Published online Feb 15, 2022. doi: 10.4251/wjgo.v14.i2.423
Peer-review started: March 18, 2021
First decision: July 16, 2021
Revised: August 1, 2021
Accepted: January 6, 2022
Article in press: January 6, 2022
Published online: February 15, 2022
Processing time: 329 Days and 0.7 Hours
The prognostic role of body composition indexes, and specifically sarcopenia, has recently been explored in different cancer types. However, conflicting results have been reported. Heterogeneity in cancer type, cancer stage or oncological treatments, as well as different methodology and definition of sarcopenia, could be accounted for different conclusions retrieved from literature. When focusing on colorectal cancer, it clearly appears that colon and rectal cancers are often treated as a single entity though they have different behaviors and treatments. Particularly, patients with advanced rectal cancer represent a peculiar group of patients that according to current guidelines are treated with neoadjuvant chemotherapy and radiotherapy followed by radical surgery. This review was restricted to a homogeneous group of patients with advanced lower rectal cancer and the aim of exploring whether there is a correlation between skeletal muscle depletion and prognosis. Literature was searched for articles related to patients with advanced rectal cancer undergoing neoadjuvant chemo-radiotherapy (NCRT) followed by radical surgery, in whom muscle mass and/or change in muscle mass during neoadjuvant treatment were measured. Eight full-text articles were selected and included in the present review. The main findings of our review were: (1) The majority of the studies defined sarcopenia as muscle mass alone over muscle strength or physical performance; (2) There was a great deal of heterogeneity in the definition and measures of sarcopenia, in the definition of cut-off values, and in the method to measure change in muscle mass; (3) There was not full agreement on the association between sarcopenia at baseline and/or after chemo-radiotherapy and prognosis, and only few studies found a significance in the multivariate analysis; and (4) It seems that a loss in skeletal muscle mass during NCRT is associated with the worst outcomes in terms of disease-free survival. In conclusion, analysis of muscle mass might provide prognostic information on patients with rectal cancer, however more robust evidence is needed to define the role of muscle depletion and/or muscle change during neoadjuvant treatments, related to this specific group of patients. If a prognostic role would be confirmed by future studies, the role of preoperative intervention aimed at modifying muscle mass could be explored in order to improve outcomes.
Core Tip: Rectal cancer is one of the most common cancers worldwide. The present review explores the potential prognostic role of muscle depletion in patients undergoing curative surgery for rectal cancer after neoadjuvant treatment. Evidence supports the hypothesis that sarcopenic patients, and patients in whom a decrement in muscle mass is detected during neoadjuvant treatment, are considered at greater risk of tumor recurrence and tumor death. Despite this observation, assessment of muscle mass is mostly neglected while it could inform on prognosis as well as guide in optimal treatment.
- Citation: De Nardi P, Giani A, Maggi G, Braga M. Relation between skeletal muscle volume and prognosis in rectal cancer patients undergoing neoadjuvant therapy. World J Gastrointest Oncol 2022; 14(2): 423-433
- URL: https://www.wjgnet.com/1948-5204/full/v14/i2/423.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i2.423
Recently, the influence of anthropometry on treatment outcome has been a matter of research in several fields of surgical oncology. Several reports identified a significant association between specific profiles of the fat and muscular compartments and short- and long-term prognosis in cancer patients.
The most investigated hallmark sign of anthropometric frailty is sarcopenia, which has been identified as a predictor of poor outcome in different gastrointestinal cancers[1-3]. Sarcopenia, initially defined as an age-related reduction in muscle mass and strength, has been otherwise associated with various chronic diseases, including cancer-related malnutrition and cachexia[4-6]. It has been suggested that it may reflect a state of increased metabolic activity of tumor biology leading to host immune functional impairment, deficient response to systemic inflammation, nutritional changes, and altered endocrine function[4,7]. These conditions enhance patient vulnerability towards stressors and lead to an increased risk of developing adverse health outcomes. Actually, skeletal muscle depletion, that is the central feature of sarcopenia, has been negatively associated with chemotherapy toxicity, complications following surgery, and impaired survival in cancer patients[8-11].
The prognostic role of body composition indexes, and specifically sarcopenia, has been broadly explored also in patients undergoing colorectal cancer resection[12-14]. Nevertheless, colonic and rectal cancers are often appraised as a single entity despite their substantial differences in surgical management, oncological strategies, and prognosis. Rectal surgery accounts for a considerably greater number of postoperative complications[15,16] and rectal tumors have a higher recurrence rate and a shorter survival than colonic ones[17-19]. In addition, preoperative neoadjuvant chemo-radiotherapy (NCRT) is the standard care for patients with advanced lower rectal cancer because of the relatively high risk of local recurrence[20]. Accordingly, it would be worthwhile to study more homogeneous patient cohorts.
The identification of patients with skeletal muscle wasting might be critical for early and tailored nutritional interventional planning that may improve long-term outcomes and treatment tolerance as reported in cases of advanced rectal cancer[21]. Thus, we conducted a review to assess whether sarcopenia could be used to predict recurrence and survival among patients with advanced lower rectal cancers who are treated with NCRT followed by surgery.
A literature review was performed through Medline/PubMed, Embase, Scopus, Web of Science, and Cochrane library databases until January 2021. Searched terms were: “Rectal cancer” OR “rectal neoplasm” and “sarcopenia” OR “muscle mass” and “neoadjuvant therapy” as term and related Medical Subject Headings. Reference lists of the selected publications were searched for identifying additional studies. Only articles in English language were included.
Studies were selected if they were related to adult patients with advanced non metastatic rectal cancer at diagnosis who underwent NCRT (any scheme) and surgery with curative intent, and if muscle mass was measured (either preoperatively and/or before and/or after NCRT) and/or change in muscle mass during NCRT was measured, and related to survival.
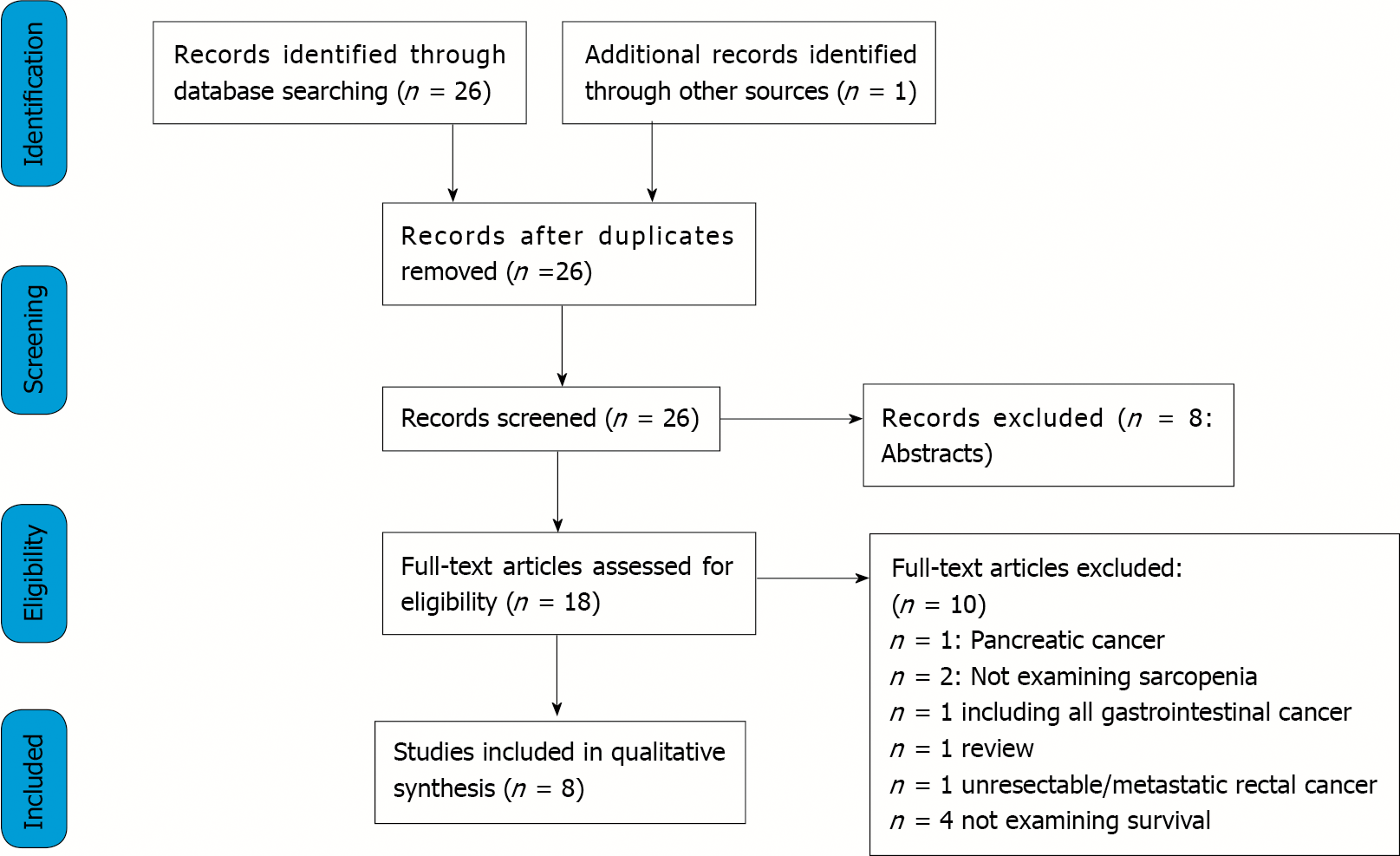
Through databases and reference lists searching, 27 articles were identified. After excluding reviews, abstracts, duplicates, and studies not providing the selected outcome measures, 8 full-text articles were selected and included in the present review[22-29].
A PRISMA flowchart reporting the studies selection process is shown in Figure 1.
Table 1 summarizes the studies included in this review along with the main outcome measures. All the studies were retrospective, with sample sizes ranging from 30 to 188; all were published between 2017 and 2019 with a median follow-up between 24.1 and 98.2 mo.
| Ref. | Type of study | Measure of muscle mass | Definition of sarcopenia | CT | Inclusion criteria | Exclusion criteria | Pts age | Mean outcome | Follow-up (mo) | Results |
| Berkel et al[22], 2019 | Retrospective | TPA and TAMA at L3 and both the superior and inferior border of L4 (+ measurement of skeletal muscle radiation attenuation) | Normalized TPA or TAMA at each level below the median (males and females) | After NCRT | cT3 or cT4 (CRM < 1 mm) and/or cN2 rectal cancer undergoing NCRT | No preoperative CT scan or of poor quality | 99; 66 (40-81) | Correlation between TAMA radiation attenuation and OS | Median: 32.9 (range: 19.4-51.1) | Sarcopenia (at TAMA L4 inferior) correlated to OS |
| Choi et al[23], 2018 | Retrospective | Skeletal muscle index at L3 level | SMI < 52.4 cm2/m2 for male and 38.5 cm2/m2 for female | Before NCRT | cT ≥ 3 or cN ≥ 1 rectal cancer undergoing NCRT | Metastasis/recurrence. No CT at initial diagnosis | 188; 61.3 (27-84) | Correlation between SMI and OS and DFS | Median: 52 (range: 5-91) | Worse OS in sarcopenic patients |
| Chung et al[24], 2020 | Retrospective | Skeletal muscle index at L3 level | SMI < 52.4 cm2/m2 for male and < 38.5 cm2/m2 for female | Before and after NCRT | Locally advanced non metastatic rectal cancer undergoing NCRT | Not reported | 93 | Correlation between SMI and skeletal muscle loss during NCRT and OS and DFS | Not found | Worse 5-yr OS in sarcopenic pts after NCRT. Worse OS in pts with severe muscle loss during NCRT. CT4 independent risk factor for severe muscle loss |
| De Nardi et al[25], 2020 | Retrospective | Skeletal muscle index at L3 level | SMI < 52 cm2/m2 for male and < 42 cm2/m2 for female | Before and after NCRT | cT ≥ 3 and N+ cancers undergoing NCRT | Not reported | 52; 63 (32-79) | Correlation between skeletal muscle change during NCRT and OS and DFS | Median: 56 (range: 32-8) | Worse DFS in pts with SML > 2%. In stage II subgroup, worse DFS in SML > 2% or > 5% |
| Fukuoka et al[26], 2019 | Retrospective | Psoas muscle index at the level of the navel | No definition | Before and after NCRT or NAC | cT3N+ rectal cancer undergoing NCRT or NAC | Distant metastasis. History of other malignancies | 47; 66 (27-88) | Correlation between change in PMI during neoadjuvant treatment and OS and DFS | Median: 24.1 | Worse OS and DFS in patients with PMI decrease > 10% |
| Levolger et al[27], 2018 | Retrospective | Skeletal muscle index at L3 level | SMI < 52 cm2/m2 for male and < 39.5 cm2/m2 for female | Before and after NCRT | cT3 and cT4 rectal cancer and/or cN+ undergoing NCRT | Not reported | 12261 (53-66.3) | Correlation between change in skeletal muscle mass during NCRT and OS, DFS, metastases | Median: 41 (range: 26-62) | Lower SMI in patients with cT4 tumors than cT3. SMI variation associated to worse DFS and metastases |
| Park et al[28], 2018 | Retrospective | Skeletal muscle index at L3 level | SMI < 55 cm2/m2 for male and < 39 cm2/m2 for female | Before NCRT | > 65-yr-old pts with rectal cancer undergoing NCRT | RT or CT alone | 30; 72 (66-87) | Correlation between sarcopenia and OS and DFS | Median: 98.2 (range 73.5-122.8) | Worse OS and DFS in sarcopenic patients |
| Takeda et al[29], 2018 | Retrospective | Skeletal muscle index at L3 level | SMI < 45 cm2/m2 for male and 33.8 | Before NCRT | cII or cIII advanced rectal cancer undergoing NCRT | Lack of baseline CT scan | 144. Sarcopenic: 65 (42-81). Not sarcopenic: 60 (32-65) | Correlation between sarcopenia and OS and DFS | Median: 67 (range: 5.7-137.1) | Worse OS and DFS in sarcopenic patients |
Irwin Rosenberg defined sarcopenia (from Greek ‘sarx’ or flesh + ‘penia’ or loss) for the first time in 1989 as an age associated decline in skeletal muscle mass[30]. However, it was not until 2010 that a Sarcopenia Working Group (SWG) developed a broadly accepted clinical definition and diagnostic criteria that would be used both in clinical practice and in research studies[31]. According to the SWG, sarcopenia was defined as a “syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with a risk of adverse outcomes”. The working group also categorized sarcopenia into primary, in which aging is the only apparent cause and secondary, among which different diseases, such as malignancy, play an important role.
The original definition was updated 10 years later as “a progressive and generalized skeletal muscle disorder that is associated with increased likelihood of adverse outcomes”[32]. One of the main insights of the revision was the prominent role of muscle strength over muscle mass as a measure that better predicts adverse outcomes. This revision also defined these two criteria: (1) Low muscle mass; and (2) Low muscle strength.
These two criteria are requested to define sarcopenia and help to resolve several questions concerning diagnostic measures and cut-off points important to clinical practice.
As a matter of fact, the majority of the studies evaluating the association of sarcopenia and clinical outcome defined sarcopenia as muscle mass alone over muscle strength or physical performance, possibly because it is based on more objective parameters that can be evaluated retrospectively in studies and rely on imaging tools that are widely used in clinical practice. On the contrary, other tests are more subjective, based on patient’s perception, or too long to be administered and thus less suited for research. All the eight studies selected for the present review used muscle mass alone as the diagnostic criterion to define sarcopenia and none employed muscle strength or physical performance.
Body muscle mass can be assessed by different techniques including imaging techniques, bio impedance analysis, anthropometric measure, or body potassium evaluation. Body imaging techniques comprise computed tomography (CT) scan, magnetic resonance imaging, and dual energy X-ray absorptiometry; CT is the most frequently used in clinical practice for its high accuracy and reproducibility[33]. The problem of the radiation exposure is bypassed in cancer patients because this exam is routinely performed for cancer staging and follow-up. As a substitute of muscle mass, the abdominal wall musculature is the most commonly assessed: Skeletal muscle cross sectional area (cm2) is generally measured with CT images at the level of the 3rd lumbar vertebra (L3). L3 vertebra level is the site most commonly used because it correlates significantly with whole-body muscle[34]. However, there could be differences depending on the exact site of measurement such as the upper, mid, or lower vertebral body. The parameter is then normalized for patient stature and designated as skeletal muscle index, skeletal muscle index (SMI) (cm2/m2). There was a substantial agreement for the choice of L3 as a reference site and only one study[22] evaluated three levels: The third lumbar vertebra and both the superior and inferior border of the fourth lumbar vertebra. Differently, Fukuoka et al[26] measured the psoas muscle index (PMI) at the level of the navel as an indicator of skeletal muscle mass; however, this approach is less standardized and since psoas is a minor muscle, it is questioned if it is representative of the overall lean body mass. The threshold of skeletal muscle radiation attenuation, to discriminate between skeletal muscle and other tissues, (mean Hounsfield Units, HU) was −29 to +150 HU[35]. The most common definition of sarcopenia in the studies considered in the present review takes into consideration gender specific cut off values, however these values vary considerably: Cut-off points of 43 cm2/m2, 52.4 cm2/m2, 52 cm2/m2, 49 cm2/m2 for men, and 41 cm2/m2, 38.5 cm2/ m2, 42 cm2/m2, 31 cm2/m2 for women are reported. Other authors[29] stratified by quartiles according to the SMI values for men and women and defined low skeletal muscle mass as the lowest quartile. A recent review found 12 different diagnostic thresholds for sarcopenia, the most common being 52.4 cm2/m2 for men and 38.5 cm2/m2 for women[36], therefore the need for further standardization is highlighted. The cut-off values may differ for different reasons: First for the reference population since, for instance, the Eastern population may have different body size with respect to the Western one[37]. The different threshold may lead to different prevalence of sarcopenia and thus to different results; moreover, it makes it difficult to compare the results of different studies. Interestingly, only one author[22] evaluated the quality of muscle assessing the skeletal muscle radiation attenuation (as mean HU of Total Abdominal Muscle Area, TAMA); according to previous studies, a lower HU corresponded to fatty infiltration of muscle myosteatosis.
The studies examining the rate of change in muscle mass during NCRT also employed different methods; De Nardi et al[25] established a 2% and 5% variation threshold[25]. Fukuoka et al[26] employed a 10% threshold, after subtracting the pre-PMI from the post-PMI and then dividing the results by the pre-PMI multiplied by 100. Chung et al[24] initially calculated (SMI_post-SMI_pre)/SMI_pre × 100 and then dichotomized the patients based on cut-off values of 4.2%/100 d; this process accounted for differences in the time elapsed between the 2 CT scans[24].
In conclusion, although the majority of the authors agreed on the tool and site to measure muscle mass, more research is needed to provide reference values in order to increase the comparability of the results.
Sarcopenia has multiple contributing factors such as age, heritability, diet, nutritional status, lifestyle, chronic diseases, hormonal changes and drug treatments. Low skeletal muscle mass is common among cancer patients. Cancer is a main cause of secondary sarcopenia because of the catabolic state caused by inflammatory reaction, possibly associated with poor nutritional status. As underlined by a recent systematic review, sarcopenia prevalence ranges from 15% to 74% in oncologic patients before cancer treatment[38]. This wide range in prevalence is partly due to different characteristics of tumor; albeit a variation in the definition of sarcopenia, as already stated, may also play a role. Among patients with colorectal cancer, a study on 3262 patients examining medical and demographic characteristics associated with sarcopenia found a prevalence of 42% with a strong correlation to older age, Caucasian race, and advanced disease stage[39]. Several other authors tried to identify patients’ characteristics or tumor factors that could be associated with sarcopenia. As expected, a relationship with older age was found since cancer and aging recognize a similar pathophysiologic mechanism[40]. In patients with colorectal cancer, sarcopenia was associated with body mass index (BMI), serum carcinoembryonic antigen level and mean number of metastatic lymph nodes[10] while in patients with colorectal liver metastasis with female sex, low BMI and a lower amount of intra-abdominal fat[41].
Several of the studies examined in the present review did not explore these correlations[22,25,28] while others[26,27] found no differences in patient demographic and clinical characteristics between sarcopenic and non-sarcopenic patients. On the other hand, an association with BMI was described by 3 studies[23,24,29] and with older age by 2[23,29]. Black et al[42] reported the data on 86 patients with colorectal cancer and although the results pertaining to rectal cancer patients only cannot be extrapolated, they described an association between sarcopenia and older age and elevated neutrophil count[42]. This last parameter reflects the systemic inflammatory response specific of cancer patients. This association has been demonstrated to be also strongly related to survival in colorectal cancer patients[43]. Interestingly, few studies recognized a relationship between sarcopenia and disease stage[44]; a possible explanation relies in the small sample size of the majority of the studies. In the studies examined here, this difference could not be found due to the homogeneity of tumor stage (only II and III stage rectal cancer). In the study by Levolger et al[27] no association was found at baseline while, after NCRT, patients with cT4 tumors had a lower SMI when compared to patients with cT3 tumors. Finally, De Nardi et al[25] examined a subgroup of patients with stage II cancer and found that poor differentiated tumors (G3) were associated with skeletal muscle loss during NCRT[25].
Body composition and functional status in cancer patients have been acknowledged as prominent factors associated with prognosis in different tumors such as liver, rectum, esophagus, stomach and kidney[38]. In patients undergoing surgery, pre-operative sarcopenia has been shown to be an independent unfavorable predicting factor for several cancers and it has been associated with worse clinical outcomes in terms of post-operative complications, hospital stay, morbidity, mortality and a lower tolerance of chemo radiation therapy[45].
The role of sarcopenia in colorectal cancer patients’ postoperative outcomes have been the topic of several works. Sarcopenia independently predicted mortality adjusted for age, sex, and previous abdominal surgery in a study on 310 patients[7]. In another study, Lieffers et al[8] reported that sarcopenic patients had significantly longer hospitalization and a higher wound infection rate[8]. A systematic review of 12 studies, including 5337 patients with non-metastatic colorectal cancer undergoing surgery, confirmed that sarcopenia was not only an independent predictor of post-operative complications, but it was also related to overall, relapse-free and progression-free survival[46]. More recently, another systematic review[47] included 44 randomized and observational studies comprising 18891 patients, to assess the prognostic value of sarcopenia on postoperative outcomes and survival rates of patients with colorectal cancer; studies involving treatment of metastatic colorectal cancer were excluded. Among the 44 studies, twenty-five, with a total of 15446 patients, reported overall survival (OS) as an outcome; the meta-analysis demonstrated an association between sarcopenia and shorter OS; furthermore, sarcopenia was negatively related to disease free survival (DFS) and cancer specific survival. However, when patients with rectal cancer were retrieved from the whole population, the authors could not find a worse survival in sarcopenic patients. Overall, 6511 patients had rectal cancer, among them a large proportion had stage I or II and the minority with advanced or metastatic disease, accordingly, only a small proportion of them underwent NCRT.
In order to analyze a more homogeneous group of patients in terms of tumor location, stage, and treatments, we restricted our review to patients with advanced rectal cancer who underwent neoadjuvant NRCT and curative surgery. In the studies analyzing the relation between skeletal muscle depletion and prognosis, we focused on two issues. Firstly, related to single time point measurement of sarcopenia and secondly to the changes in muscle mass during cancer treatment.
In univariate analysis, the majority of the authors reported an association between pre-or post-NCRT sarcopenia and OS[22-24], or both OS and DSF[28,29].
In the multivariate model, sarcopenia pre-NCRT[23,28,29], or post-NCRT[22,24], was associated with OS. Additionally, in the study by Park et al[28], sarcopenia was the only independent poor prognostic factor for OS. The DFS was also affected, in the studies by Park et al[28] and Takeda et al[29], by sarcopenia before NCRT. Takeda et al[29] identified that pathological tumor stage and sarcopenia were independently associated with poor OS and DFS in multivariate analysis. Chung et al[24] identified 51 pts (54.8%) with sarcopenia after the completion of NCRT, while they did not report the absolute number of patients with sarcopenia pre-NCRT. While there was no significant difference in OS or DFS between patients with and without sarcopenia pre-NCRT, in the patients with sarcopenia post-NCRT, the 5-year OS rate was significantly lower with respect to patients without sarcopenia.
In summary, a paucity of studies has examined, up to now, the relation between muscle depletion and prognosis in the group of patients with rectal cancer undergoing NCRT and surgery. Although several of them reported a correlation, particularly with OS, the results are inconsistent so far. The discrepancy among the studies could be due to different definitions of sarcopenia, different time points for performing CT scan, or insufficient power calculation for the analysis. Nevertheless, some interesting reports should encourage clinicians to undertake clinical trials to obtain more robust evidence.
The existing literature on sarcopenia and prognosis in cancer patients is mainly connected to evaluation of muscle mass at a single time point, while temporal changes of body composition during treatment and their impact on survival have been scarcely studied. Actually, anthropometry is generally assessed only before starting surgical or oncological programs, while a proper appraisal of changes in fat and lean body mass during therapies may be another critical prognostic tool and add value to existing literature.
Some studies demonstrated the negative impact of muscle loss during oncological treatments on prognosis of colorectal cancer patients. Nonetheless, most of those studies occurred in patients with metastatic diseases undergoing palliative chemotherapy. Miyamoto et al[48] evaluated the association between progressive skeletal muscle loss and prognosis in patients with unresectable colorectal cancer undergoing systemic first-line chemotherapy. It was found that patients who had a loss greater than 5% during chemotherapy experienced significantly shorter progression-free survival than those in the non–skeletal-muscle loss group. Similarly, a decrease in muscle area during chemotherapy of 9% or more was significantly associated with worse OS rates in metastatic colorectal cancer patients in a study by Blauwhoff-Buskermolen et al[49]. Interestingly, the static pre-treatment evaluation of skeletal muscle depletion was not a risk factor for survival in both cohorts. These results confirmed the value of depletion of skeletal muscle during chemotherapy as a prognostic factor already observed in other diseases[50,51].
Heus et al[52] measured an overall increase in skeletal muscle during neoadjuvant therapy as if chemo-radiation could lessen the inflammatory tumor state and consequently increase muscle mass.
From a biological point of view, the available data suggest that sarcopenia may reflect the increased metabolic activity of a more aggressive tumor leading to systemic inflammation and causing muscle loss. In this perspective, it might be speculated that the modification in body composition could be an expression of a different biologic response to antineoplastic therapy; thus, achieving tumor control with effective chemotherapy has the potential to reverse the catabolic processes causing cachexia. On the contrary, a significant loss of skeletal muscle during treatment suggests a more aggressive disease and potential ineffectiveness of chemotherapy.
Less evidence is available regarding changes in lean body mass specifically during neoadjuvant therapies for rectal cancer. Levolger et al[27] found that skeletal muscle loss during NCRT was associated with poor DFS and a higher risk of developing distant metastasis; however, muscle depletion did not impair OS. In addition, in their population, single time point assessment of sarcopenia, a widely adopted method was not predictive of survival. In an analogous study[25], NCRT was associated with loss of skeletal muscle in 36.5% of patients, while no variation or increased muscle mass was found in 63.5%. Muscle loss after NCRT was related to worse DFS. Additionally, even if not statistically significant, patients that experienced muscle mass depletion were more likely to have none or a poor response to neoadjuvant treatment. This last evidence, if confirmed, may support the above-mentioned theory of a relationship between treatment failure and muscle depletion. Chung et al[24] reported that 24.7% of the patients had severe muscle loss after NCRT; they found no difference in survival in sarcopenic patients, before or after NCRT, however patients with severe muscle loss during NCRT showed significant worse OS with respect to the control group. The authors also tried to identify variables that could predict severe muscle loss and found that cT4 tumors were the only risk factor. Finally, Fukuoka et al[26] reported that a > 10% decrease of muscle mass during NCRT was associated with a shorter OS and DFS[26].
Based on the little available evidence, it is not clear if an aggressive tumor biology rather than NCRT per se is more likely to be the causative factor inducing a critical catabolic state in certain patients. Further studies are needed to define the potential prognostic role of body composition changes during neoadjuvant treatments on pathological tumor response and long-term outcomes. Additionally, despite mounting evidence demonstrating a sarcopenia relationship with poor survival, it is still undefined whether targeted physical and nutritional interventions, aimed at halting or reversing cancer related muscle wasting, may improve the outcomes. Whether these regimens are effective remains to be answered, however, eventually the interval between NCRT and surgery might display a perfect opportunity to enhance the overall condition of locally advanced rectal cancer patients.
Only a few studies have been published so far on the relationship between muscle mass and prognosis in rectal cancer patients undergoing NCRT followed by surgery. Overall, these studies demonstrated an association between sarcopenia and OS; in addition, the evaluation of temporal changes in muscle mass during NCRT also showed that muscle loss during treatment was associated with a worse prognosis. Consequently, it is of paramount importance to identify patients with skeletal muscle wasting in order to plan an early and tailored intervention that may improve long-term outcomes.
Besides implementing studies examining the relationship between muscle wasting and prognosis, it would be desirable to lead studies evaluating the relationship between sarcopenia and other prognostic factors such as tumor downstaging or complete pathological response.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: ASCRS; ESCP.
Specialty type: Oncology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Hussuna A, Yoshimatsu K S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR
| 1. | Parkin E, Plumb AA, O’Reilly D, Renehan AG. Body composition and outcome in patients undergoing resection of colorectal liver metastases (Br J Surg 2012; 99: 550-557). Br J Surg. 2012;99:1021-1022; author reply 1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1822] [Cited by in RCA: 2372] [Article Influence: 139.5] [Reference Citation Analysis (0)] |
| 3. | Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 1512] [Article Influence: 126.0] [Reference Citation Analysis (0)] |
| 4. | Dodson S, Baracos VE, Jatoi A, Evans WJ, Cella D, Dalton JT, Steiner MS. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annu Rev Med. 2011;62:265-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 5. | Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:990S-991S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1263] [Cited by in RCA: 1448] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 6. | Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2908] [Cited by in RCA: 3828] [Article Influence: 273.4] [Reference Citation Analysis (0)] |
| 7. | Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewé KW, Hoofwijk AG, Stoot JH, Von Meyenfeldt MF, Beets GL, Derikx JP, Poeze M. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;261:345-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 383] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 8. | Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 610] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 9. | Giani A, Famularo S, Riva L, Tamini N, Ippolito D, Nespoli L, Conconi P, Sironi S, Braga M, Gianotti L. Association between specific presurgical anthropometric indexes and morbidity in patients undergoing rectal cancer resection. Nutrition. 2020;75-76:110779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, Hiyoshi Y, Iwagami S, Yoshida N, Yoshida M, Watanabe M, Baba H. Sarcopenia is a Negative Prognostic Factor After Curative Resection of Colorectal Cancer. Ann Surg Oncol. 2015;22:2663-2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 278] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 11. | Chemama S, Bayar MA, Lanoy E, Ammari S, Stoclin A, Goéré D, Elias D, Raynard B, Antoun S. Sarcopenia is Associated with Chemotherapy Toxicity in Patients Undergoing Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis from Colorectal Cancer. Ann Surg Oncol. 2016;23:3891-3898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Malietzis G, Currie AC, Athanasiou T, Johns N, Anyamene N, Glynne-Jones R, Kennedy RH, Fearon KC, Jenkins JT. Influence of body composition profile on outcomes following colorectal cancer surgery. Br J Surg. 2016;103:572-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 216] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 13. | Hopkins JJ, Reif RL, Bigam DL, Baracos VE, Eurich DT, Sawyer MB. The Impact of Muscle and Adipose Tissue on Long-term Survival in Patients With Stage I to III Colorectal Cancer. Dis Colon Rectum. 2019;62:549-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 14. | Brown JC, Caan BJ, Prado CM, Cespedes Feliciano EM, Xiao J, Kroenke CH, Meyerhardt JA. The Association of Abdominal Adiposity With Mortality in Patients With Stage I-III Colorectal Cancer. J Natl Cancer Inst. 2020;112:377-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Leijssen LGJ, Dinaux AM, Kunitake H, Bordeianou LG, Berger DL. The impact of postoperative morbidity on survival in patients with metastatic colon and rectal cancer. J Surg Oncol. 2019;120:460-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Alves A, Panis Y, Mathieu P, Mantion G, Kwiatkowski F, Slim K; Association Française de Chirurgie. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg. 2005;140:278-283, discussion 284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 379] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 17. | McDermott FT, Hughes ES, Pihl E, Milne BJ, Price AB. Comparative results of surgical management of single carcinomas of the colon and rectum: a series of 1939 patients managed by one surgeon. Br J Surg. 1981;68:850-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Lee YC, Lee YL, Chuang JP, Lee JC. Differences in survival between colon and rectal cancer from SEER data. PLoS One. 2013;8:e78709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Li M, Li JY, Zhao AL, Gu J. Colorectal cancer or colon and rectal cancer? Oncology. 2007;73:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Iqbal A, George TJ. Randomized Clinical Trials in Colon and Rectal Cancer. Surg Oncol Clin N Am. 2017;26:689-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Malietzis G, Aziz O, Bagnall NM, Johns N, Fearon KC, Jenkins JT. The role of body composition evaluation by computerized tomography in determining colorectal cancer treatment outcomes: a systematic review. Eur J Surg Oncol. 2015;41:186-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 22. | Berkel AEM, Klaase JM, de Graaff F, Brusse-Keizer MGJ, Bongers BC, van Meeteren NLU. Patient's Skeletal Muscle Radiation Attenuation and Sarcopenic Obesity are Associated with Postoperative Morbidity after Neoadjuvant Chemoradiation and Resection for Rectal Cancer. Dig Surg. 2019;36:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Choi MH, Oh SN, Lee IK, Oh ST, Won DD. Sarcopenia is negatively associated with long-term outcomes in locally advanced rectal cancer. J Cachexia Sarcopenia Muscle. 2018;9:53-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 24. | Chung E, Lee HS, Cho ES, Park EJ, Baik SH, Lee KY, Kang J. Prognostic significance of sarcopenia and skeletal muscle mass change during preoperative chemoradiotherapy in locally advanced rectal cancer. Clin Nutr. 2020;39:820-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | De Nardi P, Salandini M, Chiari D, Pecorelli N, Cristel G, Damascelli A, Ronzoni M, Massimino L, De Cobelli F, Braga M. Changes in body composition during neoadjuvant therapy can affect prognosis in rectal cancer patients: An exploratory study. Curr Probl Cancer. 2020;44:100510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Fukuoka T, Maeda K, Nagahara H, Shibutani M, Iseki Y, Matsutani S, Hirakawa K, Ohira M. Change in PMI During Neoadjuvant Therapy Is a Predictive Prognostic Marker in Rectal Cancer. Anticancer Res. 2019;39:5157-5163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Levolger S, van Vledder MG, Alberda WJ, Verhoef C, de Bruin RWF, IJzermans JNM, Burger JW. Muscle wasting and survival following pre-operative chemoradiotherapy for locally advanced rectal carcinoma. Clin Nutr. 2018;37:1728-1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Park SE, Hwang IG, Choi CH, Kang H, Kim BG, Park BK, Cha SJ, Jang JS, Choi JH. Sarcopenia is poor prognostic factor in older patients with locally advanced rectal cancer who received preoperative or postoperative chemoradiotherapy. Medicine (Baltimore). 2018;97:e13363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Takeda Y, Akiyoshi T, Matsueda K, Fukuoka H, Ogura A, Miki H, Hiyoshi Y, Nagasaki T, Konishi T, Fujimoto Y, Fukunaga Y, Ueno M. Skeletal muscle loss is an independent negative prognostic factor in patients with advanced lower rectal cancer treated with neoadjuvant chemoradiotherapy. PLoS One. 2018;13:e0195406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Rosenberg IH. Summary Comments in: Epidemiologic and Methodologic problems in Determining Nutritional Status of Older Patients. Am J Clin Nutr. 1989;50:1231-1233. [RCA] [DOI] [Full Text] [Cited by in Crossref: 782] [Cited by in RCA: 784] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 31. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6987] [Cited by in RCA: 8446] [Article Influence: 563.1] [Reference Citation Analysis (0)] |
| 32. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6646] [Cited by in RCA: 7757] [Article Influence: 1292.8] [Reference Citation Analysis (1)] |
| 33. | Albano D, Messina C, Vitale J, Sconfienza LM. Imaging of sarcopenia: old evidence and new insights. Eur Radiol. 2020;30:2199-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 241] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 34. | Amini B, Boyle SP, Boutin RD, Lenchik L. Approaches to Assessment of Muscle Mass and Myosteatosis on Computed Tomography: A Systematic Review. J Gerontol A Biol Sci Med Sci. 2019;74:1671-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 237] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 35. | Ceniccola GD, Castro MG, Piovacari SMF, Horie LM, Corrêa FG, Barrere APN, Toledo DO. Current technologies in body composition assessment: advantages and disadvantages. Nutrition. 2019;62:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 158] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 36. | Daly LE, Prado CM, Ryan AM. A window beneath the skin: how computed tomography assessment of body composition can assist in the identification of hidden wasting conditions in oncology that profoundly impact outcomes. Proc Nutr Soc. 2018;77:135-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 37. | Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2330] [Cited by in RCA: 2965] [Article Influence: 269.5] [Reference Citation Analysis (0)] |
| 38. | Shachar SS, Williams GR, Muss HB, Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur J Cancer. 2016;57:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 773] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 39. | Xiao J, Caan BJ, Cespedes Feliciano EM, Meyerhardt JA, Kroenke CH, Baracos VE, Weltzien E, Kwan ML, Alexeeff SE, Castillo AL, Prado CM. The association of medical and demographic characteristics with sarcopenia and low muscle radiodensity in patients with nonmetastatic colorectal cancer. Am J Clin Nutr. 2019;109:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Dolin TG, Mikkelsen M, Jakobsen HL, Nordentoft T, Pedersen TS, Vinther A, Zerahn B, Vistisen KK, Suetta C, Nielsen D, Johansen JS, Lund CM. Geriatric assessment and intervention in older vulnerable patients undergoing surgery for colorectal cancer: a protocol for a randomised controlled trial (GEPOC trial). BMC Geriatr. 2021;21:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg. 2012;99:550-557. [RCA] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 368] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 42. | Black D, Mackay C, Ramsay G, Hamoodi Z, Nanthakumaran S, Park KGM, Loudon MA, Richards CH. Prognostic Value of Computed Tomography: Measured Parameters of Body Composition in Primary Operable Gastrointestinal Cancers. Ann Surg Oncol. 2017;24:2241-2251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 43. | Watt DG, Martin JC, Park JH, Horgan PG, McMillan DC. Neutrophil count is the most important prognostic component of the differential white cell count in patients undergoing elective surgery for colorectal cancer. Am J Surg. 2015;210:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 44. | Carrara G, Pecorelli N, De Cobelli F, Cristel G, Damascelli A, Beretta L, Braga M. Preoperative sarcopenia determinants in pancreatic cancer patients. Clin Nutr. 2017;36:1649-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 45. | Zhang S, Tan S, Jiang Y, Xi Q, Meng Q, Zhuang Q, Han Y, Sui X, Wu G. Sarcopenia as a predictor of poor surgical and oncologic outcomes after abdominal surgery for digestive tract cancer: A prospective cohort study. Clin Nutr. 2019;38:2881-2888. [RCA] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 46. | Vergara-Fernandez O, Trejo-Avila M, Salgado-Nesme N. Sarcopenia in patients with colorectal cancer: A comprehensive review. World J Clin Cases. 2020;8:1188-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (1)] |
| 47. | Trejo-Avila M, Bozada-Gutiérrez K, Valenzuela-Salazar C, Herrera-Esquivel J, Moreno-Portillo M. Sarcopenia predicts worse postoperative outcomes and decreased survival rates in patients with colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2021;36:1077-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 141] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 48. | Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M, Tokunaga R, Kurashige J, Hiyoshi Y, Iwagami S, Yoshida N, Watanabe M, Baba H. Negative Impact of Skeletal Muscle Loss after Systemic Chemotherapy in Patients with Unresectable Colorectal Cancer. PLoS One. 2015;10:e0129742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 49. | Blauwhoff-Buskermolen S, Versteeg KS, de van der Schueren MA, den Braver NR, Berkhof J, Langius JA, Verheul HM. Loss of Muscle Mass During Chemotherapy Is Predictive for Poor Survival of Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2016;34:1339-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 283] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 50. | Stene GB, Helbostad JL, Amundsen T, Sørhaug S, Hjelde H, Kaasa S, Grønberg BH. Changes in skeletal muscle mass during palliative chemotherapy in patients with advanced lung cancer. Acta Oncol. 2015;54:340-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 51. | Yip C, Goh V, Davies A, Gossage J, Mitchell-Hay R, Hynes O, Maisey N, Ross P, Gaya A, Landau DB, Cook GJ, Griffin N, Mason R. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol. 2014;24:998-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 52. | Heus C, Cakir H, Lak A, Doodeman HJ, Houdijk AP. Visceral obesity, muscle mass and outcome in rectal cancer surgery after neo-adjuvant chemo-radiation. Int J Surg. 2016;29:159-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |