Published online Dec 15, 2022. doi: 10.4251/wjgo.v14.i12.2393
Peer-review started: October 3, 2022
First decision: October 21, 2022
Revised: October 26, 2022
Accepted: November 30, 2022
Article in press: November 30, 2022
Published online: December 15, 2022
Processing time: 69 Days and 16.9 Hours
Increasing evidence have shown that regional lymph node metastasis is a critical prognostic factor in gastric cancer (GC). In addition, lymph node dissection is a key factor in determining the appropriate treatment for GC. However, the association between the number of positive lymph nodes and area of lymph node metastasis in GC remains unclear.
To investigate the clinical value of regional lymph node sorting after radical gastrectomy for GC.
This study included 661 patients with GC who underwent radical gastrectomy at Tianjin Medical University General Hospital between January 2012 and June 2020. The patients were divided into regional sorting and non-sorting groups. Clinicopathological data were collected and retrospectively reviewed to deter
There were no significant differences between the groups in terms of the surgical method, tumor site, immersion depth, and degree of differentiation. The total number of lymph nodes was significantly higher in the regional sorting group (n = 324) than in the non-sorting group (n = 337) (32.5 vs 21.2, P < 0.001). There was no significant difference in the number of positive lymph nodes between the two groups. A total of 212 patients with GC had lymph node metastasis in the lymph node regional sorting group, including 89 (41.98%) cases in the first dissection station and 123 (58.02 %) cases in the second dissection station. Binary and multivariate logistic regression results showed that the number of positive lymph nodes (P < 0.001) was an independent risk factor for lymph node metastases at the second dissection station.
Regional sorting of lymph nodes after radical gastrectomy may increase the number of detected lymph nodes, thereby improving the reliability and accuracy of lymph node staging in clinical practice.
Core Tip: The lymph node metastasis rates of different groups of gastric cancer (GC) lymph nodes in different parts of GC differ. Understanding the mechanisms of lymph node metastasis to guide lymph node dissection during surgery is of great significance. Regional sorting of lymph nodes after radical gastrectomy for GC may increase the number of detected lymph nodes, thereby allowing a more accurate and reliable lymph node staging, which is helpful in clinical practice.
- Citation: Li C, Tian XJ, Qu GT, Teng YX, Li ZF, Nie XY, Liu DJ, Liu T, Li WD. Clinical value of regional lymph node sorting in gastric cancer. World J Gastrointest Oncol 2022; 14(12): 2393-2403
- URL: https://www.wjgnet.com/1948-5204/full/v14/i12/2393.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i12.2393
There has been a decline in the incidence and mortality rates of gastric cancer (GC) over the past five decades globally; however, GC remains the third leading cause of cancer-related deaths[1]. Studies have reported that 3%-20% of patients with early-stage GC have lymph node metastasis[2,3]. Therefore, lymph node dissection is a key factor in selecting an appropriate treatment for GC. Clarifying lymph node staging is also a critical evaluation in the planning of GC treatment.
Accurate lymph node staging can be used to evaluate the effectiveness of surgery and provide a reliable basis for the choice of follow-up treatment. To obtain a sufficient number of lymph nodes and accurately classify lymph node staging, Japanese scholars began to involve surgeons in the study of lymph node detection as early as 1996[4]. In China, Cao et al[5] studied patients with GC who underwent D2 lymph node dissection and found that the number of detected lymph nodes increased significantly in patients who had been regionally sorted, indicating that regional sorting improved the accuracy and reliability of lymph node staging in GC. However, it remains unclear whether regional lymph node sorting increases the number of positive lymph nodes.
Using the number of lymph nodes with metastasis alone, the current classification criteria are not sufficient for clinical and surgical guidance[6]. Therefore, it is essential to study the regions of lymph node metastasis. By knowing the metastasis rates of lymph nodes at various tumor sites preoperatively, surgeons can remove all possible positive lymph nodes, enabling a ‘root-and-branch’ effect of the operation, thus improving patient outcomes.
Lymph node staging and lymph node metastasis regions are important considerations in the diagnosis and treatment of GC. Researchers have compared the current lymph node staging system with the lymph node metastasis region system used in Japan and found that these two systems have the same advantages in determining prognosis. The authors, therefore, concluded that the lymph node metastasis system should be incorporated into the current lymph node staging system[7]. However, few studies have focused on the relationship between the current lymph node staging system and regions of lymph node metastasis. Therefore, the aim of this study was to investigate the clinical value of regional lymph node sorting after radical gastrectomy in patients with GC.
We evaluated the role of regional lymph node sorting in clinical settings by comparing the number of lymph nodes detected between regional sorting and non-sorting groups. For patients in the lymph node regional sorting group, the lymph node metastatic rate was summarized for different tumor regions, providing an analytical basis for surgical dissection of the lymph nodes. The relationship between the number of positive lymph nodes and location of lymph node metastasis was analyzed to evaluate the current lymph node staging system and provide a theoretical basis for further treatment of patients in the lymph node regional sorting group with lymph node metastasis.
Based on the inclusion and exclusion criteria, 661 patients with gastric tumors were admitted to the Department of Gastrointestinal Surgery at Tianjin Medical University General Hospital from January 2012 to June 2020. Patients were divided into two groups according to the examination method: The regional sorting group and non-sorting group. The inclusion criteria were: (1) Diagnosis of GC by imaging and pathology; (2) No history of malignant tumors; and (3) Standard radical gastrectomy for GC. The exclusion criteria were: (1) New auxiliary chemotherapy; and (2) A history of gastric resection. The study complied with the Declaration of Helsinki and was approved by the ethics committee of Tianjin Medical University General Hospital (approval number: IRB2022-WZ-167). The need for informed consent was waived by the ethics committee.
General information collected in this study included sex, age, surgical method (near-end gastrectomy, far-end gastrectomy, and total gastrectomy), tumor sites [primary lesions in the upper third of the stomach (U), primary lesions in the middle third of the stomach (M), primary lesions in the lower third of the stomach (L) (the main tumor body was considered if the tumor invaded into two regions)], immersion depth, differentiation degree [differentiated carcinoma (DCA) (highly DCA, mediated carcinoma, papilloma cancer), undifferentiated carcinoma (UCA) (low differentiation carcinoma, mucus carcinoma, anti-cell carcinoma, undifferentiated cancer)], total number of lymph nodes, number of positive lymph nodes, metastatic lymph node ratio (MLNR): The ratio of the number of positive lymph nodes to the number of lymph nodes detected, and lymph node metastatic regions (first dissection station: Group Nos. 1-7; second dissection station: group Nos. 8a-12a). The order of lymph node metastasis may vary depending on the location of the primary tumor. Additionally, if the primary tumor is more infiltrated or poorly differentiated, lymph nodes may be more prone to metastases. Gong et al[8] have indicated that the prognosis of patients with N2 stage GC is similar to that of GC patients with regional lymph node metastasis who only underwent first-stage dissection. Therefore, 6 positive lymph nodes can be used as a boundary for the analysis of the relationship between lymph node metastasis areas and current lymph node staging. Moreover, these data are intuitive and easy to analyze clinically. Therefore, tumor sites, immersion depth, differentiation degree, and number of positive lymph nodes were included as variables in our multivariate logistic regression analysis.
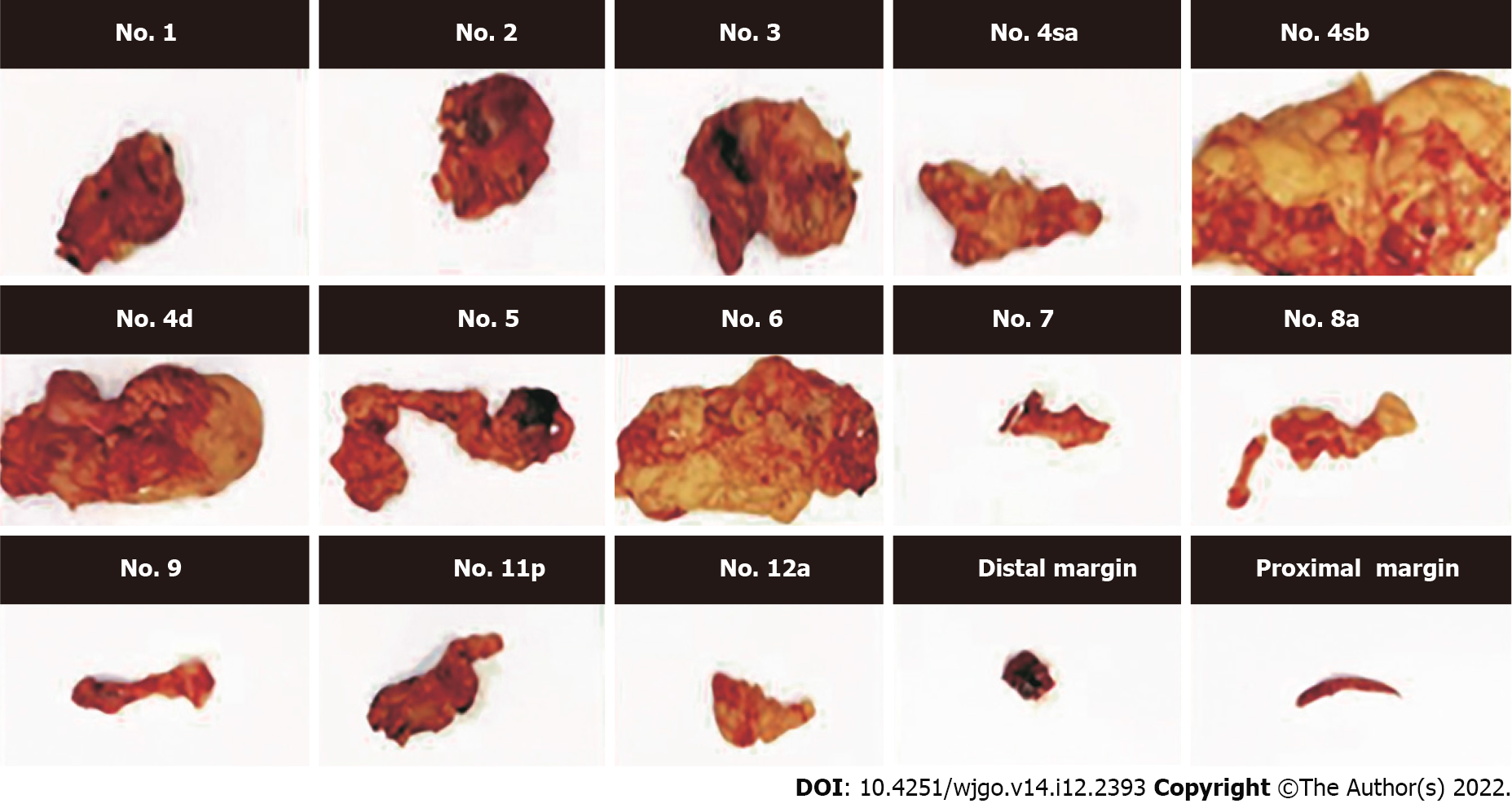
Specimens collected from gastrectomy were flushed to remove the blood and afterward dried using sterile towels. According to the original anatomical position in the body, the specimens were flattened, expanded, measured, and recorded. Lymph nodes were further sorted using the following procedure: The tissue was sequentially cut according to the dissected lymph nodes, and the staging, location, and definition of the peritoneal lymph nodes (lymph nodes on the small curved side and their surrounding soft tissues, lymph nodes on the peritoneal stem and its branch root, and lymph nodes on the large curved side and the surrounding soft tissue) were recorded and placed in the corresponding specimen bags. Afterward, the gastric wall along the opposite side (large or small curved side) was dissected, the tissue of the gastric mucosa was fully exposed, and indistinguishable tumor sites were marked with silk threads to enable the pathologist to locate the lesion. Next, the gastric tissue was unfolded, measured, and photographed. Finally, the excised stomach, peritoneal fat blood vessels, lymphoid tissue, and large omentum were fixed with 10% formaldehyde solution and sent to the Department of Pathology for further examination (Figure 1).
Statistical analysis was performed using the IBM SPSS statistical software (Version 26.0; IBM Corp., New York, United States). Categorical variables are expressed as frequencies and percentages. The chi-square test was used for intergroup comparisons (n < 40 cases with Fisher’s exact probability method). The continuous variables that conformed to the normal distribution measured in this study were expressed as mean ± SD. Independent sample t-tests were used for intergroup comparisons. Continuous variables that did not conform to a normal distribution were expressed as median (interquartile range), and the Mann-Whitney U test was used for inter-group comparisons. The MLNR in each group were expressed as percentages. Risk factors for lymph node metastasis in the second dissection station were identified using binary and multivariate logistic regression analyses. Statistical significance was set at P < 0.05. Statistical review of the study was performed by a biomedical statistician from Tianjin Medical University.
The mean patient age was 63.3 ± 10.2 (31-92), of which 457 (69.14%) were male and 204 (30.86%) were female. Three hundred and twenty-four patients (49.02%) were included in the regional sorting group and 337 (50.98%) were included in the non-sorting group. The surgical method (P = 0.422), tumor site (P = 0.254), immersion depth (P = 0.558), and degree of differentiation (P = 0.089) were not significantly different between the regional sorting and non-sorting groups (Table 1).
| Regional sorting group (n = 324) | Non-sorting group (n = 337) | χ² | P value | |
| Surgery | 1.727 | 0.422 | ||
| Proximal gastrectomy | 19 | 27 | ||
| Distal gastrectomy | 177 | 189 | ||
| Total gastrectomy | 128 | 121 | ||
| Tumor location | 2.744 | 0.254 | ||
| U | 46 | 38 | ||
| M | 92 | 85 | ||
| L | 186 | 214 | ||
| Immersion depth | 2.071 | 0.558 | ||
| T1 | 69 | 60 | ||
| T2 | 42 | 38 | ||
| T3 | 19 | 21 | ||
| T4 | 194 | 218 | ||
| Differentiation degree | 2.891 | 0.089 | ||
| DCA | 181 | 166 | ||
| UCA | 143 | 171 |
A total of 18977 lymph nodes were detected in all patients, of which 11111 (58.55%) were detected in the regional sorting group and 7866 (41.45%) in the non-sorting group. A total of 4399 positive lymph nodes were identified, of which 2264 (51.47%) were in the regional sorting group and 2135 (48.53%) in the non-sorting group. The number of detected lymph nodes in the two groups was significantly different (P < 0.001); however, there were no significant differences in the number of detected positive lymph nodes between the groups (P = 0.863) (Table 2).
| Pieces, median (interquartile range) | Z | P value | ||
| Regional sorting group | Non-sorting group | |||
| The number of lymph nodes detected | 32.5 (24.0, 42.0) | 21.0 (17.0, 28.0) | -10.775 | < 0.001 |
| Number of positive lymph nodes | 2.0 (0.0, 9.0) | 3.0 (0.0, 9.0) | -0.172 | 0.863 |
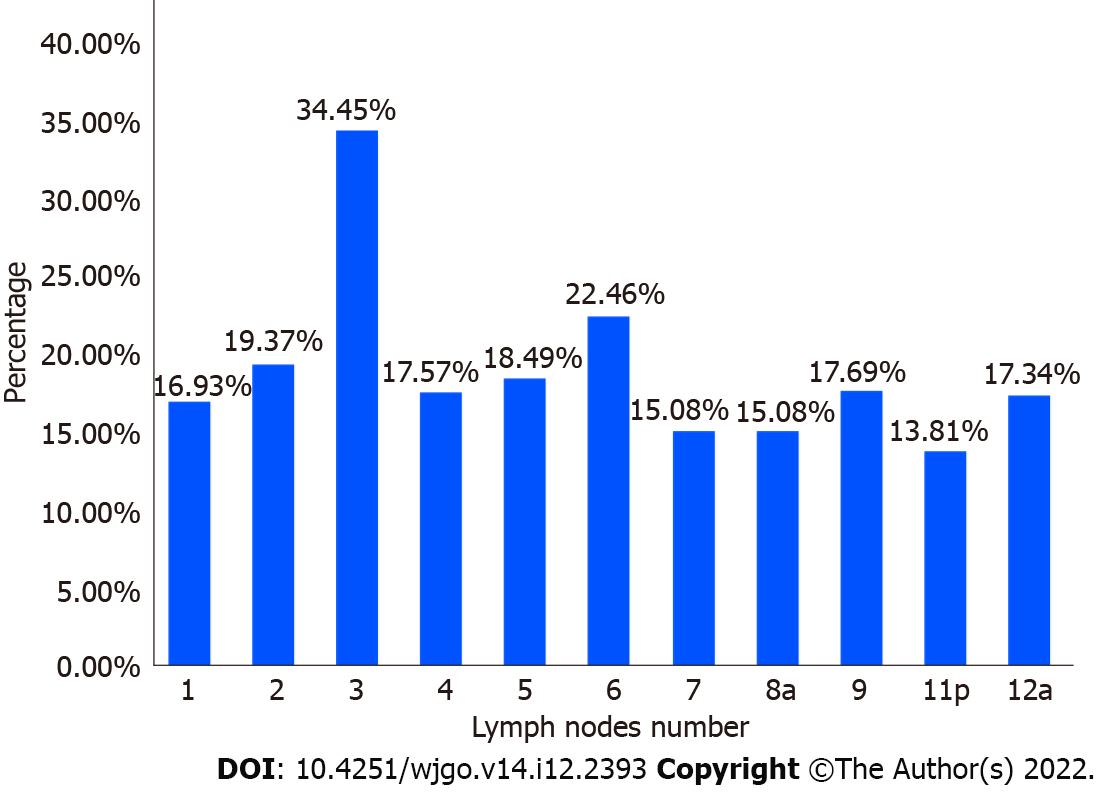
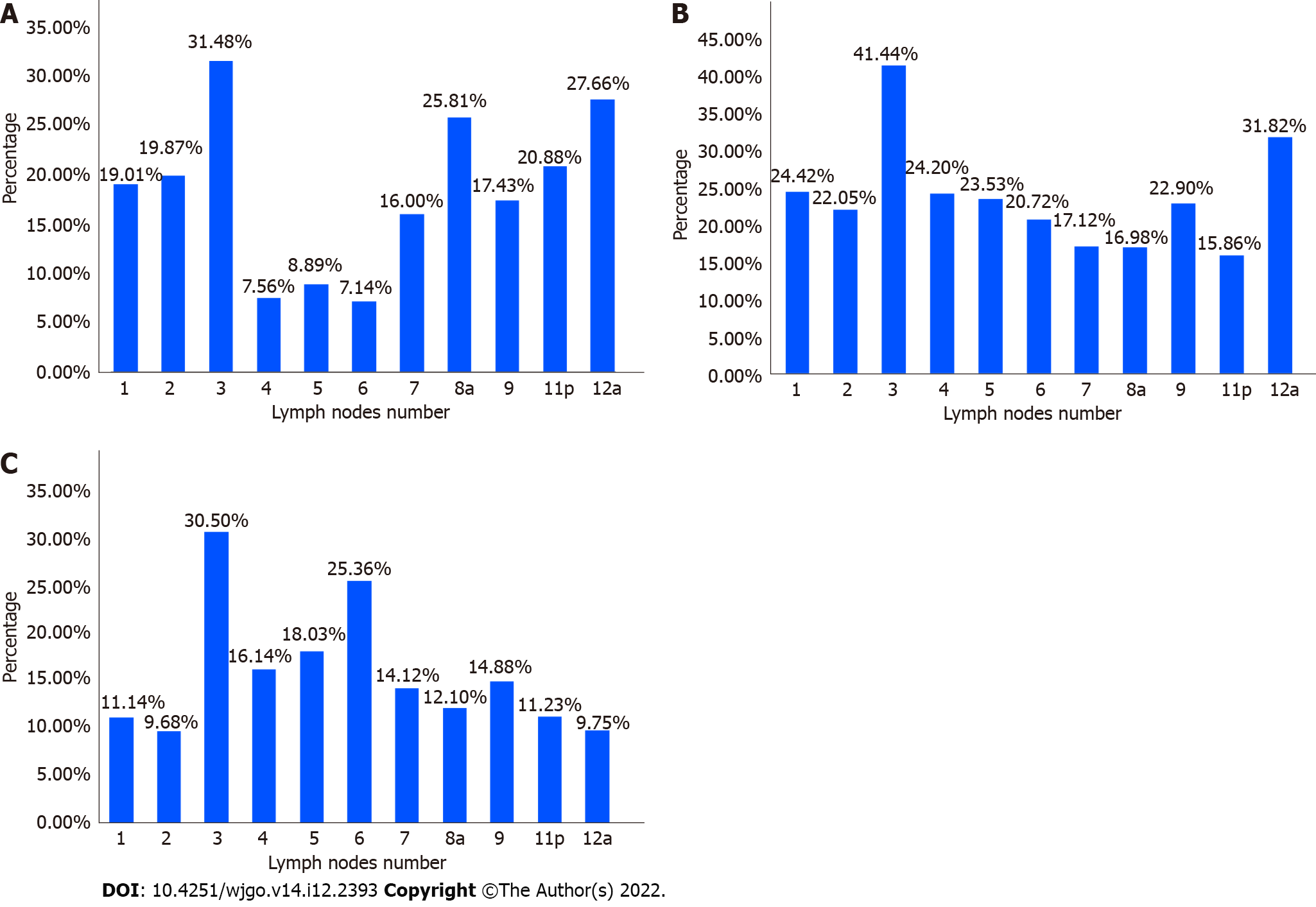
Among the 324 patients in the regional sorting group, the highest lymph node metastasis ratio was found in group 3 (34.45%), followed by group 6 (22.46%). The lowest ratio was found in group 11p (13.81%) (Figure 2). Among the 46 patients with GC, region U showed the highest lymph node metastasis ratio in group 3 (31.48%). The ratios were low in groups 4 (7.56%), 5 (8.89%), and 6 (7.14%) (Figure 3A). Among the 92 patients with GC, region M had the highest lymph node metastasis in group 3 (41.44%) and the lowest ratio in group 11p (15.56%) (Figure 3B). Among the 186 patients, the highest lymph node metastasis ratio in region L was 30.50% in group 3, followed by 25.36% in group 6 and 9.68% in group 2 (Figure 3C).
A total of 212 patients with GC in the lymph node regional sorting group had lymph node metastasis, including 89 cases (41.98%) at the first dissection station and 123 cases (58.02%) at the second dissection station. Binary and multivariate logistic regression results showed that the number of positive lymph nodes (P < 0.001) was an independent risk factor for lymph node metastases at the second dissection station (Tables 3 and 4).
| B | SE | Wald | df | P value | OR | 95%CI | ||
| Upper | Lower | |||||||
| Tumor location | ||||||||
| U | 1.067 | 2 | 0.587 | |||||
| M | -0.087 | 0.460 | 0.036 | 1 | 0.850 | 0.917 | 0.372 | 2.258 |
| L | -0.346 | 0.421 | 0.675 | 1 | 0.411 | 0.708 | 0.310 | 1.615 |
| Immersion depth | ||||||||
| T1 | 5.468 | 3 | 0.141 | |||||
| T2 | 1.872 | 0.829 | 5.092 | 1 | 0.024 | 6.500 | 1.279 | 33.034 |
| T3 | 1.504 | 0.850 | 3.132 | 1 | 0.077 | 4.500 | 0.851 | 23.801 |
| T4 | 1.464 | 0.865 | 4.567 | 1 | 0.033 | 4.324 | 1.129 | 16.557 |
| Differentiation degree | ||||||||
| DCA/UCA | 0.415 | 0.282 | 2.201 | 1 | 0.138 | 1.515 | 0.875 | 2.621 |
| The number of positive lymph nodes (pieces) | ||||||||
| ≤ 6/> 6 | 1.707 | 0.305 | 31.284 | 1 | < 0.001 | 5.514 | 3.031 | 10.029 |
| B | SE | Wald | df | P value | OR | 95%CI | ||
| Upper | Lower | |||||||
| Tumor location | ||||||||
| U | 0.601 | 2 | 0.741 | |||||
| M | -0.299 | 0.511 | 0.344 | 1 | 0.558 | 0.741 | 0.273 | 2.017 |
| L | -0.358 | 0.463 | 0.599 | 1 | 0.439 | 0.699 | 0.282 | 1.732 |
| Immersion depth | ||||||||
| T1 | 4.710 | 3 | 0.194 | |||||
| T2 | 1.817 | 0.875 | 4.316 | 1 | 0.038 | 6.153 | 1.108 | 34.160 |
| T3 | 0.956 | 0.922 | 1.075 | 0.300 | 2.601 | 0.427 | 15.852 | |
| T4 | 0.884 | 0.735 | 1.445 | 1 | 0.229 | 2.420 | 0.573 | 10.223 |
| Differentiation degree | ||||||||
| DCA/UCA | 0.124 | 0.323 | 0.147 | 1 | 0.701 | 1.132 | 0.601 | 2.130 |
| The number of positive lymph nodes (pieces) | ||||||||
| ≤ 6/> 6 | 1.718 | 0.322 | 28.541 | 1 | < 0.001 | 5.576 | 2.968 | 10.473 |
| Constant | -1197 | 0.829 | 2086 | 1 | 0.149 | 0.302 | ||
Anatomy-based GC lymph node staging cannot ensure objectivity in clinical practice[8]. Therefore, the tumor-node-metastasis staging detailed in the 5th edition published by the Union for International Cancer Control (UICC)/American Joint Committee on Cancer is no longer based on anatomy, but on the number of metastatic lymph nodes[9]. Although the methods for lymph node staging were adjusted through multiple versions of the UICC publication, the standard method based on the number of metastatic lymph nodes did not change. Furthermore, the 14th edition, published by the Japanese Gastric Cancer Association abandoned the method of determining lymph node stages based on the location of the primary lesions and lymphatic metastasis[10]. Thus, the number of lymph nodes, one of the key factors in lymph node staging, has become the focus of current research.
A previous study showed that the number of lymph nodes detected is closely related to the pathological staging and prognosis of GC[11]. Currently, it is believed that the number of lymph nodes should be > 16. With improvements in lymph node detection methods and technology, the total number of lymph nodes detected is gradually increasing. Therefore, 16 lymph nodes are now considered the minimum requirement for staging and prognosis, and their use alone cannot guarantee an accurate prognosis of patients with GC[12,13]. One study revealed that the greater the number of lymph nodes detected, the greater the credibility of lymph node staging, which in turn leads to an accurate assessment of patient prognosis and the development of appropriate follow-up treatments[14]. In the current study, more than 16 lymph nodes (median, 32.5) were detected in the regional sorting group. Therefore, we considered that the number of lymph nodes obtained from the lymph node region was sufficient for lymph node staging.
The number of detected lymph nodes can be affected by many factors, such as surgery[15,16], lymph node sorting, and detection techniques[17]. Among these, postoperative lymph node sorting methods have not been fully studied. Presently, scholars generally believe that regional lymph node sorting after surgery can increase the number of lymph nodes detected[5,18]; however, the effect of regional sorting on the detection of positive lymph nodes remains debatable. Jiang et al[19] showed that lymph node sorting can increase the number of positive lymph nodes detected after surgery in patients with GC. However, in a prospective study, Wang et al[20] proved that regional lymph node sorting did not increase the number of positive lymph nodes. In our study, significantly more lymph nodes were detected in the regional sorting group than in the non-sorting group (P < 0.001). The increased number of detected lymph nodes was due to regional sorting; a pathologist who may not be familiar with gastric circumferential anatomy can easily identify the lymph nodes, rather than striving to identify “at least 16” lymph nodes[10]. The regional sorting method used in our study largely reduced the number of undetected lymph nodes. Similar to the findings of other studies, the number of positive lymph nodes in our study did not increase with the number of lymph nodes detected in the regional sorting group. This may be due to the fact that the diameter of positive lymph nodes is usually larger than that of negative lymph nodes. Noda et al[21] showed that positive lymph nodes have an average diameter of approximately 7.80 mm, whereas negative lymph nodes have an average diameter of only 5.30 mm; therefore, positive lymph nodes are more likely to be detected by pathologists. It is reasonable to assume that, due to the smaller diameter, some negative lymph nodes may not have been detected in the past, but this did not affect lymph node staging. Regional sorting of lymph nodes increases the number of detected lymph nodes and the credibility of lymph node staging; therefore, it has important clinical benefits.
Clarifying the role of lymph node metastasis in GC can provide basic guidance for the surgical dissection of lymph nodes. In this study, we found that the most likely regions of lymph node metastases were near the lesser curvature in group 3 and near the gastric sinus in group 6, which may indicate that these sites are potential locations for GC progression. Furthermore, in studying the lymph node metastasis patterns of gastric tumors located at different sites, we found that the most easily metastasized region was group 3, regardless of the primary lesion site (U, M, and L stomach), which is consistent with the location near the lesser curvature and with the main direction of lymphatic reflux in the stomach. In region U, lymph node metastasis was mainly located in groups 1-3 and 7-12a, whereas in groups 4-6, significantly fewer positive lymph nodes were observed. In region M, the lymph nodes in groups 1-12a were susceptible to aggression. In region L, lymph node metastasis was mainly located in groups 3-6, whereas groups 2 and 12a had less aggressive metastases than those in the other groups. The results suggest that although the direction of lymphatic drainage of stomach cancer varies in different regions, lymph nodes that are closer to the tumor’s primary lesions or in the main direction of lymphatic reflux are more likely to metastasize. Therefore, for gastric tumors located at different sites, lymph nodes detected throughout the stomach can be used as a guide for further treatment.
The current lymph node staging system is based on the number of positive lymph nodes and cannot provide additional information for surgical guidance. Therefore, the standard lymph node metastasis location system for GC as a lymph node staging method has been studied and improved[22]. Duchon et al[23] showed that lymph node staging based on lymph node metastasis location is correlated with patient prognosis and that there is no difference between these two lymph node staging methods in evaluating patient prognosis. Son et al[7] studied 4043 patients with GC and found that when No. 2-7 and No. 14 lymph node metastasis occurred in patients with GC, their prognosis was worse than that of patients with only No. 1-6 lymph node metastasis. The authors suggested that inclusion of the examination of lymph node metastasis in the current lymph node staging system could more accurately predict patient prognosis. Other researchers believe that lymph node metastasis is an independent survival predictor and that lymph node metastasis sites should be considered in future staging systems[24]. However, these studies did not analyze the relationship between the location of lymph node metastasis and current lymph node staging.
Some studies have shown that the prognosis of patients with 2 stage GC in the current lymph node staging system is similar to that of patients with GC who were in the first dissection station of lymph node metastasis[8]. Therefore, we analyzed the relationship between the lymph node metastasis region system and the current lymph node staging system using a cutoff of six positive lymph nodes (Tables 3 and 4). Binary and multivariable logistic regression results showed that the number of positive lymph nodes was an independent risk factor for lymph node metastasis in the second dissection station, indicating that lymph node metastases in the second dissection station increased with the progression of lymph node staging. Inevitably, some limitations were present in our study. Firstly, the data collection in this study originated from a single surgical center over 8 years, so data bias was inevitable. In addition, the sorting of lymph nodes in surgical specimens of GC was completed by multiple people, and therefore measurement deviations were inevitable. Secondly, the Lauren classification was not included in the pathology report of Tianjin Medical University general hospital, which may have affected the richness of the analysis of this study.
Overall, with an increase in lymph node metastasis, lymph node metastasis occurred from the first to the second dissection station. Therefore, identifying the region of lymph node metastasis may increase the accuracy of lymph node staging. The inclusion of regional lymph node sorting into a lymph node staging system should be further studied in future research.
In recent years, the morbidity and mortality of gastric cancer (GC) remain high worldwide. Its incidence ranks fifth among malignant tumors, and its mortality ranks fourth. The progression of GC involves direct tumor invasion, lymph node metastasis, and organ and peritoneal metastasis. Lymph node metastasis is one of the main ways of GC metastasis. Even in patients with early GC, 3% to 20% of patients with early GC can develop lymph node metastasis. Therefore, surgical dissection of lymph nodes is the key to the treatment of GC, and obtaining accurate lymph node staging is also a non-negligible part of the treatment of GC.
Accurate lymph node staging can evaluate the therapeutic effect of surgery, and can also provide a reliable basis for patients to choose subsequent treatment options. Since the current lymph node staging takes the number of metastatic lymph nodes as the staging standard, the number of detected lymph nodes in postoperative specimens of GC is particularly important.
To explore the clinical application value of lymph node region sorting after radical gastrectomy for GC, summarize the rules of lymph node metastasis in different parts of GC around the stomach, and further explore the relationship between the number of positive lymph nodes and the lymph node metastasis area.
The clinicopathological data of patients who underwent radical gastrectomy for GC in the Gastrointestinal and Anorectal Surgery Department of Tianjin Medical University General Hospital from January 2012 to June 2020 were collected, and the number of lymph nodes, positive lymph nodes in the lymph node regional sorting group and the unsorted group were analyzed. Differences in the number of lymph nodes; GC patients who had undergone regional sorting were grouped according to tumor sites, and the lymph node metastasis rates in each group were statistically analyzed, and the relationship between the number of positive lymph nodes and the lymph node metastasis area was analyzed by logistic regression.
The number of lymph nodes sent for examination in the regional sorting group was more than that in the unsorted group (P < 0.001); there was no significant difference in the number of positive lymph nodes between the two groups (P = 0.863). The lymph nodes with higher metastasis rate in upper cancer were No. 3 group (31.48%), while No. 4 group (7.56%), No. 5 group (8.89%), and No. 6 group (7.14%). The lymph node metastasis rate is low; in the middle cancer, the lymph node metastasis rate is higher in each group; in the lower cancer, the lymph nodes with higher metastasis rate are No. 3 group (30.50%), No. 2 group (9.68%), No. 12a (9.75%) had low lymph node metastasis rate. The multivariate logistic regression results showed that the number of positive lymph nodes was positively correlated with the risk of lymph node metastasis in the second station dissection area.
Regional lymph node sorting after radical gastrectomy for GC can increase the number of detected lymph nodes and make lymph node staging more accurate and credible, which is worthy of clinical implementation. The lymph node metastasis rates of different groups of GC lymph nodes in different parts of GC are different. It is of great significance to understand the rules of lymph node metastasis to guide the lymph node dissection during operation. With the increase of the number of positive lymph nodes, the site of lymph node metastasis spreads from the first-stage dissection area to the second-stage dissection area. Identifying the location of lymph node metastasis can make lymph node staging more accurate.
The current lymph node staging has a certain degree of consistency with the location of lymph node metastasis. With the increase in the number of lymph node metastases, the location of lymph node metastasis spreads from the first-stage dissection area to the second-stage dissection area. Identifying the lymph node metastasis location can make lymph node staging more accurate. Optimization of lymph node staging by including lymph node metastases requires further study.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang HL, Japan; Wu C, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol. 2020;18:534-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 981] [Article Influence: 196.2] [Reference Citation Analysis (1)] |
| 2. | Lee JH, Choi IJ, Han HS, Kim YW, Ryu KW, Yoon HM, Eom BW, Kim CG, Lee JY, Cho SJ, Kim YI, Nam BH, Kook MC. Risk of lymph node metastasis in differentiated type mucosal early gastric cancer mixed with minor undifferentiated type histology. Ann Surg Oncol. 2015;22:1813-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Ren G, Cai R, Zhang WJ, Ou JM, Jin YN, Li WH. Prediction of risk factors for lymph node metastasis in early gastric cancer. World J Gastroenterol. 2013;19:3096-3107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Bunt AM, Hermans J, van de Velde CJ, Sasako M, Hoefsloot FA, Fleuren G, Bruijn JA. Lymph node retrieval in a randomized trial on western-type versus Japanese-type surgery in gastric cancer. J Clin Oncol. 1996;14:2289-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Cao Y, Xiong L, Deng S, Shen L, Li J, Wu K, Wang J, Tao K, Wang G, Cai K. The effect of perigastric lipolymphatic tissue grouping by surgeon on the number of pathologic sampled lymph nodes after radical gastrectomy. Medicine (Baltimore). 2018;97:e11411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Lu J, Zheng ZF, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Tu RH, Huang CM, Zheng CH, Li P. Is the 8th Edition of the AJCC TNM Staging System Sufficiently Reasonable for All Patients with Noncardia Gastric Cancer? Ann Surg Oncol. 2018;25:2002-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Son T, Hyung WJ, Kim JW, Kim HI, An JY, Cheong JH, Choi SH, Noh SH. Anatomic extent of metastatic lymph nodes: still important for gastric cancer prognosis. Ann Surg Oncol. 2014;21:899-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 8. | Gong Y, Pan S, Wang X, Zhu G, Xu H, Zhu Z. A novel lymph node staging system for gastric cancer including modified Union for cancer Control/American Joint Committee on cancer and Japanese Gastric Cancer Association criteria. Eur J Surg Oncol. 2020;46:e27-e32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803-1804. [PubMed] [DOI] [Full Text] |
| 10. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2872] [Article Influence: 205.1] [Reference Citation Analysis (0)] |
| 11. | Chen HN, Chen XZ, Zhang WH, Chen XL, Yang K, Liu JP, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. Necessity of harvesting at least 25 lymph nodes in patients with stage N2-N3 resectable gastric cancer: a 10-year, single-institution cohort study. Medicine (Baltimore). 2015;94:e620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Bouvier AM, Haas O, Piard F, Roignot P, Bonithon-Kopp C, Faivre J. How many nodes must be examined to accurately stage gastric carcinomas? Cancer. 2002;94:2862-2866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Kim YI. Does the retrieval of at least 15 lymph nodes confer an improved survival in patients with advanced gastric cancer? J Gastric Cancer. 2014;14:111-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Deng J, Zhang R, Pan Y, Wang B, Wu L, Jiao X, Bao T, Hao X, Liang H. Comparison of the staging of regional lymph nodes using the sixth and seventh editions of the tumor-node-metastasis (TNM) classification system for the evaluation of overall survival in gastric cancer patients: findings of a case-control analysis involving a single institution in China. Surgery. 2014;156:64-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Ichikura T, Ogawa T, Chochi K, Kawabata T, Sugasawa H, Mochizuki H. Minimum number of lymph nodes that should be examined for the International Union Against Cancer/American Joint Committee on Cancer TNM classification of gastric carcinoma. World J Surg. 2003;27:330-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Lee WJ, Hong RL, Lai IR, Chen CN, Lee PH, Chung KC. Reappraisal of the new UICC staging system for gastric cancer: problem in lymph node stage. Hepatogastroenterology. 2002;49:860-864. [PubMed] |
| 17. | Cai YQ, Liang YX, Yu SY, Tu RS. [Clinical value of carbon nanoparticles tracer in gastric cancer surgery to increase the number of lymph nodes retrieval]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:984-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Afaneh C, Levy A, Selby L, Ku G, Tang L, Yoon SS, Coit D, Strong VE. Ex Vivo Lymphadenectomy During Gastrectomy for Adenocarcinoma Optimizes Lymph Node Yield. J Gastrointest Surg. 2016;20:165-71; discussion 171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Jiang L, Yao Z, Zhang Y, Hu J, Zhao D, Zhai H, Wang X, Zhang Z, Wang D. Comparison of lymph node number and prognosis in gastric cancer patients with perigastric lymph nodes retrieved by surgeons and pathologists. Chin J Cancer Res. 2016;28:511-518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Wang P, Zhang K, Xi H, Liang W, Xie T, Gao Y, Wei B, Chen L. Lymph Node Yield Following Packet Submission After Isolation By Surgeon During Gastrectomy. Cancer Manag Res. 2019;11:9871-9881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Noda N, Sasako M, Yamaguchi N, Nakanishi Y. Ignoring small lymph nodes can be a major cause of staging error in gastric cancer. Br J Surg. 1998;85:831-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1336] [Article Influence: 334.0] [Reference Citation Analysis (2)] |
| 23. | Duchon R, Bernadic M Jr, Pindak D. Impact of the anatomical location and the number of metastatic lymph nodes on gastric cancer patient´s survival. Bratisl Lek Listy. 2020;121:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Anderegg MC, Lagarde SM, Jagadesham VP, Gisbertz SS, Immanuel A, Meijer SL, Hulshof MC, Bergman JJ, van Laarhoven HW, Griffin SM, van Berge Henegouwen MI. Prognostic Significance of the Location of Lymph Node Metastases in Patients With Adenocarcinoma of the Distal Esophagus or Gastroesophageal Junction. Ann Surg. 2016;264:847-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |