-5204/full/v14/i2/533.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i2.533
Endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) is emerging as a complementary therapeutic approach for the multidisciplinary treatment of pancreatic solid masses or as a single treatment in case of patients unfit for any other therapy. It has been proposed for pancreatic lesions such as pancreatic ductal adenocarcinoma, pancreatic neuroendocrine tumors (PNETs), or pancreatic cystic neoplasms[1].
The minimally invasive technique and real-time imaging guidance of a probe inside the tumor make EUS the preferred modality for the minimally invasive treatment of lesions in the pancreas. The mechanism of action of RFA is high-frequency alternating current, generating high local temperatures that induce coagulative necrosis of the tissues[2]. Beside thermal damage (direct effect), RFA has also been suggested to also act with an indirect immune modulatory effect[3,4].
Three ablation devices specifically designed for EUS are currently available. The EUS RFA System (STARMED, Koyang, South Korea) consists of an 18 G needle with a monopolar RFA electrode and a VIVA RF generator (STARmed). The device is perfused internally with circulating chilled saline solution that cools the system during the ablation. The Habib EUS monopolar RFA probe (EMcision Ltd., London, United Kingdom, recently purchased by Boston Scientific) is a 1 Fr wire (0.33 mm) that is passed through a 19 G standard EUS needle and is connected to the RITA electrosurgical RF generator (RITA Medical Systems Inc., Fremont, CA, United States). The HybridTherm bipolar flexible probe (ERBE Elektromedizin, Tübingen, Germany) is a 14 G probe that combines bipolar RF ablation with Cryotechnology. It is used in conjunction with the VIO 300 D electrosurgical unit and the ERBECRYO 3 cryosurgical unit.
Current literature[5] suggests that EUS-RFA is feasible and safe; however, the significance of the majority of the studies is hampered by a small sample size and lack of adequate follow-up. Thus, the aim of this paper was to systematically review data on EUS-RFA for solid lesions and to pool the results of the different experiences in order to have a more reliable point of view.
The methodology used was in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (commonly known as PRISMA) recommendations[6]. Our systematic review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO, www.crd.york.ac.uk/prospero/) on July 2019 (registration No. CRD42020151668).
A comprehensive systematic literature search was performed to identify full-text articles and abstracts in which patients with pancreatic solid lesions underwent EUS–guided RFA. We performed the literature search in PubMed, EMBASE, and SCOPUS (up to June 11, 2019) electronic databases. PROSPERO was searched for ongoing or recently completed systematic reviews. Electronic searches were supplemented by manual searches of references of included studies and review articles. We identified studies using the following medical subject headings (MeSH) and the keywords “pancreas,” “ablation,” and “EUS.” The search was restricted to the English language. The Medline search strategy was: “((Ultrasound-guided[tiab] OR US guided[tiab]) AND (Endoscop*[tiab] OR Endoscopy[Mesh]) OR (EUS[tiab] OR EUS-guided[tiab] OR Endosonography[Mesh] OR Endoscopic ultrasound[tiab])) AND (Pancreat*[tiab] OR Pancreas[tiab] OR Pancreas[Mesh]) AND (Ablati*[tiab] OR Ablation Techniques[Mesh]).”
For the purpose of this systematic review, we included all clinical studies enrolling patients with solid lesions treated by EUS–RFA techniques and reporting the rate of adverse events (AEs) based on the number of procedures. Prospective and retrospective studies, published as full text or presented as abstract at major international meetings (i.e. DDW, UEGW) were considered for inclusion. Relevant studies were also searched from reference lists of all identified studies. To be included, studies had to be written in the English language. Studies enrolling patients treated for either cystic or solid pancreatic lesions were excluded if outcomes of interest were not subgrouped according to lesion type. Case reports were excluded.
Two review authors (Spadaccini M; Von den Hoff D) independently screened the titles and abstracts yielded by the search against the inclusion criteria. Full reports were obtained for all titles that appeared to meet the inclusion criteria or where there was any uncertainty. Then review author pairs screened the full text and abstract reports and decided whether these met the inclusion criteria. Disagreements were resolved through discussion among all authors. The reasons for excluding trials were recorded. The review authors were not blinded to the journal titles or to the study authors or institutions. When there were multiple articles for a single study, we used the latest publication and supplemented it, if necessary, with data from the more complete version.
Using standardized forms, two reviewers (Spadaccini M; Von den Hoff D) extracted data independently and in duplicate from each eligible study. Reviewers resolved disagreements by discussion. Unresolved disagreements were resolved by two arbitrators (Carrara S; Repici A). The following data were extracted from each study including the publication status, study design and location, the number of centers involved, the enrollment period, the number of patients, the number of all lesions ablated, the number of procedures, patient characteristics (mean age, gender), mean lesion size, lesion location (head, body or tail), RF technology and needle used, technical success rate, AEs (bleeding, pancreatitis, portal vein thrombosis, ductal stenosis, abdominal pain, deaths), histology of the ablated lesions (i.e. adenocarcinoma, neuroendocrine tumor, metastasis, solid pseudopapillary tumor), number of patients with follow-up, mean follow-up period, number of patients with symptomatic success, and radiologic response (complete response [CR], partial response [PR], no response).
Quality was assessed by the modified Newcastle-Ottawa Scale for non-randomized studies, ranging from 0 (low quality) to 5 (high quality). Two reviewers (Spadaccini M; Von den Hoff D) assessed quality measures for included studies and discrepancies were adjudicated by collegial discussion.
The primary outcomes were procedure-related AEs and mortality. Secondary outcomes were technical success rate (feasibility), and the effects on primary tumor growth. In patients with hormone-producing neuroendocrine tumors (NETs), the clinical success rate was also assessed. The outcomes definitions were reported below.
AEs: Pancreatitis, bleeding, portal vein thrombosis, burn of the gastric or duodenal wall, and perforations were regarded as AEs. Any intra- or post-procedural AEs were recorded. We considered mild abdominal pain and asymptomatic ascites as “mild” AEs, while the remaining complication were classified as “serious AEs (SAEs).”
Technical success: The procedure was defined as feasible if the placement of the probe inside the target was successful and it was possible to apply the RF energy for a sufficient time.
Effects on primary tumor growth: The assessment of dimensional response was obtained by contrast-enhanced computed tomography, and/or magnetic resonance imaging, and/or contrast-enhanced EUS. Results were classified as CR (disappearance or complete necrosis), PR (significant decrease or partial necrosis), or failure (minimal decrease or no effect).
Clinical success: In patients undergoing EUS-RFA for hormone-producing NET, the procedure was defined as clinically successful if symptoms were completely resolved.
We expressed dichotomous variables as proportion with 95% confidence interval (95%CI) and continuous variables as mean with standard deviation. We calculated pooled estimates with 95%CIs in individual studies using the Freeman-Tukey Double Arcsine Transformation, which stabilizes the variances and allows the inclusion of proportions close to the margins[7]. We selected a random-effects model to summarize data from individual studies. We estimated heterogeneity between studies using the χ2 test (Cochran Q) and I2 statistics[8] . I2 values of 25%, 50%, and 75% were considered low, moderate, and high heterogeneity, respectively. We performed subgroup analyses for the outcomes of success rate, overall AEs and SAEs by publication type and technology used. We estimated differences among subgroups by the Mantel–Haenszel test and heterogeneity in subgroups by the χ2 test (Cochran Q) and I2 statistics. We also planned metaregression analyses to estimate the impact of mean pancreatic lesion size, number of lesions localized in the pancreatic head, and number of adenocarcinoma lesions on the outcomes of SAEs and overall AEs. All statistical analyses and graphics were performed using Stata version 14.0 (College Station, TX, United States) using the Metaprop[9] and Metareg[10] commands. For all calculations, a two-tailed P value < 0.05 was considered statistically significant.
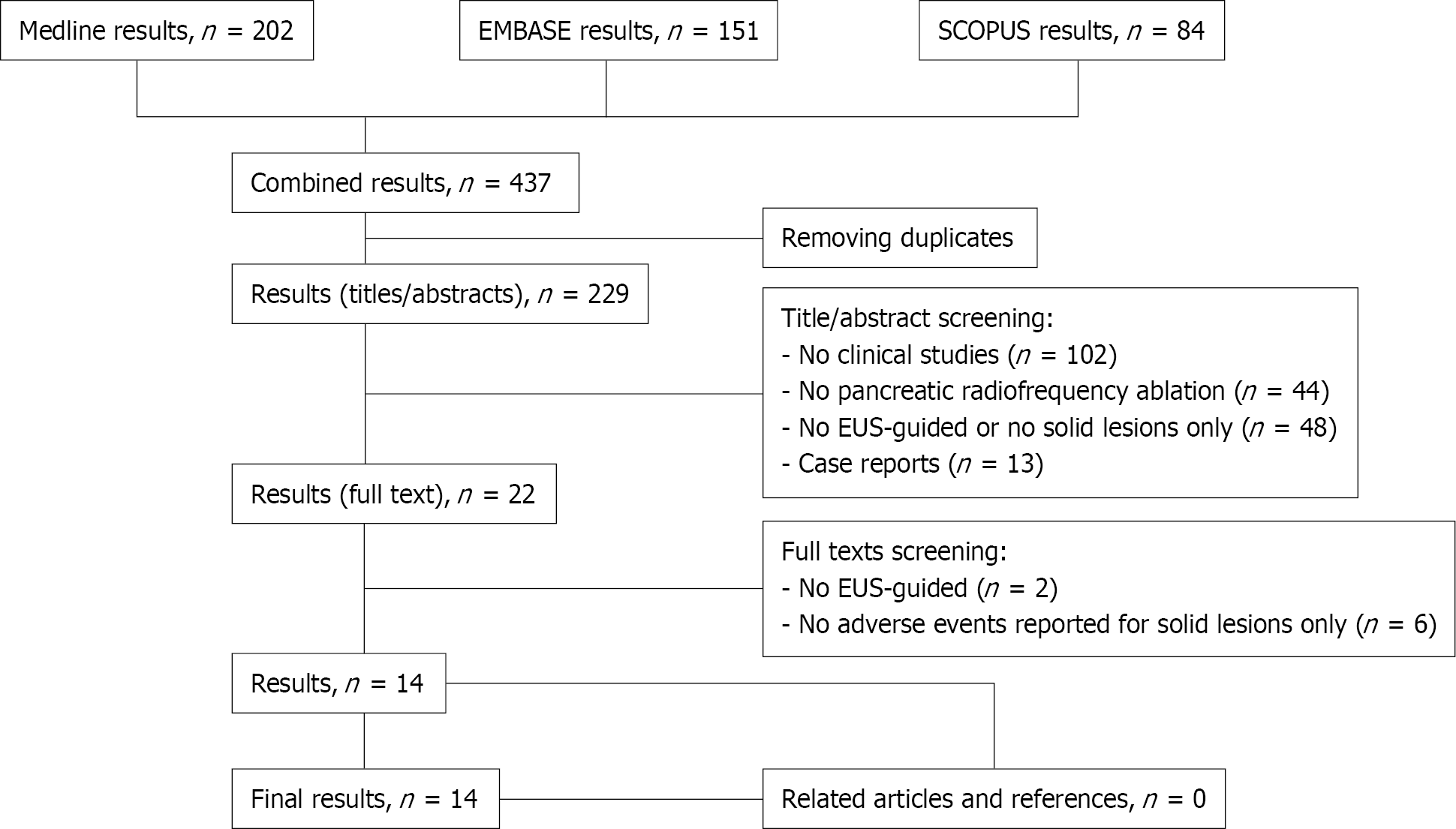
The literature search resulted in 437 articles (Figure 1). After preliminary screening of titles, 22 publications were selected to be reviewed as abstract and full text. Of these, 14 studies[11-24], published between 2012 and 2019, matched the selection criteria and were included in the final analyses (Figure 1). Nine studies (64%) were published as full text, while the remaining five (36%) were presented as abstract at international conferences (UEGW, DDW). Five studies were performed in Europe (54 patients) and the United States (29 patients), three in Asia (19 patients), and the remaining study was from Israel (22 patients). Five studies had a prospective design. All studies but four were single-center experiences. The average Newcastle Ottawa score was 4.7 (range: 3-6). Studies characteristics are summarized in Table 1.
| Author | Publication | Publication year | Country | Design | Centers involved | NOS | Patients, n | Lesions, n | Procedures, n | RF technology |
| Barthet | Full article | 2019 | France | Prospective | Multi | 6 | 12 | 14 | 14 | Starmed |
| Song | Full article | 2016 | Korea | Retrospective | Multi | 4 | 6 | 6 | 6 | Starmed |
| Oleinikov | Full article | 2019 | Israel | Retrospective | Multi | 6 | 18 | 25 | 25 | Starmed |
| Choi | Full article | 2018 | Korea | Prospective | Mono | 6 | 10 | 10 | 16 | Starmed |
| Scopelliti | Full article | 2018 | Italy | Prospective | Mono | 6 | 10 | 10 | 10 | Starmed |
| Crinò | Full article | 2018 | Italy | Retrospective | Mono | 5 | 8 | 8 | 8 | Starmed |
| Lakhtakia | Full article | 2016 | India | Retrospective | Mono | 5 | 3 | 3 | 3 | Starmed |
| Pai | Full article | 2015 | United Kingdom | Prospective | Multi | 5 | 2 | 2 | 3 | Habib |
| Arcidiacono | Full article | 2012 | Italy | Prospective | Mono | 6 | 22 | 22 | 22 | Hybrid-termal |
| Wang | Abstract | 2013 | United States | Retrospective | Mono | 3 | 3 | 3 | 5 | Habib |
| Sharma | Abstract | 2017 | United States | Retrospective | Mono | 3 | 2 | 2 | 2 | Habib |
| Ramireddy | Abstract | 2019 | United States | Retrospective | Mono | 4 | 10 | 10 | 18 | Habib |
| Malikowski | Abstract | 2017 | United States | Retrospective | Mono | 3 | 4 | 4 | 6 | Habib |
| Guider | Abstract | 2018 | United States | Retrospective | Mono | 4 | 10 | 10 | 15 | Habib |
The 14 studies reported outcomes of 120 patients who underwent 153 ablations to treat 129 solid pancreatic lesions. Of all patients, 54.4% were males (56 of 103 patients, from 11 studies) and the mean age was 58.3 ± 9.7 years (provided by 10 studies). The mean lesion size was 24.9 ± 13.3 mm (from 11 studies). Twelve studies reported the location of the treated masse, with the pancreatic head being the most frequent location (66 of 116 lesions, 56.9%), followed by the pancreatic body (39 of 116, 33.6%), and tail (11 of 116, 9.5%). In terms of histology, 68 of 129 (52.7%) lesions were locally advanced adenocarcinomas and 55 of 129 (42.6%) were NETs, of which 15 were functional tumors, 4 (3.1%) were metastatic lesions, and 2 (1.6%) were solid pseudopapillary pancreatic neoplasia. The STARmed technology was used in seven studies (76 lesions), the Habib system in six studies (31 lesions), and the HybridTherm probe in one study (22 lesions). Pre-procedural patients and lesions characteristics of each study are shown in Supplementary Table 1.
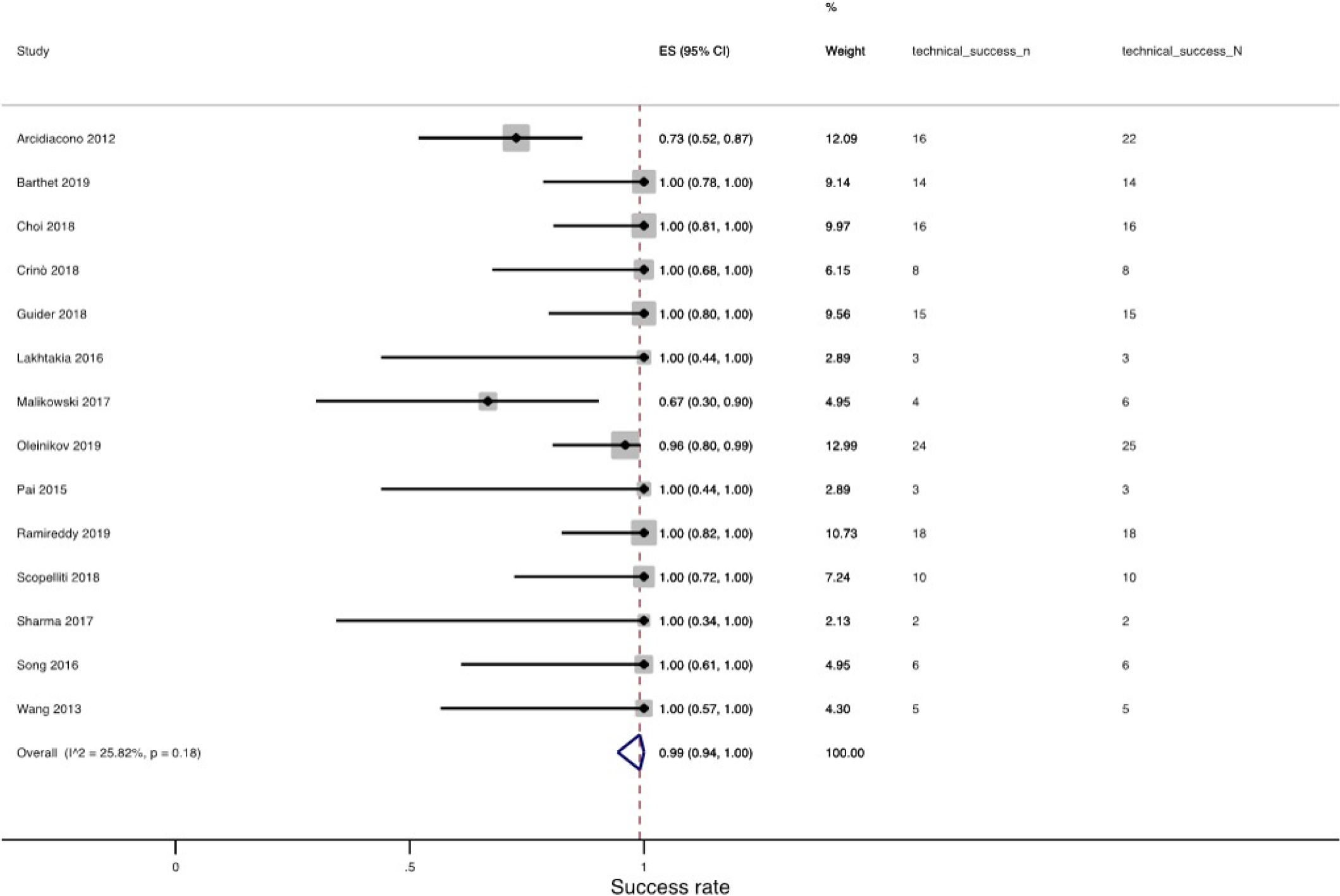
All included studies (14 studies, 120 patients) reported the technical success rate. The procedure was technically successful in 144 of 153 cases. The pooled technical success rate was 99.0% (95%CI: 94.0%-100.0%, I2: 25.82%) (Figure 2). Eleven of fourteen studies (78.6%, 100 procedures) reported a technical success rate of 100%. Arcidiacono et al[11] reported a technical success rate of 72.7% (16/22 procedures) with 6 patients in whom the procedure was not feasible because of stiffness of both the gastrointestinal wall and the tumor[11]. In the study by Oleinikov et al[18], a technically successful RFA ablation was achieved in 96% of lesions (24/25), based on the response rate visualized by EUS immediately after the procedure. A third study by Malikowski et al[17] reported a technically successful procedure in four of six RFAs. We also performed subgroup analysis by publication type and technology as shown in Supplementary Figures 1 and 2, respectively. There was no statistically significant difference between subgroups divided according to publication type (P = 0.91) or type of RFA system (P = 0.54).
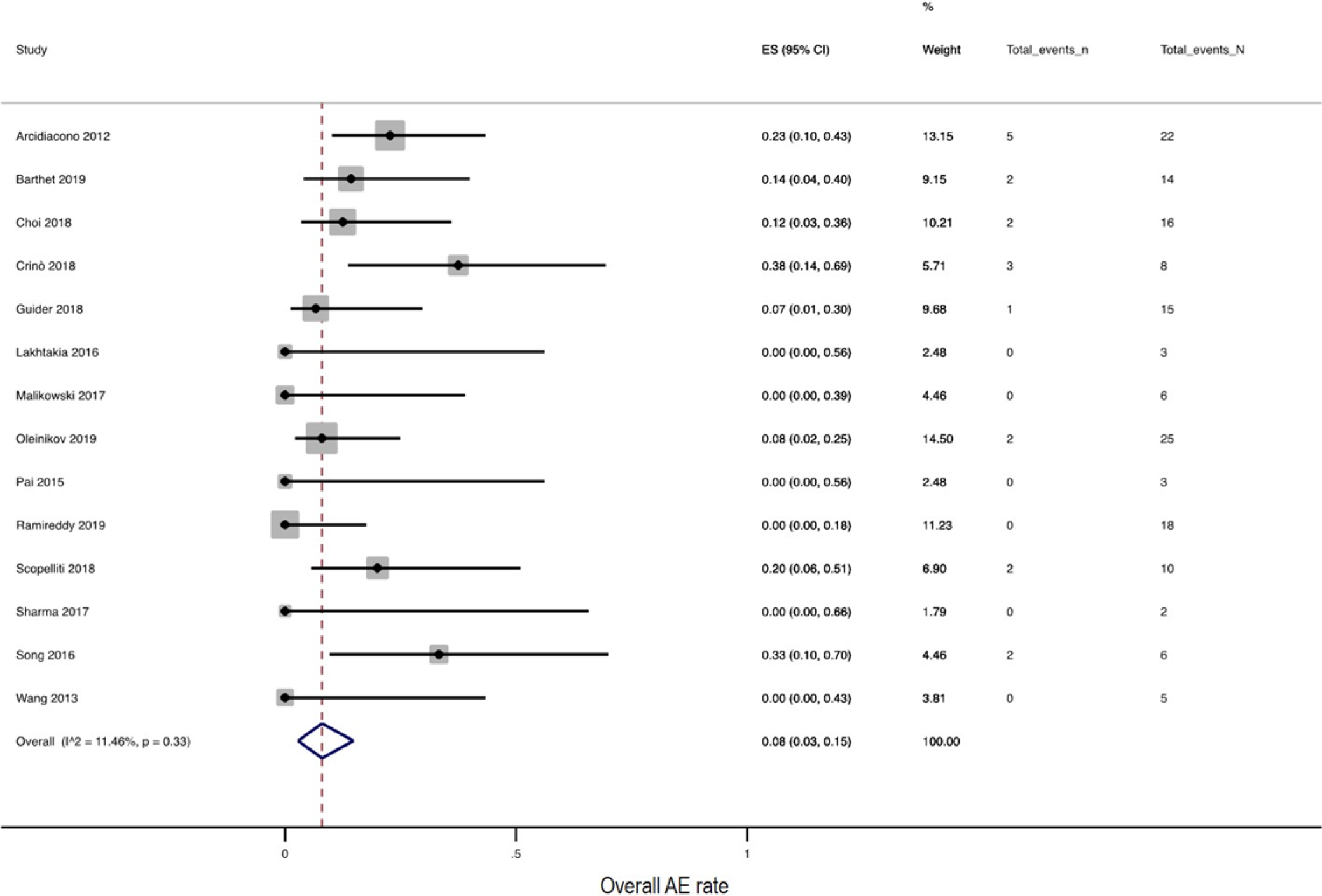
All of the included studies (14 studies, 120 patients) reported AEs related to the procedure. In 19 of 153 procedures, AEs were seen, yielding an overall pooled AE rate of 8.0% (95%CI: 3.0%-15.0%, I2: 11.46) (Figure 3). However, excluding mild AEs such as mild abdominal pain (10 cases) and asymptomatic ascites (2 cases), in 7 of 153 procedures, SAEs were reported, with a pooled SAEs rate of 1.0% (95%CI: 0.0-5.0; I2: 0%). No mortality related to the procedure was reported. Post procedural pancreatitis occurred in 1.0% of cases (5 cases, 95%CI: 0.0-4.0; I2: 0%), and one case of post procedural bleeding and one of pancreatic duct stenosis were reported. Safety outcomes of each study are shown in Supplementary Table 2.
We also performed subgroup analysis by publication type and technology. There was no statistically significant difference between subgroups divided according to publication type (P = 0.39). In the sensitivity analysis for this outcome, considering only studies published as full text articles, 14% (95%CI: 7.0%-23.0%, I2: 0%) of procedures reported AEs (Supplementary Figure 3). However, excluding mild AEs, a pooled rate of 4.0% (95%CI: 0.0-10.0, I2: 0%) was reported (details in Supple
The effect on primary tumor size was reported in nine studies. Across the five studies providing data on ablation of pancreatic NETs, 39 of 45 lesions had a CR, 4 had a PR, and 2 lesions did not respond. In all 15 patients with functional NETs, symptoms were completely resolved after a mean follow-up time ranging from 3 to 13 mo. Considering the four studies reporting outcomes on locally advanced adenocarcinoma or metastasis, 25 of 32 lesions partially responded to ablation and the remaining 7 did not significantly respond. None of these lesions were completely ablated.
To the best of our knowledge, this is the first systematic review and pooled analysis evaluating the impact of EUS-guided tissue ablation for the treatment of solid pancreatic lesions. Here, we analyzed 14 different studies that included 120 patients with a total number of 153 EUS-RFA procedures.
Our study confirmed that EUS-RFA had a high safety profile, with an overall pooled rate of 8.0% AEs. However, the pooled rate decreased to 1% when only SAEs were considered.
Furthermore, the present analysis confirmed the high technical success of EUS-RFA with a pooled technical success rate of 99.0%. Nonetheless, the study also demonstrated the lack of robust clinical data regarding the efficacy of the technique with limited information on long-term outcome. Only nine studies reported data with regard to tumor response, and there was no clear agreement on which imaging technique should be used to confirm the efficacy of the ablation. Moreover, in case of locally advanced adenocarcinoma or metastasis, none of these lesions were completely ablated. Indeed, currently, the absence of SAEs has favored the use of ablation techniques in special clinical settings, such as the local treatment of functioning PNET in patients unfit for surgery. While ablation may be a therapeutic option in these patients to control the overproduction of hormones and resolution of symptoms, its use in small asymptomatic PNETs is questionable. Due to a lack of long-term clinical outcomes, European Neuroendocrine Tumor Society guidelines suggest the use of endoscopic or percutaneous ablative therapy in patients with resectable insulinoma non-fit for surgery (Figure 4), although surgical approach remains the standard cure[25].
Although our systematic literature search is a complete review of all manuscripts published to date, some limitations are present. First, we included different ablation techniques: the STARmed technology in seven studies, the Habib system in six studies and HybridTherm probe in one study. Despite having considered different techniques and different devices, the heterogeneity among the studies was mild to moderate for all outcomes.
Second, all of the included studies are observational studies, which may have inevitably introduced a bias. Thus, the lack of controlled trials shows that the clinical value of local ablation is still insufficiently known. A multicenter randomized controlled trial named Pancreatic Locally Advanced Irresectable Cancer Ablation (PELICAN) (NTR5517) is currently ongoing and will compare chemotherapy and surgical RFA with chemotherapy alone (U.S. National Library of Medicine. Clinical Trials. PELICAN. available online: https://clinicaltrials.gov/ct2/show/NCT03690323). The results are expected in 2022 and will probably provide evidence on the additive role of RFA as part of a multimodality approach to treat patients with locally advanced pancreatic cancer.
Finally, none of the studies published to date have evaluated the indirect effects of RFA. In fact, previous results have indicated that besides thermal damage (debulking cytoreductive effect), RFA also seems to have an indirect immune modulatory effect[26,27]. A peripheral zone with sub-lethal injury is often seen around the area of necrosis created by local injury. In this area, oxidative stress and inflammation have been suggested to lead to an indirect antitumoral systemic effect, generally mediating the immune response. Moreover, the local damage causes the release of tumor antigens that can trigger an immune response and stimulate the inflammatory response, acting on distant circulating neoplastic cells. Thus, the combination of ablation techniques with immunotherapy could potentially increase the efficacy of these kind of treatments, although currently only limited experimental evidence has been published[28].
In conclusion, we conducted the first pooled analysis on EUS-RFA, confirming its emerging role as a complementary therapeutic approach for pancreatic solid masses with limited risk of serious AEs. Further studies with longer follow-up are necessary to properly evaluate the clinical efficacy as a stand-alone approach or as a part of multimodality management of pancreatic neoplasia.
Endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) is emerging as a complementary therapeutic approach for pancreatic solid masses.
Results of published data are difficult to interpret because of a retrospective design and small sample size.
The aim of this paper was to systematically review data on EUS-RFA for solid lesions and to pool the results of the different experiences in order to have a more reliable point of view.
A comprehensive systematic literature search on the main databases was performed to identify articles in which patients with pancreatic solid lesions underwent EUS-RFA.
The pooled technical success rate was 99.0% (I2: 25.82%). The pooled overall adverse event (AE) rate was 8.0% (I2: 11.46%). Excluding mild AEs, the serious AE pooled rate was 1.0% (I2: 0%). No mortality related to the procedure was reported.
The present pooled analysis confirms the safety and feasibility of the EUS-guided RFA.
Further studies with longer follow-up are necessary to properly evaluate the clinical efficacy as a stand-alone approach or as a part of multimodality management of pancreatic neoplasia.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ryozawa S S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | Lakhtakia S, Seo DW. Endoscopic ultrasonography-guided tumor ablation. Dig Endosc. 2017;29:486-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Paiella S, Salvia R, Ramera M, Girelli R, Frigerio I, Giardino A, Allegrini V, Bassi C. Local Ablative Strategies for Ductal Pancreatic Cancer (Radiofrequency Ablation, Irreversible Electroporation): A Review. Gastroenterol Res Pract. 2016;2016:4508376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Haen SP, Pereira PL, Salih HR, Rammensee HG, Gouttefangeas C. More than just tumor destruction: immunomodulation by thermal ablation of cancer. Clin Dev Immunol. 2011;2011:160250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1145] [Cited by in RCA: 1314] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 5. | Signoretti M, Valente R, Repici A, Delle Fave G, Capurso G, Carrara S. Endoscopy-guided ablation of pancreatic lesions: Technical possibilities and clinical outlook. World J Gastrointest Endosc. 2017;9:41-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7463] [Cited by in RCA: 8234] [Article Influence: 823.4] [Reference Citation Analysis (0)] |
| 7. | Freeman MF, Tukey JW. Transformations Related to the Angular and the Square Root. Ann Math Statist. 1950;21:607-611. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1674] [Cited by in RCA: 1682] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 8. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46441] [Article Influence: 2111.0] [Reference Citation Analysis (3)] |
| 9. | Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1058] [Cited by in RCA: 1617] [Article Influence: 147.0] [Reference Citation Analysis (0)] |
| 11. | Arcidiacono PG, Carrara S, Reni M, Petrone MC, Cappio S, Balzano G, Boemo C, Cereda S, Nicoletti R, Enderle MD, Neugebauer A, von Renteln D, Eickhoff A, Testoni PA. Feasibility and safety of EUS-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest Endosc. 2012;76:1142-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Barthet M, Giovannini M, Lesavre N, Boustiere C, Napoleon B, Koch S, Gasmi M, Vanbiervliet G, Gonzalez JM. Endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors and pancreatic cystic neoplasms: a prospective multicenter study. Endoscopy. 2019;51:836-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 13. | Choi JH, Seo DW, Song TJ, Park DH, Lee SS, Lee SK, Kim MH. Endoscopic ultrasound-guided radiofrequency ablation for management of benign solid pancreatic tumors. Endoscopy. 2018;50:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 14. | Crinò SF, D'Onofrio M, Bernardoni L, Frulloni L, Iannelli M, Malleo G, Paiella S, Larghi A, Gabbrielli A. EUS-guided Radiofrequency Ablation (EUS-RFA) of Solid Pancreatic Neoplasm Using an 18-gauge Needle Electrode: Feasibility, Safety, and Technical Success. J Gastrointestin Liver Dis. 2018;27:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 15. | Guider JC, Kannadath BS, Cen P, Rowe J, Wray CJ, Bynon JS, Rahimi EF, DaVee T, Thosani N. Tu1410 Feasibility, safety, and efficacy of endoscopic ultrasound guided radiofrequency ablation (EUS-RFA) of pancreatic ductal adenocarcinoma: A single center us experience. Gastrointestinal Endoscopy. 2018;87:AB585. [DOI] [Full Text] |
| 16. | Lakhtakia S, Ramchandani M, Galasso D, Gupta R, Venugopal S, Kalpala R, Reddy DN. EUS-guided radiofrequency ablation for management of pancreatic insulinoma by using a novel needle electrode (with videos). Gastrointest Endosc. 2016;83:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 17. | Malikowski T, Gleeson FC, Block M, Chari ST, Abu Dayyeh BK, Kendrick ML, Pearson RK, Petersen BT, Smoot R, Truty MJ, Topazian M, Vege SS, Levyet MJ. Mo1268 Endoscopic Ultrasound Guided Radiofrequency Ablation (EUS-RFA): Initial Assessment of Safety and Efficacy. Gastrointestinal Endoscopy. 2017;85:AB484. [DOI] [Full Text] |
| 18. | Oleinikov K, Dancour A, Epshtein J, Benson A, Mazeh H, Tal I, Matalon S, Benbassat CA, Livovsky DM, Goldin E, Gross DJ, Jacob H, Grozinsky-Glasberg S. Endoscopic Ultrasound-Guided Radiofrequency Ablation: A New Therapeutic Approach for Pancreatic Neuroendocrine Tumors. J Clin Endocrinol Metab. 2019;104:2637-2647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 19. | Pai M, Habib N, Senturk H, Lakhtakia S, Reddy N, Cicinnati VR, Kaba I, Beckebaum S, Drymousis P, Kahaleh M, Brugge W. Endoscopic ultrasound guided radiofrequency ablation, for pancreatic cystic neoplasms and neuroendocrine tumors. World J Gastrointest Surg. 2015;7:52-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 167] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 20. | Ramireddy S, Cen P, Rowe J, Wray CJ, Bynon JS, Surabhi V, Patil P, Thosani N. Tu1353 EUS RFA likely offers survival benefits in patients with unresectable pancreatic cancer: results from a single center study with long term follow-up. Gastrointestinal Endoscopy. 2019;89:AB585-AB586. [DOI] [Full Text] |
| 21. | Scopelliti F, Pea A, Conigliaro R, Butturini G, Frigerio I, Regi P, Giardino A, Bertani H, Paini M, Pederzoli P, Girelli R. Technique, safety, and feasibility of EUS-guided radiofrequency ablation in unresectable pancreatic cancer. Surg Endosc. 2018;32:4022-4028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 22. | Song TJ, Seo DW, Lakhtakia S, Reddy N, Oh DW, Park DH, Lee SS, Lee SK, Kim MH. Initial experience of EUS-guided radiofrequency ablation of unresectable pancreatic cancer. Gastrointest Endosc. 2016;83:440-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 23. | Wang D, Jin Z, Lei W, Leung JW, Li Z. Mo1524 Endoscopic Ultrasound Guided Radiofrequency Ablation for the Treatment of Advanced Pancreatic Carcinoma. Gastrointestinal Endoscopy. 2013;77:AB414. [DOI] [Full Text] |
| 24. | Sharma NR, Sharma A, Perelman A, Zelt C. Spare the Whipple: Novel Endoscopic Radiofrequency Ablation Treatment for Insulin Secreting Tumors: 2017 Presidential Poster Award: 2060. American J Gastroenter. 2017;112:S1133-S4. [DOI] [Full Text] |
| 25. | Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G, Reed N, Kianmanesh R, Jensen RT; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103:153-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 985] [Article Influence: 109.4] [Reference Citation Analysis (1)] |
| 26. | Napoletano C, Taurino F, Biffoni M, De Majo A, Coscarella G, Bellati F, Rahimi H, Pauselli S, Pellicciotta I, Burchell JM, Gaspari LA, Ercoli L, Rossi P, Rughetti A. RFA strongly modulates the immune system and anti-tumor immune responses in metastatic liver patients. Int J Oncol. 2008;32:481-490. [PubMed] |
| 27. | Zhao J, Wen X, Tian L, Li T, Xu C, Melancon MP, Gupta S, Shen B, Peng W, Li C. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat Commun. 2019;10:899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 186] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 28. | Lin M, Alnaggar M, Liang S, Wang X, Liang Y, Zhang M, Chen J, Niu L, Xu K. An important discovery on combination of irreversible electroporation and allogeneic natural killer cell immunotherapy for unresectable pancreatic cancer. Oncotarget. 2017;8:101795-101807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |