Published online Aug 15, 2021. doi: 10.4251/wjgo.v13.i8.929
Peer-review started: February 20, 2021
First decision: May 3, 2021
Revised: May 14, 2021
Accepted: July 9, 2021
Article in press: July 9, 2021
Published online: August 15, 2021
Processing time: 175 Days and 8.9 Hours
Diffuse reduction of spleen density (DROSD) is related to cancer prognosis; however, its role in intrahepatic cholangiocarcinoma (ICC) remains unclear.
To assess the predictive value of DROSD in the prognosis of ICC after curative resection.
In this multicenter retrospective cohort study, we enrolled patients with ICC who underwent curative hepatectomy between 2012 and 2019. Preoperative spleen density was measured using computed tomography. Overall survival (OS) and recurrence-free survival (RFS) rates were calculated and compared utilizing the Kaplan–Meier method. Univariable and multivariable Cox regression analyses were applied to identify independent factors for OS and RFS. A nomogram was created with independent risk factors to predict prognosis of patients with ICC.
One hundred and sixty-seven ICC patients were enrolled. Based on the diagnostic cut-off values (spleen density ≤ 45.5 Hounsfield units), 55 (32.9%) patients had DROSD. Kaplan–Meier analysis indicated that patients with DROSD had worse OS and RFS than those without DROSD (P < 0.05). Cox regression analysis revealed that DROSD, carcinoembryonic antigen level, carbohydrate antigen 19-9 level, length of hospital stay, lymph node metastasis, and postoperative complications were independent predictors for OS (P < 0.05). The nomogram created with these factors was able to predict the prognosis of patients with ICC with good reliability (OS C-index = 0.733). The area under the curve for OS was 0.79.
ICC patients with DROSD have worse OS and RFS. The nomogram is a simple and practical method to identify high-risk ICC patients with poor prognosis.
Core Tip: This study provides a new indicator for prognosis in intrahepatic cholangiocarcinoma (ICC) patients who have undergone hepatectomy. We believe that our study makes a significant contribution because it gives clinicians a tool to classify high-risk ICC patients who have a poor prognosis after hepatectomy. This tool is a nomogram that we developed using conventional indicators and diffuse reduction of spleen density. The use of this nomogram allows clinicians to take measures to improve the outcomes of patients who have been classified as high risk.
- Citation: Deng LM, Wang Y, Yang JH, Li JL, Chen ZY, Bao WM, Chen KY, Yao XF, Zheng CM, Zheng JY, Yu ZP, Jin B, Chen G. Diffuse reduction of spleen density is a novel prognostic marker for intrahepatic cholangiocarcinoma after curative resection. World J Gastrointest Oncol 2021; 13(8): 929-942
- URL: https://www.wjgnet.com/1948-5204/full/v13/i8/929.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i8.929
Intrahepatic cholangiocarcinoma (ICC) is a subtype of cholangiocarcinoma associated with poor prognosis and limited treatment options. The incidence of ICC in the United States currently is nearly 1.65 times higher than it was 30 years ago, and a similar trend has been observed worldwide, highlighting the need for immediate attention[1-3]. Surgery is the most effective treatment for ICC patients[4,5]. Previous studies have demonstrated that the 5-year overall survival (OS) rate in ICC patients with negative surgical margins is 39%-41%[6-9]. However, factors that affect long-term survival after surgical resection remain equivocal. Therefore, it is imperative to explore useful prognostic predictors for ICC patients to implement suitable therapies and follow-up strategies.
The spleen is crucial in regulating immune homeostasis[10], which is vital for a favorable prognostic outcome[11]. Studies have reported the occurrence of diffuse reduction of spleen density (DROSD) in patients with gastric cancer and acute pancreatitis, and speculated that DROSD might be associated with poor prognosis[12,13]. We have observed this phenomenon in some ICC patients; however, the predictive value of DROSD in the prognosis of ICC still needs exploration.
In this multicenter retrospective cohort study, we focused on the prognostic value of DROSD in ICC patients who underwent surgical resection. We also developed a useful predictive model for the prognosis of ICC based on DROSD to inform surgeons’ clinical decisions. To the best of our knowledge, this is the first study to explore the impact of DROSD on the long-term outcome of patients with ICC who underwent curative partial hepatectomy.
The study was based on data from two cohorts of ICC patients who underwent curative resection between August 2012 and October 2019. The two clinical cohorts included were: The First Affiliated Hospital of Wenzhou Medical University (Wenzhou, China) and Qilu Hospital of Shandong University (Jinan, China). This study was performed according to the Declaration of Helsinki, and ethical approval was obtained from the Institutional Ethics Committees of The First Affiliated Hospital of Wenzhou Medical University and Qilu Hospital Shandong University. The study participants’ clinicopathological information was reviewed with their written informed consent.
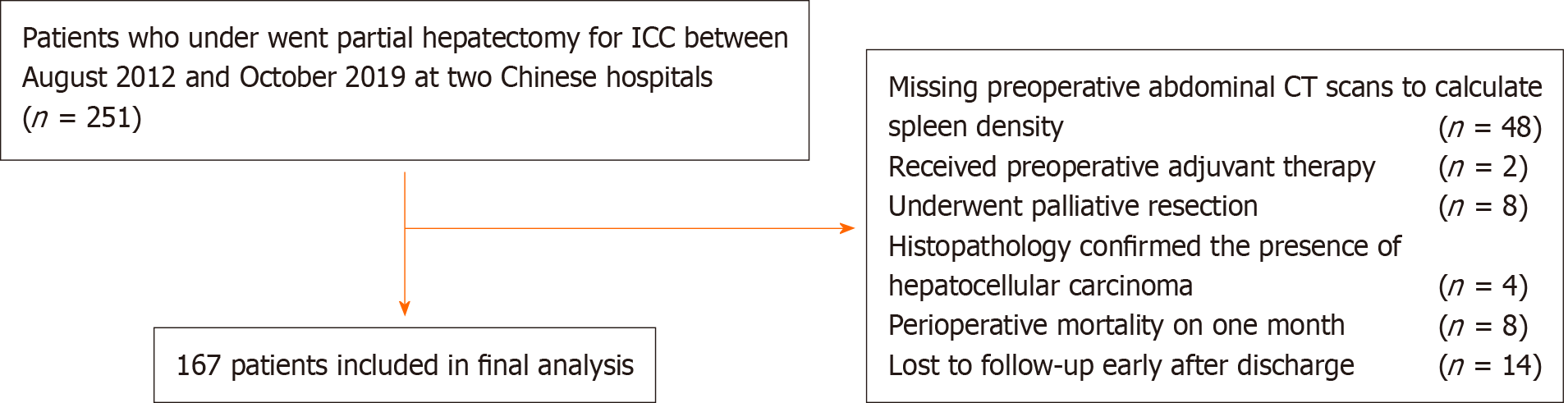
All patients enrolled were histologically confirmed to have ICC. Patient inclusion criteria were partial hepatectomy for ICC. The exclusion criteria were as follows: history of previous anticancer therapy or history of other malignancies, a history of splenic disease, presence of hematological disease, perioperative mortality, lack of preoperative abdominal computed tomography (CT) to evaluate spleen density, palliative resection, and loss to follow-up after discharge. The study flow diagram is illustrated in Figure 1.
Patients’ demographic characteristics, preoperative laboratory indicators, clinicopathological information, and operation-related variables were retrieved from the hospital database and retrospectively reviewed. Data collected included age, sex, history of abdominal surgery, length of hospital stay, comorbidities, hepatitis B surface antigen, albumin (ALB), carcinoembryonic antigen (CEA), alpha-fetoprotein, carbohydrate antigen 19-9 (CA19-9), total cholesterol, triglycerides, platelet/lymphocyte ratio (PLR), neutrophil/lymphocyte ratio (NLR), and lymphocyte/monocyte ratio (LMR). According to the Fudan score, CA19-9 cut-off values were set at 37 U/mL[14]. We also retrieved data regarding portal hypertension, tumor node metastasis (TNM) stage (8th staging system for ICC), tumor number, maximum tumor diameter, degree of tumor differentiation, lymph node metastasis, perineural invasion, vascular invasion, type of surgical procedure, extent of hepatectomy, intraoperative blood loss, postoperative complications (PCs), and operating time. Resection of three or more Couinaud liver segments was defined as major hepatectomy, while resection of fewer than three segments constituted minor hepatectomy. PCs were graded using the Clavien–Dindo classification system, which were recorded from the day of surgery until discharge[15]. Clavien–Dindo grade II or higher were considered as relevant complications.
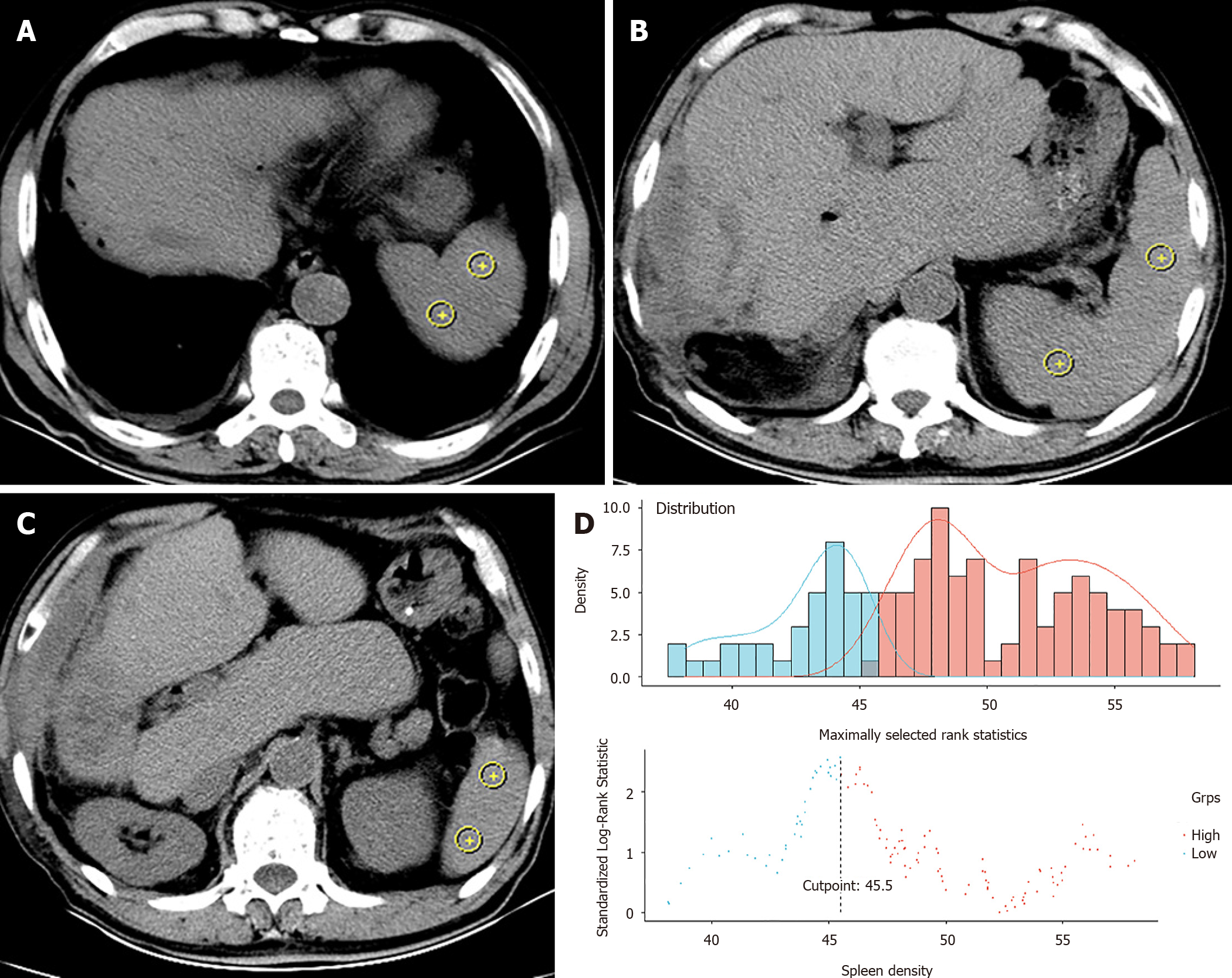
The spleen density of each patient was retrospectively reviewed and measured on cross-sectional plain CT images without contrast enhancement. The CT positioning conditions were as follows: Tube voltage 120 kV, tube rotation time 750 ms, tube current 50 mA, layer spacing 5 mm, and layer thickness 5 mm. A special processing system (version 3.0.11.3 BN1732 bit; INFINITT Healthcare Co. Ltd., Seoul, South Korea) was used to measure the CT values at the levels of the upper pole, hilum, and lower pole of the spleen (Figure 2A-C)[12,16]. On each plane, we took two points as density values for calculations. The spleen density was taken as the average value of six CT measurements. Quality control and analysis of all images were performed by two trained physicians, separately. We used the R survminer package to calculate the cut-off value of spleen density and define DROSD (Figure 2D). Based on this cut-off value, patients were then divided into DROSD and non-DROSD subgroups.
Patients were followed up once every 3 mo for the first year after surgery, once every 6 mo for the next 3 years after surgery, and once every year thereafter. OS and recurrence-free survival (RFS) were the primary endpoints. OS was calculated from the date of surgery to the date of patient death or last follow-up. RFS was defined from the date of surgery to the date of first ICC recurrence, death, or last follow-up visit. The last follow-up of the study took place on May 20, 2020.
Statistical analyses were performed using the R program (version: 3.6.1). Continuous variables are expressed as the mean ± SD or median (interquartile range). Categorical variables are expressed in terms of frequency (percentage). Missing data were imputed using multiple imputation by logistic regression. The imputation was repeated five times; the results were checked by comparing multivariable distribution of the observed and imputed data. Rubin’s rule was applied when the results were pooled. Student’s t-test was used to compare groups when a continuous variable showed normal distribution; otherwise, the Mann–Whitney U test was used. The χ2 test was applied to compare differences between independent groups of categorical variables. The optimal sex-specific cut-off value of DROSD was selected by the survminer package in R program. The Kaplan–Meier method and log-rank test were used to estimate OS and RFS among the different subgroups. Median follow-up was measured using the R survival package. Univariable and multivariable Cox regression analyses were applied to assess the prognostic factors related to OS and RFS. The R forestplot package was used to visualize the Cox regression analysis results. The multivariable analysis results were used to create a nomogram using the rms package in R software. The C-index was applied to evaluate the performance of the nomogram. The area under the receiver operating characteristic (ROC) curve (AUC) was used to evaluate prediction accuracy by using the R survival ROC package. P < 0.05 was considered statistically significant.
In total, 251 primary ICC patients who underwent partial hepatectomy were shortlisted from the multi-institutional database between August 2012 and October 2019. Of these, 167 patients who satisfied the inclusion criteria constituted the study cohort. The median follow-up time for all patients was 29.3 (95% confidence interval [CI]: 24.9-35.4) mo. The median OS was 22.3 (95%CI: 17.1-32.2) mo. The 1-, 3- and 5-year OS rates were 65.98%, 30.88% and 15.38%, respectively. The 1-, 3- and 5-year RFS rates were 51.7%, 22.06% and 12.82%, respectively. The optimal cut-off level of spleen density that stratified patients into DROSD and non-DROSD subgroups was 45.5 Hounsfield units; 55 (32.9%) patients had DROSD. The demographic and clinicopathological characteristics of patients are presented in Table 1. There were no significant differences in demographic and clinicopathological features between the DROSD and non-DROSD subgroups.
| Characteristics | All patients, n = 167 | DROSD, n = 55 | Non-DROSD, n = 112 | P value |
| Sex, n (%) | 0.062 | |||
| Female | 84 (50.3) | 22 (40.0) | 62 (55.4) | |
| Male | 83 (49.7) | 33 (60.0) | 50 (44.6) | |
| Age, years, mean ± SD | 63.35 ± 8.55 | 64.02 ± 8.64 | 63.03 ± 8.53 | 0.483 |
| BMI, kg/m2, mean ± SD | 22.61 ± 3.13 | 23.19 ± 3.27 | 22.35 ± 3.04 | 0.122 |
| Albumin, g/L, mean ± SD | 30.08 ± 5.01 | 36.4 ± 5.28 | 38.55 ± 4.86 | 0.925 |
| Hemoglobin, g/L, mean ± SD | 126.4 ± 15.36 | 121.05 ± 16.12 | 127.94 ± 14.89 | 0.446 |
| AFP, ng/mL, median (IQR) | 2.72 (2.01-3.61) | 3.09 (2.03-3.91) | 2.60 (1.99-3.53) | 0.056 |
| CEA, μg/L, median (IQR) | 2.90 (1.70-5.83) | 2.56 (2.00-7.60) | 2.95 (1.70-5.50) | 0.422 |
| CA19-9, U/mL, median (IQR) | 109.7 (21.9-852.7) | 103.1 (24.4-1000.0) | 116.7 (20.9-496.2) | 0.544 |
| PLR, median (IQR) | 141.8 (103.8-196.9) | 146.4 (99.1-236.7) | 141.8 (103.9-195.3) | 0.929 |
| NLR, median (IQR) | 2.98 (1.99-4.76) | 4.34 (1.55-6.08) | 2.78 (2.06-4.5) | 0.636 |
| LMR, median (IQR) | 2.91 (1.85-4.15) | 2.28 (1.27-3.55) | 3.14 (1.94-4.23) | 0.688 |
| Spleen density, HU, median (IQR) | 49.66 (46.19-53.92) | 43.62 (40.46-45.08) | 51.78 (48.26-54.62) | < 0.001 |
| Total cholesterol, mmol/L, mean ± SD | 4.77 ± 1.38 | 4.59 ± 1.53 | 4.85 ± 1.38 | 0.28 |
| Triglyceride, mmol/L, median (IQR) | 1.24 (0.86-1.77) | 1.19 (0.87-1.65) | 1.25 (0.85-1.77) | 0.759 |
| ASA grade, n (%) | 0.281 | |||
| 1-2 | 156 (93.4) | 53 (96.4) | 103 (92.0) | |
| 3-4 | 11 (6.6) | 2 (3.6) | 9 (8.0) | |
| Abdominal surgery history, n (%) | 0.074 | |||
| No | 122 (73.1) | 45 (81.8) | 77 (68.7) | |
| Yes | 45 (26.9) | 10 (18.2) | 35 (31.3) | |
| Hypertension, n (%) | 0.385 | |||
| No | 114 (68.3) | 40 (72.7) | 74 (66.1) | |
| Yes | 53 (31.7) | 15 (27.3) | 38 (33.9) | |
| Diabetes, n (%) | 0.096 | |||
| No | 139 (83.2) | 42 (76.4) | 97 (86.6) | |
| Yes | 28 (16.8) | 13 (23.6) | 15 (13.4) | |
| Liver Cirrhosis, n (%) | 0.847 | |||
| No | 135 (80.8) | 44 (80.0) | 91 (81.3) | |
| Yes | 32 (19.2) | 11 (20.0) | 21 (18.7) | |
| HBsAg, n (%) | 0.213 | |||
| Negative | 117 (70.1) | 42 (76.4) | 75 (67.0) | |
| Positive | 50 (29.9) | 13 (23.6) | 37 (33.0) | |
| Portal hypertension, n (%) | 0.802 | |||
| No | 156 (93.4) | 51 (92.7) | 105 (93.8) | |
| Yes | 11 (6.6) | 4 (7.3) | 7 (6.2) | |
| Surgical time, mins, median (IQR) | 150 (120-210) | 137.5 (130-200) | 150 (115-220) | 0.325 |
| Length of hospital stay in d, median (IQR) | 19 (14-25) | 17 (14-23) | 20.5 (15-26) | 0.095 |
| Intraoperative hemorrhage, mL, median (IQR) | 300 (200-600) | 300 (200-925) | 300 (200-600) | 0.304 |
| Intraoperative blood transfusion, n (%) | 0.849 | |||
| No | 120 (71.9) | 39 (70.9) | 81 (72.3) | |
| Yes | 47 (28.1) | 16 (29.1) | 31 (27.7) | |
| Extent of hepatectomy, n (%) | 0.324 | |||
| Minor | 100 (59.9) | 30 (54.5) | 70 (62.5) | |
| Major | 67 (40.1) | 25 (45.5) | 42 (37.5) | |
| Surgical procedure, n (%) | 0.834 | |||
| Laparoscopic | 20 (12.0) | 7 (12.7) | 13 (11.6) | |
| Open | 147 (80.0) | 48 (87.3) | 99 (88.4) | |
| PCs, n (%) | 0.363 | |||
| No | 117 (70.1) | 36 (65.5) | 81 (72.3) | |
| Yes | 50 (29.9) | 19 (34.5) | 31 (27.7) | |
| TNM stage, n (%) | 0.252 | |||
| I-II | 116 (69.5) | 35 (63.6) | 81 (72.3) | |
| III-IV | 51 (30.5) | 20 (36.4) | 31 (27.7) | |
| Tumor differentiation, n (%) | 0.096 | |||
| Well/Moderately | 103 (61.7) | 29 (52.7) | 74 (66.1) | |
| Poor | 61 (38.3) | 25 (47.3) | 36 (33.9) | |
| Tumor size, cm, n (%) | 0.915 | |||
| ≤ 5.0 | 86 (51.5) | 28 (50.9) | 58 (51.8) | |
| > 5.0 | 81 (48.5) | 27 (49.1) | 54 (48.2) | |
| Tumor number, n (%) | 0.103 | |||
| Single | 149(89.2) | 46 (83.6) | 103 (92.0) | |
| Multiple | 18 (10.8) | 9 (16.4) | 9 (8.0) | |
| Lymph node metastasis, n (%) | 0.959 | |||
| No | 137 (82.0) | 45 (81.8) | 92 (82.1) | |
| Yes | 30 (18.0) | 10 (18.2) | 20 (17.9) | |
| Vascular invasion, n (%) | 0.461 | |||
| No | 133 (79.6) | 42 (76.4) | 91 (81.3) | |
| Yes | 34 (20.4) | 13 (23.6) | 21 (18.7) | |
| Perineural invasion, n (%) | 0.959 | |||
| No | 137 (82.0) | 45 (81.8) | 92 (82.1) | |
| Yes | 30 (18.0) | 10 (18.2) | 20 (17.9) |
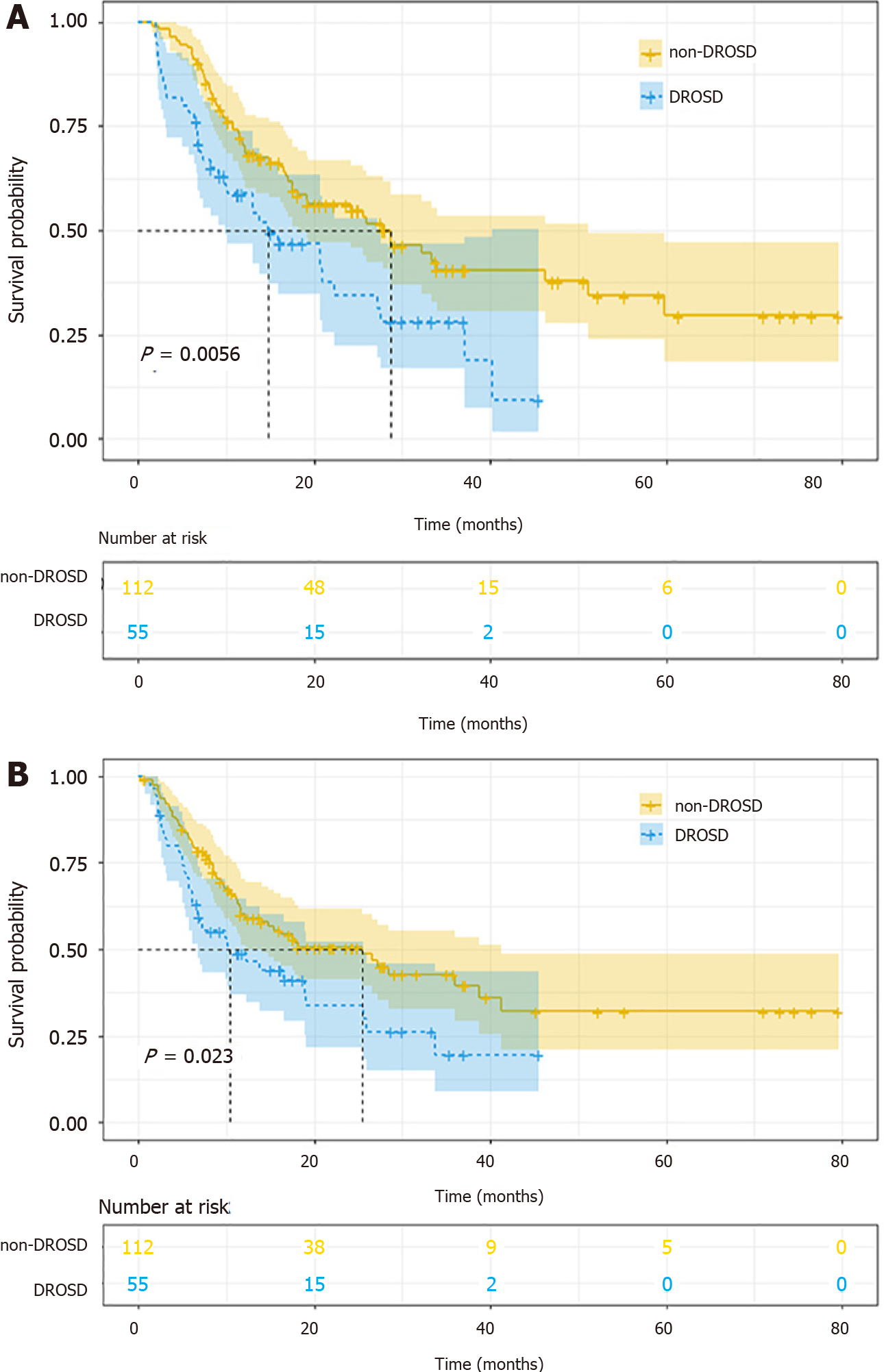
The OS rates of patients with DROSD were worse than those without DROSD. The 1- and 3-year OS rates were 69.9% and 34.69% in non-DROSD patients, and 55.56% and 21.05% in DROSD patients, respectively (P < 0.01). The median OS time in the DROSD group was lower than that in the non-DROSD group (14.8 [95%CI: 9.89-27.1] vs 28.6 [95%CI: 18.2-51.2] mo; log-rank P < 0.001) (Figure 3A). Moreover, the RFS of patients with DROSD was poorer than those without DROSD (P < 0.01). The median RFS time in the DROSD group was 10.4 (95%CI: 6.74-25.6) mo, and that in the non-DROSD group was 25.5 (95%CI: 14.0-41.3) mo (log-rank P = 0.024) (Figure 3B).
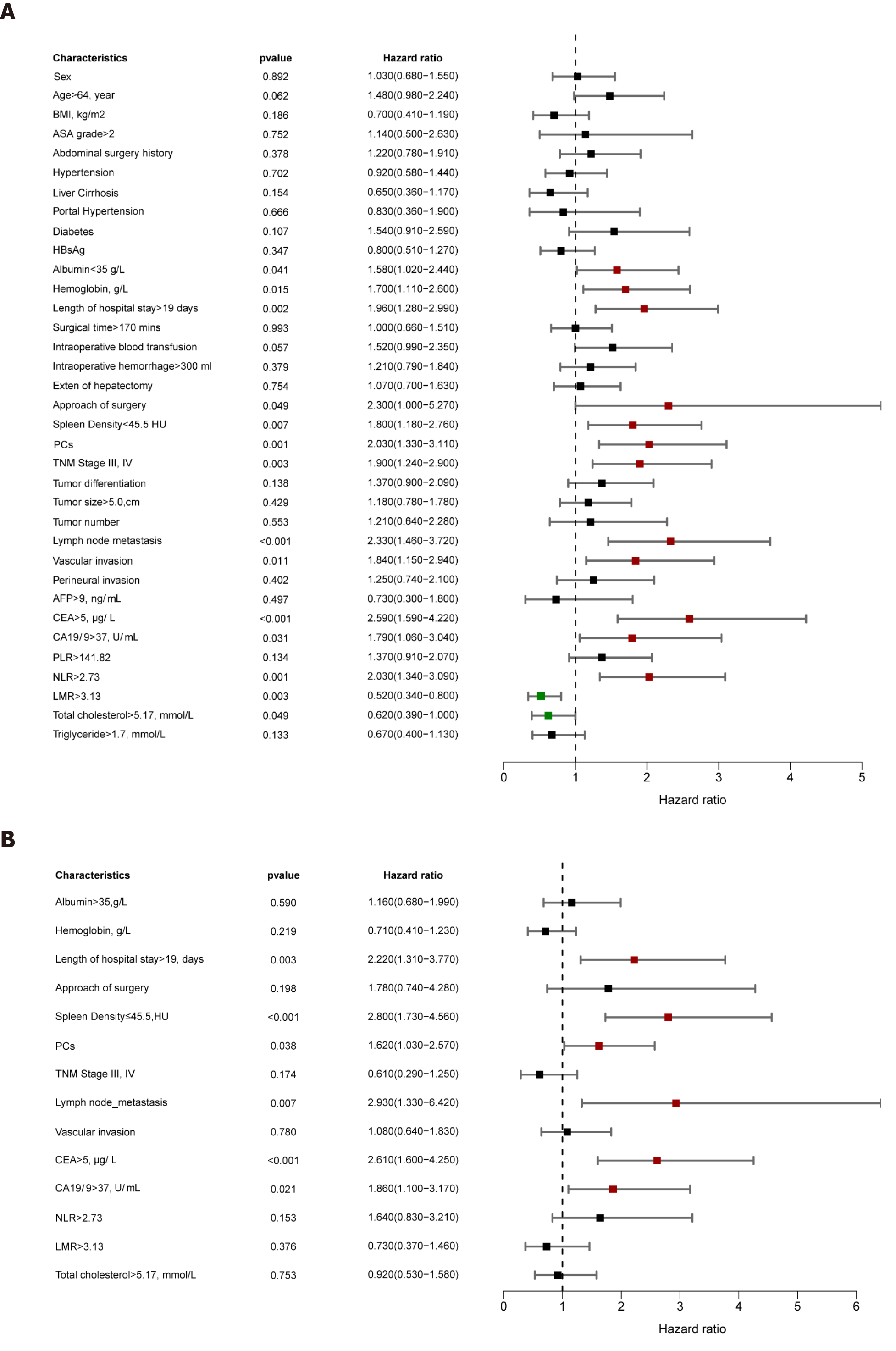
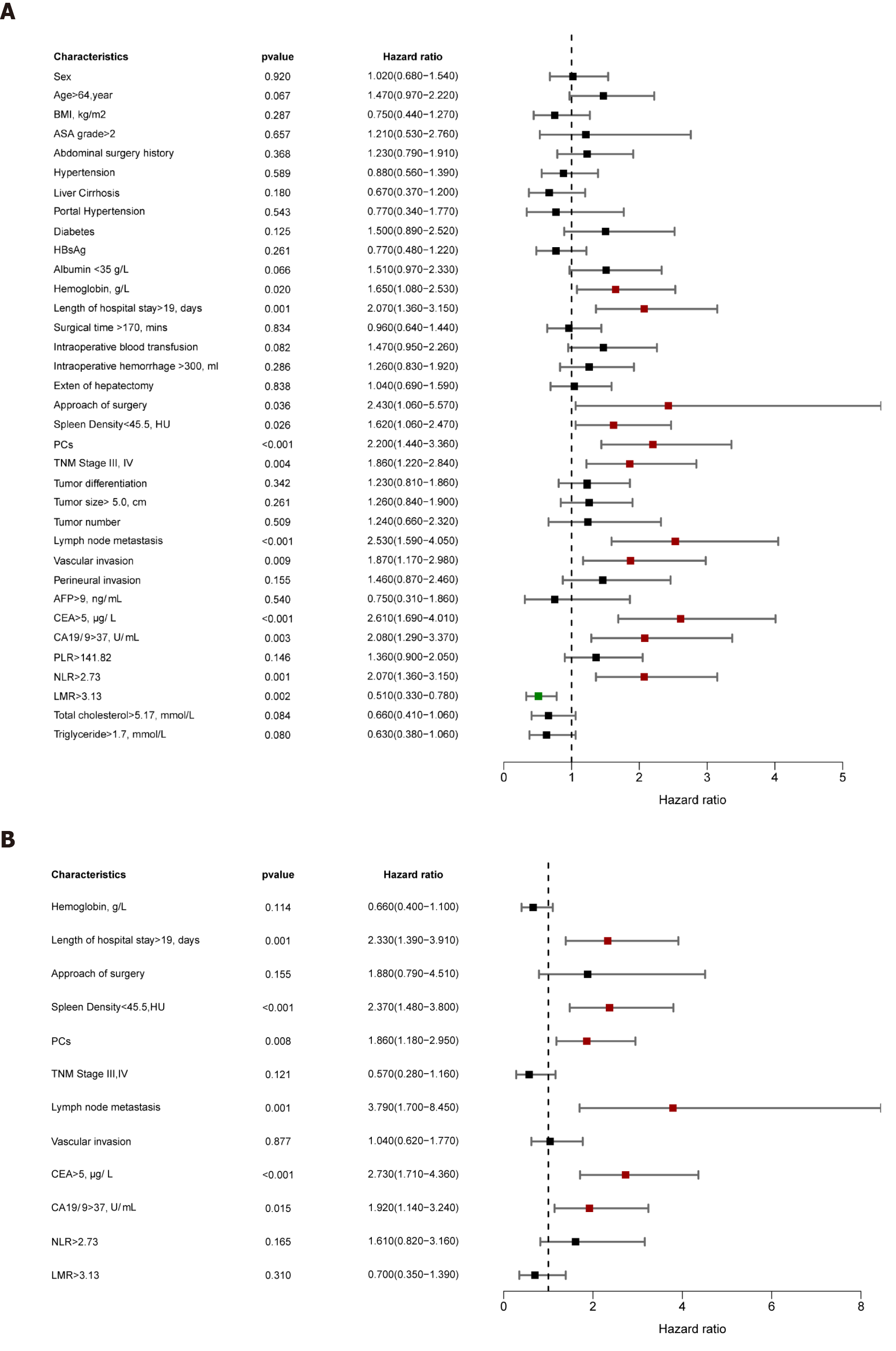
Univariable analysis demonstrated that ALB, hemoglobin, length of hospital stay, surgical procedure, DROSD, PCs, TNM stage, lymph node metastasis, vascular invasion, CEA, CA19-9, NLR, LMR, and total cholesterol were prognostic predictors for OS (P < 0.05) (Figure 4A). Multivariable analysis indicated that DROSD (hazard ratio [HR]: 2.80; 95%CI: 1.73-4.56; P < 0.001), CEA (HR: 2.61; 95%CI: 1.60-4.25; P < 0.001), CA19-9 (HR: 1.86; 95%CI: 1.1-3.17; P = 0.021), length of hospital stay (HR: 2.22; 95%CI: 1.31-3.77; P = 0.003), lymph node metastasis (HR: 2.93; 95%CI: 1.33-6.42; P = 0.007), and PCs (HR: 1.62; 95%CI: 1.03–2.57; P = 0.038) were independent prognostic factors for OS (Figure 4B).
The univariable analysis for RFS revealed that hemoglobin, length of hospital stay, surgical procedure, DROSD, PCs, TNM stage, lymph node metastasis, vascular invasion, CEA, CA19-9, NLR, and LMR were prognostic factors for RFS (P < 0.05) (Figure 5A). Multivariable analysis revealed that DROSD (HR: 2.37; 95%CI: 1.48–3.80; P < 0.001), CEA (HR: 2.73; 95%CI: 1.71–4.36; P < 0.001), CA19-9 (HR: 1.92; 95%CI; 1.14–3.24; P = 0.015), length of hospital stay (HR: 2.33; 95%CI: 1.39–3.91; P = 0.001), lymph node metastasis (HR: 3.79; 95%CI: 1.70–8.45; P = 0.001), and PCs (HR: 1.86; 95%CI: 1.18–2.95; P = 0.008) were independent prognostic factors for RFS (Figure 5B).
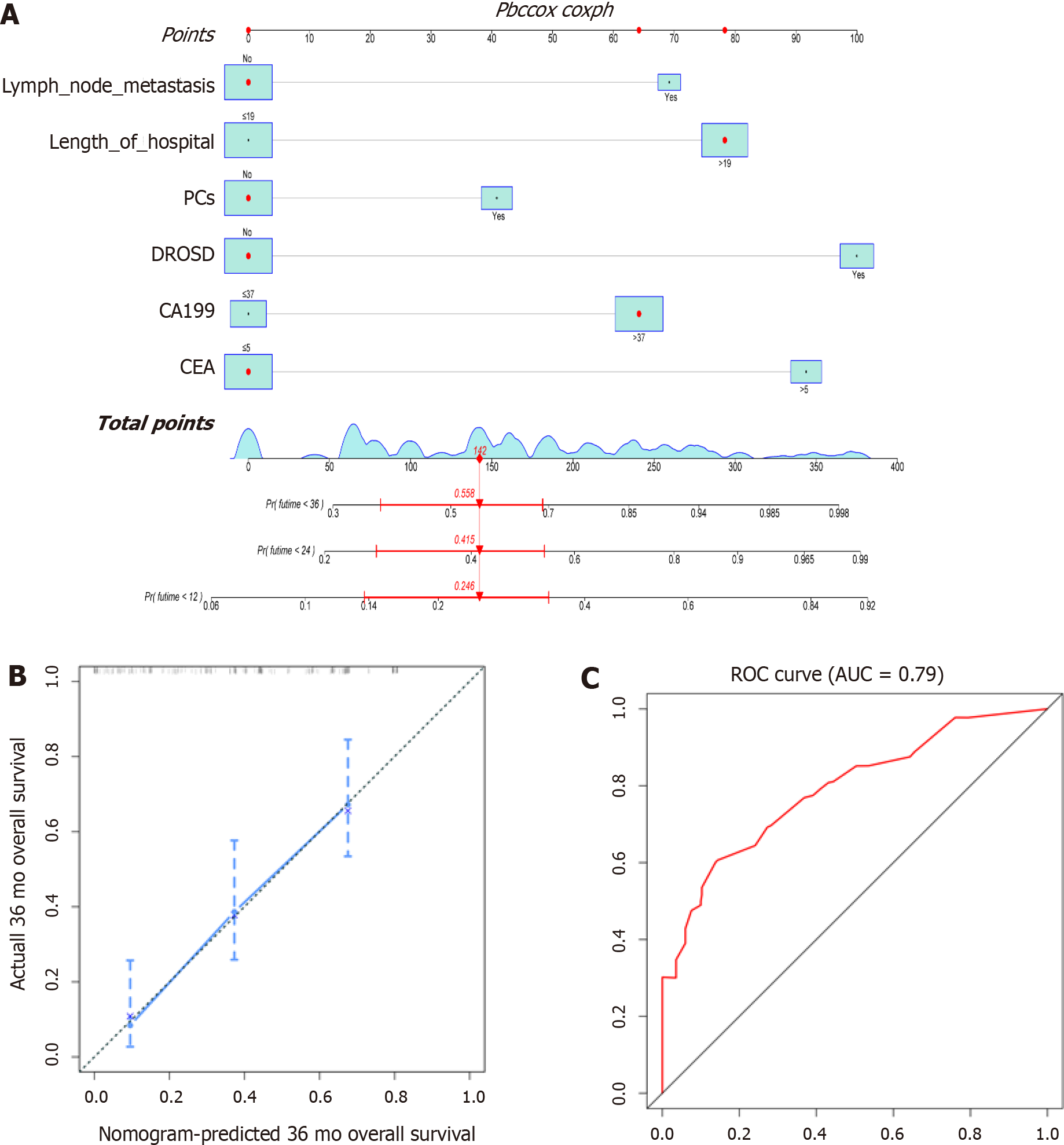
We constructed a prognostic nomogram based on the independent factors for OS identified by multivariable Cox regression analysis. The nomogram demonstrated that DROSD and CEA were the main weighting factors in the scoring system (Figure 6A). The C-index for OS prediction was 0.733 (95%CI: 0.68–0.79). The calibration chart for 3-year postoperative survival rates demonstrated satisfying coherence between nomogram predictions and actual observations (Figure 6B). The AUC of OS was 0.79 (Figure 6C).
ICC is a highly aggressive malignancy with a poor clinical prognosis. Radical hepa
Spleen density is a novel indicator of patient prognosis. It has been reported that DROSD is an excellent prognostic predictor in patients with gastric cancer and pancreatitis[12,13]. In our study, the incidence of DROSD was 32.9% in patients with ICC. For OS and RFS of patients with ICC, DROSD was an independent prognostic factor. Further studied in other patients cohorts are required to validate these findings.
The mechanism for DROSD in patients with ICC is unclear. One study revealed that the decrease of spleen density is related to lipid metabolism[17]; however, a previous study demonstrated that lipid deposition is not the cause of DROSD[13]. In our study, we found no difference in total cholesterol or triglyceride levels between DROSD and non-DROSD subgroups. Other studies have demonstrated that hemoperfusion of the spleen could impact its physical density, which is reflected as its density on CT[13,18]. The indicators of increased splenic blood perfusion include hypertension, portal hypertension, and increased hemoglobin, etc. However, in our study, no differences were found between the above-mentioned indicators in the DROSD group and the non-DROSD group, especially portal hypertension, there were 11 patients with portal hypertension, 4 patients were in the DROSD group, and 7 patients in the non-DROSD group, our data did not reflect that the spleen density of patients with external hypertension was higher. The reason may be due to less data of patients with portal hypertension, so it was not statistically significant. However, whether portal hyper
Several studies have shown that a nomogram has better predictive accuracy for survival than conventional staging systems[23-25]. Other studies have reported that CA19-9 and CEA are independent factors affecting ICC prognosis[26,27]. Our nomo
Our study had several limitations. First, as a retrospective study, selection bias was inevitable. Second, DROSD was observed on CT with no corresponding disease of the spleen. Since we were unable to explain the pathogenesis of DROSD, more research is needed to confirm the cause of this phenomenon. Third, due to the small number of patients with portal hypertension, no correlation between spleen density and portal hypertension was found in this study, which requires further study. Finally, the nomogram was created based on data collected from two institutions, and the accuracy of the model needs to be verified in more hospital settings.
DROSD is a novel prognostic marker for OS in ICC patients who underwent curative resection. The nomogram is a practical predictor and can accurately predict the prognosis of ICC patients who underwent curative resection.
Intrahepatic cholangiocarcinoma (ICC) is a malignant tumor with poor prognosis and limited treatment options. Radical surgery is the only effective method, so it is necessary to explore prognostic predictors for patients with ICC after radical surgery.
The diffuse reduction of spleen density (DROSD) is related to the prognosis of cancers; however, its role in ICC remains unclear.
This study assessed the predictive value of DROSD on the prognosis of ICC patients after curative resection.
Patients with ICC who underwent curative hepatectomy from 2012 to 2019 were enrolled. Preoperative spleen density was measured using computed tomography scans. Overall survival (OS) and recurrence-free survival (RFS) rates were calculated and compared utilizing the Kaplan–Meier method. Univariable and multivariable Cox regression analyses were applied to identify independent factors for OS and RFS. A nomogram was created with independent risk factors to predict prognosis of patients with ICC.
A total of 167 ICC patients were enrolled, and 55 (32.9%) had DROSD. Kaplan–Meier analysis indicated that patients with DROSD had worse OS and RFS than those without DROSD. Cox regression analysis revealed that DROSD, carcinoembryonic antigen level, carbohydrate antigen 19-9 level, length of hospital stay, lymph node metastasis, and postoperative complications were independent predictors for OS. The nomogram created with these factors proved to be able to predict the prognosis of ICC patients with good reliability (OS C-index = 0.733).
ICC patients with DROSD have worse OS and RFS. The nomogram is a simple and practical method to identify high-risk ICC patients with poor prognosis.
Our study found that DROSD is a novel predictor of prognosis in ICC patients after curative resection. It could best stratify patients with ICC to the appropriate screening.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mocan T S-Editor: Fan JR L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Dodson RM, Weiss MJ, Cosgrove D, Herman JM, Kamel I, Anders R, Geschwind JF, Pawlik TM. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg. 2013;217:736-750.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 543] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 3. | Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168-2179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1072] [Cited by in RCA: 1379] [Article Influence: 125.4] [Reference Citation Analysis (1)] |
| 4. | Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 582] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 5. | Buettner S, van Vugt JL, IJzermans JN, Groot Koerkamp B. Intrahepatic cholangiocarcinoma: current perspectives. Onco Targets Ther. 2017;10:1131-1142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 6. | Choi SB, Kim KS, Choi JY, Park SW, Choi JS, Lee WJ, Chung JB. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009;16:3048-3056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 236] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 7. | de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L, Ferrone CR, Zhu AX, Bauer TW, Walters DM, Gamblin TC, Nguyen KT, Turley R, Popescu I, Hubert C, Meyer S, Schulick RD, Choti MA, Gigot JF, Mentha G, Pawlik TM. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140-3145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 559] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 8. | Poultsides GA, Zhu AX, Choti MA, Pawlik TM. Intrahepatic cholangiocarcinoma. Surg Clin North Am. 2010;90:817-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Mosconi S, Beretta GD, Labianca R, Zampino MG, Gatta G, Heinemann V. Cholangiocarcinoma. Crit Rev Oncol Hematol. 2009;69:259-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 10. | Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet. 2011;378:86-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 438] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 11. | Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4947] [Cited by in RCA: 4541] [Article Influence: 324.4] [Reference Citation Analysis (0)] |
| 12. | Huang YS, Chen XD, Shi MM, Xu LB, Wang SJ, Chen WS, Zhu GB, Zhang WT, Shen X. Diffuse Reduction of Spleen Density Is an Independent Predictor of Post-Operative Outcomes After Curative Gastrectomy in Gastric Cancer: A Multi-Center Study. Front Oncol. 2020;10:1050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Shao G, Zhou Y, Song Z, Jiang M, Wang X, Jin X, Sun B, Bai X. The diffuse reduction in spleen density: an indicator of severe acute pancreatitis? Biosci Rep. 2017;37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Jiang W, Zeng ZC, Tang ZY, Fan J, Sun HC, Zhou J, Zeng MS, Zhang BH, Ji Y, Chen YX. A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: the Fudan score. Ann Oncol. 2011;22:1644-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6210] [Cited by in RCA: 8618] [Article Influence: 538.6] [Reference Citation Analysis (0)] |
| 16. | Dong J, He F, Wang L, Yue Z, Wen T, Wang R, Liu F. Iodine density Changes in Hepatic and Splenic Parenchyma in Liver Cirrhosis with Dual Energy CT (DECT): A Preliminary Study. Acad Radiol. 2019;26:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Jiang XY, Bian J, Zhang CZ, Wang SS, Nie TM, Zhang L. Transient reduction of spleen density in acute pancreatitis: case reports and literature review. J Comput Assist Tomogr. 2014;38:568-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Okuma H, Gonoi W, Ishida M, Shirota G, Kanno S, Shintani Y, Abe H, Fukayama M, Ohtomo K. Comparison of volume and attenuation of the spleen between postmortem and antemortem computed tomography. Int J Legal Med. 2016;130:1081-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Li H, Wang JJ, Zhang M, Ren B, Li JX, Xu L, Wu H. Prognostic significance of systemic immune-inflammation index in patients with intrahepatic cholangiocarcinoma undergoing hepatic resection. World J Gastrointest Oncol. 2020;12:467-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Tsilimigras DI, Moris D, Mehta R, Paredes AZ, Sahara K, Guglielmi A, Aldrighetti L, Weiss M, Bauer TW, Alexandrescu S, Poultsides GA, Maithel SK, Marques HP, Martel G, Pulitano C, Shen F, Soubrane O, Koerkamp BG, Endo I, Pawlik TM. The systemic immune-inflammation index predicts prognosis in intrahepatic cholangiocarcinoma: an international multi-institutional analysis. HPB (Oxford). 2020;22:1667-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Sakimura C, Tanaka H, Okuno T, Hiramatsu S, Muguruma K, Hirakawa K, Wanibuchi H, Ohira M. B cells in tertiary lymphoid structures are associated with favorable prognosis in gastric cancer. J Surg Res. 2017;215:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Dolan RD, Lim J, McSorley ST, Horgan PG, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: Systematic review and meta-analysis. Sci Rep. 2017;7:16717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 240] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 23. | Liang W, Zhang L, Jiang G, Wang Q, Liu L, Liu D, Wang Z, Zhu Z, Deng Q, Xiong X, Shao W, Shi X, He J. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol. 2015;33:861-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 467] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, Wan X, Liu G, Wu D, Shi L, Lau W, Wu M, Shen F. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 833] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 25. | Yang P, Qiu J, Li J, Wu D, Wan X, Lau WY, Yuan Y, Shen F. Nomograms for Pre- and Postoperative Prediction of Long-term Survival for Patients Who Underwent Hepatectomy for Multiple Hepatocellular Carcinomas. Ann Surg. 2016;263:778-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Hatzaras I, Schmidt C, Muscarella P, Melvin WS, Ellison EC, Bloomston M. Elevated CA 19-9 portends poor prognosis in patients undergoing resection of biliary malignancies. HPB (Oxford). 2010;12:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Cho SY, Park SJ, Kim SH, Han SS, Kim YK, Lee KW, Lee SA, Hong EK, Lee WJ, Woo SM. Survival analysis of intrahepatic cholangiocarcinoma after resection. Ann Surg Oncol. 2010;17:1823-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |