Published online Oct 15, 2022. doi: 10.4251/wjgo.v14.i10.2004
Peer-review started: June 22, 2022
First decision: July 12, 2022
Revised: July 18, 2022
Accepted: September 13, 2022
Article in press: September 13, 2022
Published online: October 15, 2022
Processing time: 114 Days and 4.4 Hours
The biological characteristics of gastric stromal tumors are complex, and their incidence has increased in recent years. Gastric stromal tumors (GST) have potential malignant tendencies, and the probability of transformation into malignant tumors is as high as 20%-30%.
To investigate the value of multi-slice spiral computed tomography (MSCT) in the differential diagnosis of GST and benign gastric polyps, and GST risk stratification assessment.
We included 64 patients with GST (GST group) and 60 with benign gastric polyps (control group), confirmed by pathological examination after surgery in PLA General Hospital, from January 2016 to June 2021. The differences in the MSCT imaging characteristic parameters and enhanced CT values between the two groups before surgery were compared. According to the National Institutes of Health’s standard, GST is divided into low- and high-risk groups for MSCT imaging characteristic parameters and enhanced CT values.
The incidences of extraluminal growth, blurred boundaries, and ulceration in the GST group were significantly higher than those in the control group (P < 0.05). The CT values and enhanced peak CT values in the arterial phase in the CST group were higher than those in the control group (P < 0.05). The MSCT differential diagnosis of GST and gastric polyp sensitivity, specificity, misdiagnosis rate, missed diagnosis rate, and areas under the curve (AUCs) were 73.44 %, 83.33%, 26.56%, 16.67%, 0.784, respectively. The receiver operating characteristic curves were plotted with the arterial CT value and enhanced peak CT value, with a statistical difference. The results showed that the sensitivity, specificity, misdiagnosis rate, missed diagnosis rate, and AUC value of arterial CT in the differential diagnosis of GST and gastric polyps were 80.18%, 62.20%, 19.82%, 37.80%, and 0.710, respectively. The sensitivity, specificity, misdiagnosis rate, missed diagnosis rate, and AUC value of the enhanced peak CT value in the differential diagnosis of GST and gastric polyps were 67.63%, 60.40%, 32.37%, 39.60%, and 0.710, respectively. The incidence of blurred lesion boundaries and ulceration in the high-risk group was significantly higher than that in the low-risk group (P < 0.05). The arterial phase and enhanced peak CT values in the high-risk group were significantly higher than those in the low-risk group (P < 0.05).
Presurgical MSCT examination has important value in the differential diagnosis of GST and gastric benign polyps and can effectively evaluate the risk grade of GST patients.
Core Tip: Gastric stromal tumors (GSTs) are common gastrointestinal tumors and have a certain possibility of malignant change. Therefore, surgical intervention is important. However, the signs of early patients are not obvious, and difficult to distinguish from benign gastric tumors. Imaging examinations have always been the main methods for diagnosing GSTs. The degree of risk to patients can be evaluated by performing a computed tomography (CT) examination. In this study, a CT examination was performed to analyze the difference in CT performance between GSTs and gastric polyps, to provide the corresponding basis for early diagnosis of GSTs and reasonable selection of treatment methods.
- Citation: Li XL, Han PF, Wang W, Shao LW, Wang YW. Multi-slice spiral computed tomography in differential diagnosis of gastric stromal tumors and benign gastric polyps, and gastric stromal tumor risk stratification assessment. World J Gastrointest Oncol 2022; 14(10): 2004-2013
- URL: https://www.wjgnet.com/1948-5204/full/v14/i10/2004.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i10.2004
Gastric stromal tumors (GSTs) are mesenchymal tumors originating from Cahar mesenchymal cells, with malignant potential. At present, the most effective treatment is surgical resection; however, there is a risk of postoperative recurrence and metastasis. Gastric polyps are benign tumors of gastric epithelium or gastric interstitial origin, and endoscopic resection can be performed. The two tumors have different treatment methods, but their clinical symptoms and signs are similar[1]. Imaging examination has always been a common means for clinically diagnosing GSTs, which can locate the lesion, clarify morphological characteristics, and evaluate local invasiveness. Computed tomography (CT) is a commonly used diagnostic method in clinical practice. In recent years, enhanced CT examination has been determined to evaluate the risk of GSTs. CT examination can effectively avoid the influence of gastrointestinal gas and the superposition of surrounding organs on the preliminary diagnosis of lesions and reduce the missed diagnosis rate of lesions[2]. In this study, the imaging characteristics of gastric stromal tumors and gastric polyps in this region were analyzed using multi-slice spiral CT (MSCT), and the GST risk stratification was also evaluated. The purpose of this study was to provide a basis for the early diagnosis of GST in the clinic.
Sixty-four patients (GST group), with GST confirmed by pathological examination after surgery in PLA General Hospital, from January 2016 to June 2021 and 60 patients with benign gastric polyps (control group) were selected.
The inclusion criteria were: (1) Patients aged 19–79 years were included in the study; (2) The diagnostic criteria for GST and benign gastric polyps refer to the criteria in the eighth edition of the 'Surgery' of the People's Health Press[3]; (3) All patients underwent endoscopic or surgical resection in our hospital for gastrointestinal surgery, as confirmed by postoperative pathological examination; (4) All patients underwent MSCT examination before surgery, and their imaging data were preserved completely; and (5) The research program was reviewed and approved by the medical ethics committee of our hospital. Exclusion criteria: (1) A history of chemoradiotherapy; (2) Additional with malignant tumors in other parts of the gastrointestinal tract; and (3) Patients with missing imaging data that could not be included in the statistical analysis.
Inspection instrument: Siemens 64-row dual-source CT was used to perform the whole abdominal CT plain scan + enhanced examination. The scanning parameters were set as follows: tube voltage 120 kV, tube current, using automatic mA technology; pitch, 1.0; collimation, 128 mm × 0.6 mm, scanning layer thickness 3 mm, recombination layer thickness 3 mm; and matrix, 512 × 512. In the supine position, 80–120 mL (iodine content 320 mg/mL, 1.5 mL/kg body weight) of high-pressure injector was injected intravenously, through the median elbow. The injection flow rate was 3–4 mL/s. The abdominal aorta was monitored using an injection contrast agent (trigger threshold, 100 HU) for arterial phase scanning, and portal venous phase and delayed phase scanning were delayed for 45 s and 90 s.
All images were entered into a medical imaging workstation, and image analysis was performed by two imaging physicians with more than five years of experience. The tumor location, size, growth mode, morphology, lesion necrosis, calcification, and lymph node hyperplasia were analyzed. The CT value was measured at the same level in all four stages. ROI mapping should try to avoid the surrounding blood vessels, fat spaces, calcification, and necrotic areas in the tumor, and the average value of each patient was measured three times.
The risk classification standard of gastrointestinal stromal tumors was based on the National Institutes of Health (NIH) standard, as shown in Table 1.
| GST Hazard classification | Lesion diameter (cm) | Mitosis (/50HPF) | Primary tumor location |
| Very low risk | < 2.0 | ≤ 5.0 | Any position |
| Low risk | 2.1 - 5.0 | ≤ 5.0 | Any position |
| Medium risk | 2.1 - 5.0 | > 5.0 | Stomach |
| < 5.0 | 6.0 - 1.0 | Any position | |
| 5.0 - 10.0 | ≤ 5.0 | Stomach | |
| High risk | Any case | Any case | Tumor rupture |
| > 10.0 | Any case | Any position | |
| Any case | > 10.0 | Any position | |
| > 5.0 | > 5.0 | Any position | |
| 2.1 - 5.0 | > 5.0 | Non-stomach | |
| 5.0 - 10.0 | ≤ 5.0 | Non-stomach |
The age, body mass index, lesion diameter, and other measurement indices of the patients were tested by normal distribution, which were in accordance with the approximate normal distribution or normal distribution, and expressed as mean ± SD. The t-test was used for comparisons between two groups. The non-grade count data were expressed as a percentage, and the statistical analysis was performed using the χ2 test; diagnostic analysis was performed using a 2 × 2 four-fold table, diagnostic indicators were calculated, and a receiver operating characteristic curve was drawn. The professional SPSS21.0 software was used for data processing, test level α = 0.05.
Age, BMI, lesion diameter, gender, smoking, drinking and comorbidity were compared between GST group and control group (P > 0.05, Table 2).
| Normal information | GST group (n = 64) | Control group (n = 60) | t/χ2 value | P value |
| Age (yr) | 56.9 ± 8.2 | 59.0 ± 7.5 | -1.485 | 0.140 |
| BMI (kg/m2) | 24.7 ± 2.4 | 24.4 ± 2.3 | 0.710 | 0.479 |
| Lesion diameter (cm) | 2.98 ± 0.77 | 3.05 ± 0.80 | -0.496 | 0.620 |
| Gender | 1.542 | 0.214 | ||
| Male | 37 (57.81) | 28 (46.67) | ||
| Female | 27 (42.19) | 32 (53.33) | ||
| Smoking | 1.663 | 0.197 | ||
| Yes | 24 (37.5) | 16 (26.67) | ||
| No | 40 (62.5) | 44 (73.33) | ||
| Drinking | 1.592 | 0.207 | ||
| Yes | 25 (39.06) | 17 (28.33) | ||
| No | 39 (60.94) | 43 (71.67) | ||
| Diabetes | 0.776 | 0.378 | ||
| Yes | 9 (14.06) | 12 (20.00) | ||
| No | 55 (85.94) | 48 (80.00) | ||
| Hypertension | 2.940 | 0.086 | ||
| Yes | 15 (23.44) | 7 (11.67) | ||
| No | 49 (76.56) | 53 (88.33) |
The lesion location, tumor shape, calcification and enhancement pattern of GST group and control group were compared (P > 0.05). The incidence of extraluminal growth, blurred boundary and ulcer in CST group was significantly higher than that in control group (P < 0.05) (Table 3).
| CT signs | GST group (n = 64) | Control group (n = 60) | χ2 value | P value |
| Lesion location | 4.174 | 0.383 | ||
| Fundus of stomach | 12 (18.75) | 14 (23.33) | ||
| Cardia | 6 (9.38) | 8 (13.33) | ||
| Greater curvature of the stomach | 26 (40.63) | 17 (28.33) | ||
| Lesser curvature of stomach | 11 (17.19) | 7 (11.67) | ||
| Gastric antrum | 9 (14.06) | 14 (23.33) | ||
| Tumor shape | 3.228 | 0.072 | ||
| Smooth | 50 (78.13) | 54 (90.00) | ||
| Lobulated | 14 (21.88) | 6 (10.00) | ||
| Growth pattern | 41.177 | 0.000 | ||
| Intraluminal | 22 (34.38) | 54 (90.00) | ||
| Extraluminal | 32 (50.00) | 6 (10.00) | ||
| Mixed way | 10 (15.63) | 0 (0.00) | ||
| Calcification | 1.166 | 0.280 | ||
| Yes | 5 (7.81) | 2 (3.33) | ||
| No | 59 (92.19) | 58 (96.67) | ||
| Lesion border | 31.312 | 0.000 | ||
| Clear | 11 (17.19) | 40 (66.67) | ||
| Blurry | 53 (82.81) | 20 (33.33) | ||
| Reinforcement | 3.725 | 0.054 | ||
| Uniform | 54 (84.38) | 57 (95.00) | ||
| Uneven | 10 (15.63) | 3 (5.00) | ||
| Ulcer | 9.771 | 0.002 | ||
| Yes | 18 (28.13) | 4 (6.67) | ||
| No | 46 (71.88) | 56 (93.33) |
CT values of GST group and control group in venous phase and delayed phase were compared (P > 0.05); the CT values and enhanced peak CT values in the arterial phase in the CST group were higher than those in the control group (P < 0.05) (Table 4).
| Groups | n | Arterial phase | Venous phase | Delay period | Reinforcement peak |
| GST group | 64 | 63.98 ± 14.38 | 59.04 ± 12.74 | 66.58 ± 11.47 | 75.58 ± 12.88 |
| Control group | 60 | 47.61 ± 11.04 | 56.48 ± 14.20 | 64.72 ± 9.83 | 64.46 ± 10.94 |
| t value | -7.137 | -1.054 | -0.971 | -5.192 | |
| P value | 0.000 | 0.294 | 0.333 | 0.000 |
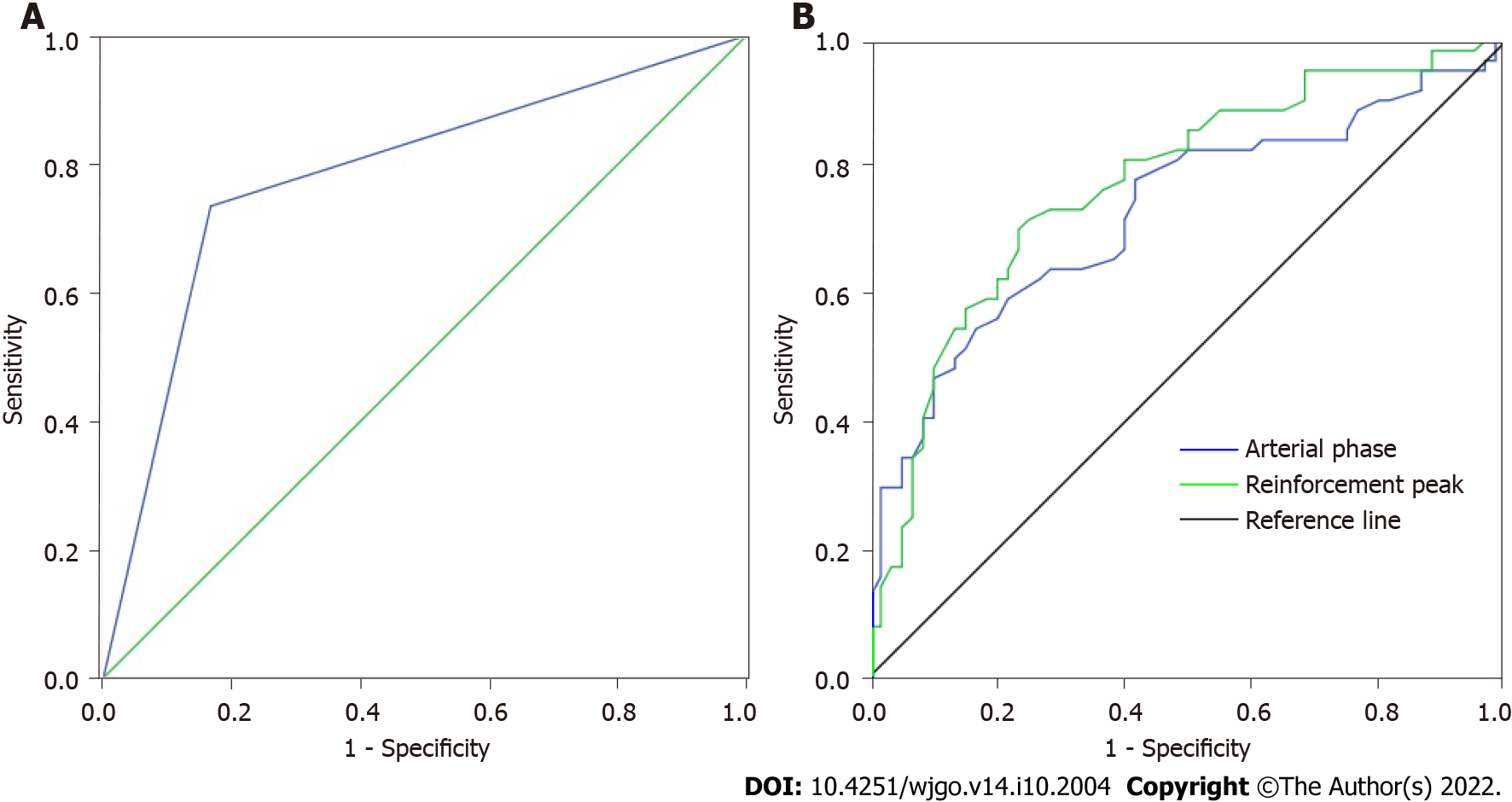
The pathological results and the diagnostic results of MSCT signs parameters were used to draw a 2 × 2 quadrangle, and the results showed that the sensitivity of MSCT in the differential diagnosis of GST and gastric polyps was 73.44%, the specificity was 83.33%, the misdiagnosis rate was 26.56%, the missed diagnosis rate was 16.67%, and the AUC value was 0.784 (Table 5, Figure 1A).
| MSCT | Pathology | Total | |
| GST | Benign polyp | ||
| GST | 47 | 10 | 57 |
| Benign polyp | 17 | 50 | 67 |
| Total | 64 | 60 | 124 |
Receiver operating characteristic (ROC) curve was drawn by arterial phase CT value and enhanced peak CT value, respectively. The results showed that the sensitivity, specificity, misdiagnosis rate, missed diagnosis rate and AUC value of arterial phase CT value in the differential diagnosis of GST and gastric polyps were 80.18%, 62.20%, 19.82%, 37.80% and 0.710, respectively. The sensitivity, specificity, misdiagnosis rate, missed diagnosis rate and AUC value of enhanced peak CT value in the differential diagnosis of GST and gastric polyps were 67.63%, 60.40%, 32.37%, 39.60% and 0.710, respectively (Figure 1B).
According to NIH classification standard, there were 23 high-risk patients, 17 middle-risk patients and 24 Low-risk patients in GST group. The incidence of blurred lesion boundary and ulceration in the high-risk group was significantly higher than that in the low-risk group (P < 0.05) (Table 6).
| CT signs | Low-intermediate-risk group (n = 41) | High-risk group (n = 23) | χ2 value | P value |
| Lesion location | 2.180 | 0.703 | ||
| Fundus of stomach | 7 (17.07) | 5 (21.74) | ||
| Cardia | 4 (9.76) | 2 (8.70) | ||
| Greater curvature of the stomach | 15 (36.59) | 11 (47.83) | ||
| Lesser curvature of stomach | 9 (21.95) | 2 (8.70) | ||
| Gastric antrum | 6 (14.63) | 3 (13.04) | ||
| Tumor shape | 1.539 | 0.215 | ||
| Smooth | 34 (82.93) | 16 (69.57) | ||
| Lobulated | 7 (17.07) | 7 (30.43) | ||
| Growth pattern | 5.520 | 0.063 | ||
| Intraluminal | 17 (41.46) | 5 (21.74) | ||
| Extraluminal | 16 (39.02) | 16 (69.57) | ||
| Mixed way | 8 (19.51) | 2 (8.70) | ||
| Calcification | 0.039 | 0.844 | ||
| Yes | 3 (7.32) | 2 (8.70) | ||
| No | 38 (92.68) | 21 (91.3) | ||
| Lesion border | 4.158 | 0.041 | ||
| Clear | 10 (24.39) | 1 (4.35) | ||
| Blurry | 31 (75.61) | 22 (95.65) | ||
| Reinforcement | 0.181 | 0.670 | ||
| Uniform | 34 (82.93) | 20 (86.96) | ||
| Uneven | 7 (17.07) | 3 (13.04) | ||
| Ulcer | 4.187 | 0.041 | ||
| Yes | 8 (19.51) | 10 (43.48) | ||
| No | 33 (80.49) | 13 (56.52) |
The arterial phase CT value and enhanced peak CT value in the high-risk group were significantly higher than those in the low-risk group (P < 0.05) (Table 7).
| Group | n | Arterial phase | Venous phase | Delay period | Reinforcement peak |
| Low-intermediate-risk group | 41 | 55.71 ± 13.77 | 57.94 ± 12.51 | 64.83 ± 11.20 | 72.66 ± 12.46 |
| High-risk group | 23 | 78.72 ± 12.66 | 61.00 ± 11.96 | 69.70 ± 10.85 | 80.79 ± 12.37 |
| t value | -6.598 | -0.954 | -1.688 | -2.511 | |
| P value | 0.000 | 0.344 | 0.097 | 0.015 |
GSTs are common mesenchymal tumors of the digestive system. Benign gastric polyps are common benign tumors of the stomach, but their clinical symptoms are not distinguished. Therefore, if an accurate diagnosis is not made prior to surgery, the treatment options will be affected[3]. CT has always been an important method for the clinical diagnosis of gastrointestinal tumors. It can distinguish the location, size, shape, and internal structure of the tumor and also distinguish the relationship between the tumor and the surrounding tissue structure. In particular, enhanced CT can be used to analyze the lesion details[4,5].
This study analyzed the differences between GSTs and benign tumors on CT scans. GSTs are rich in blood supply; therefore, they are prone to bleeding and cause cystic necrosis within the tumor, and calcification is relatively common with the progression of the disease. Benign tumors, owing to their slow growth, show homogeneous soft tissue masses with relatively clear boundaries and regular morphology. Cystic necrosis and calcification of tumors are rare[6,7]. In this study, the incidence of extraluminal growth, blurred boundaries, and ulcers in the CST group was significantly higher than that in the benign tumor group, indicating that GSTs show extraluminal growth, blurred boundaries, and ulcers, which is of great significance for the identification of GSTs and benign tumors. Some scholars have reported that malignant tumors grow rapidly and have different rates of extension in various directions, resulting in irregular shapes such as lobulation. Benign tumors are mostly round, oval, and other regular shapes, owing to the uniform expansion of the growth mode around them. The higher the risk, the more irregular the shape; the more uneven the internal density and the greater the probability of necrosis, liquefaction, and bleeding. These results are consistent with those of this study[8,9].
This study also analyzed the difference between contrast-enhanced CT in the differential diagnosis of gastric stromal and benign tumors. Previous studies have found that contrast-enhanced CT has little significance in the differential diagnosis of gastric stromal and benign tumors. The main reason is that both tumors originate from the gastric submucosa interstitial tissue, and there is little difference in blood supply between tumors, which leads to obvious enhancement in contrast-enhanced CT[10]. In this study, there was no difference in CT values between the GST and control groups in the venous and delayed phases, but the CT values and enhanced peak CT values of the CST group were higher than those of the control group in the arterial phase. We believe that the enhancement examination can reveal a cluster-like small vascular shadow around the tumor. Previous studies have suggested that enhancement may be related to the malignant degree of the tumor, and a low malignant degree of the tumor may lead to uniform and moderate enhancement, or tumor necrosis and cystic degeneration[11-14]. ROC curve analysis showed that the arterial CT value and enhanced peak CT value had a certain sensitivity and specificity in the differential diagnosis of GST and gastric polyps.
The NIH grading standard has been commonly used for assessing the risk of GSTs in clinical practice. The degree of risk is mainly divided according to mitosis, tumor size, primary site, and rupture. In this study, the lesion boundary of the high-risk group was blurred, and the incidence of ulceration in the lesion was significantly higher than that in the low-risk group[15,16]. Further analysis of the enhanced CT results showed that the arterial phase CT value and enhanced peak CT value of the high-risk group were significantly higher than those of the low-risk group, indicating that the blood supply in GSTs was rich, mainly due to the rapid proliferation of malignant tumor blood vessels. Therefore, in addition to vascular penetration, blood vessels can also be observed on CT examination[17,18]. Some scholars have reported that GSTs show mild-to-moderate homogeneous enhancement on contrast-enhanced scanning. With different degrees of malignancy, homogeneous or inhomogeneous enhancement was observed. Especially, the enhancement degree less than 15.4 Hu in the arterial phase was an important indicator for distinguishing benign tumors from GSTs, which was primarily consistent with the results of this study[19,20].
This study analyzed the differences between GSTs and benign gastric polyps on contrast-enhanced CT examination and confirmed that CT examination has a certain reference value for the identification of the two diseases. Concurrently, it also confirmed the difference in the degree of disease risk in CT examinations, which could provide the corresponding diagnostic basis for clinical differential diagnosis and risk assessment of GSTs. However, the number of samples included in this study was relatively small, and this was a single-center study, which may have regional differences. Moreover, it is not possible to analyze the CT texture differences and whether there is a difference in the size of the GSTs on CT examination. Therefore, it is necessary to expand the sample size and conduct stratified research to further demonstrate and analyze our results.
In conclusion, preoperative MSCT examination has important value in the differential diagnosis of GST and benign gastric polyps and can effectively evaluate the risk classification of GST patients.
The malignant tendency and complex features of gastric stromal tumors (GSTs) seriously threaten human health.
Multi-slice spiral computed tomography (MSCT) is widely used in clinical practice. We try to apply it in the differential diagnosis and risk stratification of GSTs and benign gastric polyps, hoping to obtain valuable clues that can guide the clinical practice.
This study aimed to clarify the manifestations of GSTs and benign gastric polyps in multi-slice computed tomography, including diagnostic value and risk stratification.
The differences and risk stratification characteristics of MSCT imaging parameters and contrast-enhanced CT between patients with GST confirmed by pathological examination after surgery and patients with benign gastric polyps were retrospectively analyzed.
There are significant differences in MSCT characteristics and enhancement characteristics between GST and gastric polyps, and the MSCT characteristics and enhancement characteristics of GST in different risk stratifications are also different. MSCT has higher value in the identification and risk stratification of GST and gastric polyps.
Preoperative application of MSCT to distinguish GST from benign gastric polyps is of high value, and it is also feasible to classify the risk level of GST patients.
We recommend preoperative MSCT to distinguish GST from benign gastric polyps and to classify GST patients at risk.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jusakul A, Thailand; Krishnan U, Australia; Shroff RT, United States S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Papke DJ Jr, Hornick JL. Recent developments in gastroesophageal mesenchymal tumours. Histopathology. 2021;78:171-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Wang MX, Devine C, Segaran N, Ganeshan D. Current update on molecular cytogenetics, diagnosis and management of gastrointestinal stromal tumors. World J Gastroenterol. 2021;27:7125-7133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Zimmer V, Bier B. Loop ligation-assisted endoscopic resection of a gastrointestinal stromal tumor in the gastric fundus. Dig Liver Dis. 2021;53:245-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Kawabata K, Takahashi T, Nakajima K, Tanaka K, Miyazaki Y, Makino T, Kurokawa Y, Yamasaki M, Mori M, Doki Y. [Laparoscopic Resection of a Huge Gastric Gastrointestinal Stromal Tumor after Neoadjuvant Chemotherapy-A Case Report]. Gan To Kagaku Ryoho. 2020;47:670-672. [PubMed] |
| 5. | Inukai M, Shibasaki S, Suzuki K, Tsuru Y, Matsuo K, Goto A, Nakamura K, Tanaka T, Kikuchi K, Suda K, Inaba K, Uyama I. [Preoperative Imatinib Therapy Followed by Laparoscopic Local Gastrectomy for a Giant Gastric Gastrointestinal Stromal Tumor-A Case Report]. Gan To Kagaku Ryoho. 2020;47:2062-2064. [PubMed] |
| 6. | Gu JY, Shi HT, Yang LX, Shen YQ, Wang ZX, Feng Q, Wang M, Cao H. [Clinical significance of the deep learning algorithm based on contrast-enhanced CT in the differential diagnosis of gastric gastrointestinal stromal tumors with a diameter ≤ 5 cm]. Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24:796-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Hamman SM, Biyyam DR, Mandell GA. Gastric Gastrointestinal Stromal Tumor Incidentally Detected With Meckel Scintigraphy. Clin Nucl Med. 2020;45:372-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Namikawa T, Maeda M, Yokota K, Tanioka N, Iwabu J, Munekage M, Uemura S, Maeda H, Kitagawa H, Nagata Y, Kobayashi M, Hanazaki K. Laparoscopic Distal Gastrectomy for Synchronous Gastric Cancer and Gastrointestinal Stromal Tumor With Situs Inversus Totalis. In Vivo. 2021;35:913-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Li C, Fu W, Huang L, Chen Y, Xiang P, Guan J, Sun C. A CT-based nomogram for predicting the malignant potential of primary gastric gastrointestinal stromal tumors preoperatively. Abdom Radiol (NY). 2021;46:3075-3085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Wu E, Son SY, Gariwala V, O'Neill C. Gastric gastrointestinal stromal tumor (GIST) with co-occurrence of pancreatic neuroendocrine tumor. Radiol Case Rep. 2021;16:1391-1394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Taguchi T, Nagase H, Noguchi K, Hirota M, Oshima K, Tanida T, Noura S, Kawase T, Imamura H, Iwazawa T, Akagi K, Andou H, Tamura Y, Adachi S, Douno K. [Gastric Gastrointestinal Stromal Tumor Appearing Nine Years after Resection of a Duodenal Gastrointestinal Stromal Tumor-A Case Report]. Gan To Kagaku Ryoho. 2020;47:144-146. [PubMed] |
| 12. | Zhao H, Chen C, Yang C, Mo S, Zhao H, Tian Y. Prefoldin and prefoldin-like complex subunits as predictive biomarkers for hepatocellular carcinoma immunotherapy. Pathol Res Pract. 2022;232:153808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Zhang Y, Zhou F, Yang H, Gong X, Gao J. Current Status of Fear of Disease Progression in Patients with Advanced Cancer and Usefulness of Dignity Therapy Intervention. J Healthc Eng. 2022;2022:6069060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Xu JX, Ding QL, Lu YF, Fan SF, Rao QP, Yu RS. A scoring model for radiologic diagnosis of gastric leiomyomas (GLMs) with contrast-enhanced computed tomography (CE-CT): Differential diagnosis from gastrointestinal stromal tumors (GISTs). Eur J Radiol. 2021;134:109395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Xiao F, Zhang L, Yang S, Peng K, Hua T, Tang G. Quantitative analysis of the MRI features in the differentiation of benign, borderline, and malignant epithelial ovarian tumors. J Ovarian Res. 2022;15:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 16. | Mazzei MA, Cioffi Squitieri N, Vindigni C, Guerrini S, Gentili F, Sadotti G, Mercuri P, Righi L, Lucii G, Mazzei FG, Marrelli D, Volterrani L. Gastrointestinal stromal tumors (GIST): a proposal of a "CT-based predictive model of Miettinen index" in predicting the risk of malignancy. Abdom Radiol (NY). 2020;45:2989-2996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Sugiyama Y, Shimbara K, Sasaki M, Kouyama M, Tazaki T, Takahashi S, Nakamitsu A. Solitary peritoneal metastasis of gastrointestinal stromal tumor: A case report. World J Gastroenterol. 2020;26:5527-5533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Li J, Jiang Y, Chen C, Tan W, Li P, Chen G, Peng Q, Yin W. Integrin β4 Is an Effective and Efficient Marker in Synchronously Highlighting Lymphatic and Blood Vascular Invasion, and Perineural Aggression in Malignancy. Am J Surg Pathol. 2020;44:681-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Wang J, Xie Z, Zhu X, Niu Z, Ji H, He L, Hu Q, Zhang C. Differentiation of gastric schwannomas from gastrointestinal stromal tumors by CT using machine learning. Abdom Radiol (NY). 2021;46:1773-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Dhali A, Ray S, Khamrui S, Dhali GK. Mucinous cystadenocarcinoma of pancreas mimicking gastrointestinal stromal tumor of stomach: Case report. Int J Surg Case Rep. 2021;85:106240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |