Published online Sep 15, 2021. doi: 10.4251/wjgo.v13.i9.1109
Peer-review started: February 22, 2021
First decision: April 19, 2021
Revised: April 29, 2021
Accepted: July 23, 2021
Article in press: July 23, 2021
Published online: September 15, 2021
Processing time: 199 Days and 23.6 Hours
Hepatopancreatobiliary tumors are challenging to treat, and the advanced or metastatic forms have a very low 5-year survival rate. Several drug combinations have been tested, and new therapeutic approaches have been introduced in the last decades, including radiofrequency and heat based methods. Hyperthermia is the artificial heating of tumors by various biophysical methods that may possess immunostimulant, tumoricidal, and chemoradiotherapy sensitizer effects. Both whole-body and regional hyperthermia studies have been conducted since the 1980s after the introduction of deep-seated tumor hyperthermia techniques. Results of the effects of hyperthermia in hepatocellular and pancreatic cancer are known from several studies. Hyperthermia in biliary cancers is a less investigated area. High local and overall responses to treatment, increased progression-free and overall survival, and improved laboratory and quality-of-life results are associated with hyperthermia in all three tumor types. With the evolution of chemotherapeutic agents and the introduction of newer techniques, the combination of adjuvant hyperthermia with those therapies is advantageous and has not been associated with an increase in alarming adverse effects. However, despite the many positive effects of hyperthermia, its use is still only known at the experimental level, and its concomitant utilization in routine cancer treatment is not certain because of the lack of thorough clinical studies.
Core Tip: Adjuvant hyperthermia is beneficial in hepatopancreatobiliary cancers because of its direct and indirect antitumor effects. Increased treatment response, prolonged survival, and improved laboratory and quality of life data have been observed in several randomized and observational clinical trials of various hyperthermia methods. The use of hyperthermia in cancer care is not yet routine. In this review, the clinical data on hyperthermia in hepatopancreatobiliary tumors is summarized, focusing mainly on results that have the most clinical and research interest.
- Citation: Herold Z, Szasz AM, Dank M. Evidence based tools to improve efficiency of currently administered oncotherapies for tumors of the hepatopancreatobiliary system. World J Gastrointest Oncol 2021; 13(9): 1109-1120
- URL: https://www.wjgnet.com/1948-5204/full/v13/i9/1109.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i9.1109
Hepatopancreatobiliary cancers are fatal diseases that can be characterized by very low 5-year survival rates[1-3]. In 2018, over 1.5 million new cases and approximately 1.4 million deaths from hepatocellular (HCC), biliary (BC) including gallbladder and cholangiocellular (CCC), and pancreatic cancers (PC) were reported[4]. Diagnosis at a more advanced stage is characteristic to all four tumor types[1-3], which is usually accompanied by other comorbidities like a cirrhotic state of the liver, which further worsens patient life expectancy[1]. In the last decades, several new techniques and possible multimodal therapies have emerged that support conventional surgical resection and facilitate chemoradiotherapy, including but not limited to various thermal ablative methods[5] including radiofrequency[6] and microwave ablation[7], laser-induced thermotherapy[8], hyperthermic intraperitoneal chemotherapy[9], percutaneous ethanol injection[10], transcatheter arterial chemoembolization[11], high-intensity focused ultrasound[12], and various types of hyperthermia[13].
The available literature on the clinical applications of hyperthermia in hepatopancreatobiliary cancers is discussed in this review, focusing on survival and safety data that might be an interest to the practicing oncologists. The presentation of in-depth technical details of hyperthermia and its effects on cells and biochemical processes is not the aim of the current review, focused publications on those subjects are available[13-18].
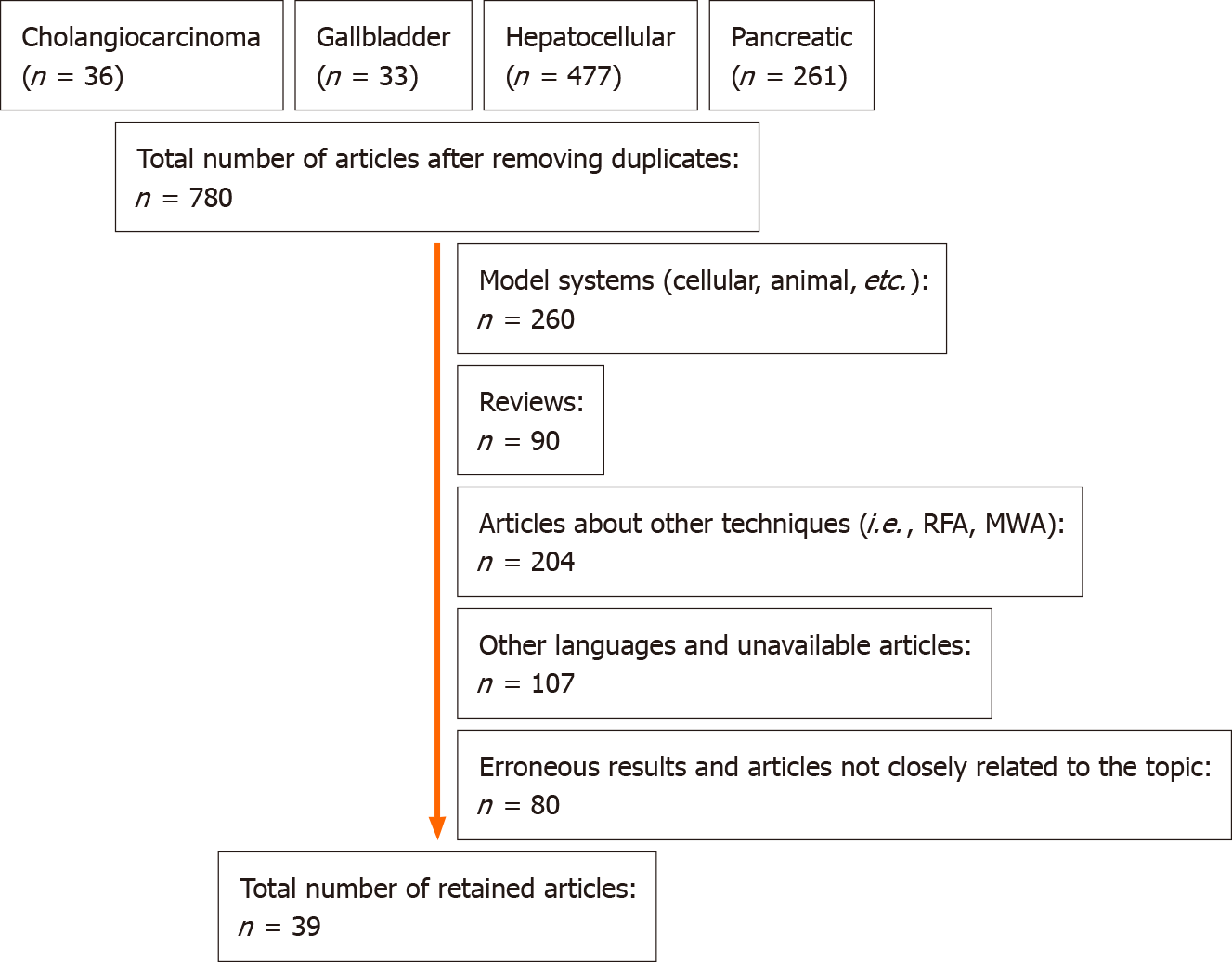
A literature search was conducted in PubMed/MEDLINE using the strings “cholangiocarcinoma hyperthermia”, “gallbladder cancer hyperthermia”, “hepatocellular hyperthermia”, and “pancreatic cancer hyperthermia”, for articles published from January 1, 1964 to January 31, 2021. After removing duplicates, a total of 780 potential articles were found, from which 39 full-text articles were selected (Figure 1). A secondary search in ClinicalTrials.gov was conducted, and an additional three finished and four currently running clinical trials were identified. Five additional studies were included from another search in meta-analysis and review citations, raising the total number of 47 publications included in this review (Table 1).
| Ref. | Study type | Type of hyperthermia | Tumor type |
| Falk et al[22], 19861 | nRCT | Regional | PC |
| Kim et al[37], 1989 | pOS | Regional | HCC |
| Kakehi et al[23], 19901 | RCT | Regional | Mixed, incl. HCC and PC |
| Nagata et al[63], 1990 | pOS | Regional | Mixed, incl. HCC and CCC |
| Akuta et al[66], 1991 | pOS | Regional | Mixed, incl. HCC |
| Hamazoe et al[51], 1991 | pOS | Regional | Mixed, incl. HCC, PC and BC |
| Maeda et al[69], 1991 | CR | Regional | HCC |
| Seong et al[65], 1991 | CR | Regional | HCC |
| Yumoto et al[38], 19911 | RCT | Regional | HCC |
| Kim et al[39], 1992 | nRCT | Regional | HCC |
| Tanaka et al[64], 1992 | pOS | Regional | Mixed, incl. HCC and CCC |
| Robins et al[25], 1993 | nRCT | Whole-body | Mixed, incl. PC |
| Maeta et al[67], 1994 | rOS | Regional | Mixed, incl. HCC |
| Seong et al[40], 1994 | pOS | Regional | HCC |
| Nagata et al[41], 1997 | pOS | Regional | Mixed, incl. HCC and CCC |
| Robins et al[26], 1997 | nRCT | Whole-body | Mixed, incl. HCC and PC |
| Dvorák et al[68], 2002 | CR | Regional | HCC |
| Kamisawa et al[74], 2005 | pOS | Regional | BC |
| Ostapenko et al[72], 20051 | pOS | Regional | HCC |
| Mambrini et al[45], 2007 | pOS | Regional | BC, incl. CCC and GC |
| Bull et al[27], 2008 | pOS | Whole-body | Mixed, incl. PC |
| Cho et al[29], 2008 | pOS | Regional | Mixed, incl. PC |
| Ishikawa et al[52], 2008 | rOS | Regional | PC |
| Ohguri et al[31], 20081 | rOS | Regional | PC |
| Zhang et al[30], 2008 | pOS | Regional | PC |
| Bakshandeh-Bath et al[28], 2009 | pOS | Whole-body | PC |
| Maluta et al[53], 20111 | pOS | Regional | PC |
| Dani et al[58], 2012 | rOS | mEHT | PC |
| Ishikawa et al[47], 20121 | nRCT | Regional | PC |
| Tschoep-Lechner et al[33], 2013 | rOS | Regional | PC |
| Wang et al[73], 2013 | pOS | Regional | HCC |
| Gadaleta-Caldarola et al[42], 2014 | pOS | mEHT | HCC |
| Volovat et al[32], 2014 | pOS | Regional | PC |
| Chen et al[46], 2016 | rOS | Regional | CCC |
| Dong et al[20], 20161 | RCT | Regional | HCC |
| Yu et al[43,44], 2016, 20171 | nRCT | Regional | HCC |
| Datta et al[62], 2017 | RCT | Regional | PC |
| Fan et al[34], 2017 | rOS | Regional | PC |
| Maebayashi et al[54], 2017 | rOS | Regional | PC |
| Pang et al[59], 2017 | RCT | mEHT | Mixed, incl. HCC and PC |
| Bonucci et al[57], 2018 | CR | Regional | PC |
| Ryu et al[75], 2018 | CR | Regional | CCC |
| Werthmann et al[56], 2018 | CR | Regional | PC |
| Fiorentini et al[36], 2019 | rOS | mEHT | PC |
| He et al[35], 2019 | rOS | Regional | PC |
| Iyikesici et al[55], 2020 | rOS | Regional | PC |
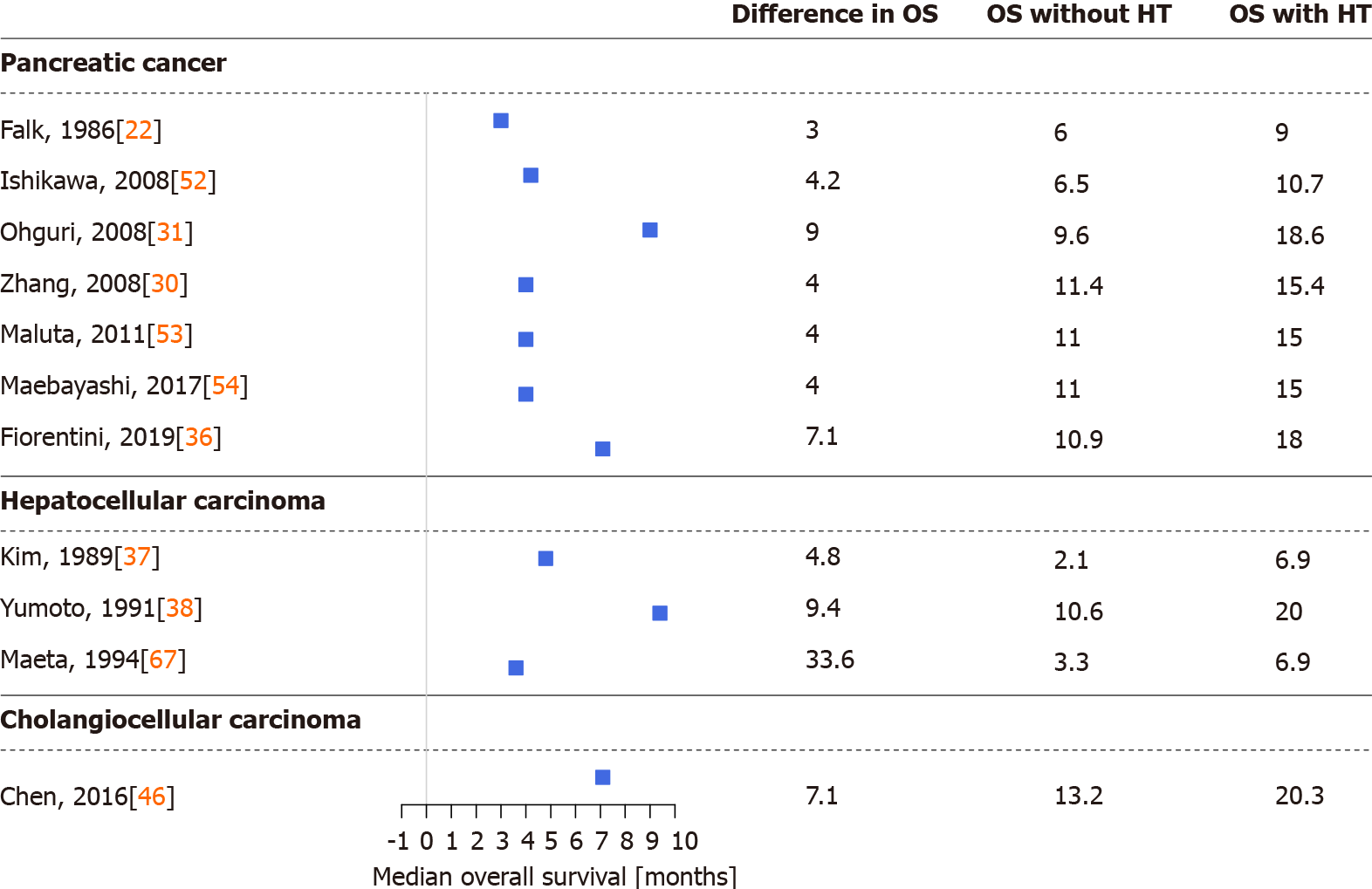
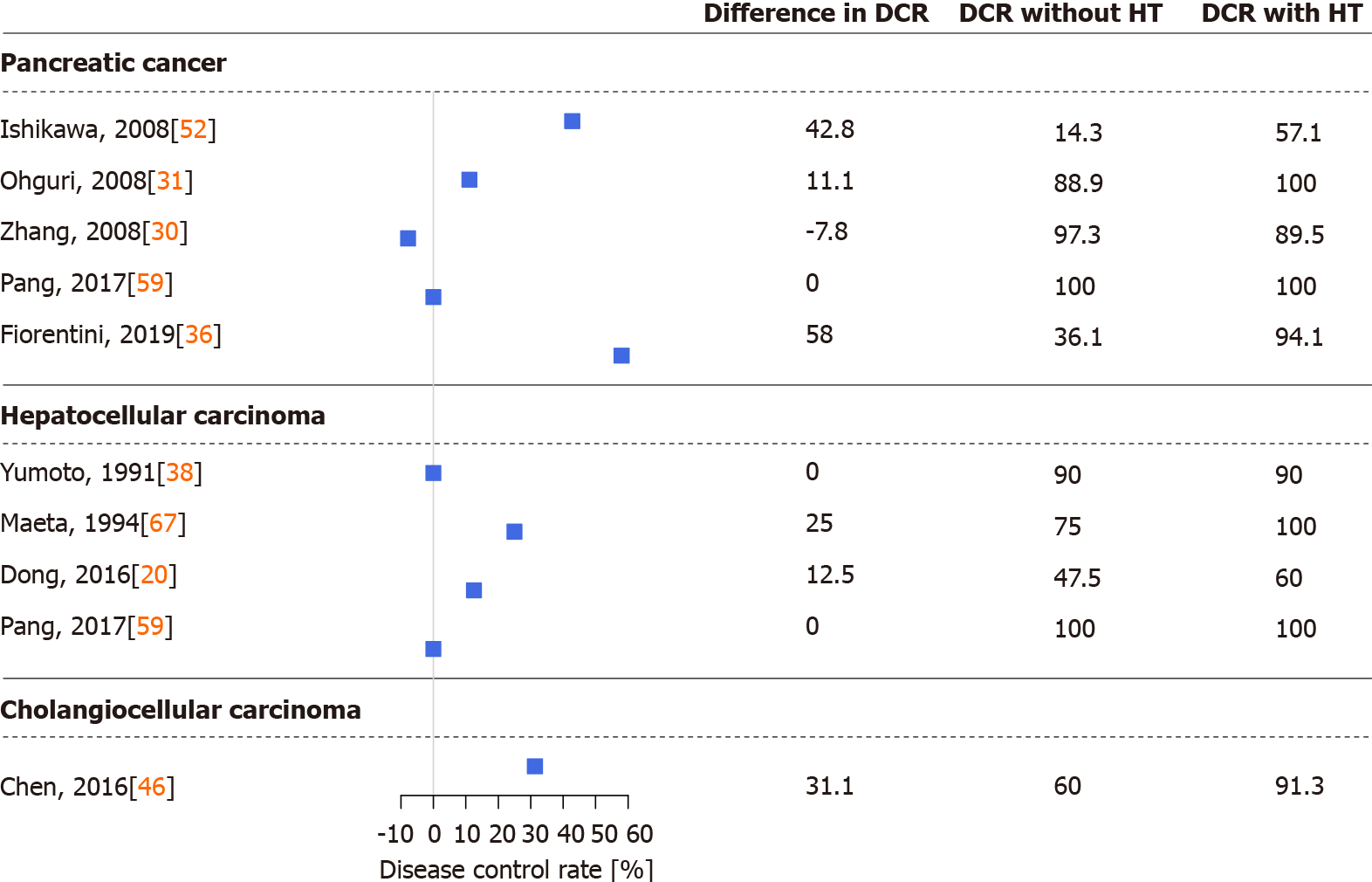
Figures 2 and 3 were drawn with R version 4.0.4 (R Foundation for Statistical Computing, Vienna, Austria, 2021) and the R package forestplot (version 1.10.1, Max Gordon and Thomas Lumley, 2020). Data were obtained from eligible articles, and a simple difference in overall survival and disease control rate (the sum of complete or partial response to treatment and stable disease) was calculated from the results of the cohorts with and without hyperthermia.
Hyperthermia in oncology is the artificial raising of the temperature of the tumor to approximately 39-45 ℃ via full body or local heating methods[18]. In brief, heating is achieved by various biophysical techniques, namely capacitive and radiative methods, which have tumoricidal effects on cancer cells through direct metabolic effects or heating or an indirect immunomodulatory impact. Radiotherapy-resistant, hypoxic, and S-phase cells can be killed. Enhancement of the radiation effect via inhibition of DNA damage repair has been observed following combined administration of adjuvant hyperthermia with radiotherapy[18-20]. Hyperthermia also has a sensitizer effect on chemotherapy via increased cytotoxicity[13,15,18].
The earliest studies on hyperthermia, published in the late 1970s investigated only superficial cancers[21]; trials on deep-seated tumors have been conducted since the 1980s[22,23]. Two major hyperthermia types can be distinguished. Whole-body hyperthermia involves the patient’s entire body surface, while in local/regional hyperthermia techniques only the tumor and its surroundings (i.e. the pelvic or abdominal region) are exposed to the electric field[18]. Early hyperthermia studies of hepatopancreatobiliary cancers included both whole-body and regional hyperthermia, but most of the latest studies evaluated only regional hyperthermia and modulated electrohyperthermia (mEHT), the latest advancement in regional hyperthermia[24]. The easier application of regional hyperthermia and a few additional adverse effects of whole-body hyperthermia, detailed below, may among the factors behind why whole-body hyperthermia has recently been used less frequently.
After whole-body hyperthermia, a significant increase in beta-endorphin, adrenocorticotropic hormone, and cortisol levels have been observed. Furthermore, the longer the hyperthermia treatment lasted, the higher the hormone levels rose, and normalization of hormone levels required at least a day following treatment[17]. Side effects of whole-body hyperthermia can be vomiting, nausea, low-grade fever ≤ 24 h after treatment, as well as a small increase in liver functions, and increased Human alphaherpesvirus 1 and urinary tract infections[25-27]. It should be also emphasized that during the procedure, patients have to be in a light conscious sedation. Moreover, whole-body hyperthermia is a longer procedure. Usually, 1-2 h are necessary to raise the body temperature, followed by maintaining the temperature reached, and a cooling phase is also necessary, resulting in a total procedure time of at least 4-6 h[25-28].
Regional hyperthermia, in comparison, does not require sedation and only takes 30-60 min. Common adverse events related to regional hyperthermia and mEHT during the procedure are thermal stress, local discomfort, hot sensation, abdominal and back pain, bolus pressure, position-related discomfort or pain, tachycardia, and grade I and II burns at the heated region[29-46]. Increased liver enzymes, skin rashes at the heated region, and occasional mild subcutaneous fat necrosis, nausea, dyspnea, and urinary urgency can also occur after the procedure[29,39-46]. Several studies have reported a higher incidence of hematotoxicity in patients treated with hyperthermia than in those without hyperthermia[30,31,47], possibly associated with the increased cytotoxicity of chemotherapeutic agents in hyperthermia[13,15,18]. Various combination of therapy options can also increase or decrease hyperthermia-related tolerability. Most of the studies reported basically 100% tolerability, but Nagata et al[41] reported that hyperthermia had to be discontinued in almost every fifth patient.
Current treatment options for PC are surgical resection with postoperative chemoradiotherapy, and systemic chemotherapy for borderline resectable and locally advanced or metastatic PCs[3]. Historically 5-fluorouracil was the most used chemotherapy agent in advanced and metastatic PCs, but was replaced by gemcitabine and FOLFIRINOX (folinic acid + 5-fluorouracil + irinotecan + oxaliplatin) starting in the mid-1990s[48,49]. Hyperthermia has been introduced as an auxiliary treatment for advanced and metastatic PCs.
Whole-body hyperthermia was shown to improve the effect of melphalan on blood cell counts in refractory cancers including PC[26], but no such effect was observed if carboplatin was administered alone[25]. A treatment regimen of monthly cisplatin + gemcitabine with whole-body hyperthermia combined with continuous low-dose interferon-α had somewhat improved survival compared with standard chemoradiotherapy in metastatic PC, and a higher partial response rate to thermo-chemotherapy[27]. In another study, advanced PC patients, who had achieved partial remission, had longer median survival than those who had not responded to the treatment (11.4 mo vs 15.8 mo)[28]. A clinical study (NCT04467593) is currently investigating the effects of whole-body hyperthermia on current chemotherapy with gemcitabine and FOLFIRINOX[50].
Several studies demonstrated that thermo-chemotherapy of locally advanced or metastatic PCs via regional hyperthermia had a positive effect on patient survival and therapeutic efficacy. During the last three decades, multiple chemo, radio, and other therapies and procedures were combined with regional hyperthermia in PC[22,23,29-36,47,51-59]. In detail, thermo-chemotherapy with selective immune stimulation by copovithane or PZ-73C resulted in longer patient survival[22]. In other studies[23,51], patients treated with chemoradiotherapy and complementary hyperthermia had better disease control rates than those without hyperthermia, doubling the observed responses to treatment. Furthermore, the partial response rate increased along with increased maximum output power of the hyperthermia device[51]. Three-dimensional conformal γ-knife radiotherapy with thermo-chemotherapy has also shown improved survival and response rates when combined with hyperthermia[30] (Figures 2 and 3).
After the introduction of routine clinical use of gemcitabine and FOLFIRINOX, several studies investigated the effect of regional hyperthermia on those treatments[29,31-35,47,52-57]. Gemcitabine alone had worse overall survival and treatment response than when used with complementary hyperthermia[29,47,52], and better progression-free and overall survival have also been reported with the concomitant use of hyperthermia in combination with radiotherapy[31,53,54]. The previous observation that increasing the power output of the hyperthermia device increases treatment response[51] was not confirmed in the case of gemcitabine[31]. Supplementation of gemcitabine thermo-chemotherapy with other chemotherapeutic agents such as cisplatin[33,34] or oxaliplatin[32] had similar results. Patients in the hyperthermia arm had better partial response rates and better survival than those without hyperthermia (Figure 2). Combined hyperthermia with metabolically supported chemotherapy (i.e. gemcitabine or FOLFIRINOX during artificial mild hypoglycemia, serum glucose < 4.0 mmol/L caused by low-dose bolus insulin), hyperbaric oxygen, and a ketogenic diet[55]; and hyperthermia with a modified FOLFIRINOX protocol (i.e. a reduced dose of irinotecan and no 5-fluorouracil)[35] have highlighted the importance of the impact of hyperthermia on overall and progression-free survival. In addition, the currently running HEAT (NCT01077427)[60] and HEATPAC (NCT02439593)[61,62] clinical trials will further broaden our knowledge of the efficacy and safety of hyperthermia combined with gemcitabine in PC.
A few case reports have been published recently where chemoradiotherapy and hyperthermia were combined with herbal remedies. One German[56] and two Italian[57] reports described treating metastatic PC patients with chemoradiotherapy and hyperthermia supplemented with subcutaneous, fever-inducing mistletoe (Viscum album) extract and other immunomodulating supplements including curcumin or shiitake (Lentinula edodes) derivatives. All three patients had survived more than 30 mo with unrestricted quality of life; no deaths were reported at the time of publication[56,57].
As with conventional regional hyperthermia, positive effects of mEHT on pro
Early-stage HCCs are treated with surgical resection or liver transplantation, radiofrequency or microwave ablation, or embolization methods with or without chemoradiotherapy. Intermediate and advanced-stage HCCs are generally treated with systemic and combination therapies[1]. Early studies of hyperthermia in HCC investigated the combination of hyperthermia with chemoradiotherapy, transarterial embolization, or transcatheter arterial chemoembolization[23,37-41,63-69]. Significantly longer survival, lower serum alpha-fetoprotein levels, and better response to treatment, even in tumors > 7 cm[23], were observed in those reports[23,37-41,63-69]. According to the results of one study[64], the best results were achieved if the intratumor temperature reached > 42 °C, while in another study[39] tumors located in the left lobe of the liver were more responsive to combined treatment with hyperthermia. The latter observation may have resulted from a technical aspect of the treatment, as noted by the authors[39]. In addition to the above, investigations of which treatment option benefits the most from hyperthermia in HCC have found that the best survival and response data have been observed in cases of immunotherapy with hyperthermia[41] (Figures 2 and 3).
Combining transcatheter arterial chemoembolization, radiotherapy and hyperther
It is known from model systems[70-72] that heating to fever-range temperatures improves the immune response against tumors, while tumoricidal temperatures (> 42 °C) inhibit host competence. Because of the latter observation, whole body hyper
Of the hepatopancreatobiliary cancers, hyperthermia is the least studied in BC. Conventional treatment options of BC are similar to those of PC and HCC; surgical resection for resectable cases and chemoradiotherapy and systematic combination therapies for advanced and metastatic cases[2]. Until the mid-2000s no studies had been specifically designed to investigate hyperthermia in BC. The results of studies investigating the effect of hyperthermia on mixed tumor types are available[26,29,41,51,63,64], in which a few biliary cases were also presented. Positive effect of hyper
Adjuvant hyperthermia with chemotherapy has increased the treatment response rate and overall survival of BC patients[74,75]. Improvements in quality of life (i.e. fewer tumor related symptoms) and laboratory results were reported in a detailed case report of a patient with unresectable hilar CCC[75]. Similarly, extending hepatic arterial infusion chemotherapy with hyperthermia in patients with advanced BC have resulted in longer progression-free and overall survival and in better disease control rate[45,46] (Figures 2 and 3). The positive effects of hyperthermia were observed after the first few treatments[46], complete responses have been reported[46], and no in
One of the major challenges in advanced and metastatic hepatopancreatobiliary cancers is to find a treatment regimen that increases therapy efficacy but has no additional adverse effects. Adjuvant hyperthermia fulfills those expectations. Patients treated with additional hyperthermia have been reported to have significantly higher local and overall response to treatment, longer progression-free and overall survival, and improved laboratory results. Several studies and case reports suggest that complementary hyperthermia also improved quality of life. Furthermore, there have been a few cases in which patient returned to his or her previous active life after treatment. As with every therapeutic method, hyperthermia also has some adverse effects, which are associated with the local heating process; all have been considered to be grade II complications at the highest. It has to be mentioned however, that regional hyper
The application of hyperthermia has not found its exact clinical indication in the setting of disease stage. For most patients, it is a palliative treatment when no other possibility is available in the traditional oncological armamentarium. Thus, a refined or subdivided stage 4 category would be most beneficial to stratify the patients according to tumor load and involved organs, supplemented by serum tumor marker levels. Tumor markers like lactic dehydrogenase as used in stage 4 melanoma might be an option[76]. Certain organs that are vital or less crucial could also be taken into account when containing metastases. To our knowledge, no such classification exists for the stratification of patients for hyperthermia treatment. Higher Eastern Coope
Summarizing all of the above, hyperthermia in advanced hepatopancreatobiliary cancers is an effective complementary treatment option with several promising positive results extending current protocols. To date, its usage in these three cancer types is mainly limited to research only. However, based on the clinical results and observations provided in this review, its routine use in advanced liver-PC care, especially with further immunomodulation, should be considered.
We are grateful to Viktor Madar-Dank for English proofreading.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Hungary
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pongcharoen S S-Editor: Zhang H L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3879] [Article Influence: 969.8] [Reference Citation Analysis (3)] |
| 2. | Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397:428-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 624] [Article Influence: 156.0] [Reference Citation Analysis (2)] |
| 3. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1670] [Article Influence: 334.0] [Reference Citation Analysis (1)] |
| 4. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55823] [Article Influence: 7974.7] [Reference Citation Analysis (132)] |
| 5. | Brace C. Thermal tumor ablation in clinical use. IEEE Pulse. 2011;2:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Ni Y, Mulier S, Miao Y, Michel L, Marchal G. A review of the general aspects of radiofrequency ablation. Abdom Imaging. 2005;30:381-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 7. | Imajo K, Ogawa Y, Yoneda M, Saito S, Nakajima A. A review of conventional and newer generation microwave ablation systems for hepatocellular carcinoma. J Med Ultrason (2001). 2020;47:265-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Mensel B, Weigel C, Hosten N. Laser-induced thermotherapy. Recent Results Cancer Res. 2006;167:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Goodman MD, McPartland S, Detelich D, Saif MW. Chemotherapy for intraperitoneal use: a review of hyperthermic intraperitoneal chemotherapy and early post-operative intraperitoneal chemotherapy. J Gastrointest Oncol. 2016;7:45-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 65] [Reference Citation Analysis (0)] |
| 10. | Lee MJ, Mueller PR, Dawson SL, Gazelle SG, Hahn PF, Goldberg MA, Boland GW. Percutaneous ethanol injection for the treatment of hepatic tumors: indications, mechanism of action, technique, and efficacy. AJR Am J Roentgenol. 1995;164:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Lencioni R, Petruzzi P, Crocetti L. Chemoembolization of hepatocellular carcinoma. Semin Intervent Radiol. 2013;30:3-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 12. | Izadifar Z, Izadifar Z, Chapman D, Babyn P. An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 229] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 13. | Rao W, Deng ZS, Liu J. A review of hyperthermia combined with radiotherapy/chemotherapy on malignant tumors. Crit Rev Biomed Eng. 2010;38:101-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 14. | Hegyi G, Szigeti GP, Szász A. Hyperthermia versus Oncothermia: Cellular Effects in Complementary Cancer Therapy. Evid Based Complement Alternat Med. 2013;2013:672873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Kampinga HH. Cell biological effects of hyperthermia alone or combined with radiation or drugs: a short introduction to newcomers in the field. Int J Hyperthermia. 2006;22:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Pettigrew RT, Galt JM, Ludgate CM, Horn DB, Smith AN. Circulatory and biochemical effects of whole body hyperthermia. Br J Surg. 1974;61:727-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 80] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Robins HI, Kalin NH, Shelton SE, Martin PA, Shecterle LM, Barksdale CM, Neville AJ, Marshall J. Rise in plasma beta-endorphin, ACTH, and cortisol in cancer patients undergoing whole body hyperthermia. Horm Metab Res. 1987;19:441-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Chicheł A, Skowronek J, Kubaszewska M, Kanikowski M. Hyperthermia – description of a method and a review of clinical applications. Rep Pract Oncol Radiother. 2007;12:267-275. [RCA] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Datta NR, Ordóñez SG, Gaipl US, Paulides MM, Crezee H, Gellermann J, Marder D, Puric E, Bodis S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: recent advances and promises for the future. Cancer Treat Rev. 2015;41:742-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 325] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 20. | Dong Y, Wu G. Analysis of short and long term therapeutic effects of radiofrequency hyperthermia combined with conformal radiotherapy in hepatocellular carcinoma. J BUON. 2016;21:407-411. [PubMed] |
| 21. | Marmor JB, Hahn GM. Combined radiation and hyperthermia in superficial human tumors. Cancer. 1980;46:1986-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Falk RE, Moffat FL, Lawler M, Heine J, Makowka L, Falk JA. Combination therapy for resectable and unresectable adenocarcinoma of the pancreas. Cancer. 1986;57:685-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Kakehi M, Ueda K, Mukojima T, Hiraoka M, Seto O, Akanuma A, Nakatsugawa S. Multi-institutional clinical studies on hyperthermia combined with radiotherapy or chemotherapy in advanced cancer of deep-seated organs. Int J Hyperthermia. 1990;6:719-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 45] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Szasz AM, Minnaar CA, Szentmártoni G, Szigeti GP, Dank M. Review of the Clinical Evidences of Modulated Electro-Hyperthermia (mEHT) Method: An Update for the Practicing Oncologist. Front Oncol. 2019;9:1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | Robins HI, Cohen JD, Schmitt CL, Tutsch KD, Feierabend C, Arzoomanian RZ, Alberti D, d'Oleire F, Longo W, Heiss C. Phase I clinical trial of carboplatin and 41.8 degrees C whole-body hyperthermia in cancer patients. J Clin Oncol. 1993;11:1787-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Robins HI, Rushing D, Kutz M, Tutsch KD, Tiggelaar CL, Paul D, Spriggs D, Kraemer C, Gillis W, Feierabend C, Arzoomanian RZ, Longo W, Alberti D, d'Oleire F, Qu RP, Wilding G, Stewart JA. Phase I clinical trial of melphalan and 41.8 degrees C whole-body hyperthermia in cancer patients. J Clin Oncol. 1997;15:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Bull JM, Scott GL, Strebel FR, Nagle VL, Oliver D, Redwine M, Rowe RW, Ahn CW, Koch SM. Fever-range whole-body thermal therapy combined with cisplatin, gemcitabine, and daily interferon-alpha: a description of a phase I-II protocol. Int J Hyperthermia. 2008;24:649-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Bakshandeh-Bath A, Stoltz AS, Homann N, Wagner T, Stölting S, Peters SO. Preclinical and clinical aspects of carboplatin and gemcitabine combined with whole-body hyperthermia for pancreatic adenocarcinoma. Anticancer Res. 2009;29:3069-3077. [PubMed] |
| 29. | Cho C, Wust P, Hildebrandt B, Issels RD, Sehouli J, Kerner T, Deja M, Budach V, Gellermann J. Regional hyperthermia of the abdomen in conjunction with chemotherapy for peritoneal carcinomatosis: evaluation of two annular-phased-array applicators. Int J Hyperthermia. 2008;24:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Zhang LP, Nie Q, Kang JB, Wang B, Cai CL, Li JG, Qi WJ. [Efficacy of whole body gamma-knife radiotherapy combined with thermochemotherapy on locally advanced pancreatic cancer]. Ai Zheng. 2008;27:1204-1207. [PubMed] |
| 31. | Ohguri T, Imada H, Yahara K, Narisada H, Morioka T, Nakano K, Korogi Y. Concurrent chemoradiotherapy with gemcitabine plus regional hyperthermia for locally advanced pancreatic carcinoma: initial experience. Radiat Med. 2008;26:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Volovat C, Volovat S, Scripcaru V, Miron L. Second-line chemotherapy with gemcitabine and oxaliplatin in combination with loco-regional hyperthermia (EHY-2000) in patients with refractory metastatic pancreatic cancer - preliminary results of a prospective trial. Rom Rep Phys. 2014;66:166-174. |
| 33. | Tschoep-Lechner KE, Milani V, Berger F, Dieterle N, Abdel-Rahman S, Salat C, Issels RD. Gemcitabine and cisplatin combined with regional hyperthermia as second-line treatment in patients with gemcitabine-refractory advanced pancreatic cancer. Int J Hyperthermia. 2013;29:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 34. | Fan YF, Qin Y, Li DG, Kerr D. Retrospective Clinical Study of Advanced Pancreatic Cancer Treated With Chemotherapy and Abdominal Hyperthermia. J Glob Oncol. 2018;4:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | He M, Sun J, Zhao D, He H, Wang B, Xu L, Shang Y, Ren S, Zhang Y, Wu T. Modified-FOLFIRINOX combined with deep regional hyperthermia in pancreatic cancer: a retrospective study in Chinese patients. Int J Hyperthermia. 2019;36:394-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 36. | Fiorentini G, Sarti D, Casadei V, Milandri C, Dentico P, Mambrini A, Nani R, Fiorentini C, Guadagni S. Modulated Electro-Hyperthermia as Palliative Treatment for Pancreatic Cancer: A Retrospective Observational Study on 106 Patients. Integr Cancer Ther. 2019;18:1534735419878505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Kim BS, Yoo HS, Park YJ, Loh JJ. Current status and future aspects on treatment of liver cancer. Cancer Chemother Pharmacol. 1989;23 Suppl:S118-S120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Yumoto Y, Jinno K, Tokuyama K, Wada T, Kobashi H, Okamoto T, Toki H, Inatsuki S, Hara K, Moriwaki S. Trans-catheter hepatic arterial injection of lipiodol soluble anti-cancer agent SMANCS and ADR suspension in lipiodol combined with arterial embolization and local hyperthermia for treatment of hepatocellular carcinoma. Int J Hyperthermia. 1991;7:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Kim BS, Chung HC, Seong JS, Suh CO, Kim GE. Phase II trial for combined external radiotherapy and hyperthermia for unresectable hepatoma. Cancer Chemother Pharmacol. 1992;31 Suppl:S119-S127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 40. | Seong J, Lee HS, Han KH, Chon CY, Suh CO, Kim GE. Combined treatment of radiotherapy and hyperthermia for unresectable hepatocellular carcinoma. Yonsei Med J. 1994;35:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Nagata Y, Hiraoka M, Nishimura Y, Masunaga S, Mitumori M, Okuno Y, Fujishiro M, Kanamori S, Horii N, Akuta K, Sasai K, Abe M, Fukuda Y. Clinical results of radiofrequency hyperthermia for malignant liver tumors. Int J Radiat Oncol Biol Phys. 1997;38:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Gadaleta-Caldarola G, Infusino S, Galise I, Ranieri G, Vinciarelli G, Fazio V, Divella R, Daniele A, Filippelli G, Gadaleta CD. Sorafenib and locoregional deep electro-hyperthermia in advanced hepatocellular carcinoma: A phase II study. Oncol Lett. 2014;8:1783-1787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Yu JI, Park HC, Oh D, Noh JM, Jung SH, Kim HY, Shin SW, Cho SK, Sinn DH, Paik YH, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC. Combination treatment of trans-arterial chemo-embolisation, radiotherapy and hyperthermia (CERT) for hepatocellular carcinoma with portal vein tumour thrombosis: Interim analysis of prospective phase II trial. Int J Hyperthermia. 2016;32:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Yu JI, Park HC, Jung SH, Choi C, Shin SW, Cho SK, Sinn DH, Paik YH, Gwak GY, Choi MS, Lee JH, Koh KC, Yoo BC, Sahinbas H, Paik SW. Combination treatment with transarterial chemoembolization, radiotherapy, and hyperthermia (CERT) for hepatocellular carcinoma with portal vein tumor thrombosis: Final results of a prospective phase II trial. Oncotarget. 2017;8:52651-52664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Mambrini A, Del Freo A, Pacetti P, Orlandi M, Torri T, Fiorentini G, Cantore M. Intra-arterial and systemic chemotherapy plus external hyperthermia in unresectable biliary cancer. Clin Oncol (R Coll Radiol). 2007;19:805-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 46. | Chen Y, Li H, Jiang X, Chen D, Ni J, Sun H, Luo J, Yao H, Xu L. Regional thermochemotherapy versus hepatic arterial infusion chemotherapy for palliative treatment of advanced hilar cholangiocarcinoma: a retrospective controlled study. Eur Radiol. 2016;26:3500-3509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (1)] |
| 47. | Ishikawa T, Kokura S, Sakamoto N, Ando T, Imamoto E, Hattori T, Oyamada H, Yoshinami N, Sakamoto M, Kitagawa K, Okumura Y, Yoshida N, Kamada K, Katada K, Uchiyama K, Handa O, Takagi T, Yasuda H, Sakagami J, Konishi H, Yagi N, Naito Y, Yoshikawa T. Phase II trial of combined regional hyperthermia and gemcitabine for locally advanced or metastatic pancreatic cancer. Int J Hyperthermia. 2012;28:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 48. | Ducreux M, Seufferlein T, Van Laethem JL, Laurent-Puig P, Smolenschi C, Malka D, Boige V, Hollebecque A, Conroy T. Systemic treatment of pancreatic cancer revisited. Semin Oncol. 2019;46:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 49. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI–PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1945] [Article Influence: 277.9] [Reference Citation Analysis (0)] |
| 50. | Peeters M. Safety study of whole body hyperthermia for advanced cancer (MATTERS). [accessed 2021 Feb 10]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT04467593 ClinicalTrials.gov Identifier: NCT04467593. |
| 51. | Hamazoe R, Maeta M, Murakami A, Yamashiro H, Kaibara N. Heating efficiency of radiofrequency capacitive hyperthermia for treatment of deep-seated tumors in the peritoneal cavity. J Surg Oncol. 1991;48:176-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Ishikawa T, Kokura S, Oyamada H, Inui T, Okita M, Isozaki Y, Nagao Y, Takagi T, Handa O, Ando T, Naito Y, Yoshida N, Yoshikawa T. Effects of a sequential combination of hyperthermia and gemcitabine in the treatment of advanced unresectable pancreatic cancer : A retrospective study. Therm Med. 2008;24:131-139. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Maluta S, Schaffer M, Pioli F, Dall'oglio S, Pasetto S, Schaffer PM, Weber B, Giri MG. Regional hyperthermia combined with chemoradiotherapy in primary or recurrent locally advanced pancreatic cancer : an open-label comparative cohort trial. Strahlenther Onkol. 2011;187:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 54. | Maebayashi T, Ishibashi N, Aizawa T, Sakaguchi M, Sato T, Kawamori J, Tanaka Y. Treatment outcomes of concurrent hyperthermia and chemoradiotherapy for pancreatic cancer: Insights into the significance of hyperthermia treatment. Oncol Lett. 2017;13:4959-4964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 55. | Iyikesici MS. Long-Term Survival Outcomes of Metabolically Supported Chemotherapy with Gemcitabine-Based or FOLFIRINOX Regimen Combined with Ketogenic Diet, Hyperthermia, and Hyperbaric Oxygen Therapy in Metastatic Pancreatic Cancer. Complement Med Res. 2020;27:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 56. | Werthmann PG, Inter P, Welsch T, Sturm AK, Grützmann R, Debus M, Sterner MG, Kienle GS. Long-term tumor-free survival in a metastatic pancreatic carcinoma patient with FOLFIRINOX/Mitomycin, high-dose, fever inducing Viscum album extracts and subsequent R0 resection: A case report. Medicine (Baltimore). 2018;97:e13243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Bonucci M, Pastore C, Ferrera V, Fiorentini C, Fabbri A. Integrated Cancer Treatment in the Course of Metastatic Pancreatic Cancer: Complete Resolution in 2 Cases. Integr Cancer Ther. 2018;17:994-999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Dani A, Varkonyi A, Magyar T, Kalden M, Szasz A. Clinical study for advanced pancreas cancer treated by oncothermia. Oncotherm J. 2012;6:11-25. |
| 59. | Pang CLK, Zhang X, Wang Z, Ou J, Lu Y, Chen P, Zhao C, Wang X, Zhang H, Roussakow SV. Local modulated electro-hyperthermia in combination with traditional Chinese medicine vs. intraperitoneal chemoinfusion for the treatment of peritoneal carcinomatosis with malignant ascites: A phase II randomized trial. Mol Clin Oncol. 2017;6:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Issels RD. Hyperthermia European Adjuvant Trial (HEAT). [accessed 2021 Feb 10]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01077427 ClinicalTrials.gov Identifier: NCT01077427. |
| 61. | Datta NR, Aarau K. Concurrent Hyperthermia and Chemoradiotherapy in LAPC: Phase II Study (HEATPAC). [accessed 2021 Feb 10]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT02439593 ClinicalTrials.gov Identifier: NCT02439593. |
| 62. | Datta NR, Pestalozzi B, Clavien PA, Siebenhüner A, Puric E, Khan S, Mamot C, Riesterer O, Knuchel J, Reiner CS, Bodis S; members of the HEATPAC Trial Group. "HEATPAC" - a phase II randomized study of concurrent thermochemoradiotherapy versus chemoradiotherapy alone in locally advanced pancreatic cancer. Radiat Oncol. 2017;12:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 63. | Nagata Y, Hiraoka M, Akuta K, Abe M, Takahashi M, Jo S, Nishimura Y, Masunaga S, Fukuda M, Imura H. Radiofrequency thermotherapy for malignant liver tumors. Cancer. 1990;65:1730-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 64. | Tanaka Y, Yamamoto K, Murata T, Nagata K. Effects of multimodal treatment and hyperthermia on hepatic tumors. Cancer Chemother Pharmacol. 1992;31 Suppl:S111-S114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 65. | Seong JS, Han EK, Han KH, Noh SH, Park CI, Loh JJ, Choi HJ. Histological studies of surgically resected hepatocellular carcinoma following combined radiotherapy and hyperthermia. Yonsei Med J. 1991;32:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 66. | Akuta K, Abe M, Kondo M, Yoshikawa T, Tanaka Y, Yoshida M, Miura T, Nakao N, Onoyama Y, Yamada T. Combined effects of hepatic arterial embolization using degradable starch microspheres (DSM) in hyperthermia for liver cancer. Int J Hyperthermia. 1991;7:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 67. | Maeta M, Kaibara N, Nakashima K, Kobayashi M, Yoshikawa T, Okamoto A, Sugiyama A. A case-matched control study of intrahepatoarterial chemotherapy in combination with or without regional hyperthermia for treatment of primary and metastatic hepatic tumours. Int J Hyperthermia. 1994;10:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 68. | Dvorák J, Zoul Z, Melichar B, Jandík P, Mergancová J, Motycková I, Kalousová D, Petera J. Pegylated liposomal doxorubicin in combination with hyperthermia in the treatment of a case of advanced hepatocellular carcinoma. J Clin Gastroenterol. 2002;34:96-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 69. | Maeda M, Watanabe N, Yamauchi N, Tsuji Y, Niitsu Y. Successful treatment of a case of hepatocellular carcinoma with tumor necrosis factor and local hyperthermia. Gastroenterol Jpn. 1991;26:774-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 70. | Shen RN, Lu L, Young P, Shidnia H, Hornback NB, Broxmeyer HE. Influence of elevated temperature on natural killer cell activity, lymphokine-activated killer cell activity and lectin-dependent cytotoxicity of human umbilical cord blood and adult blood cells. Int J Radiat Oncol Biol Phys. 1994;29:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Huang YH, Haegerstrand A, Frostegård J. Effects of in vitro hyperthermia on proliferative responses and lymphocyte activity. Clin Exp Immunol. 1996;103:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 72. | Ostapenko VV, Tanaka H, Miyano M, Nishide T, Ueda H, Nishide I, Tanaka Y, Mune M, Yukawa S. Immune-related effects of local hyperthermia in patients with primary liver cancer. Hepatogastroenterology. 2005;52:1502-1506. [PubMed] |
| 73. | Wang XP, Xu M, Gao HF, Zhao JF, Xu KC. Intraperitoneal perfusion of cytokine-induced killer cells with local hyperthermia for advanced hepatocellular carcinoma. World J Gastroenterol. 2013;19:2956-2962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Kamisawa T, Tu Y, Egawa N, Karasawa K, Matsuda T, Tsuruta K, Okamoto A. Thermo-chemo-radiotherapy for advanced bile duct carcinoma. World J Gastroenterol. 2005;11:4206-4209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Ryu J, Lee K, Joe C, Joo J, Lee N, Yoo HS. Patient With Unresectable Cholangiocarcinoma Treated With Radiofrequency Hyperthermia in Combination With Chemotherapy: A Case Report. Integr Cancer Ther. 2018;17:558-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Gao D, Ma X. Serum lactate dehydrogenase is a predictor of poor survival in malignant melanoma. Panminerva Med. 2017;59:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |