Published online Jan 15, 2022. doi: 10.4251/wjgo.v14.i1.203
Peer-review started: March 28, 2021
First decision: June 7, 2021
Revised: June 15, 2021
Accepted: December 7, 2021
Article in press: December 7, 2021
Published online: January 15, 2022
Processing time: 288 Days and 3.1 Hours
In the United States, 80%-90% of primary hepatic tumors are hepatocellular carcinomas and 10%-15% are cholangiocarcinomas (CCA), both with high mortality rate, particularly CCA, which portends a worse prognosis. Traditional management with surgery has good outcomes in appropriately selected patients; however, novel ablative treatment options have emerged, such as radiofrequency ablation (RFA), which can improve the prognosis of both hepatic and biliary tumors. RFA is aimed to generate an area of necrosis within the targeted tissue by applying thermal therapy via an electrode, with a goal to completely eradicate the tumor while preserving surrounding healthy tissue. Role of RFA in management of hepatic and biliary tumors forms the focus of our current mini-review article.
Core Tip: Radiofrequency ablation (RFA) generates an area of necrosis within the targeted tissue by applying thermal therapy via an electrode, with a goal to completely eradicate the tumor while preserving surrounding healthy tissue. RFA can maintain biliary drainage by tumor ablation within the biliary ducts or occluded metallic stents, which improves survival and quality of life in unresectable cholangiocarcinomas patients. In hepatocellular carcinoma, RFA is used alone or in combination (with hepatectomy/transcatheter arterial chemoembolization) for ablation of tumors < 2 cm, and improves local tumor progression and recurrence-free survival, and considered by some to be comparative to hepatectomy.
- Citation: Hendriquez R, Keihanian T, Goyal J, Abraham RR, Mishra R, Girotra M. Radiofrequency ablation in the management of primary hepatic and biliary tumors. World J Gastrointest Oncol 2022; 14(1): 203-215
- URL: https://www.wjgnet.com/1948-5204/full/v14/i1/203.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i1.203
Most primary hepatic tumors are found to be either Hepatocellular Carcinoma (HCC) or Cholangiocarcinoma (CCA). Specifically, within the United States, 80%-90% of these tumors are found to be HCCs, and the remaining 10%-15% being CCAs[1-9]. These hepatic tumors have a high mortality rate, particularly CCA, which portends a worse prognosis[7,10-14]. Traditionally, surgical resection has been shown to have good outcomes in appropriately selected patients. However, with the advent of novel ablative treatment options such as radiofrequency ablation (RFA), the prognosis of both hepatic and biliary tumors can be improved[12,15,16].
Radiofrequency ablation (RFA) is aimed to generate an area of necrosis within the targeted tissue by applying thermal therapy via an electrode[17-20], with a goal to eradicate the tumor while preserving surrounding healthy tissue[12,21,22]. Thermal ablation has been used for management of a wide range of lesions, from renal tumors to uterine fibroids. However, more data is emerging in its role as a curative or palliative option in those with primary and secondary hepatobiliary malignancies[11,18-20]. In this mini-review article, we discuss the role of RFA in patients with primaryhepatic and biliary tumors.
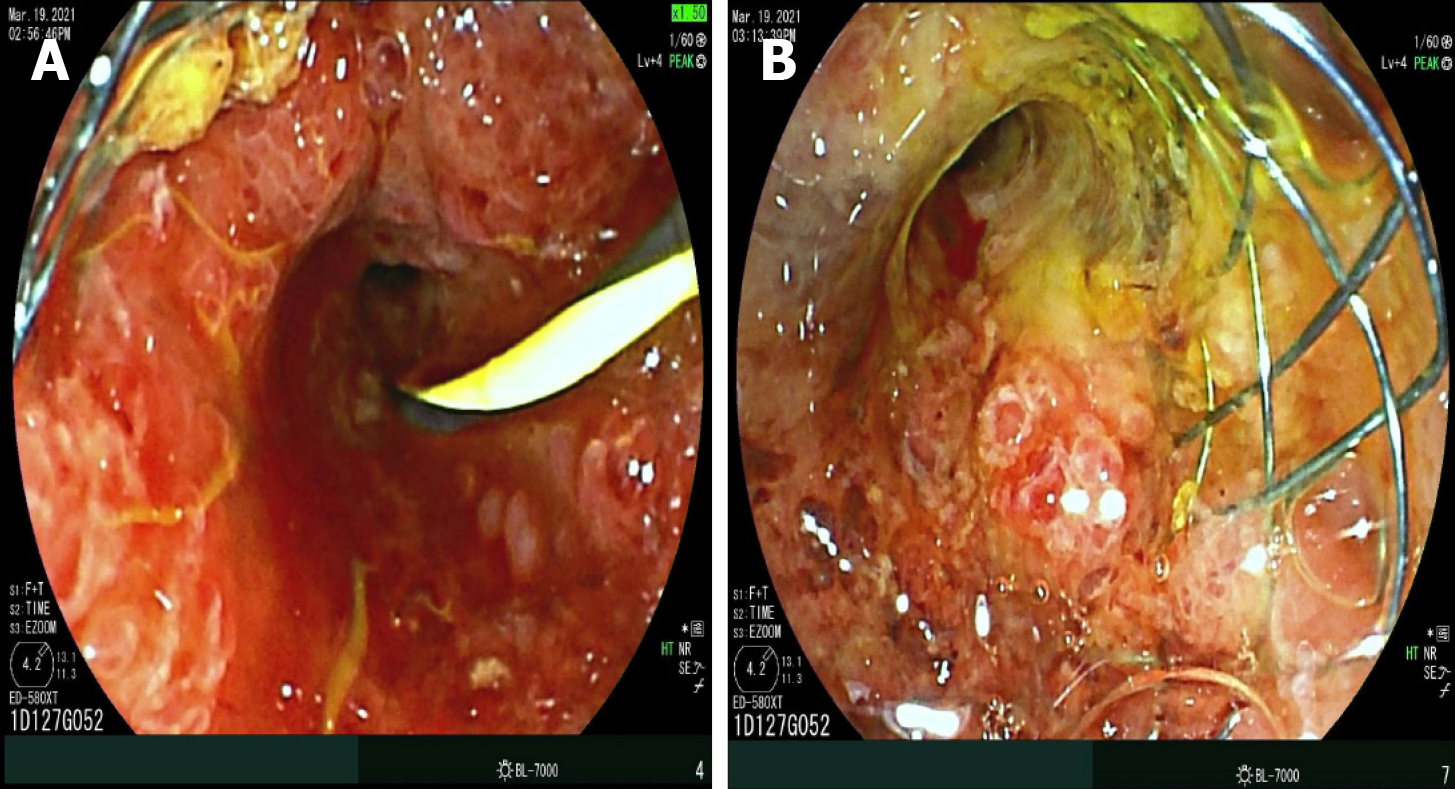
RFA utilizes electrodes to provide an alternating current, causing the ions to reverberate rapidly, thereby increasing tissue temperature[8,16,23-26]. This thermal energy induces coagulative necrosis and subsequent death of the malignant cells. RFA can be accomplished through multiple approaches, including surgical, percutaneous, and more recently, endoscopic modality[19,27,28]. Several studies have explored the safety, efficacy and feasibility of RFA for loco-regional control of tumor growth. To facilitate this, a specialized catheter named Endo Luminal RadiofrequencyAblation (ELRA) was developed (STARmed, Goyang, Korea), which is a 7-Fr bipolar catheter with a 1750 mm length, with an automatic temperature probe, allowing the user to avoid excessive heating and collateral damage to surrounding healthy tissue, thus decreasing the rates of procedure-related adverse events[29-32]. Four different exposure lengths are available (11, 18, 22 and 33 mm) to allow RFA of strictures of varying lengths, with recommended power setting of 7-10 W and target temperature of 80 °C for up to 2 min. A similar Habib EndoHPB catheter (Boston Scientific, Marlborough, MA, United States) is an 1800 mm long 8-Fr device with two distal tip electrodes placed 8 mm apart, to achieve biliary RFA. Novel devices have been developed to achieve the same via endoscopic ultrasound (EUS) approach. For example, the Habib endoscopic ultrasound (EUS) RFA device is a 1-Fr wire monopolar electrode inserted inside a standard EUS fine-needle aspiration (FNA) needle that can achieve coagulation of specific target tissue[12,21,33,34]. During endoscopic retrograde cholangiography (ERCP), after biliary cannulation a guidewire is passed through the strictured segment of bile duct, over which the RFA catheter is advanced and electrodes are positioned under fluoroscopic guidance to achieve ablation over bursts of 60 s. For longer strictures, stepwise ablation is performed to cover the entire length, or alternately catheter with varying exposure length can be utilized, if available. This modality can also be used to treat tumor/tissue ingrowth within metallic stents placed for cholangiocarcinoma (Figure 1). For strictures involving the hilum, ablation of both right and left hepatic ducts is performed after placement of two bilateral guidewires. After improvement of stricture, upstream debris removal is performed, followed by cholangiogram to assess for complications including bile leak, prior to stent placement.
CCA represents approximately 2%-3% of malignancies arising from the gastro-intestinal (GI) system, but is second most common primary liver tumor[7,34,35]. Specifically, these malignancies arise from the cells that line the biliary tree, and categorized as extra-hepatic or intra-hepatic, depending on their extent of ductal infiltration and location in relation to the cystic duct insertion; as most famously reported using the Bismuth-Corlette system[13,34]. Supplementary classifications of CCAs have been proposed, which in addition to tumor extent within the biliary system also take into account the size of the tumor, vascular (hepatic artery/portal vein) and lymph node involvement, distant metastases, and estimated post-resection hepatic volume[36], which have advantage over Bismuth-Corlette system which does not provide information on vascular encasement or metastatic disease, includes only peri-hilar CCA but not intrahepatic CCA, and does not necessarily determine local resectability, and hence of limited prognostic value. In fact, there is emerging evidence that although resection of type IV CCA is technically demanding with high morbidity, it can be performed with low mortality and offers better survival probability in selected patients[37].
When it comes to the treatment of CCAs, anatomical location and resectability play a crucial role. For those lesions that are considered resectable, surgical resection can be curable. Chemotherapy and radiation are typically utilized for unresectable lesions or can be used as neoadjuvant therapy for resectable tumors. For those tumors that cause obstruction, biliary drainage is usually the mainstay therapy with stent placement[29,32,38]. At present, extra-hepatic CCA is considered the condition most effectively treated with biliary RFA. Performance of RFA for intrahepatic CCA is challenging, and can be achieved via ERCP or EUS or percutaneous approach. RFA has also been employed to prolong the patency of stents in malignant obstructive tumors[27,38,39]. Typically the deployment of a self-expandable metallic stent (SEMS) is the mainstay palliative therapy in these patients. By prolonging the patency of stents, it improves survival and quality of life in patients with unresectable CCA.
Multiple studies have appraised the efficacy of RFA in the treatment of CCA and stent patency[33,40,41]. Cui and colleagues evaluated the effect of RFA on stent patency in malignant biliary obstruction, and while there was no significant difference in the overall survival, patency time was significantly increased in the RFA group at 7.6 mo when compared to 4.3 mo in the stent without RFA group. Another retrospective study by Li et al[29] determined hat stent patency was prolonged in those patients who underwent RFA plus stenting compared to stenting alone (81% vs 35%) with a P < 0.05. Furthermore, a meta-analysis by Sofi and colleagues, which included eight observational studies and one randomized controlled trial of RFA in malignant biliary obstruction showed not only a significantly prolonged stent patency in the RFA group when compared to the control group, but also a significant increase in overall survival in the RFA group (n = 504; 95%CI: 1.145-1.7; P < 0.01)[18]. Yang et al[20] performed a randomized control trial on patients with unresectable distal CCA and perihilar CCA; one group received RFA plus stenting (n = 32) and the other group received stenting alone (n = 33). Compared to stenting alone, the RFA plus stent group had a statistically significant increase in both patency (6.8 mo; 95%CI: 3.6-8.2 vs 3.4 mo; 95%CI: 2.4-6.5) and overall survival (13.2 mo vs 8.3 mo)[20]. These results are in contrast to previous reports, like by Wu et al[32], which has shown efficacy of RFA for stent patency, but no survival benefit. A detailed summary is provided in Table 1. Few studies have also compared Photodynamic therapy (PDT) with RFA, mostly without any statistically significant difference in overall survival between the two treatment approaches[42]. However, one of the retrospective studies did show that RFA conferred a short-term advantage in decline in bilirubin[43,44].
| Technique | Ref. | Number of Patients | Location | Stent type | Mean number of sessions | Patency of stent (d, median) | Stent occlusion | Survival | Adverse events |
| Mizandari et al[78], 2013 | 39 | CCA (17); Bismuth I (5); II (1); IIIa (4); IV (7)-Panc CA (11), GB CA (4), HCC (1), Ampullary CA (1), Metastatic CA (5) | SEMS (all) | 1 | 84.5 | 1 | 3 mo (median) | Abdominal pain (15) | |
| Wu et al[32], 2017 | 71[RFA and stenting = 35, stenting alone = 36] | Extra-hepatic distal CCA | Covered SEMS (7); uncovered SEMS (28) | 1 | Uncovered SEMS (241); covered SEMS (212) | - | Uncovered SEMS (245 d, median); covered SEMS (278 d, median) | Abdominal pain (27) | |
| Percutaneous | Li et al[29], 2015 | 26[RFA and stenting = 12, stenting alone = 14] | Hilar (2), middle and distal CBD(7), Panc CA (2), ampullary CA (1) | SEMS (all) | 1 | RFA group (0), control group (3) | RFA group 100%; control group 85% at 90 d | - | Cholangitis (3) |
| Wu et al[31], 2015 | 47 | Hilar (7), distal CBD (16);ampullary CA (8); Panc CA (6); GB CA (4); HCC(2); Metastatic disease( 4) | SEMS | 1.38 | 149 | 11 | 6 mo | Abdominal pain (21), intra-abdominal hemorrhage (1) | |
| Wang et al[28], 2016 | 9 | Bismuth IIIa (1); IIIb (1); IV (7) | SEMS | 1 (only 1 patient had 2 sessions) | 100 | - | 5.3 mo | Abdominal pain (3); Cholangitis (4) | |
| Wang et al[39], 2016 | 12 | Bismuth I (5); IIIa (1); IV (3); Gastric CA (1); HCC(1); Congenital Choledochal cyst (1) | Plastic (7); SEMs (4) | 1 | 125 | - | 7.7 mo (median) | Fever (2), pancreatitis (1) | |
| Laquière et al[81], 2016 | 12 | Bismuth I (4); II (3); III (2); IV (3) | Plastic and Metallic (does not quantify) | 1.63 | - | 4 | 12.3 mo | Sepsis (1), early stent migration (1), late stent migration(1), cholangitis (1) | |
| Endoscopic | Sharaiha et al[86], 2015 | 69 | Hilar (23); proximal CBD (7); distal CBD (7); Bismuth I (4); Bismuth III (2); Bismuth IV (5); Panc CA (19); GB CA (2); Gastric CA (1), Metastasis disease (3) | Metallic (49); Plastic (20) | 1.3 | 95% at 30 d | 3 | 17.7 ± 15.4 mo | Pancreatitis (1); Cholecystitis( 2); Haemobilia (1); abdominal pain (3) |
| Strand et al[87], 2014 | 16 | Intrahepatic/proximal (1); Hilar (13); Extrahepatic/distal (2) | Plastic (3); fully covered SEMS (3); uncovered SEMS (11) | 1.19 | - | 0.06 | 9.6 mo | Stent migration (0.02); cholangitis (0.13); hepatic abscess (0.02); need for percutaneous drainage (0.01); severe abdominal pain (0.02) (occurrence per month) | |
| Sharaiha et al[30], 2014 | 64 | CCA (18); Panc CA (8) | Covered SEMS (8); uncovered SEMS (7); Plastic (11) | 1 | 100% at 90 d | 0 | 5.9 mo | Abdominal pain(3); Pancreatitis (1); Cholecystitis (1) | |
| Alis et al[88], 2013 | 10 | Bismuth I (4); Distal CBD (6) | SEMS (all) | 1 | 270 | 0 | - | Pancreatitis (2) | |
| Figueroa Barojas et al[49], 2013 | 20 | CCA (11); Panc CA (7); Gastric Ca (1), IPMN with high grade dysplasia (1) | Plastic (6); covered SEMS (13); uncovered SEMS ( 1) | 1.25 | 100% at 30 d | 0 | - | Abdominal pain (5); Pancreatitis (1); Cholecystitis (1) | |
| Steel et al [19], 2011 | 21 | CCA (6); Panc CA (16) | Uncovered SEMS (all) | 2 | 114 (median stent patency at 9- d) | 4 | - | Pancreatitis (1); cholecystitis (2), obstructive jaundice/death (1) | |
| Percutaneous and endoscopic | Dolak et al[27], 2014 | 58 | Bismuth I (5); II (1); III (6); IV (33); distal CBD (5);Panc CA (4), central HCC,mCRC(3) | Plastic (19); SEMS (35); no stent (4) | 1.44 | 170 (Metallic stent = 218, Plastic stent = 115) | - | 10.9 mo (median) | Cholangitis (5); hemobilia (2); sepsis (2); hepatic coma (1); hepatic infarction (1) |
To summarize, RFA is a successful strategy for loco-regional management of extra-hepatic CCA, management of malignant biliary obstruction, as well as blocked metallic stents. The performance of RFA is operator dependent, and not protocol based, and hence timing and interval of RFA remains unclear, as well as choice of stents (plastic vs metallic). For intra-hepatic CCA, the access using ERCP-RFA catheters can be challenging, and alternative approaches may include EUS-RFA or percutaneous RFA by Interventional Radiology. Alternatives to RFA include Photodynamic Therapy (PDT), Microwave Ablation (MWA) and Irreversible Electroporation Ablation (IRE), all of which are complex and expensive procedures, which require highly specialized equipment, have side effects (photosensitivity with PDT) and complication profile, and hence not commonly performed worldwide. IRE is a non-thermal ablation modality, the basic principle of which is to create irreversible pores in cellular bi-lipid membranes by subjecting them to series of high intensity electrical pulses for short duration of time, resulting in cell death due to apoptosis, especially used for tumors located close to porta-hepatis. On the other hand, in MWA, tumor tissue is destroyed by direct hyperthermic injury produced by electromagnetic waves emitted from non-insulated portions of antenna, resulting in larger volume of active heating resulting in shorter procedure times, higher tissue temperatures beyond the threshold of water vaporization and less susceptibility to the heat sink effect of blood flow. Detailed discussion regarding these modalities is beyond the scope of this mini-review manuscript.
HCC is the most common primary liver cancer and has the third-highest cancer-related mortality worldwide, exceeding 700000 deaths per year[2,3,5,6,45-47]. There are different causes of HCC, which vary worldwide; in Africa, aflatoxin B1 and chronic Hepatitis B infection seem to account for the most incidence of HCC, whereas cases in North America, Japan, and Europe are related to alcoholism and Hepatitis C infection[2,3,5,6,45-47]. Currently, the curative management options for HCC include liver transplantation, hepatectomy, or ablative therapies. Most patients diagnosed with HCC are not surgical candidates due to the advanced tumor size, invasion, or presence of metastasis[1-4,48]. Most management algorithms worldwide employ a specific scoring system named Barcelona Clinic Liver Cancer guidelines[1-6,48-50], to aids clinicians in determining the most appropriate management modality. Patients with early-stage HCC without any vascular invasion are classified as BCLC-A, which are suitable candidates for resection, ablation, or transplantation. On the other end of the treatment spectrum, patients with extra-hepatic tumor spread or vascular invasion are classified as BCLC-C, and are best managed with systemic therapies such as Sorafenib[1-6,48-50].
With the introduction of the Milan criteria, an increase in liver transplantation has been witnessed, ushering in a new era of curative treatment for HCC[51,52]. However, transplantation is dependent on donor availability, and since there are a limited number of donors, only a finite number of patients can undergo successful treatment. More importantly, patients may spend long periods of time awaiting transplant, allowing cancer to progress, which may disqualify formerly eligible patients from transplantation. To avoid this clinical dilemma, ablative techniques such as RFA become important for the crucial role they can play in delaying the malignancy progression[24,53-56]. A distinct advantage of these ablation techniques is that they can be performed safely on suboptimal surgical candidates.
RFA is the most widely used thermal ablative procedure used in patients with HCC[49,54,57,58], the success of which is inversely related to tumor size. Complete remission is achieved in approximately 90%-92% in those with tumor size < 2 cm whereas remission rates decrease to 20%-40% in those ≥ 2 cm in size[59]. While theoretically, multipolar electrodes may expand the ablation zone of RFA, this has not panned out in clinical studies. Cartier et al[60] compared traditional monopolar electrode RFA with multipolar electrodes in patients with tumors > 2.5 cm, and found no difference in residual tumor or recurrence. RFA seems to be a safe treatment option with procedure-related mortality of approximately 0.2% and an overall complication rate of 2.2%[61,62]. A novel RFA technique being studied is the "no-touch RFA protocol," which involves inserting multiple electrodes within the tissue that surrounds the tumor[62], which avoids direct contact with the tumor, allowing thermal ablation to be conducted with decreased risk of tumor seeding by the probe.
Several studies have investigated the effectiveness of RFA in HCC (Table 2). Liao et al[63] randomized 96 patients into those undergoing wide margin (WM ≥ 10 mm) ablation (n = 48) and normal margin (NM: ≥ 5 but < 10 mm) ablation (n = 48), and followed for mean period of 38.3 ± 4.8 mos. When analyzed based on intention-to-treat strategy, the 3-year incidences of local tumor progression (LTP) (14.9% vs 30.2%), intrahepatic recurrence (IHR) (15.0% vs 32.7%), and recurrence-free survival (RFS) (31.7 ± 12.1 vs 24.0 ± 11.7 mo) for WM group were significantly improved compared to NM group[63]. Getting recurrence-free survival advantage with RFA is a major success, for which RFA is adopted widely worldwide for smaller HCC, especially in non-resectable candidates. In regards to the “no-touch RFA protocol," a multicenter retrospective study of HCC < 5 cm in diameter (n = 362) showed effectiveness of this approach over monopolar RFA in terms of recurrence rates[62,63], but no statistical difference in 5-year survival rates (monopolar 37.2% vs no-touch multipolar 46.4% P = 0.378). Some investigators have proposed that stereotactic body radiotherapy (SBRT) was more effective than RFA, which has been challenged in recent studies[64,65]. In 2018, Rajyaguru et al[64] compared the effectiveness of RFA (n = 3684) against SBRT (n = 296), and their analysis support superior survival with RFA for non-surgically managed patients with stage I or II HCC. Various studies have investigated predictive factors to achieve improved outcomes in HCC when utilizing RFA. In a recent meta-analysis by Giardini et al[61,65-68] (34 studies; n = 11,216), alpha-fetoprotein (AFP) < 20 ng/mL, Child-Pugh class A and albumin-bilirubin index of 1 were noted to confer increased survival benefit. In addition, survival also increased in patients with single tumor < 2 cm in diameter and preserved hepatic function[61,69-71].
| Ref. | Type | N | Technique | Survival | Recurrence | Adverse Events | Outcome |
| Zhang et al[89], 2013 | Retrospective | 155 | RFA (78- 93 sessions) and MWA (77-91 sessions) | 1-, 3-, and 5-year overall survival rates: RFA: 91.0%, 64.1% and 41.3%; MWA: 92.2%, 51.7%, and 38.5% | RFA: 11/93 (11.8%) and MWA: 11/105 (10.5%) | RFA group: persistent jaundice (n = 1) and biliary fistula (n = 1). MWA group: hemothorax and intrahepatic hematoma (n = 1) and peritoneal hemorrhage (n = 1) | No significant differences LTP, DR, and overall survival |
| Karla et al[90], 2017 | Prospective | 50 | RFA alone (25) and RFA + alcohol ablation (25) | RFA alone 84%; RFA + alcohol (80%) (at 6 month) | Local recurrence (11); Distant intrahepatic tumor recurrence (4) | Hemoperitoneum (1) | Combined use of RFA and alcohol did not improve the local tumor control and survival |
| Abdelaziz et al[91], 2017 | Retrospective | 67 | TACE-RFA (22) and TACE-MWA (45) | Survival at 1, 2 and 3 years: TACE-MWA: 83.3%, 64.7%, 64.7%; TACE-RFA: 73.1%, 40.6% and 16.2% (P = 0.08) | TACE-RFA: 4 (18.2%); TACE-MWA: 8 (17.8%) | TACE-RFA: bone metastases 1 (4.5%), Ascites 3 (13.6%), variceal bleeding 5 (22.7%); TACE-MWA: portal vein thrombosis: 1 (2.2%), ascites 6 (13.3%), variceal bleeding: 4 (8.9%) | No significant difference in overall survival was observed |
| Gyori et al[92], 2017 | Retrospective | 150 | 54% (n = 81) received TACE-based LRT, 26% (n = 39) PEI/RFA regimen, and 17% (n = 26) had no treatment while on the waiting list | No difference in overall survival after liver transplantation when comparing TACE- and RFA-based regimens. | TACE- and RFA-based regimens showed equal outcomes in terms of transplantation rate, tumor response, and post-transplant survival. Lower survival in recipients of Multimodality LRT. | ||
| Hao et al[93], 2017 | Retrospective | 237 | 50 pathologically early HCCs, 187 typical HCCs | LTP observed in 1 Early HCC (2%); 46 Typical HCC (24.6%) | Fever, abdominal pain and elevated liver enzyme levels. | Rate of LTP for early HCCs after RFA was significantly lower than typical HCCs. | |
| Liao et al[63], 2017 | Prospective randomized | 96 | 48 patients wide margin (WM) ablation (≥ 10 mm) and 48 normal margin (NM) ablation (≥ 5 mm but < 10 mm ) | The 1-, 2-, and 3-year survival rates: WM: 95.8%, 91.6%, and 74.6%; NM: 95.8%, 78.4%, and 60.2% | 3-year LTP: WM: 14.9%; NM: 30.2% Intrahepatic recurrence (IHR): WM: 15.0% NM: 32.7% | Perihepatic bile collection (1); intrahepatic hemorrhage(1); fever(1); liver infarction (1); thermal skin injury (1); pleural effusion (1) | WM-RFA may reduce the incidence of tumor recurrence among cirrhotic patients with small HCCs |
| Rajyaguru et al[64], 2018 | Observational | 3980 | RFA (3,684) and SBRT (296) | 5 yr overall survival: RFA: 29.8% (95%CI: 24.5-35.3%); SBRT: 19.3% (95%CI: 13.5-25.9%) | Treatment with RFA yields superior survival compared with SBRT for nonsurgically managed patients with stage I or II HCC | ||
| Parick et al[65], 2018 | Retrospective cohort | 440 | RFA (408) and SBRT (32) | RFA patients had better overall survival (P < 0.001) | SBRT (HR 1.80; 95%CI: 1.15-2.82) associated with worse survival | ||
| Santambrogio et al[94], 2018 | Prospective controlled | 264 | Laparoscopic hepatic resection (LHR = 59) vs laparoscopic ablation therapy (LAT = 205) | Survival rates LHR at 1, 3, and 5 years were 93, 82, and 56%. In LAT = 91%, 62%, and 40% | LHR = 24/59 (41%); LAT = 135/205 (66%) | LAT found to be adequate alternative |
Several studies have also explored the comparative success rates of RFA vs hepatectomy in HCC. A meta-analysis by Xu et al[72] indicated that RFA and surgical hepatectomy had similar overall survival at 1 year (relative risk [RR], 1.39; 95% confidence interval [CI]: 0.36, 5.33; P = 0.63) and 3 years (RR, 1.40; 95%CI: 0.75, 2.62; P = 0.29), whereas RFA resulted in decreased overall survival compared with HR at 5 years (RR: 1.91; 95%CI: 1.32, 2.79; P = 0.001)[72]. However, closer analysis of subgroup data, results showed no difference in survival between the groups in tumors less than 2.0 cm in size[72]. The Surveillance, Epidemiology and End Results (SEER) database explored the same question further stratified by age[65], and noted that patients older than 65 years with tumors less than 2 cm had similar survival to their propensity-matched group age less than 65 years. Interestingly, those < 65 years and tumors >3.0 cm had an increased overall survival with hepatectomy compared to RFA. However, large-scale studies have not been able to incorporate the novel RFA techniques previously discussed compared to hepatectomy[59,73,74]. Further studies will need to be conducted to answer this question.
Several studies have explored the role of combination therapy with RFA. In a recent meta-analysis, the pooled results showed that the 1-, 3-, 5-year overall survival rate in the combined RFA+hepatectomy group were comparable with those in the hepatectomy alone group (OR = 0.77, 0.96, 0.88; P = 0.33, 0.88, 0.70, respectively). Similarly, there was no significant difference in 1-, 3-, 5-year disease free survival rate between the combined group and the surgical alone group (OR = 0.57, 0.83, 0.72; P = 0.17, 0.37, 0.32, respectively). These results indicated that the hepatectomy combined with RFA could reach a long-term survival outcome similar to curative surgical resection for multifocal HCC patients, and this approach may be a promising alternative for patients with marginal liver function or complicated tumor distribution[75]. But, RFA+hepatectomy is limited due to its increased rate of post-op complications such as liver failure and death. Transcatheter arterial chemoembolization (TACE) is another commonly used percutaneous non-ablative treatment for HCC[76], which when combined with RFA yields a feasible treatment strategy with promising outcomes. In study by Kim et al[77], 1 mo, 6 mo, and 1-year tumor responses of TACE-RFA were similar to those of RFA and better than those of TACE. A distinct advantage of this combination therapy may be in patients with tumors located close to major vessels, wherein TACE occludes the hepatic artery flow, allowing a larger area for RFA ablation. This strategy minimizes the “heat-sink” effect associated with RFA. Regardless, the TACE-RFA group showed longer hospital stay and more frequent patient discomfort requiring medication than TACE or RFA monotherapy groups (P < 0.001), as well as the frequency of overall complications after TACE-RFA was higher than TACE (P = 0.006) or RFA (P = 0.009)[52,74,76-78]. Finally, RFA is also being utilized in combination with Sorafenib for management of HCC. A recent meta-analysis (15 studies, 2227 patients) showed that compared to RFA-alone, the patients in RFA+Sorafenib had longer 1-, 2- and 3-year overall survival (P < 0.05), better overall efficacy (P < 0.0001), longer RFA interval (P < 0.001) and lower 2-year recurrence rate (P = 0.02). However, this came at the cost of higher adverse reactions compared to RFA-alone group, including hand-foot skin reactions (P < 0.001), diarrhea and constipation (P < 0.0001), hypertension (P = 0.009) and alopecia (P < 0.001)[79]. Therefore, cognizance of overall adverse events is necessary while choosing the most optimal strategy. Despite these limitations, overall improvements in technology under development show promising prospects in the treatment of HCC.
Several adverse events have been associated with RFA, the most common being post-procedure mild abdominal pain following either endoscopic or percutaneous RFA approaches. There seems to be a higher incidence of bleeding with percutaneous RFA, whereas a higher association of pancreatitis with the endoscopic approach[59,80]. Other post-procedure complications, such as hemobilia and hepatic artery pseudoaneurysms, have been postulated to be due to thermal injury[38,81]. This can be avoided with the newer ELRA RF catheter, which has a temperature probe. Further complications have been listed in Tables 1 and 2.
RFA does have its limitations. The therapeutic efficacy of RFA is inversely associated with tumor size and location[59]. RFA needs direct contact with the tissue, which can pose a challenge to treat tumors in inaccessible sites. Furthermore, tumors in close proximity to large vessels pose interesting therapeutic challenges[10,18,22,41,82,83]. Tumors located near large portal and hepatic vein branches can result in a "heat-sink" effect, which results in the inability to reach maximal ablation temperatures, thereby causing incomplete cell death[84]. It is important to keep in mind that RFA cannot be used in pregnancy or patients with cardiac devices or coagulopathy[24,73,82,83,85].
RFA has been established as novel and safe minimally invasive management tool for HCC. While multiple studies optimizing these techniques have shown promising results in patients with CCA, the low incidence of these biliary tumors makes it challenging to coordinate high-powered RCTs comparing various techniques and treatment strategies. It is paramount that future studies are coordinated through collaboration between various institutions of excellence for the progress of this still novel technique.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Gastroenterological Association; American College of Gastroenterology; American Society for Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lang SA S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Avolio AW, Cillo U, Salizzoni M, De Carlis L, Colledan M, Gerunda GE, Mazzaferro V, Tisone G, Romagnoli R, Caccamo L, Rossi M, Vitale A, Cucchetti A, Lupo L, Gruttadauria S, Nicolotti N, Burra P, Gasbarrini A, Agnes S; Donor-to-Recipient Italian Liver Transplant (D2R-ILTx) Study Group. Balancing donor and recipient risk factors in liver transplantation: the value of D-MELD with particular reference to HCV recipients. Am J Transplant. 2011;11:2724-2736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 2. | Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La Vecchia C. Trends in mortality from hepatocellular carcinoma in Europe, 1980-2004. Hepatology. 2008;48:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 3. | Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 1106] [Article Influence: 100.5] [Reference Citation Analysis (1)] |
| 4. | Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4507] [Article Influence: 225.4] [Reference Citation Analysis (0)] |
| 5. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6569] [Article Influence: 469.2] [Reference Citation Analysis (1)] |
| 6. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3594] [Article Influence: 276.5] [Reference Citation Analysis (4)] |
| 7. | Green BL, House MG. Nonsurgical Approaches to Treat Biliary Tract and Liver Tumors. Surg Oncol Clin N Am. 2019;28:573-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 628] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 9. | Loriaud A, Denys A, Seror O, Vietti Violi N, Digklia A, Duran R, Trillaud H, Hocquelet A. Hepatocellular carcinoma abutting large vessels: comparison of four percutaneous ablation systems. Int J Hyperthermia. 2018;34:1171-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Buerlein RCD, Wang AY. Endoscopic Retrograde Cholangiopancreatography-Guided Ablation for Cholangiocarcinoma. Gastrointest Endosc Clin N Am. 2019;29:351-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Chahal P, Baron TH. Endoscopic palliation of cholangiocarcinoma. Curr Opin Gastroenterol. 2006;22:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Labib PL, Davidson BR, Sharma RA, Pereira SP. Locoregional therapies in cholangiocarcinoma. Hepat Oncol. 2017;4:99-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Qureshi K, Jesudoss R, Al-Osaimi AM. The treatment of cholangiocarcinoma: a hepatologist's perspective. Curr Gastroenterol Rep. 2014;16:412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013;11:13-21.e1; quiz e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 216] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 15. | Nault JC, Sutter O, Nahon P, Ganne-Carrié N, Séror O. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol. 2018;68:783-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 283] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 16. | Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y, Goto T, Yoshida H, Omata M, Koike K. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569-77; quiz 578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 577] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 17. | Lee EW, Chen C, Prieto VE, Dry SM, Loh CT, Kee ST. Advanced hepatic ablation technique for creating complete cell death: irreversible electroporation. Radiology. 2010;255:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 18. | Sofi AA, Khan MA, Das A, Sachdev M, Khuder S, Nawras A, Lee W. Radiofrequency ablation combined with biliary stent placement vs stent placement alone for malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2018;87:944-951.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Steel AW, Postgate AJ, Khorsandi S, Nicholls J, Jiao L, Vlavianos P, Habib N, Westaby D. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011;73:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 20. | Yang J, Wang J, Zhou H, Zhou Y, Wang Y, Jin H, Lou Q, Zhang X. Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma: a randomized trial. Endoscopy. 2018;50:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 21. | Larghi A, Rimbaș M, Tringali A, Boškoski I, Rizzatti G, Costamagna G. Endoscopic radiofrequency biliary ablation treatment: A comprehensive review. Dig Endosc. 2019;31:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Lee DH, Lee JM. Recent Advances in the Image-Guided Tumor Ablation of Liver Malignancies: Radiofrequency Ablation with Multiple Electrodes, Real-Time Multimodality Fusion Imaging, and New Energy Sources. Korean J Radiol. 2018;19:545-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, Gwak GY, Yoo BC. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 24. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 825] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 25. | Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 594] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 26. | Yan K, Chen MH, Yang W, Wang YB, Gao W, Hao CY, Xing BC, Huang XF. Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur J Radiol. 2008;67:336-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Dolak W, Schreiber F, Schwaighofer H, Gschwantler M, Plieschnegger W, Ziachehabi A, Mayer A, Kramer L, Kopecky A, Schrutka-Kölbl C, Wolkersdörfer G, Madl C, Berr F, Trauner M, Püspök A; Austrian Biliary RFA Study Group. Endoscopic radiofrequency ablation for malignant biliary obstruction: a nationwide retrospective study of 84 consecutive applications. Surg Endosc. 2014;28:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 28. | Wang F, Li Q, Zhang X, Jiang G, Ge X, Yu H, Nie J, Ji G, Miao L. Endoscopic radiofrequency ablation for malignant biliary strictures. Exp Ther Med. 2016;11:2484-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Li TF, Huang GH, Li Z, Hao CF, Ren JZ, Duan XH, Zhang K, Chen C, Han XW, Jiao DC, Zhang MF, Wang YL. Percutaneous transhepatic cholangiography and intraductal radiofrequency ablation combined with biliary stent placement for malignant biliary obstruction. J Vasc Interv Radiol. 2015;26:715-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Sharaiha RZ, Natov N, Glockenberg KS, Widmer J, Gaidhane M, Kahaleh M. Comparison of metal stenting with radiofrequency ablation vs stenting alone for treating malignant biliary strictures: is there an added benefit? Dig Dis Sci. 2014;59:3099-3102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 31. | Wu TT, Li HC, Li WM, Ao GK, Lin H, Zheng F, Song JY. Percutaneous Intraluminal Radiofrequency Ablation for Malignant Extrahepatic Biliary Obstruction: A Safe and Feasible Method. Dig Dis Sci. 2015;60:2158-2163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Wu TT, Li WM, Li HC, Ao GK, Zheng F, Lin H. Percutaneous Intraductal Radiofrequency Ablation for Extrahepatic Distal Cholangiocarcinoma: A Method for Prolonging Stent Patency and Achieving Better Functional Status and Quality of Life. Cardiovasc Intervent Radiol. 2017;40:260-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Cui W, Fan W, Lu M, Zhang Y, Yao W, Li J, Wang Y. The safety and efficacy of percutaneous intraductal radiofrequency ablation in unresectable malignant biliary obstruction: A single-institution experience. BMC Cancer. 2017;17:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 848] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 35. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11832] [Article Influence: 845.1] [Reference Citation Analysis (4)] |
| 36. | Deoliveira ML, Schulick RD, Nimura Y, Rosen C, Gores G, Neuhaus P, Clavien PA. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53:1363-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 207] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 37. | Ebata T, Mizuno T, Yokoyama Y, Igami T, Sugawara G, Nagino M. Surgical resection for Bismuth type IV perihilar cholangiocarcinoma. Br J Surg. 2018;105:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 38. | Tal AO, Vermehren J, Friedrich-Rust M, Bojunga J, Sarrazin C, Zeuzem S, Trojan J, Albert JG. Intraductal endoscopic radiofrequency ablation for the treatment of hilar non-resectable malignant bile duct obstruction. World J Gastrointest Endosc. 2014;6:13-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 39. | Wang Y, Cui W, Fan W, Zhang Y, Yao W, Huang K, Li J. Percutaneous intraductal radiofrequency ablation in the management of unresectable Bismuth types III and IV hilar cholangiocarcinoma. Oncotarget. 2016;7:53911-53920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Cui W, Wang Y, Fan W, Lu M, Zhang Y, Yao W, Li J. Comparison of intraluminal radiofrequency ablation and stents vs stents alone in the management of malignant biliary obstruction. Int J Hyperthermia. 2017;33:853-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Lee YN, Jeong S, Choi HJ, Cho JH, Cheon YK, Park SW, Kim YS, Lee DH, Moon JH. The safety of newly developed automatic temperature-controlled endobiliary radiofrequency ablation system for malignant biliary strictures: A prospective multicenter study. J Gastroenterol Hepatol. 2019;34:1454-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Yang J, Shen H, Jin H, Lou Q, Zhang X. Treatment of unresectable extrahepatic cholangiocarcinoma using hematoporphyrin photodynamic therapy: A prospective study. Photodiagnosis Photodyn Ther. 2016;16:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Patel J, Rizk N, Kahaleh M. Role of photodynamic therapy and intraductal radiofrequency ablation in cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Schmidt A, Bloechinger M, Weber A, Siveke J, von Delius S, Prinz C, Schmitt W, Schmid RM, Neu B. Short-term effects and adverse events of endoscopically applied radiofrequency ablation appear to be comparable with photodynamic therapy in hilar cholangiocarcinoma. United European Gastroenterol J. 2016;4:570-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Ikenaga N, Chijiiwa K, Otani K, Ohuchida J, Uchiyama S, Kondo K. Clinicopathologic characteristics of hepatocellular carcinoma with bile duct invasion. J Gastrointest Surg. 2009;13:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5305] [Article Influence: 182.9] [Reference Citation Analysis (0)] |
| 47. | Murray KF, Carithers RL Jr; AASLD. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 516] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 48. | Arii S, Teramoto K, Kawamura T, Okamoto H, Kaido T, Mori A, Imamura M. Characteristics of recurrent hepatocellular carcinoma in Japan and our surgical experience. J Hepatobiliary Pancreat Surg. 2001;8:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Figueroa-Barojas P, Bakhru MR, Habib NA, Ellen K, Millman J, Jamal-Kabani A, Gaidhane M, Kahaleh M. Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: a novel palliation technique. J Oncol. 2013;2013:910897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 50. | Freeman RB, Edwards EB, Harper AM. Waiting list removal rates among patients with chronic and malignant liver diseases. Am J Transplant. 2006;6:1416-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 51. | Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 641] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 52. | Liu W, Zheng Y, He W, Zou R, Qiu J, Shen J, Yang Z, Zhang Y, Wang C, Wang Y, Zuo D, Li B, Yuan Y. Microwave vs radiofrequency ablation for hepatocellular carcinoma within the Milan criteria: a propensity score analysis. Aliment Pharmacol Ther. 2018;48:671-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 53. | DuBay DA, Sandroussi C, Kachura JR, Ho CS, Beecroft JR, Vollmer CM, Ghanekar A, Guba M, Cattral MS, McGilvray ID, Grant DR, Greig PD. Radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. HPB (Oxford). 2011;13:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Fisher RA, Maluf D, Cotterell AH, Stravitz T, Wolfe L, Luketic V, Sterling R, Shiffman M, Posner M. Non-resective ablation therapy for hepatocellular carcinoma: effectiveness measured by intention-to-treat and dropout from liver transplant waiting list. Clin Transplant. 2004;18:502-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Lu DS, Yu NC, Raman SS, Lassman C, Tong MJ, Britten C, Durazo F, Saab S, Han S, Finn R, Hiatt JR, Busuttil RW. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41:1130-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 269] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 56. | Toso C, Dupuis-Lozeron E, Majno P, Berney T, Kneteman NM, Perneger T, Morel P, Mentha G, Combescure C. A model for dropout assessment of candidates with or without hepatocellular carcinoma on a common liver transplant waiting list. Hepatology. 2012;56:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 57. | Fontana RJ, Hamidullah H, Nghiem H, Greenson JK, Hussain H, Marrero J, Rudich S, McClure LA, Arenas J. Percutaneous radiofrequency thermal ablation of hepatocellular carcinoma: a safe and effective bridge to liver transplantation. Liver Transpl. 2002;8:1165-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 58. | Hansen PD, Cassera MA, Wolf RF. Ablative technologies for hepatocellular, cholangiocarcinoma, and metastatic colorectal cancer of the liver. Surg Oncol Clin N Am. 2015;24:97-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Jiang YQ, Wang ZX, Deng YN, Yang Y, Wang GY, Chen GH. Efficacy of Hepatic Resection vs Radiofrequency Ablation for Patients With Very-Early-Stage or Early-Stage Hepatocellular Carcinoma: A Population-Based Study With Stratification by Age and Tumor Size. Front Oncol. 2019;9:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 60. | Cartier V, Boursier J, Lebigot J, Oberti F, Fouchard-Hubert I, Aubé C. Radiofrequency ablation of hepatocellular carcinoma: Mono or multipolar? J Gastroenterol Hepatol. 2016;31:654-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Casadei Gardini A, Marisi G, Canale M, Foschi FG, Donati G, Ercolani G, Valgiusti M, Passardi A, Frassineti GL, Scarpi E. Radiofrequency ablation of hepatocellular carcinoma: a meta-analysis of overall survival and recurrence-free survival. Onco Targets Ther. 2018;11:6555-6567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | Hocquelet A, Aubé C, Rode A, Cartier V, Sutter O, Manichon AF, Boursier J, N'kontchou G, Merle P, Blanc JF, Trillaud H, Seror O. Comparison of no-touch multi-bipolar vs monopolar radiofrequency ablation for small HCC. J Hepatol. 2017;66:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 63. | Liao M, Zhong X, Zhang J, Liu Y, Zhu Z, Wu H, Zeng Y, Huang J. Radiofrequency ablation using a 10-mm target margin for small hepatocellular carcinoma in patients with liver cirrhosis: A prospective randomized trial. J Surg Oncol. 2017;115:971-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 64. | Rajyaguru DJ, Borgert AJ, Smith AL, Thomes RM, Conway PD, Halfdanarson TR, Truty MJ, Kurup AN, Go RS. Radiofrequency Ablation Versus Stereotactic Body Radiotherapy for Localized Hepatocellular Carcinoma in Nonsurgically Managed Patients: Analysis of the National Cancer Database. J Clin Oncol. 2018;36:600-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 65. | Parikh ND, Marshall VD, Green M, Lawrence TS, Razumilava N, Owen D, Singal AG, Feng M. Effectiveness and cost of radiofrequency ablation and stereotactic body radiotherapy for treatment of early-stage hepatocellular carcinoma: An analysis of SEER-medicare. J Med Imaging Radiat Oncol. 2018;62:673-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 66. | Nicoli N, Casaril A, Marchiori L, Mangiante G, Hasheminia AR. Treatment of recurrent hepatocellular carcinoma by radiofrequency thermal ablation. J Hepatobiliary Pancreat Surg. 2001;8:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 67. | Pai M, Spalding D, Jiao L, Habib N. Use of bipolar radiofrequency in parenchymal transection of the liver, pancreas and kidney. Dig Surg. 2012;29:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 68. | Pulvirenti A, Garbagnati F, Regalia E, Coppa J, Marchiano A, Romito R, Schiavo M, Fabbri A, Burgoa L, Mazzaferro V. Experience with radiofrequency ablation of small hepatocellular carcinomas before liver transplantation. Transplant Proc. 2001;33:1516-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 69. | Brillet PY, Paradis V, Brancatelli G, Rangheard AS, Consigny Y, Plessier A, Durand F, Belghiti J, Sommacale D, Vilgrain V. Percutaneous radiofrequency ablation for hepatocellular carcinoma before liver transplantation: a prospective study with histopathologic comparison. AJR Am J Roentgenol. 2006;186:S296-S305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 70. | Chan AC, Poon RT, Cheung TT, Chok KS, Chan SC, Fan ST, Lo CM. Survival analysis of re-resection vs radiofrequency ablation for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. World J Surg. 2012;36:151-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 71. | Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 336] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 72. | Xu XL, Liu XD, Liang M, Luo BM. Radiofrequency Ablation vs Hepatic Resection for Small Hepatocellular Carcinoma: Systematic Review of Randomized Controlled Trials with Meta-Analysis and Trial Sequential Analysis. Radiology. 2018;287:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 173] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 73. | Majumdar A, Roccarina D, Thorburn D, Davidson BR, Tsochatzis E, Gurusamy KS. Management of people with early- or very early-stage hepatocellular carcinoma: an attempted network meta-analysis. Cochrane Database Syst Rev. 2017;3:CD011650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 74. | Mohkam K, Dumont PN, Manichon AF, Jouvet JC, Boussel L, Merle P, Ducerf C, Lesurtel M, Rode A, Mabrut JY. No-touch multibipolar radiofrequency ablation vs surgical resection for solitary hepatocellular carcinoma ranging from 2 to 5 cm. J Hepatol. 2018;68:1172-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 75. | Xu LL, Zhang M, Yi PS, Zheng XB, Feng L, Lan C, Tang JW, Ren SS, Xu MQ. Hepatic resection combined with radiofrequency ablation vs hepatic resection alone for multifocal hepatocellular carcinomas: A meta-analysis. J Huazhong Univ Sci Technolog Med Sci. 2017;37:974-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Martin AN, Wilkins LR, Das D, Johnston LE, Bauer TW, Adams RB, Zaydfudim VM. Efficacy of Radiofrequency Ablation versus Transarterial Chemoembolization for Patients with Solitary Hepatocellular Carcinoma ≤ 3 cm. Am Surg. 2019;85:150-155. [PubMed] |
| 77. | Kim W, Cho SK, Shin SW, Hyun D, Lee MW, Rhim H. Combination therapy of transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) for small hepatocellular carcinoma: comparison with TACE or RFA monotherapy. Abdom Radiol (NY). 2019;44:2283-2292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 78. | Mizandari M, Pai M, Xi F, Valek V, Tomas A, Quaretti P, Golfieri R, Mosconi C, Guokun A, Kyriakides C, Dickinson R, Nicholls J, Habib N. Percutaneous intraductal radiofrequency ablation is a safe treatment for malignant biliary obstruction: feasibility and early results. Cardiovasc Intervent Radiol. 2013;36:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 79. | Jin M, Yu Q, Liu Y, Xu W, Fu X, Ji B. Safety and Efficacy of Physical Thermal Ablation Combined Sorafenib for Hepatocellular Carcinoma: A Meta-analysis. J Clin Transl Hepatol. 2021;9:149-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 80. | Germani G, Pleguezuelo M, Gurusamy K, Meyer T, Isgrò G, Burroughs AK. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol. 2010;52:380-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 81. | Laquière A, Boustière C, Leblanc S, Penaranda G, Désilets E, Prat F. Safety and feasibility of endoscopic biliary radiofrequency ablation treatment of extrahepatic cholangiocarcinoma. Surg Endosc. 2016;30:1242-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 82. | Kang TW, Lim HK, Cha DI. Percutaneous ablation for perivascular hepatocellular carcinoma: Refining the current status based on emerging evidence and future perspectives. World J Gastroenterol. 2018;24:5331-5337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 83. | Lurje I, Czigany Z, Bednarsch J, Roderburg C, Isfort P, Neumann UP, Lurje G. Treatment Strategies for Hepatocellular Carcinoma ⁻ a Multidisciplinary Approach. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 84. | Weber JC, Navarra G, Jiao LR, Nicholls JP, Jensen SL, Habib NA. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236:560-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 198] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 85. | Koda M, Ueki M, Maeda Y, Mimura KI, Okamoto K, Matsunaga Y, Kawakami M, Hosho K, Murawaki Y. The influence on liver parenchymal function and complications of radiofrequency ablation or the combination with transcatheter arterial embolization for hepatocellular carcinoma. Hepatol Res. 2004;29:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 86. | Sharaiha RZ, Sethi A, Weaver KR, Gonda TA, Shah RJ, Fukami N, Kedia P, Kumta NA, Clavo CM, Saunders MD, Cerecedo-Rodriguez J, Barojas PF, Widmer JL, Gaidhane M, Brugge WR, Kahaleh M. Impact of Radiofrequency Ablation on Malignant Biliary Strictures: Results of a Collaborative Registry. Dig Dis Sci. 2015;60:2164-2169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 87. | Strand DS, Cosgrove ND, Patrie JT, Cox DG, Bauer TW, Adams RB, Mann JA, Sauer BG, Shami VM, Wang AY. ERCP-directed radiofrequency ablation and photodynamic therapy are associated with comparable survival in the treatment of unresectable cholangiocarcinoma. Gastrointest Endosc. 2014;80:794-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 88. | Alis H, Sengoz C, Gonenc M, Kalayci MU, Kocatas A. Endobiliary radiofrequency ablation for malignant biliary obstruction. Hepatobiliary Pancreat Dis Int. 2013;12:423-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 89. | Zhang L, Wang N, Shen Q, Cheng W, Qian GJ. Therapeutic efficacy of percutaneous radiofrequency ablation vs microwave ablation for hepatocellular carcinoma. PLoS One. 2013;8:e76119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 90. | Kalra N, Kang M, Duseja AK, Bhatia A, Singh V, Dhiman RK, Rajwanshi A, Chawla YK, Khandelwal N. Comparison of radiofrequency ablation alone and in combination with percutaneous ethanol injection for management of hepatocellular carcinoma. Indian J Med Res. 2017;146:S30-S37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 91. |
Abdelaziz AO, Abdelmaksoud AH, Nabeel MM, Shousha HI, Cordie AA, Mahmoud ShH, Medhat E, Omran D, Elbaz TM.
Transarterial Chemoembolization Combined with Either Radiofrequency or Microwave Ablation in Management of Hepatocellular Carcinoma |
| 92. | Györi GP, Felsenreich DM, Silberhumer GR, Soliman T, Berlakovich GA. Multimodality locoregional treatment strategies for bridging HCC patients before liver transplantation. Eur Surg. 2017;49:236-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 93. | Hao Y, Numata K, Ishii T, Fukuda H, Maeda S, Nakano M, Tanaka K. Rate of local tumor progression following radiofrequency ablation of pathologically early hepatocellular carcinoma. World J Gastroenterol. 2017;23:3111-3121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 94. | Santambrogio R, Barabino M, Bruno S, Mariani N, Maroni N, Bertolini E, Franceschelli G, Opocher E. Surgical Resection vs Ablative Therapies Through a Laparoscopic Approach for Hepatocellular Carcinoma: a Comparative Study. J Gastrointest Surg. 2018;22:650-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |