Published online Aug 15, 2021. doi: 10.4251/wjgo.v13.i8.959
Peer-review started: June 9, 2021
First decision: June 15, 2021
Revised: June 18, 2021
Accepted: July 5, 2021
Article in press: July 5, 2021
Published online: August 15, 2021
Processing time: 66 Days and 9.6 Hours
Duodenal gastrointestinal stromal tumor (DGIST) is a rare tumor with a specific anatomic site and biological characteristics. As the incidence of lymph node metastasis is very low, the main treatment method is surgery. Two main surgical techniques (local resection and Whipple) are performed in patients with DGISTs. The critical question is which surgical technique to choose.
To identify factors influencing the choice of surgery for DGISTs.
The clinicopathological data of patients with DGISTs who underwent surgery between January 1999 and January 2021 were analyzed. We used the Student’s t-test or Mann-Whitney U-test and the χ2 test or Fisher’s exact test to determine the differences between the two groups of patients. Furthermore, we used logistic analysis to identify the relevant factors and independent factors related to the type of surgery. The Kaplan-Meier method was used to analyze the patient’s survival information and Cox regression analysis was performed to determine prognostic risk factors.
Overall, 86 patients were analyzed, including 43 men (50%) and 43 women (50%). We divided the patients into two groups based on surgical technique (local resection or Whipple surgery). There were no differences in the age, mitotic figures, and complications between the two groups; however, the tumor size, tumor location, risk grade, postoperative hospital stay, and abdominal drainage time were significantly different. Based on univariate logistic analysis, the Whipple procedure was chosen if the tumor size was ≥ 5.0 cm, the tumor was located in the descending part of the duodenum, or the risk grade was medium or high. In our research, the five-year overall survival rate of patients was more than 90%. We also describe two DGIST patients with liver metastases at first diagnosis and analyzed their management in order to provide advice on complicated cases.
The Whipple procedure was performed if the primary tumor was in the descending part of the duodenum, tumor size was ≥ 5.0 cm, or the tumor risk grade was medium or high.
Core Tip: We investigated the factors influencing the surgical treatment of duodenal gastrointestinal stromal tumors, and found that if the primary tumor was in the descending part of the duodenum, tumor size was ≥ 5.0 cm and tumor risk grade was medium or high, Whipple surgery was performed.
- Citation: Wu YZ, Li Y, Wu M, Zheng XH, Tian YT, Xie YB. Investigation of the factors influencing surgical treatment of duodenal gastrointestinal stromal tumors. World J Gastrointest Oncol 2021; 13(8): 959-969
- URL: https://www.wjgnet.com/1948-5204/full/v13/i8/959.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i8.959
Duodenal gastrointestinal stromal tumors (DGISTs) account for 12%–18% of small intestinal stromal tumors and 1%–4% of all gastrointestinal stromal tumors[1,2]. DGISTs rarely break the tumor capsule and seldom cause lymph node metastasis; therefore, surgery is the best method to cure this disease. However, the duodenum is located near the pancreas and biliary tract. A critical question in the surgical treatment of these tumors is which surgical procedure to choose. Two types of surgery are performed in patients with DGISTs: Local resection and Whipple surgery. Traditional Whipple surgery results in severe surgical injury; therefore, local resection is performed to preserve more healthy tissue[3,4].
In recent years, there have been some reports on DGISTs; however, there is a lack of large-scale reports as the incidence of DGISTs is low[5,6]. In the present study, we excluded patients with liver metastasis. Therefore, understanding which surgical procedure preserves more normal tissue, especially normal anatomical structures is the main focus of our study. In this study, we investigated the factors influencing DGIST surgery and provide advice regarding the choice of surgery.
All DGISTs were referred to the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Science and Peking Union Medical College, between January 1999 and January 2021. This study was approved by the Ethics Committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Science, and Peking Union Medical College. The diagnosis of DGIST was confirmed by immunohistochemical staining. Inclusion criteria are as follows: (1) Patients who underwent laparotomy; (2) Patients with DGISTs, as proven by pathology or immunohistochemistry (CD117, CD34, DOG1 and Ki67); (3) The tumor was located in the duodenum, as confirmed by preoperative abdominal computed tomography scan, ultrasound, endoscopy, upper gastrointestinal barium swallow, and surgery; and (4) Patients with GIST synchronous with other malignancies were excluded[7]. The risk grade of DGIST was assessed by a pathologist. Clinicopathological parameters (age, sex, tumor size, and tumor location) were retrospectively reviewed and documented. The patients were divided into the local resection group and Whipple group.
According to the type of distribution, continuous variables are expressed as average (range) or median (quartile) and were compared using the Student’s t-test or Mann-Whitney U-test. Categorical variables are expressed as percentages and were compared using the χ2 test or Fisher’s exact test. Furthermore, univariate and multivariate logistic analyses were used to assess the type of surgery. Kaplan-Meier survival analysis was performed using GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA, United States) to compare overall survival. Cox proportional hazards regression analysis was used to identify risk factors for mortality. Statistical analyses were two-sided, and the threshold for statistical significance was defined as P < 0.05. Retrospective analyses were performed using SPSS v26 (IBM Inc., Armonk, NY, United States).
During the study period of 21 years, 86 patients with histopathologically confirmed DGISTs underwent surgery at our hospital. Their clinical features are summarized in Table 1, and their pathological characteristics are summarized in Table 2. The patients included 43 men and 43 women, with a median age of 62.15 years (range, 26–87 years). The tumors ranged from 2 cm to 28 cm in size and were distributed in different sections of the duodenum with the predominant location being the descending section (55.8%). Fifty-six (65.1%) patients underwent local resection, and 30 patients (34.9%) underwent the Whipple procedure. According to the GIST guidelines of the Chinese Society of Clinical Oncology, immunohistochemical parameters, such as CD34, CD117, DOG1, and SDHB, support the diagnosis of DGIST.
| Clinical characteristics | Duodenal gastrointestinal stromal tumors |
| Age (yr) | 62.15 (26-87) |
| Gender | |
| Male | 43 (50) |
| Female | 43 (50) |
| Mitotic figures | |
| ≤ 5/50 HPF | 47 (60.3) |
| > 5/50 HPF | 31 (39.7) |
| Tumor size (cm) | 6.674 (2.0-28.0) |
| Surgery type | |
| Local resection | 56 (65.1) |
| Whipple | 30 (34.9) |
| Location | |
| Duodenal bulb | 17 (19.8) |
| Descending part | 48 (55.8) |
| Horizontal part | 16 (18.6) |
| Ascending part | 6 (7.0) |
| Risk grade | |
| Low | 27 (33.8) |
| Medium | 11 (13.8) |
| High | 4 2 (52.5) |
| Lymph node number | 13.72 (1-40) |
| Positive lymph node number | 0 |
| Postoperative hospital time (days) | 20.96 (5-81) |
| Abdominal drainage time (days) | 16.08 (3-78) |
| Complications | |
| Yes | 20 (23.3) |
| No | 66 (76.7) |
| Antigen | Negative and weakly positive | Strongly positive |
| Ki-67 | 65/77 (84.4) | 12/77 (15.6) |
| CD117 | 3/82 (3.7) | 79/82 (96.3) |
| CD34 | 49/83 (59.0) | 34/83 (41.0) |
| DOG1 | 2/53 (3.8) | 51/53 (96.2) |
| Desmin | 68/69 (98.6) | 1/69 (1.4) |
| SMA | 68/75 (90.7) | 7/75 (9.3) |
| S100 | 78/80 (97.5) | 2/80 (2.5) |
| SHDB | 3/16 (18.8) | 13/16 (81.2) |
| AE1/AE3 | 41/41 (100.00) | 0/41 (0.00) |
Patients were allocated to two groups according to the surgical procedure they underwent. The patients’ clinicopathological characteristics were also compared. There were no differences in age, sex, mitotic figures, and incidence of complications between the two groups; however, differences in tumor size, location, and risk grade were statistically significant. These results indicated that we were inclined to choose Whipple surgery when the tumor was in the descending part of the duodenum (P = 0.017), had a medium/high risk score (P = 0.004), or was ≥ 5.0 cm in size (P = 0.015). Furthermore, patients who underwent Whipple surgery had a longer postoperative hospital stay (P = 0.000) and abdominal drainage time (P = 0.001, Table 3).
| Clinical characteristics | Local resection (n = 56) | Whipple (n = 30) | χ2 | P value |
| Age (yr) | 62.2 (26-83) | 62.07 (39-87) | 0.960 | |
| Gender | 1.843 | 0.175 | ||
| Male | 25 (44.6) | 18 (60.0) | ||
| Female | 31 (55.4) | 12 (40.0) | ||
| Mitotic figures | 0.815 | 0.367 | ||
| ≤ 5/50 HPF | 32 (64.0) | 15 (53.6) | ||
| > 5/50 HPF | 18 (36.0) | 13 (46.4) | ||
| Tumor size (cm) | 6.461 (2.0-28.0) | 7.073 (3.0-15) | 0.0151 | |
| Location | 5.734 | 0.0171 | ||
| Other parts | 30 (53.5) | 8 (26.6) | ||
| Descending part | 26 (46.4) | 22 (73.3) | ||
| Risk grade | 8.103 | 0.0041 | ||
| Very low and low | 23 (45.1) | 4 (13.8) | ||
| Medium and high | 28 (54.9) | 25 (86.2) | ||
| Complications | 1.174 | 0.279 | ||
| No | 45 (80.4) | 21 (70.0) | ||
| Yes | 11 (19.6) | 9 (30.0) | ||
| Postoperative hospital time (days) | 15.64 (5-66) | 24.08 (4-78) | 0.0001 | |
| Abdominal drainage time (days) | 11.92 (3-42) | 31.07 (12-81) | 0.0011 |
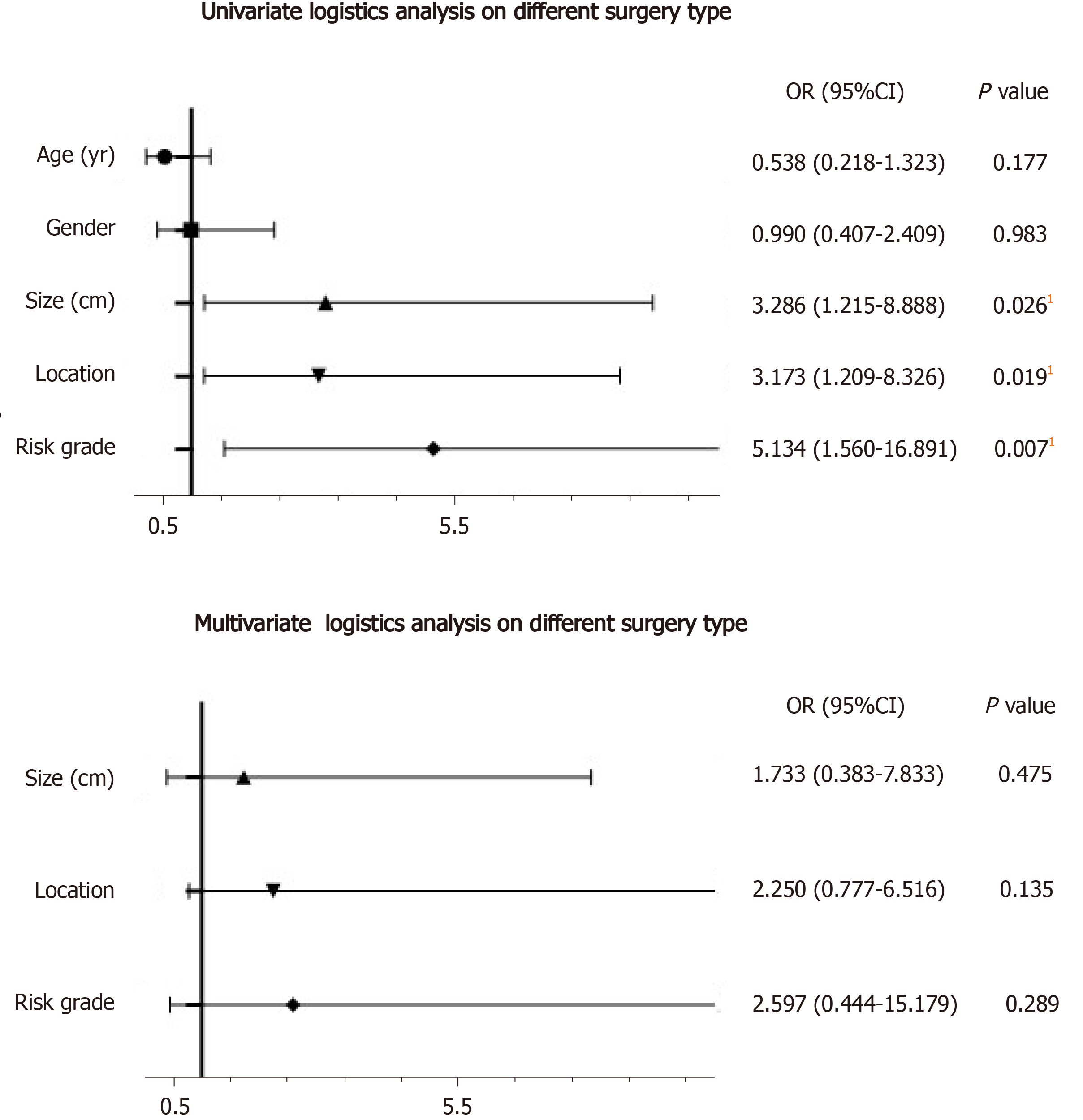
Logistic analysis was used to determine the factors that influenced the type of surgery (Figure 1). In univariate logistic analysis (Table 4), the risk factors for treatment included tumor size ≥ 5.0 cm (P = 0.026), tumor location in the descending part of the duodenum (P = 0.019), and medium/high risk score (P = 0.007). We then used these three parameters to build a multivariate logistic analysis model (Table 4). None of these parameters were independent factors for the type of surgery.
| Characteristics | Univariate logistics analysis | Multivariate analysis | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age (yr) | ||||||
| < 62 | Reference | |||||
| ≥ 62 | 0.538 | 0.218-1.323 | 0.177 | |||
| Gender | ||||||
| Male | Reference | |||||
| Female | 0.990 | 0.407-2.409 | 0.983 | |||
| Tumor size (cm) | ||||||
| < 5.0 | Reference | Reference | ||||
| ≥ 5.0 | 3.286 | 1.215-8.888 | 0.0261 | 1.733 | 0.383-7.833 | 0.475 |
| Location | ||||||
| Other parts | Reference | Reference | ||||
| Descending part | 3.173 | 1.209-8.326 | 0.0191 | 2.250 | 0.777-6.516 | 0.135 |
| Risk grade | ||||||
| Very low and low | Reference | Reference | ||||
| Medium and high | 5.134 | 1.560-16.891 | 0.0071 | 2.597 | 0.444-15.179 | 0.289 |
It is known that compared to local resection, Whipple surgery results in serious surgical injury and can result in a higher incidence of postoperative complications. In this study, we found no difference between patients who underwent Whipple surgery and those who underwent local resection (Table 5).
| Local resection (n = 56) | Whipple (n = 30) | χ2 | P value | |
| Fistula (n = 4) | 2.882 | 0.09 | ||
| Yes | 1 | 4 | ||
| No | 55 | 26 | ||
| Hemorrhage (n = 5) | 0.305 | 0.887 | ||
| Yes | 4 | 4 | ||
| No | 52 | 26 | ||
| Infection (n = 8) | 3.044 | 0.081 | ||
| Yes | 3 | 6 | ||
| No | 53 | 24 | ||
| Gastroparesis (n = 4) | 0.925 | 0.336 | ||
| Yes | 4 | 0 | ||
| No | 52 | 30 |
Overall, 48 patients had tumors in the descending part of the duodenum. We determined the best surgical option for tumors in the descending part of the duodenum. We compared the clinical characteristics of patients who underwent the two types of surgery. Of these, only the duration of postoperative hospital stay (P = 0.000) and abdominal drainage time (P = 0.001) were statistically significant (Table 6).
| Clinical characteristics | Local resection (n = 26) | Whipple (n = 22) | χ2 | P value |
| Age (yr) | 64.5 (53.25-77) | 62 (58.25-67) | 0.621 | |
| Gender | 0 | 1 | ||
| Male | 13 (50.0) | 11 (50.0) | ||
| Female | 13 (50.0) | 11 (50.0) | ||
| Mitotic figures | 0.467 | 0.494 | ||
| ≤ 5/50 HPF | 15 (65.2) | 11 (55.0) | ||
| > 5/50 HPF | 8 (34.8) | 9 (45.0) | ||
| Tumor size (cm) | 5.25 (4.0-6.4) | 6 (4.625-7.75) | 0.164 | |
| Risk grade | 0.467 | 0.494 | ||
| Very low and low | 15 (65.2) | 11 (55.0) | ||
| Medium and high | 8 (34.8) | 9 (45.0) | ||
| Complications | 0.138 | 0.710 | ||
| No | 19 (73.1) | 15 (68.2) | ||
| Yes | 7 (26.9) | 7 (31.8) | ||
| Postoperative hospital time (days) | 13.5 (11-16) | 25.5 (18.5-40.75) | 0.0001 | |
| Abdominal drainage time (days) | 10 (9-13) | 20 (10.5-33.5) | 0.0021 |
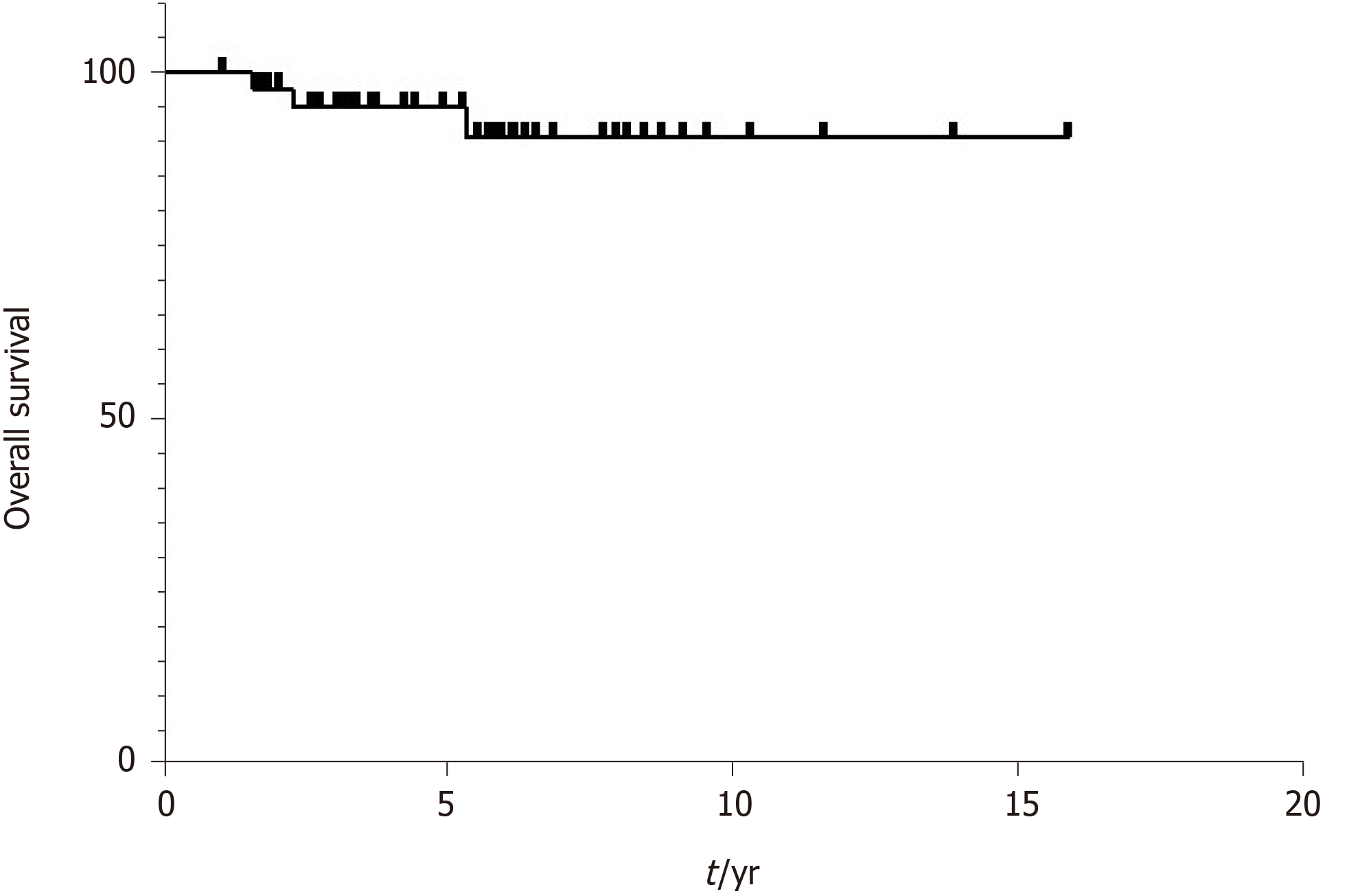
Consistent with previous reports, DGIST is a low-grade malignant tumor with a good prognosis. We used the Kaplan-Meier method to assess the survival curves. The results showed that more than 90% of all patients lived longer than 5 years (Figure 2). Cox regression analysis was used to identify risk factors for mortality. The differences were not statistically significant (Table 7).
| Characteristics | Univariate Cox analysis | ||
| Hazard ratio | 95%CI | P value | |
| Age (yr) | |||
| < 62 | Reference | ||
| ≥ 62 | 79.97 | 0.057-1.12 × 105 | 0.236 |
| Gender | |||
| Male | Reference | ||
| Female | 0.69 | 0.115-4.142 | 0.682 |
| Tumor size (cm) | |||
| < 5 | Reference | ||
| ≥ 5 | 3.725 | 0.416-33.351 | 0.240 |
| Location | |||
| Other parts | Reference | ||
| Descending part | 0.617 | 0.103-3.695 | 0.597 |
| Risk grade | |||
| Very low and low | Reference | ||
| Medium and high | 41.935 | 0.009-2.002 × 105 | 0.387 |
Although the biological characteristics of DGISTs include low-grade malignancy, some patients still develop synchronous liver metastases. We describe the treatment of two patients with synchronous liver metastases (Table 8). Patient 1 was diagnosed with duodenal stromal tumor with liver metastasis. Due to the large metastatic liver tumor, he first received liver resection and gastro-jejunal circuit to relieve symptoms followed by imatinib for 2 years after surgery. Tumor shrinkage was observed in the patient and he then underwent the Whipple procedure for radical treatment of the tumor. Patient 2 was diagnosed with duodenal stromal tumor with multiple liver metastases which were located in the left liver. Considering that the surgical risk in this patient was high, he received imatinib for 16 mo. The primary DGIST and all the liver tumors reduced in size and he then underwent the Whipple procedure and left liver resection followed by imatinib treatment after surgery.
| No. | Age | Gender | Primary tumor size (cm) | Liver tumor size (cm) | Mitotic index | Risk grade | Gene mutation |
| 1 | 60 | Male | 4.5 | 11 | 1-3/50 HPF | Low | c-kit |
| 2 | 39 | Male | 16 | 4.5 | 8-10/50 HPF | High | c-kit |
In this study, we retrospectively analyzed 86 patients with DGISTs treated between 1999 and 2021. We also described two patients with DGISTs and synchronous liver metastases. Previous studies have demonstrated that local resection has obvious advantages over pancreaticoduodenectomy in terms of surgical trauma, surgical complications, and postoperative recovery; however, there was no difference in the long-term oncological efficacy between the two surgical methods in the treatment of DGISTs[8-10]. Additionally, postoperative adjuvant treatment can result in a better prognosis[11-13]. In recent years, several clinical studies and meta-analyses have suggested that surgical resection of GISTs should adhere to the principle of local resection[14,15].
Based on the results of this study, the clinicopathological parameters of our patients were similar to the basic characteristics of DGISTs in China[16,17]. There were no differences in the incidence rate between different genders with a median age of 62.15 years (range, 26–87 years). The tumors ranged from 2 cm to 28 cm in size and were distributed in different parts of the duodenum with the predominant location being the descending section (55.8%). Fifty-six (65.1%) patients underwent local resection, and 30 patients (34.9%) underwent the Whipple procedure. Almost 90% of patients lived longer than five years. Furthermore, by comparing the difference between the two groups of patients, we suggest performing the Whipple procedure if the primary tumor is in the descending part of the duodenum, tumor size is ≥ 5.0 cm, or the tumor risk grade is medium or high. We believe that the use of extended radical surgery (Whipple procedure) in patients with DGISTs will not improve their prognosis; rather, it will increase surgical trauma and postoperative complications, and reduce the quality of life of patients postoperatively.
GISTs have unique biological characteristics, and they rarely result in lymph node metastasis; therefore, surgical resection of GISTs does not require excessive tumor margins and resection of lymph nodes. Surgical resection of DGIST does not need to involve the scope of surgical resection for duodenal cancer or require pancreaticoduodenectomy and peripheral lymph node dissection. Some patients diagnosed with synchronous liver metastases underwent surgery followed by the administration of imatinib.
Due to the long operation time related to Whipple surgery, extensive trauma, and high surgical risk, Whipple surgery will inevitably increase the number of postope
Even if the tumor is located in the descending part of the duodenum, Whipple surgery seems to be appropriate. However, in most instances, to promote speedy recovery, local resection should be the first choice with administration of postoperative adjuvant treatment as soon as possible[18,19]. However, the premise is the definitive diagnosis of DGISTs. Percutaneous or endoscopic ultrasonography-guided fine-needle aspiration can be used before surgery[20-22]. When histopathological diagnosis is confirmed, preoperative GIST treatment can be administered in order to increase surgical safety. When the tumor has reduced in size[23], local resection of the intestine can eliminate the tumor with less trauma.
Due to the low incidence rate of DGISTs, the number of cases available for analysis is limited. Multicenter analysis and high standard meta-analysis are necessary. As reported in previous research[24,25], based on our results, primary tumor size, pri
There are some limitations in this study. First, the sample size was relatively small, and may influence the clinical application value. Second, this was a retrospective study, and a prospective study should be performed in the future.
If the primary tumor is in the descending part of the duodenum, tumor size is ≥ 5.0 cm, and tumor risk grade is medium or high, Whipple surgery should be performed.
Duodenal gastrointestinal stromal tumors (DGISTs) rarely break the tumor capsule and lymph node metastases seldom occur. However, the duodenum is located near the pancreas and biliary tract. Traditional Whipple surgery results in severe injury; thus, it is necessary to preserve as much normal tissue as possible.
The present study attempted to identify the factors influencing the surgical treatment of DGISTs and to determine the best surgical procedure.
This study aimed to investigate the factors influencing DGIST surgery and provide advice regarding the best surgical technique.
The clinicopathological data of patients with DGISTs who underwent surgery from January 1999 to January 2021 were analyzed. The Student t test or Mann-Whitney U-test and χ2 test or Fisher’s exact test were used to identify differences between the two groups of patients treated with different surgical techniques. Logistic regression analysis was carried out to assess the relevant factors influencing the choice of surgical procedure.
There were no differences in age, mitotic figures and complications between these two groups, while the tumor size, tumor location, risk grade, postoperative hospital time, and abdominal drainage time showed statistically significant differences. Using univariate logistic analysis, if the tumor was ≥ 5.0 cm in size, located in the descending part of the duodenum or the risk grade was medium or high, Whipple surgery was performed.
If the primary tumor was in the descending part of the duodenum, was ≥ 5.0 cm in size and the risk grade was medium or high, Whipple surgery was performed.
Although we investigated the factors influencing the surgical treatment of duodenal gastrointestinal stromal tumors and assessed the best surgical procedure for these patients, a prospective study should be performed to confirm these findings.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ibrahim R, Lee KG, Yasukawa K S-Editor: Wang JL L-Editor: Webster JR P-Editor: Li JH
| 1. | Hoeppner J, Kulemann B, Marjanovic G, Bronsert P, Hopt UT. Limited resection for duodenal gastrointestinal stromal tumors: Surgical management and clinical outcome. World J Gastrointest Surg. 2013;5:16-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Johnston FM, Kneuertz PJ, Cameron JL, Sanford D, Fisher S, Turley R, Groeschl R, Hyder O, Kooby DA, Blazer D 3rd, Choti MA, Wolfgang CL, Gamblin TC, Hawkins WG, Maithel SK, Pawlik TM. Presentation and management of gastrointestinal stromal tumors of the duodenum: a multi-institutional analysis. Ann Surg Oncol. 2012;19:3351-3360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Bourgouin S, Hornez E, Guiramand J, Barbier L, Delpero JR, Le Treut YP, Moutardier V. Duodenal gastrointestinal stromal tumors (GISTs): arguments for conservative surgery. J Gastrointest Surg. 2013;17:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Qian HR, Lin TY. [Tough choice of surgical treatment for duodenal gastrointestinal stromal tumor: pancreaticoduodenectomy or local resection? Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:861-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Colombo C, Ronellenfitsch U, Yuxin Z, Rutkowski P, Miceli R, Bylina E, Hohenberger P, Raut CP, Gronchi A. Clinical, pathological and surgical characteristics of duodenal gastrointestinal stromal tumor and their influence on survival: a multi-center study. Ann Surg Oncol. 2012;19:3361-3367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Liang X, Yu H, Zhu LH, Wang XF, Cai XJ. Gastrointestinal stromal tumors of the duodenum: surgical management and survival results. World J Gastroenterol. 2013;19:6000-6010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Du H, Ning L, Li S, Lou X, Chen H, Hu F, Shan G, Zhang F, Xu G. Diagnosis and Treatment of Duodenal Gastrointestinal Stromal Tumors. Clin Transl Gastroenterol. 2020;11:e00156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 8. | Shen C, Chen H, Yin Y, Chen J, Han L, Zhang B, Chen Z. Duodenal gastrointestinal stromal tumors: clinicopathological characteristics, surgery, and long-term outcome. BMC Surg. 2015;15:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Yang F, Jin C, Du Z, Subedi S, Jiang Y, Li J, Di Y, Zhou Z, Tang F, Fu D. Duodenal gastrointestinal stromal tumor: clinicopathological characteristics, surgical outcomes, long term survival and predictors for adverse outcomes. Am J Surg. 2013;206:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Lee SY, Goh BK, Sadot E, Rajeev R, Balachandran VP, Gönen M, Kingham TP, Allen PJ, D'Angelica MI, Jarnagin WR, Coit D, Wong WK, Ong HS, Chung AY, DeMatteo RP. Surgical Strategy and Outcomes in Duodenal Gastrointestinal Stromal Tumor. Ann Surg Oncol. 2017;24:202-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Crown A, Biehl TR, Rocha FG. Local resection for duodenal gastrointestinal stromal tumors. Am J Surg. 2016;211:867-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Chen P, Song T, Wang X, Zhou H, Zhang T, Wu Q, Kong D, Cui Y, Li H, Li Q. Surgery for Duodenal Gastrointestinal Stromal Tumors: A Single-Center Experience. Dig Dis Sci. 2017;62:3167-3176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Lv A, Qian H, Qiu H, Wu J, Li Y, Li Z, Hao C. Organ-preserving surgery for locally advanced duodenal gastrointestinal stromal tumor after neoadjuvant treatment. Biosci Trends. 2017;11:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Lee SJ, Song KB, Lee YJ, Kim SC, Hwang DW, Lee JH, Shin SH, Kwon JW, Hwang SH, Ma CH, Park GS, Park YJ, Park KM. Clinicopathologic Characteristics and Optimal Surgical Treatment of Duodenal Gastrointestinal Stromal Tumor. J Gastrointest Surg. 2019;23:270-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Zhou Y, Wang X, Si X, Wang S, Cai Z. Surgery for duodenal gastrointestinal stromal tumor: A systematic review and meta-analysis of pancreaticoduodenectomy vs local resection. Asian J Surg. 2020;43:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Zhang S, Tian Y, Chen Y, Zhang J, Zheng C, Wang C. Clinicopathological Characteristics, Surgical Treatments, and Survival Outcomes of Patients with Duodenal Gastrointestinal Stromal Tumor. Dig Surg. 2019;36:206-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Du CY, Zhou Y, Song C, Wang YP, Jie ZG, He YL, Liang XB, Cao H, Yan ZS, Shi YQ. Is there a role of surgery in patients with recurrent or metastatic gastrointestinal stromal tumours responding to imatinib: a prospective randomised trial in China. Eur J Cancer. 2014;50:1772-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Marano L, Boccardi V, Marrelli D, Roviello F. Duodenal gastrointestinal stromal tumor: From clinicopathological features to surgical outcomes. Eur J Surg Oncol. 2015;41:814-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Etherington MS, DeMatteo RP. Tailored management of primary gastrointestinal stromal tumors. Cancer. 2019;125:2164-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Castro-Poças FM, Araújo TP, Silva JD, Lopes CA, M Saraiva M. Duodenal gastrointestinal stromal tumor and endoscopic ultrasound. Rev Esp Enferm Dig. 2015;107:759-760. [PubMed] |
| 21. | Jung H, Lee SM, Kim YC, Byun J, Kwon MJ. A pictorial review on clinicopathologic and radiologic features of duodenal gastrointestinal stromal tumors. Diagn Interv Radiol. 2020;26:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Spiel A, Patel R, Minter R, Rahnemai Azar AA, Agni R, Bosch A, Gopal D. Endoscopic Ultrasound in Guiding Local Resection and Ampullary Preservation of a High-Risk Periampullary GIST. Case Rep Gastrointest Med. 2020;2020:8418905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Wang SY, Wu CE, Lai CC, Chen JS, Tsai CY, Cheng CT, Yeh TS, Yeh CN. Prospective Evaluation of Neoadjuvant Imatinib Use in Locally Advanced Gastrointestinal Stromal Tumors: Emphasis on the Optimal Duration of Neoadjuvant Imatinib Use, Safety, and Oncological Outcome. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Wei YZ, Cai ZB, Zhu CL, Zhou YM, Zhang XF. Impact of Surgical Modalities on Long-term Survival Outcomes of Patients with Duodenal Gastrointestinal Stromal Tumor. Ann Surg Oncol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Gu L, Khadaroo PA, Chen M, Qian H, Zhu H, Li X, Pan J, Zhong X, Wang X. Surgical management and outcomes of duodenal gastrointestinal stromal tumors. Acta Gastroenterol Belg. 2019;82:11-18. [PubMed] |
| 26. | Lin Y, Wang M, Jia J, Wan W, Wang T, Yang W, Li C, Chen X, Cao H, Zhang P, Tao K. Development and validation of a prognostic nomogram to predict recurrence in high-risk gastrointestinal stromal tumour: A retrospective analysis of two independent cohorts. EBioMedicine. 2020;60:103016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Li J, Ye Y, Wang J, Zhang B, Qin S, Shi Y, He Y, Liang X, Liu X, Zhou Y, Wu X, Zhang X, Wang M, Gao Z, Lin T, Cao H, Shen L; Chinese Society Of Clinical Oncology Csco Expert Committee On Gastrointestinal Stromal Tumor. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res. 2017;29:281-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 28. | Voss RK, Massarweh NN, Chiang YJ, Somaiah N, Feig BW, Roland CL. National Utilization of Imatinib in the Management of Resected Gastrointestinal Stromal Tumors. Ann Surg Oncol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Marqueen KE, Moshier E, Buckstein M, Ang C. Neoadjuvant therapy for gastrointestinal stromal tumors: A propensity score-weighted analysis. Int J Cancer. 2021;149:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Vassos N, Jakob J, Kähler G, Reichardt P, Marx A, Dimitrakopoulou-Strauss A, Rathmann N, Wardelmann E, Hohenberger P. Preservation of Organ Function in Locally Advanced Non-Metastatic Gastrointestinal Stromal Tumors (GIST) of the Stomach by Neoadjuvant Imatinib Therapy. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (1)] |