Published online Feb 15, 2022. doi: 10.4251/wjgo.v14.i2.511
Peer-review started: March 16, 2021
First decision: May 7, 2021
Revised: July 1, 2021
Accepted: December 25, 2022
Article in press: December 25, 2021
Published online: February 15, 2022
Processing time: 331 Days and 13.1 Hours
Chemotherapy has long been shown to confer a survival benefit in patients with metastatic esophageal cancer. However, not all patients with metastatic disease receive chemotherapy.
To evaluate a large cancer database of metastatic esophageal cancer cases to identify predictors of receipt to chemotherapy and survival.
We interrogated the National Cancer Database (NCDB) between 2004-2015 and included patients with M1 disease who had received or did not receive chemotherapy. A logistic regression model was used to examine the associations between chemotherapy and potential confounders and a Cox proportional hazards model was employed to examine the effect of chemotherapy on overall survival (OS). Propensity score analyses were further performed to balance measurable confounders between patients treated with and without chemotherapy.
A total of 29182 patients met criteria for inclusion in this analysis, with 21911 (75%) receiving chemotherapy and 7271 (25%) not receiving chemotherapy. The median follow-up was 69.45 mo. The median OS for patients receiving chemotherapy was 9.53 mo (9.33-9.72) vs 2.43 mo (2.27-2.60) with no chemotherapy. Year of diagnosis 2010-2014 [odds ratio (OR): 1.29, 95% confidence interval (CI): 1.17-1.43, P value < 0.001], median income > $46000 (OR: 1.49, 95%CI: 1.27-1.75, P value < 0.001), and node-positivity (OR: 1.35, 95%CI: 1.20-1.52, P < 0.001) were independent predictors of receiving chemotherapy, while female gender (OR: 0.86, 95%CI: 0.76-0.98, P = 0.019), black race (OR: 0.76, 95%CI: 0.67-0.93, P = 0.005), uninsured status (OR: 0.41, 95%CI: 0.33-0.52, P < 0.001), and high Charlson Comorbidity Index (CCI) (OR for CCI ≥ 2: 0.61, 95%CI: 0.50-0.74, P < 0.001) predicted for lower odds of receiving chemotherapy. Modeling the effect of chemotherapy on OS using a time-dependent coefficient showed that chemotherapy was associated with improved OS up to 10 mo, after which there is no significant effect on OS. Moreover, uninsured status [hazard ratio (HR): 1.20, 95%CI: 1.09-1.31, P < 0.001], being from the geographic Midwest (HR: 1.07, 95%CI: 1.01-1.14, P = 0.032), high CCI (HR for CCI ≥ 2: 1.16, 95%CI: 1.07-1.26, P < 0.001), and higher tumor grade (HR for grade 3 vs grade 1: 1.28, 95%CI: 1.14-1.44, P < 0.001) and higher T stage (HR for T1 vs T4: 0.89, 95%CI: 0.84-0.95, P < 0.001) were independent predictors of worse OS on multivariable analyses.
In this large, retrospective NCDB analysis, we identified several socioeconomic and clinicopathologic predictors for receiving chemotherapy and OS in patients with metastatic esophageal cancer. The benefit of chemotherapy on OS is time-dependent and favors early initiation. Focused outreach in lower income and underinsured patients is critical as receipt of chemotherapy is associated with improved OS.
Core Tip: We evaluated a large cancer database of metastatic esophageal cancer cases to identify predictors of receipt to chemotherapy and survival. We confirmed that although palliative, receipt of chemotherapy in metastatic esophageal cancer conferred an overall survival (OS) benefit over no chemotherapy. However, the benefit of this OS benefit with chemotherapy is time-dependent and favors early initiation. Furthermore, several socioeconomic and clinicopathologic factors were predictive for receipt of chemotherapy and OS in this cohort.
- Citation: Midthun L, Kim S, Hendifar A, Osipov A, Klempner SJ, Chao J, Cho M, Guan M, Placencio-Hickok VR, Gangi A, Burch M, Lin DC, Waters K, Atkins K, Kamrava M, Gong J. Chemotherapy predictors and a time-dependent chemotherapy effect in metastatic esophageal cancer. World J Gastrointest Oncol 2022; 14(2): 511-524
- URL: https://www.wjgnet.com/1948-5204/full/v14/i2/511.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i2.511
Esophageal cancer is the eighth most common cancer throughout the world and the sixth most common global cause of cancer-related mortality[1,2]. In 2020 there will be a projected total of 18440 new cases affecting approximately 14350 men and 4090 women in the United States[3]. This is rising from an estimated 17290 new cases in 2018[4]. In terms of histology, there is an increasing incidence of adenocarcinoma in men and women, reflecting in part the increased rates of obesity-related comorbidities such as gastroesophageal reflux disease in the developed world[1,2,5]. Resec
While palliative chemotherapy has been shown to prolong survival and improve quality of life in stage IV esophageal cancer[6], not all patients receive it. As an example, in one study only 18% of patients with advanced gastroesophageal cancer patients received chemotherapy, with the most common treatment being supportive care alone (21%)[7]. The adverse effects of chemotherapy in metastatic esophageal cancer can be significant, with grade 3-5 toxicity rates as high as 33%-48% with platinum-based doublet regimens[8,9]. Current National Comprehensive Cancer Network (NCCN) guidelines recommend systemic therapy for those with favorable Eastern Cooperative Oncology Group (ECOG) or Karnofsky performance status[10].
Since the decision to offer chemotherapy is individualized to each patient with metastatic esophageal cancer, it is helpful to identify those demographic factors that can impact the receipt of chemotherapy by these patients in the United States. In addition, evaluation of the patient and disease characteristics that affect overall survival (OS) can help identify candidates for chemotherapy who may have poorer prognoses in advanced disease. In this study, we reviewed a large data set of United States patients with metastatic esophageal cancer, seeking to identify predictors for the receipt of chemotherapy and variables affecting OS.
We interrogated the National Cancer Database (NCDB) between 2004-2015 and included patients with stage 4 esophageal cancer (any T + any N + M1 disease) who had known chemotherapy status (received or did not receive chemotherapy). Patients were categorized demographically by age, race, geographic region, treatment site (academic vs non-academic hospital), type of residence (including urban, rural or metropolitan), form of insurance (Medicare, Medicaid, private or other), income bracket and presence of 1 or more other comorbidities according to the Charlson Comorbidity Index (CCI) as modified by Deyo et al[11]. Cancer-based variables included T and N classification as well as histologic grade of tumor.
Data are presented as frequency (percentage, %) for categorical variables and mean ± SD or median (IQR, interquartile range) for continuous variables. The primary endpoint was OS calculated from diagnosis to the date of death or censor at last follow-up. A logistic regression model was employed to estimate the effect of chemotherapy with and without adjustment for potential confounding factors. Median follow-up was calculated using the reverse Kaplan-Meier method[12]. Survival functions were estimated by the Kaplan-Meier method and compared using a log-rank test[13]. Univariate and multivariable survival analyses were carried out using a Cox proportional hazards model[14]. Multivariable analyses were performed using a stepwise variable selection procedure based on Akaike Information Criterion (AIC)[15] while the main predictor variable was forced into the model. Final multivariable models were returned by the lowest AIC value. The proportional hazards assumption was assessed with scaled Schoenfeld residuals[16]. A violation of proportional hazards was addressed by time-dependent coefficient models. Possibility of multicollinearity was assessed by tolerance and the variance inflation factor.
To further balance measurable confounders between patients treated with and without chemotherapy, propensity score was estimated for each patient using a multivariable logistic regression model to predict the receipt of chemotherapy based on patient demographic, clinical, and facility characteristics including age, gender, race, insurance type, income level, treatment site, geographic location, residence area type, number of comorbidity, year of diagnosis, grade, T stage, and N stage[17,18]. Then, the propensity score was incorporated into a Cox regression model in the following four approaches[19]: (1) Regression adjustment by including the estimated propensity score as a covariate in the model; (2) Propensity score was used to calculate stabilized weights (i.e., normalized inverse probability of treatment weighting, IPTW)[20,21], which were then used to weight patients; (3) Patients were stratified into 4 subclasses based on quartiles of the estimated propensity score as recommended in[22,23], and Cox models were employed separately within each stratum to compare OS between patients treated with and without chemotherapy, and then four estimated hazard ratios (HRs) were combined into an overall HR for the entire cohort; and (4) 1:3 ratio optimal matching without replacement, which finds matched patients with the smallest average absolute distance across all the matched pairs[24]. With propensity score stratification and matching approaches, the quality of the estimated propensity scores was evaluated by comparing the distributions of the propensity score between patients treated with and without chemotherapy using box plots within each quartile and histograms for pre- and post-matched samples, respectively. The degree of balance in baseline characteristics between patients treated with and without chemotherapy pre- and post-propensity score adjustment was assessed by calculating the standardized differences[25].
Analyses were performed using SAS 9.4 (SAS Institute, Inc., Cary, North Carolina) and R package version 3.5.3 with two-sided tests at a significance level of 0.05.
A total of 29182 patients met criteria for inclusion in this analysis, with 21911 (75%) receiving chemotherapy and 7271 (25%) not receiving chemotherapy (Table 1). Median age in patients undergoing chemo was 63 years (range 55-71). Median follow-up was 69.45 mo [95% confidence interval (CI): 66.56-72.57] with median OS of 7.16 mo (95%CI: 7.03-7.26) for all patients. In the overall cohort, majority of patients were male (82%) with the most common race being white (84%), followed by black (9.9%) and other racial groups (5.7%). More patients were treated at non-academic sites (54%) vs academic (44%) and treated in the following geographic regions (South 33.1%, Midwest 28.8%, Northeast 23.6%, and West 14.6%). Eighty percent reported living in metropolitan areas, followed by 17.6% in urban and 2.3% in rural communities. Eighty three percent of patients earned more than $30000 annually and 47% reported Medicare as their insurance type. In terms of tumor features, more cases were (60.5%) poorly differentiated (grade 3) and 77% had node-positive disease. A similar breakdown of patient and disease characteristics from the overall cohort was seen in those who did and did not receive chemotherapy (Table 1).
| Variable | All patients (n = 29182) | Chemotherapy received (n = 21911) | No chemo received (n = 7271) |
| Age | |||
| Median (IQR) | 64 (56-73) | 63 (55-71) | 69 (59-79) |
| Gender | |||
| Female | 5126 (17.57) | 3569 (16.29) | 1557 (21.41) |
| Male | 24056 (82.43) | 18342 (83.71) | 5714 (78.59) |
| Race | |||
| Black | 2878 (9.95) | 1936 (8.9) | 942 (13.12) |
| Other | 1669 (5.77) | 1245 (5.72) | 424 (5.9) |
| White | 24389 (84.29) | 18574 (85.38) | 5815 (80.98) |
| Insurance type | |||
| Medicaid | 2415 (8.44) | 1752 (8.14) | 663 (9.36) |
| Medicare | 13412 (46.87) | 9263 (43.02) | 4149 (58.58) |
| Not insured | 1404 (4.91) | 959 (4.45) | 445 (6.28) |
| Other government | 459 (1.6) | 323 (1.5) | 136 (1.92) |
| Private | 10923 (38.17) | 9233 (42.88) | 1690 (23.86) |
| Income quartiles for place of residence | |||
| Less than $30000 | 3918 (13.99) | 2687 (12.79) | 1231 (17.61) |
| $30000-$34999 | 5362 (19.15) | 3942 (18.77) | 1420 (20.31) |
| $35000-$45999 | 8066 (28.81) | 6081 (28.95) | 1985 (28.39) |
| $46000+ | 10652 (38.05) | 8297 (39.5) | 2355 (33.69) |
| Treatment site | |||
| Academic | 12955 (45.07) | 9856 (45.75) | 3099 (43.05) |
| Non-academic | 15786 (54.93) | 11686 (54.25) | 4100 (56.95) |
| Geographic location in United States | |||
| Midwest | 8278 (28.8) | 6388 (29.65) | 1890 (26.25) |
| Northeast | 6772 (23.56) | 5178 (24.04) | 1594 (22.14) |
| South | 9509 (33.09) | 6977 (32.39) | 2532 (35.17) |
| West | 4182 (14.55) | 2999 (13.92) | 1183 (16.43) |
| Residence area type | |||
| Metro | 22465 (80.15) | 16883 (80.25) | 5582 (79.88) |
| Rural | 641 (2.29) | 467 (2.22) | 174 (2.49) |
| Urban | 4921 (17.56) | 3689 (17.53) | 1232 (17.63) |
| Number of comorbidities1 | |||
| 0 | 22009 (75.42) | 17017 (77.66) | 4992 (68.66) |
| 1 | 5401 (18.51) | 3789 (17.29) | 1612 (22.17) |
| ≥ 2 | 1772 (6.07) | 1105 (5.04) | 667 (9.17) |
| Year of diagnosis | |||
| 2004-2009 | 15715 (53.85) | 11588 (52.89) | 4127 (56.76) |
| 2010-2014 | 13467 (46.15) | 10323 (47.11) | 3144 (43.24) |
| Grade2 | |||
| 1 | 633 (2.77) | 475 (2.73) | 158 (2.89) |
| 2 | 7926 (34.65) | 6099 (35.02) | 1827 (33.46) |
| 3 | 13849 (60.54) | 10497 (60.28) | 3352 (61.38) |
| 4 | 467 (2.04) | 343 (1.97) | 124 (2.27) |
| AJCC T stage | |||
| T0 | 51 (0.32) | 31 (0.24) | 20 (0.58) |
| T1 | 2624 (16.26) | 1889 (14.86) | 735 (21.45) |
| T2 | 1751 (10.85) | 1442 (11.35) | 309 (9.02) |
| T3 | 7128 (44.18) | 6105 (48.04) | 1023 (29.86) |
| T4 | 4570 (28.33) | 3233 (25.44) | 1337 (39.03) |
| pIS | 10 (0.06) | 8 (0.06) | 2 (0.06) |
| AJCC N stage | |||
| Negative | 4983 (22.23) | 3562 (20.36) | 1421 (28.86) |
| Positive | 17435 (77.77) | 13933 (79.64) | 3502 (71.14) |
Univariate and multivariable analyses of receipt of chemotherapy are presented in Table 2. Of 29182, 12370 patients with complete data were included in multivariable analyses. In multivariable analysis, older age [odds ratio (OR) 0.95, 95%CI: 0.95-0.96, P < 0.001], black race compared to white race (OR 0.79, 95%CI: 0.67-0.93, P = 0.005) and women were less likely to receive chemotherapy (OR 0.86, 95%CI: 0.76-0.98, P = 0.019). Patients with Medicare (OR 0.84, 95%CI: 0.73-0.97, P = 0.017) or Medicaid insurance (OR 0.52, 95%CI: 0.43-0.64, P < 0.001), along with the uninsured group (OR 0.41, 95%CI: 0.33-0.52, P < 0.001), all had lower likelihood of receiving chemotherapy compared to those with private insurance. Those who were diagnosed recently, between 2010-2014, were more likely to be treated with chemotherapy than those diagnosed between 2004-09 (OR 1.29, 95%CI: 1.17-1.43, P < 0.001). Patients in the Northeast (OR 1.45, 95%CI: 1.22-1.72, P < 0.001), Midwest (OR 1.43, 95%CI: 1.22-1.68, P < 0.001) and Southern regions (OR 1.22, 95%CI: 1.04-1.43, P = 0.015) were all significantly more likely to receive chemotherapy than those in the West. Having higher income quartile (OR for $35000-$45999 1.31, 95%CI: 1.12-1.54, P = 0.001; for more than $46000 1.49, 95%CI: 1.27-1.75, P < 0.001) were more likely associated with receipt of chemotherapy than those with < $30000 income quartile. Besides, on univariate analysis, receiving treatment at an academic site (OR 1.12, 95%CI 1.06-1.18, P < 0.001) was more likely to receive chemotherapy than those treated at non-academic sites.
| Variable | Univariate | Multivariable1 | |||
| n | Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value | |
| Age | 29182 | 0.96 (0.95-0.96) | < 0.001 | 0.95 (0.94-0.96) | < 0.001 |
| Gender | |||||
| Female | 5126 | 0.71 (0.67-0.76) | < 0.001 | 0.86 (0.76-0.98) | 0.019 |
| Male | 24056 | 1 (reference) | 1 (reference) | ||
| Race (combined) | |||||
| Black | 2878 | 0.64 (0.59-0.70) | < 0.001 | 0.79 (0.67-0.93) | 0.005 |
| Other | 1669 | 0.92 (0.82-1.03) | 0.148 | 1.12 (0.89-1.40) | 0.322 |
| White | 24389 | 1 (reference) | 1 (reference) | ||
| Insurance type | |||||
| Medicaid | 2415 | 0.48 (0.44-0.54) | < 0.001 | 0.52 (0.43-0.64) | < 0.001 |
| Medicare | 13412 | 0.41 (0.38-0.44) | < 0.001 | 0.84 (0.73-0.97) | 0.017 |
| Other government | 459 | 0.43 (0.35-0.53) | < 0.001 | 0.74 (0.50-1.09) | 0.123 |
| Not insured | 1404 | 0.39 (0.35-0.45) | < 0.001 | 0.41 (0.33-0.52) | < 0.001 |
| Private | 10923 | 1 (reference) | 1 (reference) | ||
| Income quartiles for place of residence | |||||
| Less than $30000 | 3918 | 1 (reference) | 1 (reference) | ||
| $30000-$34999 | 5362 | 1.27 (1.16-1.39) | < 0.001 | 1.12 (0.95-1.33) | 0.172 |
| $35000-$45999 | 8066 | 1.40 (1.29-1.53) | < 0.001 | 1.31 (1.12-1.54) | 0.001 |
| $46000+ | 10652 | 1.61 (1.49-1.75) | < 0.001 | 1.49 (1.27-1.75) | < 0.001 |
| Treatment site | |||||
| Academic | 12955 | 1.12 (1.06-1.18) | < 0.001 | Dropped out of the model | |
| Non-academic | 15786 | 1 (reference) | |||
| Geographic location in United States | |||||
| Northeast | 6772 | 1.28 (1.17-1.40) | < 0.001 | 1.45 (1.22-1.72) | < 0.001 |
| Midwest | 8278 | 1.33 (1.23-1.45) | < 0.001 | 1.43 (1.22-1.68) | < 0.001 |
| South | 9509 | 1.09 (1.00-1.18) | 0.044 | 1.22 (1.04-1.43) | 0.015 |
| West | 4182 | 1 (reference) | 1 (reference) | ||
| Residence area type | |||||
| Metro | 22465 | 1.13 (0.94-1.34) | 0.185 | Dropped out of the model | |
| Urban | 4921 | 1.12 (0.93-1.34) | 0.248 | ||
| Rural | 641 | 1 (reference) | |||
| Number of comorbidities2 | |||||
| 1 | 5401 | 0.69 (0.65-0.74) | < 0.001 | 0.79 (0.70-0.90) | < 0.001 |
| ≥ 2 | 1772 | 0.49 (0.44-0.54) | < 0.001 | 0.61 (0.50-0.74) | < 0.001 |
| 0 | 22009 | 1 (reference) | 1 (reference) | ||
| Year of diagnosis | |||||
| 2010-2014 | 13467 | 1.17 (1.11-1.23) | < 0.001 | 1.29 (1.17-1.43) | < 0.001 |
| 2004-2009 | 15715 | 1 (reference) | 1 (reference) | ||
| Grade3 | |||||
| 1 | 633 | 1 (reference) | 1 (reference) | ||
| 2 | 7926 | 1.11 (0.92-1.34) | 0.273 | 1.08 (0.78-1.48) | 0.648 |
| 3 | 13849 | 1.04 (0.87-1.25) | 0.664 | 0.96 (0.70-1.31) | 0.786 |
| 4 | 467 | 0.92 (0.70-1.21) | 0.550 | 0.69 (0.44-1.08) | 0.105 |
| AJCC T stage | |||||
| pIS | 10 | 1.65 (0.35-7.80) | 0.525 | 20075.87 (0.00-NA) | 0.948 |
| T0 | 51 | 0.64 (0.36-1.13) | 0.123 | 0.63 (0.29-1.40) | 0.261 |
| T1 | 2624 | 1.06 (0.96-1.18) | 0.262 | 1.10 (0.96-1.27) | 0.181 |
| T2 | 1751 | 1.93 (1.68-2.22) | < 0.001 | 1.87 (1.56-2.25) | < 0.001 |
| T3 | 7128 | 2.47 (2.25-2.71) | < 0.001 | 2.29 (2.03-2.59) | < 0.001 |
| T4 | 4570 | 1 (reference) | 1 (reference) | ||
| AJCC N stage | |||||
| Positive | 17435 | 1.59 (1.48-1.71) | < 0.001 | 1.35 (1.20-1.52) | < 0.001 |
| Negative | 4983 | 1 (reference) | 1 (reference) |
A total of 12370 patients with metastatic esophageal cancer were included in the multivariable analyses of OS (Table 3). Baseline characteristics of these 12370 patients did not largely differ from the overall population (Supplementary Table 1). The median follow-up was 72.8 mo (95%CI: 68.5-77.9) and the median OS was 7.95 mo (95%CI: 7.75-8.11) in this cohort. Here, women had better OS than men (HR 0.9, 95%CI: 0.86-0.95, P < 0.001), while survival of black patients was not significantly different than that of whites (HR 1.02, 95%CI: 0.96-1.09, P = 0.457), but patients in other racial/ethnic groups had significantly better OS than whites (HR 0.87, 95%CI: 0.80-0.95, P = 0.002). Uninsured patients (HR 1.2, 95%CI: 1.09-1.31, P < 0.001) and those with Medicaid (HR 1.21, 95%CI: 1.13-1.31, P < 0.001) had worse OS than those with private insurance. Receiving treatment at an academic center (HR 0.91, 95%CI: 0.87-0.94, P < 0.001) was associated with improved survival. OS did not differ significantly among geographic subgroups, with the exception of patients in the Midwest, who had slightly worse survival (HR 1.07, 95%CI: 1.01-1.14, P = 0.032) when compared to those located in the geographic West.
| Variable | Univariate | Multivariable1 | ||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| Chemotherapy | ||||
| Chemotherapy received vs not received | ||||
| If 0 < time ≤ 10 mo2 | - | < 0.001 | - | < 0.001 |
| If time > 10 mo | 0.95 (0.88-1.03) | 0.204 | 0.98 (0.86-1.11) | 0.699 |
| Age | 1.01 (1.01-1.01) | < 0.001 | 1.01 (1.00-1.01) | < 0.001 |
| Gender | ||||
| Female | 0.99 (0.96-1.02) | 0.420 | 0.90 (0.86-0.95) | < 0.001 |
| Male | 1 (reference) | 1 (reference) | ||
| Race (combined) | ||||
| Black | 1.09 (1.05-1.14) | < 0.001 | 1.02 (0.96-1.09) | 0.457 |
| Other | 0.86 (0.82-0.91) | < 0.001 | 0.87 (0.80-0.95) | 0.002 |
| White | 1 (reference) | 1 (reference) | ||
| Insurance type | ||||
| Medicaid | 1.28 (1.22-1.34) | < 0.001 | 1.21 (1.13-1.31) | < 0.001 |
| Medicare | 1.30 (1.26-1.33) | < 0.001 | 1.04 (0.99-1.10) | 0.119 |
| Other government | 1.15 (1.04-1.26) | 0.006 | 0.95 (0.82-1.10) | 0.493 |
| Not insured | 1.37 (1.29-1.45) | < 0.001 | 1.20 (1.09-1.31) | < 0.001 |
| Private | 1 (reference) | 1 (reference) | ||
| Income quartiles for place of residence | ||||
| $30000-$34999 | 0.94 (0.90-0.98) | 0.007 | Dropped out of the model | |
| $35000-$45999 | 0.93 (0.90-0.97) | < 0.001 | ||
| $46000+ | 0.87 (0.83-0.90) | < 0.001 | ||
| Less than $30000 | 1 (reference) | |||
| Treatment site | ||||
| Academic | 0.87 (0.85-0.89) | < 0.001 | 0.91 (0.87-0.94) | < 0.001 |
| Non-academic | 1 (reference) | 1 (reference) | ||
| Geographic location in United States | ||||
| Northeast | 0.92 (0.89-0.96) | < 0.001 | 0.96 (0.90-1.02) | 0.180 |
| Midwest | 1.02 (0.98-1.06) | 0.340 | 1.07 (1.01-1.14) | 0.032 |
| South | 1.03 (0.99-1.07) | 0.142 | 1.05 (0.99-1.11) | 0.138 |
| West | 1 (reference) | 1 (reference) | ||
| Residence area type | ||||
| Metro | 0.93 (0.86-1.01) | 0.086 | Dropped out of the model | |
| Urban | 0.95 (0.87-1.03) | 0.193 | ||
| Rural | 1 (Reference) | |||
| Number of comorbidities | ||||
| 1 | 1.20 (1.17-1.24) | < 0.001 | 1.09 (1.04-1.14) | < 0.001 |
| ≥ 2 | 1.45 (1.37-1.52) | < 0.001 | 1.16 (1.07-1.26) | < 0.001 |
| 0 | 1 (reference) | 1 (reference) | ||
| Year of diagnosis | ||||
| 2010-2014 | 0.99 (0.96-1.01) | 0.286 | Dropped out of the model | |
| 2004-2009 | 1 (Reference) | |||
| Grade | ||||
| 2 | 1.08 (1.00-1.18) | 0.065 | 1.06 (0.95-1.20) | 0.297 |
| 3 | 1.30 (1.20-1.41) | < 0.001 | 1.28 (1.14-1.44) | < 0.001 |
| 4 | 1.30 (1.15-1.48) | < 0.001 | 1.21 (1.02-1.45) | 0.032 |
| 1 | 1 (reference) | 1 (reference) | ||
| AJCC T stage | ||||
| pIS | 0.57 (0.30-1.09) | 0.090 | 1.11 (0.46-2.66) | 0.821 |
| T0 | 0.94 (0.69-1.26) | 0.665 | 0.86 (0.59-1.25) | 0.424 |
| T1 | 0.88 (0.84-0.93) | < 0.001 | 0.89 (0.84-0.95) | < 0.001 |
| T2 | 0.66 (0.62-0.69) | < 0.001 | 0.69 (0.65-0.74) | < 0.001 |
| T3 | 0.65 (0.63-0.68) | < 0.001 | 0.73 (0.70-0.76) | < 0.001 |
| T4 | 1 (reference) | 1 (reference) | ||
| AJCC N stage | ||||
| Positive | 0.94 (0.91-0.97) | < 0.001 | Dropped out of the model | |
| Negative | 1 (reference) |
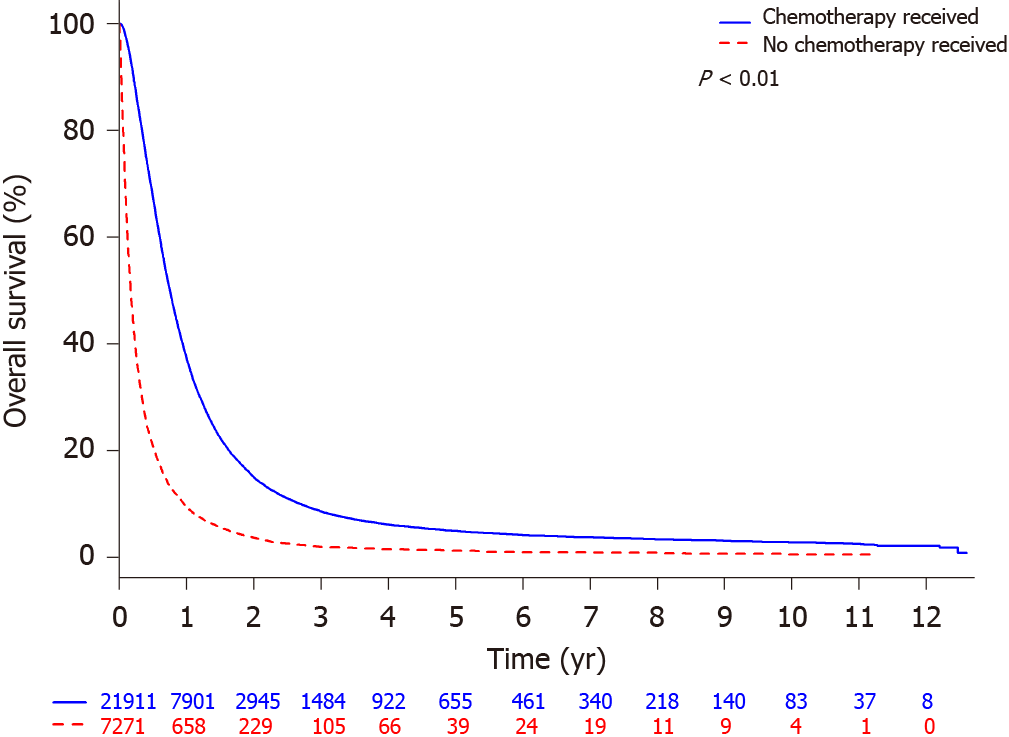
OS was higher for those receiving chemotherapy with median OS of 9.03 mo (95%CI: 8.90-9.20) than those who did not receive chemotherapy with median OS of 2.07 mo (95%CI: 2-2.14; Figure 1). Out of 21911 patients that received chemotherapy, the estimated 1-year OS rate was 37.4% (95%CI: 36.7-38.0) compared to 9.7% (95%CI: 9.0-10.4) for the 7271 patients who did not received chemotherapy.
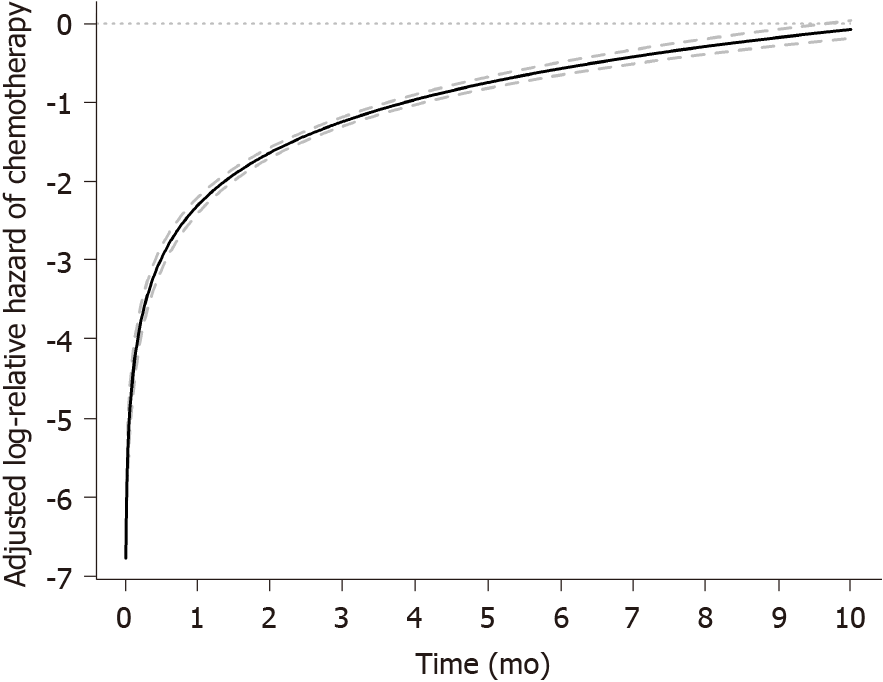
Modeling the effect of chemotherapy on OS using a time-dependent coefficient showed that the receipt of chemotherapy was associated with improved OS up to 10 mo while its benefit decreases over time, after which there was no significant chemotherapy effect on OS on both univariate and multivariable analyses (Table 3 and Figure 2). Propensity score-adjusted log relative HR for chemotherapy compared with no chemotherapy showed that propensity score-adjusted analysis results are consistent with findings from multivariable analyses whereby the effect of chemotherapy on OS similarly varied with time from diagnosis and is associated with improved OS up until 10 mo, after which there is no chemotherapy effect on OS (Supplementary Figure 1).
In this retrospective, analysis of a large NCDB dataset of metastatic esophageal cancer patients, we first demonstrated that chemotherapy, although palliative, does improve survival (median OS 9.0 mo) compared to those who do not receive chemotherapy (2 mo), which is consistent with canonical data that have structured our framework of how we treat this disease in the systemic setting[6]. Our median OS in the chemotherapy cohort is comparable to the median OS in the chemotherapy control arms of modern phase III trials in advanced gastroesophageal cancer[26].
However, not all patients with metastatic esophageal cancer can receive chemotherapy[7] and we sought to explore patient and disease factors that predicted likelihood of receipt to chemotherapy on multivariable analyses of 10799 metastatic esophageal cancer cases (Table 2). Older age, female gender, black race, not having private insurance, lower income quartiles, geographic location West, greater number of comorbidities (CCI ≥ 1), higher T stage, and node negative were significantly associated with a decreased likelihood of receiving chemotherapy in patients with stage IV esophageal cancer.
To identify at-risk patient subgroups and clinicopathologic characteristics with poorer mortality in the setting of metastatic esophageal cancer, we also performed multivariable analyses of the same variables but now in association with OS in our 12370 patient cohort (Table 3). Factors that were associated with poor OS included older age, male gender, white race compared to other races, being uninsured or having Medicaid compared to private insurance, non-academic site, residing in the geographic Midwest, having a CCI ≥ 1, higher tumor grade, and higher T stage.
Our findings are largely consistent with population-based studies identifying racial, gender and socioeconomic disparities in treatment and mortality rates among United States patients with esophageal cancer. In terms of gender, male gender has historically been associated with more advanced disease and poorer survival in esophageal cancer[27]. Female gender was an independent predictor of improved OS in our cohort, but it remains unclear why female gender was an independent predictor of lower likelihood for chemotherapy receipt. This might reflect the lower incidence and prevalence of esophageal cancer in women, however the gender gap is closing[2].
Those in lower socioeconomic status (SES) brackets are also less likely to receive optimal treatment for esophageal cancer. This has been attributed to a variety of factors including education, perceived lack of confidence in healthcare providers, financial strain and fear of losing employment, and minimizing time spent in healthcare settings even in the face of life-threatening illness[28]. Patients of lower SES with stage IV cancer of any kind are less likely to participate in clinical trials[29]. Those who are uninsured or have Medical (as opposed to Medicaid) tend to present for treatment at a later stage and, once they do, have lower chances of receiving mul
Historically, patients of black race with esophageal cancer have been described to have significantly worse survival than white patients with respect to esophageal cancer-related death and lower probability of receiving cancer therapy[31]. Others have reported that black patients were significantly less likely to undergo esophagectomy for potentially curable disease[32]. However, there is data to suggest that once patients receive an esophagectomy, OS is no longer dependent on race[5]. Reassuringly, on multivariable analysis when adjusted for other variables, we found that the OS of patients of black race was not significantly different from those of white race with metastatic esophageal cancer. This finding is consistent with growing evidence in the literature showing that when controlled for other factors, SES remains the most significant contributor to survival for esophageal cancer patients[27,33].
Our findings suggest that several patient and disease factors independently predict for likelihood of receiving chemotherapy and OS in stage IV esophageal cancer. Importantly, many of these predictors are socioeconomic-related factors that underscore the urgent need for further study to better identify and address the multilevel disparities that we have shown which can significantly impact likelihood of receiving chemotherapy and survival in metastatic esophageal cancer.
Chemotherapy significantly improved OS in our cohort, but the magnitude of this benefit decreased over time and was not seen past 10 mo of treatment when the chemotherapy effect was modeled as a time-dependent coefficient and plotted as the adjusted HR of chemotherapy vs no chemotherapy from time of diagnosis (Table 3 and Figure 2). Other groups have described a median first symptom onset to treatment delay for esophageal cancer of 2.1 mo (range 0.5 to 24) with a significantly shorter symptom-to-treatment delay for stage I-II than stage III-IV esophageal cancer (P = 0.0177)[34]. In early-stage esophageal cancer, a longer hospital delay between diagnosis and surgery resulted in worse short-term outcomes but did not affect long-term outcomes such as OS[35]. Our novel findings suggest that the magnitude of benefit of chemotherapy is potentially greatest with early initiation of chemotherapy, as the benefit decreases over time. When tied to our earlier findings on impact of SES to receipt of chemotherapy and survival, it would be prudent to develop strategies to improve access to timely therapy for patients with at-risk SES including the underinsured as these factors have been shown to be associated with healthcare delays and treatment in esophageal cancer[36].
In this large, retrospective analysis of metastatic esophageal cancer patients, we identified a survival benefit for chemotherapy that decreases over time and not seen beyond 10 mo from time of diagnosis. We also identified several clinicopathologic and socioeconomic factors associated with likelihood of receiving chemotherapy and survival in metastatic esophageal cancer. Together, these findings point to the need for early initiation of chemotherapy and increased multidisciplinary efforts to identify and address disparities that can adversely affect patient access chemotherapy and the survival benefits it can confer in metastatic esophageal cancer.
Palliative chemotherapy has long been known to improve overall survival (OS) in metastatic esophageal cancer, but not all patients with advanced disease receive chemotherapy.
As not all patients with metastatic esophageal cancer are able to receive the benefits of chemotherapy, we evaluated a large cancer database of metastatic esophageal cancer cases to better understand predictors of chemotherapy and survival.
The objectives of this study were to investigate the patient and disease characteristics associated with receipt of palliative chemotherapy in metastatic esophageal cancer. We evaluated the impact of chemotherapy on OS compared to no chemotherapy in our cohort. We also investigated independent predictors of OS on multivariable analyses. Lastly, we investigated whether the effect of chemotherapy on OS in metastatic esophageal cancer patients was time dependent.
We identified cases of M1 esophageal cancer in the National Cancer Database (NCDB) between 2004-2015 who had received or did not receive chemotherapy. A logistic regression model was used to examine the associations between chemotherapy and patient factors, and a Cox proportional hazards model was employed to examine the effect of chemotherapy on OS.
We included 21911 (75%) metastatic esophageal cancer cases receiving chemotherapy and 7271 (25%) not receiving chemotherapy with a median follow-up of 69.45 mo. Several factors were independent predictors of chemotherapy including year of diagnosis 2010-2014, median income > $46000, and node-positivity, while female gender, black race, uninsured status, and high Charlson Comorbidity Index predicted for lower odds of receiving chemotherapy. Although the median OS for patients receiving chemotherapy was 9.53 mo (9.33-9.72) vs 2.43 mo (2.27-2.60) with no chemotherapy, modeling the effect of chemotherapy on OS using a time-dependent coefficient showed that chemotherapy was associated with improved OS up to 10 mo, after which there is no significant effect on OS.
Palliative chemotherapy confers a significant OS benefit in those with metastatic esophageal cancer. However, the benefit of chemotherapy in this setting is time-dependent and emphasizes the importance of early initiation of chemotherapy.
Several socioeconomic and clinicopathologic predictors for receiving chemotherapy and OS exist in patients with metastatic esophageal cancer. Future studies should focus on outreach in lower income and underinsured patients to improve receipt of chemotherapy, which is associated with improved OS when initiated in a timely fashion.
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ma J S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Abbas G, Krasna M. Overview of esophageal cancer. Ann Cardiothorac Surg. 2017;6:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 2. | Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598-5606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 736] [Cited by in RCA: 747] [Article Influence: 62.3] [Reference Citation Analysis (8)] |
| 3. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15320] [Article Influence: 3064.0] [Reference Citation Analysis (4)] |
| 4. | Patel N, Benipal B. Incidence of Esophageal Cancer in the United States from 2001-2015: A United States Cancer Statistics Analysis of 50 States. Cureus. 2018;10:e3709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Nassri A, Zhu H, Muftah M, Ramzan Z. Epidemiology and Survival of Esophageal Cancer Patients in an American Cohort. Cureus. 2018;10:e2507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | van Rossum PSN, Mohammad NH, Vleggaar FP, van Hillegersberg R. Treatment for unresectable or metastatic oesophageal cancer: current evidence and trends. Nat Rev Gastroenterol Hepatol. 2018;15:235-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 7. | Opstelten JL, de Wijkerslooth LR, Leenders M, Bac DJ, Brink MA, Loffeld BC, Meijnen-Bult MJ, Minderhoud IM, Verhagen MA, van Oijen MG, Siersema PD. Variation in palliative care of esophageal cancer in clinical practice: factors associated with treatment decisions. Dis Esophagus. 2017;30:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Baumgartner R, Taghizadeh H, Jomrich G, Schoppmann SF, Preusser M, Ilhan-Mutlu A. Utilization and Efficacy of Palliative Chemotherapy for Locally Advanced or Metastatic Gastroesophageal Carcinoma. Anticancer Res. 2020;40:965-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Dijksterhuis WPM, Verhoeven RHA, Slingerland M, Haj Mohammad N, de Vos-Geelen J, Beerepoot LV, van Voorthuizen T, Creemers GJ, van Oijen MGH, van Laarhoven HWM. Heterogeneity of first-line palliative systemic treatment in synchronous metastatic esophagogastric cancer patients: A real-world evidence study. Int J Cancer. 2020;146:1889-1901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | National Comprehensive Cancer Network. Esophageal and Esophagogastric Junction Cancers. NCCN Guidelines. [cited 9 October 2020]. In: National Comprehensive Cancer Network [Internet]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf. |
| 11. | Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7532] [Cited by in RCA: 8646] [Article Influence: 262.0] [Reference Citation Analysis (0)] |
| 12. | Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 2149] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 13. | Kalbfleisch J, Prentice R. The statistical analysis of failure time data. Wiley series in probability and mathematical statistics. New York: John Wiley & Sons, 1980. |
| 14. | Cox D. Regression Models and Life Tables. J Royal Stat Society. 1972;B34:187-220. |
| 15. | Yamashita T, Yamashita K, Kamimura R. A Stepwise AIC Method for Variable Selection in Linear Regression. Commun Stat Theory Methods. 2007;36. |
| 16. | Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515-526. |
| 17. | D'Agostino RB Jr, D'Agostino RB Sr. Estimating treatment effects using observational data. JAMA. 2007;297:314-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 260] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 18. | PR R, DB R. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41-55. |
| 19. | Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46:399-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6382] [Cited by in RCA: 7530] [Article Influence: 537.9] [Reference Citation Analysis (0)] |
| 20. | Lunceford JK, Davidian M. Stratification and weighting via the propensity score in estimation of causal treatment effects: a comparative study. Stat Med. 2004;23:2937-2960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 921] [Cited by in RCA: 906] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 21. | Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3270] [Cited by in RCA: 3539] [Article Influence: 141.6] [Reference Citation Analysis (0)] |
| 22. | Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516-524. |
| 23. | Wg C. The effectiveness of adjustment by subclassification in removing bias in observational studies. Biometrics. 1968;24:295-313. |
| 24. | Gu X, Rosenbaum P. Comparison of multivariate matching methods: Structures, distances, and algorithms. J Comput Graph Stat. 1993;2:405-420. |
| 25. | Austin PC. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Making. 2009;29:661-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 319] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 26. | Kato K, Sun JM, Shah MA, Enzinger PC, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, Cho BCC, Mansoor W, Li SH, Sunpaweravong P, Maqueda MA, Goekkurt E, Liu Q, Shah S, Bhagia P, Shen L. LBA8_PR Pembrolizumab plus chemotherapy vs chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase 3 KEYNOTE-590 study. Ann Oncol. 2020;31:S1192-S1193. |
| 27. | Tran PN, Taylor TH, Klempner SJ, Zell JA. The impact of gender, race, socioeconomic status, and treatment on outcomes in esophageal cancer: A population-based analysis. J Carcinog. 2017;16:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Lineback CM, Mervak CM, Revels SL, Kemp MT, Reddy RM. Barriers to Accessing Optimal Esophageal Cancer Care for Socioeconomically Disadvantaged Patients. Ann Thorac Surg. 2017;103:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Jimenez R, Zhang B, Joffe S, Nilsson M, Rivera L, Mutchler J, Lathan C, Paulk ME, Prigerson HG. Clinical trial participation among ethnic/racial minority and majority patients with advanced cancer: what factors most influence enrollment? J Palliat Med. 2013;16:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Walker GV, Grant SR, Guadagnolo BA, Hoffman KE, Smith BD, Koshy M, Allen PK, Mahmood U. Disparities in stage at diagnosis, treatment, and survival in nonelderly adult patients with cancer according to insurance status. J Clin Oncol. 2014;32:3118-3125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 248] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 31. | Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, McGinn TG, Wisnivesky JP. Racial disparities in esophageal cancer treatment and outcomes. Ann Surg Oncol. 2008;15:881-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Revels SL, Morris AM, Reddy RM, Akateh C, Wong SL. Racial disparities in esophageal cancer outcomes. Ann Surg Oncol. 2013;20:1136-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Erhunmwunsee L, Gulack BC, Rushing C, Niedzwiecki D, Berry MF, Hartwig MG. Socioeconomic Status, Not Race, Is Associated With Reduced Survival in Esophagectomy Patients. Ann Thorac Surg. 2017;104:234-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Wang J, Liu F, Gao H, Wei W, Zhang X, Liang Y, Cheng Y. The symptom-to-treatment delay and stage at the time of treatment in cancer of esophagus. Jpn J Clin Oncol. 2008;38:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Grotenhuis BA, van Hagen P, Wijnhoven BP, Spaander MC, Tilanus HW, van Lanschot JJ. Delay in diagnostic workup and treatment of esophageal cancer. J Gastrointest Surg. 2010;14:476-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Wang N, Cao F, Liu F, Jia Y, Wang J, Bao C, Wang X, Song Q, Tan B, Cheng Y. The effect of socioeconomic status on health-care delay and treatment of esophageal cancer. J Transl Med. 2015;13:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |