Published online Jan 15, 2022. doi: 10.4251/wjgo.v14.i1.278
Peer-review started: March 18, 2021
First decision: July 3, 2021
Revised: July 4, 2021
Accepted: November 24, 2021
Article in press: October 24, 2021
Published online: January 15, 2022
Processing time: 298 Days and 10.9 Hours
Digestive cancer has traditionally been thought of as a disease that mainly occurs in elderly individuals, and it has been ignored in young adults by both patients and physicians.
To describe the worldwide profile of digestive cancer incidence, mortality and corresponding trends among 20–39-year-olds, with major patterns highlighted by age, sex, development level, and geographical region.
I performed a population-based study to quantify the burden of young adult digestive cancers worldwide. Global, regional, sex, and country-specific data estimates of the number of new cancer cases and cancer-associated deaths that occurred in 2020 were extracted from the GLOBOCAN Cancer Today database. To assess long-term trends in young adult digestive cancer, cancer incidence data and mortality data were obtained from the Cancer in Five Continents Plus database and the World Health Organization mortality database, respectively. The associations between the human development index (HDI) and digestive cancer burden in young adults were evaluated by linear regression analyses.
In 2020, there were an estimated 19292789 new cancer cases, resulting in 9958133 deaths worldwide, which equated to an age-standardized incidence rate (ASIR) of 5.16 and age-standardized mortality rate (ASMR) of 3.04, accounting for 12.24% of all new cancer cases and 25.26% of all cancer deaths occurring in young adults. The burden was disproportionally greater among males, with male: female ratios of 1.34 for incidence and 1.58 for mortality. The ASIRs were 2.1, 1.4, and 1.0 per 100000 people per year, whereas the ASMRs were 0.83, 1.1, and 0.62 per 100000 people per year for colorectal, liver, and gastric cancer, respectively. When assessed by geographical region and HDI levels, the cancer profile varied substantially, and a strong positive correlation between the mortality-to-incidence ratio of digestive cancer and HDI ranking was found (R2 = 0.7388, P < 0.001).
The most common digestive cancer types are colorectal, liver and gastric cancer. The global digestive cancer burden among young adults is greater among males and exhibits a positive association with socioeconomic status. The digestive cancer burden is heavy in young adults, reinforcing the need for primary and secondary prevention strategies.
Core Tip: This study is the first to explore the global burden of digestive cancer among young adults. By assessing 6 major digestive cancer types, I provide up-to-date estimates across levels of sex, geographical region, and human development. Furthermore, this study investigates the long-term trends in digestive cancer in young adults, serving as the latest report to aid oncology studies and increase awareness of digestive cancer among this underserved subpopulation. Through continuous prevention, screening, and early detection programs, the digestive cancer burden in young adults can be reduced.
- Citation: Li J. Digestive cancer incidence and mortality among young adults worldwide in 2020: A population-based study. World J Gastrointest Oncol 2022; 14(1): 278-294
- URL: https://www.wjgnet.com/1948-5204/full/v14/i1/278.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i1.278
Cancer is a leading cause of morbidity and mortality and is an important barrier to increasing life expectancy across all age groups globally. According to estimates from the GLOBOCAN database, there were an estimated 19.3 million new cases and 10 million cancer deaths worldwide in 2020[1]. Cancer has traditionally been thought to be a disease that mainly occurs in elderly individuals, and studies have focused on cancers at older ages. Therefore, in relative terms, cancers in young adults have been ignored by both patients and physicians.
Digestive cancer represents approximately 30% of the global cancer incidence and 40% of all cancer-related deaths worldwide[1]. Studies on cancer trends have demonstrated that the incidence of digestive cancers has increased significantly in young adults over the last few decades[2-5]. Digestive cancer is difficult to diagnose in a timely manner because of the lack of symptoms and signs at an early stage. It is more serious among young adults, which can be attributed to the relatively lower health consciousness and lack of screening programs in this age group[6]. However, young adults represent the main proportion of contributors to the economy and their family care[7]. Thus, to improve digestive cancer-associated outcomes among young adults, it is important to investigate the accurate profile of disease burden in this age group. This will help in not only determining the screening population to identify cancers early but also in developing preventative programs against them.
Therefore, I here presented a comprehensive assessment of the digestive cancer burden in young adult globally. I reported the incidence and mortality estimates of young adult digestive cancer in 2020, describing variations by sex and geographical region as well as the correlation between young adult digestive cancer burden and human development level.
I performed a population-based study to assess the global burden of young adult digestive cancer incidence and mortality in 2020 and to investigate incidence and mortality trends over specific periods for selected countries. The study population comprised young adults diagnosed with digestive cancers, classified according to the International Classification of Diseases (ICD), tenth revision (ICD-10), as esophagus (C15), stomach (C16), colorectum (including anus, C18–21), liver (C22), gallbladder (C23), and pancreas (C25).
Age is a continuous variable. Variation exists among individuals of the same age, and any predefined age range is an arbitrary rather than an unequivocal definition. However, to facilitate clarity, consensus, simplicity and data collection and comparison, it is necessary to define different populations by upper and lower age limits. Although 0-19 years is broadly used to define childhood and adolescent cancer, the age range used to define young adult cancer are not always consistent in the literature or guidelines. Nevertheless, two large groups, the Adolescent and Young Adult Health Outcomes and Patient Experience (AYA HOPE) study and the Adolescent and Young Adult Oncology Progress Review Group (AYAO PRG), were in favor of the upper limit of 39 years of age in their studies[8,9]. As the AYAO PRG states, to apply this age range is based on the biological and physiological maturity and relative stability during the 20s and 30s, and these individuals have not yet experienced the effects of hormonal and immune response decline or chronic medical conditions that can influence oncologic decision-making and care of older patients. Furthermore, cancer survivorship studies have similarly used age 39 to define the upper limit of young adults[10,11]. For these reasons, the young adult digestive cancer in this analysis encompasses individuals diagnosed between 20–39 years.
GLOBOCAN held at the International Agency for Research on Cancer (IARC) provides estimates of the incidence and mortality of 36 cancer types for 185 countries or territories by sex and age group, with the most recent estimates applying to 2020 (https://gco.iarc.fr/today/). To quantify the digestive cancer burden in young adults, global, regional, and country-specific cancer incidence and mortality estimates for 2020 were obtained from the GLOBOCAN Cancer Today database. I reported the numbers of new cases, deaths, age-standardized incidence rates (ASIRs), and age-standardized mortality rates (ASMRs) for digestive cancer among adults aged 20-39 years globally. Estimates are also presented and compared based on cancer type, world area, sex, country, and human development index (HDI). To make comparisons of the cancer type spectrum with younger and older age groups, I extracted the incidence and mortality data of all cancer types that were classified as digestive, brain and central nervous system (CNS), breast, lung, hematological, head and neck, genitourinary, and others (eight cancer groups) among individuals aged 0-19 years, 40-59 years, and 60 years and older. To make comparisons of the cancer type spectrum within young adults with digestive cancer, I also extracted the incidence and mortality data of all six digestive cancer types in four 5-year bands.
Furthermore, I used the GLOBOCAN data to examine the correlations of young adult digestive cancer burden with HDI ranking, a socioeconomic development indicator that was created by the United Nations Development Program, to highlight the importance of national policy decisions beyond economic growth in assessing development outcomes. The latest edition is the Human Development Report 2020 (http://hdr.undp.org). The association between the mortality-to-incidence ratio (MIR) and HDI ranking was also examined. The MIR, which was calculated as the ratio of all-age death counts and all-age incidence counts of the mean estimates, was employed as a proxy for 5-year survival rates to measure the severity of a disease. Because the number of new cases and deaths of young adult digestive cancer in most countries was small, I focused primarily on heavily burdened countries and regions with HDI rankings.
To assess long-term trends in young adult digestive cancer, I used cancer incidence data from IARC’s Cancer in Five Continents Plus database, which compiles high-quality recorded cancer incidence data from cancer registries worldwide, with 2012 being the last available year (https://ci5.iarc.fr/CI5plus/Default.aspx). Mortality data were obtained from the World Health Organization mortality database compiled by IARC for countries with different available time periods (https://www-dep.iarc.fr/WHOdb/WHOdb.htm). To include countries with representative geographies and developmental levels that spanned globally, I used the 14-year period of 1999–2012 to estimate trends in incidence and the 32-year period of 1985-2016 to estimate trends in mortality. Because of the volatile age-standardized rates in most countries, I only analyzed the overall trends of available countries based on the number of cases and deaths in young adults.
Detailed descriptions of the applied methods used to generate incidence and mortality estimates for each country are available on the GLOBOCAN website. In the current report, I used the Pearson correlation method to assess the correlation between ASIR, ASMR, and MIR for young adult digestive cancer and HDI ranking. Statistical analyses and graphs were performed using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, United States) and Excel 2013. For all analyses, P values less than 0.05 were considered statistically significant, and all P values reported in this study were two-sided.
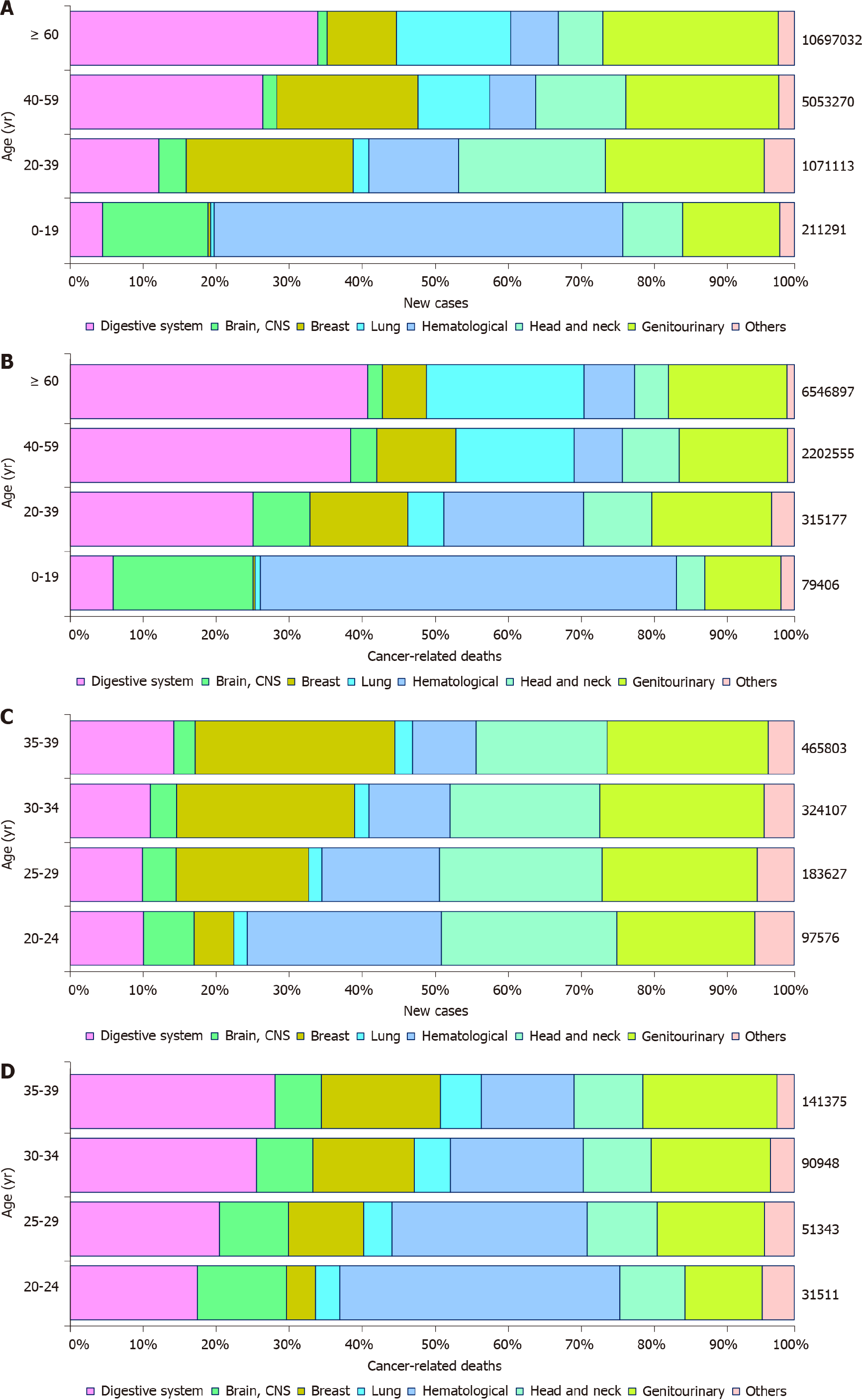
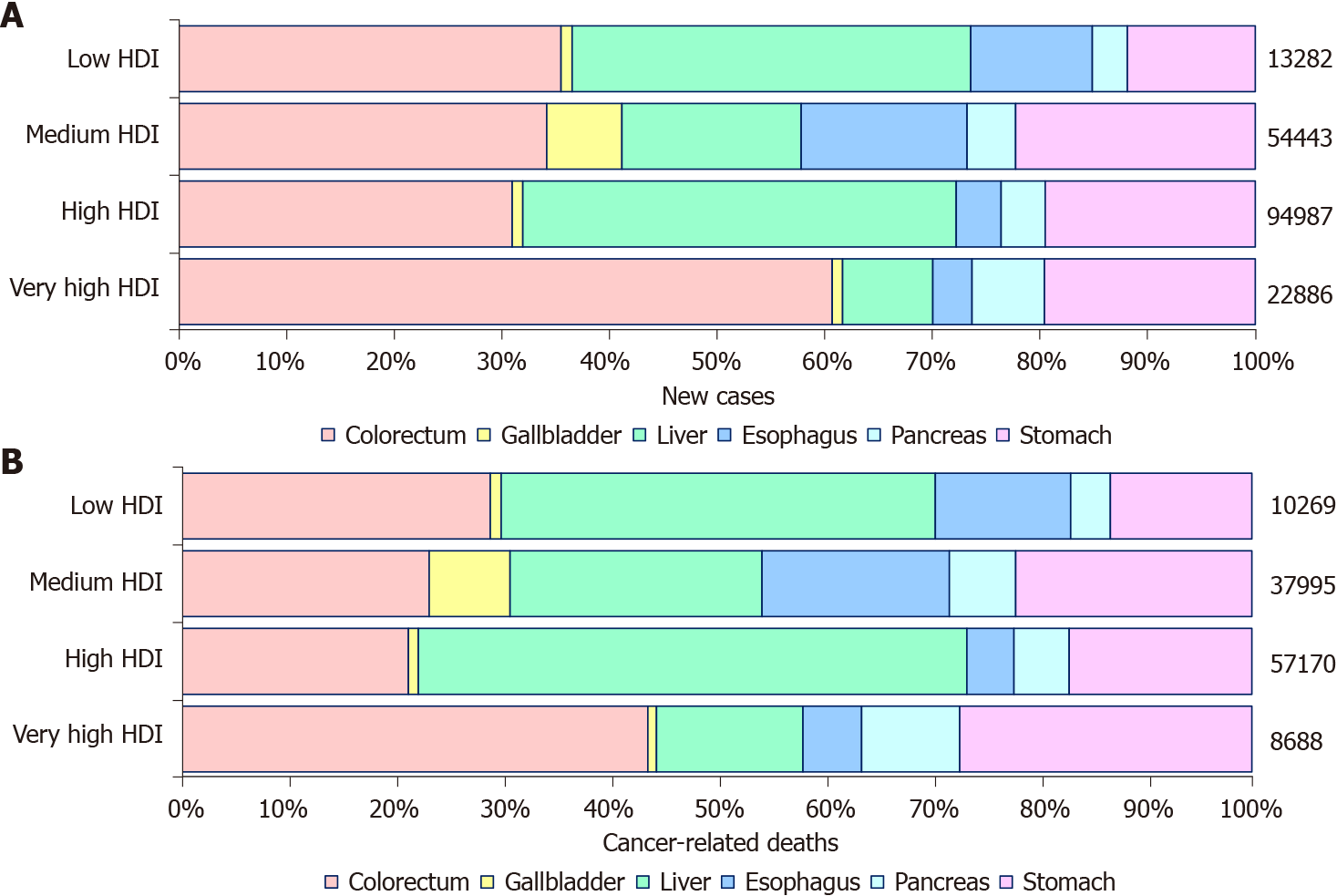
In 2020, there were an estimated 19292789 new cancer cases, resulting in 9958133 deaths worldwide. The spectrum of cancers differed among age groups. Common tumor types in ages 0-19 (children and adolescents) included hematological tumors and cancers of the CNS. Epithelial tumors, including digestive cancers, were the majority of malignancies in other age groups with heterogeneous profiles. As age increased, the incidence and mortality of digestive cancers increased. The new cases and cancer-associated deaths of digestive cancer among young adults were 14 and 17 times that among children and adolescents, but markedly less than that in middle age (40–59 years) and elderly (60+ years) (Figure 1A and B). For digestive cancer, young adults accounted for approximately 2.55% (131068/5142192) of new cases and 2.19% (79614/3628920) of cancer-associated deaths. Among young adults, the cancer profile varied obviously, with the proportion of hematological cancer decreased and breast cancer increased with increasing age. However, the difference in digestive cancers was not significant, with 12.24% (131068/1071113) of all new cancer cases and 25.26% (79614/315177) of all cancer deaths occurring in the digestive system (Figure 1C and D).
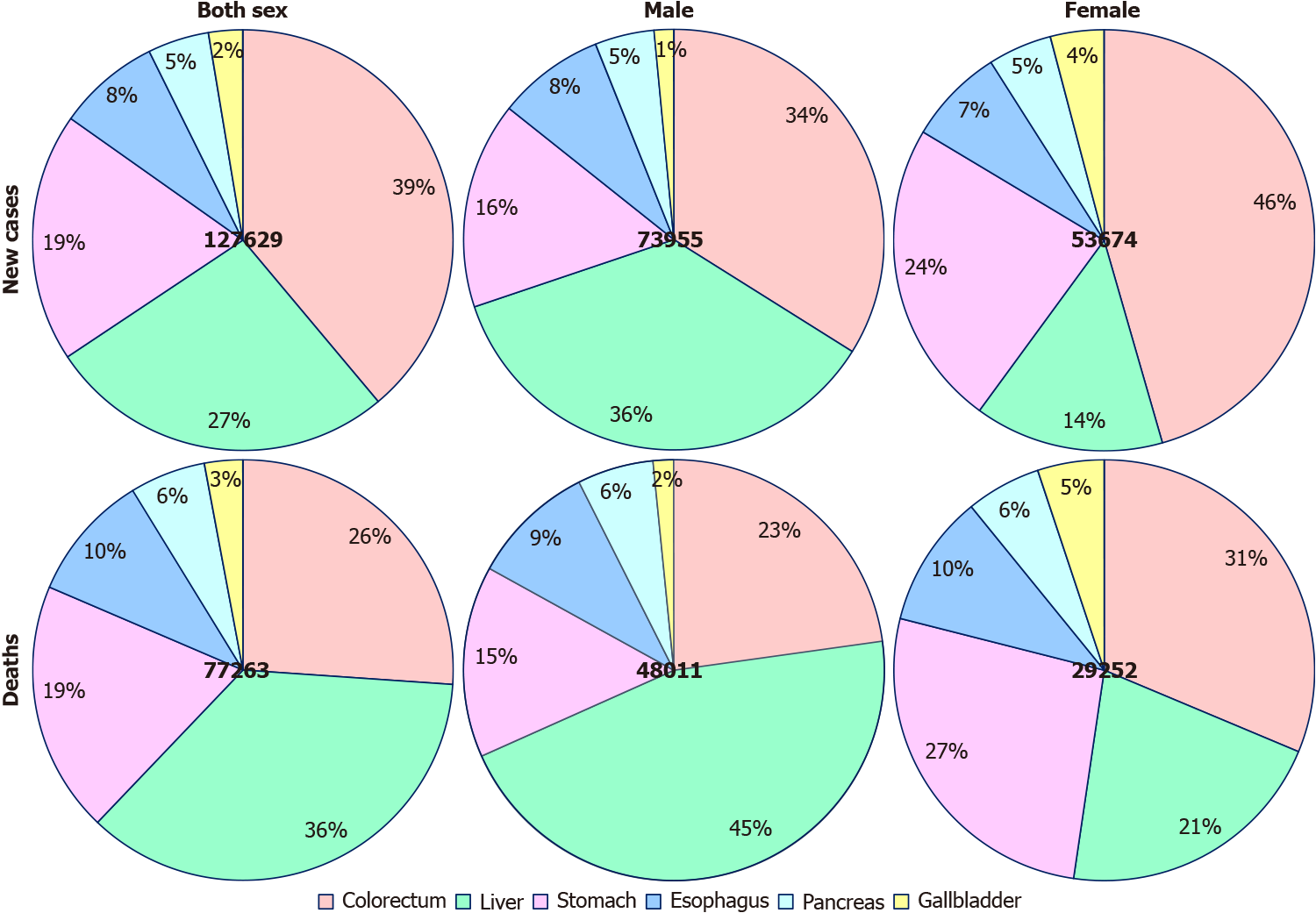
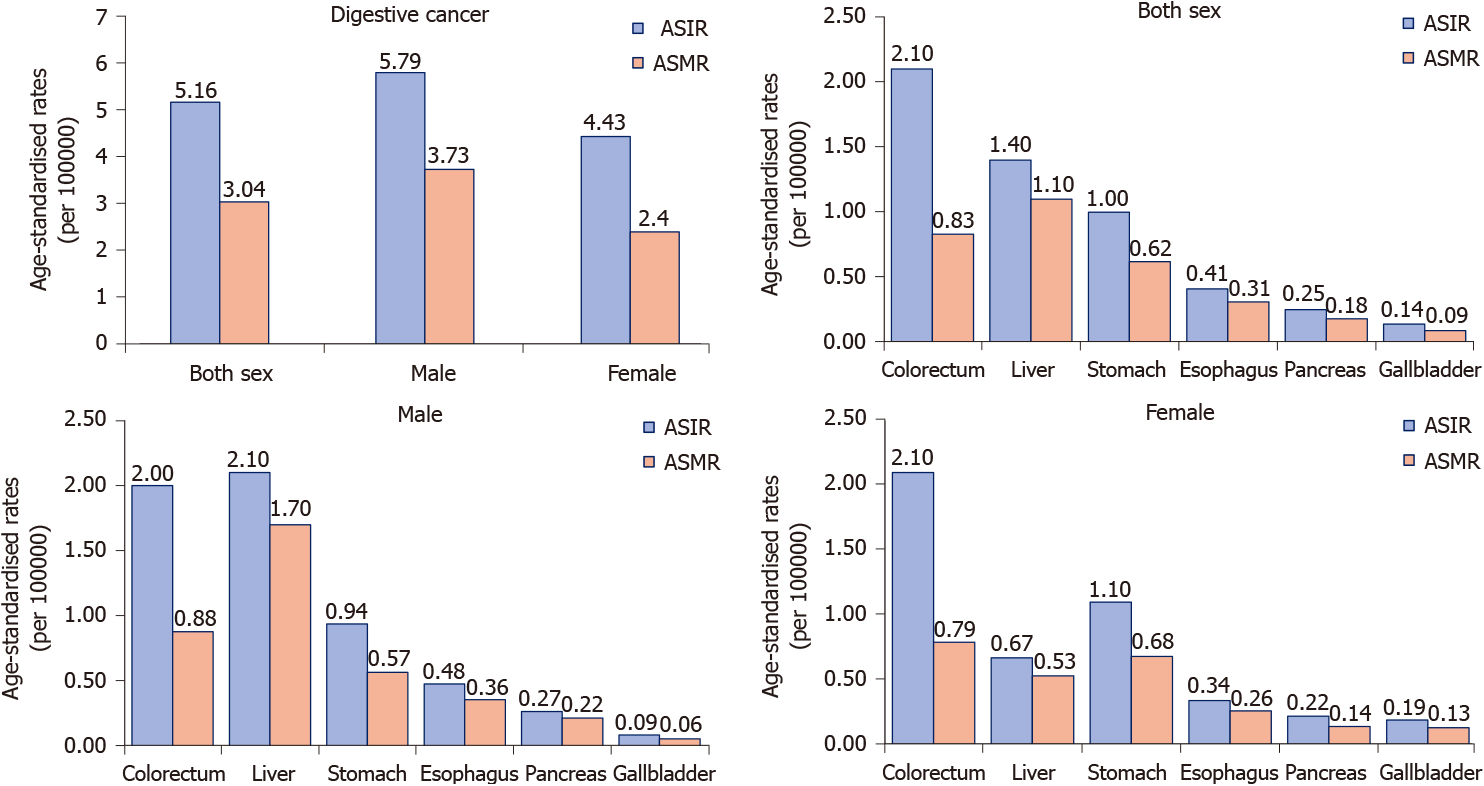
Among young adults, it is estimated that there were 131068 new digestive cancer cases and 79614 digestive cancer-associated deaths in 2020. The ASIR and ASMR were 5.16 and 3.04 per 100000 people per year, respectively. Colorectal, liver, and gastric cancer together accounted for 111137 (86.04%) of the total new cases and 64843 (81.45%) of the total deaths (Figure 2). The ASIRs were 2.1, 1.4, and 1.0 per 100000 people per year, whereas the ASMRs were 0.83, 1.1, and 0.62 per 100000 people per year for colorectal, liver, and gastric cancer, respectively. Notably, digestive cancers were more frequent among men (male: female ratios of 1.34 for incidence and 1.58 for mortality), with liver cancer being more than 3 times more common in men than in women with regard to both incidence and mortality. However, the incidence and mortality of gastric and gallbladder cancer were lower in men than in women (Figure 3).
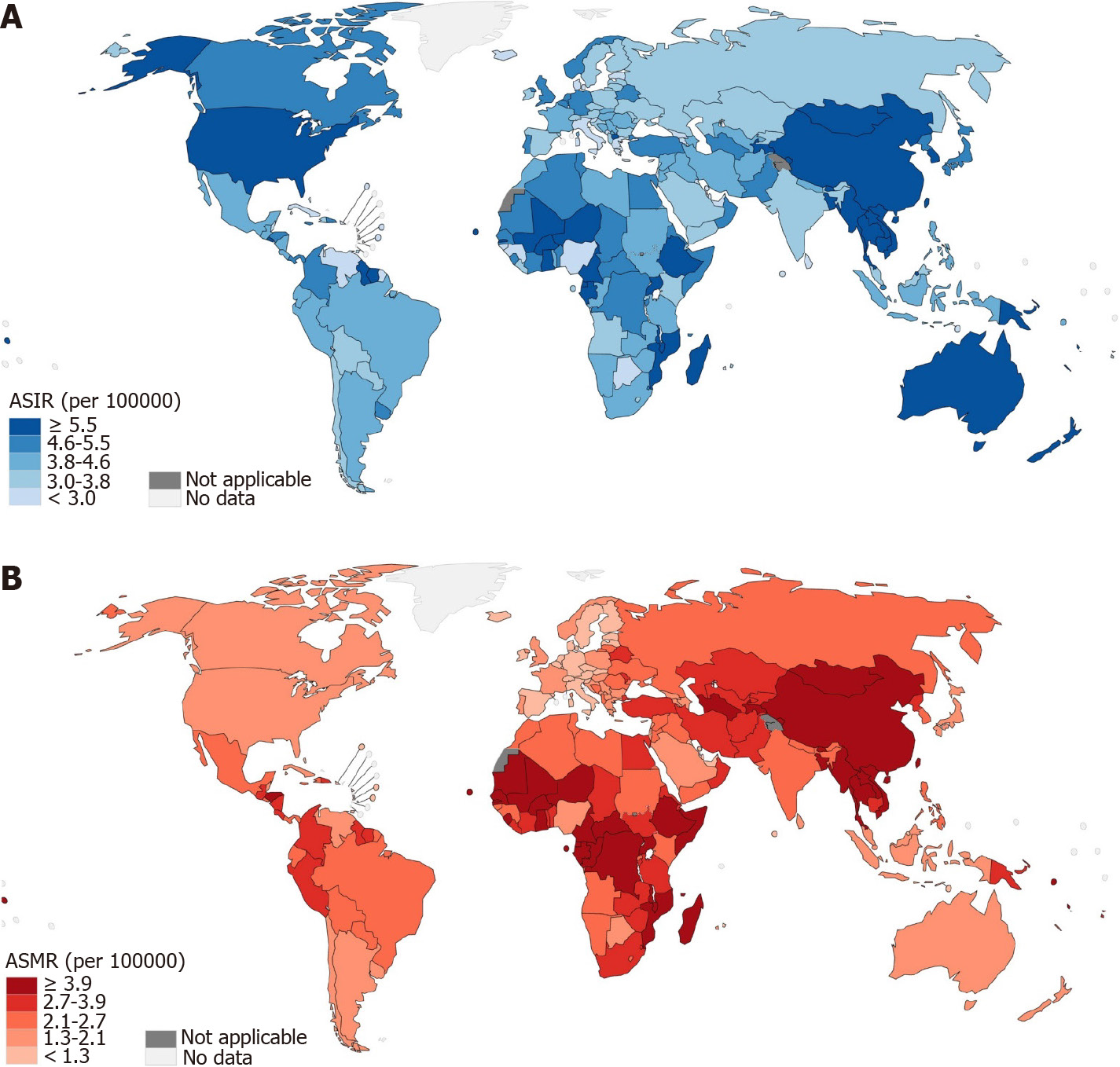
Based on the estimates in the 21 world areas, the incidence rate of digestive cancer among young adults was the greatest in Australia and New Zealand, followed by Eastern Asia, whereas other parts of Oceania, Southern Europe, and the Caribbean had the lowest incidence. There was substantial variability in the incidence and mortality rates across the geographical regions. For instance, the ASIR for colorectal cancer was 1.3 per 100000 in Middle Africa, whereas it was 6.2 per 100000 in Australia and New Zealand. The incidence of liver cancer showed the greatest variation, with the highest incidence in Melanesia, which was 24 times that in Western Europe. Despite the incidence being highest in Australia and New Zealand, the region conversely had a lower mortality rate (1.8 per 100000 people per year). In contrast, Africa and Asia contributed the majority of deaths. With regard to the estimated number of incident cases and deaths, 65.64% and 67.59% of digestive cancers occurred in Asia, with the majority occurring in Eastern Asia (Table 1, Supplementary Table 1).
| World areas | Incidence | Mortality | MIR | ||||
| Cases | % | ASIR | Deaths | % | ASMR | ||
| Asia | |||||||
| Eastern Asia | 40966 | 31.26 | 7.70 | 24006 | 30.15 | 4.40 | 0.59 |
| South-Central Asia | 29100 | 22.20 | 4.20 | 20045 | 25.18 | 2.90 | 0.69 |
| South-Eastern Asia | 12048 | 9.19 | 5.40 | 7639 | 9.60 | 3.40 | 0.63 |
| Western Asia | 3919 | 2.99 | 3.90 | 2123 | 2.67 | 2.10 | 0.54 |
| Americas and Caribbean | |||||||
| Northern America | 6690 | 5.10 | 6.10 | 2118 | 2.66 | 1.90 | 0.32 |
| South America | 6321 | 4.82 | 4.30 | 3626 | 4.55 | 2.50 | 0.57 |
| Central America | 2570 | 1.96 | 4.40 | 1472 | 1.85 | 2.50 | 0.57 |
| Caribbean | 485 | 0.37 | 3.60 | 293 | 0.37 | 2.20 | 0.60 |
| Europe | |||||||
| Central and Eastern Europe | 3374 | 2.57 | 3.60 | 2136 | 2.68 | 2.20 | 0.63 |
| Western Europe | 2513 | 1.92 | 4.70 | 549 | 0.69 | 0.97 | 0.22 |
| Southern Europe | 1344 | 1.03 | 3.20 | 441 | 0.55 | 1.00 | 0.33 |
| Northern Europe | 1356 | 1.03 | 4.40 | 400 | 0.50 | 1.30 | 0.29 |
| Africa | |||||||
| Eastern Africa | 7378 | 5.63 | 5.80 | 5724 | 7.19 | 4.50 | 0.78 |
| Northern Africa | 3722 | 2.84 | 4.70 | 2328 | 2.92 | 3.00 | 0.63 |
| Western Africa | 4751 | 3.62 | 4.40 | 3686 | 4.63 | 3.40 | 0.78 |
| Middle Africa | 2483 | 1.89 | 5.20 | 2037 | 2.56 | 4.30 | 0.82 |
| Southern Africa | 1092 | 0.83 | 4.50 | 691 | 0.87 | 2.80 | 0.63 |
| Oceania | |||||||
| Australia and New Zealand | 721 | 0.55 | 7.80 | 165 | 0.21 | 1.80 | 0.23 |
| Melanesia | 227 | 0.17 | 6.60 | 132 | 0.17 | 3.80 | 0.58 |
| Polynesia | 5 | 0.00 | 2.70 | 3 | 0.00 | 1.80 | 0.60 |
| Micronesia | 3 | 0.00 | 2.00 | 0 | 0.00 | 0.00 | 0.00 |
Country-wise, China and India were the two leading countries in terms of new cases, with 36723 and 17819 cases, respectively, in 2020. Together, they accounted for 41.6% of the total estimated new cases, followed by the United States, Pakistan, Bangladesh, and Indonesia, with estimated 6069, 3811, 3779, and 3462 new cases, respectively. In terms of death counts, China was the leading country, with 22345 deaths, followed by India, with an estimated 12166 deaths in 2020. Together, they accounted for 43.35% of the total deaths. Vietnam was ranked third in terms of death counts, with 2953 deaths, which was higher than the number of deaths in Bangladesh (2795), Pakistan (2520), and the United States (1900). The top three countries in terms of ASIR were The Republic of the Gambia (16.9), Malawi (12.6), and Ghana (11.7). The ASMR mostly followed the patterns of ASIR, with The Republic of the Gambia (15.0), Malawi (1.4), and Ghana (8.9) as the three leading countries. In terms of individual cancer types, China had the largest counts of new colorectal, gastric, liver, and pancreatic cancer, and India had the largest number of new cases of esophageal and gallbladder cancer. The deaths of individual digestive cancer types mostly followed the patterns of new cases (Figure 4, Table 2, and Supplementary Tables 2-6).
| Country | Incidence | Mortality | MIR | HDI | HDI ranking | ||||
| Cases | % | ASIR | Deaths | % | ASMR | ||||
| China | 36723 | 28.02 | 7.8 | 22345 | 28.07 | 4.6 | 0.61 | 0.761 | 87 |
| India | 17819 | 13.60 | 3.8 | 12166 | 15.28 | 2.6 | 0.68 | 0.645 | 130 |
| United States of America | 6069 | 4.63 | 6.2 | 1900 | 2.39 | 1.9 | 0.31 | 0.926 | 17 |
| Pakistan | 3811 | 2.91 | 5.4 | 2520 | 3.17 | 3.6 | 0.66 | 0.557 | 154 |
| Bangladesh | 3779 | 2.88 | 6.4 | 2795 | 3.51 | 4.7 | 0.74 | 0.632 | 134 |
| Indonesia | 3462 | 2.64 | 3.8 | 1645 | 2.07 | 1.8 | 0.48 | 0.718 | 110 |
| Viet Nam | 3440 | 2.62 | 9.9 | 2953 | 3.71 | 8.3 | 0.86 | 0.704 | 118 |
| Brazil | 3398 | 2.59 | 4.6 | 1801 | 2.26 | 2.4 | 0.53 | 0.765 | 84 |
| Ethiopia | 1966 | 1.50 | 5.9 | 1516 | 1.90 | 4.6 | 0.77 | 0.485 | 174 |
| Mexico | 1826 | 1.39 | 4.3 | 975 | 1.22 | 2.3 | 0.53 | 0.779 | 76 |
| Russian Federation | 1736 | 1.32 | 3.7 | 1193 | 1.50 | 2.4 | 0.69 | 0.824 | 49 |
| Egypt | 1647 | 1.26 | 5.1 | 1203 | 1.51 | 3.7 | 0.73 | 0.707 | 117 |
| Philippines | 1540 | 1.17 | 4.4 | 773 | 0.97 | 2.2 | 0.50 | 0.718 | 111 |
| Iran, Islamic Republic of | 1520 | 1.16 | 4.6 | 1003 | 1.26 | 3 | 0.66 | 0.783 | 70 |
| Turkey | 1445 | 1.10 | 5.2 | 775 | 0.97 | 2.8 | 0.54 | 0.82 | 54 |
| Japan | 1440 | 1.10 | 4.7 | 541 | 0.68 | 1.7 | 0.38 | 0.919 | 20 |
| Korea, Republic of | 1404 | 1.07 | 8.7 | 342 | 0.43 | 2.1 | 0.24 | 0.916 | 22 |
| Thailand | 1322 | 1.01 | 6.4 | 864 | 1.09 | 4.1 | 0.65 | 0.777 | 80 |
| Germany | 1212 | 0.92 | 5.3 | 195 | 0.24 | 0.79 | 0.16 | 0.947 | 4 |
| Congo, Democratic Republic of | 1171 | 0.89 | 5.1 | 965 | 1.21 | 4.2 | 0.82 | 0.48 | 174 |
| Myanmar | 1143 | 0.87 | 6.4 | 768 | 0.96 | 4.2 | 0.67 | 0.583 | 148 |
| Ghana | 1106 | 0.84 | 11.7 | 841 | 1.06 | 8.9 | 0.76 | 0.611 | 138 |
| Nigeria | 1001 | 0.76 | 1.8 | 719 | 0.90 | 1.3 | 0.72 | 0.539 | 161 |
| South Africa | 993 | 0.76 | 4.6 | 630 | 0.79 | 2.9 | 0.63 | 0.709 | 115 |
| United Kingdom | 985 | 0.75 | 4.9 | 300 | 0.38 | 1.5 | 0.30 | 0.932 | 14 |
| Uganda | 922 | 0.70 | 7.5 | 730 | 0.92 | 6 | 0.79 | 0.544 | 160 |
| Colombia | 889 | 0.68 | 5.1 | 550 | 0.69 | 3.2 | 0.62 | 0.767 | 83 |
| Mozambique | 812 | 0.62 | 9.6 | 649 | 0.82 | 7.7 | 0.80 | 0.456 | 181 |
| Algeria | 772 | 0.59 | 4.9 | 364 | 0.46 | 2.4 | 0.47 | 0.748 | 91 |
| Tanzania, United Republic of | 749 | 0.57 | 4.5 | 615 | 0.77 | 3.7 | 0.82 | 0.529 | 164 |
| France | 738 | 0.56 | 4.3 | 259 | 0.33 | 1.4 | 0.35 | 0.901 | 26 |
| Malawi | 663 | 0.51 | 12.6 | 548 | 0.69 | 10.4 | 0.83 | 0.483 | 174 |
| Australia | 644 | 0.49 | 8.1 | 140 | 0.18 | 1.8 | 0.22 | 0.944 | 7 |
| Kenya | 625 | 0.48 | 3.7 | 440 | 0.55 | 2.6 | 0.70 | 0.601 | 141 |
| Canada | 621 | 0.47 | 5.4 | 218 | 0.27 | 1.9 | 0.35 | 0.929 | 14 |
| Argentina | 593 | 0.45 | 4.2 | 291 | 0.37 | 2.1 | 0.49 | 0.845 | 46 |
| Cameroon | 572 | 0.44 | 7.3 | 473 | 0.59 | 6.1 | 0.83 | 0.563 | 153 |
| Saudi Arabia | 542 | 0.41 | 3.7 | 264 | 0.33 | 1.8 | 0.49 | 0.854 | 40 |
| Morocco | 528 | 0.40 | 4.3 | 293 | 0.37 | 2.4 | 0.55 | 0.686 | 121 |
| Iraq | 504 | 0.38 | 4.1 | 289 | 0.36 | 2.4 | 0.57 | 0.674 | 123 |
| Uzbekistan | 491 | 0.37 | 4.1 | 363 | 0.46 | 3 | 0.74 | 0.72 | 107 |
| Madagascar | 490 | 0.37 | 6.2 | 374 | 0.47 | 4.7 | 0.76 | 0.528 | 163 |
| Ukraine | 487 | 0.37 | 3.4 | 326 | 0.41 | 2.2 | 0.67 | 0.779 | 78 |
| Burkina Faso | 486 | 0.37 | 8.6 | 432 | 0.54 | 7.7 | 0.89 | 0.452 | 183 |
| Afghanistan | 484 | 0.37 | 4.4 | 368 | 0.46 | 3.3 | 0.76 | 0.511 | 169 |
| Sudan | 467 | 0.36 | 3.8 | 316 | 0.40 | 2.6 | 0.68 | 0.51 | 171 |
| Peru | 465 | 0.35 | 4.1 | 356 | 0.45 | 3.2 | 0.77 | 0.777 | 78 |
| Mali | 465 | 0.35 | 9.2 | 349 | 0.44 | 6.9 | 0.75 | 0.434 | 184 |
| Italy | 425 | 0.32 | 2.6 | 138 | 0.17 | 0.87 | 0.32 | 0.892 | 29 |
| Spain | 417 | 0.32 | 3.3 | 80 | 0.10 | 0.56 | 0.19 | 0.904 | 25 |
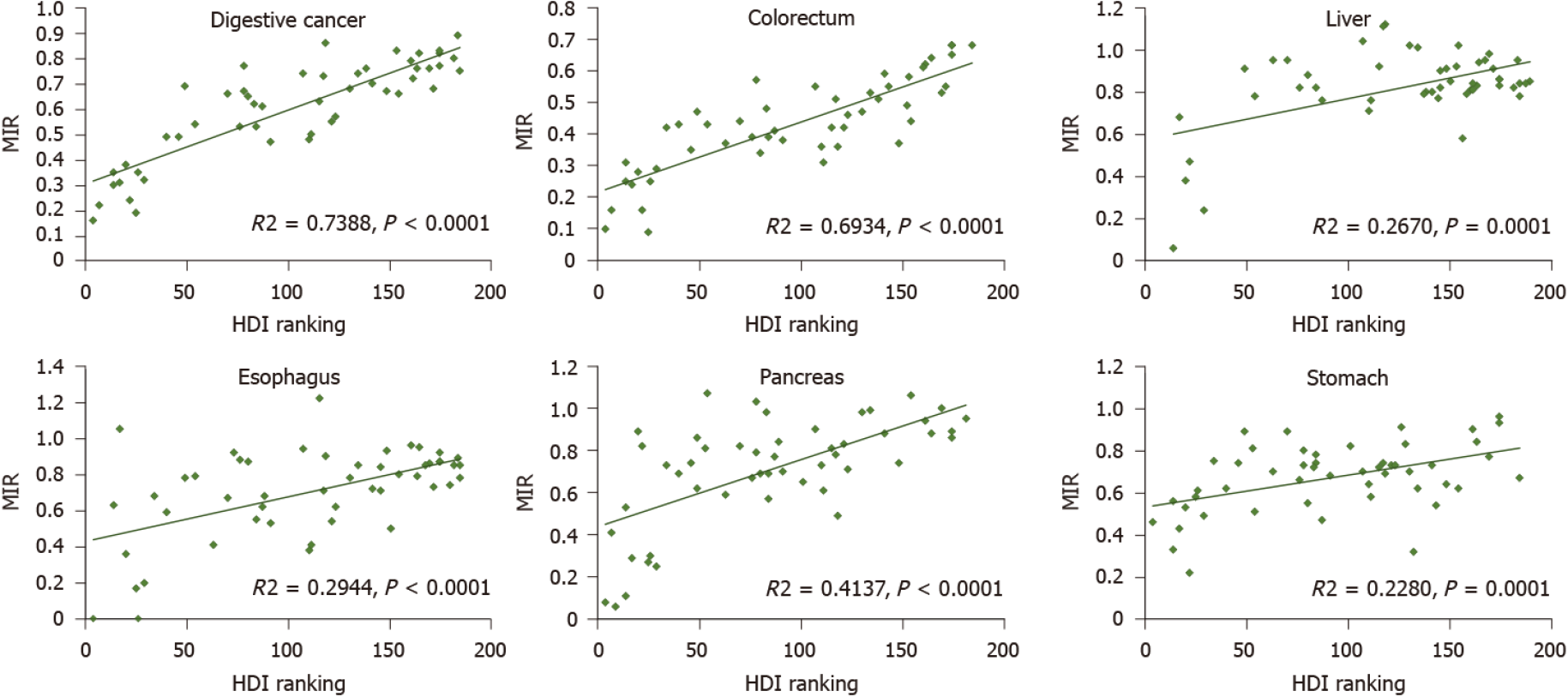
The burden of young adult digestive cancer varied substantially according to the HDI ranking. The four HDI level-based analysis showed that colorectal cancer was the most frequent digestive cancer in the most developed regions (60.72% of all new digestive cancer cases, 43.55% of all digestive cancer-related deaths). At high and low HDI levels, the most common digestive cancers were colorectal and liver cancer, with more new cases of colorectal cancer and more deaths from liver cancer. The proportions of colorectal, liver, and stomach cancer were similar in the median HDI level both for new cases and for deaths (Figure 5). The Pearson correlation analysis reflected a weak positive correlation between the ASIR of digestive cancer and HDI ranking (R2 = 0.0791, P = 0.0478), whereas a positive and significant correlation was observed between the ASMR of digestive cancer and HDI ranking (R2 = 0.4252, P < 0.001). The results also demonstrated a strong positive correlation between the MIR of digestive cancer and HDI ranking (R2 = 0.7388, P < 0.001). The positive correlation between MIR and HDI ranking was shared equally by individual digestive cancer types (Figure 6). Except for both ASIR and ASMR of stomach cancer and ASMR of pancreatic cancer, the correlation was significant for individual digestive cancer types, with a negative correlation between HDI ranking and ASIR of colorectal and pancreatic cancer (Supplementary Figures 1 and 2).
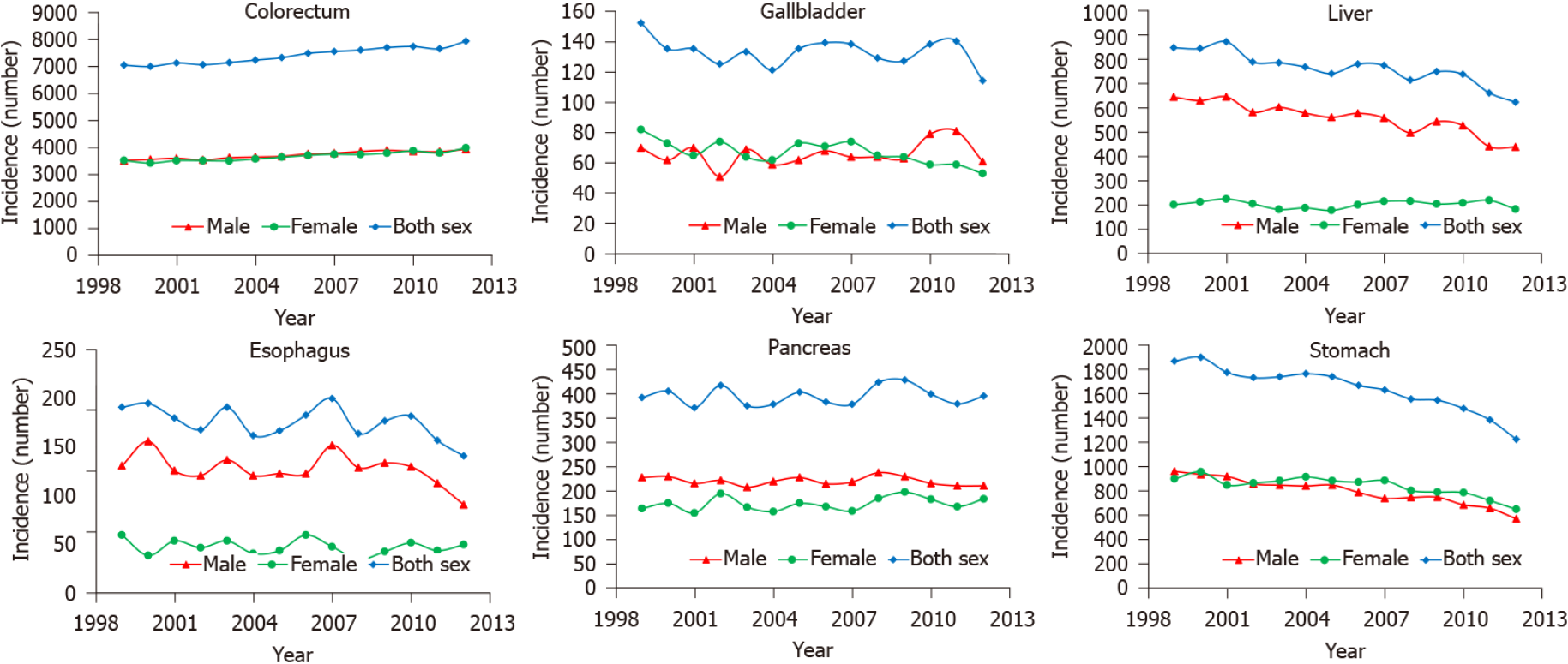
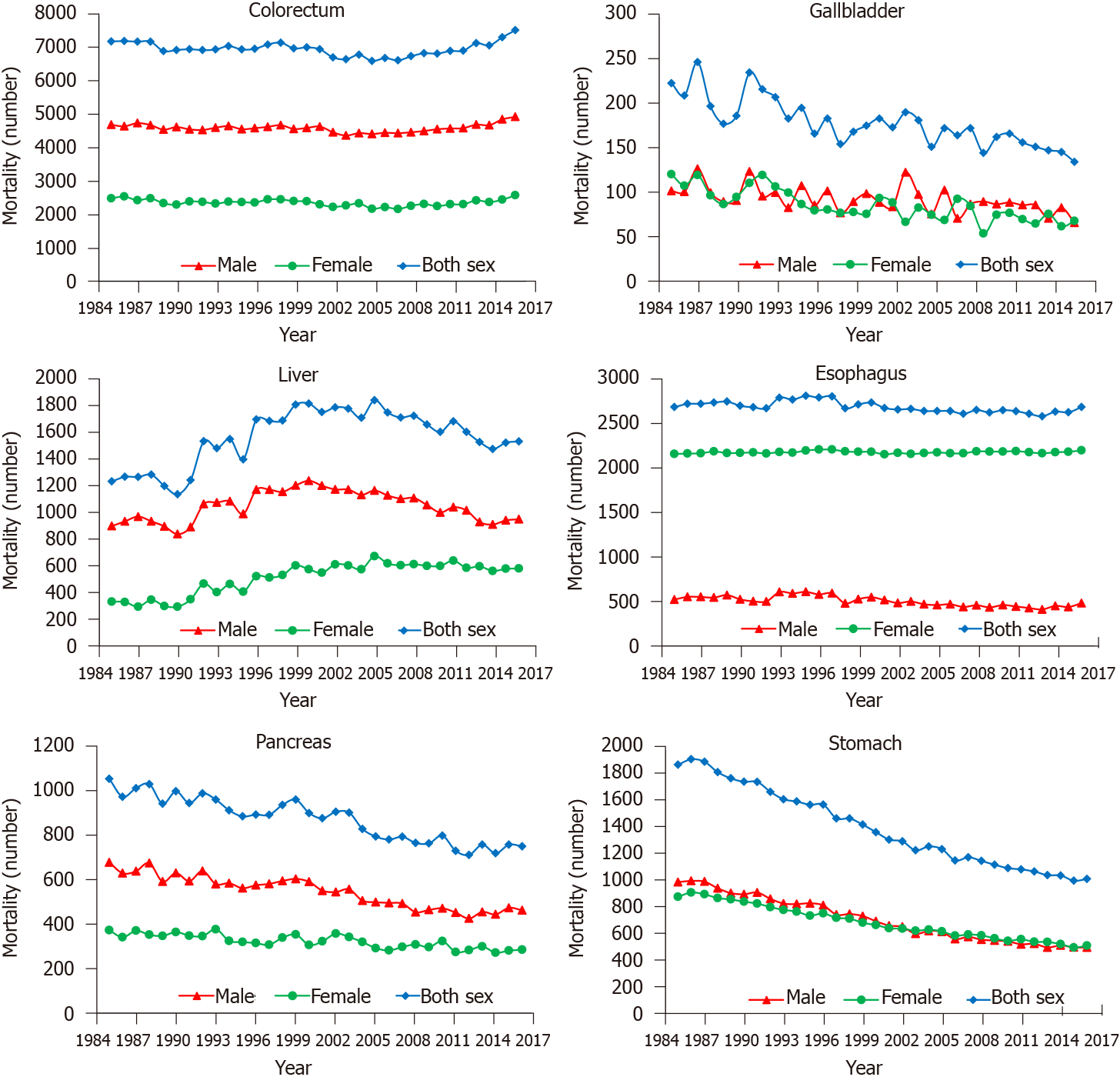
The 14-year (1999-2012) overall incidence trends showed a persistent slow increase for colorectal cancer, whereas obvious decreases were observed for liver and gastric cancer. The overall incidence trend curves of gallbladder, esophageal, and pancreatic cancer were volatile (Figure 7). The temporal variations (1985-2016) in mortality of colorectal and esophageal cancer were not obvious. The deaths resulting from liver cancer showed an unstable upward trend, especially in women. The number of deaths from gallbladder, pancreatic, and gastric cancer decreased from 1985 to 2016, especially for gastric cancer (Figure 8).
Worldwide, the incidence of digestive cancer in young adults in 2020 was 5.16 per 100000 people per year with an estimated 131068 new cases, and the corresponding mortality was 3.04 per 100000 people per year with an estimated 79614 deaths in 2020. Although only 2.55% of new digestive cancer cases and 2.19% of digestive cancer-associated deaths occurred in young adults, digestive cancer accounted for 12.24% of all new cancer cases and 25.26% of all cancer-associated deaths in this age group. This indicates that the disease burden is heavier and the outcome is much worse for digestive cancer than cancers arising from other systems in young adults[1,8]. Cancers of the colorectum, liver, and stomach were the most common digestive cancer types, together accounting for 86.04% of the total estimated new cases and 81.45% of the total estimated cancer-associated deaths, which is consistent with that in the general population[12]. However, the burden of young adult digestive cancer varied substantially across levels of sex, geographical region, and human development, which was related to differences in cancer screening and detection modalities, genetic backgrounds, and carcinogenic exposures[6].
The distribution of digestive cancer between young males and females was clear, with higher incidence and mortality in men, mainly contributed by liver cancer, which was over 3 times more common in men than in women with regard to both incidence and mortality. The majority of primary liver cancer was hepatocellular carcinoma (HCC), which has been mainly associated with chronic infection with hepatitis B or C virus (HBV or HCV) as well as exposure to aflatoxin, excessive alcohol consumption, obesity, type 2 diabetes, and smoking. All of these risk factors are prevalent in males[12,13]. However, the incidence and mortality of gastric cancer were lower in men than in women. Many studies on gastric cancer in young adults have reported a higher female proportion, and the incidence in females gradually changes from a higher to a lower level than that in males at 40 years of age, which indicates that estrogen may play an important role in gastric cancer development in young females[14].
The burden of digestive cancer in young adults varies across geographical regions. This can be explained by the population size; therefore, most young adult digestive cancer cases and deaths occurred in Asia, especially in China and India, the world's two largest populations. This can also be explained by the development levels. A greater proportion of colorectal cancer occurred in developed countries, the incidence increased as the HDI ranking improved, and the incidence trends showed a persistent slow increase for colorectal cancer in young adults. These results are consistent with the established conclusion that colorectal cancer can be considered a marker of socioeconomic development, and incidence rates tend to rise uniformly as countries continue to undergo socioeconomic transition[2,15]. In contrast, liver cancer is generally more prominent in low- and middle-income countries (LMICs), with the aforementioned risk factors for HCC mainly prevalent in these countries and regions[12,13]. However, new cases of liver and gastric cancer showed a stable decrease from 1999 to 2012, reflecting the improvement in the environment and prevention of chronic infections in countries with a high incidence of these two cancer types[13,16]. Despite the increased incidence of colorectal cancer in young adults, deaths caused by this disease were stable. In addition, the number of deaths from gallbladder, pancreatic, and gastric cancer decreased from 1985 to 2016, especially for gastric cancer, which might be due to the improvement in the outcomes of these cancer types. In addition, variations in mortality and MIR across HDI rankings were significant, with mortality and MIR being higher in LMICs, implying that the worse outcomes are probably due to underdeveloped health systems, limitations in diagnostic capacity, and paucity of health insurance in less developed countries[6].
With the heavy burden and worse outcomes of digestive cancer in young adults and given that they are expected to have a long life remaining and they represent the main proportion of contributors to the economy and their family care, effective strategies are urgently needed to decrease the burden in these settings. For a long time, young adult patients with digestive cancer have been neglected by global health stakeholders[17,18]. However, in the past two decades, many studies and programs have been initiated to address this issue; landmarks of these programs are reports by AYAO PRG, National Comprehensive Cancer Network, and IARC[6,8,19]. Globally, however, there are still a lot of unknown fields about young adult oncology, and what is known is mainly recognized in high-income countries. Expanding these recommendations to LICMs remains a challenge, but it is more important for these countries, as young populations are the main contributors to productivity growth[6]. Therefore, considering the greater loss of life-years in this younger population, I need strategies to decrease digestive cancer burden in young adults. However, locally advanced or distant metastatic digestive cancer is more frequently diagnosed in young patients, which is influenced by highly aggressive growth patterns of these cancers in young individuals, physicians’ lack of familiarity with these cancers, and diagnosis delay[17,20,21]. Currently, the treatment protocols offered to young adults with cancer are extrapolated from younger and older populations[22,23]. Although young adults can always tolerate more aggressive treatment because of fewer comorbidities, the outcomes are poor for young patients with digestive cancers[10]. Therefore, before the emergence of a treatment that can cure any patient with cancer, prevention and diagnosis at a relatively early stage are the two most effective ways to lower the burden of young adult digestive cancer.
Prevention is the most effective way to decrease the digestive cancer incidence in young populations because majority of them are caused by amenable risk factors[12]. Environmental and lifestyle factors, such as poor dietary habits, alteration of the microbiome, chronic infection, sedentary lifestyle, and obesity, contribute to most digestive cancer cases[24]. Given that liver cancer contributes a large proportion of young adult digestive cancer, a global HBV vaccination program of HBV-naive people would likely have a significant effect in decreasing cancer burden in young populations worldwide. In China, the full embedment of HBV vaccination into the neonatal immunization program and mandatory HCV screening for blood transfusions have largely prevented new HBV and HCV infections, which may decrease the incidence of liver cancer[25]. Similarly, the prevalence of chronic Helicobacter pylori (H. pylori) infection is extraordinarily high, infecting approximately half of the world's population[26]. Chronic H. pylori infection is considered the principal cause of gastric cancer[27]. Eradication of H. pylori and improvements in the preservation and storage of foods have led to a steady decline in the incidence and mortality rates of noncardia gastric cancer over the last half century in most populations[28]. Adequate treatment of these bacteria could decrease the cancer incidence of gastric cancer, particularly in East Asia, where the infection rate is the highest. As previously mentioned, the incidence of colorectal cancer in young adults has increased over the last decades, likely reflecting changes in lifestyle factors and diet, leading to decreased physical activity and an increased prevalence of obesity[29]. These unhealthy lifestyles are changeable, and this change has led to declines in colorectal cancer incidence in some high-incidence countries[12,30].
Although most of the young adult digestive cancers are caused by lifestyle and environmental factors, some of them are strongly correlated to hereditary factors. Approximately 20% of colorectal cancer and 5% to 10% of gastric cancer are associated with familial clustering, and most of them are associated with inherited cancer predisposition syndromes. A family history of hereditary diffuse gastric cancer (HDGC), familial adenomatous polyposis, and Lynch syndrome predisposes young people to develop gastric cancer and colorectal cancer[31,32]. HDGC is an autosomal dominant syndrome arising from germline mutations in the tumor suppressor gene CDH1 (encoding the cell-to-cell adhesion protein E cadherin). HDGC is characterized by the development of gastric cancers, predominantly the diffuse type, at a young age[31]. Genetic susceptibility also appears to be more prevalent among young colorectal patients, with a prevalence of germline mutations of 16%–33% among those diagnosed before age 50[32]. Therefore, for hereditary factors, which are unchangeable with current technological capabilities, the prevention approach is precursor lesion detection by endoscopic surveillance and prophylactic total gastrectomy or colectomy[31,32].
Another opportunity to improve the outcomes of young adult patients with digestive cancer is screening and ensuring timely diagnosis[7,17]. However, no age-specific screening tests are currently available for young adults, and the screening targets are limited to individuals 40 years or older[33,34]. Increased colonoscopy screening and the removal of precursor lesions have decreased the incidence of colorectal cancer in adults older than 50 years in some countries, whereas the incidence in young adults continues to increase[3,35]. Therefore, studies examining such programs to improve the effective screening and avoid potential risks in young populations are needed. For all cancers, including digestive cancer, patients with early-stage disease have a far better prognosis than those with advanced-stage cancers, whereas young patients experience more delays than patients in other age groups, leading to diagnosis at late stages[17,20,21]. Although the factors leading to diagnosis delay of young adult digestive cancer vary across countries and regions and may be associated with psychosocial factors, cultural norms, geographic accessibility, and limitations in diagnostic capacity, the results of this study indicate that screening and early diagnosis programs may significantly reduce the burden of young adult digestive cancer. For hepatobiliary neoplasms, screening is mainly based on ultrasound and alpha fetoprotein, which can be easily carried out[13]. In contrast, for cancers of the gastrointestinal tract, the effective approach is endoscopy, which has skill limitations and is expensive in some countries; therefore, overuse of endoscopy is associated with a low yield rate in young patients and is not cost-effective[34]. However, as previously mentioned, the young adult population has a long-life expectancy remaining and represents the most important creator of the social material wealth. Endoscopic screening among young people in regions with a higher incidence is worthwhile.
This study has several limitations which have also been described in many studies using data from the GLOBOCAN database[36-38]. First, although the estimates of cancer burden were produced based on the best available data, they might not be accurate, particularly for countries where data are not available or of poor quality. Second, the number of digestive cancer cases was small in some countries, and the estimates may lack sufficient statistical power. Thus, the results should be interpreted with caution if they are used to inform cancer control policies. Third, the MIR was employed as a proxy indicator of the 5-year survival rate in this study; however, the MIR is not an exact measure of the survival rate and only serves as a proxy for the survival rate of digestive cancer in young adults in the absence of a country-wise exact measure. Fourth, due to concerns about generating misleading MIRs, I focused primarily on the heavily burdened countries and regions with HDI rankings, which makes the results incomplete and in the global context. Fifth, the HDI grading system was established in 2000, and it may not precisely reflect the current status of health care systems in different countries. The diagnosed stage and risk factors, such as smoking, obesity, and chronic infection, which play crucial roles in explaining the incidence and mortality rates, were not documented in this study. Therefore, the association between young digestive cancer burden and HDI ranking must be interpreted with caution in these countries. Despite these limitations, because of the small proportion of digestive cancer in young adults, individual studies with small sample sizes cannot reflect the whole picture with regard to the epidemiological characteristics. This study involved data retrieval from the GLOBOCAN database, the best currently available worldwide data, and these findings highlight the health burden of young adult digestive cancer and provide a direction to increase awareness and re-allocate resources for this neglected subpopulation.
In summary, the global digestive cancer burden among young adults differs from that in children and adolescents or individuals older in age, but it also varies significantly by sex, geographical region, and HDI level. Although the burden of young adult digestive cancer is much small when compared with digestive cancer in older populations, its effects remain substantial because young adults have a long-life expectancy remaining, and this age group represents the most important creator of the social material wealth. Estimating the burden of digestive cancer in young adults might help to raise awareness at both public and professional levels, inform recommendations for the aforementioned measures for prevention and timely diagnosis, and lead to improvements in outcomes of these specific cancer types across all resource levels.
Studies on cancer trends have demonstrated that the incidence of digestive cancers has increased significantly in young adults over the last few decades. However, digestive cancer has traditionally been thought of as a disease that mainly occurs in elderly individuals, and studies have focused on cancers at older ages. Therefore, cancers in young adults have been relatively ignored by both patients and physicians.
Young adults represent the main proportion of contributors to the economy and their family care. Thus, it is important to investigate the specific issues unique to this age group of cancer patients.
To describe the worldwide profile of digestive cancer incidence, mortality and corresponding trends among 20–39-year-olds, with major patterns highlighted by age, sex, development level, and geographical region.
I performed a population-based study to quantify the burden of young adult digestive cancers worldwide. Global, regional, sex, and country-specific data estimates of the number of new cancer cases and cancer-associated deaths that occurred in 2020 were extracted from the GLOBOCAN Cancer Today database. To assess long-term trends in young adult digestive cancer, cancer incidence data and mortality data were obtained from the Cancer in Five Continents Plus database and the World Health Organization mortality database, respectively. The associations between the human development index (HDI) and digestive cancer burden in young adults were also evaluated by linear regression analyses.
A total of 131068 new digestive cancer cases and 79614 cancer-associated deaths occurred among young adults worldwide in 2020, which equated to an age-standardized incidence rate (ASIR) of 5.16 and age-standardized mortality rate (ASMR) of 3.04, accounting for 12.24% of all new cancer cases and 25.26% of all cancer deaths occurring in young adults. The burden was disproportionally greater among males, with male: female ratios of 1.34 for incidence and 1.58 for mortality. The ASIRs were 2.1, 1.4, and 1.0 per 100000 people per year, whereas the ASMRs were 0.83, 1.1, and 0.62 per 100000 people per year for colorectal, liver, and gastric cancer, respectively. When assessed by geographical region and HDI levels, the cancer profile varied substantially, and a strong positive correlation between the mortality-to-incidence ratio of digestive cancer and HDI ranking was found (R2 = 0.7388, P < 0.001).
The most common digestive cancer types were colorectal, liver and gastric cancer. The global digestive cancer burden among young adults was greater among males and exhibited a positive association with socioeconomic status. The digestive cancer burden is heavy in this age group, and the societal and economic effects remain great.
Although the burden of young adult digestive cancer is much small when compared with digestive cancer in older populations, its effects remain substantial. Estimating the burden of young adult digestive cancer might help to raise awareness at both public and professional levels. Through continuous prevention, screening, and early detection programs, the digestive cancer burden in young adults can be reduced.
The author thanks the Health Commission of Mianyang City and the Science and Education Department of the Third Hospital of Mianyang for their support. The author also thanks the researchers, online database builders and cancer registries for their work in data sharing.
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Luchini C S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64442] [Article Influence: 16110.5] [Reference Citation Analysis (176)] |
| 2. | Sung H, Siegel RL, Rosenberg PS, Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019;4:e137-e147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 389] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 3. | Vuik FE, Nieuwenburg SA, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ, Zadnik V, Pellisé M, Esteban L, Kaminski MF, Suchanek S, Ngo O, Májek O, Leja M, Kuipers EJ, Spaander MC. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68:1820-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 476] [Cited by in RCA: 531] [Article Influence: 88.5] [Reference Citation Analysis (1)] |
| 4. | Lee J, Lee MA, Kim IH, Roh SY. Clinical characteristics of young-age onset gastric cancer in Korea. BMC Gastroenterol. 2016;16:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Marcano-Bonilla L, Mohamed EA, Mounajjed T, Roberts LR. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin Clin Oncol. 2016;5:61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 6. | Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova-Foucher E, Bray F. Cancer incidence and mortality among young adults aged 20-39 years worldwide in 2012: a population-based study. Lancet Oncol. 2017;18:1579-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 386] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 7. | Magrath I, Epelman S. Cancer in adolescents and young adults in countries with limited resources. Curr Oncol Rep. 2013;15:332-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Adolescent and Young Adult Oncology Progress Review Group. Closing the gap: research and care imperatives for adolescents and young adults with cancer. [cited 2021 Mar 18]. Available from: https://www.cancer.gov/types/aya/research/ayao-august-2006.pdf. |
| 9. | Smith AW, Bellizzi KM, Keegan TH, Zebrack B, Chen VW, Neale AV, Hamilton AS, Shnorhavorian M, Lynch CF. Health-related quality of life of adolescent and young adult patients with cancer in the United States: the Adolescent and Young Adult Health Outcomes and Patient Experience study. J Clin Oncol. 2013;31:2136-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 262] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 10. | Hydeman JA, Uwazurike OC, Adeyemi EI, Beaupin LK. Survivorship needs of adolescent and young adult cancer survivors: a concept mapping analysis. J Cancer Surviv. 2019;13:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Nichols HB, Baggett CD, Engel SM, Getahun D, Anderson C, Cannizzaro NT, Green L, Gupta P, Laurent CA, Lin PC, Meernik C, Moy LM, Wantman E, Xu L, Kwan ML, Mersereau JE, Chao CR, Kushi LH. The Adolescent and Young Adult (AYA) Horizon Study: An AYA cancer survivorship cohort. Cancer Epidemiol Biomarkers Prev. 2021;30:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 12. | Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020; 159: 335-349. e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1223] [Article Influence: 244.6] [Reference Citation Analysis (0)] |
| 13. | Sharma R. Descriptive epidemiology of incidence and mortality of primary liver cancer in 185 countries: evidence from GLOBOCAN 2018. Jpn J Clin Oncol. 2020;50:1370-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Li J. Gastric Cancer in Young Adults: A Different Clinical Entity from Carcinogenesis to Prognosis. Gastroenterol Res Pract. 2020;2020:9512707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Fidler MM, Soerjomataram I, Bray F. A global view on cancer incidence and national levels of the human development index. Int J Cancer. 2016;139:2436-2446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 16. | Arnold M, Karim-Kos HE, Coebergh JW, Byrnes G, Antilla A, Ferlay J, Renehan AG, Forman D, Soerjomataram I. Recent trends in incidence of five common cancers in 26 European countries since 1988: Analysis of the European Cancer Observatory. Eur J Cancer. 2015;51:1164-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 341] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 17. | Bleyer A. Young adult oncology: the patients and their survival challenges. CA Cancer J Clin. 2007;57:242-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 233] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 18. | Stevens MC. The 'Lost Tribe' and the need for a promised land: the challenge of cancer in teenagers and young adults. Eur J Cancer. 2006;42:280-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | National Comprehensive Cancer Network. The NCCN adolescent and young adult (AYA) oncology clinical guidelines (version 1, 2021). [cited 2021 Mar 18]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/aya.pdf. |
| 20. | Zeng Y, Ruan W, Liu J, Liang W, He J, Cui F, Pan H. Esophageal cancer in patients under 50: a SEER analysis. J Thorac Dis. 2018;10:2542-2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Tricoli JV, Boardman LA, Patidar R, Sindiri S, Jang JS, Walsh WD, McGregor PM 3rd, Camalier CE, Mehaffey MG, Furman WL, Bahrami A, Williams PM, Lih CJ, Conley BA, Khan J. A mutational comparison of adult and adolescent and young adult (AYA) colon cancer. Cancer. 2018;124:1070-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Muffly L, Lichtensztajn D, Shiraz P, Abrahão R, McNeer J, Stock W, Keegan T, Gomez SL. Adoption of pediatric-inspired acute lymphoblastic leukemia regimens by adult oncologists treating adolescents and young adults: A population-based study. Cancer. 2017;123:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Albritton KH, Wiggins CH, Nelson HE, Weeks JC. Site of oncologic specialty care for older adolescents in Utah. J Clin Oncol. 2007;25:4616-4621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Tasneem AA, Luck NH. Digestive tract neoplasms in young individuals: Demographics, staging and risk factors. Cancer Rep (Hoboken). 2021;4:e1319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 942] [Article Influence: 85.6] [Reference Citation Analysis (4)] |
| 26. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2047] [Article Influence: 255.9] [Reference Citation Analysis (0)] |
| 27. | Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 695] [Article Influence: 69.5] [Reference Citation Analysis (0)] |
| 28. | Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 544] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 29. | Conti L, Del Cornò M, Gessani S. Revisiting the impact of lifestyle on colorectal cancer risk in a gender perspective. Crit Rev Oncol Hematol. 2020;145:102834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | GBD 2017 Colorectal Cancer Collaborators. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:913-933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 279] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 31. | Gamble LA, Heller T, Davis JL. Hereditary Diffuse Gastric Cancer Syndrome and the Role of CDH1: A Review. JAMA Surg. 2021;156:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Valle L, Vilar E, Tavtigian SV, Stoffel EM. Genetic predisposition to colorectal cancer: syndromes, genes, classification of genetic variants and implications for precision medicine. J Pathol. 2019;247:574-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 33. | Fan X, Qin X, Zhang Y, Li Z, Zhou T, Zhang J, You W, Li W, Pan K. Screening for gastric cancer in China: Advances, challenges and visions. Chin J Cancer Res. 2021;33:168-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 34. | Chang HS, Park EC, Chung W, Nam CM, Choi KS, Cho E, Cho WH. Comparing endoscopy and upper gastrointestinal X-ray for gastric cancer screening in South Korea: a cost-utility analysis. Asian Pac J Cancer Prev. 2012;13:2721-2728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK, Jemal A. Global patterns and trends in colorectal cancer incidence in young adults. Gut. 2019;68:2179-2185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 538] [Article Influence: 89.7] [Reference Citation Analysis (1)] |
| 36. | Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia MM. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health. 2020;8:e1027-e1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 500] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 37. | Sharma R. An examination of colorectal cancer burden by socioeconomic status: evidence from GLOBOCAN 2018. EPMA J. 2020;11:95-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 38. | Sung WW, Wang SC, Hsieh TY, Ho CJ, Huang CY, Kao YL, Chen WJ, Chen SL. Favorable mortality-to-incidence ratios of kidney Cancer are associated with advanced health care systems. BMC Cancer. 2018;18:792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |