Published online Oct 15, 2022. doi: 10.4251/wjgo.v14.i10.2014
Peer-review started: June 8, 2022
First decision: June 23, 2022
Revised: July 6, 2022
Accepted: August 22, 2022
Article in press: August 22, 2022
Published online: October 15, 2022
Processing time: 128 Days and 6.9 Hours
Multiple classes of molecular biomarkers have been studied as potential predictors for rectal cancer (RC) response. Carcinoembryonic antigen (CEA) is the most widely used blood-based marker of RC and has proven to be an effective predictive marker. Cancer antigen 19-9 (CA19-9) is another tumor biomarker used for RC diagnosis and postoperative monitoring, as well as monitoring of the therapeutic effect. Using a panel of tumor markers for RC outcome prediction is a practical approach.
To assess the predictive effect of pre-neoadjuvant chemoradiotherapy (NCRT) CEA and CA19-9 levels on the prognosis of stage II/III RC patients.
CEA and CA19-9 levels were evaluated 1 wk before NCRT. According to the receiver operating characteristic curve analysis, the optimal cut-off point of CEA and CA19-9 levels for the prognosis were 3.55 and 19.01, respectively. The novel serum tumor biomarker (NSTB) scores were as follows: score 0: Pre-NCRT CEA < 3.55 and CA19-9 < 19.01; score 2: Pre-NCRT CEA > 3.55 and CA19-9 > 19.01; score 1: Other situations. Pathological information was recorded according to histopathological reports after the operation.
In the univariate analysis, pre-NCRT CEA < 3.55 [P = 0.025 for overall survival (OS), P = 0.019 for disease-free survival (DFS)], pre-NCRT CA19-9 < 19.01 (P = 0.014 for OS, P = 0.009 for DFS), a lower NSTB score (0-1 vs 2, P = 0.009 for OS, P = 0.005 for DFS) could predict a better prognosis. However, in the multivariate analysis, only a lower NSTB score (0-1 vs 2; for OS, HR = 0.485, 95%CI: 0.251-0.940, P = 0.032; for DFS, HR = 0.453, 95%CI: 0.234-0.877, P = 0.019) and higher pathological grade, node and metastasis stage (0-I vs II-III; for OS, HR = 0.363, 95%CI: 0.158-0.837, P = 0.017; for DFS, HR = 0.342, 95%CI: 0.149-0.786, P = 0.012) were independent predictive factors.
The combination of post-NCRT CEA and CA19-9 was a predictive factor for clinical stage II/III RC patients receiving NCRT, and the combined index had a stronger predictive effect.
Core Tip: Tumor microenvironment (TME) combined with neoadjuvant chemotherapy (NCRT) is the standard treatment for resectable stage II/III rectal cancer (RC). Multiple classes of molecular biomarkers have been studied as potential predictors for RC response but there is no sufficient evidence for any of them to be introduced into clinical practice. By retrospectively evaluating clinical stage II/III RC patients undergoing NCRT followed by standard TME, we found that the combination of NCRT carcinoembryonic antigen and carbohydrate antigen 19-9 levels could be a prognostic predictor for clinical stage II/III RC patients receiving NCRT, and the combined indexes had a stronger predictive effect than the index alone.
- Citation: Zhao JY, Tang QQ, Luo YT, Wang SM, Zhu XR, Wang XY. Predictive value of a serum tumor biomarkers scoring system for clinical stage II/III rectal cancer with neoadjuvant chemoradiotherapy. World J Gastrointest Oncol 2022; 14(10): 2014-2024
- URL: https://www.wjgnet.com/1948-5204/full/v14/i10/2014.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i10.2014
In the United States, tumor microenvironment (TME) combined with neoadjuvant chemotherapy (NCRT) is the standard treatment for resectable stage II/III rectal cancer (RC)[1-3]. Although numerous studies have shown that NCRT can reduce the rate of local recurrence, it is difficult to improve overall survival (OS)[4-6]. Multiple classes of molecular biomarkers have been studied as potential predictors for RC response but there is no sufficient evidence for any of them to be introduced into clinical practice[7]. Moreover, additional systematic chemotherapy could increase the toxicity [8,9]. Therefore, it is critical to identify predictive factors for clinical stage II/III patients and give additional chemotherapy or more aggressive treatment strategies.
Pathological indicators are generally considered to be the most effective predictive factors[10,11]. Unfortunately, pathological characteristics are difficult to obtain and quantitate and are usually affected by the operation and specimen-processing quality[8,9]. Moreover, the pathological indicators, which can only be obtained after surgery, do not assist in judging whether patients need additional chemotherapy before undergoing NCRT or surgery.
A glycoprotein, carcinoembryonic antigen (CEA), is the most widely used blood-based marker of RC and has proven to be an effective predictive marker[12-14]. According to You et al[15], the increment in postoperative serum CEA levels (CEA < 5 vs > 5) was an independent predictor of a poor prognosis. However, the major problem with the use of CEA as a marker of RC is its association with other types of cancer and benign diseases (inflammatory bowel disease)[16-18]. Cancer antigen 19-9 (CA19-9) is another tumor biomarker used for RC diagnosis and postoperative monitoring, as well as monitoring of the therapeutic effect[19,20]. Due to the highly heterogeneous nature of RC, a single tumor marker is unlikely to become a stand-alone predictive factor. Using a panel of tumor markers for RC outcome prediction is a practical approach.
In this study, we analyzed the predictive value of the combination of pre-NCRT serum tumor markers (CEA and CA19-9) in clinical stage II/III RC patients.
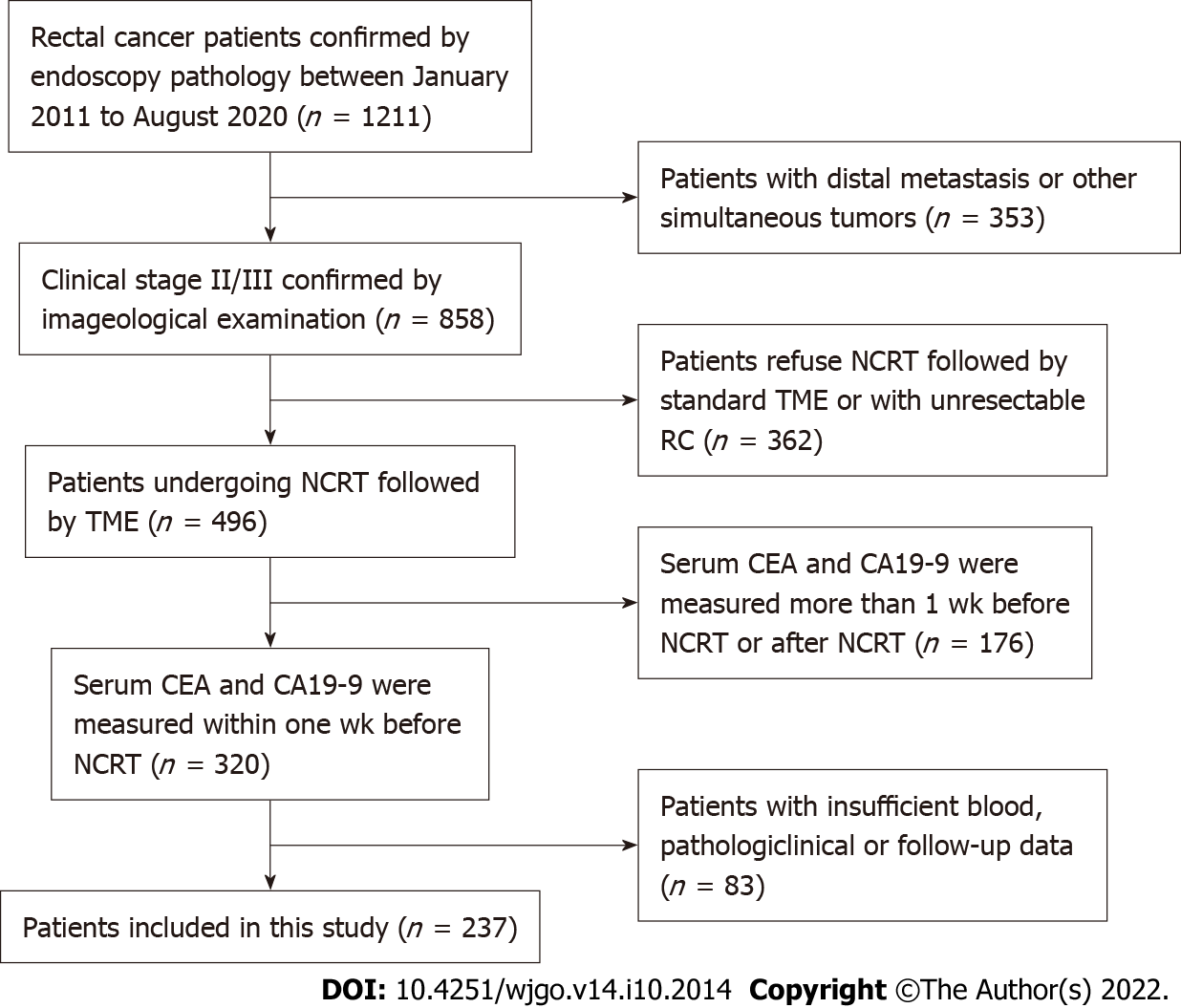
We retrospectively evaluated clinical stage II/III RC patients undergoing NCRT followed by standard TME in our hospital from February 2011 to August 2020. We included the following categories of patients: (1) Patients receiving preoperative NCRT; (2) patients with colorectal adenocarcinoma confirmed by pathological biopsy; (3) patients whose serum CEA and CA19-9 levels were measured within one week before NCRT; and (4) patients undergoing NCRT followed by standard TME. We excluded the following categories of patients: (1) Patients with distal metastasis; (2) patients with other concomitant tumors; (3) patients with insufficient blood, clinicopathological, or follow-up data; and (4) patients with unresectable RC. The patient-screening flowchart is shown in Figure 1.
This retrospective study was approved by the ethics committee of our hospital. The requirement for patients’ informed consent was waived due to the retrospective nature of the study.
All patients in this study received NCRT followed by standard TME. Their CEA and CA19-9 levels were evaluated within 1 wk pre-NCRT. Pathological, node and metastasis (TNM) stages and histological grades were noted according to histopathological reports. The receiver operating characteristic (ROC) curve was adopted to determine the best cut-off values of pre-NCRT CEA and CA19-9 levels for predicting OS. The novel serum tumor biomarker (NSTB) scores were as follows: score 0: Pre-NCRT CEA < 3.55 and CA19-9 < 19.01; score 2: Pre-NCRT CEA > 3.55 and CA19-9 > 19.01; score 1: Pre-NCRT CEA < 3.55 and CA19-9 > 19.01 or pre-NCRT CEA > 3.55 and CA19-9 < 19.01.
Postoperative follow-up was performed according to the National Comprehensive Cancer Network guidelines[13]. Generally, patients were followed up clinically and radiographically at three-month intervals in the first 2 years after surgery, then every 6 mo for 3 years, and annually thereafter[13]. Follow-up data were obtained from medical records, telephone follow-ups, out-patient clinics, or visits.
OS was defined as the survival time until death by any reason[21]. DFS was defined as the time-lapse between surgery and either RC recurrence or death[22]. Patients lost to follow-up or still alive at the final follow-up were included in the analysis as censored data[21].
Data were analyzed using SPSS for Windows (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.). Continuous data were described in terms of the median and interquartile range (IQR) whereas categorical variables were described in terms of frequencies and percentages. Significant parameters identified in the univariate analysis (P < 0.05) were entered into the multivariate Cox regression analysis to determine independent predictive factors[23,24]. All statistical tests were two-sided, and a P value of < 0.05 was considered statistically significant[25].
In general, pathological characteristics have the strongest predictive value for patient outcomes[21]. To compare the predictive effect of the NSTB score, several pathological indicators were included. To prevent the effects of pre-NCRT CEA and CA19-9 levels on the NSTB score, two models, one including and the other excluding the NSTB score in the multivariate analysis, were established.
Eighty-seven (36.7%) patients were female and 150 (63.3%) were male. The distribution of the patients according to pathological evaluation was as follows: vascular invasion was detected in 13 (5.5%) patients, lymphatic invasion in 13 (5.5%) patients, perineural invasion in 41 (17.3%) patients, and circumferential resection margin (CRM) positivity in 8 (3.8%) patients. Regarding the pathological TNM classification, 45 (19.0%) patients were in stage 0, 57 (24.1%) were in stage I, 72 (30.4%) were in stage II, and 63 (26.6%) were in stage III (Table 1). A total of 118 (49.8%) patients were in pT stage 0-2 while 119 (50.2%) were in pT stage 3-4. Sixty (25.3%) patients had pN metastases while 177 (74.7%) did not have pN metastases. The median (IQR) level of pre-NCRT CEA was 4.15 (2.18-10.07) and that of pre-NCRT CA19-9 was 13.56 (7.80-25.40).
| Features | Median (IQR) |
| Pre-NCRT CEA | 4.15 (2.18-10.07) |
| Pre-NCRT CA19-9 | 13.56 (7.80-25.40) |
| Age in yr | 57.0 (50.0-66.5) |
| Sex | |
| Male | 150 |
| Female | 87 |
| Pathological T stage | |
| T0-2 | 118 |
| T3-4 | 119 |
| Pathological N stage | |
| N0 | 177 |
| N+ | 60 |
| Pathological TNM stage | |
| 0 | 45 |
| I | 57 |
| II | 72 |
| III | 63 |
| Pathological vascular invasion | |
| Yes | 13 |
| No | 224 |
| Pathological lymphatic invasion | |
| Yes | 13 |
| No | 224 |
| Pathological perineural invasion | |
| Yes | 41 |
| No | 196 |
| Pathological CRM | |
| Positive | 8 |
| Negative | 229 |
During follow-up, 9 (3.8%) patients were lost to follow-up and 36 (15.2%) developed cancer recurrence and died.
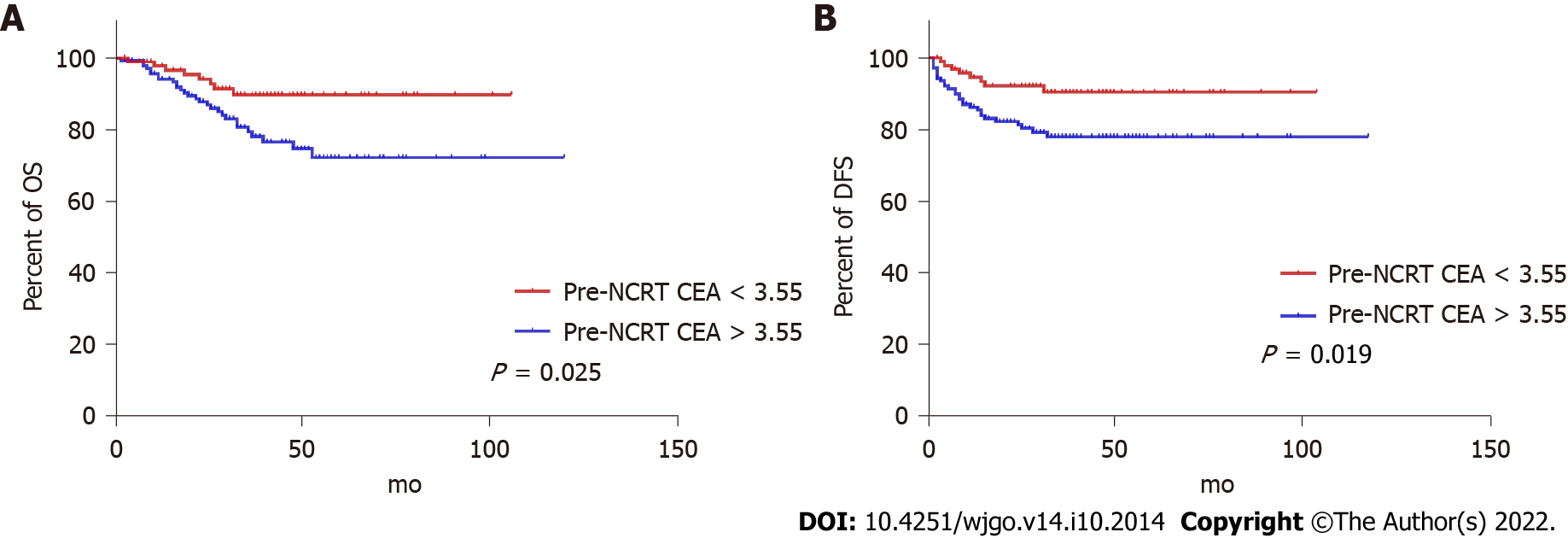
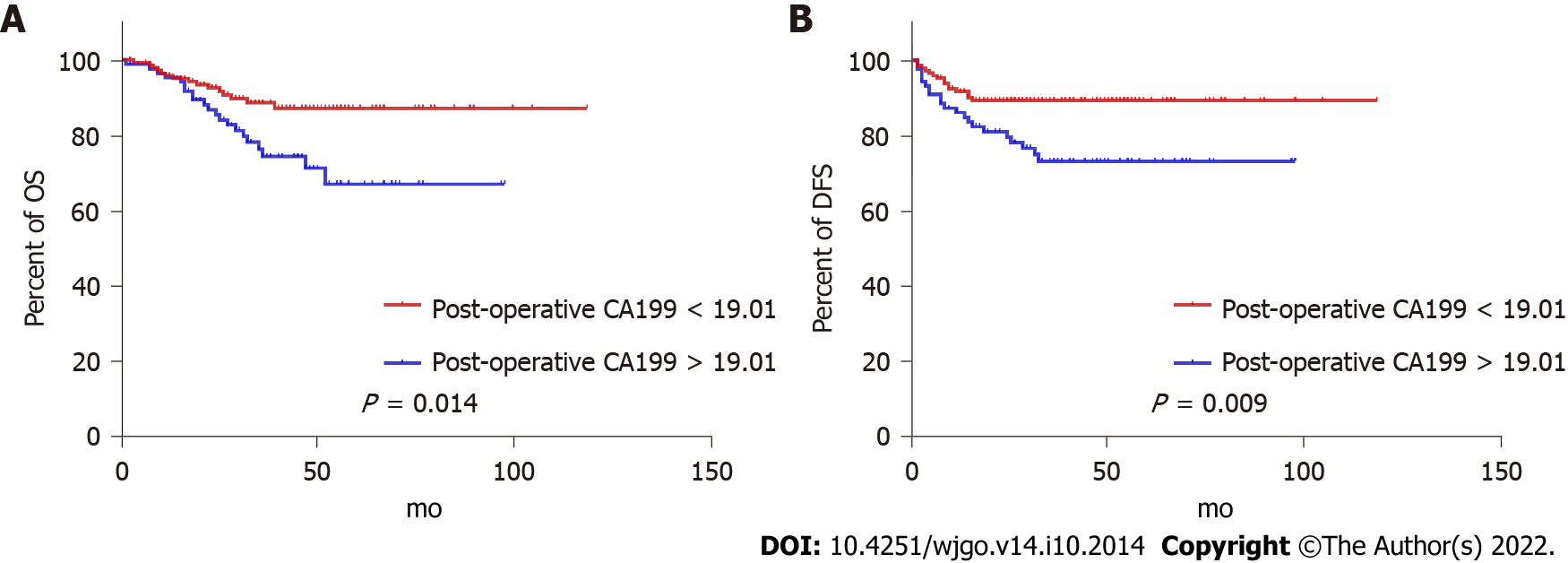
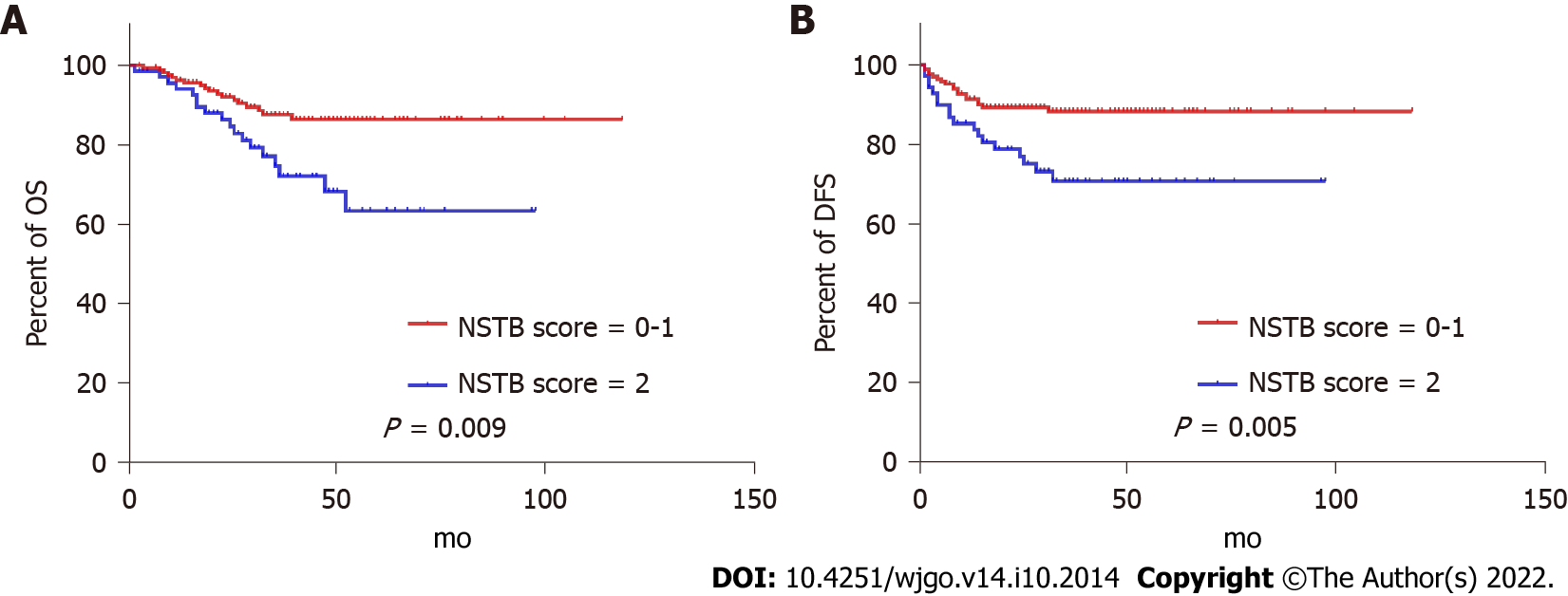
ROC curves identified the optimal cut-off for survival prediction by pre-NCRT CEA and CA19-9 were 3.55 and 19.01, respectively. They divided patients into different groups. Figures 2-4A show the OS of included patients stratified by pre-NCRT CEA, CA19-9, and the NSTB score, respectively, and Figures 2-4B show their DFS stratified by the same parameters. According to the Kaplan–Meier curves, increased pre-NCRT CEA and CA19-9 levels and higher NSTB scores were all associated with decreased OS and DFS.
Possible clinicopathological parameters that may predict patient outcome were reviewed. In the univariate analysis, pre-NCRT CEA > 3.55, pre-CA19-9 > 19.01, a higher pathological TNM stage, and a higher NSTB score were significantly associated with decreased OS (Table 2) and DFS (Table 3).
| Characteristics | HR (95%CI) | P value |
| Pre-NCRT CEA (< 3.55/> 3.55) | 0.407 (0.185-0.893) | 0.025 |
| Pre-NCRT CA19-9 (< 19.01/> 19.01) | 0.437 (0.225-0.849) | 0.014 |
| Sex (male/female) | 0.478 (0.218-1.049) | 0.066 |
| Pathological TNM stage (0-I/II-III) | 0.321 (0.141-0.732) | 0.007 |
| Pathological vascular invasion (absent/present) | 0.556 (0.170-1.821) | 0.332 |
| Pathological lymphatic invasion (absent/present) | 0.400 (0.141-1.136) | 0.085 |
| Pathological perineural invasion (absent/present) | 0.534 (0.250-1.141) | 0.105 |
| Pathological CRM (negative/positive) | 0.826 (0.198-3.449) | 0.793 |
| NSTB score (0-1/2) | 0.416 (0.217-0.800) | 0.009 |
| Characteristic | HR (95%CI) | P value |
| Pre-NCRT CEA (< 3.55/> 3.55) | 0.391 (0.178-0.859) | 0.019 |
| Pre-NCRT CA19-9 (< 19.01/> 19.01) | 0.413 (0.213-0.802) | 0.009 |
| Sex (male/female) | 0.466 (0.213-1.023) | 0.057 |
| Pathological TNM stage (0-I/II-III) | 0.302 (0.132-0.690) | 0.005 |
| Pathological vascular invasion (absent/present) | 0.571 (0.175-1.863) | 0.353 |
| Pathological lymphatic invasion (absent/present) | 0.435 (0.154-1.231) | 0.117 |
| Pathological perineural invasion (absent/present) | 0.595 (0.279-1.265) | 0.177 |
| Pathological CRM (negative/positive) | 0.657 (0.158-2.738) | 0.564 |
| NSTB score (0-1/2) | 0.391 (0.203-0.751) | 0.005 |
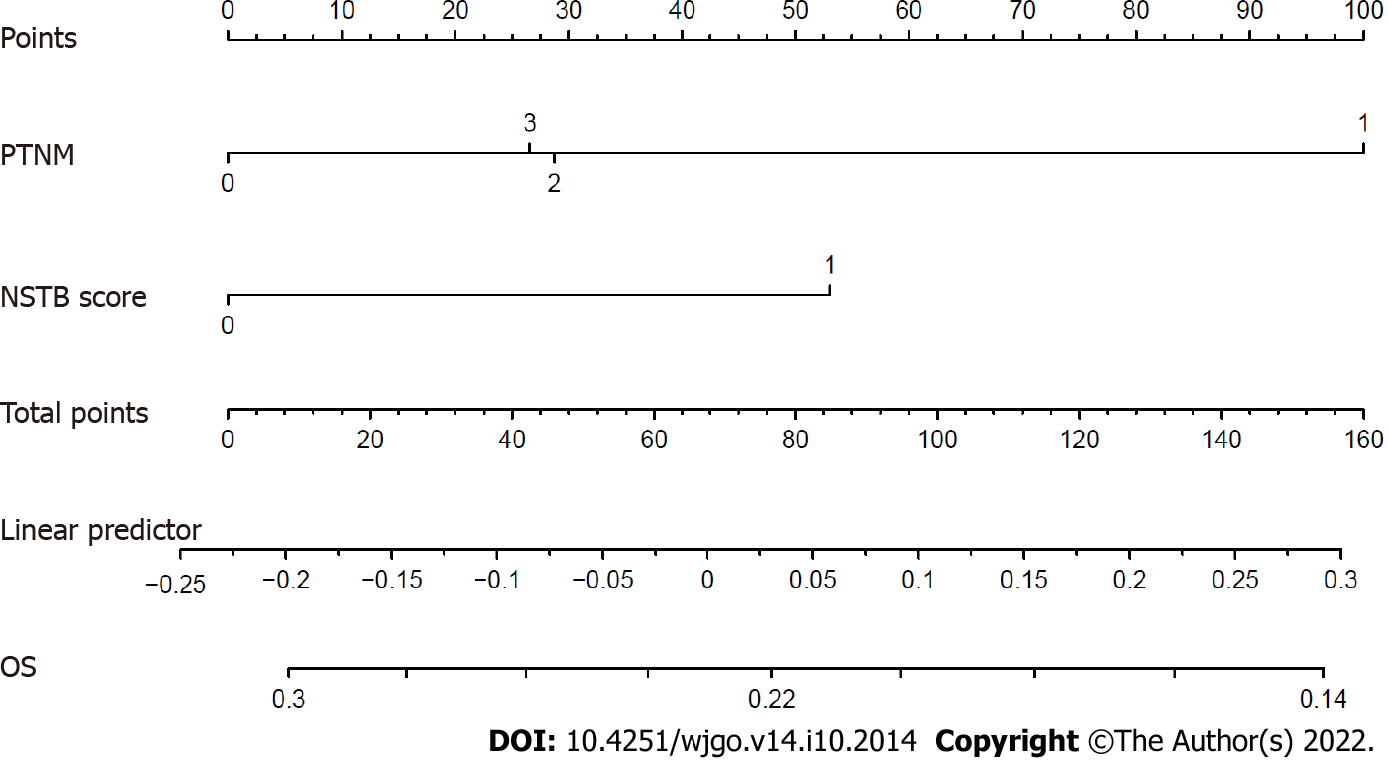
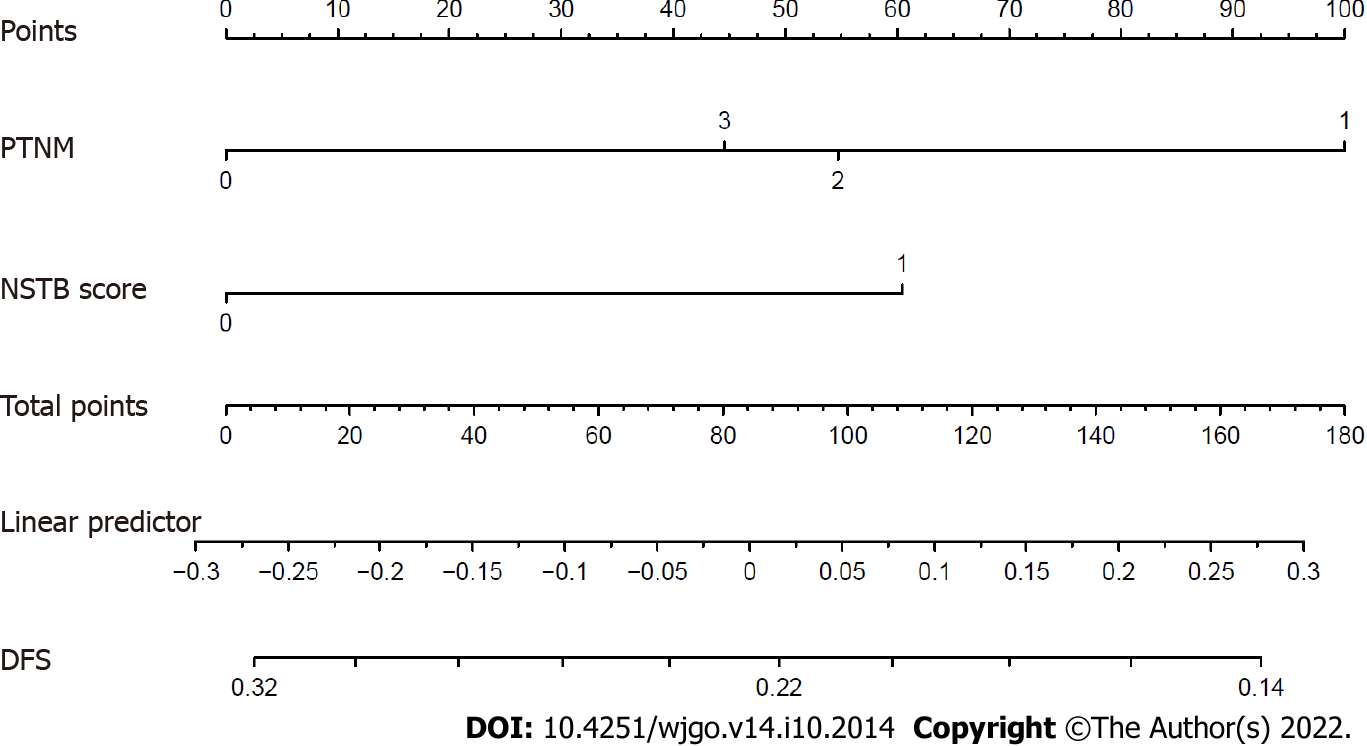
In the multivariate analysis of OS (Table 4), only a lower pathological TNM stage (stage 0-I vs II-III, HR = 0.363, 95%CI: 0.158-0.837, P = 0.017) and the NSTB score (score 0-1 vs 2, HR = 0.485, 95%CI: 0.251-0.940, P = 0.032) were significant predictors of a better outcome while pre-NCRT CEA < 3.55 (HR = 0.529, 95%CI: 0.23-1.205, P = 0.130) and CA19-9 < 19.01 (HR = 0.604, 95%CI: 0.300-1.215, P = 0.158) were not. In the multivariate analysis of DFS (Table 5), a lower pathological TNM stage (stage 0-I vs II–III, HR = 0.342, 95%CI: 0.149-0.786, P = 0.012) and the NSTB score (score 0-1 vs 2, HR = 0.453, 95%CI: 0.234-0.877, P = 0.019) could also predict a better outcome while pre-NCRT CEA < 3.55 (HR = 0.521, 95%CI: 0.226-1.162, P = 0.109) and CA19-9 < 19.01 (HR = 0.570, 95%CI: 0.284-1.141, P = 0.112) could not. The nomogram of OS (Figure 5) and DFS (Figure 6) shows the precise prognosis.
| Characteristic | Multivariate analysis | |||
| Model 1 | Model 2 | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Pre-NCRT CEA (< 3.55/> 3.55) | 0.529 (0.232-1.205) | 0.130 | ||
| Pre-NCRT CA19-9 (< 19.01/> 19.01) | 0.604 (0.300-1.215) | 0.158 | ||
| Pathological TNM stage (0-I/II-III) | 0.373 (0.162-0.859) | 0.020 | 0.363 (0.158-0.837) | 0.017 |
| NSTB score (0-1/2) | 0.485 (0.251-0.940) | 0.032 | ||
| Characteristic | Multivariate analysis | |||
| Model 1 | Model 2 | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Pre-NCRT CEA (< 3.55/> 3.55) | 0.512 (0.226-1.162) | 0.109 | ||
| Pre-NCRT CA19-9 (< 19.01/> 19.01) | 0.570 (0.284-1.141) | 0.112 | ||
| Pathological TNM stage (0-I/II-III) | 0.350 (0.152-0.806) | 0.014 | 0.342 (0.149-0.786) | 0.012 |
| NSTB score (0-1/2) | 0.453 (0.234-0.877) | 0.019 | ||
Our data showed that the combination of pre-NCRT tumor markers had better predictive value than a single marker. Although univariate analyses demonstrated that lower pre-NCRT CEA and CA19-9 levels were potential indicators of a better prognosis, the multivariate analysis proved that only the NSTB score and pathological TNM stage could independently determine the prognosis. In general, pathological indicators had a more robust predictive value than other indicators in determining the prognosis[8]; however, the multivariate analysis revealed that the NSTB score could predict outcomes better than pathological characteristics of lymphatic invasion, vascular invasion, nerve infiltration, and CRM invasion. Thus, we propose that the NSTB score should be used to guide the treatment and determine the prognosis of patients with RC of clinical stage II/III.
Pathological findings were generally recognized as the most effective indicators to predict the prognosis[8]. A previous study revealed that lymphatic invasion, perineural invasion, vascular invasion, CRM invasion, LN metastasis, and a higher tumor invasion stage can predict a worse outcome[4]. However, pathological characteristics were difficult to identify as they are usually affected by the quality of surgery and specimen-processing, and their analysis is significantly subjective and difficult to quantitate[15]. Moreover, pathological indicators could only be obtained after surgery, which means that it was impossible to judge whether patients needed additional chemotherapy before undergoing NCRT or surgery. Moreover, the NSTB score could be obtained before NCRT and surgery.
Some molecules or proteins can determine a patient’s prognosis. Lin et al[26] reported that the high expression of EphA4 served as an independent adverse predictor for DFS. Rödel et al[27] found that an increase in survivin levels was a significant risk factor for local recurrence and decreased DFS. Hiyoshi et al[28] demonstrated that serum miR-143 was a non-invasive and novel predictive marker for locally advanced rectal cancer (LARC) patients. Unfortunately, all of these molecular or protein markers had the following disadvantages: First, the detection cost of these markers was high, which increased the economic burden for patients; second, these novel markers could only be detected in large medical centers, which made them difficult to be used widely in clinical practice; finally, these new indicators lack uniform standards, and the test results may vary a lot in different medical centers. CEA and CA19-9 levels are widely used clinically because they are cheap, convenient to detect, and have uniform standards in different hospitals.
CEA is currently one of the primary markers for the diagnosis and follow-up of RC[2,18,19]. We found that lower CEA levels could predict a better prognosis in a univariate analysis. CA19-9 has shown great value for the differential diagnosis of malignant tumors and disease monitoring and evaluation[19]. Compared to either CA19-9 or CEA alone, an essential advantage of the combination was that it could reduce the interference of other factors and increase the predictive effectiveness. Although some studies also focused on the influence of CEA and CA19-9 levels on the prognosis, the two markers were studied separately[2,18-20,29]. Consequently, they failed to identify CEA and CA19-9 as predictive factors, which was similar to our findings. However, the predictive value increased significantly and was even stronger than that of several pathological factors when they were combined.
Our study had a few strengths: Firstly, to the best of our knowledge, this is the first study that combined CEA and CA19-9 to evaluate the prognosis of clinical stage II/III patients undergoing NCRT. Secondly, we adopted an ROC curve to determine the cut-off point of CEA and CA19-9 instead of just evaluating whether they were higher than the normal values, which optimized the efficiency of the OS prediction. Ultimately, the NSTB score was cheap and easily accessible before treatment.
Our study also had some shortcomings. First, this was a retrospective study conducted in a single medical center. Second, the cut-off points of pre-NCRT CEA and CA19-9 levels in our center may not always be reproducible in other centers.
This study established a NSTB score by combining pre-NCRT CEA and CA19-9 levels. The NSTB score can independently predict the prognosis of patients with LARC of clinical stage II/III who underwent NCRT. Its predictive value was stronger than that of either marker alone, and even some pathologic characteristics.
Multiple classes of molecular biomarkers were studied as potential predictors for rectal cancer (RC) response but there was no sufficient evidence for any of them to be introduced into clinical practice.
To assess the predictive effect of pre-neoadjuvant chemoradiotherapy (NCRT) carcinoembryonic antigen (CEA) and cancer antigen 19-9 (CA19-9) levels on the prognosis of stage II/III RC patients.
The objective of this study is to establish a novel serum tumor biomarker score by combining pre-NCRT CEA and CA19-9 levels. The novel serum tumor biomarker (NSTB) score may predict the prognosis of patients with locally advanced rectal cancer (LARC) of clinical stage II/III who underwent NCRT independently.
A total of 237 patients were included in this study. CEA and CA 19-9 levels were evaluated 1 wk before NCRT. The NSTB score was as follows: score 0: pre-NCRT CEA < 3.55 and CA19-9 < 19.01; score 2: pre-NCRT CEA > 3.55 and CA19-9 > 19.01; score 1: other situations. Pathological information was recorded according to histopathological reports after the operation.
In the univariate analysis, pre-NCRT CEA < 3.55, pre-NCRT CA19-9 < 19.01, and a lower NSTB score could predict a better prognosis. However, in the multivariate analysis, only a lower NSTB score and higher pathological tumor-node-metastasis (TNM) stage were independent predictive factors.
We established a novel serum tumor biomarker score by combining pre-NCRT CEA and CA19-9 levels. The NSTB score can independently predict the prognosis of patients with LARC of clinical stage II/III who underwent NCRT.
More accurate prediction models need to be established by studies with a larger number of patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dulskas A, Lithuania; Elkady N, Egypt S-Editor: Zhang H L-Editor: Ma JY-MedE P-Editor: Zhang H
| 1. | Naffouje S, Sabesan A, Powers BD, Dessureault S, Sanchez J, Schell M, Imanirad I, Sahin I, Xie H, Felder S. Patient Risk Subgroups Predict Benefit of Adjuvant Chemotherapy in Stage II Rectal Cancer Patients Following Neoadjuvant Chemoradiation and Total Mesorectal Excision. Clin Colorectal Cancer. 2021;20:e155-e164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Zhang S, Zhang R, Li RZ, Wang QX, Chang H, Ding PR, Li LR, Wu XJ, Chen G, Zeng ZF, Xiao WW, Gao YH. Beneficiaries of radical surgery among clinical complete responders to neoadjuvant chemoradiotherapy in rectal cancer. Cancer Sci. 2021;112:3607-3615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Zhou S, Jiang Y, Pei W, Zhou H, Liang J, Zhou Z. Neoadjuvant chemoradiotherapy followed by lateral pelvic lymph node dissection for rectal cancer patients: A retrospective study of its safety and indications. J Surg Oncol. 2021;124:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Diefenhardt M, Ludmir EB, Hofheinz RD, Ghadimi M, Minsky BD, Rödel C, Fokas E. Association of Treatment Adherence With Oncologic Outcomes for Patients With Rectal Cancer: A Post Hoc Analysis of the CAO/ARO/AIO-04 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:1416-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Kroon HM, Malakorn S, Dudi-Venkata NN, Bedrikovetski S, Liu J, Kenyon-Smith T, Bednarski BK, Ogura A, van de Velde CJH, Rutten HJT, Beets GL, Thomas ML, Kusters M, Chang GJ, Sammour T. Local recurrences in western low rectal cancer patients treated with or without lateral lymph node dissection after neoadjuvant (chemo)radiotherapy: An international multi-centre comparative study. Eur J Surg Oncol. 2021;47:2441-2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Zhang YZ, Song M, Geng JH, Zhu XG, Li S, Li YH, Cai Y, Wang WH. Patterns of failure and implications for clinical target volume definition of locally advanced T4b rectal cancer identified with magnetic resonance imaging and treated using neoadjuvant chemoradiotherapy and surgery. Radiother Oncol. 2021;161:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Smolskas E, Mikulskytė G, Sileika E, Suziedelis K, Dulskas A. Tissue-Based Markers as a Tool to Assess Response to Neoadjuvant Radiotherapy in Rectal Cancer-Systematic Review. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Bauer PS, Chapman WC Jr, Atallah C, Makhdoom BA, Damle A, Smith RK, Wise PE, Glasgow SC, Silviera ML, Hunt SR, Mutch MG. Perioperative Complications After Proctectomy for Rectal Cancer: Does Neoadjuvant Regimen Matter? Ann Surg. 2022;275:e428-e432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Petrelli F, Trevisan F, Cabiddu M, Sgroi G, Bruschieri L, Rausa E, Ghidini M, Turati L. Total Neoadjuvant Therapy in Rectal Cancer: A Systematic Review and Meta-analysis of Treatment Outcomes. Ann Surg. 2020;271:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 245] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 10. | Xu T, Zhang L, Yu L, Zhu Y, Fang H, Chen B, Zhang H. Log odds of positive lymph nodes is an excellent prognostic factor for patients with rectal cancer after neoadjuvant chemoradiotherapy. Ann Transl Med. 2021;9:637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Zhao Q, Wan L, Zou S, Zhang C, E T, Yang Y, Ye F, Zhao X, Ouyang H, Zhang H. Prognostic risk factors and survival models for T3 locally advanced rectal cancer: what can we learn from the baseline MRI? Eur Radiol. 2021;31:4739-4750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Cheong C, Shin JS, Suh KW. Prognostic value of changes in serum carcinoembryonic antigen levels for preoperative chemoradiotherapy response in locally advanced rectal cancer. World J Gastroenterol. 2020;26:7022-7035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | You YN, Hardiman KM, Bafford A, Poylin V, Francone TD, Davis K, Paquette IM, Steele SR, Feingold DL; On Behalf of the Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer. Dis Colon Rectum. 2020;63:1191-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 14. | Zheng Z, Wang X, Huang Y, Lu X, Huang Z, Chi P. Defining and predicting early recurrence in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. Eur J Surg Oncol. 2020;46:2057-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | You W, Yan L, Cai Z, Xie L, Sheng N, Wang G, Wu X, Wang Z. Clinical Significances of Positive Postoperative Serum CEA and Post-preoperative CEA Increment in Stage II and III Colorectal Cancer: A Multicenter Retrospective Study. Front Oncol. 2020;10:671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Doos WG, Wolff WI, Shinya H, DeChabon A, Stenger RJ, Gottlieb LS, Zamcheck N. CEA levels in patients with colorectal polyps. Cancer. 1975;36:1996-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Moore TL, Kantrowitz PA, Zamcheck N. Carcinoembryonic antigen (CEA) in inflammatory bowel disease. JAMA. 1972;222:944-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Rule AH, Goleski-Reilly C, Sachar DB, Vandevoorde J, Janowitz HD. Circulating carcinoembryonic antigen (CEA): relationship to clinical status of patients with inflammatory bowel disease. Gut. 1973;14:880-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Huang D, Lin Q, Song J, Xu B. Prognostic Value of Pretreatment Serum CA199 in Patients with Locally Advanced Rectal Cancer Treated with CRT Followed by TME with Normal Pretreatment Carcinoembryonic Antigen Levels. Dig Surg. 2021;38:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Mizuno H, Miyake H, Nagai H, Yoshioka Y, Shibata K, Asai S, Takamizawa J, Yuasa N. Optimal cutoff value of preoperative CEA and CA19-9 for prognostic significance in patients with stage II/III colon cancer. Langenbecks Arch Surg. 2021;406:1987-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Dossa F, Acuna SA, Rickles AS, Berho M, Wexner SD, Quereshy FA, Baxter NN, Chadi SA. Association Between Adjuvant Chemotherapy and Overall Survival in Patients With Rectal Cancer and Pathological Complete Response After Neoadjuvant Chemotherapy and Resection. JAMA Oncol. 2018;4:930-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 22. | Zhang XY, Wang S, Li XT, Wang YP, Shi YJ, Wang L, Wu AW, Sun YS. MRI of Extramural Venous Invasion in Locally Advanced Rectal Cancer: Relationship to Tumor Recurrence and Overall Survival. Radiology. 2018;289:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 23. | Goh TS, Lee JS, Il Kim J, Park YG, Pak K, Jeong DC, Oh SO, Kim YH. Prognostic scoring system for osteosarcoma using network-regularized high-dimensional Cox-regression analysis and potential therapeutic targets. J Cell Physiol. 2019;234:13851-13857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Li Z, Wang Q, Qiao Y, Wang X, Jin X, Wang A. Incidence and associated predictors of adverse pregnancy outcomes of maternal syphilis in China, 2016-19: a Cox regression analysis. BJOG. 2021;128:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Hsu CH, Yu M. Cox regression analysis with missing covariates via nonparametric multiple imputation. Stat Methods Med Res. 2019;28:1676-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Lin CY, Lee YE, Tian YF, Sun DP, Sheu MJ, Lin CY, Li CF, Lee SW, Lin LC, Chang IW, Wang CT, He HL. High Expression of EphA4 Predicted Lesser Degree of Tumor Regression after Neoadjuvant Chemoradiotherapy in Rectal Cancer. J Cancer. 2017;8:1089-1096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Rödel F, Hoffmann J, Distel L, Herrmann M, Noisternig T, Papadopoulos T, Sauer R, Rödel C. Survivin as a radioresistance factor, and prognostic and therapeutic target for radiotherapy in rectal cancer. Cancer Res. 2005;65:4881-4887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 212] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 28. | Hiyoshi Y, Akiyoshi T, Inoue R, Murofushi K, Yamamoto N, Fukunaga Y, Ueno M, Baba H, Mori S, Yamaguchi T. Serum miR-143 levels predict the pathological response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer. Oncotarget. 2017;8:79201-79211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Chen Y, Gao SG, Chen JM, Wang GP, Wang ZF, Zhou B, Jin CH, Yang YT, Feng XS. Serum CA242, CA199, CA125, CEA, and TSGF are Biomarkers for the Efficacy and Prognosis of Cryoablation in Pancreatic Cancer Patients. Cell Biochem Biophys. 2015;71:1287-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |