Published online Feb 15, 2022. doi: 10.4251/wjgo.v14.i2.498
Peer-review started: August 12, 2021
First decision: September 4, 2021
Revised: September 6, 2021
Accepted: January 14, 2022
Article in press: January 14, 2022
Published online: February 15, 2022
Processing time: 182 Days and 5.1 Hours
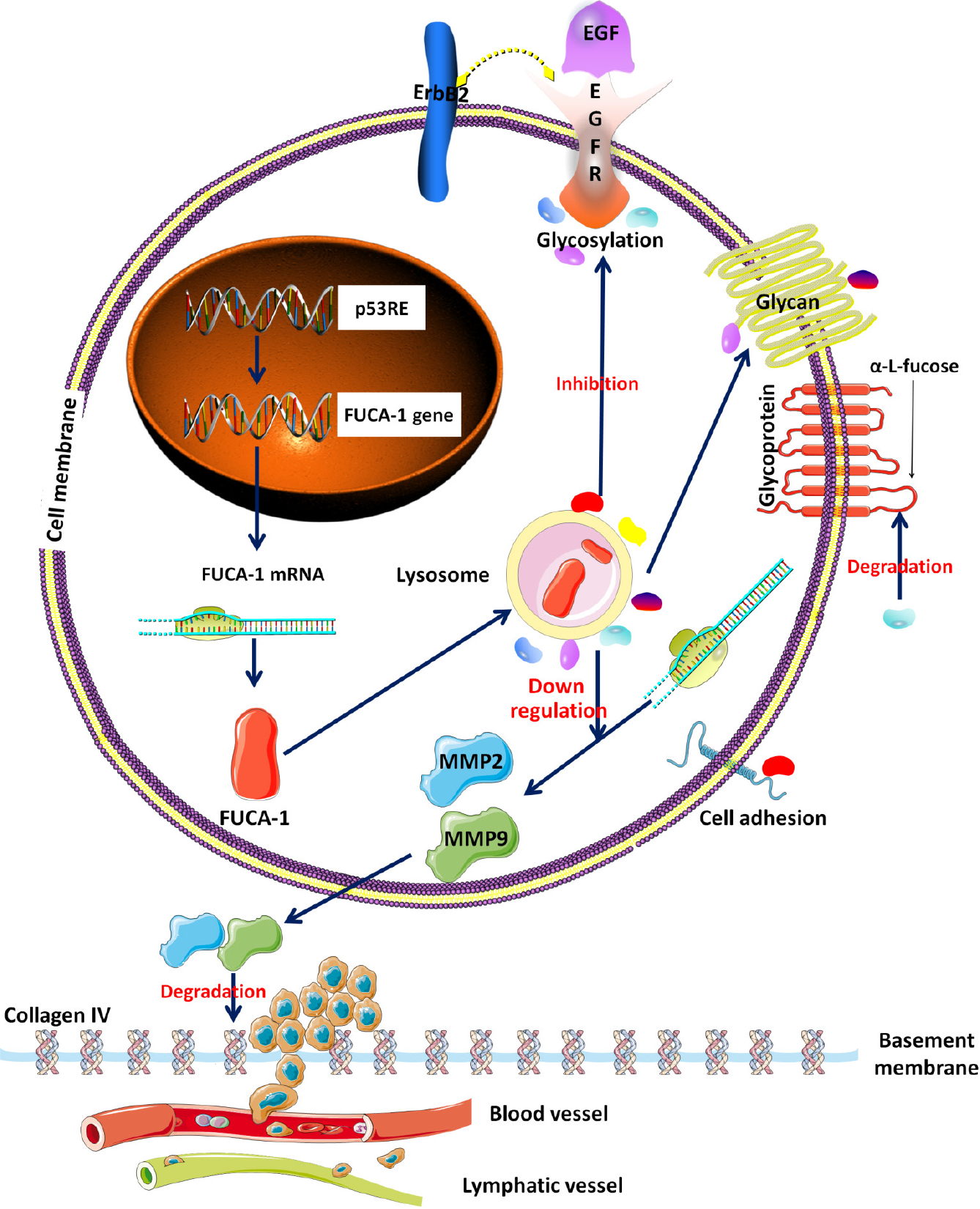
Alpha-L-fucosidase-1 (FUCA1) has been demonstrated to play opposing regula
To evaluate the status, association, and prognostic value of FUCA1 and MMP-9 expression in ESCC.
Patients who underwent esophagectomy for ESCC between January 1, 2014, and December 31, 2014 at Sun Yat-Sen University Cancer Center were enrolled. The expression status of FUCA1 and MMP-9 in cancerous tissues was detected using immunohistochemistry. In addition, the expression profiles of the FUCA1 and MMP-9 genes in non-metastatic ESCC were extracted from The Cancer Genome Atlas (TCGA) database.
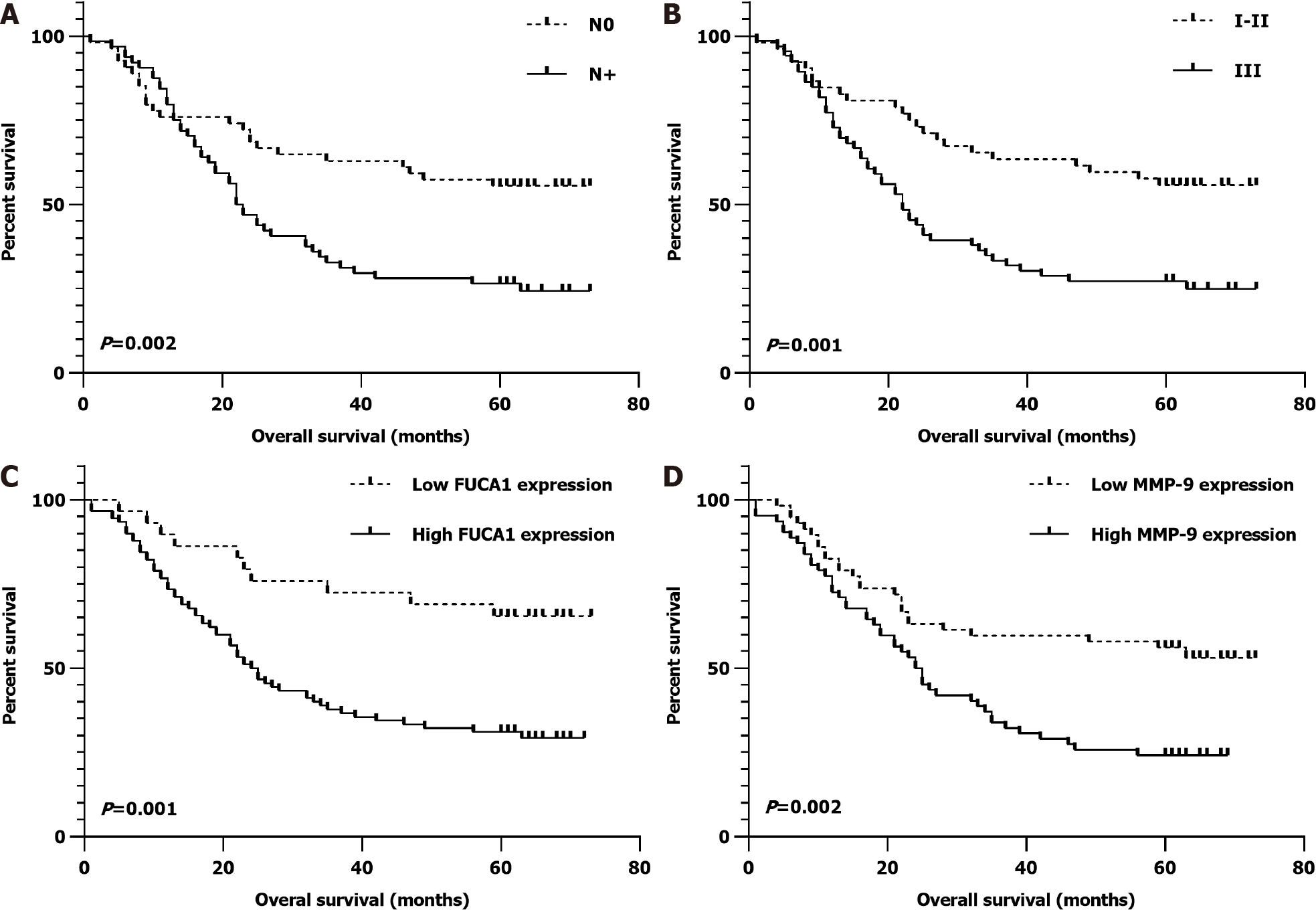
High expression of FUCA1 and MMP-9 was found in 90 patients (75.6%) and 62 patients (52.1%), respectively. In the high FUCA1 expression group, the constituent ratios of patients with stage III disease (61.1% vs 37.9%, P = 0.029), lymphatic invasion (62.2% vs 31.0%, P = 0.003), and high MMP-9 expression (60.0% vs 27.6%, P = 0.002) were significantly higher than those in the low FUCA1 expression group. In Kaplan-Meier univariate analysis, advanced tumor-node-metastasis stage (III, P = 0.001), positive regional lymph node metastasis (N+, P = 0.002), high FUCA1 expression (P = 0.001), and high MMP-9 expression (P = 0.002) were potential predictors of shorter overall survival (OS), which was similar to the results analyzed based on the TCGA database. Further Cox multivariate regression analyses still demonstrated that FUCA1 and MMP-9 expression levels were independent prognostic factors of OS [hazard ratio (HR): 0.484, 95% confidence interval (CI): 0.239-0.979; P = 0.044; and HR: 0.591, 95%CI: 0.359-0.973, P = 0.039, respectively].
FUCA1 cooperation with MMP-9 may have a major role in affecting the ESCC invasion and metastatic capability, and serve as a valuable prognostic biomarker in ESCC.
Core Tip: Our results demonstrated that high alpha-L-fucosidase-1 (FUCA1) expression was significantly associated with a worse overall survival, which illustrated that FUCA1 may have the ability to promote tumor cell invasion and metastasis among patients with esophageal squamous cell carcinoma (ESCC). Moreover, this study provides additional evidence that the molecular mechanisms of FUCA1 in ESCC are entirely different.
- Citation: Yu XY, Lin SC, Zhang MQ, Guo XT, Ma K, Wang LX, Huang WT, Wang Z, Yu X, Wang CG, Zhang LJ, Yu ZT. Association and prognostic significance of alpha-L-fucosidase-1 and matrix metalloproteinase 9 expression in esophageal squamous cell carcinoma. World J Gastrointest Oncol 2022; 14(2): 498-510
- URL: https://www.wjgnet.com/1948-5204/full/v14/i2/498.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i2.498
Esophageal squamous cell carcinoma (ESCC) is the predominantly diagnosed histological subtype in China, accounting for approximately 90% of all esophageal carcinomas (EC)[1]. Although more advances in early screening and multimodal therapy have been approved for use in patients with ESCC, the long-term survival rates even after curative surgery remain unsatisfactory[2-4]. This undesirable prognosis appears to be triggered mostly by aggressive tumor cell invasion and metastasis. Novel modalities for ESCC treatment that target molecular pathways are required to change the prognostic dilemma[5]. In addition, patients diagnosed with ESCC of the same stage show varied prognoses[2,3]. Hence, it is also necessary to explore the potential molecular mechanisms of ESCC as prognostic factors to distinguish those patients with a high-risk of local recurrence and/or distant metastasis[2].
The alpha-L-fucosidase-1 (FUCA1) gene, targeted by the p53 tumor suppression gene, encodes a lysosomal enzyme named FUCA1[6]. Its main biological function in human cells is to degrade alpha-L-fucose-containing glycoproteins and glycolipids to inhibit cell growth and induce cell death[5-7]. Moreover, a recent study elaborated that FUCA1 could inhibit the activation and fucosylation of epidermal growth factor receptor (EGFR), thereby blocking the EGFR signaling pathway[6]. Therefore, in theory, the molecular function of FUCA1 appears to diminish the invasion capacity of tumor cells (Figure 1). The association of high FUCA1 expression in serum or tumor tissue with a favorable prognosis in triple-negative breast cancer and intrahepatic cholangiocarcinoma has been confirmed[8,9]. However, probably due to molecular mechanistic heterogeneity, some studies have reported that elevated FUCA1 content predicts worse survival outcomes in hepatocellular carcinoma and glioma[7,10]. To our knowledge, it is unknown whether FUCA1 is expressed in ESCC and whether the expression status of FUCA1 is related to the prognostic outcome of ESCC.
The matrix metalloproteinase (MMP) family, consisting of a group of zinc-containing enzymes that can degrade the extracellular matrix and destroy the basement membrane, plays a critical role in epithelial and mesenchymal tumor invasion and metastasis (Figure 1)[11,12]. Many previous studies and meta-analyses have proven that overexpression of MMP family proteins in ESCC is associated with an unfavorable survival[11-13]. Notably, further studies discovered that FUCA1 could downregulate MMP-9 expression and activity, thereby diminishing the invasive ability of intrahepatic cholangiocarcinoma and breast cancer[9,14]. However, no relationship between FUCA1 expression and MMP-9 expression in ESCC has been reported.
Therefore, the main aim of this study was to evaluate the prognostic significance of FUCA1 and MMP-9 expression in ESCC and investigate the correlation of FUCA1 expression with MMP-9 expression.
The gene expression profiles in tumor tissues, clinical information, survival times, and outcomes of patients diagnosed with ESCC without distant metastasis were obtained from the public The Cancer Genome Atlas (TCGA) database (https://tcga-data.nci.nih.gov/tcga/). The skewed data of FUCA1 and MMP-9 expression profiles were log-transformed to reduce skewness. Then, on the basis of the best cutoff value determined by using X-tile 3.6.3 software (Copyright Yale University 2003), the log-transformed gene expression levels were divided into two groups (high/low).
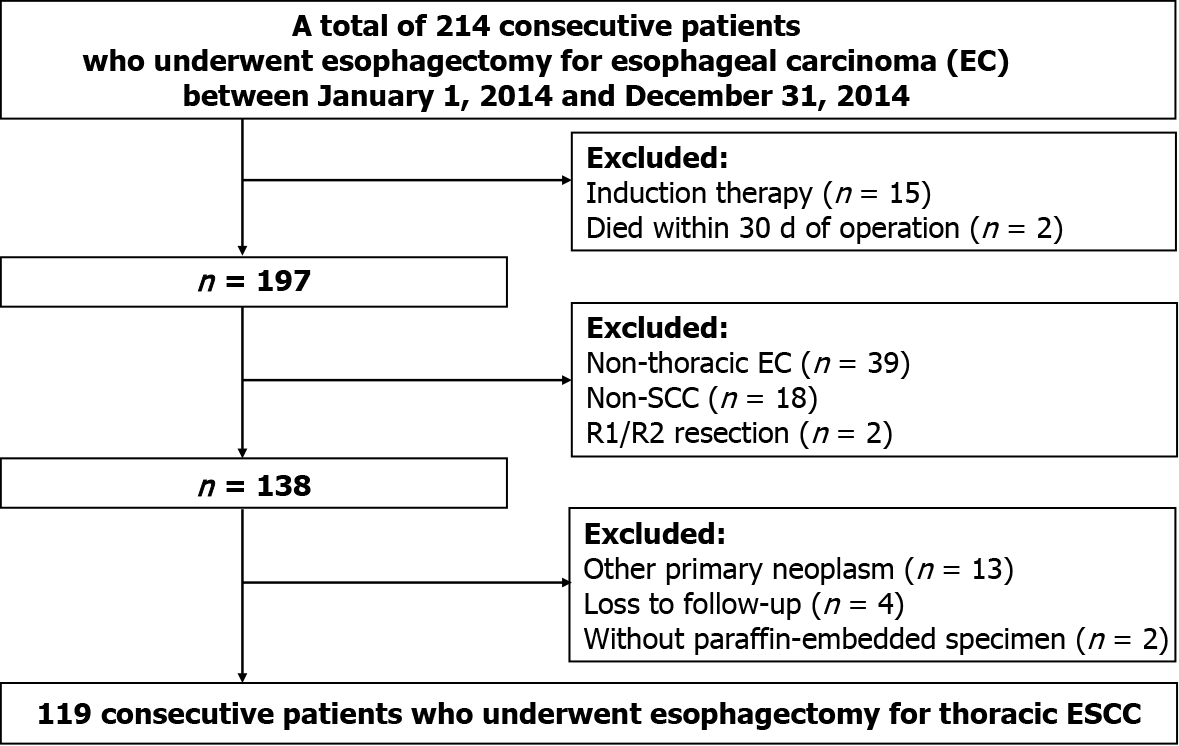
The clinical, pathological, and follow-up information of all patients who underwent curative esophagectomy for EC from January 1, 2014 to December 31, 2014 at the Sun Yat-Sen University Cancer Center (SYSUCC) was retrospectively collected from the Hospital Information System. All tumor-node-metastasis (TNM) staging was reclassified according to the 8th edition of the American Joint Committee on Cancer Staging Manual. Subsequently, only patients who met the following criteria were retained: (1) Diagnosed with thoracic ESCC; (2) Underwent complete removal (R0); (3) No induction therapy; (4) No death occurred within 30 d after operation; (5) No other primary neoplasm; (6) Had adequate paraffin-embedded specimens for immunohis
Written informed consent was obtained from all patients themselves during preoperative conversations. This retrospective study was approved by the Research Ethics Committee at the Sun Yat-Sen University Cancer Center (No. 308–2015–012).
Detection of FUCA1 and MMP-9 in ESCC tissues was carried out by immunohistochemistry (IHC), as described in related studies[6,8]. Specifically, the paraffin slices were incubated with a rabbit anti-human FUCA1 polyclonal antibody (dilution, 1:400; Abcam, Cambridge, United Kingdom) and a rabbit anti-human MMP-9 monoclonal antibody (dilution, 1:300; D603H, Cell Signaling Technology, Danvers, MA, United States). The expression of FUCA1 and MMP-9 was independently evaluated by two pathologists (Zhang MQ and Huang WT) who all engaged in pathological diagnosis over 5 years. If there was inconsistent interpretation, reevaluation under a double-head microscope was performed to obtain a consistently reliable result.
The staining intensity was graded on a four-step scale: Negative, weak, moderate, and strong, scored as 0, 1, 2, and 3, respectively. The percentage of the chromogenic reaction, counted in five random fields per section using 100 × magnification, was grouped into < 25%, 25%-50%, 50%-75%, and ≥ 75% (scored 1, 2, 3, and 4, respectively). The case was interpreted as high expression of FUCA1 and MMP-9 if moderate to strong cytoplasmic staining was observed in ≥ 25% of ESCC cells with reference to previous studies[13,15]. In addition, the total immunoreaction score was calculated according to the staining intensity (scores 0-3) multiplied by the percentage of stained ESCC cells (scores 1-4) to generate a total score from 0 to 12[13,15].
All statistical analyses were performed using SPSS 24.0 software (IBM, Chicago, IL, United States), and a two-sided P value less than 0.05 was defined as a statistically significant difference. Student’s t test and χ2 test were used to compare the differences in continuous variables and categorical variables, respectively, between the high expression group and the low expression group. Overall survival (OS) months were counted from the date of ESCC diagnosis to the date of death or the last follow-up (December 31, 2020). The Kaplan-Meier method was applied to identify the potential prognostic variables using the log-rank test. Subsequently, all of the above statistically significant variables were retained in the Cox proportional hazards model. In addition, scatter plots and survival curves were drawn using GraphPad Prism 8.0 software (San Diego, CA, United States).
Of the 119 patients from the SYSUCC, 21 (17.6%) were women, and the mean (standard deviation, SD) age of all patients was 59.0 (8.9) years. The majority of patients were smokers at the time of diagnosis (80, 67.2%) and had a history of alcohol use (74, 62.2%). The middle third of the thoracic esophagus was the most common site of ESCC (69, 58.0%), followed by the lower third (36, 30.3%). Upon pathological examination of resected specimens, the majority of tumors were limited to location between the mucosa and adventitia (pT1, 6.7%; pT2, 16.8%; pT3, 52.1%) under the microscope and had regional lymph node metastasis (pN1, 35.3%; pN2, 10.9%; pN3, 8.4%). The constituent ratios of pathological TNM stages I, II, and III were 5.0%, 39.5%, and 55.5%, respectively. In addition, 67.2% of all patients (80/119) received post
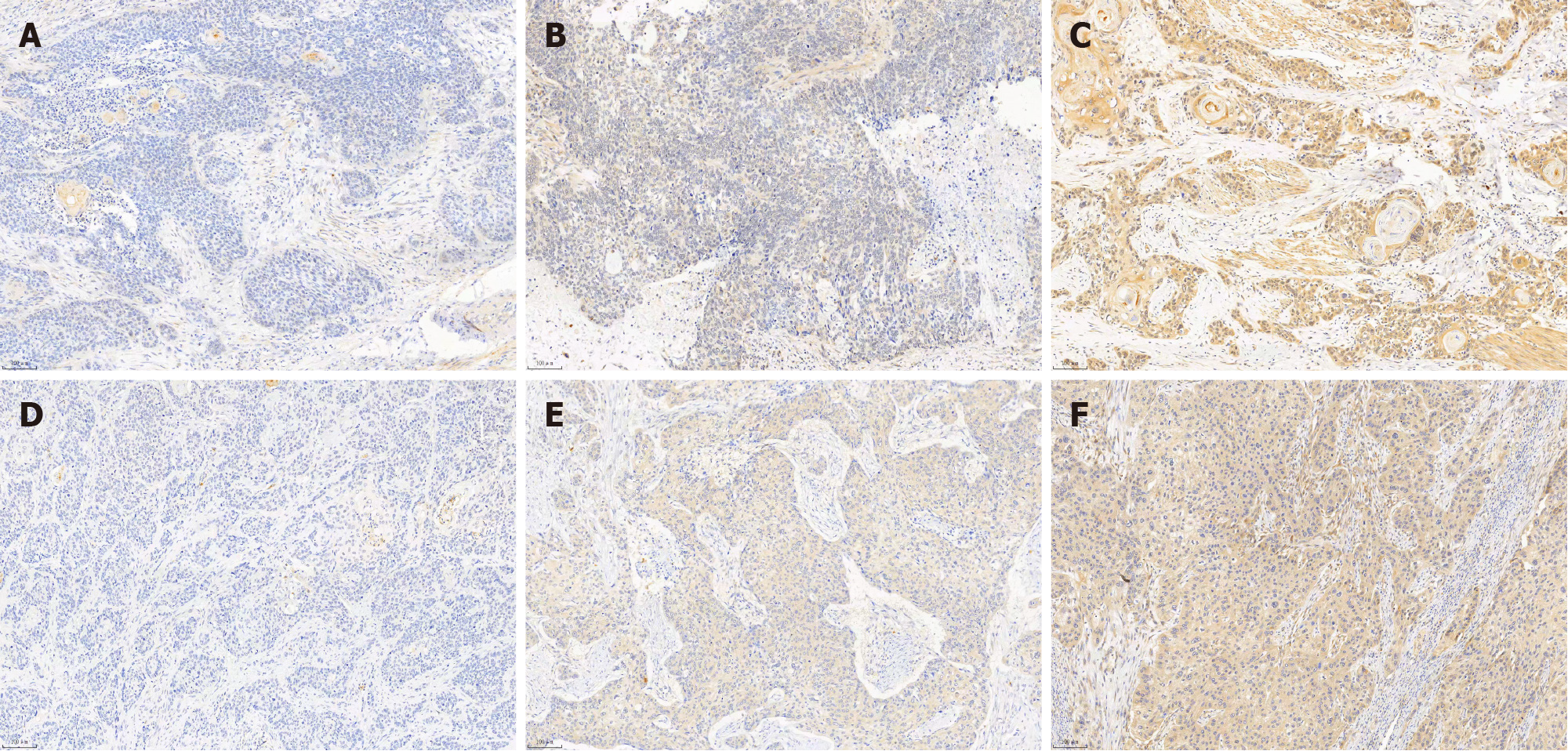
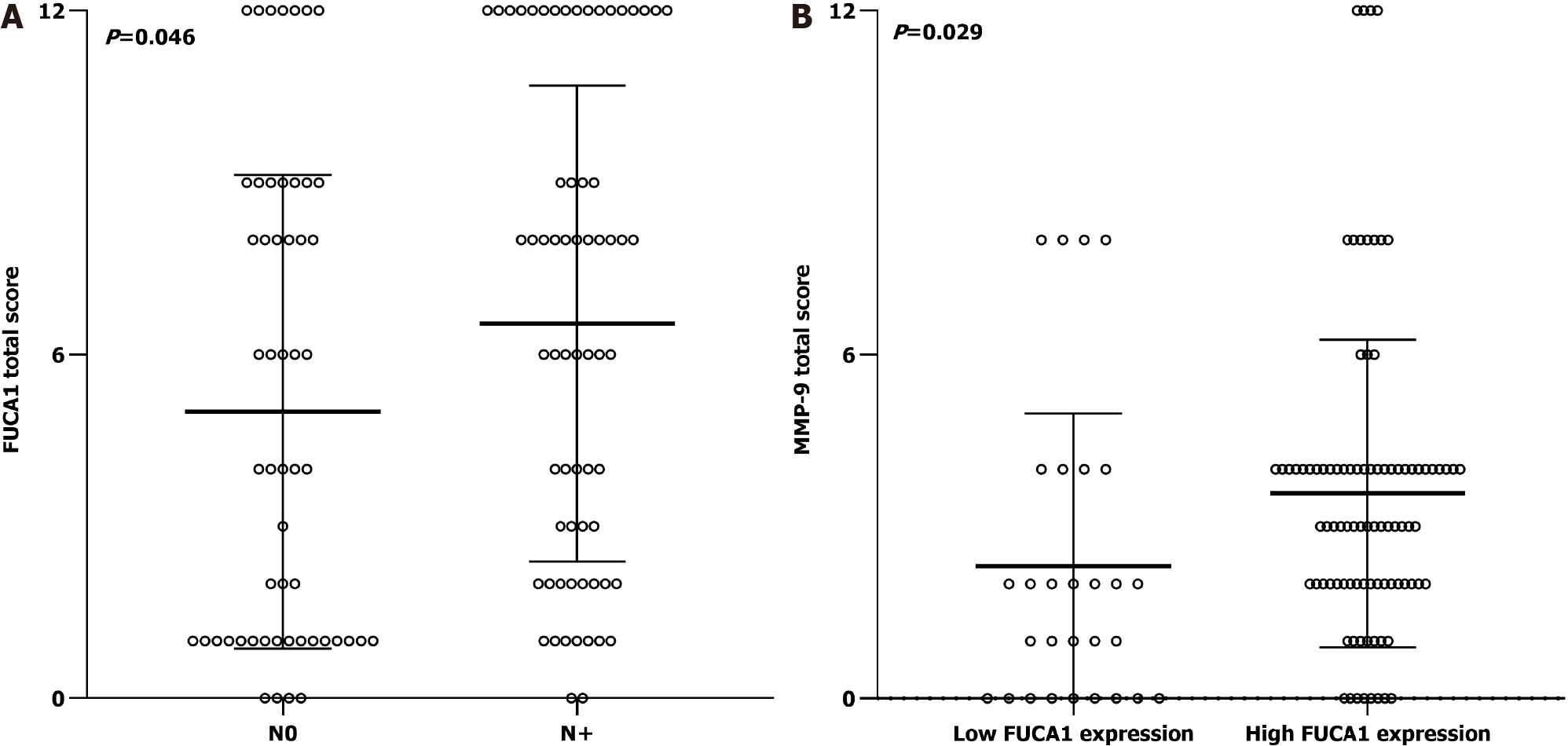
The clinicopathologic features of patients from the SYSUCC and TCGA databases according to the expression status of FUCA1 and MMP-9 are summarized in Table 1 and Supplementary Table 1, respectively. High expression of FUCA1 and MMP-9 in ESCC tumor cells was observed in 90 (75.6%, with a median total score of 6) and 62 patients (52.1%, with a median total score of 3), respectively. The IHC staining for FUCA1 and MMP-9 is shown in Figure 3. High FUCA1 expression was more frequent in patients with positive regional lymph node metastasis (P = 0.003, Figure 4A) and advanced stage tumors (P = 0.029). In the high MMP-9 expression group, patients showed a lower proportion of smoking history (56.5% vs 78.9%, P = 0.009) and alcohol use (53.2% vs 71.9%, P = 0.036). The FUCA1 expression status and total score were positively related to the MMP-9 expression status (P = 0.002) and total score (r = 0.258, P = 0.005, Figure 4B), respectively. However, in TCGA data analysis, we only found that male patients (P = 0.035) and patients without lymph node metastasis (P = 0.050) had a relatively higher proportion of low MMP-9 gene expression (Supplementary Table 1).
| Variable | FUCA1 expression | MMP-9 expression | ||||
| Low expression (n = 29) | High expression (n = 90) | P value | Low expression (n = 57) | High expression (n = 62) | P value | |
| Age (yr, mean ± SD) | 61.2 ± 7.6 | 58.3 ± 9.2 | 0.137 | 59.5 ± 8.4 | 58.6 ± 9.4 | 0.587 |
| Female, n (%) | 3 (10.3) | 18 (20.0) | 0.236 | 6 (10.5) | 15 (24.2) | 0.058 |
| History of smoking, n (%) | 6 (20.7) | 33 (36.7) | 0.111 | 45 (78.9) | 35 (56.5) | 0.009a |
| History of alcohol use, n (%) | 10 (34.5) | 35 (38.9) | 0.670 | 41 (71.9) | 33 (53.2) | 0.036 |
| Localization, n (%) | ||||||
| Upper | 2 (6.9) | 12 (13.3) | 0.360 | 5 (8.8) | 9 (14.5) | 0.555 |
| Middle | 20 (69.0) | 49 (54.4) | 33 (57.9) | 36 (58.1) | ||
| Lower | 7 (24.1) | 29 (32.2) | 19 (33.3) | 17 (27.4) | ||
| Grade, n (%) | ||||||
| Well | 4 (13.8) | 19 (21.1) | 0.648 | 12 (21.1) | 11 (17.7) | 0.820 |
| Moderately | 16 (55.2) | 48 (53.3) | 31 (54.4) | 33 (53.2) | ||
| Poorly | 9 (31.0) | 23 (25.6) | 14 (24.6) | 18 (29.0) | ||
| T stage, n (%) | ||||||
| T1-2 | 6 (20.7) | 22 (24.4) | 0.678 | 12 (21.1) | 16 (25.8) | 0.541 |
| T3-4a | 23 (79.3) | 68 (75.6) | 45 (78.9) | 46 (74.2) | ||
| Lymphatic invasion, n (%) | ||||||
| Negative (N0) | 20 (69.0) | 34 (47.8) | 0.003a | 31 (54.4) | 23 (37.1) | 0.067 |
| Positive (N+) | 9 (31.0) | 56 (62.2) | 26 (45.6) | 39 (62.9) | ||
| TNM stage, n (%) | ||||||
| I-II | 18 (62.1) | 35 (38.9) | 0.029a | 29 (50.9) | 24 (38.7) | 0.182 |
| III | 11 (37.9) | 55 (61.1) | 28 (49.1) | 38 (61.3) | ||
| Postoperative complication, n (%) | ||||||
| Arrhythmia | 5 (17.2) | 22 (24.4) | 0.421 | 16 (28.1) | 11 (17.7) | 0.179 |
| Pneumonia | 1 (3.4) | 11 (12.2) | 0.172 | 5 (8.8) | 7 (11.3) | 0.649 |
| Anastomotic leak | 0 (0) | 11 (12.2) | 0.048a | 4 (7.0) | 7 (11.3) | 0.421 |
| Adjuvant therapy, n (%) | 19 (65.5) | 61 (67.8) | 0.822 | 36 (63.2) | 44 (71.0) | 0.365 |
| FUCA1 total score (mean ± SD) | - | - | - | 4.3 ± 3.8 | 7.3 ± 4.1 | 0.000a |
| High FUCA1 expression, n (%) | - | - | - | 36 (63.2) | 54 (87.1) | 0.002a |
| MMP-9 total score (mean ± SD) | 3.6 ± 2.7 | 2.3 ± 2.6 | 0.029a | - | - | - |
| High MMP-9 expression, n (%) | 8 (27.6) | 54 (60.0) | 0.002a | - | - | - |
In the SYSUCC cohort, the median OS time was 32.2 mo (range, 1-73 mo) and the 5-year OS rate was 39.5%. In univariate survival analysis, no regional lymph node metastasis (N0, P = 0.002), earlier TNM stage (I-II, P = 0.001), low FUCA1 expression (P = 0.001), and low MMP-9 expression (P = 0.002) were significantly associated with a favorable OS (Table 2). After further adjustment in the Cox proportional hazards model, the above four variables still had strong prognostic value for OS (Table 2 and Figure 5).
| Variable | No. | Univariate analysis | Multivariate analysis | ||
| 5-yr overall survival rate (%) | P value | Hazard ratio (95% confidence interval) | P value | ||
| Age (yr) | |||||
| < 60 | 61 | 44.3 | 0.133 | ||
| ≥ 60 | 58 | 34.5 | |||
| Sex | |||||
| Female | 21 | 28.6 | 0.510 | ||
| Male | 98 | 41.8 | |||
| History of smoking | |||||
| Yes | 39 | 42.5 | 0.533 | ||
| No | 80 | 33.3 | |||
| History of alcohol use | |||||
| Yes | 74 | 40.5 | 0.831 | ||
| No | 45 | 37.8 | |||
| Localization | |||||
| Upper | 14 | 21.4 | 0.426 | ||
| Middle | 69 | 42.0 | |||
| Lower | 36 | 41.7 | |||
| Grade | |||||
| Well | 23 | 43.5 | 0.222 | ||
| Moderately | 64 | 43.8 | |||
| Poorly | 32 | 28.1 | |||
| T stage | |||||
| T1-2 | 28 | 39.3 | 0.890 | ||
| T3-4a | 91 | 39.6 | |||
| Lymphatic invasion | |||||
| Negative (N0) | 54 | 55.6 | 0.002a | 0.584 (0.351-0.972) | 0.039a |
| Positive (N+) | 65 | 23.5 | Reference | ||
| TNM stage | |||||
| I-II | 53 | 54.7 | 0.001a | 0.520 (0.316-0.856) | 0.010a |
| III | 66 | 27.3 | Reference | ||
| Arrhythmia | |||||
| Yes | 27 | 29.6 | 0.210 | ||
| No | 92 | 42.4 | |||
| Pneumonia | |||||
| Yes | 12 | 33.3 | 0.110 | ||
| No | 107 | 40.2 | |||
| Anastomotic leak | |||||
| Yes | 11 | 36.4 | 0.295 | ||
| No | 108 | 39.8 | |||
| Adjuvant therapy | |||||
| Yes | 80 | 33.7 | 0.159 | ||
| No | 39 | 51.3 | |||
| FUCA1 expression | |||||
| Low | 29 | 65.5 | 0.001a | 0.484 (0.239-0.979) | 0.044a |
| High | 90 | 31.1 | Reference | ||
| MMP-9 expression | |||||
| Low | 57 | 56.1 | 0.002a | 0.591 (0.359-0.973) | 0.039a |
| High | 62 | 24.2 | Reference | ||
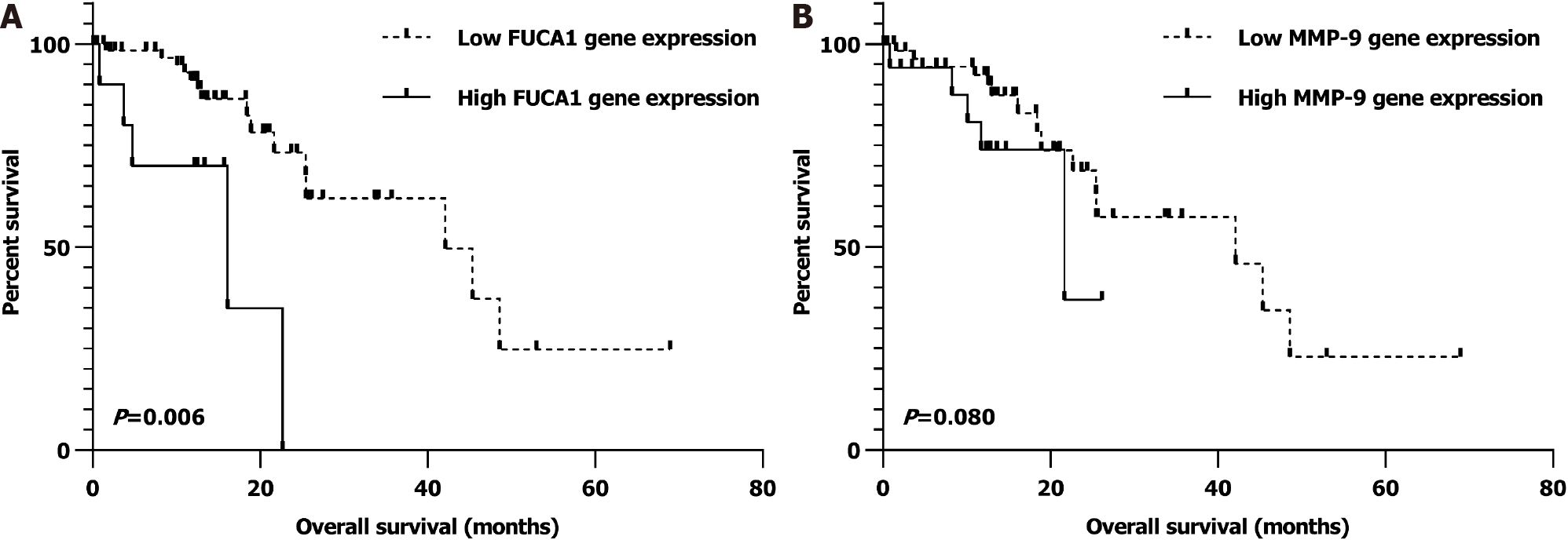
Similarly, after univariate and multivariate analyses, we found that nonmetastatic ESCC patients (I-III) with low FUCA1 gene expression in the TCGA database were also significantly related to a better OS (P = 0.006; Supplementary Table 2 and Figure 6A). However, the relationship of low MMP-9 expression with favorable prognosis was observed as a trend but with no statistical significance (P = 0.080; Supplemen
In this study, we detected FUCA1 protein overexpression in most ESCC tissues by IHC for the first time and found that high FUCA1 expression was positively associated with high MMP-9 expression, regional lymph node metastasis (pN+), and advanced TNM stage (III). Additionally, multivariable survival analysis showed that the FUCA1 and MMP-9 expression status could independently predict the postoperative survival of patients with resected ESCC.
In 2015, Tzu-Chun Cheng and his colleagues reported that upregulation of FUCA1 expression in triple-negative breast cancer (adenocarcinoma) conferred a favorable OS by degrading cell surface glycoproteins and glycolipids to inhibit cell growth and induce cell death[8]. Subsequently, the association of higher FUCA expression (≥ 20.85 U/L) with a better prognosis in patients with intrahepatic cholangiocarcinoma (adenocarcinoma) was also confirmed. Further mechanistic investigations revealed that FUCA could diminish the invasive ability of tumor cells by downregulating MMP-9 expression[9]. In addition, another recent study on thyroid cancer demon
Many studies observed MMP-9 overexpression detected by IHC staining in ESCC (34.8%-90.0%), and the related mechanistic studies elaborated that MMP-9 could participate in the development and progression of ESCC mainly by degrading type IV collagen to promote tumor cell metastasis and resulted in poor prognosis, which was completely consistent with our present study[11]. However, few studies have focused on the association between FUCA1 and MMP-9 expression. Shuang et al[9] reported that AFU (another abbreviation form for FUCA1) significantly downregulated the expression of MMP-9 in intrahepatic cholangiocarcinoma (adenocarcinoma) in vitro. Another cytological experiment carried out by Yuan et al[14] also demonstrated that AFU could significantly reduce MMP-9 activity and expression in human breast cancer cell lines (adenocarcinoma). Conversely, our study found that FUCA1 staining was positively associated with MMP-9 staining in ESCC, which was first reported in squamous cell carcinoma. The detailed molecular mechanisms need to be explored in the future to obtain more effective therapeutic targets in ESCC.
Although our present study clarified the prognostic significance of FUCA1 expression and the correlation of FUCA1 and MMP-9 expression in ESCC for the first time, several limitations cannot be ignored when extrapolating these results. First, this retrospective study was carried out in a single-center, small sample size cohort, which inevitably caused selection bias. Moreover, external validation in other patient cohorts was also lacking. Second, due to intratumoral heterogeneity, the paraffin section used for IHC staining may not represent the entire tumor mass. To obtain the most representative staining, all paraffin slices used in this study included ESCC tissues and corresponding normal esophageal tissues without necrotic tissue. Third, the antibodies selected in this study were not completely the same as those used in previous studies[12,13,15,18]. Undeniably, different antibodies may vary the IHC staining results. Fourth, the consensus cutoff values for FUCA1 and MMP-9 staining interpretation had not been determined; thus, there may be variation in the statistical results in which different cutoff values were used. In this study, the threshold value was selected for interpreting high and low FUCA1/MMP-9 expression with reference to previous large cohort studies[12,13,15,18].
FUCA1 cooperation with MMP-9 may have a major role in affecting the ESCC invasion and metastatic capability and serve as a valuable prognostic biomarker in ESCC.
Fundamental studies discovered that alpha-L-fucosidase-1 (FUCA1) could down
To explore the prognostic significance of FUCA1 and MMP-9 expression in esophageal squamous cell carcinoma (ESCC) and investigate the correlation of FUCA1 expression with MMP-9 expression.
A total of 119 consecutive patients who underwent esophagectomy for ESCC between January 1, 2014 and December 31, 2014 at the Sun Yat-Sen University Cancer Center (SYSUCC) were enrolled in the final analysis. In addition, the FUCA1 and MMP-9 gene expression profiles of 76 patients diagnosed with ESCC without distant metastasis were obtained from the public The Cancer Genome Atlas (TCGA) database.
Student’s t test and χ2 test were used to compare the differences in continuous variables and categorical variables, respectively. The Kaplan-Meier method was applied to identify the potential prognostic variables using the log-rank test. Subsequently, all of the above statistically significant variables were retained in the Cox proportional hazards model.
In the SYSUCC cohort, the FUCA1 expression status (high/low) and total IHC score were positively related to the MMP-9 expression status (high/low, P = 0.002) and total IHC score (r = 0.258, P = 0.005). Moreover, after further adjusting in the Cox proportional hazards model, low FUCA1 expression (P = 0.001) and low MMP-9 expression (P = 0.002) still showed significant associations with a favorable overall survival (OS). Similarly, after univariate and multivariate analysis, we found that nonmetastatic ESCC patients (I-III) with low FUCA1 gene expression in the TCGA database also had a significantly better OS (P = 0.006). However, the relationship of low MMP-9 expression with a favorable prognosis was observed as a trend but with no statistical significance (P = 0.080).
FUCA1 cooperation with MMP-9 may have a major role in affecting ESCC invasion and metastasis capability and serve as a valuable prognostic biomarker in ESCC.
The present study offers a future research direction in which FUCA1 cooperation with MMP-9 may be a potential regulator in ESCC progression, which needs to be explored in fundamental studies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: China Anti-Cancer Association; Chinese Medical Association; Chinese Medical Doctor Association; Chinese Society of Clinical Oncology.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chien CR, Dalal N S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Yuan YY
| 1. | Yang S, Lin S, Li N, Deng Y, Wang M, Xiang D, Xiang G, Wang S, Ye X, Zheng Y, Yao J, Zhai Z, Wu Y, Hu J, Kang H, Dai Z. Burden, trends, and risk factors of esophageal cancer in China from 1990 to 2017: an up-to-date overview and comparison with those in Japan and South Korea. J Hematol Oncol. 2020;13:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 2. | Yu X, Gao S, Xue Q, Tan F, Gao Y, Mao Y, Wang D, Zhao J, Li Y, He J, Mu J. Development of a predictive nomogram for cause-specific mortality in surgically resected early-stage oesophageal cancer: a Surveillance, Epidemiology, and End Results (SEER) analysis. J Thorac Dis. 2020;12:2583-2594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Yu X, Wen Y, Lin Y, Zhang X, Chen Y, Wang W, Wang G, Zhang L. The value of preoperative Glasgow Prognostic Score and the C-Reactive Protein to Albumin Ratio as prognostic factors for long-term survival in pathological T1N0 esophageal squamous cell carcinoma. J Cancer. 2018;9:807-815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Kakeji Y, Oshikiri T, Takiguchi G, Kanaji S, Matsuda T, Nakamura T, Suzuki S. Multimodality approaches to control esophageal cancer: development of chemoradiotherapy, chemotherapy, and immunotherapy. Esophagus. 2021;18:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 5. | Yu X, Zhang R, Yang T, Zhang M, Xi K, Lin Y, Wen Y, Wang G, Huang Z, Zhang X, Zhang L. Alpha-l-fucosidase: a novel serum biomarker to predict prognosis in early stage esophageal squamous cell carcinoma. J Thorac Dis. 2019;11:3980-3990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Ezawa I, Sawai Y, Kawase T, Okabe A, Tsutsumi S, Ichikawa H, Kobayashi Y, Tashiro F, Namiki H, Kondo T, Semba K, Aburatani H, Taya Y, Nakagama H, Ohki R. Novel p53 target gene FUCA1 encodes a fucosidase and regulates growth and survival of cancer cells. Cancer Sci. 2016;107:734-745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Wang K, Guo W, Li N, Shi J, Zhang C, Lau WY, Wu M, Cheng S. Alpha-1-fucosidase as a prognostic indicator for hepatocellular carcinoma following hepatectomy: a large-scale, long-term study. Br J Cancer. 2014;110:1811-1819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Cheng TC, Tu SH, Chen LC, Chen MY, Chen WY, Lin YK, Ho CT, Lin SY, Wu CH, Ho YS. Down-regulation of α-L-fucosidase 1 expression confers inferior survival for triple-negative breast cancer patients by modulating the glycosylation status of the tumor cell surface. Oncotarget. 2015;6:21283-21300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Shuang Z, Mao Y, Lin G, Wang J, Huang X, Chen J, Duan F, Li S. Alpha-L-Fucosidase Serves as a Prognostic Indicator for Intrahepatic Cholangiocarcinoma and Inhibits Its Invasion Capacity. Biomed Res Int. 2018;2018:8182575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Xu L, Li Z, Song S, Chen Q, Mo L, Wang C, Fan W, Yan Y, Tong X, Yan H. Downregulation of α-l-fucosidase 1 suppresses glioma progression by enhancing autophagy and inhibiting macrophage infiltration. Cancer Sci. 2020;111:2284-2296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Zeng R, Duan L, Kong Y, Liang Y, Wu X, Wei X, Yang K. Clinicopathological and prognostic role of MMP-9 in esophageal squamous cell carcinoma: a meta-analysis. Chin J Cancer Res. 2013;25:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 12. | Li Y, Ma J, Guo Q, Duan F, Tang F, Zheng P, Zhao Z, Lu G. Overexpression of MMP-2 and MMP-9 in esophageal squamous cell carcinoma. Dis Esophagus. 2009;22:664-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Xia T, Tong S, Fan K, Zhai W, Fang B, Wang SH, Wang JJ. XBP1 induces MMP-9 expression to promote proliferation and invasion in human esophageal squamous cell carcinoma. Am J Cancer Res. 2016;6:2031-2040. [PubMed] |
| 14. | Yuan K, Listinsky CM, Singh RK, Listinsky JJ, Siegal GP. Cell surface associated alpha-L-fucose moieties modulate human breast cancer neoplastic progression. Pathol Oncol Res. 2008;14:145-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Vecchio G, Parascandolo A, Allocca C, Ugolini C, Basolo F, Moracci M, Strazzulli A, Cobucci-Ponzano B, Laukkanen MO, Castellone MD, Tsuchida N. Human a-L-fucosidase-1 attenuates the invasive properties of thyroid cancer. Oncotarget. 2017;8:27075-27092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Otero-Estévez O, Martínez-Fernández M, Vázquez-Iglesias L, Páez de la Cadena M, Rodríguez-Berrocal FJ, Martínez-Zorzano VS. Decreased expression of alpha-L-fucosidase gene FUCA1 in human colorectal tumors. Int J Mol Sci. 2013;14:16986-16998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Zhou Y, Ma X, Wu J, Zhang C, Wang B, Song B, Guo W, Pan B. Preoperative serum α-1-fucosidase as an early-recurrent indicator for hepatocellular carcinoma following curative resection. Zhonghua Yi Xue Za Zhi. 2014;94:3623-3628. [PubMed] |
| 18. | Ishida S, Kayamori K, Sakamoto K, Yukimori A, Kugimoto T, Harada H, Ikeda T. Alpha-L-fucosidase-1 is a diagnostic marker that distinguishes mucoepidermoid carcinoma from squamous cell carcinoma. Pathol Int. 2019;69:76-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |