Published online Jan 15, 2022. doi: 10.4251/wjgo.v14.i1.265
Peer-review started: September 6, 2021
First decision: November 8, 2021
Revised: November 18, 2021
Accepted: December 8, 2021
Article in press: December 8, 2021
Published online: January 15, 2022
Processing time: 126 Days and 10.8 Hours
Gastric cardia adenocarcinoma (GCA), which has been classified as type II adenocarcinoma of the esophagogastric junction in western countries, is of similar geographic distribution with esophageal squamous cell carcinoma in China, and even referred as "sister cancer" by Chinese oncologists. The molecular mechanism for GCA is largely unknown. Recent studies have shown that decreased expression of E-cadherin is associated with the invasion and metastasis of multiple cancers. However, the E-cadherin expression has not been well characterized in gastric cardia carcinogenesis and its effect on GCA prognosis.
To characterize E-cadherin expression in normal gastric cardia mucosa, dysplasia and GCA tissues, and its influence on prognosis for GCA.
A total of 4561 patients with GCA were enrolled from our previously established GCA and esophageal cancer databases. The enrollment criteria included radical surgery for GCA, but without any radio- or chemo-therapy before operation. The GCA tissue from 4561 patients and matched adjacent normal epithelial tissue (n = 208) and dysplasia lesions (n = 156) were collected, and processed as tissue microarray for immunohistochemistry. The clinicopathological characteristics were retrieved from the medical records in hospital and follow-up was carried out through letter, telephone or home interview. E-cadherin protein expression was determined by two step immunohistochemistry. Kaplan–Meier and Cox regression analyses were used to correlate E-cadherin protein expression with survival of GCA patients.
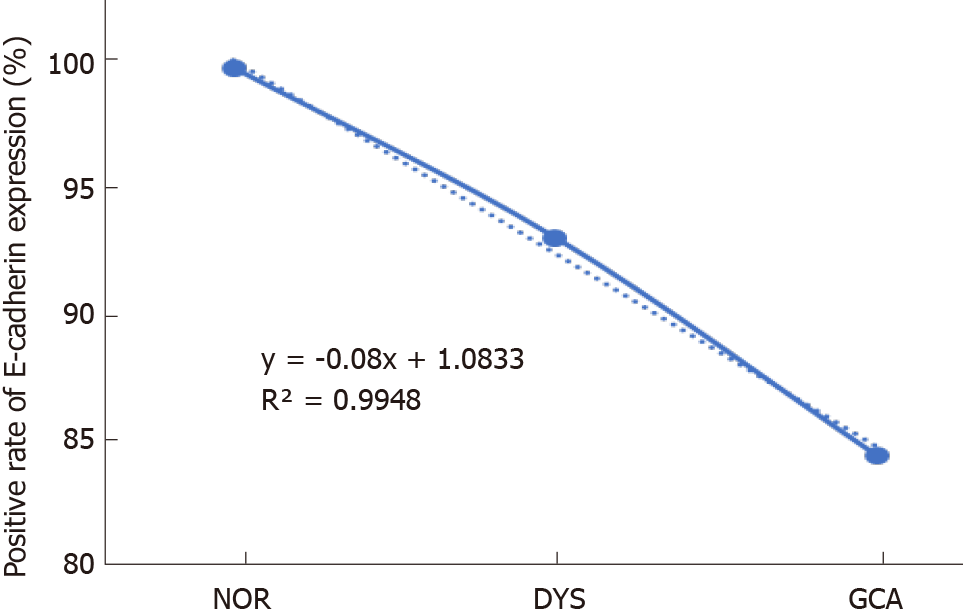
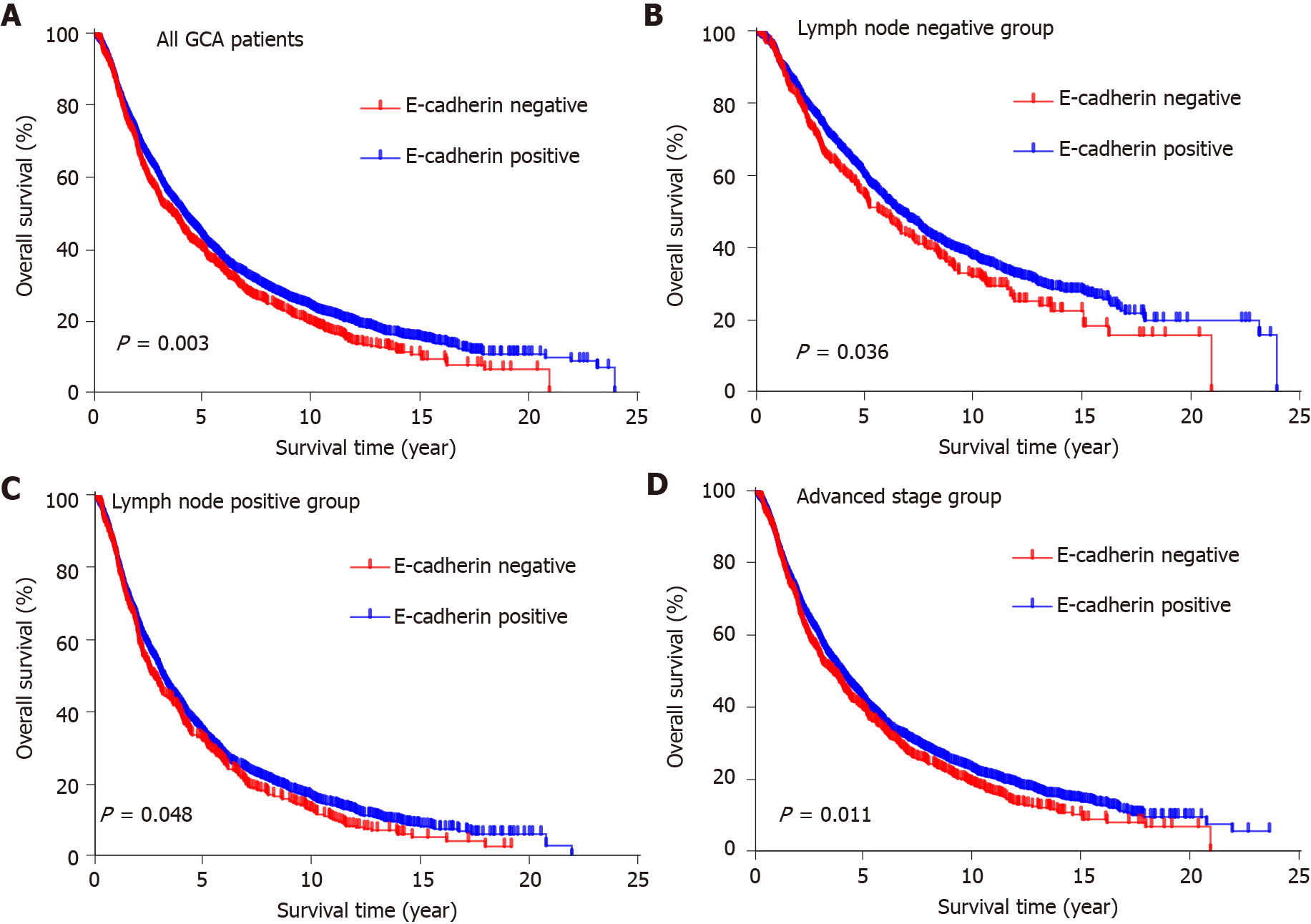
Of the 4561 GCA patients, there were 3607 males with a mean age of 61.6 ± 8.8 and 954 females with a mean age of 61.9 ± 8.6 years, respectively. With the lesions progressed from normal gastric cardia mucosa to dysplasia and GCA, the positive immunostaining rates for E-cadherin decreased significantly from 100% to 93.0% and 84.1%, respectively (R2 = 0.9948). Furthermore, E-cadherin positive immunostaining rate was significantly higher in patients at early stage (0 and I) than in those at late stage (II and III) (92.7% vs 83.7%, P = 0.001). E-cadherin positive expression rate was significantly associated with degree of differentiation (P = 0.001) and invasion depth (P < 0.001). Multivariate analysis showed that the GCA patients with positive E-cadherin immunostaining had better survival than those with negative (P = 0.026). It was noteworthy that E-cadherin positive expression rate was similar in patients with positive and negative lymph node metastasis. However, in patients with negative lymph node metastasis, those with positive expression of E-cadherin had better survival than those with negative expression (P = 0.036). Similarly, in patients with late stage GCA, those with positive expression of E-cadherin had better survival than those with negative expression (P = 0.011).
E-cadherin expression may be involved in gastric cardia carcinogenesis and low expression of E-cadherin may be a promising early biomarker and overall survival predictor for GCA.
Core Tip: In previous reports, there is no consistent conclusion on the association between E-cadherin expression and gastric cardia carcinogenesis and its effect on prognosis with gastric cardia adenocarcinoma (GCA) patients. It was notable that the positive immunostaining rates of E-cadherin decreased significantly from normal mucosa to dysplasia and GCA, as well as higher in early stage than those in advanced stage of GCA. Moreover, we found high expression of E–cadherin represented a better survival, especially for patients with negative lymph node metastasis. In conclusion, E-cadherin may be involved in carcinogenesis and may be a predictor on prognosis for GCA.
- Citation: Wang HL, Zhao XK, Zhou FY, Song X, Li LY, Huang GR, Bao QD, Lei LL, Yang HJ, Li L, Xu RH, Li AL, Wang XZ, Han WL, Ren JL, Wang LD. Characterization of E-cadherin expression in normal mucosa, dysplasia and adenocarcinoma of gastric cardia and its influence on prognosis. World J Gastrointest Oncol 2022; 14(1): 265-277
- URL: https://www.wjgnet.com/1948-5204/full/v14/i1/265.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i1.265
Gastric cardia adenocarcinoma (GCA), which has been classified as type II adenocarcinoma of the esophagogastric junction in western countries[1], is of similar geographic distribution with esophageal squamous cell carcinoma in China[2], and even referred as "sister cancer" by Chinese oncologists. In contrast to esophageal squamous cell carcinoma, the incidence for GCA is increasing worldwide[3,4]. Most GCA patients lack early warning symptoms, and > 90% of patients are diagnosed at an advanced stage, resulting in poor prognosis, with < 20% 5-year survival[5,6]. Obviously, early detection for GCA is crucial in decreasing the high mortality. Identification of unique molecular biomarkers at the early stage of GCA is crucial for screening high-risk individuals and early detection of GCA. Unfortunately, the molecular mechanism of human gastric cardia carcinogenesis is largely unknown.
Accumulated evidence indicates that E-cadherin protein, a member of the cadherin family encoded by the CDH1 gene, may play an important role in intercellular adhesion, maintaining the stability of epithelial structure and function, cell polarity, and regulating intracellular signaling pathways[7]. Reduced expression of E-cadherin has been reported as a molecular biomarker of a cellular process called epithelial-mesenchymal transition, which is often associated with cancer progression[8]. The latest studies have indicated that decreased expression of E-cadherin is involved in many different types of cancer[9-11]. However, E-cadherin expression in GCA has not been well characterized.
In the present study, we detected the expression of E-cadherin in GCA, precancerous lesions and normal mucosa. We also evaluated the relationship of E-cadherin expression and survival of GCA.
All the patients were enrolled from the 500000 esophageal and gastric cardia carcinoma databases (1973–2020) established by the State Key Laboratory for Esophageal Cancer Prevention & Treatment and Henan Key Laboratory for Esophageal Cancer Research of the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China). GCA patients were enrolled in the present study according to the following criteria: (1) Patients were diagnosed with GCA by postoperative histopathology; (2) Patients had tumors located in the esophagogastric junction; (3) Patients had no other malignant tumors except for GCA; (4) Patients received no chemotherapy or/and radiotherapy before surgery; and (5) The tissue samples of the patients were available. The exclusion criteria were: (1) Pathological type was not adenocarcinoma; (2) Clinicopathological information was incomplete; and (3) patients had received preoperative radiation or chemotherapy. A total of 4561 patients with GCA were enrolled in the study (Table 1). In addition, 208 matched adjacent normal epithelial tissue and 156 dysplasia lesions were selected.
| Variables | Cases, n (%) | |
| Gender | ||
| Female | 954 | 20.9 |
| Male | 3607 | 79.1 |
| Age at diagnosis (yr) | ||
| < 60 | 1717 | 37.6 |
| ≥ 60 | 2844 | 62.4 |
| Family history | ||
| Negative | 3366 | 73.8 |
| Positive | 1195 | 26.2 |
| Cigarette smoking | ||
| No | 2166 | 47.5 |
| Yes | 2395 | 52.5 |
| Alcohol consumption | ||
| No | 3206 | 70.3 |
| Yes | 1355 | 29.7 |
| Differentiation | ||
| Well | 133 | 2.9 |
| Moderate | 2039 | 44.7 |
| Poor | 2389 | 52.4 |
| T status | ||
| T1 | 71 | 1.6 |
| T2 | 308 | 6.8 |
| T3 | 3044 | 66.7 |
| T4 | 1138 | 25.0 |
| Lymph node metastasis | ||
| Negative | 1637 | 35.9 |
| Positive | 2924 | 64.1 |
| Staging | ||
| Early stage | 191 | 4.2 |
| Advanced stage | 4370 | 95.8 |
The staged of patients with GCA were based on the 8th edition of the American Joint Committee. Positive smoking history was defined as having smoked continuously or accumulatively for 6 mo or more in one's lifetime and negative drinking history was defined less than 20 g of alcohol per day. Family history positive was defined as more than two patients with GCA in two consecutive generations.
Histopathological diagnoses for normal mucosa, dysplasia and adenocarcinoma of the gastric cardia were made according to established criteria[12]. The normal gastric cardia mucosa, composing of a single columnar epithelium and mucous glands composed only of mucous cells; dysplasia, neoplastic feature including nuclear atypia and/or architectural abnormalities confined to the gastric cardia epithelium, without invasion; GCA, invasion of neoplastic gastric cardia cells through the basement membrane.
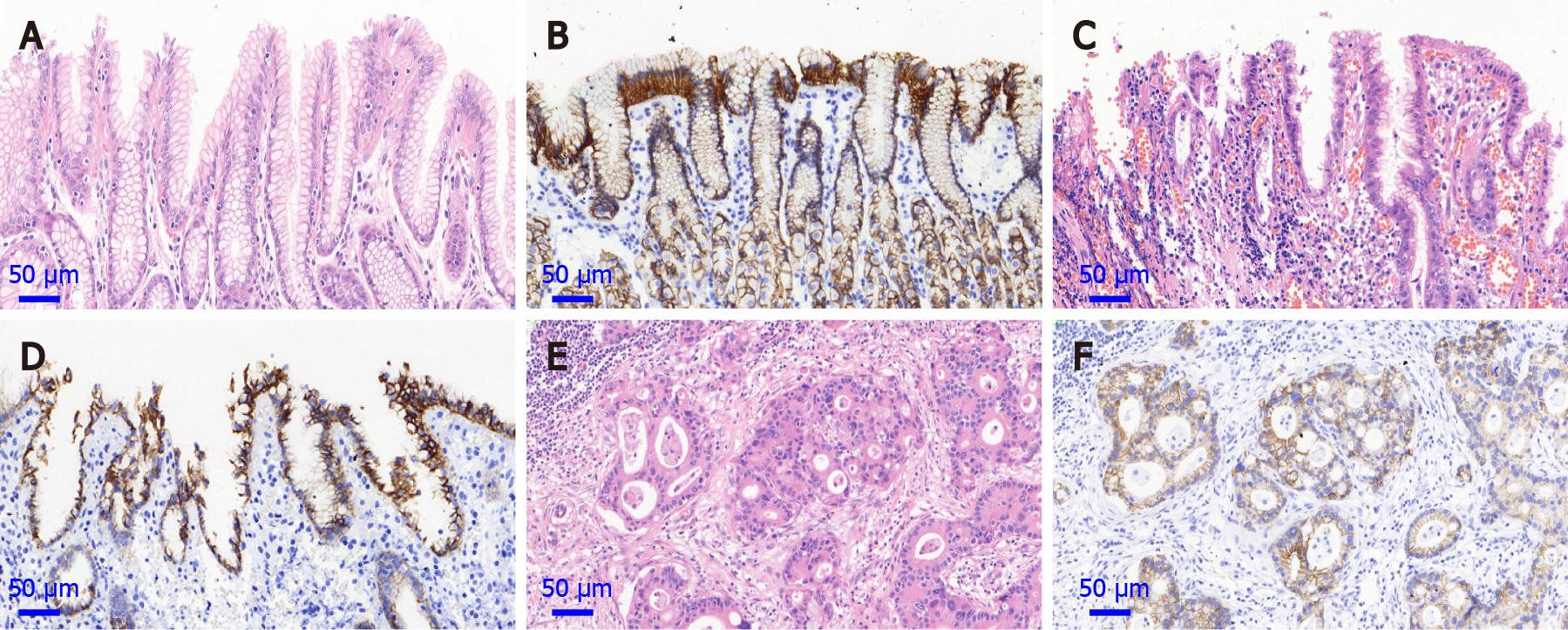
E-cadherin protein expression was detected by immunohistochemical staining on normal mucosa, dysplasia and GCA with tissue microarray. The focal area of the cancer tissue was marked on the paraffin-embedded specimens, and a 7 × 16 microarray was designed. Punch holes with a diameter of 1.5 mm were made in the samples. The tissue chip model was then made and fixed. Immunohistochemistry was carried out by a two-step protocol using the Roche Benchmark XT. In brief, the paraffin-embedded tissue sections were deparaffinized with xylene and anhydrous ethanol for rehydration and heated in citrate buffer (G1202, pH 6.0) for 25 min at 95 ℃ for antigen repair. The sections were then cooled for 60 min at room temperature, and immersed in 3% hydrogen peroxide solution (G0115) to neutralize endogenous peroxidase. A mouse monoclonal anti-E-cadherin antibody was used (cat. no. GB13083-1; dilution 1:500; Wuhan Servicebio Technology Co., Ltd, Wuhan, China). The anti-E-cadherin antibody was added and incubated overnight at 4 ℃. The secondary antibody was then added (cat. no. G1210-2). Between each incubation step, the slides were washed with phosphate buffered saline (PBS, pH 7.4, G0002) three times. Immunostaining was performed using the Roche Benchmark XT with diaminobenzidine (DAB, G1212-200) according to the manufacturer’s instructions and the sections were subsequently counterstained with hematoxylin (G1004). The known positive sections were used as the positive control, and PBS was used as the negative control instead of the primary antibody. Observation was performed using a microscope at a magnification of 400 ×. The positive cells for E-cadherin protein expression showed yellow or brown staining in the cell membrane.
According to the staining intensity, the results were categorized as: 0 points, no staining; 1 point, light yellow; 2 points, brown yellow; and 3 points, tan. According to the ratio of the positive cell number, they were scored as 0 (< 10%), 1 (11%-25%), 2 (26%-50%) or 3 (> 50%). The two scores were multiplied, and the results were classified as negative (< 3) or positive (≥ 3). Immunohistochemical results were independently assessed by two pathologists. If the results were inconsistent, they were evaluated by the two pathologists together until a consensus was reached.
All the patients were followed up by letter, telephone or home interview every 3–6 mo after initial diagnosis and treatment. Before the 1990s, patients were usually followed up through letters. The data were saved in medical records. The patients who survived for > 5 years were followed up once a year until the end event (death) occurred. The last follow-up was on June 30, 2020. The median follow-up time was 5.4 [interquartile range (IQR) 3.4–7.6] years.
SPSS statistical software (version 25.0, IBM, Chicago, IL) and GraphPad Prism version 8.0 (GraphPad Software, San Diego, California United States) were used to analyze the data. Variables with abnormal distribution were represented by a median (IQR). The χ2 test and Fisher tests were used for the differences in clinicopathological characteristics and the protein expression of E-cadherin between the groups. The correlation of E-cadherin expression in normal mucosa, dysplasia and adenocarcinoma of the gastric cardia was evaluated by linear regression analysis (R2-value). The effect of a single factor on survival was analyzed by Kaplan–Meier method and log-rank test. Independent risk factors affecting survival were analyzed by Cox regression model. All the test levels were α = 0.05. The statistical review of the study was performed by the biomedical statistician from Zhengzhou University.
From the clinical records, we retrieved the baseline clinical parameters for this group of GCA patients (Table 1). It shows the distribution of all GCA patients by gender, age, family history, cigarette smoking, alcohol consumption and histopathology. Among the 4561 patients with GCA, there were 954 women and 3607 men with a mean age of 61.6 ± 8.8 and 61.9 ± 8.6, respectively. The number of male patients was 3.8 times that of female patients. Family aggregation for GCA patients was evident with a positive family history in 26.2% of the patients. In addition, 2395 (52.5%) patients had a history of cigarette smoking and 1355 (29.7%) patients had a history of alcohol consumption. Among male patients, 65.7% (2370/3607) had a history of smoking and 36.8% (1327/3607) had alcohol consumption. The depth of invasion and lymph node metastasis were also classified. There were 2924 (64.1%) patients with positive lymph node metastasis in postoperative pathology. There were 191 (4.2%) patients at early stage (0 and I) and 4370 (95.8%) patients at advanced stage (II and III).
The positive immunostaining reaction of E-cadherin protein expression was mainly located in the cell membrane (Figure 1). With the lesions progressed from normal gastric cardia mucosa to dysplasia and GCA, the positive immunostaining rates for E-cadherin decreased significantly from 100.0% (208/208), to 93.0% (145/156) and 84.1% (3836/4561), respectively (χ2 = 47.439, P < 0.001; Table 2). In the linear analysis of E-cadherin protein expression in normal mucosa, dysplasia and GCA, the decreasing tendency was observed (y = -0.08x + 1.0833, R2 = 0.9948, Figure 2).
| Lesion type | Total | E-cadherin protein expression | χ2 | P value | |
| n | Positive, n (%) | Negative, n (%) | |||
| Normal | 208 | 208 (100.0) | 0 (0) | 47.439 | < 0.001 |
| DYS | 156 | 145 (93.0) | 11 (7.0) | ||
| GCA | 4561 | 3836 (84.1) | 725 (15.9) | ||
By comparing the relationship between E-cadherin expression and clinicopathological characteristics, the expression rate of E-cadherin in male patients was lower than that in female patients (83.5 vs 86.4%, χ2 = 4.645, P = 0.031, Table 3). It was found that the positive rate of E-cadherin expression gradually decreased with the degree of differentiation (92.5% vs 85.4% vs 82.5%, χ2 = 14.259, P = 0.001, Table 3). E-cadherin expression differed according to degree of tumor invasion (χ2 = 22.490, P < 0.001, Table 3). The E-cadherin positive immunostaining rate was significantly higher in the patients at early stage (0 and I) than advanced stage (II and III) (92.7% vs 83.7%, χ2 = 10.941, P = 0.001, Table 3). There was no significant difference in the expression of E-cadherin protein according to age at diagnosis, family history, cigarette smoking, alcohol consumption and lymph node metastasis (P > 0.05, Table 3).
| Variables | Total | E-cadherin protein expression | χ2 | P value | |
| n | Positive, n (%) | Negative, n (%) | |||
| Gender | 4.645 | 0.031 | |||
| Female | 954 | 824 (86.4) | 130 (13.6) | ||
| Male | 3607 | 3012 (83.5) | 595 (16.5) | ||
| Age at diagnosis (yr) | 0.709 | > 0.05 | |||
| < 60 | 1717 | 1434 (83.5) | 283 (16.5) | ||
| ≥ 60 | 2844 | 2402 (84.5) | 442 (15.5) | ||
| Family history | 1.018 | > 0.05 | |||
| Negative | 3366 | 2820 (83.8) | 546 (16.2) | ||
| Positive | 1195 | 1016 (85.0) | 179 (15.0) | ||
| Cigarette smoking | 1.408 | > 0.05 | |||
| No | 2166 | 1841 (85.0) | 325 (15.0) | ||
| Yes | 2395 | 2005 (83.7) | 390 (16.3) | ||
| Alcohol consumption | 0.011 | > 0.05 | |||
| No | 3206 | 2706 (84.4) | 500 (15.6) | ||
| Yes | 1355 | 1142 (84.3) | 213 (15.7) | ||
| Differentiation | 14.259 | 0.001 | |||
| Well | 133 | 123 (92.5) | 10 (7.5) | ||
| Moderate | 2039 | 1742 (85.4) | 297 (14.6) | ||
| Poor | 2389 | 1971 (82.5) | 418 (17.5) | ||
| T status | 22.490 | < 0.001 | |||
| pT1 | 71 | 63 (88.7) | 8 (11.3) | ||
| pT2 | 308 | 278 (90.3) | 30 (9.7) | ||
| pT3 | 3044 | 2580 (84.8) | 464 (15.2) | ||
| pT4 | 1138 | 915 (80.4) | 223 (19.6) | ||
| Lymph node metastasis | 0.481 | > 0.05 | |||
| Negative | 1637 | 1385 (84.6) | 252 (15.4) | ||
| Positive | 2924 | 2451 (83.8) | 473 (16.2) | ||
| Staging | 10.941 | 0.001 | |||
| Early stage | 191 | 177 (92.7) | 14 (7.3) | ||
| Advanced stage | 4370 | 3659 (83.7) | 711 (16.3) | ||
To evaluate the potential association of clinical factors with overall survival, we performed univariate Cox regression analysis. In univariate analysis, age at diagnosis (P < 0.001), degree of differentiation (P < 0.001), invasion depth (P < 0.001), lymph node metastasis (P < 0.001) and E-cadherin expression (P = 0.003) were survival factors (Table 4). There was no significant difference in overall survival among patients with different gender, family history, cigarette smoking and alcohol consumption (Table 4). Kaplan–Meier analysis showed that positive E-cadherin expression predicted better overall survival (P = 0.003; Figure 3A). Similarly, it showed that age < 60 years at diagnosis, well differentiation, T1 and negative lymph node metastasis predicted better overall survival (P < 0.001; Supplementary Figure 1). In multivariate analysis, E-cadherin expression was an independent factor of GCA survival (P = 0.026; Table 4).
| Variables | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Gender | > 0.05 | |||||
| Female | 1 | |||||
| Male | 1.063 | 0.979-1.153 | ||||
| Age at diagnosis (yr) | < 0.001 | < 0.001 | ||||
| < 60 | 1 | 1 | ||||
| ≥ 60 | 1.335 | 1.246-1.431 | 1.352 | 1.262-1.449 | ||
| Family history | > 0.05 | |||||
| Negative | 1 | |||||
| Positive | 1.060 | 0.983-1.143 | ||||
| Cigarette smoking | > 0.05 | |||||
| No | 1 | |||||
| Yes | 1.004 | 0.929-1.084 | ||||
| Alcohol consumption | > 0.05 | |||||
| No | 1 | |||||
| Yes | 0.947 | 0.869-1.031 | ||||
| Differentiation | < 0.001 | < 0.001 | ||||
| Well | 1 | 1 | ||||
| Moderate | 1.316 | 1.067-1.623 | 1.234 | 1.000-1.522 | ||
| Poor | 1.791 | 1.454-2.206 | 1.480 | 1.199-1.827 | ||
| T status | < 0.001 | < 0.001 | ||||
| pT1 | 1 | 1 | ||||
| pT2 | 1.916 | 1.289-2.849 | 1.604 | 1.078-2.387 | ||
| pT3 | 2.829 | 1.949-4.107 | 2.074 | 1.426-3.018 | ||
| pT4 | 3.390 | 2.328-4.936 | 2.272 | 1.555-3.320 | ||
| Lymph node metastasis | < 0.001 | < 0.001 | ||||
| Negative | 1 | 1 | ||||
| Positive | 1.952 | 1.815-2.099 | 1.805 | 1.676-1.944 | ||
| E-cadherin | 0.003 | 0.026 | ||||
| Positive | 1 | 1 | ||||
| Negative | 1.144 | 1.048-1.248 | 1.104 | 1.012-1.206 | ||
According to the clinicopathological features, the patients were divided into different groups. In the group with negative lymph node metastasis, survival was better in patients with positive E-cadherin expression than negative expression (P = 0.036; Figure 3B). A similar result was found in the group with positive lymph node metastases (P = 0.048; Figure 3C). With regard to the patients at advanced stage (II and III), patients with positive E-cadherin expression survived better than those with negative expression (P = 0.011; Figure 3D).
As we know, the present study is the first report about the E-cadherin protein expression in the lesions progressed from normal gastric cardia mucosa to dysplasia and GCA, and the largest sample study of the expression of E-cadherin protein and its influence on survival with GCA[13,14].
It is well known that loss of E-cadherin expression resulting from CDH1 gene alterations is the primary carcinogenetic event in hereditary diffuse gastric cancer[15]. However, there was few report concerning the expression of E-cadherin in the gastric cardia carcinogenesis, progressed from normal gastric cardia to dysplasia and GCA. It is showed that, in our study, the significantly decreased immunostaining rate of E-cadherin protein presented from normal gastric cardia to dysplasia and GCA, which indicated that E-cadherin protein may be involved in the gastric cardia carcinogenesis and low expression of E-cadherin protein may accelerate the process. The result in our study was consistent with those reported on gastric cancer[16].
It was found that the positive rate of E-cadherin expression gradually decreased with the decline of the degree of differentiation (92.5% vs 85.4% vs 82.5%, χ2 = 14.259, P = 0.001). The worse the differentiation, the lower the positive expression rate of E-cadherin. This is consistent with previous studies[17,18]. We think that E-cadherin may be a differentiation marker.
The present study demonstrated that patients with positive expression of E-cadherin protein had better survival than those with negative expression. Cox regression analysis indicated that positive expression of E-cadherin protein was an independent factor for better prognosis of patients with GCA, considered together with age at diagnosis, degree of differentiation, invasion depth and lymph node metastasis. The mechanism for the E-cadherin expression and cancer prognosis is largely unknown. E-cadherin gene, also known as CDH1, has been recognized as a tumor suppressor gene. Decreased expression of E-cadherin is reported to be related to prognosis in breast, colorectal and hepatocellular cancers[19,20]. However, less research has been conducted on GCA and controversial results have been observed in gastric cancer[21,22]. A meta-analysis of E-cadherin expression in 4383 patients with gastric cancer showed that the down-regulation of E-cadherin expression was significantly correlated with TNM stage, tumor invasion depth, lymph node metastasis, tumor differentiation, vascular invasion, tissue type and distant metastasis[23]. This study showed that negative E-cadherin was associated with poor differentiation and deep invasion of tumors, which suggested that tumor differentiation was related to cell adhesion, while tumors lacking adhesion were prone to regional lymph node or distant metastasis and had a relatively poor prognosis. The results of our study did not indicate that E-cadherin was associated with lymph node metastasis of GCA, which still needs to be confirmed by further studies.
Disruption of the cell adhesion molecule E-cadherin causes dysregulation of cell–cell adhesion properties. E-cadherin expression may be associated with epithelial–mesenchymal transition through activating the Akt and mitogen-activated protein kinase signaling pathways[24], and negative expression of E-cadherin could lead to a decline of proliferation and metastasis. Medicines for CDH1 mutations are being developed and it has been suggested that non-steroidal anti-inflammatory drugs can inhibit CDH1 methylation in human gastric mucosa[25].
Another interesting finding in the present study was that positive expression of E-cadherin protein in GCA patients at dysplasia lesion was higher than in GCA stage (93% vs 84.1%, P = 0.003), which indicates that E-cadherin protein may be an early potential biomarker for gastric cardia carcinogenesis. Accumulated evidence demonstrates that the germline mutations of E-cadherin gene are highly correlated with hereditary diffuse gastric cancer and lobular breast cancer, and are considered to be promising biomarkers, combined with endoscopy, for early detection of hereditary diffuse gastric cancer and breast cancer[26-29].
Lastly, we found that in the GCA patients with negative lymph node metastasis, positive expression of E-cadherin protein indicated better survival than negative expression. The difference in E-cadherin expression can further stratify the prognosis of patients with negative lymph node metastasis, indicating that E-cadherin protein expression may be a promising prognostic biomarker for non-surgical GCA patients. It is well known that lymph node metastasis is a useful indicator for poor survival in almost all cancer patients, including GCA. However, clinically, GCA patients with negative lymph node metastasis also showed different survival. E-cadherin protein expression may shed a light on these phenomena.
This study has some limitations. Firstly, there might be some missing data about clinical information. Secondly, the patients’ time span was long and the fact that they came from different hospitals also might have caused some bias. Further studies are needed to confirm the new findings.
E-cadherin plays an important role in carcinogenesis of GCA. E-cadherin may be a promising biomarker for early warning and overall survival predictor for GCA patients. E-cadherin protein expression may also shed light on the clinical phenomena for the GCA patients with negative lymph node metastasis with different survival.
Gastric cardia adenocarcinoma (GCA), which has been classified as type II adenocarcinoma of the esophagogastric junction in western countries, is of similar geographic distribution with esophageal squamous cell carcinoma in China, and even referred as "sister cancer" by Chinese oncologists. The molecular mechanism for GCA is largely unknown. Recent studies have shown that decreased expression of E-cadherin is associated with the invasion and metastasis of multiple cancers. However, the E-cadherin expression has not been well characterized in gastric cardia carcinogenesis and its effect on GCA prognosis.
In previous reports, there is no consistent conclusion on the association between E-cadherin expression and gastric cardia carcinogenesis and its effect on prognosis with GCA.
This study aimed to characterize E-cadherin expression in normal gastric cardia epithelium, dysplasia lesions and GCA tissues, and its influence on prognosis for GCA.
Immunochemistry stating of E-cadherin was performed on GCA and matched adjacent normal epithelial tissue and dysplasia. The correlation on E-cadherin protein expression and prognosis of patients with GCA were analyzed using Kaplan–Meier and Cox regression test.
With the lesions progressed from normal gastric cardia mucosa to dysplasia and GCA, the positive immunostaining rates for E-cadherin decreased significantly from 100% to 93.0% and 84.1%, respectively (R2 = 0.9948). E-cadherin had better survival than those with negative expression (P = 0.026). In the group with negative lymph node metastasis, survival was better in patients with positive E-cadherin expression than negative expression (P = 0.036). Similarly, in patients with late stage GCA, those with positive expression of E-cadherin had better survival than those with negative expression (P = 0.011).
E-cadherin expression may be involved in gastric cardia carcinogenesis and low expression of E-cadherin may be a promising early biomarker and overall survival predictor for GCA.
E-cadherin protein expression is expected to be a molecular marker for early detection and prognosis prediction for GCA.
We thank Professor Xue-Zhong Shi (Department of Epidemiology and Biostatistics, College of Public Health, Zhengzhou University) for help in statistical analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Association for Cancer Research; International Society for Diseases of the Esophagus.
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Andrejic-Visnjic B, Micsik T S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55781] [Article Influence: 7968.7] [Reference Citation Analysis (132)] |
| 2. | Chen H, Wang LD, Guo M, Gao SG, Guo HQ, Fan ZM, Li JL. Alterations of p53 and PCNA in cancer and adjacent tissues from concurrent carcinomas of the esophagus and gastric cardia in the same patient in Linzhou, a high incidence area for esophageal cancer in northern China. World J Gastroenterol. 2003;9:16-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Arnold M, Laversanne M, Brown LM, Devesa SS, Bray F. Predicting the Future Burden of Esophageal Cancer by Histological Subtype: International Trends in Incidence up to 2030. Am J Gastroenterol. 2017;112:1247-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 303] [Article Influence: 37.9] [Reference Citation Analysis (2)] |
| 4. | Dubecz A, Solymosi N, Stadlhuber RJ, Schweigert M, Stein HJ, Peters JH. Does the Incidence of Adenocarcinoma of the Esophagus and Gastric Cardia Continue to Rise in the Twenty-First Century? J Gastrointest Surg. 2013;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Liu K, Yang K, Zhang W, Chen X, Zhang B, Chen Z, Chen J, Zhao Y, Zhou Z, Chen L, Hu J. Changes of Esophagogastric Junctional Adenocarcinoma and Gastroesophageal Reflux Disease Among Surgical Patients During 1988-2012: A Single-institution, High-volume Experience in China. Ann Surg. 2016;263:88-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | Buas MF, Vaughan TL. Epidemiology and risk factors for gastroesophageal junction tumors: understanding the rising incidence of this disease. Semin Radiat Oncol. 2013;23:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 7. | Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol. 2003;19:207-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 495] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 8. | Bure IV, Nemtsova MV, Zaletaev DV. Roles of E-cadherin and Noncoding RNAs in the Epithelial-mesenchymal Transition and Progression in Gastric Cancer. Int J Mol Sci. 2019;20:2870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Ishiguro H, Wakasugi T, Terashita Y, Sakamoto N, Tanaka T, Mizoguchi K, Sagawa H, Okubo T, Takeyama H. Decreased expression of CDH1 or CTNNB1 affects poor prognosis of patients with esophageal cancer. World J Surg Oncol. 2016;14:240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Lin Y, Shen LY, Fu H, Dong B, Yang HL, Yan WP, Kang XZ, Dai L, Zhou HT, Yang YB, Liang Z, Chen KN. P21, COX-2, and E-cadherin are potential prognostic factors for esophageal squamous cell carcinoma. Dis Esophagus. 2017;30:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Bruun J, Kolberg M, Nesland JM, Svindland A, Nesbakken A, Lothe RA. Prognostic Significance of β-Catenin, E-Cadherin, and SOX9 in Colorectal Cancer: Results from a Large Population-Representative Series. Front Oncol. 2014;4:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Giroux V, Rustgi AK. Metaplasia: tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat Rev Cancer. 2017;17:594-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 230] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 13. | Polkowski WP, Skomra DG, Mielko J, Wallner GT, Szumiło J, Zinkiewicz K, Korobowicz EM, van Lanschot JJ. E-cadherin expression as predictive marker of proximal resection line involvement for advanced carcinoma of the gastric cardia. Eur J Surg Oncol. 2004;30:1084-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Wijnhoven BP, Tucker ET, Dinjens WN, Tilanus HW, Pignatelli M. Biochemical analysis and subcellular distribution of E-cadherin-catenin in adenocarcinomas of the gastro-oesophageal junction. Anticancer Res. 2004;24:1369-1375. [PubMed] |
| 15. | Yakirevich E, Resnick MB. Pathology of gastric cancer and its precursor lesions. Gastroenterol Clin North Am. 2013;42:261-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | Zhou YN, Xu CP, Han B, Li M, Qiao L, Fang DC, Yang JM. Expression of E-cadherin and beta-catenin in gastric carcinoma and its correlation with the clinicopathological features and patient survival. World J Gastroenterol. 2002;8:987-993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Corso G, Figueiredo J, De Angelis SP, Corso F, Girardi A, Pereira J, Seruca R, Bonanni B, Carneiro P, Pravettoni G, Guerini Rocco E, Veronesi P, Montagna G, Sacchini V, Gandini S. E-cadherin deregulation in breast cancer. J Cell Mol Med. 2020;24:5930-5936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 18. | Graziano F, Humar B, Guilford P. The role of the E-cadherin gene (CDH1) in diffuse gastric cancer susceptibility: from the laboratory to clinical practice. Ann Oncol. 2003;14:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Padmanaban V, Krol I, Suhail Y, Szczerba BM, Aceto N, Bader JS, Ewald AJ. E-cadherin is required for metastasis in multiple models of breast cancer. Nature. 2019;573:439-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 551] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 20. | Wong SHM, Fang CM, Chuah LH, Leong CO, Ngai SC. E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit Rev Oncol Hematol. 2018;121:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 263] [Article Influence: 37.6] [Reference Citation Analysis (1)] |
| 21. | Sun S, Gong Q. The expressions and prognostic implications of Twist and E-cadherin in adenocarcinomas of the gastroesophageal junction and proximal gastric carcinoma. Medicine (Baltimore). 2019;98:e18449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Schizas D, Moris D, Michalinos A, Kanavidis P, Oikonomou D, Papalampros A, Machairas A, Liakakos T. E-cadherin in gastric carcinomas: Relations with histological parameters and its prognostic value. J BUON. 2017;22:383-389. [PubMed] |
| 23. | Hu L, Li HL, Li WF, Chen JM, Yang JT, Gu JJ, Xin L. Clinical significance of expression of proliferating cell nuclear antigen and E-cadherin in gastric carcinoma. World J Gastroenterol. 2017;23:3721-3729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Reddy P, Liu L, Ren C, Lindgren P, Boman K, Shen Y, Lundin E, Ottander U, Rytinki M, Liu K. Formation of E-cadherin-mediated cell-cell adhesion activates AKT and mitogen activated protein kinase via phosphatidylinositol 3 kinase and ligand-independent activation of epidermal growth factor receptor in ovarian cancer cells. Mol Endocrinol. 2005;19:2564-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Tahara T, Shibata T, Nakamura M, Yamashita H, Yoshioka D, Okubo M, Maruyama N, Kamano T, Kamiya Y, Fujita H, Nagasaka M, Iwata M, Takahama K, Watanabe M, Hirata I, Arisawa T. Chronic aspirin use suppresses CDH1 methylation in human gastric mucosa. Dig Dis Sci. 2010;55:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Xing X, Tang YB, Yuan G, Wang Y, Wang J, Yang Y, Chen M. The prognostic value of E-cadherin in gastric cancer: a meta-analysis. Int J Cancer. 2013;132:2589-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Curtin BF, Gamble LA, Schueler SA, Ruff SM, Quezado M, Miettinen M, Fasaye GA, Passi M, Hernandez JM, Heller T, Koh C, Davis JL. Enhanced endoscopic detection of occult gastric cancer in carriers of pathogenic CDH1 variants. J Gastroenterol. 2021;56:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Pilonis ND, Tischkowitz M, Fitzgerald RC, di Pietro M. Hereditary Diffuse Gastric Cancer: Approaches to Screening, Surveillance, and Treatment. Annu Rev Med. 2021;72:263-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Memni H, Macherki Y, Klayech Z, Ben-Haj-Ayed A, Farhat K, Remadi Y, Gabbouj S, Mahfoudh W, Bouzid N, Bouaouina N, Chouchane L, Zakhama A, Hassen E. E-cadherin genetic variants predict survival outcome in breast cancer patients. J Transl Med. 2016;14:320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |