Published online Jan 15, 2022. doi: 10.4251/wjgo.v14.i1.110
Peer-review started: April 29, 2021
First decision: June 6, 2021
Revised: June 19, 2021
Accepted: November 24, 2021
Article in press: November 24, 2021
Published online: January 15, 2022
Processing time: 256 Days and 13.5 Hours
Statins inhibit 3-hydroxy-3-methylglutaryl-CoA reductase, the rate-limiting enzyme of the mevalonate pathway, and are widely used as an effective and safe approach handle hypercholesterolemia. The mevalonate pathway is a vital metabolic pathway that uses acetyl-CoA to generate isoprenoids and sterols that are crucial to tumor growth and progression. Multiple studies have indicated that statins improve patient prognosis in various carcinomas. Basic research on the mechanisms underlying the antitumor effects of statins is underway. The development of new anti-cancer drugs is progressing, but increasing medical costs from drug development have become a major obstacle. Readily available, inexpensive and well-tolerated drugs like statins have not yet been successfully repurposed for cancer treatment. Identifying the cancer patients that may benefit from statins is key to improved patient treatment. This review summarizes recent advances in statin research in cancer and suggests important considerations for the clinical use of statins to improve outcomes for cancer patients.
Core Tip: Novel pharmacological therapies for cancer are in development, but the expense of new drug development has increased medical costs and placed a heavy financial burden on governments worldwide. Therefore, drug repositioning has become a major focus for new drug development because of reliability and cost effectiveness. Statins are one of the most studied drugs with potential drug repositioning for cancer treatment, but they have not reached clinical application. This review summarizes the results of recent research and clinical studies of statins in cancer, suggests strategies for clinical trial planning, and discusses the potential clinical application of statins for cancer treatment.
- Citation: Uemura N, Hayashi H, Baba H. Statin as a therapeutic agent in gastroenterological cancer. World J Gastrointest Oncol 2022; 14(1): 110-123
- URL: https://www.wjgnet.com/1948-5204/full/v14/i1/110.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i1.110
Since the clinical application of statins in the late 1980s, statins have dramatically improved the clinical management of high cholesterol and ischemic heart disease, and their use has become widespread worldwide. Statins are specified inhibitors of the mevalonate (MVA) pathway, that is involved in the de novo synthesis of cholesterol and other nonsterol isoprenoids. The rate-limiting enzyme in MVA synthesis is 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR)[1,2]. Statins function by inhibiting HMGCR and are effective in the management of hypercholesterolemia.
In addition to their functional role in normal physiology, the MVA pathway is noted to support tumorigenesis and be dysregulated in cancers[3-5]. The MVA pathway is a vital metabolic pathway that uses acetyl-CoA to generate isoprenoids and sterols, which are crucial to tumor growth and progression. Therefore, there is a great deal of interest in repurposing statins as anticancer drugs. Numerous cohort studies have announced that statin use is linked with lower risk of cancer development, lower cancer grade at diagnosis, and lower recurrence and cancer-related death[6]. Several randomized clinical trials have investigated the advantages of adding statins to anti-cancer agents. However, most of the trials did not show an improvement in prognosis and have not led to the clinical application of statins. The development of new anti-cancer drugs is progressing but increasing medical costs from drug development have become a major obstacle. Readily available, inexpensive and well-tolerated drugs like statins have not yet been successfully repurposed for cancer treatment. Planning clinical trials is difficult, and it is possible that the previous clinical trials were poorly designed[7]. In the age of precision medicine, defining the cancer patients that may benefit from statins is critical.
This review summarizes the results of recent basic research and clinical studies on statins in cancer and suggests strategies for future clinical trial planning. In addition, the potential for the clinical application of statins in cancer treatment is discussed.
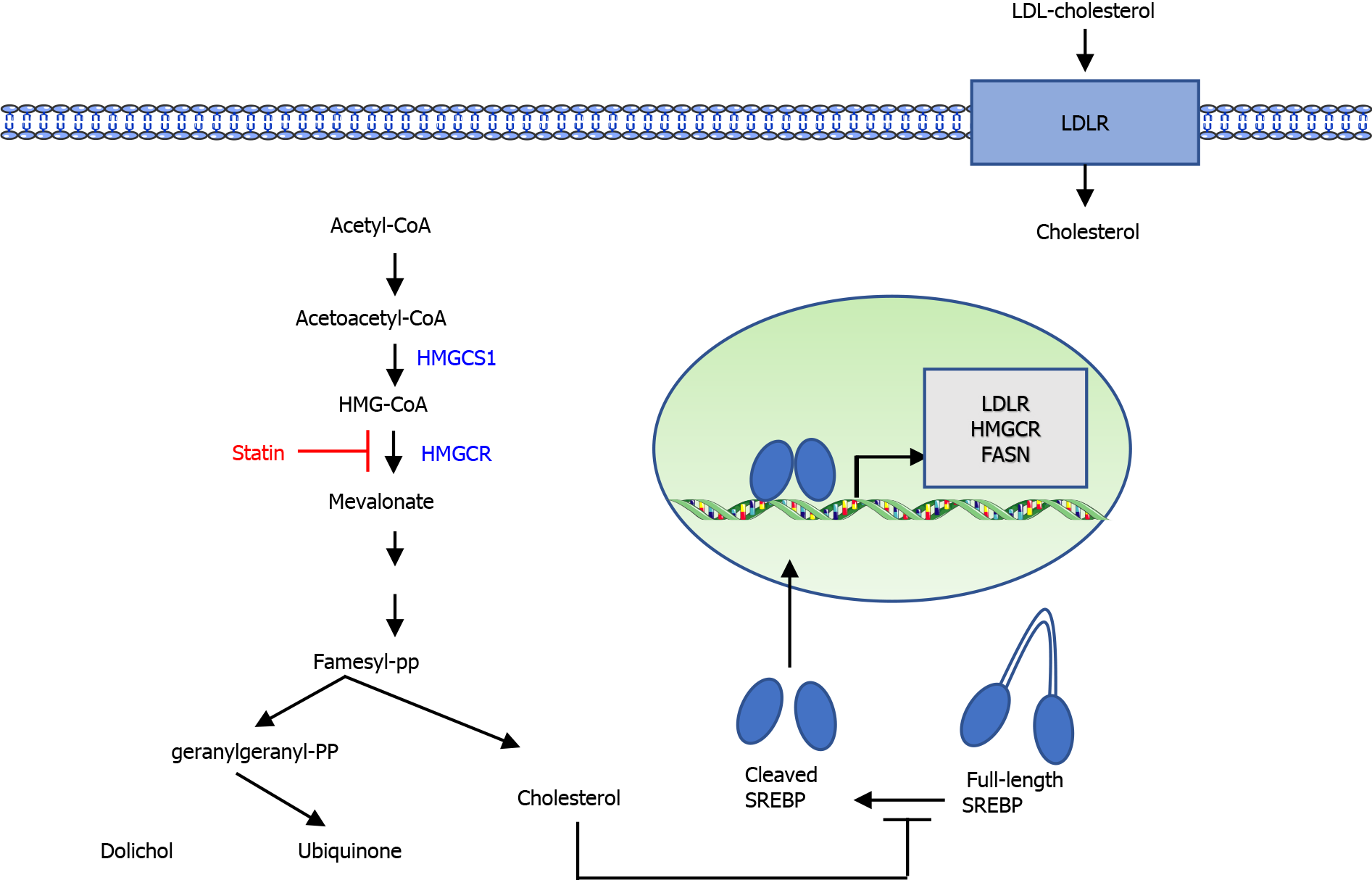
The MVA pathway is a vital metabolic pathway that uses acetyl-CoA to generate isoprenoids and sterols, which are crucial to tumor growth and progression. In the first step of the MVA pathway, the rate-limiting enzyme HMGCR converts HMG-CoA to MVA (Figure 1). MVA is further metabolized to farnesyl pyrophosphate (FPP). FPP is the precursor in cholesterol and steroid biosynthesis as well as in the biosynthesis of dolichols. Intracellular cholesterol preserves sterol regulatory element-binding proteins (SREBPs) as an inactive form in their full-length. In a situation of cholesterol depletion, SREBP proteins are cleaved, releasing the active transcription factors involved in the MVA pathway and cholesterol transport.
Statins bind to the active site of HMGCR, compete with HMG-CoA, and reduce MVA synthesis. Hence, statins exhaust intracellular cholesterol, causing a homeostatic feedback machinery by the SREBP family of transcription factors. Activation of SREBPs increases the gene expression of low density lipoprotein (LDL) receptor (LDLR). Increased membrane expression of LDLR promotes the uptake of LDL cholesterol from the blood circulation and efficiently lowers serum cholesterol levels. Statins are generally prescribed to lower blood cholesterol, decrease the risk of cardiovascular disease, or enhance the survival rate of cases with cardiovascular disease.
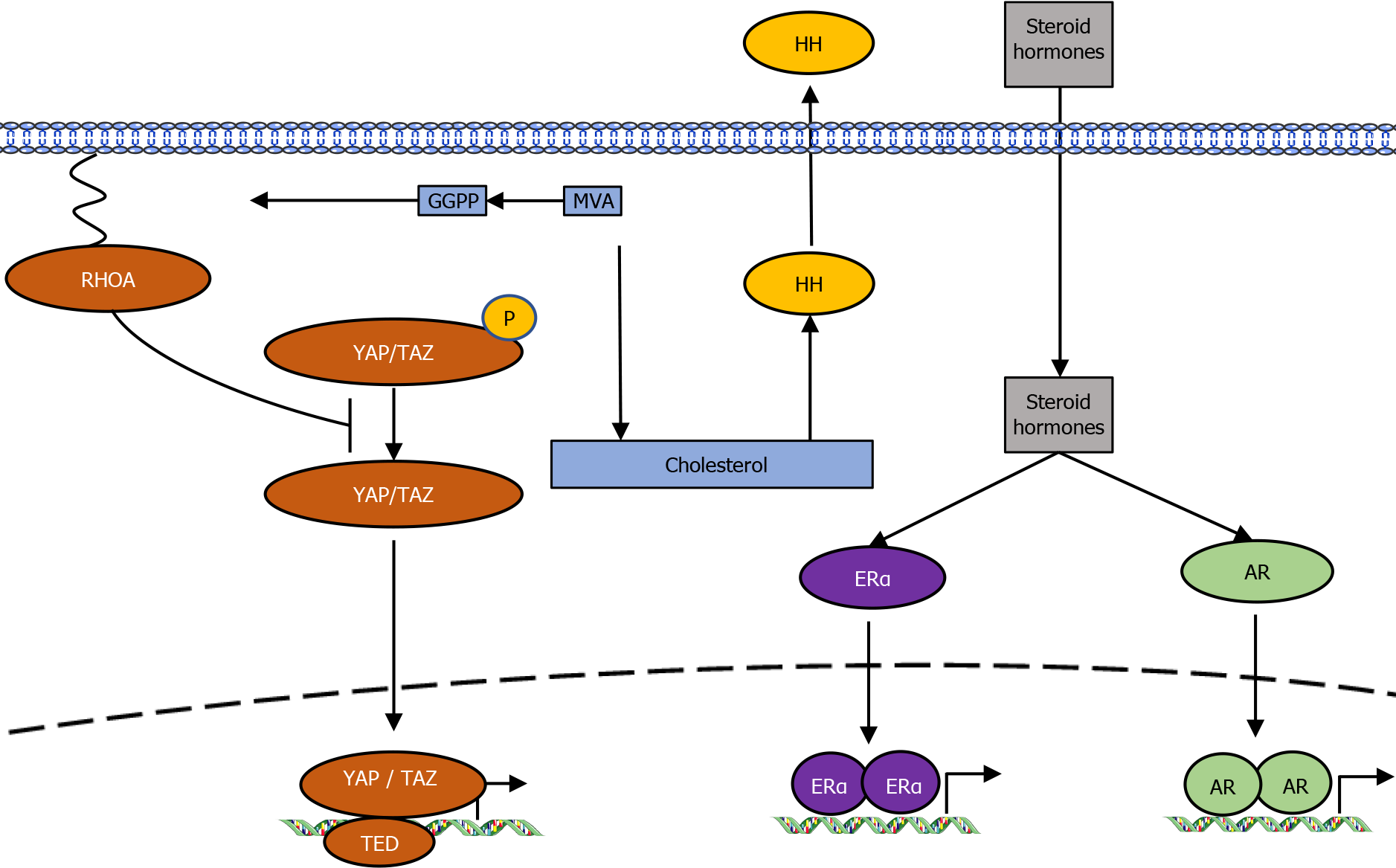
The MVA pathway has been shown to play a multifaceted role in tumorigenesis[4,8]. The PI3K/AKT pathway is a critical regulator of cell proliferation and cell survival in response to growth factors. PI3K/AKT signaling activates the MVA pathway through increasing the expression of SREBPs. The increase in lipid and cholesterol generation regulated by the PI3K/AKT/SREBPs axis enhances the tumorigenesis and cancer growth[9,10]. Conversely, inhibition of the MVA pathway decreases PI3K activity through decreased RAS isoprenylation[11].
Two p53 mutants with gain-of-function mutations were shown to interact with nuclear SREBP2 and enhance the gene transcription of MVA pathway[12]. In contrast, wild-type p53 reduces lipid production by increasing LPIN1 expression under conditions of glucose starvation[13]. The tumor suppressor protein RB has also been involved as a MVA pathway regulator by interacting with SREBPs and reducing their binding to promoters of target genes[14,15]. The oncoproteins Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ), both mediators of the Hippo pathway, are controlled by the SREBPs/MVA pathway[16]. The geranylgeranyl pyrophosphate generated by the MVA cascade is essential for activation of Rho GTPases that, in turn, activate YAP/TAZ by inhibiting their phosphorylation and promoting their nuclear accumulation (Figure 2).
The Hedgehog (HH) signaling pathway, which has crucial roles in tumorigenesis, is controlled by cholesterol. Cholesterol and cholesterol-derived oxysterols activate HH signal transduction[17], whereas inhibition of the MVA pathway or downstream sterol biosynthesis decreases HH signaling and reduces cell proliferation.
Cholesterol is the precursor for steroid hormones such as estrogen and androgen. These hormones are implicated in hormone-driven breast cancers and prostate cancers via the activation of estrogen receptor-α (ERα) and androgen receptor, respectively[18,19]. Perhaps because of these functions, research into the antitumor effects of statins is the most advanced in the fields of breast cancer, ovarian cancer, and prostate cancer.
A recent report showed that the MVA pathway is involved in T lymphocyte metabolism and regulates T cell differentiation[20]. Improved understanding of MVA metabolism will enhance more efficient T cell manipulation for immunotherapy in cancer treatment.
Three meta-analyses have been conducted on the effects of statins on esophageal cancer. In a meta-analysis of five cohort studies comprising 24576 patients, Zhou et al[21] reported that statin use in esophageal cancer patients was associated with a 26% improved overall survival [OS; 95% confidence interval (CI): 0.75–0.94] and disease-free survival (95%CI: 0.75–0.96)[22-26]. Deng et al[27] reported that statin use was considerably associated with decreased all-cause [random effects: Hazard ratio (HR) = 0.81, 95%CI: 0.75–0.89, P < 0.001] and cancer-specific mortality (fixed effects: HR = 0.84, 95%CI: 0.78–0.89, P < 0.001) in esophageal cancer from four cohort studies involving a total of 20435 patients[25]. In the subgroup analysis, both meta-analyses showed an effect of statins on improving prognosis regardless of the histological type of squamous cell carcinoma and adenocarcinoma. Thomas et al[28] insisted that statins might play a protective role against esophageal cancer development in cases with or without Barrett’s esophagus.
Two randomized controlled trials (RCTs) have examined the effects of statin combination therapy on gastric cancer. A phase III study that examined simvastatin (40 mg/d) plus capecitabine-cisplatin compared with capecitabin-cisplatin alone did not show increased progression-free survival[29]. A phase II study that examined pravastatin (40 mg/d) plus standard chemotherapy revealed no improvement of the progression-free survival rate at 6 mo compared with standard chemotherapy alone[30]. A matched case-control study reported that statin use in patients who underwent radical gastrectomy for stage II and III gastric cancer was associated with good prognosis. No significant differences were shown in relapse-free survival or OS between statin users and non-users. On the other hand, subgroup analysis revealed that patients who used statins for more than 6 mo showed better prognostic outcomes than non-users or those who used statins for less than 6 mo[31]. A population-based cohort study including 3833 patients with gastric cancer showed that statin use was linked with decreased cancer-specific mortality (adjusted HR = 0.83, 95%CI: 0.74–0.92)[32]. Several studies have shown that the use of statins reduces the risk of gastric cancer[33-35].
Many epidemiologic and clinical studies have been performed on statins and colorectal cancer (CRC). However, the results have been inconsistent. One notable observational study from Israel showed that 5 or more years of statin use was linked with a 45% decrease in CRC risk (95%CI: 0.40–0.74)[36]. An another study of United States veterans also revealed a 35% decrease in CRC risk with statin use (95%CI: 0.55–0.78)[37]. On the other hand, several meta-analyses of case-control and cohort studies have revealed smaller risk decreases[38,39], or no relationship[40,41]. The unconvincing results from observational studies could be due to healthier behaviors in statin users compared with nonusers, the different durations of statin intake[39], different hydrophilicity of specific statins[42], or different effects of statins on colon or rectal cancers[43,44].
A nationwide population-based nested case-control study of patients with diabetes indicated a dose-dependent reduction of hepatocellular carcinoma (HCC) incidence with statin treatment[45]. In this study, statin users had a dose-dependent [cumulative defined daily dose (cDDD)] reduced risk of developing HCC [odds ratios (ORs) = 0.53, 0.36, 0.32, and 0.26 in ≤ 60, 60–180, 181–365, and > 365 cDDD, respectively; P < 0.0001]. The study also suggested that risk reduction was apparent in the presence of liver diseases such as chronic viral hepatitis, liver cirrhosis, alcoholic liver disease, and previous cancer (OR = 0.27, 95%CI: 0.14–0.50), but not significant in cases without liver disease (OR = 0.64, 95%CI: 0.32–1.29). Similar reports from Taiwan showed a dose-response relationship between statin use and the risk of HCV and HBV in an HCV cohort (HR = 0.66, 0.41, and 0.34 in 28–90, 91–365, and > 365 cDDD, respectively; P < 0.0001) and in an HBV cohort (HR = 0.66, 0.47, and 0.33 in 28–89, 90–180, and > 180 cDDD, respectively; P < 0.0001)[46,47]. In a cohort of 7248 HCV-infected patients in the United States ERCHIVES database, statin use was linked with a 44% decrease in the development of cirrhosis and a 49% decrease in incident HCC. Atorvastatin and fluvastatin were associated with more significant antifibrotic effects than other statins[48], and in 18080 patients with nonalcoholic fatty liver disease without cirrhosis, even higher HCC suppressive effects were suggested (HR = 0.29)[49]. Several reports have indicated that statins prevent liver fibrosis, and statins may delay the development of HCC by preventing fibrosis and inflammation of the liver[50]. A phase II trial to investigate the efficiency of a simvastatin vs placebo on the change in serum AFP-L3% from baseline to 6 mo following treatment initiation in cirrhotic patients with end-stage liver disease (NCT02968810) is currently underway. Atorvastatin is being investigated for tertiary prevention after curative resection or ablation for HCC (SHOT trial, NCT03024684).
A meta-analysis of 26 studies showed a considerable reduction in pancreatic cancer risk with statin use [relative risk (RR) = 0.84, 95%CI: 0.73–0.97; P < 0.001][51]. In subgroup analyses of the study, a non-significant relation was found between long-term statin use and the risk of pancreatic cancer (RR = 0.98, 95%CI: 0.86–1.11; P = 0.718). There was a non-significant relation between the use of lipophilic statins and the risk of pancreatic cancer (RR = 0.98, 95%CI: 0.84–1.15; P = 0.853). On the other hand, several studies revealed a reduced risk of pancreatic cancer among statin users[52-54], other reports showed no evidence of an association between statin use and pancreatic cancer[52,55]. A retrospective study of 2427 pancreatic cancer patients showed a 31% reduction in mortality in the group taking simvastatin and a 39% reduction in the group taking atorvastatin[56,57]. In another study of 1761 pancreatic cancer patients, the 5-year OS rate was 16.6% for statin users and 8.9% for nonusers (P = 0.012)[57]. Among 226 patients undergoing resection for pancreatic cancer, active use of moderate- to high-dose simvastatin was linked with favorable OS and disease-free survival[58].
Many retrospective cohort studies have identified a reduced risk of cancer mortality in patients taking statins to control cholesterol. However, prospective clinical studies have mostly not been successful (Table 1)[29,30,59-61]. Several causes might interpret these differences, including interpatient differences in the type of statins and the dose and duration of statin use. Besides, it is possible that not all cases benefit equally from statin treatment.
| Cancer type | Study type | Statin (dose) | Combination therapies | Outcome |
| Gastric cancer | Phase III | Simvastatin (40 mg/d) | Capecitabine andcisplatin | Simvastatin + capecitabine-cisplatin did not increase progression-free survival compared with capecitabine-cisplatin alone |
| Phase II | Pravastatin (40 mg/d) | Epirubicin, cisplatinand capecitabine | Pravastatin + standard chemotherapy was well tolerated, but did not improve progression-free survival at 6 months compared with chemotherapy alone | |
| Colorectal | Phase III | Simvastatin (40 mg/d) | FOLFIRI/XELIRI | Simvastatin + FOLFIRI/XELIRI did not increase progression-free survival compared with FOLFIRI/XELIRI alone |
| Hepatocellular | Phase III | Pravastatin (40 mg/d) | Sorafenib | Pravastatin + sorafenib did not improve overall or progression-free survival compared with sorafenib alone |
| Phase II | Pravastatin (40 mg/d) | Transcatheter arterialembolization followedby fluorouracil | Pravastatin + standard therapy prolonged overall survival compared with standard therapy alone | |
| Pancreatic | Phase II | Simvastatin (40 mg/d) | Gemcitabine | Simvastatin + gemcitabine was well tolerated, but did not decrease time to progression compared with gemcitabine alone |
There are seven types of statins (simvastatin, atorvastatin, fluvastatin, lovastatin, pitavastatin, rosuvastatin, and pravastatin) that can be prescribed for hypercholesterolemia worldwide (Table 2). However, which statins are most effective against cancer remains unclear. In many in vitro studies, lipophilic statins are more effective in anti-proliferation ability. Because lipophilic statins can cross biological membranes without requiring specific transporters, they have greater intracellular access and are thought to have more effective mechanisms than hydrophilic statins. One report examined differences in the effect of statins on pancreatic cancer using in vivo studies[62]. While simvastatin exerted the highest tumor suppressive effects in vitro, rosuvastatin and fluvastatin were the most potent compounds in an animal model. A retrospective cohort study examining the effects of different types of statins on advanced prostate cancer treated with androgen deprivation therapy found that atorvastatin, pravastatin, rosuvastatin, or pitavastatin showed a stronger effect on reduction in mortality compared with other statins[63]. It is necessary to determine the type of statin most effective against cancer to plan an optimal RCT.
| Statin | Solubility[3]
| Metabolism[3]
| Human dose to lower cholesterol (mg) | ||
| Low | Moderate | High | |||
| Simvastatin | Lipophilic | CYP3A4 | 10 | 20-40 | - |
| Atorvastatin | Lipophilic | CYP3A4/2C9 | - | 10-20 | 40-80 |
| Fluvastatin | Lipophilic | CYP2C9 | 20-40 | 80 | - |
| Pitavastatin | Lipophilic | Non-CYP450 | - | 1-4 | - |
| Lovastatin | Lipophilic | CYP3A4/2C9 | 20 | 40-80 | - |
| Rosuvastatin | Hydrophilic | Non-CYP450 | - | 5-10 | 20-40 |
| Pravastatin | Hydrophilic | Non-CYP450 | 10-20 | 40-80 | - |
Previous RCTs used simvastatin and pravastatin at 40 mg/d, which are moderate-intensity prescriptions (Table 1), and therefore higher doses or prescription of a higher-intensity statin might have provided enhanced responses in these studies. Drug combination strategies to reinforce the anti-cancer effect of statins should also be evaluated for future RCTs.
The members of the SREBP family of transcription factors control the upregulation of HMGCR and other lipid metabolism genes and are activated to restore homeostasis in response to cholesterol depletion (Figure 1). A subset of cell lines and primary cells from multiple myeloma patients were unable to provoke the expression of SREBP target genes by statin treatment and readily undergo apoptosis[64]. On the contrary, cell lines with potent statin-induced activation of SREBPs were resistive to statin treatment. In prostate cancer, this sterol-regulated feedback loop may modulate statin sensitivity, and a combination therapy of statins and SREBP inhibitors has a synergistic effect in prostate cancer[65]. Although it is theoretically convincing that feedback dysregulation of the MVA pathway is involved in statin sensitivity, further research is required to verify whether SREBPs can be clinically useful biomarkers.
HMGCR is directly inhibited by statins, and SREBPs increase HMGCR expression through a feedback mechanism that is induced when intracellular cholesterol is depleted (Figure 1). High HMGCR protein expression is associated with poor prognosis in various cancers[65-67]. The efficacy of statins for cancer is inversely linked with high expression of cholesterol biosynthesis genes, including the HMGCR gene[64,68]. However, other reports suggested that HMGCR expression alone could not accurately predict the effect of statins[65,69]. Whether HMGCR expression alone can accurately predict statin susceptibility remains unclear. One possible inter
A population-based case-control study of incident CRC in northern Israel showed that specific polymorphisms in the HMGCR gene modify the protective association between statins and CRC risk. Compared with non-statin users, the unadjusted OR of CRC among statin users with the A/A genotype of rs12654264 in HMGCR was 0.3 (95%CI: 0.18–0.51) and 0.66 among statin users with the T/T genotype (95%CI: 0.41–1.06; P = 0.0012)[71].
Several studies have demonstrated that tumor cells with higher vimentin expression (mesenchymal cell marker) and lower E-cadherin expression (epithelial cell marker) are highly sensitive to statin treatment[72-74]. Total vimentin and E-cadherin expression are not appropriate markers for the sensitivity of statins, but abundant cytosolic vimentin and absent cell surface E-cadherin expression indicate sensitivity to statins[73]. HRAS-induced epithelial-to-mesenchymal transition (EMT) through activation of zinc finger E-box binding homeobox 1 sensitized tumor cells to the antiproliferative activity of statins[74]. These studies also showed that statins preferentially kill cells induced to undergo EMT, suggesting that statins may be more effective against metastatic disease and prevent metastasis.
Wild-type p53 represses the MVA pathway[12], while loss of TP53 and two gain-of-function TP53 mutants have been reported to enhance the expression of MVA pathway genes[11,75]. Tumors with loss of TP53 or the two gain-of-function mutations are particularly vulnerable to statin treatment[76-78].
The FPP and geranylgeranyl pyrophosphate produced by the MVA pathway serve as substrates for the post-translational prenylation of RAS. Therefore, RAS mutations have been hypothesized to be potential biomarkers of statin sensitivity. However, pre-clinical studies have shown that RAS mutation alone cannot predict statin susceptibility[74,79]. In a subgroup analysis of retrospective studies of CRC, statins were shown to have a higher prognostic effect in cancers with KRAS mutations[80]. However, other studies have reported no association between statin effects on CRC and KRAS status[39,81]. Further studies are needed to evaluate the utility of KRAS mutation status to predict the effect of statins on cancer.
In breast cancer, the effect of statins has been linked with ER status, in which ER-negative breast cancer cells are notably sensitive to statin treatment[67]. These pre-clinical findings are further strengthened by clinical data demonstrating greater tumor cell apoptosis after fluvastatin treatment in women with ER-negative breast cancer[82].
Several clinical trials have examined the introduction of simvastatin to epidermal growth factor receptor (EGFR) inhibitors therapy for KRAS-mutated CRC patients. The hypothesis behind these clinical trials is that the statin-induced depletion of MVA will inhibit KRAS prenylation, which will inhibit membrane localization and enhance the effectiveness of EGFR inhibitors[83,84]. Unfortunately, most trials have failed to show significant survival benefits from statins[85-87]. These results may suggest that KRAS mutation status is not a predictive biomarker of response to statin treatment. Another clinical trial showed that the addition of simvastatin to a cetuximab/irinotecan regimen overcame cetuximab resistance[88]. In this clinical trial, the therapeutic benefit of statin was only detectable in patients bearing tumors with mutant KRAS and a low Ras signature[88]. The Ras signature score is derived from the expression of Ras pathway-related genes across multiple databases and reflects other possible aberrations such as BRAF and PI3KCA mutations. Hence, factors other than KRAS mutation must be considered to predict the efficiency of statins in overcoming resistance to anti-EGFR therapy.
Statins may have synergistic effects with radiation therapy (RT) on cancer and may reduce inflammation and the gut and skin toxicities induced by RT. In retrospective cohort studies, patients taking statins during RT or chemo-RT for rectal, bladder, or prostate cancer treatment showed considerably higher rates of pathological complete response, local control and progression-free survival[89-93]. However, no study has shown an apparent benefit[94]. Furthermore, statins significantly reduced RT-induced bowel toxicity and skin injury[95-97]. However, a single-arm phase II trial of 53 prostate cancer patients taking lovastatin showed no reduced incidence of grade 2 or higher rectal toxicity compared with historical controls[98]. A RCT of simvastatin combined with standard chemotherapy and radiation in preoperative treatment for rectal cancer is underway.
Mevalonic acid metabolism is involved in controlling T cell activation[19,20,99,100]. Statins inhibit the geranylgeranylation of small GTPases, resulting in arrested endosomal maturation, prolonged antigen retention, enhanced antigen presentation, and T cell activation. It has been reported in multiple mouse cancer models that MVA pathway inhibitors are vigorous for cancer vaccinations and synergize with anti-PD-1 antibodies[101]. The tumor microenvironment is enriched with cholesterol. The high cholesterol in the tumor microenvironment induces CD8+ T cell exhaustion and upregulates the immune checkpoints PD-1, 2B4, TIM-3, and LAG-3[102]. Furthermore, lowering cholesterol levels in the tumor microenvironment by simvastatin restores the antitumor activity of CD8+ T cells. Many preclinical studies have demonstrated that the MVA pathway is involved in immune regulation. Future research into the immunomodulatory properties of statins has important clinical implications for cancer immunotherapy.
Clinical data that evaluated the utility of statins as anticancer agents have shown responses in some but not all cancers. Optimizing the type, dose, and duration of statins, as well as detecting biomarkers to recognize responders and developing combination therapies, will heighten the value of statins in cancer treatment.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Meng FZ S-Editor: Liu M L-Editor: A P-Editor: Liu M
| 1. | Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3933] [Cited by in RCA: 3999] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 2. | Sinensky M. Recent advances in the study of prenylated proteins. Biochim Biophys Acta. 2000;1484:93-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 155] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 3. | Duncan RE, El-Sohemy A, Archer MC. Mevalonate promotes the growth of tumors derived from human cancer cells in vivo and stimulates proliferation in vitro with enhanced cyclin-dependent kinase-2 activity. J Biol Chem. 2004;279:33079-33084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Mullen PJ, Yu R, Longo J, Archer MC, Penn LZ. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer. 2016;16:718-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 486] [Article Influence: 54.0] [Reference Citation Analysis (2)] |
| 5. | Carrer A, Trefely S, Zhao S, Campbell SL, Norgard RJ, Schultz KC, Sidoli S, Parris JLD, Affronti HC, Sivanand S, Egolf S, Sela Y, Trizzino M, Gardini A, Garcia BA, Snyder NW, Stanger BZ, Wellen KE. Acetyl-CoA Metabolism Supports Multistep Pancreatic Tumorigenesis. Cancer Discov. 2019;9:416-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 6. | Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 770] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 7. | Abdullah MI, de Wolf E, Jawad MJ, Richardson A. The poor design of clinical trials of statins in oncology may explain their failure - Lessons for drug repurposing. Cancer Treat Rev. 2018;69:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Yamauchi Y, Furukawa K, Hamamura K. Positive feedback loop between PI3K-Akt-mTORC1 signaling and the lipogenic pathway boosts Akt signaling: induction of the lipogenic pathway by a melanoma antigen. Cancer Res. 2011;71:4989-4997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Yi J, Zhu J, Wu J, Thompson CB, Jiang X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc Natl Acad Sci U S A. 2020;117:31189-31197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 643] [Article Influence: 128.6] [Reference Citation Analysis (0)] |
| 10. | Kusama T, Mukai M, Iwasaki T, Tatsuta M, Matsumoto Y, Akedo H, Inoue M, Nakamura H. 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors reduce human pancreatic cancer cell invasion and metastasis. Gastroenterology. 2002;122:308-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Freed-Pastor WA, Mizuno H, Zhao X, Langerød A, Moon SH, Rodriguez-Barrueco R, Barsotti A, Chicas A, Li W, Polotskaia A, Bissell MJ, Osborne TF, Tian B, Lowe SW, Silva JM, Børresen-Dale AL, Levine AJ, Bargonetti J, Prives C. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell. 2012;148:244-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 700] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 12. | Assaily W, Rubinger DA, Wheaton K, Lin Y, Ma W, Xuan W, Brown-Endres L, Tsuchihara K, Mak TW, Benchimol S. ROS-mediated p53 induction of Lpin1 regulates fatty acid oxidation in response to nutritional stress. Mol Cell. 2011;44:491-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Shamma A, Takegami Y, Miki T, Kitajima S, Noda M, Obara T, Okamoto T, Takahashi C. Rb Regulates DNA damage response and cellular senescence through E2F-dependent suppression of N-ras isoprenylation. Cancer Cell. 2009;15:255-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio R, Piazza S, Rosato A, Piccolo S, Del Sal G. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 613] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 15. | Higashi T, Hayashi H, Kitano Y, Yamamura K, Kaida T, Arima K, Taki K, Nakagawa S, Okabe H, Nitta H, Imai K, Hashimoto D, Chikamoto A, Beppu T, Baba H. Statin attenuates cell proliferative ability via TAZ (WWTR1) in hepatocellular carcinoma. Med Oncol. 2016;33:123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Corcoran RB, Scott MP. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci U S A. 2006;103:8408-8413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 250] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 17. | Nguyen VT, Barozzi I, Faronato M, Lombardo Y, Steel JH, Patel N, Darbre P, Castellano L, Győrffy B, Woodley L, Meira A, Patten DK, Vircillo V, Periyasamy M, Ali S, Frige G, Minucci S, Coombes RC, Magnani L. Differential epigenetic reprogramming in response to specific endocrine therapies promotes cholesterol biosynthesis and cellular invasion. Nat Commun. 2015;6:10044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Huang WC, Li X, Liu J, Lin J, Chung LW. Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol Cancer Res. 2012;10:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 19. | Thurnher M, Gruenbacher G. T lymphocyte regulation by mevalonate metabolism. Sci Signal. 2015;8:re4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 1018] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 21. | Zhou C, Zhong X, Gao P, Wu Z, Shi J, Guo Z, Wang Z, Song Y. Statin use and its potential therapeutic role in esophageal cancer: a systematic review and meta-analysis. Cancer Manag Res. 2019;11:5655-5663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Nguyen T, Khan A, Liu Y, El-Serag HB, Thrift AP. The Association Between Statin Use After Diagnosis and Mortality Risk in Patients With Esophageal Cancer: A Retrospective Cohort Study of United States Veterans. Am J Gastroenterol. 2018;113:1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Nimako GK, Wintrob ZA, Sulik DA, Donato JL, Ceacareanu AC. Synergistic Benefit of Statin and Metformin in Gastrointestinal Malignancies. J Pharm Pract. 2017;30:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Cardwell CR, Spence AD, Hughes CM, Murray LJ. Statin use after esophageal cancer diagnosis and survival: A population based cohort study. Cancer Epidemiol. 2017;48:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Alexandre L, Clark AB, Bhutta HY, Chan SS, Lewis MP, Hart AR. Association Between Statin Use After Diagnosis of Esophageal Cancer and Survival: A Population-Based Cohort Study. Gastroenterology. 2016;150:854-865.e1; quiz e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Lacroix O, Couttenier A, Vaes E, Cardwell CR, De Schutter H, Robert A. Statin use after diagnosis is associated with an increased survival in esophageal cancer patients: a Belgian population-based study. Cancer Causes Control. 2019;30:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Deng HY, Lan X, Zheng X, Zha P, Zhou J, Wang RL, Jiang R, Qiu XM. The association between statin use and survival of esophageal cancer patients: A systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e16480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Thomas T, Loke Y, Beales ILP. Systematic Review and Meta-analysis: Use of Statins Is Associated with a Reduced Incidence of Oesophageal Adenocarcinoma. J Gastrointest Cancer. 2018;49:442-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Kim ST, Kang JH, Lee J, Park SH, Park JO, Park YS, Lim HY, Hwang IG, Lee SC, Park KW, Lee HR, Kang WK. Simvastatin plus capecitabine-cisplatin vs placebo plus capecitabine-cisplatin in patients with previously untreated advanced gastric cancer: a double-blind randomised phase 3 study. Eur J Cancer. 2014;50:2822-2830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Konings IR, van der Gaast A, van der Wijk LJ, de Jongh FE, Eskens FA, Sleijfer S. The addition of pravastatin to chemotherapy in advanced gastric carcinoma: a randomised phase II trial. Eur J Cancer. 2010;46:3200-3204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Nam DH, Lee H, Park JC, Shin SK, Lee SK, Hyung WJ, Lee YC, Kang MW, Noh SH. Long-term statin therapy improves oncological outcome after radical gastrectomy for stage II and III gastric cancer. Anticancer Res. 2014;34:355-361. [PubMed] |
| 32. | Spence AD, Busby J, Hughes CM, Johnston BT, Coleman HG, Cardwell CR. Statin use and survival in patients with gastric cancer in two independent population-based cohorts. Pharmacoepidemiol Drug Saf. 2019;28:460-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Singh PP, Singh S. Statins are associated with reduced risk of gastric cancer: a systematic review and meta-analysis. Ann Oncol. 2013;24:1721-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 34. | Wu XD, Zeng K, Xue FQ, Chen JH, Chen YQ. Statins are associated with reduced risk of gastric cancer: a meta-analysis. Eur J Clin Pharmacol. 2013;69:1855-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 35. | Cheung KS, Chan EW, Wong AYS, Chen L, Seto WK, Wong ICK, Leung WK. Statins Were Associated with a Reduced Gastric Cancer Risk in Patients with Eradicated Helicobacter Pylori Infection: A Territory-Wide Propensity Score Matched Study. Cancer Epidemiol Biomarkers Prev. 2020;29:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | Poynter JN, Gruber SB, Higgins PD, Almog R, Bonner JD, Rennert HS, Low M, Greenson JK, Rennert G. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 595] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 37. | Farwell WR, Scranton RE, Lawler EV, Lew RA, Brophy MT, Fiore LD, Gaziano JM. The association between statins and cancer incidence in a veterans population. J Natl Cancer Inst. 2008;100:134-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 38. | Taylor ML, Wells BJ, Smolak MJ. Statins and cancer: a meta-analysis of case-control studies. Eur J Cancer Prev. 2008;17:259-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Li Y, He X, Ding Y, Chen H, Sun L. Statin uses and mortality in colorectal cancer patients: An updated systematic review and meta-analysis. Cancer Med. 2019;8:3305-3313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 40. | Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol. 2004;22:2388-2394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 416] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 41. | Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA. 2006;295:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 449] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 42. | Liu Y, Tang W, Wang J, Xie L, Li T, He Y, Deng Y, Peng Q, Li S, Qin X. Association between statin use and colorectal cancer risk: a meta-analysis of 42 studies. Cancer Causes Control. 2014;25:237-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 43. | Lee JE, Baba Y, Ng K, Giovannucci E, Fuchs CS, Ogino S, Chan AT. Statin use and colorectal cancer risk according to molecular subtypes in two large prospective cohort studies. Cancer Prev Res (Phila). 2011;4:1808-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 44. | Ibáñez-Sanz G, Guinó E, Pontes C, Quijada-Manuitt MÁ, de la Peña-Negro LC, Aragón M, Domínguez M, Rodríguez-Alonso L, Blasco A, García-Rodríguez A, Morros R, Moreno V. Statin use and the risk of colorectal cancer in a population-based electronic health records study. Sci Rep. 2019;9:13560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Kim G, Jang SY, Han E, Lee YH, Park SY, Nam CM, Kang ES. Effect of statin on hepatocellular carcinoma in patients with type 2 diabetes: A nationwide nested case-control study. Int J Cancer. 2017;140:798-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 46. | Tsan YT, Lee CH, Ho WC, Lin MH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis C virus infection. J Clin Oncol. 2013;31:1514-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 47. | Tsan YT, Lee CH, Wang JD, Chen PC. Statins and the risk of hepatocellular carcinoma in patients with hepatitis B virus infection. J Clin Oncol. 2012;30:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 217] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 48. | Simon TG, Bonilla H, Yan P, Chung RT, Butt AA. Atorvastatin and fluvastatin are associated with dose-dependent reductions in cirrhosis and hepatocellular carcinoma, among patients with hepatitis C virus: Results from ERCHIVES. Hepatology. 2016;64:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 49. | Simon TG, King LY, Zheng H, Chung RT. Statin use is associated with a reduced risk of fibrosis progression in chronic hepatitis C. J Hepatol. 2015;62:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 50. | Chang FM, Wang YP, Lang HC, Tsai CF, Hou MC, Lee FY, Lu CL. Statins decrease the risk of decompensation in hepatitis B virus- and hepatitis C virus-related cirrhosis: A population-based study. Hepatology. 2017;66:896-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 51. | Zhang Y, Liang M, Sun C, Qu G, Shi T, Min M, Wu Y, Sun Y. Statin Use and Risk of Pancreatic Cancer: An Updated Meta-analysis of 26 Studies. Pancreas. 2019;48:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 52. | Bonovas S, Filioussi K, Sitaras NM. Statins are not associated with a reduced risk of pancreatic cancer at the population level, when taken at low doses for managing hypercholesterolemia: evidence from a meta-analysis of 12 studies. Am J Gastroenterol. 2008;103:2646-2651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 53. | Walker EJ, Ko AH, Holly EA, Bracci PM. Statin use and risk of pancreatic cancer: results from a large, clinic-based case-control study. Cancer. 2015;121:1287-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 54. | Archibugi L, Arcidiacono PG, Capurso G. Statin use is associated to a reduced risk of pancreatic cancer: A meta-analysis. Dig Liver Dis. 2019;51:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 55. | Kirkegård J, Lund JL, Mortensen FV, Cronin-Fenton D. Statins and pancreatic cancer risk in patients with chronic pancreatitis: A Danish nationwide population-based cohort study. Int J Cancer. 2020;146:610-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Huang BZ, Chang JI, Li E, Xiang AH, Wu BU. Influence of Statins and Cholesterol on Mortality Among Patients With Pancreatic Cancer. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 57. | Lee HS, Lee SH, Lee HJ, Chung MJ, Park JY, Park SW, Song SY, Bang S. Statin Use and Its Impact on Survival in Pancreatic Cancer Patients. Medicine (Baltimore). 2016;95:e3607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 58. | Wu BU, Chang J, Jeon CY, Pandol SJ, Huang B, Ngor EW, Difronzo AL, Cooper RM. Impact of statin use on survival in patients undergoing resection for early-stage pancreatic cancer. Am J Gastroenterol. 2015;110:1233-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Jouve JL, Lecomte T, Bouché O, Barbier E, Khemissa Akouz F, Riachi G, Nguyen Khac E, Ollivier-Hourmand I, Debette-Gratien M, Faroux R, Villing AL, Vergniol J, Ramee JF, Bronowicki JP, Seitz JF, Legoux JL, Denis J, Manfredi S, Phelip JM; PRODIGE-11 investigators/collaborators. Pravastatin combination with sorafenib does not improve survival in advanced hepatocellular carcinoma. J Hepatol. 2019;71:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 60. | Kawata S, Yamasaki E, Nagase T, Inui Y, Ito N, Matsuda Y, Inada M, Tamura S, Noda S, Imai Y, Matsuzawa Y. Effect of pravastatin on survival in patients with advanced hepatocellular carcinoma. A randomized controlled trial. Br J Cancer. 2001;84:886-891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 264] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 61. | Hong JY, Nam EM, Lee J, Park JO, Lee SC, Song SY, Choi SH, Heo JS, Park SH, Lim HY, Kang WK, Park YS. Randomized double-blinded, placebo-controlled phase II trial of simvastatin and gemcitabine in advanced pancreatic cancer patients. Cancer Chemother Pharmacol. 2014;73:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 62. | Gbelcová H, Lenícek M, Zelenka J, Knejzlík Z, Dvoráková G, Zadinová M, Poucková P, Kudla M, Balaz P, Ruml T, Vítek L. Differences in antitumor effects of various statins on human pancreatic cancer. Int J Cancer. 2008;122:1214-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 63. | Wu SY, Fang SC, Shih HJ, Wen YC, Shao YJ. Mortality associated with statins in men with advanced prostate cancer treated with androgen deprivation therapy. Eur J Cancer. 2019;112:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 64. | Clendening JW, Pandyra A, Li Z, Boutros PC, Martirosyan A, Lehner R, Jurisica I, Trudel S, Penn LZ. Exploiting the mevalonate pathway to distinguish statin-sensitive multiple myeloma. Blood. 2010;115:4787-4797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 65. | Longo J, Mullen PJ, Yu R, van Leeuwen JE, Masoomian M, Woon DTS, Wang Y, Chen EX, Hamilton RJ, Sweet JM, van der Kwast TH, Fleshner NE, Penn LZ. An actionable sterol-regulated feedback loop modulates statin sensitivity in prostate cancer. Mol Metab. 2019;25:119-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 66. | Chang WC, Cheng WC, Cheng BH, Chen L, Ju LJ, Ou YJ, Jeng LB, Yang MD, Hung YC, Ma WL. Mitochondrial Acetyl-CoA Synthetase 3 is Biosignature of Gastric Cancer Progression. Cancer Med. 2018;7:1240-1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 67. | Goard CA, Chan-Seng-Yue M, Mullen PJ, Quiroga AD, Wasylishen AR, Clendening JW, Sendorek DH, Haider S, Lehner R, Boutros PC, Penn LZ. Identifying molecular features that distinguish fluvastatin-sensitive breast tumor cells. Breast Cancer Res Treat. 2014;143:301-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 68. | Kimbung S, Lettiero B, Feldt M, Bosch A, Borgquist S. High expression of cholesterol biosynthesis genes is associated with resistance to statin treatment and inferior survival in breast cancer. Oncotarget. 2016;7:59640-59651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 69. | Bjarnadottir O, Romero Q, Bendahl PO, Jirström K, Rydén L, Loman N, Uhlén M, Johannesson H, Rose C, Grabau D, Borgquist S. Targeting HMG-CoA reductase with statins in a window-of-opportunity breast cancer trial. Breast Cancer Res Treat. 2013;138:499-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 70. | Clendening JW, Pandyra A, Boutros PC, El Ghamrasni S, Khosravi F, Trentin GA, Martirosyan A, Hakem A, Hakem R, Jurisica I, Penn LZ. Dysregulation of the mevalonate pathway promotes transformation. Proc Natl Acad Sci U S A. 2010;107:15051-15056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 310] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 71. | Lipkin SM, Chao EC, Moreno V, Rozek LS, Rennert H, Pinchev M, Dizon D, Rennert G, Kopelovich L, Gruber SB. Genetic variation in 3-hydroxy-3-methylglutaryl CoA reductase modifies the chemopreventive activity of statins for colorectal cancer. Cancer Prev Res (Phila). 2010;3:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 72. | Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada K, Aguirre AJ, Viswanathan SR, Chattopadhyay S, Tamayo P, Yang WS, Rees MG, Chen S, Boskovic ZV, Javaid S, Huang C, Wu X, Tseng YY, Roider EM, Gao D, Cleary JM, Wolpin BM, Mesirov JP, Haber DA, Engelman JA, Boehm JS, Kotz JD, Hon CS, Chen Y, Hahn WC, Levesque MP, Doench JG, Berens ME, Shamji AF, Clemons PA, Stockwell BR, Schreiber SL. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 1370] [Article Influence: 171.3] [Reference Citation Analysis (0)] |
| 73. | Warita K, Warita T, Beckwitt CH, Schurdak ME, Vazquez A, Wells A, Oltvai ZN. Statin-induced mevalonate pathway inhibition attenuates the growth of mesenchymal-like cancer cells that lack functional E-cadherin mediated cell cohesion. Sci Rep. 2014;4:7593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 74. | Yu R, Longo J, van Leeuwen JE, Mullen PJ, Ba-Alawi W, Haibe-Kains B, Penn LZ. Statin-Induced Cancer Cell Death Can Be Mechanistically Uncoupled from Prenylation of RAS Family Proteins. Cancer Res. 2018;78:1347-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Moon SH, Huang CH, Houlihan SL, Regunath K, Freed-Pastor WA, Morris JP 4th, Tschaharganeh DF, Kastenhuber ER, Barsotti AM, Culp-Hill R, Xue W, Ho YJ, Baslan T, Li X, Mayle A, de Stanchina E, Zender L, Tong DR, D'Alessandro A, Lowe SW, Prives C. p53 Represses the Mevalonate Pathway to Mediate Tumor Suppression. Cell. 2019;176:564-580.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 322] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 76. | Turrell FK, Kerr EM, Gao M, Thorpe H, Doherty GJ, Cridge J, Shorthouse D, Speed A, Samarajiwa S, Hall BA, Griffiths M, Martins CP. Lung tumors with distinct p53 mutations respond similarly to p53 targeted therapy but exhibit genotype-specific statin sensitivity. Genes Dev. 2017;31:1339-1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 77. | Parrales A, Ranjan A, Iyer SV, Padhye S, Weir SJ, Roy A, Iwakuma T. DNAJA1 controls the fate of misfolded mutant p53 through the mevalonate pathway. Nat Cell Biol. 2016;18:1233-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 78. | Tutuska K, Parrilla-Monge L, Di Cesare E, Nemajerova A, Moll UM. Statin as anti-cancer therapy in autochthonous T-lymphomas expressing stabilized gain-of-function mutant p53 proteins. Cell Death Dis. 2020;11:274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 79. | Wong WW, Clendening JW, Martirosyan A, Boutros PC, Bros C, Khosravi F, Jurisica I, Stewart AK, Bergsagel PL, Penn LZ. Determinants of sensitivity to lovastatin-induced apoptosis in multiple myeloma. Mol Cancer Ther. 2007;6:1886-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Ling Y, Yang L, Huang H, Hu X, Zhao C, Ying Y. Prognostic Significance of Statin Use in Colorectal Cancer: A Systematic Review and Meta-Analysis. Medicine (Baltimore). 2015;94:e908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Voorneveld PW, Reimers MS, Bastiaannet E, Jacobs RJ, van Eijk R, Zanders MMJ, Herings RMC, van Herk-Sukel MPP, Kodach LL, van Wezel T, Kuppen PJK, Morreau H, van de Velde CJH, Hardwick JCH, Liefers GJ. Statin Use After Diagnosis of Colon Cancer and Patient Survival. Gastroenterology. 2017;153:470-479.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 82. | Garwood ER, Kumar AS, Baehner FL, Moore DH, Au A, Hylton N, Flowers CI, Garber J, Lesnikoski BA, Hwang ES, Olopade O, Port ER, Campbell M, Esserman LJ. Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Res Treat. 2010;119:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 83. | Shimoyama S. Statins are logical candidates for overcoming limitations of targeting therapies on malignancy: their potential application to gastrointestinal cancers. Cancer Chemother Pharmacol. 2011;67:729-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 84. | Krens LL, Baas JM, Gelderblom H, Guchelaar HJ. Therapeutic modulation of k-ras signaling in colorectal cancer. Drug Discov Today. 2010;15:502-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 85. | Baas JM, Krens LL, ten Tije AJ, Erdkamp F, van Wezel T, Morreau H, Gelderblom H, Guchelaar HJ. Safety and efficacy of the addition of simvastatin to cetuximab in previously treated KRAS mutant metastatic colorectal cancer patients. Invest New Drugs. 2015;33:1242-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 86. | Baas JM, Krens LL, Bos MM, Portielje JE, Batman E, van Wezel T, Morreau H, Guchelaar HJ, Gelderblom H. Safety and efficacy of the addition of simvastatin to panitumumab in previously treated KRAS mutant metastatic colorectal cancer patients. Anticancer Drugs. 2015;26:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Krens LL, Simkens LH, Baas JM, Koomen ER, Gelderblom H, Punt CJ, Guchelaar HJ. Statin use is not associated with improved progression free survival in cetuximab treated KRAS mutant metastatic colorectal cancer patients: results from the CAIRO2 study. PLoS One. 2014;9:e112201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 88. | Lee J, Hong YS, Hong JY, Han SW, Kim TW, Kang HJ, Kim TY, Kim KP, Kim SH, Do IG, Kim KM, Sohn I, Park SH, Park JO, Lim HY, Cho YB, Lee WY, Yun SH, Kim HC, Park YS, Kang WK. Effect of simvastatin plus cetuximab/irinotecan for KRAS mutant colorectal cancer and predictive value of the RAS signature for treatment response to cetuximab. Invest New Drugs. 2014;32:535-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 89. | Tsai HK, Katz MS, Coen JJ, Zietman AL, Kaufman DS, Shipley WU. Association of statin use with improved local control in patients treated with selective bladder preservation for muscle-invasive bladder cancer. Urology. 2006;68:1188-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 90. | Mace AG, Gantt GA, Skacel M, Pai R, Hammel JP, Kalady MF. Statin therapy is associated with improved pathologic response to neoadjuvant chemoradiation in rectal cancer. Dis Colon Rectum. 2013;56:1217-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 91. | Armstrong D, Raissouni S, Price Hiller J, Mercer J, Powell E, MacLean A, Jiang M, Doll C, Goodwin R, Batuyong E, Zhou K, Monzon JG, Tang PA, Heng DY, Cheung WY, Vickers MM. Predictors of Pathologic Complete Response After Neoadjuvant Treatment for Rectal Cancer: A Multicenter Study. Clin Colorectal Cancer. 2015;14:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 92. | Katz MS, Minsky BD, Saltz LB, Riedel E, Chessin DB, Guillem JG. Association of statin use with a pathologic complete response to neoadjuvant chemoradiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62:1363-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 93. | Kollmeier MA, Katz MS, Mak K, Yamada Y, Feder DJ, Zhang Z, Jia X, Shi W, Zelefsky MJ. Improved biochemical outcomes with statin use in patients with high-risk localized prostate cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 94. | Hardie C, Jung Y, Jameson M. Effect of statin and aspirin use on toxicity and pathological complete response rate of neo-adjuvant chemoradiation for rectal cancer. Asia Pac J Clin Oncol. 2016;12:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 95. | Nübel T, Damrot J, Roos WP, Kaina B, Fritz G. Lovastatin protects human endothelial cells from killing by ionizing radiation without impairing induction and repair of DNA double-strand breaks. Clin Cancer Res. 2006;12:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 96. | Holler V, Buard V, Gaugler MH, Guipaud O, Baudelin C, Sache A, Perez Mdel R, Squiban C, Tamarat R, Milliat F, Benderitter M. Pravastatin limits radiation-induced vascular dysfunction in the skin. J Invest Dermatol. 2009;129:1280-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 97. | Teo MT, Sebag-Montefiore D, Donnellan CF. Prevention and Management of Radiation-induced Late Gastrointestinal Toxicity. Clin Oncol (R Coll Radiol). 2015;27:656-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 98. | Jameson MB, Gormly K, Espinoza D, Hague W, Asghari G, Jeffery GM, Price TJ, Karapetis CS, Arendse M, Armstrong J, Childs J, Frizelle FA, Ngan S, Stevenson A, Oostendorp M, Ackland SP. SPAR - a randomised, placebo-controlled phase II trial of simvastatin in addition to standard chemotherapy and radiation in preoperative treatment for rectal cancer: an AGITG clinical trial. BMC Cancer. 2019;19:1229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 99. | Wang R, Green DR. Metabolic checkpoints in activated T cells. Nat Immunol. 2012;13:907-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 376] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 100. | Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 962] [Cited by in RCA: 1231] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 101. | Xia Y, Xie Y, Yu Z, Xiao H, Jiang G, Zhou X, Yang Y, Li X, Zhao M, Li L, Zheng M, Han S, Zong Z, Meng X, Deng H, Ye H, Fa Y, Wu H, Oldfield E, Hu X, Liu W, Shi Y, Zhang Y. The Mevalonate Pathway Is a Druggable Target for Vaccine Adjuvant Discovery. Cell. 2018;175:1059-1073.e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 170] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 102. | Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, Wang Q, Yang M, Kalady MF, Qian J, Zhang A, Gupte AA, Hamilton DJ, Zheng C, Yi Q. Cholesterol Induces CD8+ T Cell Exhaustion in the Tumor Microenvironment. Cell Metab. 2019;30:143-156.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 654] [Article Influence: 109.0] [Reference Citation Analysis (0)] |